Abstract
Background:
Although mortality has decreased considerably in pediatric heart transplantation, waitlist and post-transplant death rates remain notable. End-of-life focused research in this population, however, is very limited. This Pediatric Heart Transplant Society study aimed to describe the circumstances surrounding death of pediatric heart transplant patients.
Methods:
A retrospective analysis of the multi-institutional, international, Pediatric Heart Transplant Society registry was conducted. Descriptive statistics and univariate analyses were performed to 1) describe end-of-life in pediatric pre- and post-heart transplant patients and 2) examine associations between location of death and technological interventions at end-of-life with demographic and disease factors.
Results:
Of 9,217 patients (0–18 years) enrolled in the registry between 1993–2018, 2,804 (30%) deaths occurred; 1,310 while awaiting heart transplant and 1,494 post-heart transplant. The majority of waitlist deaths (89%) occurred in the hospital, primarily in ICU (74%) with most receiving mechanical ventilation (77%). Fewer post-transplant deaths occurred in the hospital (22%). Out of hospital death was associated with older patient age (p<0.01).
Conclusions:
ICU deaths with high use of technological interventions at end of life were common, particularly in patients awaiting heart transplant. In this high mortality population, findings raise challenging considerations for clinicians, families, and policy makers on how to balance quality of life amidst high risk for hospital-based death.
Introduction
Medical advancements have led to overall longer median survival in pediatric heart transplant recipients. However, mortality rates remain high both pre- and post-heart transplantation. Approximately 25% of infants and 13% of children on the heart transplant waitlist do not survive to transplantation.1 Among those who go on to receive a donor heart, approximately 1 in 10 pediatric heart transplant recipients die in the first year.1,2 Beyond the high risk early post-transplant period, approximately 20% experience death 5 years post-transplant.2,3 While the adult heart failure and transplant communities have provided scoping reviews,4 practice guidelines,5–7 and palliative care focused interventions8,9 to improve care across the course of heart failure, heart transplant and at end-of-life, the pediatric community has given considerably less attention to this important aspect of care.10
To date, much of what we know about circumstances surrounding death in pediatric advanced heart disease and heart transplantation is based on single-center experiences, which indicate high rates of hospital-based deaths with intubation frequently performed near end-of-life.11,12 Building upon these initial studies, increased understanding of circumstances surrounding death, including location of death and interventions performed at end-of-life in a large, multi-site sample, will enable the pediatric transplant community to move forward with guidelines and interventions to improve the quality of care patients and families receive at a child’s end-of-life. Thus, in response to calls for increased research study,13 this Pediatric Heart Transplant Society (PHTS) registry analysis study aimed to 1) describe the circumstances surrounding death of pediatric heart transplant patients, including transplant status at time of death, primary and contributing causes of death, location of death, and use of technological interventions at end-of-life and 2) examine associations between location of death and technological interventions at end-of-life with demographic, disease, and transplant-related factors.
Patients and Methods
Patients
PHTS maintains an international pediatric heart transplant data registry with 56 contributing centers from four countries providing demographic and clinical data at the time of heart transplant listing and in the years to follow. Inclusion criteria for the current study included: pediatric heart transplant patient aged 0–18 years (waitlist or post-transplant) enrolled in PHTS between 1993 and 2018. Patients were stratified into two cohorts: 1) pre-transplant (waitlist) and 2) post-transplant.
Methods
All data forms collected by PHTS from contributing sites can be found on the PHTS website (https://pediatrichearttransplantsociety.org/2015-forms/). To accomplish the aims of this study, data was utilized from the following PHTS forms: Demographics, Listing, Re-Listing, Transplant, and Death. Descriptive statistics were presented as frequency and percentage (%) for categorical variables and median and interquartile range (IQR) for continuous variables. Univariate associations between location of death and technological interventions at end-of-life with demographic, disease, and transplant-related factors were assessed using Chi-square test, Fisher’s exact test, or Wilcoxon rank sum test, as appropriate. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). A statistical significance of 0.05 was used for two-sided tests.
Results
Sample Characteristics
Patient demographics, transplant characteristics, primary causes of death, and contributing causes of death are detailed in Table 1. Of the 9,217 registry entries, 2,804 (30%) deaths occurred; 1,310 while awaiting heart transplant and 1,494 post-heart transplant (Figure 1). Location of death was recorded for 1,113 patients (40% of total deaths; Figure 1). Sensitivity analyses showed that patients without location of death recorded were more likely to be Caucasian/non-Hispanic (p=0.03) and have private insurance (p=0.001) compared to those with death location. Diagnosis was similar between those with location of death recorded and those without.
Table 1.
Demographics, Transplant characteristics, and Primary/contributing causes of death (N=9,217)
| Patient demographics | N=9,217 |
| Male sex | 5,146 (55.8) |
| Caucasian/White | 6,446 (69.9) |
| African American/Black | 1,652 (17.9) |
| American Indian or Alaskan Native | 88 (1.0) |
| Asian | 298 (3.2) |
| Hawaiian or other Pacific islander | 47 (0.5) |
| Other | 581 (6.3) |
| Unknown/Undisclosed | 280 (3.0) |
| Hispanic | 1,623 (17.6) |
| Non-Hispanic | 6,386 (69.3) |
| Unknown | 1,208 (13.1) |
| Charitable Donation | 9 (0.1) |
| Free | 222 (2.4) |
| Government | 4,394 (47.7) |
| Private | 3,484 (37.8) |
| Self Pay | 79 (0.9) |
| Other | 193 (2.1) |
| Unknown/Not reported | 836 (9.1) |
| Cardiac Tumor | 32 (0.3) |
| Cardiomyopathy | 4,026 (43.7) |
| Congenital Heart Disease | 4,840 (52.5) |
| Myocarditis | 313 (3.4) |
| Other | 6 (0.1) |
| Transplant/Post-transplant characteristics | N=6,793 |
| Age at Transplant, years | 4.1 (0.6–12.7) |
| 1993–2000 | 1,140 (16.8) |
| 2001–2010 | 2,296 (33.8) |
| 2011–2019 | 3,357 (49.4) |
| Time since transplant, years | 4.1 (1.4–8.3) |
| Patient seen for follow-up (anytime during post-transplant) | 5,644 (96.6) |
| Death | N=2,804 |
| Cardiac | 1,300 (46.4) |
| GI/Intestinal Complications | 24 (0.9) |
| Hepatic Failure | 7 (0.2) |
| Infection | 227 (8.1) |
| Major bleeding (non-neurological) | 82 (2.9) |
| Malignancy/Cancer | 53 (1.9) |
| Neurologic | 169 (6.0) |
| Pulmonary embolism | 9 (0.3) |
| Pulmonary hypertension/RV failure | 40 (1.4) |
| Rejection | 222 (7.9) |
| Renal Failure | 31 (1.1) |
| Respiratory failure | 170 (6.1) |
| Suicide | 1 (0.0) |
| Trauma/Accidental | 105 (3.7) |
| Other | 248 (9.8) |
| Unknown | 114 (4.1) |
| Not reported | 2 (0.1) |
| Cardiac | 430 (15.3) |
| Family decision to withdraw of support | 124 (4.4) |
| Hepatic Failure | 34 (1.2) |
| Infection | 252 (9.0) |
| Major bleeding (non-neurological) | 81 (2.9) |
| Malignancy/Cancer | 31 (1.1) |
| Neurologic | 156 (5.6) |
| Non-compliance | 96 (3.4) |
| Poor donor preservation | 4 (0.1) |
| Primary graft failure (onset < 24 hours post-transplant) | 11 (0.4) |
| Pulmonary embolism | 18 (0.6) |
| Pulmonary hypertension/RV failure | 85 (3.0) |
| Rejection | 187 (6.7) |
| Renal Failure | 337 (12.0) |
| Respiratory failure | 94 (3.4) |
| Suicide | 0 (0.0) |
| Trauma/Accidental | 57 (2.0) |
| Other | 754 (26.9) |
| Unknown | 67 (2.4) |
Data are presented as N (%) for categorical variables and Median (interquartile range) for continuous variables.
Figure 1.
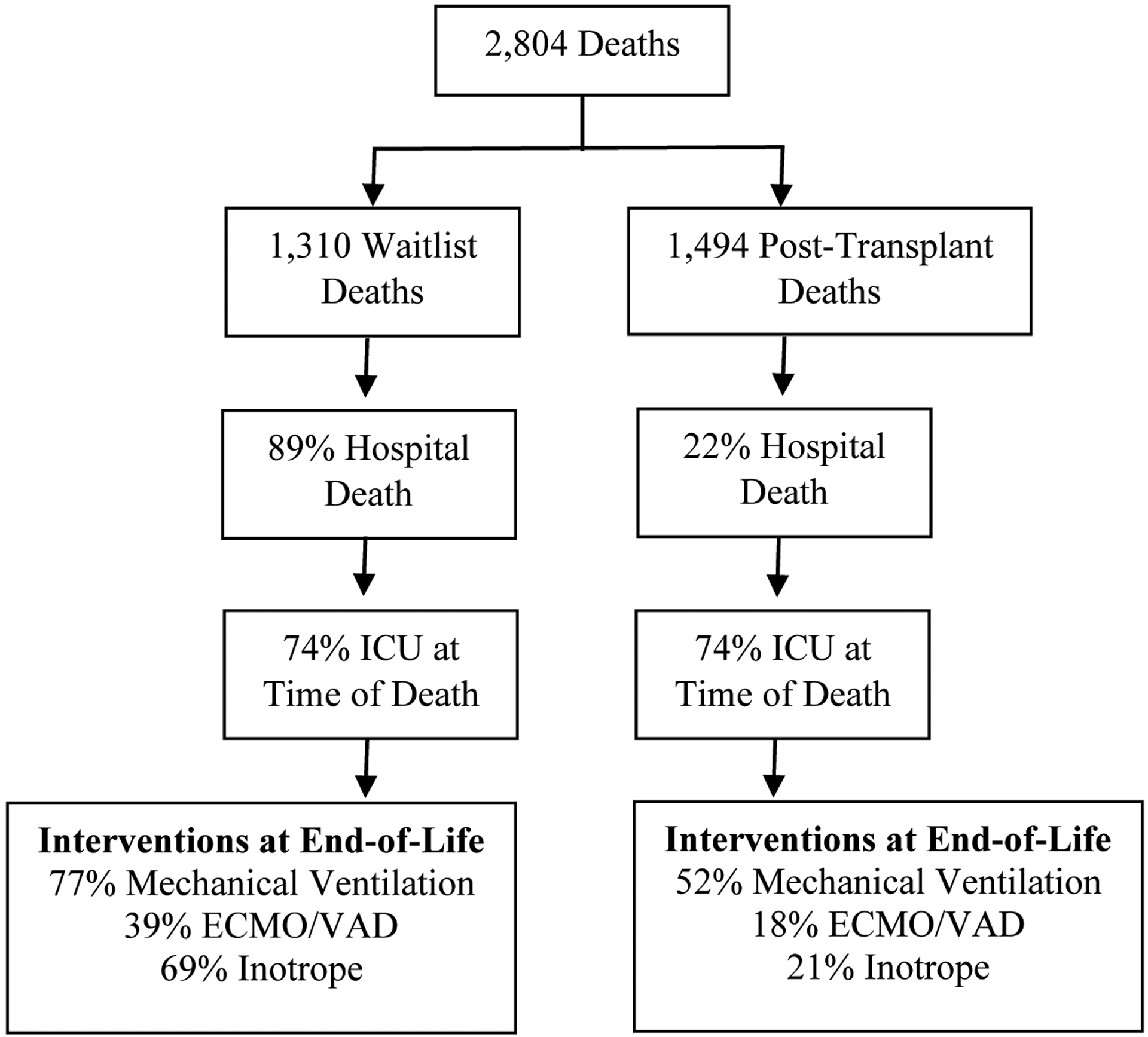
Circumstances Surrounding End-of-Life in Pediatric Waitlist and Post-Heart Transplant Patients
Location of Death
Of the 804 waitlist deaths with location of death indicated, 89% occurred in the hospital, primarily in ICU settings (74%). Location of death was captured for proportionately fewer post-heart transplant recipients, with only 309 post-heart transplant patients having death location known or recorded. Fewer than a quarter (22%) died in a hospital, however, hospital deaths were predominately within an ICU setting (74%).
Location of death was unrelated to patient sex, race, ethnicity, or insurance type in waitlist and post-transplant patients (Table 2). Older patients were more likely to die out of the hospital among both waitlist (p=0.01) and post-transplant patients (p=0.001). Waitlist patients with a primary etiology of congenital heart disease were more likely to experience an in-hospital death compared to those with primary cardiac tumor, cardiomyopathy, or myocarditis (p=0.04). Primary etiology was unrelated to location of death in post-transplant patients. Year of transplant was also associated with location of death, with considerably more out of hospital deaths occurring in those transplanted between the years of 2011–2019 (64%; p<.0001) compared to transplanted between 1993–2000 (7%) and 2001–2010 (29%).
Table 2.
Associations of Demographics and Transplant and Post-transplant characteristics by Location of Death (N=1,113)
| Characteristics | While awaiting heart transplant | Post-heart transplant | ||||||
|---|---|---|---|---|---|---|---|---|
| All (N=804) | In-hospital at time of death | P-value¥ | All (N=309) | In-hospital at time of death | P-value¥ | |||
| Yes (N=718) | No (N=86) | Yes (N=69) | No (N=240) | |||||
| Patient demographics | ||||||||
| Male sex | 453 (56.3) | 407 (56.7) | 46 (53.5) | 0.57 | 177 (57.3) | 33 (47.8) | 144 (60.0) | 0.07 |
| Age at death, years | 0.8 (0.2–3.1) | 0.7 (0.2–2.9) | 1.2 (0.5–5.5) | 0.01 | 11.1 (3.1–17.1) | 6.9 (1.3–13.8) | 12.3 (3.8–17.8) | 0.001 |
| Caucasian/White | 527 (65.5) | 478 (66.6) | 49 (57.0) | 191 (61.8) | 43 (62.3) | 148 (61.7) | ||
| African American/Black | 169 (21.0) | 154 (21.4) | 15 (17.4) | 77 (24.9) | 18 (26.1) | 59 (24.6) | ||
| American Indian or Alaskan Native | 9 (1.1) | 7 (1.0) | 2 (2.3) | 3 (1.0) | 1 (1.4) | 2 (0.8) | ||
| Asian | 24 (3.0) | 19 (2.6) | 5 (5.8) | 10 (3.2) | 2 (2.9) | 8 (3.3) | ||
| Hawaiian or other Pacific islander | 6 (0.7) | 5 (0.7) | 1 (1.2) | 1 (0.3) | 0 (0.0) | 1 (0.4) | ||
| Other | 56 (7.0) | 44 (6.1) | 12 (14.0) | 21 (6.8) | 5 (7.2) | 16 (6.7) | ||
| Unknown/Undisclosed | 29 (3.6) | 22 (3.1) | 7 (8.1) | 12 (3.9) | 2 (2.9) | 10 (4.2) | ||
| Hispanic | 159 (19.8) | 139 (19.4) | 20 (23.3) | 68 (22.0) | 15 (21.7) | 53 (22.1) | ||
| Non-Hispanic | 537 (66.8) | 479 (66.7) | 58 (67.4) | 213 (68.9) | 40 (58.0) | 173 (72.1) | ||
| Unknown/Not reported | 108 (13.4) | 100 (13.9) | 8 (9.3) | 28 (9.1) | 14 (20.3) | 14 (5.8) | ||
| Charitable Donation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Free | 19 (2.4) | 17 (2.4) | 2 (2.3) | 9 (2.9) | 2 (2.9) | 7 (2.9) | ||
| Government | 426 (53.0) | 374 (52.1) | 52 (60.5) | 163 (52.8) | 33 (47.8) | 130 (54.2) | ||
| Private | 253 (31.5) | 223 (31.1) | 30 (34.9) | 105 (34.0) | 20 (29.0) | 85 (35.4) | ||
| Self Pay | 5 (0.6) | 5 (0.7) | 0 (0.0) | 1 (0.3) | 1 (1.4) | 0 (0.0) | ||
| Other | 11 (1.4) | 9 (1.3) | 2 (2.3) | 11 (3.6) | 1 (1.4) | 10 (4.2) | ||
| Unknown/Not reported | 90 (11.2) | 90 (12.5) | 0 (0.0) | 20 (6.5) | 12 (17.4) | 8 (3.3) | ||
| Cardiac Tumor | 4 (0.5) | 3 (0.4) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Cardiomyopathy | 221 (27.5) | 189 (26.3) | 32 (37.2) | 117 (37.9) | 30 (43.5) | 87 (36.3) | ||
| Congenital Heart Disease | 553 (68.7) | 502 (69.9) | 51 (59.3) | 185 (59.9) | 37 (53.6) | 148 (61.7) | ||
| Myocarditis | 26 (3.2) | 24 (3.3) | 2 (2.3) | 7 (2.3) | 2 (2.9) | 5 (2.1) | ||
| Transplant/Post-transplant characteristics | ||||||||
| Age at Transplant, years | 4.1 (0.7–11.5) | 1.7 (0.5–7.4) | 5.2 (0.7–12.4) | 0.02 | ||||
| Year of Transplant | ||||||||
| 1993–2000 | 36 (11.7) | 20 (29.0) | 16 (6.7) | |||||
| 2001–2010 | 104 (33.7) | 34 (49.3) | 70 (29.2) | |||||
| 2011–2019 | 169 (54.7) | 15 (21.7) | 154 (64.2) | |||||
| Time since transplant, years | 2.0 (0.3–6.9) | 1.5 (0.1–6.0) | 2.6 (0.4–7.0) | 0.02 | ||||
| Patient seen for follow-up (anytime during post-transplant) | 200 (64.7) | 36 (52.2) | 164 (68.3) | 0.01 | ||||
Data are presented as N (%) for categorical variables and Median (interquartile range) for continuous variables.
P-value from Chi-square test for categorical variables and Wilcoxon rank sum test for continuous variables.
Comparison was made as Caucasian/White vs. all others (except Unknown/Undisclosed) and p-value came from Chi-square test.
Comparison was made as Government vs. Private and p-value came from Chi-square test.
Comparison was made as Congenital Heart Disease vs. all others and p-value came from Chi-square test.
Technological Interventions at End-of-Life
Among waitlist deaths, technological interventions at end-of-life were common. The majority (77%) received continuous invasive mechanical ventilation near time of death. A subset (39%) were supported by ECMO/VAD at time of death and 69% were receiving inotrope support. Although a smaller proportion of post-transplant patients died in the hospital when compared to waitlist patients, use of technological interventions at end-of-life were still common in hospital-based deaths. Approximately half (52%) received mechanical ventilation near end-of-life, while 18% and 21% were supported by ECMO/VAD and inotropes, respectively.
Discussion
Despite considerable improvements in both waitlist and post-transplant survival in pediatric heart transplant, death occurred in ~1/3 of patients captured in this international registry over a 25-year period. Of the registry entries with complete death data, ICU-based deaths with high use of technological interventions were common, particularly in waitlisted patients. Although dying at home is the preference of most adolescents with other life-threating conditions,14 the majority of pediatric heart transplant waitlist patients die in an ICU. This is not particularly surprising since survival on the transplant waitlist is likely to require close medical monitoring and continued advanced therapy interventions, as demonstrated by study findings. However, for some patients, this raises challenging, and potentially conflicting, clinical implications for clinicians, families, and policy makers. To qualify for highest 1A pediatric heart transplant listing status in the United State, a pediatric waitlist patient must be hospitalized or receiving mechanical circulatory support. Across many other pediatric conditions, patients and families can opt to receive concurrent care, which includes access to both life-prolonging treatments and hospice care. Within pediatric heart transplant, however, those at highest risk of death on the waitlist must choose between greatest likelihood for donor offer by being hospitalized, even if care could be provided via outpatient home-based services, and a potential end-of-life care aligned with one’s wishes, such as a home-based death.
Consistent with previous work,11,12,15 findings also demonstrated high use of technological interventions at end-of-life. Use of these potentially life-saving technological interventions near end-of-life may indeed be the preference of some patients and families. However, unlike adult heart failure and other serious pediatric conditions, little is known about pediatric heart transplant patients’ desires for their end-of-life care, despite their high mortality risks. Emerging research suggests that the end-of-life care needs of pediatric heart transplant patients are unmet. For example, in a pilot study of adolescents and young adults pre-heart transplant, the majority stated a desire to discuss their prognosis and end-of-life preferences, but fewer than half had.16
Both findings and limitations of the current study highlight important future directions for research and practice. First, missing death data was notable. This was an unexpected, yet important limitation to highlight. For 60% of deaths in the most robust pediatric transplant database available, location of death was unknown or unreported. The development of interventions specific to end-of-life hinges on a foundational understanding of what occurs. Through PHTS and other cardiac registries, we may enhance understanding of patients’ end-of-life with improved data collection methods and the inclusion of other important variables. For example, data specific to palliative care team involvement, advance care planning documentation (i.e., do not resuscitate orders), or patient/family preferred location of death were not available for this study, but would have improved our understanding of the end-of-life care experience for patients and families. The use of patient- and caregiver-proxy reported outcome (PRO) measures may be an additional route for improving data collection specific to end-of-life. For example, a multisite electronic PRO-based study in pediatric advanced cancer, the Pediatric Quality of Life and Evaluation of Symptoms Technology (PediQUEST) Study, yielded important insights about both physical and psychological symptoms in young oncologic patients in the 12 weeks prior to their death.17 Recognizing the hesitation of clinicians and researchers to conduct end-of-life focused research due to concerns of inducing unnecessary burden on patients and families, a recent systematic review of the pediatric literature suggests that patients and parents perceive more benefits from participating in palliative and end-of-life care focused research than burdens.18
Second, given the high rate of mortality with invasive interventions near end-of-life observed in the current study, we must broaden our understanding of transplant patients and families’ wants for end-of-life discussions and intervention. Pediatric transplant clinicians have spoken to the challenges of balancing the hopefulness of transplant with the risks of morbidity and mortality on the waitlist and post-transplant. Among multi-organ transplant clinicians, only ~20% reported that they often or very often discuss dying or advance care planning with adolescent solid organ transplant patients.19 However, given findings from the current study, as well as a single-center experience underscoring the unexpectedness of death in pediatric post-transplant recipients,12 clinicians are encouraged to systematically assess both patient and family preferences specific to end-of-life communication and preferences as part of routine pre- and post-transplant care. Research from pediatric oncology has demonstrated that more prognostic disclosure from clinicians, even when likelihood of cure from cancer was low, resulted in greater communication-based hope in parents.20 The most common barriers to optimal end-of-life care for pediatric oncology patients per bereaved caregivers have been identified as 1) delayed or no communication about prognosis, limiting care specific to comfort and quality of life, 2) limited emotional support for both patients and caregivers, and 3) lack of home-based, concurrent care models, where both life-prolonging and palliative care interventions are provided.21,22 Delayed or no discussion of prognosis with medical team or child has contributed to regret in bereaved caregivers of pediatric patients.21,23 Parents of children who die of advanced heart disease recognized no chance for survival only an average of two days prior to death.15 Thus, taken together with current study findings and what has been demonstrated across other pediatric critical illness populations, the field of pediatric heart failure/transplant must begin to engage patients and parents in stakeholder-led research and intervention specific to prognostic and end-of-life care focused communication.
Lastly, primary palliative care training for heart transplant multidisciplinary providers is imperative.10 Primary palliative care is the practice of providing palliative care services, including symptom management and goals of care discussions, within the regular care of the patient and family. Research has demonstrated that pediatric cardiologists who have received some didactic training in palliative care feel more competent in integrating palliative care based practices.24 A pilot study demonstrated that an intervention focused on advance care planning documentation for young adults seen in a pediatric heart failure/transplant clinic was well received and effective,25 pointing to the value of wider-spread integration of this practice in pediatric heart transplant. Further, continuing to describe how pediatric heart transplant and pediatric palliative care teams can best work together to serve our patients and families who often have concurrent care goals will be important.26
Acknolwedgments and Funding
The funder/sponsor did not participate in the work. This work was supported by funding provided by the University of Michigan Palliative Care Pilot Grant. Dr. Cousino’s research is supported by the National Heart, Lung, and Blood Institute (K23HL145096) of the National Institutes of Health.
Abbreviations:
- HTx
heart transplant
- ICU
intensive care unit
- PHTS
Pediatric Heart Transplant Society
Footnotes
Disclosures
The authors have no financial or industry relationships specific to this research to disclose.
References
- 1.Rossano JW, Singh TP, Cherikh WS, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-second pediatric heart transplantation report–2019; Focus theme: Donor and recipient size match. The Journal of Heart and Lung Transplantation. 2019;38(10):1028–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossano JW, Cherikh WS, Chambers DC, et al. The Registry of the International Society for Heart and Lung Transplantation: twentieth pediatric heart transplantation report— 2017; focus theme: allograft ischemic time. The Journal of Heart and Lung Transplantation. 2017;36(10):1060–1069. [DOI] [PubMed] [Google Scholar]
- 3.Miller R, Tumin D, Cooper J, Hayes D Jr, Tobias JD. Prediction of mortality following pediatric heart transplant using machine learning algorithms. Pediatric transplantation. 2019;23(3):e13360. [DOI] [PubMed] [Google Scholar]
- 4.Kavalieratos D, Gelfman LP, Tycon LE, et al. Palliative care in heart failure: rationale, evidence, and future priorities. Journal of the American College of Cardiology. 2017;70(15):1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang JC, Ewald GA, Allen LA, et al. Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. Journal of cardiac failure. 2015;21(6):519–534. [DOI] [PubMed] [Google Scholar]
- 6.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. The Journal of Heart and Lung Transplantation. 2013;32(2):157–187. [DOI] [PubMed] [Google Scholar]
- 7.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;62(16):e147–e239. [DOI] [PubMed] [Google Scholar]
- 8.Rogers JG, Patel CB, Mentz RJ, et al. Palliative care in heart failure: the PAL-HF randomized, controlled clinical trial. Journal of the American College of Cardiology. 2017;70(3):331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarz ER, Baraghoush A, Morrissey RP, et al. Pilot study of palliative care consultation in patients with advanced heart failure referred for cardiac transplantation. Journal of palliative medicine. 2012;15(1):12–15. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman BD, Cohen HJ. Palliative care in pediatric heart failure and transplantation. Current opinion in pediatrics. 2019;31(5):611–616. [DOI] [PubMed] [Google Scholar]
- 11.Morell E, Wolfe J, Scheurer M, et al. Patterns of care at end of life in children with advanced heart disease. Archives of pediatrics & adolescent medicine. 2012;166(8):745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollander SA, Dykes JC, Chen S, et al. The end-of-life experience of pediatric heart transplant recipients. Journal of pain and symptom management. 2017;53(5):927–931. [DOI] [PubMed] [Google Scholar]
- 13.Cousino MK, Lord BT, Blume ED. State of the science and future research directions in palliative and end-of-life care in paediatric cardiology: a report from the Harvard Radcliffe Accelerator Workshop. Cardiology in the Young. 2021:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feudtner C, Feinstein JA, Satchell M, Zhao H, Kang TI. Shifting place of death among children with complex chronic conditions in the United States, 1989–2003. Jama. 2007;297(24):2725–2732. [DOI] [PubMed] [Google Scholar]
- 15.Blume ED, Balkin EM, Aiyagari R, et al. Parental perspectives on suffering and quality of life at end-of-life in children with advanced heart disease: an exploratory study. Pediatric Critical Care Medicine| Society of Critical Care Medicine. 2014;15(4):336–342. [DOI] [PubMed] [Google Scholar]
- 16.Cousino MK, Miller VA, Smith C, et al. Medical and end-of-life decision making in adolescents’ pre-heart transplant: A descriptive pilot study. Palliative medicine. 2020;34(3):272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfe J, Orellana L, Ullrich C, et al. Symptoms and distress in children with advanced cancer: prospective patient-reported outcomes from the PediQUEST study. Journal of Clinical Oncology. 2015;33(17):1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver MS, Mooney-Doyle K, Kelly KP, et al. The benefits and burdens of pediatric palliative care and end-of-life research: a systematic review. Journal of palliative medicine. 2019;22(8):915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cousino MK, Schumacher KR, Magee JC, et al. Communication about prognosis and end‐of‐life in pediatric organ failure and transplantation. Pediatric transplantation. 2019;23(3):e13373. [DOI] [PubMed] [Google Scholar]
- 20.Mack JW, Wolfe J, Cook EF, Grier HE, Cleary PD, Weeks JC. Hope and prognostic disclosure. Journal of Clinical Oncology. 2007;25(35):5636–5642. [DOI] [PubMed] [Google Scholar]
- 21.Mack JW, Currie ER, Martello V, et al. Barriers to Optimal End-of-Life Care for Adolescents and Young Adults With Cancer: Bereaved Caregiver Perspectives. Journal of the National Comprehensive Cancer Network. 2021;19(5):528–533. [DOI] [PubMed] [Google Scholar]
- 22.Sedig LK, Spruit JL, Paul TK, Cousino MK, Pituch K, Hutchinson R. Experiences at the end of life from the perspective of bereaved parents: Results of a qualitative focus group study. American Journal of Hospice and Palliative Medicine®. 2020;37(6):424–432. [DOI] [PubMed] [Google Scholar]
- 23.Kreicbergs U, Valdimarsdóttir U, Onelöv E, Henter J-I, Steineck G. Talking about death with children who have severe malignant disease. New England Journal of Medicine. 2004;351(12):1175–1186. [DOI] [PubMed] [Google Scholar]
- 24.Balkin EM, Kirkpatrick JN, Kaufman B, et al. Pediatric cardiology provider attitudes about palliative care: a multicenter survey study. Pediatric cardiology. 2017;38(7):1324–1331. [DOI] [PubMed] [Google Scholar]
- 25.Edwards LA, Bui C, Cabrera AG, Jarrell JA. Improving outpatient advance care planning for adults with congenital or pediatric heart disease followed in a pediatric heart failure and transplant clinic. Congenital heart disease. 2018;13(3):362–368. [DOI] [PubMed] [Google Scholar]
- 26.Wan A, Weingarten K, Rapoport A. Palliative Care?! But This Child’s Not Dying: The Burgeoning Partnership Between Pediatric Cardiology and Palliative Care. Canadian Journal of Cardiology. 2020;36(7):1041–1049. [DOI] [PubMed] [Google Scholar]


