Abstract
Near-infrared spectroscopy has revealed considerable heterogeneity of delivery-to-uptake () as identified by disparate deoxygenation (deoxy[Hb+Mb]) values in the exercising quadriceps. However, whether this represents a recruitment phenomenon or contrasting vascular-metabolic control, as seen among fiber types, has not been established. We utilized knee extension (KE) and cycling (CE) incremental exercise paradigms to examine whether differential muscle activation profiles could account for the heterogeneity of deoxy[Hb+Mb] and microvascular hemoconcentration (i.e., total[Hb+Mb]). Using time-resolved near-infrared spectroscopy for the quadriceps femoris (vastus lateralis [VL] and rectus femoris [RF]) during exhaustive ramp exercise in eight participants, we tested the hypotheses that: 1) the deoxy[Hb+Mb] (i.e., fractional extraction) would relate to muscle activation levels across exercise paradigms, and 2) KE would induce greater total[Hb+Mb] (i.e., diffusive potential) at task failure (i.e., ) than CE irrespective of muscle site. At a given level of muscle activation, as assessed by relative integrated electromyography normalized to maximal voluntary contraction (%iEMGmax), the VL deoxy[Hb+Mb] profile was not different between exercise paradigms. However, at and until 20 %iEMGmax for CE, RF exhibited a lower deoxy[Hb+Mb] (83.2±15.5 vs. 98.2±19.4 μM) for KE than CE (P<0.05). The total[Hb+Mb] at was not different between exercise paradigms for either muscle site. These data support that the contrasting patterns of convective and diffusive transport correspond to different muscle activation patterns in VL but not RF. Thus, the differential deoxygenation profiles for RF across exercise paradigms may be dependent upon specific facets of muscle architecture and functional hemodynamic events.
Keywords: Near-infrared spectroscopy, Muscle deoxygenation, Knee extension exercise, Quadriceps femoris
INTRODUCTION
Muscle metabolic rate () rises progressively with work rate during an incremental or ramp exercise test with fractional extraction increasing hyperbolically as effluent venous content decreases (Richardson et al., 1993; Whipp & Ward, 1982; reviewed by Poole et al., 2011). As muscles may regulate their delivery ratio as a function of fiber type (Behnke et al., 2003; McDonough et al., 2005), the muscle(s) effluent venous concentration may conceal considerable heterogeneity (Spencer et al., 2014).
The two investigations in humans that have addressed this question reached diametrically opposed conclusions. Specifically, Vogiatzis et al. (2015) used near-infrared spectroscopy (NIRS) to relate multi-site blood flow and deoxygenation () profiles in the superficial vastus lateralis during constant-load cycle ergometry and concluded that was well matched to across sites. In contrast, Richardson et al. (2001) combined spatially-defined magnetic resonance spectroscopy (change of phosphocreatine, ΔPCr and thus ) with arterial spin labelling () and found evidence for substantial heterogeneity within and across calf muscles during plantar flexion exercise. Moreover, the latter observation was also reported in several studies using magnetic resonance imaging and positron emission tomography (Kalliokoski et al., 2005; Heinonen et al., 2010; Laaksonen et al., 2010). It is pertinent that neither study investigated the relationship between the muscle deoxygenation and electromyography (EMG) profiles, either within or across muscles. Thus, the presence and extent of heterogeneity across human muscles and whether it relates specifically to different muscle activation patterns is unknown.
The present investigation selected two distinct exercise paradigms to evoke contrasting profiles of muscle activation (Richardson et al., 1998; Krustrup et al., 2009; Chin et al., 2011; Cannon et al., 2013) and blood flow (Knight et al., 1992; Richardson et al., 1993). Namely, large muscle mass cycling exercise (CE) where and muscle mass specific perfusive conductance () are constrained by the cardiac output ceiling (Calbet et al., 2004; Mortensen et al., 2005) and small muscle mass knee extensor exercise (KE) where they are not (Andersen et al., 1985; Richardson et al., 1993; Richardson et al., 1995; Richardson et al., 1999). In addition to differential perfusive conductances, these two exercise paradigms also may exhibit contrasting patterns of diffusive transport (Richardson et al., 1999). Diffusive transport is facilitated by hemoglobin-dependent (i.e., capillary hematocrit, red blood cell [RBC] spacing and transit time, and off-loading kinetics) and independent components (i.e., perfused muscle capillary beds and longitudinal capillary recruitment) (Federspiel & Popel, 1986; Groebe & Thews, 1990; Kurdak et al., 1995; Poole et al., 2013; Hirai et al., 2018). As capillary hemoglobin concentration (i.e., capillary hematocrit) increases as a function of red blood cell flux (Damon & Duling, 1987; Kindig et al., 2002; Poole et al., 2011; Poole et al., 2013), it is expected that KE will induce greater diffusive conductance in quadriceps femoris muscles than CE.
Morphometric analysis of skeletal muscle showed that 85% of intramuscular blood volume was placed within the capillary bed (Poole & Mathieu-Costello, 1992; Mathieu-Costello, 1994). Near-infrared spectroscopy (NIRS) is generally considered to only measure small blood vessels (e.g., Barstow, 2019). Especially, time-resolved NIRS (TR-NIRS) was used to noninvasively investigate matching (via deoxy[Hb+Mb] and tissue saturation, St) and microvascular hematocrit (via total[Hb+Mb]) within specific muscle regions (Federspiel & Popel, 1986; Groebe & Thews, 1990; Koga et al., 2012; Okushima et al., 2016; Koga et al., 2017). Crucially, TR-NIRS measures in vivo optical path length distribution facilitating resolution of absolute [Hb+Mb]. As [Mb] is not expected to change in the sampled region, any alteration of the [Hb+Mb] signal reflects that of [Hb]. Thus, in the present investigation, we measured muscle deoxygenation and hemoglobin profiles in the quadriceps femoris (vastus lateralis [VL] and rectus femoris [RF] muscle) during ramp incremental KE and CE using multi-channel TR-NIRS for investigating the presence and extent of function influencing heterogeneity across human muscles. We tested the hypothesis that 1) the deoxy[Hb+Mb] (i.e., fractional O2 extraction) profile would be determined by the extent of muscle activation, as quantified by integrated electromyography, across exercise paradigms, and 2) KE would induce greater total[Hb+Mb] at task failure compared to CE, irrespective of muscle site.
METHODS
Ethical approval
This study was approved by the human subjects committee at Kobe Design University, Japan (approval number, 2015–2). All participants signed an informed consent form after explanation of experimental procedures and the potential risks and benefits of study participation. Participants provided written informed consent in accordance with the latest revision of the Declaration of Helsinki, except for registration in a database.
Experimental Design
Eight young males (age, 21 ± 3 yrs; height, 175 ± 3 cm; and weight, 65 ± 7 kg; mean ± standard deviation, SD) participated in this study. All participants were nonsmokers and free of known cardiovascular, respiratory, and metabolic disease. Participants were instructed to maintain their normal diet, continue normal daily activities, and to refrain from any strained exercise on the day before testing until the completion of the study. Cycling exercise (CE) tests were performed in the upright position on an electronically braked cycle ergometer (75XL-III, Combi, Tokyo, Japan). In cycling exercise, the range of knee extension and flexion angle was established between ≈90–170°. On a separate day, upright knee extension exercise (KE) tests were performed on a double leg knee extension/flexion ergometer. This ergometer consists of an adjustable chair mounted behind an electrically braked cycle ergometer, with the leg-locking arm connected by metal bars to the cranks of the cycle ergometer (Koga et al., 2005; Koga et al., 2019). Exercise was performed through 40° of knee extension and flexion (knee angle: 90–130°) in an alternating kicking pattern (i.e., while one leg was extended, the other was flexed). The ergometer also had an adjustable backrest that allowed the participants to sit with a hip angle up to 100°. Participants were familiarized with the ergometer prior to performing KE exercise testing procedures. Participants started the experimental procedure at least 2 h postprandial and refrained from caffeine, alcohol, and intense exercise for 24 h before testing. All experiments were conducted in environmental chambers (FLC-2700S; Fuji Medical Science, Chiba, Japan) maintained at an ambient temperature of 22°C and relative humidity of 50 %.
The exercise protocol began with 2 min of rest, followed by 4 min of 20 W baseline exercise and a ramp-incremental exercise test. The ramp increase was 20 W min−1 for the CE test and 10 W min−1 for the KE test. The same rate of contraction between CE and KE was achieved by asking participants to maintain a pedaling frequency of 60 rpm for CE and kicking frequency at 120 contractions min−1 (i.e., being equivalent to 60 contractions leg−1 min−1) for KE throughout the exercise test. The test was terminated when participants could no longer maintain the specified revolution rate despite strong verbal encouragement, concomitant with a leveling off in the breath-by-breath gas exchange data.
Measurements
Pulmonary .
Gas exchange was measured using the same methods as in previous studies (Okushima et al., 2016; Koga et al., 2017). The breath-by-breath gas exchange system (AE-300S, Minato-Medical, Osaka, Japan) was calibrated according to the manufacturer’s recommendation before each exercise test. Participants breathed through a low resistance mouthpiece containing a hot-wire flowmeter for measurement of inspiratory and expiratory flows and volumes. Inspired and expired gases were continuously sampled from the mouth and and CO2 fractional concentration was measured by fast-responding paramagnetic and infrared analyzers, respectively. Gas volume and concentration signals were time-aligned to account for the time lag between the signals to calculate on a breath-by-breath basis. Alveolar gas exchange variables were calculated according to the algorithms of Beaver et al. (1981).
Near-infrared spectroscopy.
Absolute values of oxygenated (oxy[Hb+Mb]), deoxygenated (deoxy[Hb+Mb]), and total hemoglobin and myoglobin concentration (total[Hb+Mb]) were sampled from the distal side of the vastus lateralis (VL) and rectus femoris (RF) on the dominant leg by a TR-NIRS instrument (TRS-20, Hamamatsu photonics KK, Hamamatsu, Japan). Measurement algorithms are the same as previous studies (Hamaoka et al., 2000; Ijichi et al., 2005; Chin et al., 2011). Before applying the probes, the skin under the probes was cleaned and shaved. The NIRS optodes were housed in black rubber holders and fixed to the skin with adhesive tape to minimize incidental movement and intrusion of ambient light on the NIRS detectors. The interoptode spacing between irradiation and detection probes was 3 cm for all measured sites. The sample rate was set to 1 Hz. StO2 was calculated as oxy[Hb+Mb]/total[Hb+Mb]*100.
Ultrasonographic imaging.
The adipose tissue thickness (ATT) over the muscles of interest were measured at rest with the participant in an upright-seated position using B-mode ultrasound (Logiq 400; GE-Yokogawa Medical Systems, Tokyo, Japan) before the first ramp exercise test. ATT was measured at the same sites where the TR-NIRS optodes were placed on the VL and RF muscles. Ultrasound images were collected with care to prevent pressure and distortion of the skin and adipose tissue thickness under the probe. To quantify the influence of ATT on dynamic changes in the TR-NIRS signals, we used the ATT correction method of our previous studies (Okushima et al., 2015; Okushima et al., 2016). This normalization process allowed absolute values of both deoxy- and total[Hb+Mb] to be compared among participants and muscle sites differing in ATT.
Surface electromyography.
Two separate bipolar electromyography (EMG) sensors were connected to a multi-channel data acquisition system (MP100; Biopac systems, California, USA) through the EMG amplifier (Polyam 4; NIHON SANTEKU Co., Osaka, Japan), with a sampling frequency of 1 kHz. Electrodes (Bluesensor T-00-S; Ambu, Ballerup, Denmark) were positioned just proximal to the NIRS optodes assembly on the VL and RF, and all sites were prepared by shaving, abrading, and cleaning the skin with alcohol and preconditioning agent (Skin pure; NIHON KODEN, Tokyo, Japan). Before data collection, EMG signals were tested for movement artifact and the EMG sensor body was then secured to the leg with surgical tape to help minimize any movement during exercise.
On a separate day, participants performed three repetitions of maximal voluntary contractions (MVC) while seated upright in a chair with 90° and 100° of knee and hip joint angle, respectively. The MVCs were performed to induce a maximal activation of the knee-extensor muscle for assessing maximal EMG activity associated with this maximal recruitment, which was then used to normalize the EMG response during ramp incremental cycling. Previously, Alkner et al. (2000) reported that isometric single-joint knee extension demonstrates a close-to-linear relationship between force and EMG signals. Moreover, we have investigated the variability/reproducibility of the EMG signals during an MVC by collecting additional data on 12 subjects across separate days (unpublished findings). We found a high value of intraclass correlation coefficient of 0.962. The MVC was measured during a 7-s maximal contraction, with participants extending their dominant leg against an immovable bar. Participants rested for at least 3 min before repeating the MVC test.
Data analysis
Pulmonary was determined as the mean value of the last 30 s during the ramp exercise test. Maximal power output was determined as the power output attained when participants reached exhaustion. Analysis of deoxy- and total[Hb+Mb] was performed after correction for ATT to a thickness of 0 mm using linear regression of the relationship between total[Hb+Mb] and ATT for all participants at rest (Bowen et al., 2013; Okushima et al., 2015). For NIRS measurements (deoxy- and total[Hb+Mb] and StO2), 1 Hz optical measurements were averaged over 6 data points, resulting in one NIRS datum calculation every 6 s. The baseline of each NIRS measurement was calculated as the mean value of 60 s prior to the start of ramp exercise. The absolute value of each NIRS measurement during ramp exercise was calculated every 20 W from baseline to the maximal power output achieved by each participant (KE, up to 80 W; CE up to 220 W). Submaximal NIRS variables were calculated by averaging three NIRS datum (i.e., 18-s interval values) centered with the time at which that specific exercise intensity was attained. Maximal NIRS variables were calculated as the mean of the last 18 s during ramp exercise. Raw EMG signals were band-pass-filtered (8–500 Hz) and rectified (Labchart pro ver. 8.1.6, ADinstruments, Sydney, Australia). The integrated EMG (iEMG) signals were averaged into 6-s bins (i.e., every 2 W for CE and 1 W for KE respectively) and normalized to the highest iEMG from the MVC trial (the highest 1-s iEMG value during the highest iEMG trial of the three efforts), and expressed as % iEMGmax. Each NIRS measurement was also averaged by 5 % iEMGmax from the mean interpolated iEMG to peak exercise.
Statistical analysis
All values were expressed as mean ± SD. Comparisons of , and maximal power output were analyzed by paired t-test. Comparisons of temporal profiles for pulmonary , muscle EMG and all NIRS measurements were analyzed by two-way repeated-measures ANOVA, with main effects of exercise paradigm (KE and CE) and intensity (absolute power output: every 20 W from 20 W baseline to maximal power output). All NIRS measurements across normalized muscle activation were also analyzed by two-way repeated-measures ANOVA, with main effects of exercise paradigm (KE and CE) and muscle activations (VL: 5 to 25 % iEMGmax, RF: 5 to 20 % iEMGmax). With regards to the two-way repeated-measures ANOVA, a significant F ratio was analyzed using Bonferroni’s post-hoc test. For indices of effect size, Cohen’s d (for post-hoc tests) were also calculated. The relationships of deoxy[Hb+Mb] and StO2 during KE to those during CE at 20 % iEMGmax were evaluated by calculating the average bias and the 95 % limits of agreement using Bland-Altman analysis. Significance was accepted at P < 0.05. A statistical software (GraphPad Prism ver. 7.02, GraphPad Software, San Diego, USA) was used for each test.
RESULTS
Two-legged KE exhibited a considerably lower maximal power output (112 ± 20 vs. 281 ± 37 W, P < 0.001) and (1784 ± 513 vs. 3041 ± 434 ml·min−1, P < 0.001, Fig. 1) compared with CE. Two-legged KE had greater submaximal compared with CE from 50 to 80 W (all P < 0.01) (Fig. 1). Mean ATTs overlying VL and RF muscles were 4.1 ± 1.0 and 5.6 ± 1.5 mm, respectively. There was a significant correlation between ATT and total[Hb+Mb] as described by: f(x) = −17.0x + 203 (R2 = 0.754, P < 0.001). After the ATT correction, the total[Hb+Mb] concentrations in VL and RF muscles at rest were 204.2 ± 13.4 and 201.1 ± 7.9 μM in CE and 201.2 ± 11.5 and 203.5 ± 11.9 μM in KE, respectively.
Figure 1.
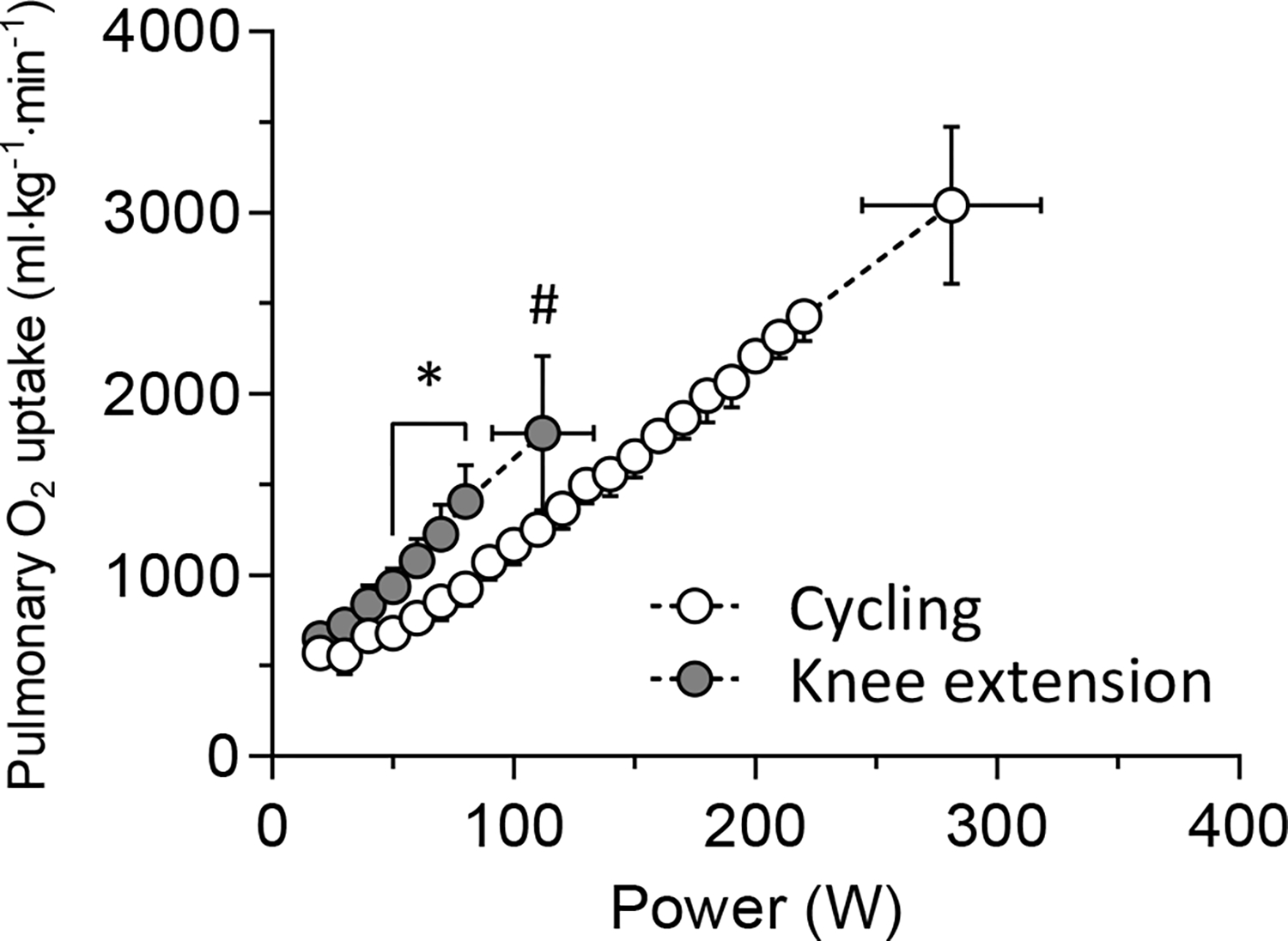
Mean pulmonary uptake across power output during each exercise paradigm. * showed the significant differences between exercise modes at the same absolute power output (P < 0.05). # showed the significant difference between exercise modes at maximal power output (P < 0.05).
At the same absolute submaximal power output, iEMG in VL was not significantly different between the two exercise paradigms (Fig. 2). However, peak iEMG in VL was greater for CE than KE in concert with the far higher work rate achieved in CE versus KE (P < 0.001, Cohen’s d = 1.01). In contrast, in the RF muscle, the iEMG was greater at 60–80 W and task failure during KE than CE (all P < 0.01, Cohen’s d = 1.25–3.54) (Fig. 2).
Figure 2.
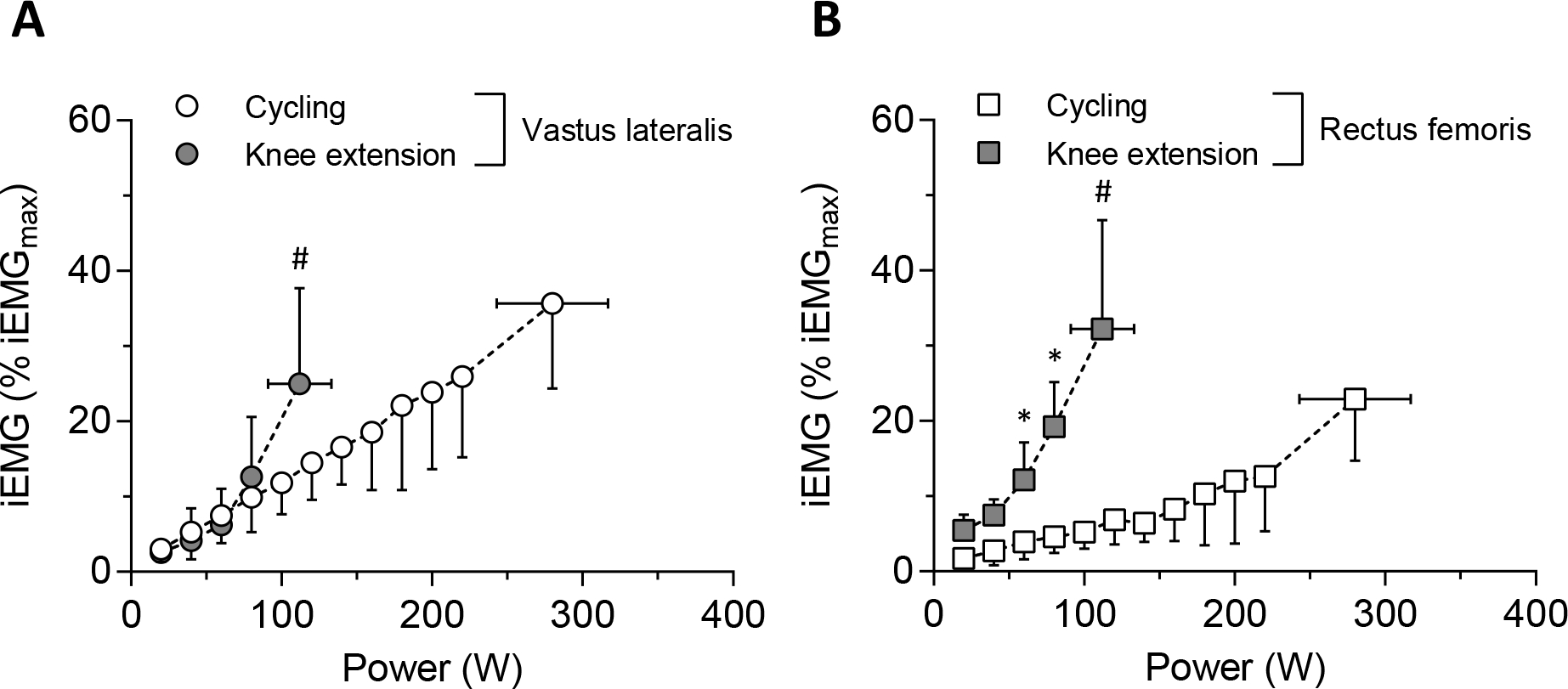
Mean normalized muscle activation patterns across absolute power output in each muscle site (A, vastus lateralis [VL]; B, rectus femoris [RF]). * showed the significant differences between exercise modes at the same absolute power output (P < 0.05). # showed the significant differences between exercise modes at maximal power output (P < 0.05).
At an absolute power output of 80 W, StO2 in VL was lower during KE than CE (P = 0.016, Cohen’s d = 1.10) (Fig. 3), but neither deoxy[Hb+Mb] nor StO2 in VL were different between exercise paradigms at task failure. The RF muscle had greater deoxy- and total[Hb+Mb] and lower StO2 at 20–80 W during KE than CE (all P < 0.01, Cohen’s d = 0.40–1.83) (Fig. 3). At task failure, lower deoxy[Hb+Mb] and higher StO2 was present in RF muscle during KE versus CE (all P < 0.001, Cohen’s d = 0.87–0.96) (Fig. 3).
Figure 3.
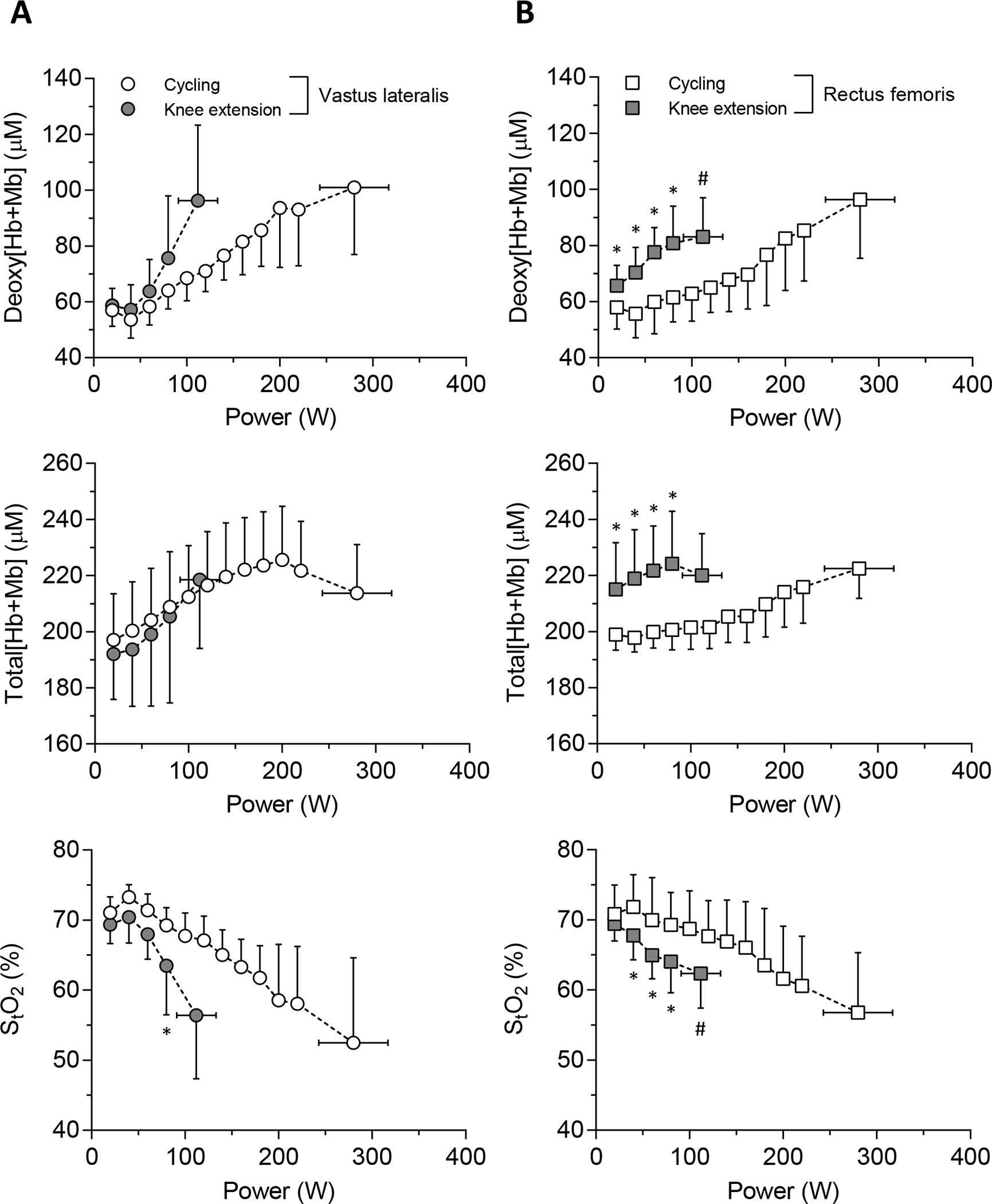
Mean absolute value of NIRS measurements (top, deoxy[Hb+Mb]; middle, total[Hb+Mb]; bottom, StO2) across absolute power output in each muscle site (A, VL; B, RF). * showed the significant differences between exercise modes at same absolute power output (P < 0.05). # showed the significant differences between exercise modes at maximal power output (P < 0.05).
For VL across the range of muscle activation levels achieved (i.e., iEMG normalized to MVC), the response profile for each NIRS variable was not different between exercise paradigms (Fig. 4). This was not the case for the RF muscle at 15 and 20 % iEMGmax where the deoxy[Hb+Mb] was lower (i.e., at 20 % iEMGmax, 98.2 ± 19.4 vs. 81.6 ± 13.2 μM) and StO2 greater (i.e., at 20 % iEMGmax, 56.0 ± 7.9 vs. 62.6 ± 4.6 %) for KE (all P < 0.001, Cohen’s d = 0.63–1.02) (Fig. 4). Specifically, with respect to the divergent observation at 20 % iEMGmax, the average bias of deoxy[Hb+Mb] was lower (−14.6 vs. 0.9 μM) and that of StO2 greater (6.6 vs. −1.7 %) for KE (Fig. 5). Moreover, total[Hb+Mb] in the RF muscle during KE was greater at both 5 and 10 % iEMGmax (both P < 0.001, Cohen’s d = 0.76–1.23) but not for 15 or 20 % iEMGmax (Fig. 4).
Figure 4.
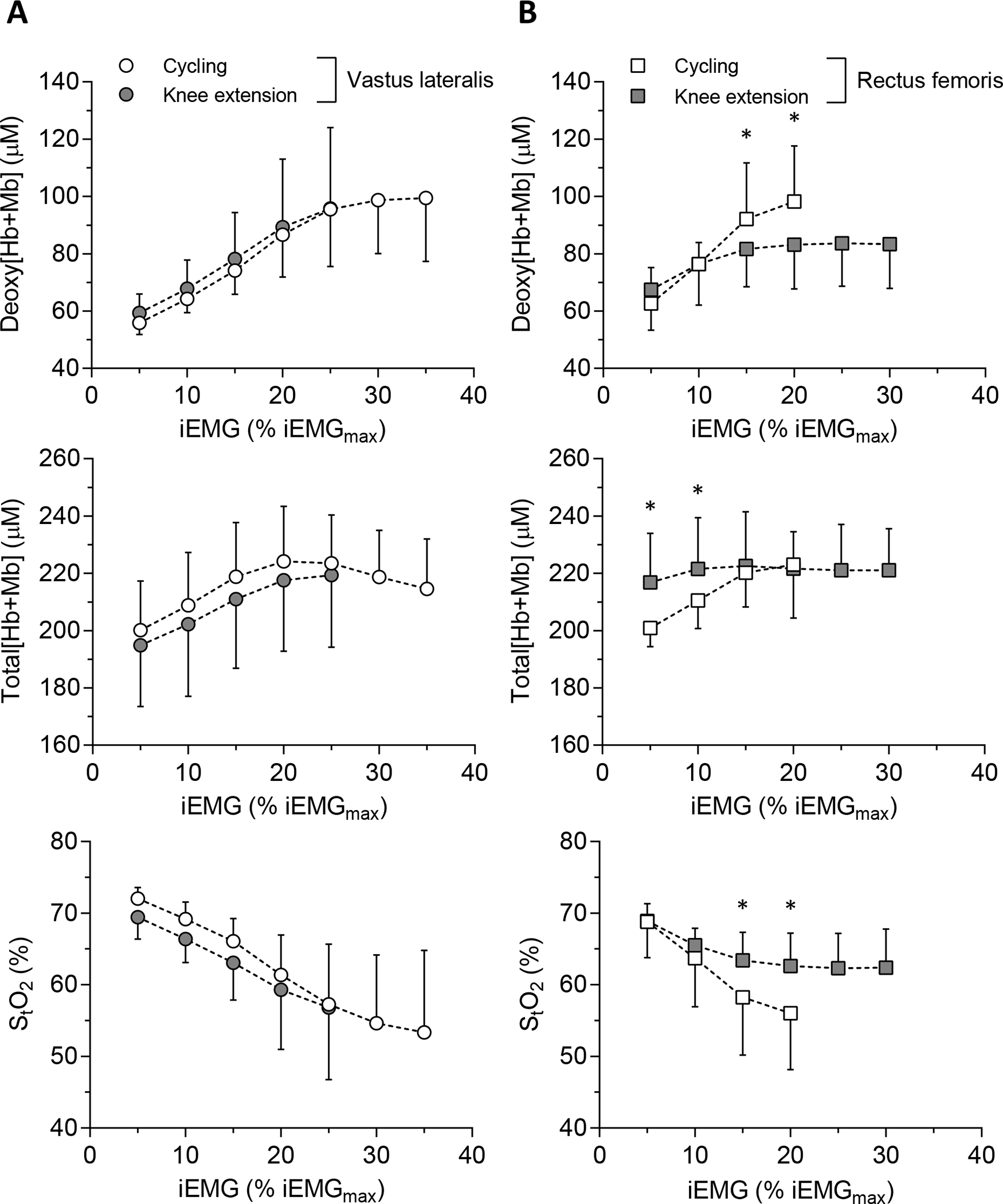
The relationship of NIRS measurements (top, deoxy[Hb+Mb]; middle, total[Hb+Mb]; bottom, StO2) to normalized iEMG level in each muscle site (A, VL; B, RF). * showed the significant differences between exercise paradigms at the same iEMG level.
Figure 5.
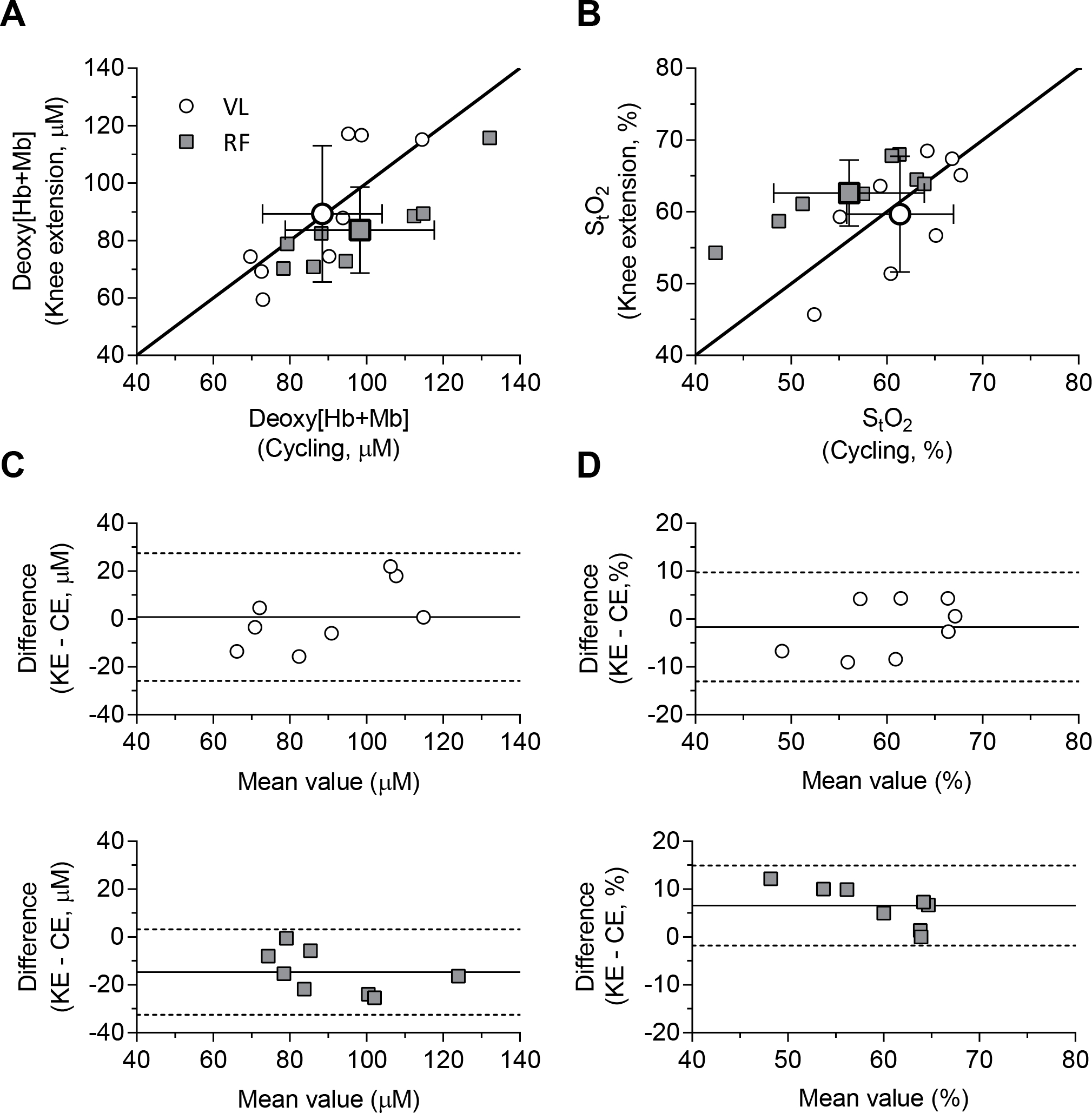
The relationship of deoxy[Hb+Mb] (A) and StO2 (B) concentration between exercise paradigm (x-axis, cycling; y-axis, knee extension) at 20 % iEMGmax for iEMG, and Bland-Altman plot of deoxy[Hb+Mb] (C) and StO2 (D) for each muscle site. Large and small symbols show the relationship of mean and individual values, respectively. Solid line in (A, B) shows line of identity (y = x, reflecting that deoxy[Hb+Mb] and StO2 is a function of muscle activation). Solid and dotted lines in (C, D) show the average bias and range of 95 % limits of agreement, respectively.
DISCUSSION
This investigation explored the degree to which contrasting muscle activation patterns evoked by KE and CE could explain heterogeneities in muscle oxygenation, deoxygenation and total[Hb+Mb] during ramp incremental exercise in VL and RF muscles. The principal original findings are that: 1) the deoxy- and total[Hb+Mb], and StO2 at a given level of muscle activation had common absolute values and profiles across exercise paradigms in the VL but not the RF (Fig. 4), and 2) total[Hb+Mb] concentration at task failure was not different between exercise paradigms irrespective of muscle or recruitment level (Fig. 3).
The relationship of NIRS measurements to muscle activation pattern
In the VL muscle, the deoxy[Hb+Mb] and StO2 values at task failure were not different between exercise paradigms despite the greater normalized iEMG achieved during CE compared to KE (Figs. 2 and 3). Moreover, the deoxygenation responses in the VL exhibited closely similar curvilinear profiles across the range of muscle activations achieved for both exercise paradigms (Fig. 4). Despite very different mass-specific metabolic rates for KE and CE (Knight et al., 1993; Richardson et al., 1993; Richardson et al., 1995; Richardson et al., 1999) it is notable that VL muscle deoxy- and total[Hb+Mb], and StO2 changed in concert with the muscle activation profile (Fig. 4). An original aspect of the present investigation was that NIRS measurements were made in the same muscle (i.e., invariant muscle fiber composition) across different contraction-to-relaxation knee angles incurred by the two exercise paradigms. Despite the opportunity for different muscle utilization-to-delivery relationships resulting from differential activation and/or contraction patterns as dictated by the exercise paradigm (i.e., additional muscle recruitment from a limited number of motor units), the NIRS measurements (deoxy- and total[Hb+Mb], and StO2) were ultimately a function of iEMG (Fig. 4). The question as to whether this relationship is fortuitous in balancing among substrate-level and oxidative phosphorylation processes to power muscle energetics or, rather, reflects control of the local relationship at a given activation level is intriguing.
In contrast, for the RF muscle, the change in deoxy[Hb+Mb] and StO2 values from rest to task failure was significantly less during KE than CE despite the greater muscle activation (Figs. 2 and 3). In addition, the deoxygenation responses in the RF at equivalent levels of muscle activation (i.e., 15 and 20 % iEMGmax) were markedly lower in KE than CE (Fig. 4). These findings suggest that convective transport in specific muscle regions have a different relationship to muscle activation per se. Previously, Cannon et al. (2013) documented the spatial heterogeneity of substrate phosphorylation across the quadriceps femoris muscles and found the greatest dephosphorylation in RF muscle during KE with prone posture (knee movement: ~35°). Previous and present observations suggest that RF contractions during KE depend proportionally more on substrate phosphorylation than oxidative phosphorylation compared with the VL. One putative explanation is that a relative deficiency of convective transport constrains oxidative phosphorylation in the RF. It is pertinent that Laaksonen et al. (2006) reported the absence of any significant correlation between muscle activation and blood flow during dynamic KE in the RF. The mechanism for this phenomenon deserves investigation.
For VL during CE, the deoxy[Hb+Mb] demonstrated a prolonged plateau at ~100 μM from 25 to 35 % iEMGmax which was a considerably higher level of muscle activation than achieved during KE (i.e., 20 % iEMGmax, Fig. 4). In marked contrast, for RF during KE, deoxy[Hb+Mb] plateaued at ~80 μM from 15 to 30 % iEMGmax whilst CE produced a systematic increase in deoxy[Hb+Mb] to ~100 μM at 20 % iEMGmax (i.e., task failure). Mass balance dictates that the increase of muscle deoxy[Hb+Mb] at progressively higher levels of muscle activation (i.e., % iEMGmax) will be determined by the extant fractional extraction and its interdependence with augmented blood flow (Okushima et al., 2015; Okushima et al., 2016). In addition, mitochondrial activation and affinity (p50) also alters this point (Cardinale et al., 2018). Should mitochondrial volume and respiratory capacity be different between muscle groups, this would affect mitochondrial p50 and contribute to the different deoxy[Hb+Mb] patterns observed between muscles. As post-exercise ischemic intervention has demonstrated (Morales-Alamo et al., 2015; Inglis et al., 2017; Iannetta et al., 2018), this is not because fractional extraction cannot go higher but rather that it is limited under the particular perfusive and diffusive conductances extant during high intensity exercise. The mechanistic bases for the differential patterns of deoxy[Hb+Mb] and StO2 identified between muscles using the two exercise paradigms herein is deserving of future attention.
At given submaximal power output, deoxy[Hb+Mb] was greater and StO2 lower in RF muscle for KE compared with CE (Fig. 3). However, as apparent from Figure 4, because the mass of the exercising musculature is far smaller for KE than CE, the achieved RF recruitment was far greater (i.e., 30 versus 20 % iEMGmax). As mentioned above, this lower muscle deoxygenation at high muscle activation levels (i.e., the greater muscle demands) in RF muscle during KE is consequent to a higher ratio. Specifically, with respect to the divergent observation at 20 % iEMGmax seen in Figure 5, the RF deoxy[Hb+Mb] and StO2 exhibited far less muscle deoxygenation compared to that in VL. The most logical interpretations for this phenomenon are that, during KE, the RF muscle can uncouple fractional extraction from metabolic rate possibly due to restricted availability/utilization and a relatively large contribution by substrate phosphorylation as previously mentioned (Cannon et al., 2013).
At submaximal power outputs, RF total[Hb+Mb] was greater during KE than CE (Fig. 3) but these differences were abolished at higher muscle activation levels (seen most clearly in Fig. 4). This observation supports that some facet of the KE exercise paradigm that initially induced the high total[Hb+Mb] concentration was impacted by hemodynamic events independent of muscle activation per se. Putative mechanistic bases for this phenomenon include high shear stress inducing microvascular dilation and also blood pooling in venules/veins consequent to less effective muscle pumping action; both of which would act to increase muscle [Hb].
Effect of exercise paradigm on microvascular hemoconcentration.
Capillary hematocrit in non-contracting skeletal muscle is substantially below systemic levels (i.e., 15–20 %, refs. Damon & Duling, 1987; Poole et al., 1997), and this increases during hyperemic states such as vasodilation with or without contractions (Kindig et al., 2002; Richardson et al., 2003; Poole et al., 2011; Poole et al., 2013). Thus, it is theoretically possible that capillary hematocrit and therefore [Hb] can increase 2–3 fold from rest to exercise (Kindig et al., 2002; Copp et al., 2009), and this effect has been attributed, in part, to the mechanical interactions between the RBCs and endothelial surface layer that decreases the disparity in mean RBC and plasma velocities with higher “blood” flow rates. As demonstrated in animal muscles, differential changes in RBC velocity and flux during contractions impacts capillary hematocrit (Berg et al., 1997; Kindig et al., 2002). However, measurement of capillary hematocrit directly using intravital microscopy in animal muscles during maximal exercise is presently intractable. Thus, it is not known how high capillary hematocrit, and therefore muscle [Hb] can go during the prodigious mass-specific blood flows generated by KE (Richardson et al., 1993). This is unfortunate as capillary hematocrit is considered to be a, or the, primary determinant of muscle diffusive transport (Federspiel & Popel, 1986; Groebe & Thews, 1990; Hogan et al., 1991; Kurdak et al., 1995). pressure (PO2) measurements in the microvascular and interstitial spaces demonstrate sustained PO2 gradients across the microvascular walls during metabolic transients (Hirai et al., 2018). This latter observation supports that the increased diffusional conductance seen with elevated metabolic rates occurs primarily in the microcirculation and intracellularly within the myocytes. The present investigation offers some insights into events within the capillaries across the range from rest to maximal exercise and the results contrast with our hypotheses in that: 1) There was only a modest (< 20 %) increase in total[Hb+Mb] from rest to maximal exercise, and 2) At task failure total[Hb+Mb] was not different between KE and CE regardless of muscle site and what must have been substantially higher blood flows during KE (refs. Knight et al., 1993; Richardson et al., 1993, Fig. 3). These results suggest, by default, that the intramyocyte component of diffusive transport is more important than previously considered. Interestingly, Groebe and Thews (1990) pointed out that for high RBC velocities (and metabolic rates), their model will overestimate PO2 drops across the microvascular wall as there is an increase in effective carrier-free layer conductance, much of which resides within the myocyte. Present and recent observations (Hirai et al., 2018) support this conclusion. An additional consideration is that high RBC velocities and fractional extractions increase the effective surface area for diffusion along the length of capillaries; a process termed longitudinal recruitment (Poole et al., 2011; Hirai et al., 2018).
Investigating the microcirculatory hemodynamics during muscle contractions in humans is important for elucidating the mechanisms of convective and diffusive matching and the consequences of pathologically-induced mismatching. Diffusive transport is believed to be defined by the product of RBC-perfused capillaries and their hematocrit (Federspiel & Popel, 1986; Groebe & Thews, 1990; Kurdak et al., 1995; Hirai et al., 2018) and there are clear reductions in this measurement in disease (e.g., heart failure, Richardson et al., 2003). That there was no difference in total[Hb+Mb] at task failure between exercise paradigms herein provides novel evidence that challenges our understanding of microvascular [Hb] as a primary determinant of diffusive transport.
Experimental limitations
In the present investigation, CE and KE induced a different range of knee extension (CE vs. KE, 90–130 vs. 90–170 degree). This mandates that we interpret our observations within the context of the range of knee movement and muscle stretch/tension potentially impacting NIRS measurements differently between exercise paradigms in that there may have been more sampled area movement for KE or the magnitude of capillary tortuosity may be different due to sarcomere lengths and the range of muscle contractions (Mathieu-Costello et al., 1989; Ellis et al., 1990) achieved with CE vs. KE.
In addition, muscle blood volume content may change during rhythmic exercise if blood is “squeezed out” of the muscle during contractions. In this instance, the average blood volume in the exercising muscle would depend on the duty-cycle of the exercise (i.e., the duration of the contraction relative to relaxation). However, contrary to this expectation, Lutjemeier et al. (2008) reported that the total[Hb+Mb] signal does not differ significantly between relaxation and contraction phases during even heavy knee extension exercise. Moreover, since the resistance for the two-legged KE was provided via two long metal bars attached to the cranks of the cycle ergometer positioned behind the seated subject, the kicking frequency at 120 contractions min−1 is equivalent to 60 rpm during cycle ergometry (Koga et al., 2005; Koga et al., 2019). Therefore, the duty-cycle of CE corresponds closely to that of KE (i.e., ~40–50 % herein). Accordingly, contraction duty-cycle differences between KE and CE are not expected to impact NIRS measurements.
Activation of muscle groups other than those measured in this study may also contribute to the observed NIRS responses for the CE and KE paradigm. For instance, increased activity from the biceps femoris, rectus femoris, and other lower thigh muscles may occur during CE (Hug & Dorel, 2009) when the pedal is pulled up from the bottom of the pedal stroke. This change in gross efficiency of CE pedaling, however, does not occur unless subjects are specifically instructed to pull up actively on the pedal (Korff et al., 2007). Therefore, muscles other than the quadriceps femoris may contribute to the muscle activation patterns observed, however these contributions are likely to be very small.
CONCLUSIONS
For the VL but not RF muscle, deoxy- and total[Hb+Mb] profiles were a function of muscle activation patterns across KE and CE exercise paradigms. This suggests that specific exercise paradigms may influence convective and diffusive delivery in specific muscle regions, independently from recruitment. That total[Hb+Mb] at task failure was not exercise paradigm-dependent runs counter to the notion that higher mass specific blood flows in human muscles invariably produce greater capillary hematocrits. Collectively, these data support the notion that apparent differences of convective and diffusive delivery in VL muscle during KE vs. CE, were the product of differential muscle activation patterns. Conversely, those divergent patterns of muscle deoxygenation in the RF between KE and CE may be dependent upon specific facets of muscle architecture and functional hemodynamic events (e.g. arteriolar vascular regulation and control of RBC flux) that remain to be defined.
New Findings.
What is the central question of this study?
Does the presence and extent of heterogeneity across human muscles relate specifically to different muscle activation patterns?
What is the main finding and its importance?
During ramp incremental knee extension and cycling exercise, the profiles of muscle deoxygenation (deoxy[Hb+Mb]) and diffusive potential (total[Hb+Mb]) in the vastus lateralis corresponded to different muscle activation strategies. However, this was not the case for the rectus femoris where muscle activation and deoxygenation profiles were dissociated and may therefore be determined by other structural and/or functional attributes (e.g. arteriolar vascular regulation and control of RBC flux).
Acknowledgement
The authors express our gratitude to all participants in this study. We also express our gratitude to Drs. T. Amano, T. S. Bowen and N. Gerrett for very helpful insights during the preparation of the manuscript. This study was partly supported by a Grant-in-Aid for Scientific Research (KAKENHI-15K16476 and -17J09854 to D.O., KAKENHI-26560362 and -24247046 to S.K.) from the Japan Society for the Promotion of Science from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest concerning this article.
REFERENCES
- Alkner BA, Tesch PA & Berg HE (2000). Quadriceps EMG/force relationship in knee extension and leg press. Medicine and Science in Sports and Exercise 32, 459–463. [DOI] [PubMed] [Google Scholar]
- Andersen P, Adams RP, Sjogaard G, Thorboe A & Saltin B (1985). Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol (1985) 59, 1647–1653. [DOI] [PubMed] [Google Scholar]
- Barstow TJ (2019). Understanding near infrared spectroscopy and its application to skeletal muscle research. J Appl Physiol (1985) 126, 1360–1376. [DOI] [PubMed] [Google Scholar]
- Beaver WL, Lamarra N & Wasserman K (1981). Breath-by-breath measurement of true alveolar gas exchange. J Appl Physiol Respir Environ Exerc Physiol 51, 1662–1675. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, McDonough P, Padilla DJ, Musch TI & Poole DC (2003). Oxygen exchange profile in rat muscles of contrasting fibre types. Journal of Physiology 549, 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg BR, Cohen KD & Sarelius IH (1997). Direct coupling between blood flow and metabolism at the capillary level in striated muscle. American Journal of Physiology 272, H2693–2700. [DOI] [PubMed] [Google Scholar]
- Bowen TS, Rossiter HB, Benson AP, Amano T, Kondo N, Kowalchuk JM & Koga S (2013). Slowed oxygen uptake kinetics in hypoxia correlate with the transient peak and reduced spatial distribution of absolute skeletal muscle deoxygenation. Experimental Physiology 98, 1585–1596. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Jensen-Urstad M, van Hall G, Holmberg HC, Rosdahl H & Saltin B (2004). Maximal muscular vascular conductances during whole body upright exercise in humans. Journal of Physiology 558, 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DT, Howe FA, Whipp BJ, Ward SA, McIntyre DJ, Ladroue C, Griffiths JR, Kemp GJ & Rossiter HB (2013). Muscle metabolism and activation heterogeneity by combined 31P chemical shift and T2 imaging, and pulmonary O2 uptake during incremental knee-extensor exercise. J Appl Physiol (1985) 115, 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale DA, Larsen FJ, Jensen-Urstad M, Rullman E, Sondergaard H, Morales-Alamo D, Ekblom B, Calbet JAL & Boushel R (2018). Muscle mass and inspired oxygen influence oxygen extraction at maximal exercise: Role of mitochondrial oxygen affinity. Acta Physiol, e13110. [DOI] [PubMed] [Google Scholar]
- Chin LM, Kowalchuk JM, Barstow TJ, Kondo N, Amano T, Shiojiri T & Koga S (2011). The relationship between muscle deoxygenation and activation in different muscles of the quadriceps during cycle ramp exercise. J Appl Physiol (1985) 111, 1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Ferreira LF, Herspring KF, Musch TI & Poole DC (2009). The effects of aging on capillary hemodynamics in contracting rat spinotrapezius muscle. Microvascular Research 77, 113–119. [DOI] [PubMed] [Google Scholar]
- Damon DH & Duling BR (1987). Are physiological changes in capillary tube hematocrit related to alterations in capillary perfusion heterogeneity? International Journal of Microcirculation: Clinical and Experimental 6, 309–319. [PubMed] [Google Scholar]
- Ellis CG, Mathieu-Costello O, Potter RF, MacDonald IC & Groom AC (1990). Effect of sarcomere length on total capillary length in skeletal muscle: in vivo evidence for longitudinal stretching of capillaries. Microvascular Research 40, 63–72. [DOI] [PubMed] [Google Scholar]
- Federspiel WJ & Popel AS (1986). A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvascular Research 32, 164–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groebe K & Thews G (1990). Calculated intra- and extracellular PO2 gradients in heavily working red muscle. American Journal of Physiology 259, H84–92. [DOI] [PubMed] [Google Scholar]
- Hamaoka T, Katsumura T, Murase N, Nishio S, Osada T, Sako T, Higuchi H, Kurosawa Y, Shimomitsu T, Miwa M & Chance B (2000). Quantification of ischemic muscle deoxygenation by near infrared time-resolved spectroscopy. Journal of Biomedical Optics 5, 102–105. [DOI] [PubMed] [Google Scholar]
- Heinonen I, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos MM, Oikonen V, Nuutila P, Knuuti J, Hellsten Y, Boushel R & Kalliokoski KK (2010). Comparison of exogenous adenosine and voluntary exercise on human skeletal muscle perfusion and perfusion heterogeneity. J Appl Physiol (1985) 108, 378–386. [DOI] [PubMed] [Google Scholar]
- Hirai DM, Craig JC, Colburn TD, Eshima H, Kano Y, Sexton WL, Musch TI & Poole DC (2018). Skeletal muscle microvascular and interstitial PO2 from rest to contractions. Journal of Physiology 596, 869–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Bebout DE & Wagner PD (1991). Effect of hemoglobin concentration on maximal O2 uptake in canine gastrocnemius muscle in situ. J Appl Physiol (1985) 70, 1105–1112. [DOI] [PubMed] [Google Scholar]
- Hug F & Dorel S (2009). Electromyographic analysis of pedaling: a review. Journal of Electromyography and Kinesiology 19, 182–198. [DOI] [PubMed] [Google Scholar]
- Iannetta D, Okushima D, Inglis EC, Kondo N, Murias JM & Koga S (2018). Blood flow occlusion-related O2 extraction “reserve” is present in different muscles of the quadriceps but greater in deeper regions after ramp-incremental test. J Appl Physiol (1985) 125, 313–319. [DOI] [PubMed] [Google Scholar]
- Ijichi S, Kusaka T, Isobe K, Islam F, Okubo K, Okada H, Namba M, Kawada K, Imai T & Itoh S (2005). Quantification of cerebral hemoglobin as a function of oxygenation using near-infrared time-resolved spectroscopy in a piglet model of hypoxia. Journal of Biomedical Optics 10, 24–26. [DOI] [PubMed] [Google Scholar]
- Inglis EC, Iannetta D & Murias JM (2017). The plateau in the NIRS-derived [HHb] signal near the end of a ramp incremental test does not indicate the upper limit of O2 extraction in the vastus lateralis. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 313, R723–R729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliokoski KK, Knuuti J & Nuutila P (2005). Relationship between muscle blood flow and oxygen uptake during exercise in endurance-trained and untrained men. J Appl Physiol (1985) 98, 380–383. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Richardson TE & Poole DC (2002). Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer. J Appl Physiol (1985) 92, 2513–2520. [DOI] [PubMed] [Google Scholar]
- Knight DR, Poole DC, Schaffartzik W, Guy HJ, Prediletto R, Hogan MC & Wagner PD (1992). Relationship between body and leg VO2 during maximal cycle ergometry. J Appl Physiol (1985) 73, 1114–1121. [DOI] [PubMed] [Google Scholar]
- Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE & Wagner PD (1993). Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol (1985) 75, 2586–2594. [DOI] [PubMed] [Google Scholar]
- Koga S, Kano Y, Barstow TJ, Ferreira LF, Ohmae E, Sudo M & Poole DC (2012). Kinetics of muscle deoxygenation and microvascular PO2 during contractions in rat: comparison of optical spectroscopy and phosphorescence-quenching techniques. J Appl Physiol (1985) 112, 26–32. [DOI] [PubMed] [Google Scholar]
- Koga S, Okushima D, Barstow TJ, Rossiter HB, Kondo N & Poole DC (2017). Near-infrared spectroscopy of superficial and deep rectus femoris reveals markedly different exercise response to superficial vastus lateralis. Physiol Rep 5, e13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga S, Okushima D, Poole DC, Rossiter HB, Kondo N & Barstow TJ (2019). Unaltered Vo2 kinetics despite greater muscle oxygenation during heavy-intensity two-legged knee extension versus cycle exercise in humans. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 317, R203–R213. [DOI] [PubMed] [Google Scholar]
- Koga S, Poole DC, Shiojiri T, Kondo N, Fukuba Y, Miura A & Barstow TJ (2005). Comparison of oxygen uptake kinetics during knee extension and cycle exercise. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 288, R212–220. [DOI] [PubMed] [Google Scholar]
- Korff T, Romer LM, Mayhew I & Martin JC (2007). Effect of pedaling technique on mechanical effectiveness and efficiency in cyclists. Medicine and Science in Sports and Exercise 39, 991–995. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Soderlund K, Relu MU, Ferguson RA & Bangsbo J (2009). Heterogeneous recruitment of quadriceps muscle portions and fibre types during moderate intensity knee-extensor exercise: effect of thigh occlusion. Scandinavian Journal of Medicine and Science in Sports 19, 576–584. [DOI] [PubMed] [Google Scholar]
- Kurdak SS, Grassi B, Wagner PD & Hogan MC (1995). Effect of [Hb] on blood flow distribution and O2 transport in maximally working skeletal muscle. J Appl Physiol (1985) 79, 1729–1735. [DOI] [PubMed] [Google Scholar]
- Laaksonen MS, Bjorklund G, Heinonen I, Kemppainen J, Knuuti J, Kyrolainen H & Kalliokoski KK (2010). Perfusion heterogeneity does not explain excess muscle oxygen uptake during variable intensity exercise. Clinical Physiology and Functional Imaging 30, 241–249. [DOI] [PubMed] [Google Scholar]
- Laaksonen MS, Kyrolainen H, Kalliokoski KK, Nuutila P & Knuuti J (2006). The association between muscle EMG and perfusion in knee extensor muscles. Clinical Physiology and Functional Imaging 26, 99–105. [DOI] [PubMed] [Google Scholar]
- Lutjemeier BJ, Ferreira LF, Poole DC, Townsend D & Barstow TJ (2008). Muscle microvascular hemoglobin concentration and oxygenation within the contraction-relaxation cycle. Respiratory Physiology & Neurobiology 160, 131–138. [DOI] [PubMed] [Google Scholar]
- Mathieu-Costello O (1994). Morphometry of the size of the capillary-to-fiber interface in muscles. Advances in Experimental Medicine and Biology 345, 661–668. [DOI] [PubMed] [Google Scholar]
- Mathieu-Costello O, Hoppeler H & Weibel ER (1989). Capillary tortuosity in skeletal muscles of mammals depends on muscle contraction. J Appl Physiol (1985) 66, 1436–1442. [DOI] [PubMed] [Google Scholar]
- McDonough P, Behnke BJ, Padilla DJ, Musch TI & Poole DC (2005). Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. Journal of Physiology 563, 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Alamo D, Losa-Reyna J, Torres-Peralta R, Martin-Rincon M, Perez-Valera M, Curtelin D, Ponce-Gonzalez JG, Santana A & Calbet JA (2015). What limits performance during whole-body incremental exercise to exhaustion in humans? Journal of Physiology 593, 4631–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Dawson EA, Yoshiga CC, Dalsgaard MK, Damsgaard R, Secher NH & Gonzalez-Alonso J (2005). Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. Journal of Physiology 566, 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima D, Poole DC, Barstow TJ, Rossiter HB, Kondo N, Bowen TS, Amano T & Koga S (2016). Greater VO2peak is correlated with greater skeletal muscle deoxygenation amplitude and hemoglobin concentration within individual muscles during ramp-incremental cycle exercise. Physiol Rep 4, e13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima D, Poole DC, Rossiter HB, Barstow TJ, Kondo N, Ohmae E & Koga S (2015). Muscle deoxygenation in the quadriceps during ramp incremental cycling: Deep vs. superficial heterogeneity. J Appl Physiol (1985) 119, 1313–1319. [DOI] [PubMed] [Google Scholar]
- Poole DC, Copp SW, Ferguson SK & Musch TI (2013). Skeletal muscle capillary function: contemporary observations and novel hypotheses. Experimental Physiology 98, 1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC, Copp SW, Hirai DM & Musch TI (2011). Dynamics of muscle microcirculatory and blood-myocyte O2 flux during contractions. Acta Physiol 202, 293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC & Mathieu-Costello O (1992). Capillary and fiber geometry in rat diaphragm perfusion fixed in situ at different sarcomere lengths. J Appl Physiol (1985) 73, 151–159. [DOI] [PubMed] [Google Scholar]
- Poole DC, Musch TI & Kindig CA (1997). In vivo microvascular structural and functional consequences of muscle length changes. American Journal of Physiology 272, H2107–2114. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Frank LR & Haseler LJ (1998). Dynamic knee-extensor and cycle exercise: functional MRI of muscular activity. International Journal of Sports Medicine 19, 182–187. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J & Wagner PD (1999). Evidence of O2 supply-dependent VO2max in the exercise-trained human quadriceps. J Appl Physiol (1985) 86, 1048–1053. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Haseler LJ, Nygren AT, Bluml S & Frank LR (2001). Local perfusion and metabolic demand during exercise: a noninvasive MRI method of assessment. J Appl Physiol (1985) 91, 1845–1853. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Knight DR, Poole DC, Kurdak SS, Hogan MC, Grassi B & Wagner PD (1995). Determinants of maximal exercise VO2 during single leg knee-extensor exercise in humans. American Journal of Physiology 268, H1453–1461. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK & Wagner PD (1993). High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol (1985) 75, 1911–1916. [DOI] [PubMed] [Google Scholar]
- Richardson TE, Kindig CA, Musch TI & Poole DC (2003). Effects of chronic heart failure on skeletal muscle capillary hemodynamics at rest and during contractions. J Appl Physiol (1985) 95, 1055–1062. [DOI] [PubMed] [Google Scholar]
- Spencer MD, Amano T, Kondo N, Kowalchuk JM & Koga S (2014). Muscle O2 extraction reserve during intense cycling is site-specific. J Appl Physiol (1985) 117, 1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzis I, Habazettl H, Louvaris Z, Andrianopoulos V, Wagner H, Zakynthinos S & Wagner PD (2015). A method for assessing heterogeneity of blood flow and metabolism in exercising normal human muscle by near-infrared spectroscopy. J Appl Physiol (1985) 118, 783–793. [DOI] [PubMed] [Google Scholar]
- Whipp BJ & Ward SA (1982). Cardiopulmonary coupling during exercise. Journal of Experimental Biology 100, 175–193. [DOI] [PubMed] [Google Scholar]


