Highlights
-
•
Heart failure induces skeletal muscle atrophy and dysfunction, which decreases regular treatment and patient quality of life.
-
•
To date, no effective treatment for heart failure-induced muscle pathology is established, apart from exercise training.
-
•
Aerobic and resistance exercise training are both effective treatments in patients with heart failure for conferring health benefits, higher quality of life, and improved survival.
Keywords: Calcium, Exercise training, Heart failure, Satellite cells, Skeletal muscle wasting
Abstract
This review highlights some established and some more contemporary mechanisms responsible for heart failure (HF)-induced skeletal muscle wasting and weakness. We first describe the effects of HF on the relationship between protein synthesis and degradation rates, which determine muscle mass, the involvement of the satellite cells for continual muscle regeneration, and changes in myofiber calcium homeostasis linked to contractile dysfunction. We then highlight key mechanistic effects of both aerobic and resistance exercise training on skeletal muscle in HF and outline its application as a beneficial treatment. Overall, HF causes multiple impairments related to autophagy, anabolic-catabolic signaling, satellite cell proliferation, and calcium homeostasis, which together promote fiber atrophy, contractile dysfunction, and impaired regeneration. Although both wasting and weakness are partly rescued by aerobic and resistance exercise training in HF, the effects of satellite cell dynamics remain poorly explored.
Graphical Abstract
1. Introduction
Skeletal muscle is the most abundant tissue in the human body and is involved in various fundamental functions such as mobility (locomotion and posture), inspiratory function, thermoregulation, metabolism of macronutrients such as glucose, lipids, and amino acids,1 and it has also been described as an endocrine organ.2 Skeletal muscle tissue has a remarkable capacity to adapt to different stimuli (i.e., plasticity), dramatically changing its mass and function according to each situation. While an increase in muscle mass (i.e., hypertrophy) occurs in response to intense resistance exercise training (RET) or the presence of certain hormones,3 loss of muscle mass and strength are often observed in specific scenarios, including physical inactivity, disuse, aging, and following chronic diseases such as cancer and heart failure (HF).4
Chronic disease-related muscle wasting at its most severe is often termed cachexia. Cachexia is defined as a complex, multifactorial metabolic syndrome underpinned by an underlying illness and associated with a significant reduction in body mass derived from muscle tissue loss with or without adipose tissue loss, and which cannot be reversed by conventional nutritional interventions.5 Cachexia impairs quality of life in patients by reducing the effectiveness of treatments; indeed, evidence indicates that patients with cachexia exhibit shorter survival than non-cachectic patients.6,7 In addition, cachexia also affects the main muscle of respiration, the diaphragm,8 the wasting of which exacerbates symptoms of breathlessness and impairs ventilation, leading to life-threatening respiratory failure.9 Nevertheless, it is important to recognize a large proportion of patients may not present with overt cachexia or wasting yet lose muscle strength due to intrinsic muscle dysfunction (i.e., loss of function independent of mass). Accordingly, it is important to appreciate both aspects as key factors limiting quality of life in patients.10
Therefore, the recognition that chronic diseases inducing both muscle mass loss and dysfunction as a widespread condition affect millions of people has stimulated the search for treatments able to attenuate this and improve the quality of life of patients. While no effective pharmacological treatments are clearly established at present, exercise training has been proposed as a potential therapeutic approach due to its various effects on both the systemic and local muscle levels (i.e., anti-inflammatory, immunological,11 anti-atrophic,12,13 and pro-oxidative metabolism14). In terms of chronic diseases, HF warrants important consideration because this condition continues to increase in prevalence, is one of the most common causes of hospitalizations15 and global deaths,16 and there is no cure. Of note, cardiac dysfunction in HF poorly correlates to symptoms and skeletal muscle dysfunction, which indicates that a more complex situation is at play.17,18
Therefore, in this brief review, we outline selected mechanisms underpinning limb and diaphragm muscle loss and weakness in HF, with a specific focus on fiber atrophy, regeneration, and contractile dysfunction. We then highlight the important benefits associated with exercise training for attenuating skeletal muscle impairments (as summarized in Fig. 1). Unless otherwise noted, we focus mostly on studies of HF with reduced rather than preserved ejection fraction given that more evidence is available, which allows for more robust conclusions.
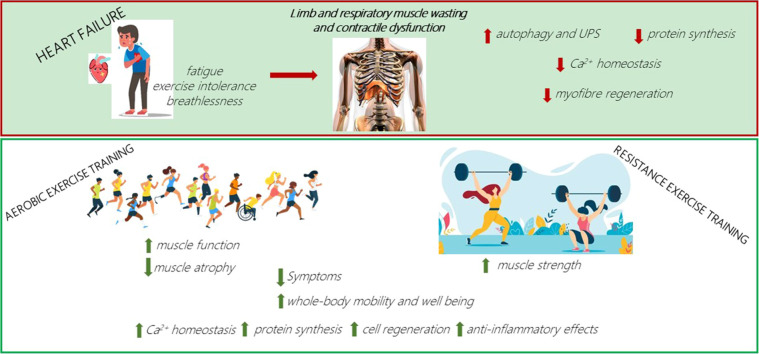
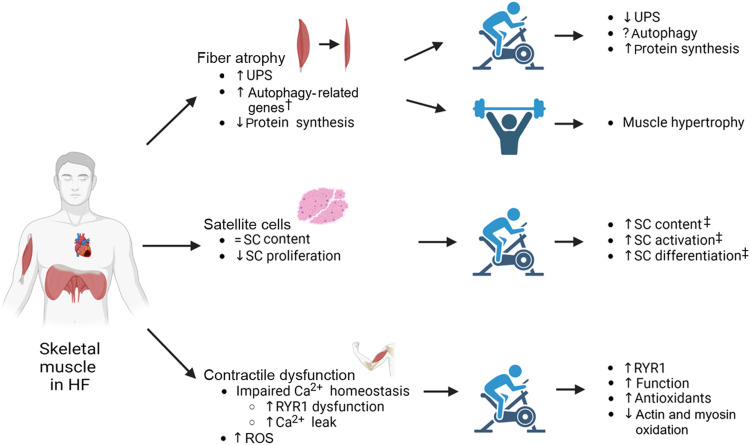
Fig. 1.
Summary of the primary effects of heart failure on skeletal muscle fiber atrophy, satellite cells, and contractile dysfunction, as well as the secondary impact following prolonged exercise training in patients and animal models. † means in animal models only; ‡ means in healthy individuals. ↑ means increase; ↓ means decrease; = means no change; ? means lacking information yet. HF = heart failure; ROS = reactive oxygen species; RT = resistance training; RYR1 = ryanodine receptor 1; SC = satellite cell; UPS = ubiquitin proteasome system.
2. Effects of HF on fiber atrophy
The regulation of muscle mass and function reflects protein turnover (i.e., the balance between protein synthesis and degradation). The 2 major proteolytic systems involved in muscle wasting are the ubiquitin proteasome system (UPS) and autophagy-lysosomal system (for a full review see Singh et al.19), although the calpain and caspase systems can also play important roles.20 Whereas the UPS specifically degrades myofibrillar proteins, autophagy is responsible for the clearance of damaged cellular components via autophagosome formation. The UPS system is regulated by ubiquitin enzymes E1, E2, and E3, which respectively activate, carry, and bind ubiquitin to target proteins before degradation at the proteasome complex. The UPS is involved in a number of cachectic conditions, displaying high levels of E3-ligases as well as proteasome activity. Indeed, leg muscle samples (of the vastus lateralis (VL)) from HF patients display an increase in the protein content of E3-ligase muscle RING-finger protein-1 (MuRF-1),21,22 with concomitant increases in proteasome activity.23 This finding was mirrored in a rat model of myocardial infarction (MI)-induced HF, where proteasome activity was higher in plantaris and soleus muscles.24 However, given the limited access to patient samples, the role of autophagy in HF-induced cachexia remains less clear. It is known, however, that some autophagy-associated markers, such as the expression of microtubule-associated proteins 1A/1B light chain 3B and B-cell lymphoma-2 interacting protein 3, are upregulated in skeletal muscle during starvation periods, with forkhead box protein O3 recognized as the most important transcription factor controlling autophagy.25 Despite evidence from experimental models of HF with preserved ejection fraction (HFpEF) and MI indicating autophagy may be dysregulated,26,27 clinical patient data is limited. However, evidence indicates no difference in skeletal muscle mRNA expression or protein content of lysosomal proteolysis marker cathepsin L in patient tissue.22
As the underlying mechanisms of muscle wasting and HF progression remain poorly understood, an elegant study suggested the dysregulation of myokine expression from wasting muscles impairs HF severity.28 The study observed that musclin expression is reduced in HF and the muscle-specific disruption of musclin in mice contributes to the progression of HF, while elevated musclin levels improved cardiac function.28 This is one of the first studies to suggest a link between skeletal-cardiac muscle cross talk in HF-induced muscle wasting that could suggest a promising therapeutic strategy.
Another mechanism that contributes to muscle wasting is an inability to activate pro-hypertrophic pathways, which is a condition known as anabolic resistance. In this sense, the key mediator of myofibrillar protein synthesis and muscle growth is the mechanistic target of rapamycin complex 1 (mTORC1) pathway. The activation of mTORC1 by upstream factors insulin-like growth factor-1 (IGF-1) and protein kinase B (Akt) phosphorylates downstream targets ribosomal protein S6 kinase beta-1, eukaryotic translation initiation factor 4E-binding protein 1, and eukaryotic initiation factor 4E to activate protein translation.29 It has been shown that HF patients with reduced or preserved ejection fraction alike displayed reduced skeletal muscle mRNA expression and protein content of IGF-1.21,30 In line with this, phosphorylated Akt protein content is also lower in the skeletal muscle of HF patients,31 which is perhaps indicative of impaired translational activity as it is in other conditions such as cancer.13 One other key study in mice post-MI showed that muscle-specific overexpression of IGF-1 blocked atrophy via normalizing Akt phosphorylation in line with inhibiting E3-ligase expression and proteasome activity.32 Interestingly, despite past evidence suggesting a poor link between cardiac dysfunction and skeletal muscle changes in HF, a study using dual X-ray absorptiometry showed left ventricular assist device recipients gained muscle mass within 6 months of surgery.33 However, it remains unclear whether the increased muscle mass in HF patients was caused by increased blood flow or improvements in physical activity given the expected reduction in symptoms. Unfortunately, no muscle biopsies were taken to further investigate mechanistic signaling. Thus, it is important to consider 2 mechanistic angles in HF, both the hypertrophic and atrophic signaling nexus.
Alongside the mTORC1 pathway, the key upstream regulator of muscle protein balance is cell metabolism. Mitochondria regulate energy metabolism by integrating key cell signaling pathways related to oxidative stress and energy production.34 Mitochondrial dynamics have been shown to regulate muscle mass in aging. It was observed that physical inactivity contributes to age-related decline in the activity of optic atrophy gene 1 (OPA1), one of the genes regulating mitochondrial dynamics and biogenesis, which are associated with muscle atrophy.35 It was also observed that a muscle-specific deletion of OPA1 alters mitochondrial morphology and function, leading to endoplasmic reticulum stress, which then induces a catabolic program via the unfolded protein response and forkhead box Os.35 The role of mitochondria dynamics in the context of HF remains to be explored. However, evidence has shown that mRNA expression of OPA1 and peroxisome proliferator-activated receptor-gamma coactivator-1α were downregulated in female HF patients who also present impaired in situ mitochondrial function.36 Interestingly, these changes were not observed in male patients. Instead, male HF patients present functional impairments related to complex I oxidative phosphorylation, indicating an important divergence in phenotype between sexes.36
Accordingly, HF patients present a number of structural abnormalities in the mitochondria, including a reduction in size37 and content36,38 as well as fluid accumulation and membrane disruption.37 In addition, HF patients with diabetes also present impairments in mitochondrial function in situ, including reduced oxygen flux and coupling efficiency as well as a concomitant increase in reactive oxygen species (ROS) production.39 Please refer to Lv et al.34 for an in-depth review of mitochondrial dynamics in HF.
3. Effects of HF on satellite cells (SCs) and muscle regeneration
The changes in protein turnover leading to muscle hypertrophy or atrophy do not occur according to the simplistic balance between protein synthesis and degradation but may be affected by nuclear turnover. Hypertrophy can occur by accretion of new myonuclei by muscle stem cell or SC fusion, which in turn helps expand cytoplasmic volume,40 while loss of myonuclei by cell apoptosis can lead to muscle atrophy.41 Therefore, impairments to SCs may also contribute to reduced skeletal muscle mass in HF (Fig. 1).
SCs are located between the sarcolemma and basal lamina of muscle fibers42 and usually reside in a resting state known as quiescence, which is characterized by the expression of transcription factor paired box 7 (Pax7).43 In response to exercise and/or muscle injury, Pax7 is downregulated, and SCs enter an activated state.44 In turn, SCs proliferate and differentiate under the control of a group of transcription factors termed myogenic regulatory factors, with a proportion also returning to quiescence to repopulate the SC pool.45 Differentiated cells fuse with one another to form new myofibers or with damaged myofibers to facilitate repair and myonuclear turnover. The understanding of the role of SCs in muscle has improved with the development of the Pax7-diptheria toxin A mouse model, which allows conditional ablation of SCs upon tamoxifen administration,46 although this is yet to be tested in the context of HF. Despite proving critical for injury-induced skeletal muscle regeneration,47,48 the role of SCs in muscle growth remains controversial.49,50 Evidence suggests that SCs are required for optimal hypertrophy in aged muscle51 and in response to chronic overload,52 while impairments in SC function have been identified in patients and in various experimental models of muscle wasting.53,54
In the context of HF, only a handful of studies have explored the link between muscle atrophy and SCs. In a transgenic dilated cardiomyopathy mouse model, the plantar flexors were stimulated in vivo before their ability to regenerate following eccentric forced dorsiflexion contraction-induced damage was assessed.55 The study showed that the plantar torque recovered by 95% within 2 weeks of injury in controls, but that it was attenuated in HF mice with the number of centrally located nuclei substantially elevated.55 While these results suggest that HF impairs skeletal muscle regeneration after injury, the study concluded this was SC-independent given that the number of Pax7 positive cells was unchanged between groups. Clearly further experiments directly assessing SC function in HF will be needed to verify this assumption. Additional data confirmed that the SC number per 100 myofibers in the tibialis anterior muscle of obese HFpEF rats at baseline was not different when compared to lean controls.56 In contrast, fiber size and SC abundance have been reported to be reduced in the gastrocnemius muscle post cardio toxin-induced muscle injury following 7 days of treatment with angiotensin II (i.e., a known peptide hormone playing a significant role in the development of cardiovascular disease).57 Furthermore, to aid clinical translation and better explore the effects of HF on SC dynamics, the study confirmed that following MI in mice, the number of SCs in the gastrocnemius muscle was lower in comparison to that of the respective sham control group, while pharmacological blockade of the angiotensin Type I receptor prevented this. This further supports the viewpoint that angiotensin II could be an upstream trigger of myofiber atrophy in HF not only by regulating the transcription factor EB–MuRF-1 axis58 but also by modulating SC function.
While an SC abundance issue remains unclear in HF, impairment of SC function and myogenic progression could play an important role in driving muscle wasting, and its key effect on proliferation has been identified. In support of this understanding, myoblast determination protein 1 and myogenin mRNA expression were attenuated 3 days post injury in MI-induced HF vs. control mice.57 Furthermore, isolated and cultured SCs treated with angiotensin II showed impairments in proliferation as confirmed via bromodeoxyuridine incorporation.57 Similarly, in humans, primary cultures of skeletal muscle myoblasts isolated from the VL of 8 HF patients (with reduced ejection fraction) and 8 healthy matched controls showed proliferation kinetics were delayed at 90 h into the growth phase.57,59 In addition, mRNA expression of proliferation factors interleukin-6 and tumor necrosis factor receptor 2 were attenuated in myoblasts derived from muscle samples from HF patients with reduced ejection fraction. Therefore, these findings suggest that SC proliferation is impaired in HF, which may attenuate muscle repair and contribute to atrophy. However, because it remains poorly understood whether HF substantially impacts muscle regeneration, further study in this area is warranted.
4. Effects of HF on contractile function
As presented above, muscle fatigue and weakness are key features of HF. These are determined not only by muscle wasting but also by intrinsic fiber dysfunction evident via a reduction in specific force, which is often termed contractile dysfunction and is consequent to impaired excitation–contraction coupling (ECC).60 Impaired ECC increases motor unit firing frequency to meet muscular demands, thereby accelerating muscle fatigue and heightening symptoms of ventilation/breathlessness.61 It is often underappreciated how the loss of contractile function, which is a major clinical problem in HF patients because it limits their daily activities and quality of life, may also be caused by sarcopenia.62 Sarcopenia is regularly used to define the loss of muscle mass and strength associated with aging,63 with studies demonstrating that low muscle force production is more predictive of falls than is low lean mass.64,65
One contributing factor to impaired ECC in skeletal muscle is decreased Ca2+ homeostasis (Fig. 1). In HF, cytosolic Ca2+ fluxes during muscle contraction are reduced in both limb muscles66,67 and the diaphragm.68 Alterations in Ca2+ homeostasis have profound effects on muscle performance, and it seems that reduced sarcoplasmic reticulum (SR) release and reuptake both contribute to muscle weakness in HF. Post-MI, HF rats showed prolonged Ca2+ transients and reduced SR release in extensor digitorum longus, accompanied by lower twitch and tetanic tension as well as fatigue resistance;69 a similar pattern was found in diaphragm fibers.68 Sarcoplasmic or endoplasmic reticulum Ca2+ ATPase (SERCA) is largely responsible for Ca2+ uptake in cardiac and skeletal muscle (predominantly in the adult SERCA1a and neonatal SERCA1b isoforms in skeletal muscle, with SERCA2a also present in slow skeletal muscle70). HF rats have decreased SERCA1a protein expression in limb and respiratory muscle, which likely impairs Ca2+ reuptake to blunt ECC.70 A similar trend was also observed in the VL of HF patients, whose biopsies presented lower SERCA2a protein content and diminished levels of phosphorylated phospholamban, both of which reduce Ca2+ sequestration into the SR.71 Interestingly, expression of SERCA1 and SERCA2a were higher in diaphragm biopsies from HF patients when compared to controls with coronary heart disease, indicating a divergence between limb and respiratory muscle.72 Strong evidence supports the idea that the impairment of SR Ca2+ release dynamics in HF is caused by dysfunction of the ryanodine receptor 1 (RYR1) complex (i.e., the main channel responsible for SR Ca2+ release in skeletal muscle), which also disrupts basal fiber homoestasis.67,73, 74, 75 For example, binding of FK506 binding protein 12 (FKBP12, also termed calstabin) to RYR1 in order to stabilize the closed state is diminished in HF.74 This is partly due to hyperphosphorylation of RYR1 by protein kinase A due to chronic β-adrenergic signaling,75 which promotes Ca2+ leakage from the RYR1 into the cytoplasm. VL biopsies from HF patients demonstrate hyper phosphorylation of RYR1 and depleted FKBP12 binding76 as well as lower 1,4 dihydopyridine receptor (DHPR) protein content,71 while the diaphragm in HF patients also showed lower incidence of FKBP12 binding to the RYR1 complex.72 In addition, sensitivity of single diaphragm muscle fibers to cytoplasmic Ca2+ concentrations is decreased in experimental HF, while single muscle fibers in HF patients demonstrate reduced actomyosin ATPase activity regardless of fiber type.77 These conditions likely combine to further reduce contractile function.78 Other factors that play a role include reduced contractile protein content, per se, and the associated post-translational oxidative modifications, with increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and mitochondrial ROS identified as contributing to diaphragm dysfunction during experimental HF.79,80 Studies have further determined that neuromuscular junction (NMJ) fragmentation occurs in HF,81 as it does in aging,82 but it remains unclear whether this reduces muscle function.83
Disruptions to Ca2+ homeostasis in HF may also exacerbate atrophy in both limb and respiratory muscle. In an experimental HF model, greater calpain activity has been found in limb muscle84 alongside raised resting Ca2+ levels in atrophied diaphragm muscle.85 High cytosolic Ca2+ concentrations can activate calpains in skeletal muscle,86 which may accelerate proteolytic activity via UPS to drive fiber atrophy.60,84 Calpains also induce sarcomere disorganization through the degradation of the Z-disk, leading to a reduction in isometric force. Calpain inhibition preserves sarcomere structure, indicating the key role of calpains in contractile dysfunction.87 Furthermore, greater cytosolic Ca2+ concentration leads to increased ROS production from the key sources, including mitochondria, NADPH oxidase (Nox), and xanthine oxidase, in both limb and diaphragm muscle in experimental HF.60,72,80,88, 89, 90 Upregulation of Nox has been found in diaphragm biopsies of HF patients, alongside greater protein oxidation, in spite of increases in antioxidant enzymes.79 This increase in ROS can lead to the upregulation of key catabolic factors, such as E3-ligases, resulting in muscle atrophy and post-translational oxidative modifications of sarcomeric proteins, which contribute to impaired function.60 Targeting these sources of ROS may prove beneficial in the treatment of exercise intolerance in HF, a notion that is supported by various studies. For example, reduction in mitochondrial ROS through the use of a mitochondrial-targeted antioxidant80 and a neutral sphingomyelinase inhibitor91 preserved diaphragm dysfunction in HF rats post MI. Additionally, inhibition of xanthine oxidase in mice with HF prevented the atrophy of type I and type II fibers92 in limb muscle and preserved exercise capacity. Interestingly, only certain isoforms of Nox seem to play a role in diaphragm abnormalities in HF; knockout of a subunit necessary for Nox2 activity restored diaphragm function,88 whereas Nox4 knockout had no impact on acute MI.93 While complex, ROS may also facilitate the dissociation of FKBP12 from the RYR1, destabilizing the closed state and perpetuating further Ca2+ leaks.94,95
Muscle force production is affected by mitochondrial dysfunction and oxidative stress. However, the underlying mechanisms by which oxidative stress contributes to HF-related muscle wasting remain poorly understood. The role of chronic oxidative stress in a mouse model lacking the antioxidant enzyme copper-zinc-superoxide dismutase shows a progressive decline in mitochondrial function and an increase in ROS production caused muscle atrophy.96 When aged mice were evaluated, a striking increase in muscle mitochondrial content near the NMJs was found. However, the function of mitochondria was impaired and an increase in denervated NMJs leading to a reduction in force production was observed. This study suggested that NMJ degeneration and mitochondrial dysfunction are potential mechanisms of sarcopenia.96
Given the greater prevalence of HF in older people97 and the negative effects of aging on skeletal muscle, quantifying the independent contribution of aging vs. HF to skeletal muscle dysfunction is a complex task. In aging, uncoupling of DHPR and RYR1 occurs, and Ca2+ spark duration is reduced, both of which likely contribute to a reduction in specific force generation.98,99 Similarly, HF risk is increased with sedentary behavior, and exercise intolerance may limit physical activity in HF patients,100 again complicating the roles of HF and inactivity in muscle dysfunction. Indeed, inactivity has been found to decrease specific force in young humans and old rats.101,102 Therefore, it is likely that abnormal Ca2+ homeostasis and mitochondrial dysfunction in HF collectively contribute to weakness not only via intrinsic fiber dysfunction but also by promoting fiber atrophy.
5. The effects of exercise training on HF-induced muscle wasting and dysfunction
In the past few years, research groups worldwide have tried to uncover ways to prevent chronic disease-related muscle wasting and dysfunction. Numerous pharmacological and non-pharmacological interventions have been tested, but they have shown limited efficacy.103 Therefore, a combination of interventions emphasizing the importance of a healthy lifestyle, diet, and physical activity have been proposed. Exercise training, specifically aerobic exercise training (AET), is associated with improved quality of life, reduced hospitalizations, and prolonged survival104 and should be considered an adjuvant therapy to counteract muscle defects in HF.
AET can act in a preventive and/or therapeutic way for a number of non-communicable chronic diseases.105,106 Among several abnormalities observed in HF, one of the main features is early muscle fatigue leading to exercise intolerance, and this is related to reduced peak oxygen consumption.107,108 In fact, lower aerobic capacity is strongly related with precocious death in healthy subjects and those with cardiovascular disease.109 More than a decade ago, high-intensity AET was proposed as an alternative to moderate-intensity AET for stimulating higher levels of peak oxygen consumption in HF patients,110 but the effects of both AET protocols on muscle indices are similar.111 Therefore, while AET plays an important role as an adjuvant therapy for counteracting skeletal muscle defects, an intriguing question remains whether RET might be a more effective strategy. Therefore, we will briefly review how AET and RET could benefit HF patients by impacting muscle mass, regeneration, and function, as summarized in Fig. 1.
5.1. Muscle mass
Protein synthesis is essential for maintaining muscle mass, and this seems to be modulated by exercise training in HF. Previous studies showed that 8 weeks of moderate AET (treadmill) activated the Akt/mTORC1 signaling pathway to counteract muscle wasting in an experimental model of HF.12 The same type of exercise modulated that pathway in VL muscle samples from patients (e.g., IGF-1 expression was higher 6 months after training).112
It has been widely reported that the HF-related overactivation of UPS in skeletal muscle is due to increased oxidative stress levels.113, 114, 115 In HF patients and animal models, AET has been found to induce anti-inflammatory effects in addition to improving antioxidant defenses, mainly by reducing the pro-inflammatory cytokines of tumor necrosis factor-α and interleukin-6 muscle expression116,117 and by increasing glutathione peroxidase 1 and catalase enzyme activities.117 It was also shown that MuRF-1 expression decreased after 12 weeks of AET in HF patients, which was strongly correlated with lower proteasome activity and decreased myofiber size compared to non-trained HF patients.22,118 In addition, moderate AET has been shown to help re-establish proteasome homeostasis to attenuate muscle wasting in both animal models and patients.119 Regarding other key proteolytic systems, such as autophagy, further studies will be necessary to clarify the impact of AET on HF.
Previously, RET was avoided by cardiac patients because it was considered to be a potential cause of adverse ventricular remodeling due to high-pressure loads during weightlifting.120 However, evidence from the past 2 decades points to the contrary, and recommends RET (Fig. 1) across a range of clinical populations.121,122 Indeed, RET provides many beneficial effects not only in terms of muscle strength and function,123 but in terms of overall full-body mobility124 and mental health125 as well. HF patients may also experience skeletal muscle hypertrophy at the whole-muscle level126 although a lack of evidence remains available to firmly support this suggestion (especially at the myofiber level) indicative of anabolic resistance.127, 128, 129 In an MI model, 4 weeks of RET was found to restore limb muscle weight (relative to body mass) and muscle fiber area to that of sham operated animals.130 This was associated with the reduction of MuRF-1 and muscle atrophy F-box mRNA expression to control levels, decreases in myostatin protein expression, and increases in factors associated with muscle growth.130 Interestingly, AET also restored muscle mass and fiber area in the same study, and both RET and AET were able to re-establish antioxidant capacity and then reduce oxidative stress.130 Similarly, it was found that high- and moderate-intensity AET restored cross sectional area, mitochondrial function, antioxidant activity, and reversed proteolytic signaling in an MI experimental model.24 Likewise, maintaining mitochondrial function through targeted anti-oxidant treatment prevented immobilization-induced limb muscle atrophy.131 Collectively, therefore, these studies suggest that both aerobic and resistance exercise may prevent atrophy by reducing oxidative stress, in turn blunting catabolic signaling.132
5.2. Contractile dysfunction
While most studies have focused on AET in HF, a study where HF patients performed 18 weeks of RET showed improvement in muscle strength despite a lack of myofiber hypertrophy.31 This could have resulted from improvements in force production for a given level of Ca2+, as is seen with aging.133 Thus, it is important to realize that RET or AET may be of benefit to the contractile function of muscle in HF patients independent of muscle mass gains. Alternatively, blood flow restriction exercise in the form of resistance exercise and aerobic exercise134 showed benefits (e.g., in functional capacity, isometric strength, endurance, and quality of life) in HF patients after 6 weeks without concomitant increases in mass.135
The mechanisms by which exercise training benefits contractile function in HF are still being revealed, but one of them appears to be related to HF-induced NMJ fragmentation, which has negative effects on muscle mass.81 AET has been shown to reduce the proportion of fragmented NMJs in aged mice,136 and as such, this type of exercise may play a role in preserving muscle mass in HF through the same mechanism.
Another probable mechanism is related to the HF-induced Ca2+ dysfunction of myofibers. It is known that exercise training increases expression of DHPR, RYR1, and SERCA proteins,137 with experimental models suggesting a link between improved exercise tolerance in HF with AET and restored expression of Ca2+-related proteins, in particular DHPR, RYR1, SERCA1, and SERCA2.138 Studies have shown that restoring Ca2+ homeostasis in skeletal muscle in HF may be achievable via pharmacological treatment that mimics exercise-related benefits. For example, the use of RYR1 stabilizing agent S107 improves exercise tolerance in diaphragm139 and limb muscles94 by reducing Ca2+ leaks through improved FKBP12 binding. A rat model of HF using abdominal aortic coarctation improved limb muscle fatigue resistance and perfusion after 4 weeks of AET (voluntary wheel running); these same markers also improved in rats subject to 2 weeks of overload (a potent angiogenic stimulus akin to RET).140 This suggests a close link between peripheral vascular and contractile function in HF. Improved mitochondrial function also occurs after AET (and blood flow restriction exercise in the form of resistance exercise135), likely improving oxidative capacity to enhance fatigue resistance while reducing ROS production to alleviate associated myofilament damage. Reducing ROS production through exercise may result in reductions in mitochondrial ROS through the use of a mitochondrial-targeted antioxidant-maintained maximal specific force in the diaphragm of mice with experimental HF.80
HF is also associated with respiratory muscle weakness, and the effects of both AET and inspiratory muscle training in HF patients were investigated by this. It showed that both protocols are safe and effective in HF for improving quality of life and enhancing muscle mass, leg blood flow, and overall functional capacity.141 More direct studies assessing fiber contractile function in the diaphragm have used HF experimental models. For example, 9 weeks of AET prevented diaphragm dysfunction in post-MI HF mice, and such effects were associated with attenuated proteolytic pathway expression (UPS and calpain) and oxidative contractile protein modifications (actin and creatine kinase), likely via the upregulation of antioxidant enzyme expression.60 Similar findings have been reported in pre-HF animal models of hypertension where 4 weeks of high intensity interval training prevented diaphragm dysfunction.142
5.3. Satellite cells
Limited evidence exists connecting the effects of exercise training and SC dynamics in HF. SCs are activated in response to exercise, which is concomitant with an increase in gene transcription of myogenic regulatory factors,143, 144, 145, 146 and an increase in SC content is typically observed.147 In line with this, endurance exercise training has been shown to alleviate declines in SC content as well as impairments in proliferation and differentiation capacity in aged rodents.148,149 Running performance is also positively correlated with SC content in the rats’ muscle.150 Whether similar exercise interventions can alleviate SC impairments and thus exercise intolerance in HF is unknown. One study proposed the importance of myofiber capillarization for the SC response to RET-induced muscle hypertrophy; thus, healthy young men and women underwent aerobic conditioning for 6 weeks followed by 10 weeks of RET in order to investigate how prior aerobic conditioning alters SC content, activity, and myofiber hypertrophy.151 Those with the greatest capillary-to-fiber perimeter exchange index before RET had the greatest change in muscle hypertrophy. Importantly, SC content, activation, and differentiation increased more in the Type I myofiber, which may in part be modulated by enhanced capillarity given the close relationship between the SC and the endothelial niche.151 Moreover, baseline capillarization has been found to be predictive of hypertrophic response in older people,152,153 who are thought to demonstrate anabolic resistance.154 HF patients are well known to have reduced capillarity, and a link between blunted hypertrophy and lower capillarization was shown in experimental HF rat models.140 This suggests that aerobic conditioning prior to RET can improve muscle adaption by increasing capillarization, thus reinforcing the idea that engagement in a regular exercise training program involving both aerobic and strength conditioning can be a reliable strategy to counteract HF-induced muscle wasting and dysfunction.
6. Contribution of aging and physical inactivity to the skeletal muscle phenotype in HF
Given the greater prevalence of HF in older people97 and the negative effects of aging on skeletal muscle, it is difficult to separate out the contribution of HF per se to skeletal muscle dysfunction. Indeed, in both aging and HF, the diaphragm seems to demonstrate a reduction in fiber cross-sectional area85,155 (although this isn't always true in HF78). Similarly, the isometric force of limb and diaphragm muscle is decreased in both aging (i.e., sarcopenia)98,99 and HF,78 but reductions in myofibrillar protein content do not account for all impairments in function.156 Interestingly, skeletal muscle fatigue resistance is maintained157 or improved with age but impaired in HF,158 and unfortunately, HF risk is increased with sedentary behavior even while exercise intolerance limits the physical activity of HF patients.100 However, while many of the symptoms of HF may be attributable to inactivity,159 a number of studies have confirmed the effects of HF are independent of inactivity160,161 and age. For example, most animal studies of HF use young animals, who still develop muscle dysfunction, and data indicate muscle alterations are induced independent of age, with young and old patients responding similarly to exercise training.22 Therefore, some but not all muscle alterations in HF can be explained by disuse and aging, which clearly indicates the existence of a muscle pathology.
7. Conclusion and future perspectives
In this review, we have demonstrated promising progress in understanding the basic mechanisms that underpin perturbed skeletal muscle health in HF. This knowledge is pertinent given that HF is one of the most common causes of hospitalization15 and that low skeletal muscle mass is an independent risk factor for mortality in HF.162 The problem is compounded in societies with aging populations as HF is more prevalent in older people,163 of whom 10% are estimated to have sarcopenia.164 The mechanisms involved include impairments in SC proliferation, anabolic–catabolic signaling, and myofiber calcium homeostasis. Importantly, we have also shown that exercise, particularly AET, attenuates a number of these impairments in the context of HF (Fig. 1). Despite this, future research is required to investigate the specific role played by SCs in skeletal muscle dysfunction. Moreover, while it is well established that exercise can reverse some skeletal muscle deficits in HF, we have a poor understanding of how this is achieved, which limits the potential benefits of exercise prescription. Greater scientific understanding of the mechanisms by which exercise improves skeletal muscle health in HF would provide targets for pharmacological mimetics for bedridden patients unable to perform physical activity, which at present can only provide limited benefit.165
Acknowledgments
Acknowledgments
This work was supported by Heart Research UK (Grant number 119191) and British Heart Foundation (Grant number 124055). Fig. 1 was created using BioRender.com (https://www.biorender.com/).
Authors’ contributions
HG, PWH, MGP, and TSB conceived, planned, drafted, edited, and revised the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Data access statement
The authors declare that no original data are associated with this article.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials
References
- 1.Gonzalez-Freire M, Semba RD, Ubaida-Mohien C, et al. The human skeletal muscle proteome project: A reappraisal of the current literature. J Cachexia Sarcopenia Muscle. 2017;8:5–18. doi: 10.1002/jcsm.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Severinsen MCK, Pedersen BK. Muscle–organ crosstalk: The emerging roles of myokines. Endocr Rev. 2020;41:594–609. doi: 10.1210/endrev/bnaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hakkinen K, Newton RU, Walker S, et al. Effects of upper body eccentric versus concentric strength training and detraining on maximal force, muscle activation, hypertrophy and serum hormones in women. J Sports Sci Med. 2022;21:200–213. doi: 10.52082/jssm.2022.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lena A, Anker MS, Springer J. Muscle wasting and sarcopenia in heart failure—The current state of science. Int J Mol Sci. 2020;21:6549. doi: 10.3390/ijms21186549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans WJ, Morley JE, Argiles J, et al. Cachexia: A new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Kimura M, Naito T, Kenmotsu H, et al. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support Care Cancer. 2015;23:1699–1708. doi: 10.1007/s00520-014-2534-3. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T, Palus S, Springer J. Skeletal muscle wasting in chronic heart failure. ESC Heart Fail. 2018;5:1099–1107. doi: 10.1002/ehf2.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nosacka RL, Delitto AE, Delitto D, et al. Distinct cachexia profiles in response to human pancreatic tumours in mouse limb and respiratory muscle. J Cachexia Sarcopenia Muscle. 2020;11:820–837. doi: 10.1002/jcsm.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts BM, Ahn B, Smuder AJ, et al. Diaphragm and ventilatory dysfunction during cancer cachexia. FASEB J. 2013;27:2600–2610. doi: 10.1096/fj.12-222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyart E, Bindels LB, Mina E, Menga A, Stanga S, Porporato PE. Cachexia, a systemic disease beyond muscle atrophy. Int J Mol Sci. 2020;21:8592. doi: 10.3390/ijms21228592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen L, Idorn M, Olofsson GH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23:554–562. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Bacurau AV, Jannig PR, de Moraes WM, et al. Akt/mTOR pathway contributes to skeletal muscle anti-atrophic effect of aerobic exercise training in heart failure mice. Int J Cardiol. 2016;214:137–147. doi: 10.1016/j.ijcard.2016.03.071. [DOI] [PubMed] [Google Scholar]
- 13.Pereira MG, Voltarelli VA, Tobias GC, et al. Aerobic exercise training and in vivo Akt activation counteract cancer cachexia by inducing a hypertrophic profile through eIF-2α modulation. Cancers (Basel) 2021;14:28. doi: 10.3390/cancers14010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alves CRR, Neves WD, de Almeida NR, et al. Exercise training reverses cancer-induced oxidative stress and decrease in muscle COPS2/TRIP15/ALIEN. Mol Metab. 2020;39 doi: 10.1016/j.molmet.2020.101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salah HM, Minhas AMK, Khan MS, et al. Causes of hospitalization in the USA between 2005 and 2018. Eur Heart J Open. 2021;1:oeab001. doi: 10.1093/ehjopen/oeab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rana JS, Khan SS, Lloyd-Jones DM, Sidney S. Changes in mortality in top 10 causes of death from 2011 to 2018. J Gen Intern Med. 2021;36:2517–2518. doi: 10.1007/s11606-020-06070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benge W, Litchfield RL, Marcus ML. Exercise capacity in patients with severe left ventricular dysfunction. Circulation. 1980;61:955–959. doi: 10.1161/01.cir.61.5.955. [DOI] [PubMed] [Google Scholar]
- 18.Franciosa JA, Park M, Levine TB. Lack of correlation between exercise capacity and indexes of resting left ventricular performance in heart failure. Am J Cardiol. 1981;47:33–39. doi: 10.1016/0002-9149(81)90286-1. [DOI] [PubMed] [Google Scholar]
- 19.Singh A, Yadav A, Phogat J, Dabur R. Dynamics and interplay between autophagy and ubiquitin-proteasome system coordination in skeletal muscle atrophy. Curr Mol Pharmacol. 2022;15:475–486. doi: 10.2174/1874467214666210806163851. [DOI] [PubMed] [Google Scholar]
- 20.Talbert EE, Smuder AJ, Min K, Kwon OS, Powers SK. Calpain and caspase-3 play required roles in immobilization-induced limb muscle atrophy. J Appl Physiol (1985) 2013;114:1482–1489. doi: 10.1152/japplphysiol.00925.2012. [DOI] [PubMed] [Google Scholar]
- 21.Bekfani T, Bekhite Elsaied M, Derlien S, et al. Skeletal muscle function, structure, and metabolism in patients with heart failure with reduced ejection fraction and heart failure with preserved ejection fraction. CircHeart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007198. [DOI] [PubMed] [Google Scholar]
- 22.Gielen S, Sandri M, Kozarez I, et al. Exercise training attenuates MuRF-1 expression in the skeletal muscle of patients with chronic heart failure independent of age: The randomized Leipzig Exercise Intervention in Chronic Heart Failure and Aging catabolism study. Circulation. 2012;125:2716–2727. doi: 10.1161/CIRCULATIONAHA.111.047381. [DOI] [PubMed] [Google Scholar]
- 23.Adams V, Wunderlich S, Mangner N, et al. Ubiquitin-proteasome-system and enzymes of energy metabolism in skeletal muscle of patients with HFpEF and HFrEF. ESC Heart Fail. 2021;8:2556–2568. doi: 10.1002/ehf2.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreira JB, Bechara LR, Bozi LH, et al. High- vs. moderate-intensity aerobic exercise training effects on skeletal muscle of infarcted rats. J Appl Physiol (1985) 2013;114:1029–1041. doi: 10.1152/japplphysiol.00760.2012. [DOI] [PubMed] [Google Scholar]
- 25.Milan G, Romanello V, Pescatore F, et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun. 2015;6:6670. doi: 10.1038/ncomms7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowen TS, Herz C, Rolim NPL, et al. Effects of endurance training on detrimental structural, cellular, and functional alterations in skeletal muscles of heart failure with preserved ejection fraction. J Card Fail. 2018;24:603–613. doi: 10.1016/j.cardfail.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Jannig PR, Moreira JB, Bechara LR, et al. Autophagy signaling in skeletal muscle of infarcted rats. PLoS One. 2014;9:e85820. doi: 10.1371/journal.pone.0085820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szaroszyk M, Kattih B, Martin-Garrido A, et al. Skeletal muscle derived musclin protects the heart during pathological overload. Nat Commun. 2022;13:149. doi: 10.1038/s41467-021-27634-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hambrecht R, Schulze PC, Gielen S, et al. Reduction of insulin-like growth factor-I expression in the skeletal muscle of noncachectic patients with chronic heart failure. J Am Coll Cardiol. 2002;39:1175–1181. doi: 10.1016/s0735-1097(02)01736-9. [DOI] [PubMed] [Google Scholar]
- 31.Toth MJ, Ward K, van der Velden J, et al. Chronic heart failure reduces Akt phosphorylation in human skeletal muscle: Relationship to muscle size and function. J Appl Physiol (1985) 2011;110:892–900. doi: 10.1152/japplphysiol.00545.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulze PC, Fang J, Kassik KA, et al. Transgenic overexpression of locally acting insulin-like growth factor-1 inhibits ubiquitin-mediated muscle atrophy in chronic left-ventricular dysfunction. Circ Res. 2005;97:418–426. doi: 10.1161/01.RES.0000179580.72375.c2. [DOI] [PubMed] [Google Scholar]
- 33.Vest AR, Wong WW, Chery J, et al. Skeletal muscle mass recovery early after left ventricular assist device implantation in patients with advanced systolic heart failure. Circ Heart Fail. 2022;15 doi: 10.1161/CIRCHEARTFAILURE.121.009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lv J, Li Y, Shi S, et al. Skeletal muscle mitochondrial remodeling in heart failure: An update on mechanisms and therapeutic opportunities. Biomed Pharmacother. 2022;155 doi: 10.1016/j.biopha.2022.113833. [DOI] [PubMed] [Google Scholar]
- 35.Tezze C, Romanello V, Desbats MA, et al. Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab. 2017;25:1374–1389. doi: 10.1016/j.cmet.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garnham JO, Roberts LD, Caspi T, et al. Divergent skeletal muscle mitochondrial phenotype between male and female patients with chronic heart failure. J Cachexia Sarcopenia Muscle. 2020;11:79–88. doi: 10.1002/jcsm.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guzmán Mentesana G, Báez AL, Lo Presti MS, et al. Functional and structural alterations of cardiac and skeletal muscle mitochondria in heart failure patients. Arch Med Res. 2014;45:237–246. doi: 10.1016/j.arcmed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Esposito F, Mathieu-Costello O, Wagner PD, Richardson RS. Acute and chronic exercise in patients with heart failure with reduced ejection fraction: Evidence of structural and functional plasticity and intact angiogenic signalling in skeletal muscle. J Physiol. 2018;596:5149–5161. doi: 10.1113/JP276678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garnham JO, Roberts LD, Espino-Gonzalez E, et al. Chronic heart failure with diabetes mellitus is characterized by a severe skeletal muscle pathology. J Cachexia Sarcopenia Muscle. 2020;11:394–404. doi: 10.1002/jcsm.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundberg TR, Martinez-Aranda LM, Sanz G, et al. Early accentuated muscle hypertrophy is strongly associated with myonuclear accretion. Am J Physiol Regul Integr Comp Physiol. 2020;319:R50–R58. doi: 10.1152/ajpregu.00061.2020. [DOI] [PubMed] [Google Scholar]
- 41.Jejurikar SS, Kuzon Jr WM. Satellite cell depletion in degenerative skeletal muscle. Apoptosis. 2003;8:573–578. doi: 10.1023/A:1026127307457. [DOI] [PubMed] [Google Scholar]
- 42.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zammit PS, Relaix F, Nagata Y, et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- 44.Snijders T, Verdijk LB, Beelen M, et al. A single bout of exercise activates skeletal muscle satellite cells during subsequent overnight recovery. Exp Physiol. 2012;97:762–773. doi: 10.1113/expphysiol.2011.063313. [DOI] [PubMed] [Google Scholar]
- 45.Zammit PS. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell Dev Biol. 2017;72:19–32. doi: 10.1016/j.semcdb.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Fry CS, Lee JD, Mula J, et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med. 2015;21:76–80. doi: 10.1038/nm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sambasivan R, Yao R, Kissenpfennig A, et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 49.Egner IM, Bruusgaard JC, Gundersen K. Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development. 2016;143:2898–2906. doi: 10.1242/dev.134411. [DOI] [PubMed] [Google Scholar]
- 50.McCarthy JJ, Mula J, Miyazaki M, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murach KA, White SH, Wen Y, et al. Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice. Skelet Muscle. 2017;7:14. doi: 10.1186/s13395-017-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Englund DA, Murach KA, Dungan CM, et al. Depletion of resident muscle stem cells negatively impacts running volume, physical function, and muscle fiber hypertrophy in response to lifelong physical activity. Am J Physiol Cell Physiol. 2020;318:C1178–C1188. doi: 10.1152/ajpcell.00090.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.García-Prat L, Martínez-Vicente M, Perdiguero E, et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529:37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- 54.Pomiès P, Rodriguez J, Blaquière M, et al. Reduced myotube diameter, atrophic signalling and elevated oxidative stress in cultured satellite cells from COPD patients. J Cell Mol Med. 2015;19:175–186. doi: 10.1111/jcmm.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song T, Manoharan P, Millay DP, et al. Dilated cardiomyopathy-mediated heart failure induces a unique skeletal muscle myopathy with inflammation. Skelet Muscle. 2019;9:4. doi: 10.1186/s13395-019-0189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdellatif M, Trummer-Herbst V, Koser F, et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci Transl Med. 2021;13:eabd7064. doi: 10.1126/scitranslmed.abd7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshida T, Galvez S, Tiwari S, et al. Angiotensin II inhibits satellite cell proliferation and prevents skeletal muscle regeneration. J Biol Chem. 2013;288:23823–23832. doi: 10.1074/jbc.M112.449074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Du Bois P, Pablo Tortola C, Lodka D, et al. Angiotensin ii induces skeletal muscle atrophy by activating TFEB-mediated MuRF1 expression. Circ Res. 2015;117:424–436. doi: 10.1161/CIRCRESAHA.114.305393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sente T, Van Berendoncks AM, Jonckheere AI, et al. Primary skeletal muscle myoblasts from chronic heart failure patients exhibit loss of anti-inflammatory and proliferative activity. BMC Cardiovasc Disord. 2016;16:107. doi: 10.1186/s12872-016-0278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mangner N, Bowen TS, Werner S, et al. Exercise training prevents diaphragm contractile dysfunction in heart failure. Med Sci Sports Exerc. 2016;48:2118–2124. doi: 10.1249/MSS.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 61.Kelley RC, Ferreira LF. Diaphragm abnormalities in heart failure and aging: Mechanisms and integration of cardiovascular and respiratory pathophysiology. Heart Fail Rev. 2017;22:191–207. doi: 10.1007/s10741-016-9549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrington D, Anker SD, Chua TP, et al. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30:1758–1764. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 63.Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177–180. [PMC free article] [PubMed] [Google Scholar]
- 64.Schaap LA, van Schoor NM, Lips P, Visser M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: The Longitudinal Aging Study Amsterdam. J Gerontol A Biol Sci Med Sci. 2018;73:1199–1204. doi: 10.1093/gerona/glx245. [DOI] [PubMed] [Google Scholar]
- 65.Yang NP, Hsu NW, Lin CH, et al. Relationship between muscle strength and fall episodes among the elderly: The Yilan study, Taiwan, China. BMC Geriatr. 2018;18:90. doi: 10.1186/s12877-018-0779-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lunde PK, Sejersted OM, Thorud HM, et al. Effects of congestive heart failure on Ca2+ handling in skeletal muscle during fatigue. Circ Res. 2006;98:1514–1519. doi: 10.1161/01.RES.0000226529.66545.e5. [DOI] [PubMed] [Google Scholar]
- 67.Ward CW, Reiken S, Marks AR, Marty I, Vassort G, Lacampagne A. Defects in ryanodine receptor calcium release in skeletal muscle from post-myocardial infarct rats. FASEB J. 2003;17:1517–1519. doi: 10.1096/fj.02-1083fje. [DOI] [PubMed] [Google Scholar]
- 68.MacFarlane NG, Darnley GM, Smith GL. Cellular basis for contractile dysfunction in the diaphragm from a rabbit infarct model of heart failure. Am J Physiol Cell Physiol. 2000;278:C739–C746. doi: 10.1152/ajpcell.2000.278.4.C739. [DOI] [PubMed] [Google Scholar]
- 69.Perreault CL, Gonzalez-Serratos H, Litwin SE, Sun X, Franzini-Armstrong C, Morgan JP. Alterations in contractility and intracellular Ca2+ transients in isolated bundles of skeletal muscle fibers from rats with chronic heart failure. Circ Res. 1993;73:405–412. doi: 10.1161/01.res.73.2.405. [DOI] [PubMed] [Google Scholar]
- 70.Peters DG, Mitchell HL, McCune SA, Park S, Williams JH, Kandarian SC. Skeletal muscle sarcoplasmic reticulum Ca(2+)-ATPase gene expression in congestive heart failure. Circ Res. 1997;81:703–710. doi: 10.1161/01.res.81.5.703. [DOI] [PubMed] [Google Scholar]
- 71.Middlekauff HR, Vigna C, Verity MA, et al. Abnormalities of calcium handling proteins in skeletal muscle mirror those of the heart in humans with heart failure: A shared mechanism? J Card Fail. 2012;18:724–733. doi: 10.1016/j.cardfail.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mangner N, Garbade J, Heyne E, et al. Molecular mechanisms of diaphragm myopathy in humans with severe heart failure. Circ Res. 2021;128:706–719. doi: 10.1161/CIRCRESAHA.120.318060. [DOI] [PubMed] [Google Scholar]
- 73.Agrawal A, Suryakumar G, Rathor R. Role of defective Ca(2+) signaling in skeletal muscle weakness: Pharmacological implications. J Cell Commun Signal. 2018;12:645–659. doi: 10.1007/s12079-018-0477-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wehrens XH, Lehnart SE, Reiken S, et al. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc Natl Acad Sci U S A. 2005;102:9607–9612. doi: 10.1073/pnas.0500353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reiken S, Lacampagne A, Zhou H, et al. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: Defective regulation in heart failure. J Cell Biol. 2003;160:919–928. doi: 10.1083/jcb.200211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rullman E, Andersson DC, Melin M, et al. Modifications of skeletal muscle ryanodine receptor type 1 and exercise intolerance in heart failure. J Heart Lung Transplant. 2013;32:925–929. doi: 10.1016/j.healun.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szentesi P, Bekedam MA, van Beek-Harmsen BJ, et al. Depression of force production and ATPase activity in different types of human skeletal muscle fibers from patients with chronic heart failure. J Appl Physiol (1985) 2005;99:2189–2195. doi: 10.1152/japplphysiol.00542.2005. [DOI] [PubMed] [Google Scholar]
- 78.van Hees HW, van der Heijden HF, Ottenheijm CA, et al. Diaphragm single-fiber weakness and loss of myosin in congestive heart failure rats. Am J Physiol Heart Circ Physiol. 2007;293:H819–H828. doi: 10.1152/ajpheart.00085.2007. [DOI] [PubMed] [Google Scholar]
- 79.Ahn B, Coblentz PD, Beharry AW, et al. Diaphragm abnormalities in patients with end-stage heart failure: NADPH oxidase upregulation and protein oxidation. Front Physiol. 2017;7:686. doi: 10.3389/fphys.2016.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laitano O, Ahn B, Patel N, et al. Pharmacological targeting of mitochondrial reactive oxygen species counteracts diaphragm weakness in chronic heart failure. J Appl Physiol (1985) 2016;120:733–742. doi: 10.1152/japplphysiol.00822.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steinbeck L, Ebner N, Valentova M, et al. Detection of muscle wasting in patients with chronic heart failure using C-terminal agrin fragment: Results from the studies investigating co-morbidities aggravating heart failure (SICA-HF) Eur J Heart Fail. 2015;17:1283–1293. doi: 10.1002/ejhf.400. [DOI] [PubMed] [Google Scholar]
- 82.Willadt S, Nash M, Slater CR. Age-related fragmentation of the motor endplate is not associated with impaired neuromuscular transmission in the mouse diaphragm. Sci Rep. 2016;6:24849. doi: 10.1038/srep24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Minotti JR, Pillay P, Chang L, Wells L, Massie BM. Neurophysiological assessment of skeletal muscle fatigue in patients with congestive heart failure. Circulation. 1992;86:903–908. doi: 10.1161/01.cir.86.3.903. [DOI] [PubMed] [Google Scholar]
- 84.Seiler M, Bowen TS, Rolim N, et al. Skeletal muscle alterations are exacerbated in heart failure with reduced compared with preserved ejection fraction: Mediated by circulating cytokines? Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.116.003027. [DOI] [PubMed] [Google Scholar]
- 85.Dominguez JF, Howell S. Compartmental analysis of steady-state diaphragm Ca2+ kinetics in chronic congestive heart failure. Cell Calcium. 2003;33:163–174. doi: 10.1016/s0143-4160(02)00208-7. [DOI] [PubMed] [Google Scholar]
- 86.Huang J, Forsberg NE. Role of calpain in skeletal-muscle protein degradation. Proc Natl Acad Sci U S A. 1998;95:12100–12105. doi: 10.1073/pnas.95.21.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salazar JJ, Michele DE, Brooks SV. Inhibition of calpain prevents muscle weakness and disruption of sarcomere structure during hindlimb suspension. J Appl Physiol (1985) 2010;108:120–127. doi: 10.1152/japplphysiol.01080.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ahn B, Beharry AW, Frye GS, Judge AR, Ferreira LF. NAD(P)H oxidase subunit p47phox is elevated, and p47phox knockout prevents diaphragm contractile dysfunction in heart failure. Am J Physiol Lung Cell Mol Physiol. 2015;309:L497–L505. doi: 10.1152/ajplung.00176.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bowen TS, Mangner N, Werner S, et al. Diaphragm muscle weakness in mice is early-onset post-myocardial infarction and associated with elevated protein oxidation. J Appl Physiol (1985) 2015;118:11–19. doi: 10.1152/japplphysiol.00756.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bowen TS, Rolim NP, Fischer T, et al. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur J Heart Fail. 2015;17:263–272. doi: 10.1002/ejhf.239. [DOI] [PubMed] [Google Scholar]
- 91.Coblentz PD, Ahn B, Hayward LF, Yoo JK, Christou DD, Ferreira LF. Small-hairpin RNA and pharmacological targeting of neutral sphingomyelinase prevent diaphragm weakness in rats with heart failure and reduced ejection fraction. Am J Physiol Lung Cell Mol Physiol. 2019;316:L679–L690. doi: 10.1152/ajplung.00516.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nambu H, Takada S, Maekawa S, et al. Inhibition of xanthine oxidase in the acute phase of myocardial infarction prevents skeletal muscle abnormalities and exercise intolerance. Cardiovasc Res. 2021;117:805–819. doi: 10.1093/cvr/cvaa127. [DOI] [PubMed] [Google Scholar]
- 93.Hahn D, Kumar RA, Muscato DR, Ryan TE, Schröder K, Ferreira LF. Nox4 knockout does not prevent diaphragm atrophy, contractile dysfunction, or mitochondrial maladaptation in the early phase post-myocardial infarction in mice. Cell Physiol Biochem. 2021;55:489–504. doi: 10.33594/000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bellinger AM, Mongillo M, Marks AR. Stressed out: The skeletal muscle ryanodine receptor as a target of stress. J Clin Invest. 2008;118:445–453. doi: 10.1172/JCI34006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dridi H, Yehya M, Barsotti R, et al. Mitochondrial oxidative stress induces leaky ryanodine receptor during mechanical ventilation. Free Radic Biol Med. 2020;146:383–391. doi: 10.1016/j.freeradbiomed.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 96.Jang YC, Lustgarten MS, Liu Y, et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24:1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Christiansen MN, Kober L, Weeke P, et al. Age-specific trends in incidence, mortality, and comorbidities of heart failure in Denmark, 1995 to 2012. Circulation. 2017;135:1214–1223. doi: 10.1161/CIRCULATIONAHA.116.025941. [DOI] [PubMed] [Google Scholar]
- 98.Delbono O, O'Rourke KS, Ettinger WH. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J Membr Biol. 1995;148:211–222. doi: 10.1007/BF00235039. [DOI] [PubMed] [Google Scholar]
- 99.Weisleder N, Ma J. Altered Ca2+ sparks in aging skeletal and cardiac muscle. Ageing Res Rev. 2008;7:177–188. doi: 10.1016/j.arr.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pandey A, LaMonte M, Klein L, et al. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol. 2017;69:1129–1142. doi: 10.1016/j.jacc.2016.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim JH, Thompson LV. Inactivity, age, and exercise: Single-muscle fiber power generation. J Appl Physiol (1985) 2013;114:90–98. doi: 10.1152/japplphysiol.00525.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Larsson L, Li X, Berg HE, Frontera WR. Effects of removal of weight-bearing function on contractility and myosin isoform composition in single human skeletal muscle cells. Pflugers Arch. 1996;432:320–328. doi: 10.1007/s004240050139. [DOI] [PubMed] [Google Scholar]
- 103.Sartori R, Romanello V, Sandri M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat Commun. 2021;12:330. doi: 10.1038/s41467-020-20123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Belardinelli R, Georgiou D, Cianci G, Purcaro A. 10-year exercise training in chronic heart failure: A randomized controlled trial. J Am Coll Cardiol. 2012;60:1521–1528. doi: 10.1016/j.jacc.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 105.Neilson HK, Friedenreich CM, Brockton NT, Millikan RC. Physical activity and postmenopausal breast cancer: Proposed biologic mechanisms and areas for future research. Cancer Epidemiol Biomarkers Prev. 2009;18:11–27. doi: 10.1158/1055-9965.EPI-08-0756. [DOI] [PubMed] [Google Scholar]
- 106.Schmidt SF, Rohm M, Herzig S, Berriel Diaz M. Cancer cachexia: More than skeletal muscle wasting. Trends Cancer. 2018;4:849–860. doi: 10.1016/j.trecan.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 107.Gademan MG, van der Laarse A, Swenne CA, van der Wall EE. Oxygen uptake in heart failure: How much, how fast? Neth Heart J. 2009;17:224–225. doi: 10.1007/BF03086251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kemps HM, Schep G, Hoogsteen J, et al. Oxygen uptake kinetics in chronic heart failure: Clinical and physiological aspects. Neth Heart J. 2009;17:238–244. doi: 10.1007/BF03086254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kokkinos P, Myers J, Kokkinos JP, et al. Exercise capacity and mortality in black and white men. Circulation. 2008;117:614–622. doi: 10.1161/CIRCULATIONAHA.107.734764. [DOI] [PubMed] [Google Scholar]
- 110.Wisløff U, Støylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 111.Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999;129(Suppl.1):S227–S237. doi: 10.1093/jn/129.1.227S. [DOI] [PubMed] [Google Scholar]
- 112.Heineke J, Auger-Messier M, Xu J, et al. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010;121:419–425. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007;35:411–429. doi: 10.1002/mus.20743. [DOI] [PubMed] [Google Scholar]
- 114.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: Role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R337–R344. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- 115.Tisdale MJ. The ubiquitin-proteasome pathway as a therapeutic target for muscle wasting. J Support Oncol. 2005;3:209–217. [PubMed] [Google Scholar]
- 116.Gielen S, Adams V, Möbius-Winkler S, et al. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42:861–868. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 117.Linke A, Adams V, Schulze PC, et al. Antioxidative effects of exercise training in patients with chronic heart failure: Increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111:1763–1770. doi: 10.1161/01.CIR.0000165503.08661.E5. [DOI] [PubMed] [Google Scholar]
- 118.Hollriegel R, Beck EB, Linke A, et al. Anabolic effects of exercise training in patients with advanced chronic heart failure (NYHA IIIb): Impact on ubiquitin-protein ligases expression and skeletal muscle size. Int J Cardiol. 2013;167:975–980. doi: 10.1016/j.ijcard.2012.03.083. [DOI] [PubMed] [Google Scholar]
- 119.Cunha TF, Bacurau AV, Moreira JB, et al. Exercise training prevents oxidative stress and ubiquitin-proteasome system overactivity and reverse skeletal muscle atrophy in heart failure. PLoS One. 2012;7:e41701. doi: 10.1371/journal.pone.0041701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Price KJ, Gordon BA, Bird SR, Benson AC. A review of guidelines for cardiac rehabilitation exercise programmes: Is there an international consensus? Eur J Prev Cardiol. 2016;23:1715–1733. doi: 10.1177/2047487316657669. [DOI] [PubMed] [Google Scholar]
- 121.Fisher S, Smart NA, Pearson MJ. Resistance training in heart failure patients: A systematic review and meta-analysis. Heart Fail Rev. 2022;27:1665–1682. doi: 10.1007/s10741-021-10169-8. [DOI] [PubMed] [Google Scholar]
- 122.Levinger I, Bronks R, Cody DV, Linton I, Davie A. The effect of resistance training on left ventricular function and structure of patients with chronic heart failure. Int J Cardiol. 2005;105:159–163. doi: 10.1016/j.ijcard.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 123.Schoenfeld BJ, Ogborn D, Krieger JW. Dose–response relationship between weekly resistance training volume and increases in muscle mass: A systematic review and meta-analysis. J Sports Sci. 2017;35:1073–1082. doi: 10.1080/02640414.2016.1210197. [DOI] [PubMed] [Google Scholar]
- 124.Jadczak AD, Makwana N, Luscombe-Marsh N, Visvanathan R, Schultz TJ. Effectiveness of exercise interventions on physical function in community-dwelling frail older people: An umbrella review of systematic reviews. JBI Database System Rev Implement Rep. 2018;16:752–775. doi: 10.11124/JBISRIR-2017-003551. [DOI] [PubMed] [Google Scholar]
- 125.Gordon BR, McDowell CP, Hallgren M, Meyer JD, Lyons M, Herring MP. Association of efficacy of resistance exercise training with depressive symptoms: Meta-analysis and meta-regression analysis of randomized clinical trials. JAMA Psychiatry. 2018;75:566–576. doi: 10.1001/jamapsychiatry.2018.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Magnusson G, Gordon A, Kaijser L, et al. High intensity knee extensor training, in patients with chronic heart failure, Major skeletal muscle improvement. Eur Heart J. 1996;17:1048–1055. doi: 10.1093/oxfordjournals.eurheartj.a015001. [DOI] [PubMed] [Google Scholar]
- 127.Pu CT, Johnson MT, Forman DE, et al. Randomized trial of progressive resistance training to counteract the myopathy of chronic heart failure. J Appl Physiol (1985) 2001;90:2341–2350. doi: 10.1152/jappl.2001.90.6.2341. [DOI] [PubMed] [Google Scholar]
- 128.Jankowska EA, Wegrzynowska K, Superlak M, et al. The 12-week progressive quadriceps resistance training improves muscle strength, exercise capacity and quality of life in patients with stable chronic heart failure. Int J Cardiol. 2008;130:36–43. doi: 10.1016/j.ijcard.2007.07.158. [DOI] [PubMed] [Google Scholar]
- 129.Toth MJ, Miller MS, VanBuren P, et al. Resistance training alters skeletal muscle structure and function in human heart failure: Effects at the tissue, cellular and molecular levels. J Physiol. 2012;590:1243–1259. doi: 10.1113/jphysiol.2011.219659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cai M, Wang Q, Liu Z, Jia D, Feng R, Tian Z. Effects of different types of exercise on skeletal muscle atrophy, antioxidant capacity and growth factors expression following myocardial infarction. Life Sci. 2018;213:40–49. doi: 10.1016/j.lfs.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 131.Min K, Smuder AJ, Kwon OS, Kavazis AN, Szeto HH, Powers SK. Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J Appl Physiol (1985) 2011;111:1459–1466. doi: 10.1152/japplphysiol.00591.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ren W, Xu Z, Pan S, et al. Irisin and ALCAT1 mediated aerobic exercise-alleviated oxidative stress and apoptosis in skeletal muscle of mice with myocardial infarction. Free Radic Biol Med. 2022;193:526–537. doi: 10.1016/j.freeradbiomed.2022.10.321. [DOI] [PubMed] [Google Scholar]
- 133.Godard MP, Gallagher PM, Raue U, Trappe SW. Alterations in single muscle fiber calcium sensitivity with resistance training in older women. Pflugers Arch. 2002;444:419–425. doi: 10.1007/s00424-002-0821-1. [DOI] [PubMed] [Google Scholar]
- 134.Cahalin LP, Formiga MF, Owens J, Anderson B, Hughes L. Beneficial role of blood flow restriction exercise in heart disease and heart failure using the muscle hypothesis of chronic heart failure and a growing literature. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.924557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Groennebaek T, Sieljacks P, Nielsen R, et al. Effect of blood flow restricted resistance exercise and remote ischemic conditioning onfunctional capacity and myocellular adaptations in patients with heart failure. Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.119.006427. [DOI] [PubMed] [Google Scholar]
- 136.Valdez G, Tapia JC, Kang H, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ferreira JC, Bacurau AV, Bueno Jr CR, et al. Aerobic exercise training improves Ca2+ handling and redox status of skeletal muscle in mice. Exp Biol Med (Maywood) 2010;235:497–505. doi: 10.1258/ebm.2009.009165. [DOI] [PubMed] [Google Scholar]
- 138.Bueno CR, Jr, Ferreira JC, Pereira MG, Bacurau AV, Brum PC. Aerobic exercise training improves skeletal muscle function and Ca2+ handling-related protein expression in sympathetic hyperactivity-induced heart failure. J Appl Physiol (1985) 2010;109:702–709. doi: 10.1152/japplphysiol.00281.2010. [DOI] [PubMed] [Google Scholar]
- 139.Andersson DC, Betzenhauser MJ, Reiken S, et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14:196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tickle PG, Hendrickse PW, Weightman A, Nazir MH, Degens H, Egginton S. Impaired skeletal muscle fatigue resistance during cardiac hypertrophy is prevented by functional overload- or exercise-induced functional capillarity. J Physiol. 2021;599:3715–3733. doi: 10.1113/JP281377. [DOI] [PubMed] [Google Scholar]
- 141.Antunes-Correa LM, Trevizan PF, Bacurau AVN, et al. Effects of aerobic and inspiratory training on skeletal muscle microRNA-1 and downstream-associated pathways in patients with heart failure. J Cachexia Sarcopenia Muscle. 2020;11:89–102. doi: 10.1002/jcsm.12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bowen TS, Brauer D, Rolim NPL, et al. Exercise training reveals inflexibility of the diaphragm in an animal model of patients with obesity-driven heart failure with a preserved ejection fraction. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Caldow MK, Thomas EE, Dale MJ, Tomkinson GR, Buckley JD, Cameron-Smith D. Early myogenic responses to acute exercise before and after resistance training in young men. Physiol Rep. 2015;3:e12511. doi: 10.14814/phy2.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Psilander N, Damsgaard R, Pilegaard H. Resistance exercise alters MRF and IGF-I mRNA content in human skeletal muscle. J Appl Physiol (1985) 2003;95:1038–1044. doi: 10.1152/japplphysiol.00903.2002. [DOI] [PubMed] [Google Scholar]
- 145.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J Appl Physiol (1985) 2006;101:53–59. doi: 10.1152/japplphysiol.01616.2005. [DOI] [PubMed] [Google Scholar]
- 146.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol (1985) 2005;98:1745–1752. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]
- 147.Snijders T, Nederveen JP, McKay BR, et al. Satellite cells in human skeletal muscle plasticity. Front Physiol. 2015;6:283. doi: 10.3389/fphys.2015.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cisterna B, Giagnacovo M, Costanzo M, et al. Adapted physical exercise enhances activation and differentiation potential of satellite cells in the skeletal muscle of old mice. J Anat. 2016;228:771–783. doi: 10.1111/joa.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shefer G, Rauner G, Yablonka-Reuveni Z, Benayahu D. Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PLoS One. 2010;5:e13307. doi: 10.1371/journal.pone.0013307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kurosaka M, Naito H, Ogura Y, Kojima A, Goto K, Katamoto S. Effects of voluntary wheel running on satellite cells in the rat plantaris muscle. J Sports Sci Med. 2009;8:51–57. [PMC free article] [PubMed] [Google Scholar]
- 151.Thomas ACQ, Brown A, Hatt AA, et al. Short-term aerobic conditioning prior to resistance training augments muscle hypertrophy and satellite cell content in healthy young men and women. FASEB J. 2022;36:e22500. doi: 10.1096/fj.202200398RR. [DOI] [PubMed] [Google Scholar]
- 152.Moro T, Brightwell CR, Phalen DE, et al. Low skeletal musclecapillarization limits muscle adaptation to resistance exercise training in older adults. Exp Gerontol. 2019;127 doi: 10.1016/j.exger.2019.110723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Snijders T, Nederveen JP, Joanisse S, et al. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle. 2017;8:267–276. doi: 10.1002/jcsm.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. 2018;47:123–132. doi: 10.1016/j.arr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol. 2013;48:881–887. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Coirault C, Guellich A, Barbry T, Samuel JL, Riou B, Lecarpentier Y. Oxidative stress of myosin contributes to skeletal muscle dysfunction in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H1009–H1017. doi: 10.1152/ajpheart.00438.2006. [DOI] [PubMed] [Google Scholar]
- 157.Gosselin LE, Johnson BD, Sieck GC. Age-related changes in diaphragm muscle contractile properties and myosin heavy chain isoforms. Am J Respir Crit Care Med. 1994;150:174–178. doi: 10.1164/ajrccm.150.1.8025746. [DOI] [PubMed] [Google Scholar]
- 158.Lunde PK, Verburg E, Eriksen M, Sejersted OM. Contractile properties of in situ perfused skeletal muscles from rats with congestive heart failure. J Physiol. 2002;540:571–580. doi: 10.1113/jphysiol.2001.013324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Toth MJ, Miller MS, Ward KA, Ades PA. Skeletal muscle mitochondrial density, gene expression, and enzyme activities in human heart failure: Minimal effects of the disease and resistance training. J Appl Physiol (1985) 2012;112:1864–1874. doi: 10.1152/japplphysiol.01591.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Duscha BD, Annex BH, Green HJ, Pippen AM, Kraus WE. Deconditioning fails to explain peripheral skeletal muscle alterations in men with chronic heart failure. J Am Coll Cardiol. 2002;39:1170–1174. doi: 10.1016/s0735-1097(02)01740-0. [DOI] [PubMed] [Google Scholar]
- 161.Simonini A, Long CS, Dudley GA, Yue P, McElhinny J, Massie BM. Heart failure in rats causes changes in skeletal muscle morphology and gene expression that are not explained by reduced activity. Circ Res. 1996;79:128–136. doi: 10.1161/01.res.79.1.128. [DOI] [PubMed] [Google Scholar]
- 162.Lopez PD, Nepal P, Akinlonu A, et al. Low skeletal muscle mass independently predicts mortality in patients with chronic heart failure after an acute hospitalization. Cardiology. 2019;142:28–36. doi: 10.1159/000496460. [DOI] [PubMed] [Google Scholar]
- 163.Rodgers JL, Jones J, Bolleddu SI, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. 2019;6:19. doi: 10.3390/jcdd6020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: A systematic review and meta-analysis of general population studies. J Diabetes Metab Disord. 2017;16:21. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hawley JA, Joyner MJ, Green DJ. Mimicking exercise: What matters most and where to next? J Physiol. 2021;599:791–802. doi: 10.1113/JP278761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.