Abstract
A total 68 types of marine algae oligosaccharides and polysaccharides were prepared and used to study the structure-activity relationship of oligosaccharides and polysaccharides in their interactions with fibroblast growth factors (FGF) 1 and 2. Factors considered include different types of algae, extraction methods, molecular weight, sulfate content and fractions. In the case of low molecular weight polysaccharide (SJ-D) from Saccharina japonica and its fractions eluting from anion exchange column, both 1.0 M NaCl fraction (SJ-D-I) and 2.0 M NaCl fraction (SJ-D-S) had stronger binding affinity than the parent SJ-D, suggesting that sulfated galactofucans represented the major tight binding component. Nuclear magnetic resonance showed that SJ-D-I was a typical sulfated galactofucan, composed of four units: 1, 3-linked 4-sulfated α-L-fucose (Fuc); 1, 3-linked 2, 4-disulfated α-L-Fuc; 1, 6-linked 4-sulfated β-D-Gal and/or 1, 6-linked 3, 4-sulfated β-D-Gal. Modification by autohydrolysis to oligosaccharides and desulfation decreased the FGF binding affinity while oversulfation increased the affinity. The solution-based affinities of SJ-D-I to FGF1 and FGF2 were 69 nM and 3.9 nM, suggesting that SJ-D-I showed better preferentially binding to FGF1 than a natural ligand, heparin, suggesting that sulfated galactofucan might represent a good regulator of FGF1.
Keywords: structure-activity relationship, sulfated galactofucan, fibroblast growth factors
1. Introduction
Growth factors (GFs) play key roles in cell development, such as survival, proliferation, differentiation, and some cell behavior is influenced by the balance between stimulatory and inhibitory signals [1]. An abnormal activity of growth factor signaling pathway can lead to many diseases, such as cancer, aging, intervertebral disc degeneration, metabolic disorders and heart disease [2–8]. Growth factors are divided into several different superfamilies/families such as: transforming growth factor β (TGF-β) superfamily, fibroblast growth factor (FGF) family, platelet-derived growth factor (PDGF) family, vascular endothelial growth factor (VEGF) family, epidermal growth factor (EGF) family, hepatocyte growth factor (HGF) family and neurotrophins family [5, 9]. FGF1 and FGF2 are two of the prominent members belonging to fibroblast growth factor (FGF) family [8, 10], which is comprised of eighteen secreted proteins that interact with four signaling tyrosine kinase FGF receptors (FGFRs). For example, importin α1 protein reportedly binds directly to FGF1 and FGF2, and the treatment with an anti-importin α1 antibody could result in the suppression of cancer cell proliferation by regulating FGF1 signaling [11].
Most of growth factors functions are attributed to the ability to bind with heparan sulfate (HS), which is the important component of heparan sulfate proteoglycans (HSPGs) on the cell surface [5]. Hundreds of proteins have the capacity to interact with HS and these interactions are critical in the many physiological/pathological processes, including cell attachment, migration, invasion and differentiation, morphogenesis, organogenesis, blood coagulation, lipid metabolism, inflammation and responses to injury [12]. For example, the FGF family, relays on HS as co-receptor, has essential functions in embryogenesis and post-natal growth [9]. Any modifications in HS/heparin structures, such as sulfation patterns, domain length and conformation can be discriminated by FGF and result in the different potential binding abilities between FGF and HS/heparin, leading to the alteration of FGF signaling pathway. On the other hand, many other sulfated glycans (such as chitosan sulfate, sulfated fucans and sulfated galactans) have demonstrated the ability to mimic HS to interact and modulate the function of growth factors [13–14]. In recent years, there is a growing interest in the development of glycomimetics as therapeutics targeting FGF signaling pathway in glycoscience research.
Polysaccharides from Sargassum thunbergii and Saccharina japonica contain three major types of polysaccharides, fucoidans, laminarans and alginates [15]. Fucoidan, a family of heteropolysaccharides, differs in different seaweeds, with harvest times and using different extraction methods [15–20]. There are many excellent reviews on the structure of fucoidan [21–33]. After purification by anion exchange chromatography, fucoidan can be divided into two fractions, sulfated heteropolysaccharides and sulfated galactofucan or fucan. Sulfated heteropolysaccharides have a backbone of alternating 2-linked α-D mannopyranose (Manp) residues and 4-linked β-D glucopyranuronosyl (GlcpA) residues, sulfated at C6 of Manp and branched with sulfated galactofucan, xylan and glucuronan. Sulfated galactofucan (or fucan) contains 3-linked fucopyranose (Fucp) residues, 4-linked Fucp, or a mixture of 3-linked Fucp and 4-linked Fucp, sulfated at C2, C4 or C2/C4 and branched with galactan, accompanied with different sulfation patterns. Polysaccharides from Enteromorpha prolifera belongs to sulfated glucurono-xylo-rhamnan, which is comprised of different types of major monosaccharides including glucuronic acid, xylose, and rhamnose. The structure of glucuronic-xylo-rhamnan contains many units including α-L 4-linked rhamnopyranose (Rha) residues sulfated at C3 or C2, 4-linked β-D GlcpA and 4-linked β-D xylopyranose (Xyl) residue.
In this study, 68 polysaccharides and oligosaccharides (Abbreviation and structural type of polysaccharides and oligosaccharides were summarized in Table S1) were prepared to elucidate the structure-activity relationship between marine algae polysaccharides and their binding affinities towards FGF1 and FGF2. Many factors including extraction methods, different algae, molecular weight, fractions and sulfate content were examined to understand their binding affinity to FGF1 and FGF2.
2. Materials and methods
2.1. Preparation of polysaccharides and oligosaccharides
Crude polysaccharides were from Sargassum thunbergii. STW polysaccharide was extracted by hot water, STA polysaccharide was extracted by 0.1 M HCl, STJ polysaccharide was extracted by 5 % Na2CO3. All S. thunbergii polysaccharides were prepared according to a previous study [17]. Crude polysaccharide from Saccharina japonica (SJ) was prepared according to the previous study by hot water extraction [15]. Enteromorpha prolifera (EP) polysaccharide was similarly extracted by hot water [15].
Crude polysaccharides (0.5 g of STW, STA, STJ, SJ and EP) were dissolved in 10 mL 0.2 M HCOOH at 70 °C for 24 h after which the solution was neutralized by NH4HCO3. Five types of ultrafiltration devices, Ultracel 100 kDa membrane, Ultracel 50 kDa membrane, Ultracel 30 kDa membrane, Ultracel 10 kDa membrane, and Ultracel 3 kDa membrane were used to prepare 100 kD, 50 kD, 30 kD, 10 kD and 3 kD polysaccharides, respectively. All fractions were lyophilized.
Crude polysaccharides (8.0 g of STW, STA, STJ, SJ and EP) were purified by anion exchange chromatography on a DEAE-Bio Gel Agarose FF gel (6 cm × 40 cm) eluted with water (5 L), 0.5 M (5 L) (Fraction W: STW-W, STA-W, STJ-W, SJ-W and EP-W), 1 M (5 L) (Fraction I: STW-I, STA-I, STJ-I, SJ-I and EP-I) and 2 M NaCl (5 L) (Fraction S: STW-S, STA-S, STJ-S, SJ-S and EP-S). The polysaccharides were then dialyzed, concentrated and precipitated with ethanol.
Low molecular weight polysaccharide (SJ-D-I) was prepared according to a previous study [34–36]. Briefly, 50 g SJ was dissolved in 10 L water and 50 mM hydrogen peroxide and 50 mM ascorbic acid were added at room temperature for 2 h. The partially depolymerized fucoidans were ultra-filtered and precipitated with ethanol and the low molecular weight polysaccharide (SJ-D-I) was fractionated by anion-exchange chromatography on a DEAE-Bio Gel agarose FF (6 cm × 40 cm) eluted with water (5 L), 0.5 M (5 L) (SJ-D-W), 1 M (5 L) (SJ-D-I) and 2 M NaCl (5 L) (SJ-D-S). The resulting polysaccharide fractions were then dialyzed, concentrated and precipitated with ethanol.
Modification by autohydrolysis was performed based on a previous study [37]. Briefly, SJ-D-I and SJ-D-S (0.8 g) were converted to the H+-form using a cation exchange column and maintained at room temperature for 72 h. The mixture was neutralized with 5% NH4OH solution in water, concentrated, and precipitated with ethanol. The resulting precipitates were fractionated on a Bio-Gel P-10 column (2.6 × 100 cm) eluted with 0.5 M NH4HCO3 into higher molecular weight, H-type fractions (SJ-D-I-H and SJ-D-S-H), and lower molecular weight, L-type fractions (SJ-D-I-L and SJ-D-S-L).
Modification by desulfation and oversulfation were performed according to the previous methods [15, 17]. Briefly, for desulfation 10 mg polysaccharide samples, SJ-I, SJ-S, SJ-D-I and SJ-D-S, were converted to the H+-form and neutralized with pyridine and lyophilized. The resulting pyridinium salts were dissolved in 20 mL dimethylsulfoxide: methanol (9:1) at 80 °C for 5 h. The solutions of the resulting desulfated polysaccharides were then dialyzed and lyophilized to give desulfated products, SJ-I-DS, SJ-S-DS, SJ-D-I-DS and SJ-D-S-DS. Oversulfation reaction was accomplished using sulfur trioxide-pyridine. Briefly, 20 mg samples, SJ-I, SJ-S, SJ-D-I, SJ-D-S and SJ-D-S-L and 100 mg sulfur trioxide-pyridine were added into dimethylformamide (DMF) and reacted at 60 °C for 24 h, after which the reaction was neutralized, dialyzed, concentrated and lyophilized. The persulfated polysaccharides obtained from SJ-I, SJ-S, SJ-D-I, SJ-D-S and SJ-D-S-L were SJ-I-PS, SJ-S-PS, SJ-D-I-PS, SJ-D-S-PS and SJ-D-S-L-PS, respectively.
Glucuronomannan oligomers (G2, G4 and G6 disaccharide through hexasaccharide) were prepared in our laboratory by previously described methods [38–39]. Low molecular weight (7.0 kDa) glucuronomannan polysaccharide (Gn) was prepared from the crude polysaccharide by degradation with sulfuric acid, neutralized, fractionated by anion exchange chromatography, desalted by Sephadex G10 chromatography.
2.2. Solution competition study between heparin on chip surface and polysaccharides or oligosaccharides in solution using surface plasmon resonance (SPR)
Solution competition SPR measurements were performed on a BIAcore 3000 (GE Healthcare, Uppsala, Sweden). Heparin chip was prepared according to the previous studies [5] by the immobilization of biotinylated heparin on a streptavidin (SA) chip. FGF1 or FGF2 was pre-mixed with different concentrations of polysaccharides or oligosaccharides and injected over the heparin chip at 30 μL/min to measure the inhibition of polysaccharides or oligosaccharides on fibroblast growth factors (FGF1 and FGF2) binding to heparin surface. After each run, a dissociation period and regeneration with 2 M NaCl was performed.
2.3. Compositional analysis and NMR spectroscopy
The molar ratio of monosaccharides and the fucose (Fuc) and galactose (Gal) contents were determined as described by Zhang et al [40]. Sulfation was determined by the method of Dodgson and Price [41]. The molecular weights of the polysaccharides were evaluated by GPC-HPLC on TSK G3000 PWxl column (7 μm 7.8 × 300 mm) with elution in 0.05 M Na2SO4 at a flow rate of 0.5 mL/min at 40 °C with refractive index detection. Ten different molecular weight dextrans, purchased from the National Institute for the Control of Pharmaceutical and Biological Products (China), were used as molecular weight standards.
NMR spectra were recorded at a Hudson-Bruker SB 800 MHz spectrometer (Bruker BioSpin, Billerica, MA, USA) at 25 °C.
3. Results
3.1. SPR solution competition study of different polysaccharides obtained by different extraction methods and from different seaweeds
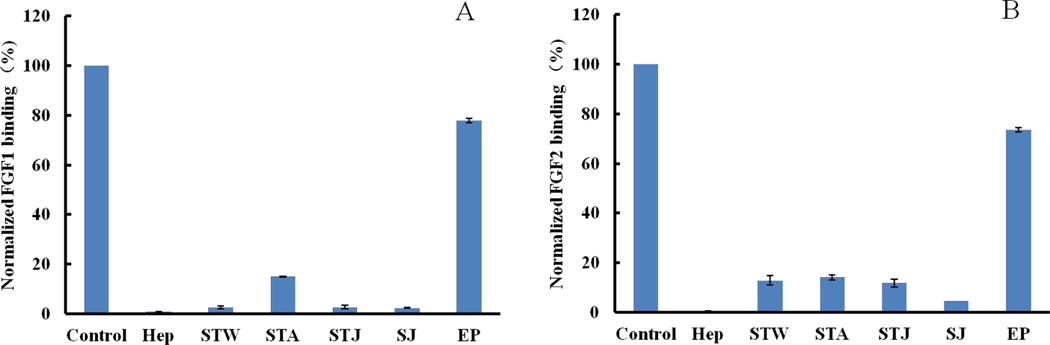
Crude polysaccharides from Sargassum thunbergii (STW polysaccharide extracted by hot water, STA polysaccharide extracted by 0.1 M HCl, STJ polysaccharide extracted by 5 % Na2CO3) and Saccharina japonica (SJ polysaccharide was extracted by hot water) showed very strong competition with heparin (>80%) for binding of FGF1 and FGF2 in Fig 1. In contrast, crude polysaccharide from Enteromorpha prolifera (EP polysaccharide extracted by hot water) competed poorly with heparin for binding of FGF1 and FGF2. The extraction methods appear to influence competition of binding of FGF1 (STW and STJ > STA), more than towards FGF2.
Figure 1.
Bar graphs of normalized different growth factors binding preference to surface heparin by competing with different polysaccharides in solution. (A) FGF1 and (B) FGF2. Concentrations were 100 and 33 nM for FGF1 and FGF2, respectively, and concentrations of different polysaccharides were 1000 nM. All bar graphs are based on triplicate experiments and std. dev. is displayed.
3.2. SPR Solution competition study of different molecular weight polysaccharides obtained by membrane separation techniques
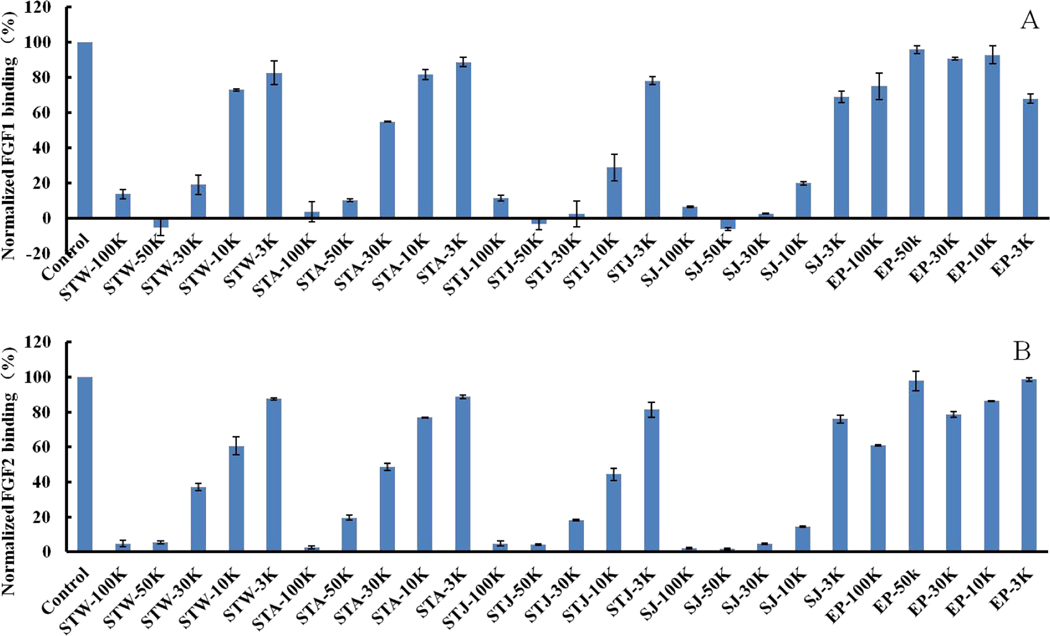
Five MWCO membranes (100K, 50K, 30K, 10K and 3K) were used in ultrafiltration to fractionate the partially degraded polysaccharides. All of the resulting size fractionated polysaccharides from E. Prolifera showed relatively low competitive affinity towards FGF1 (4~32%) and FGF2 (2~39%) in Fig 2. Very strong competitive affinity (>80%) towards FGF1 were observed for STW-100K, STW-50K, STW-30K, STA-100K, STA-50K, STJ-100K, STJ-50K and STJ-30K. In addition, very strong inhibitory activities (>80%) towards FGF2 were observed for STW-100K, STW-50K, STA-100K, STA-50K, STJ-100K, STJ-50K and STJ-30K. Thus, polysaccharides with certain molecular weight characteristics (>50 kDa) from S. thunbergii had very strong affinity towards FGF1 and FGF2. Additionally, polysaccharides with certain molecular weight characteristics (>10 kDa) from S. japonica exhibited very strong inhibitory affinity towards FGF1 and FGF2.
Figure 2.
Bar graphs of normalized different growth factors binding preference to surface heparin by competing with different polysaccharides in solution. (A) FGF1 and (B) FGF2. Concentrations were 100 and 33 nM for FGF1 and FGF2, respectively, and concentrations of different polysaccharides were 1000 nM. All bar graphs based on triplicate experiments and std. dev. is shown.
3.3. SPR solution competition study of different polysaccharides fractions obtained by anion exchange chromatography
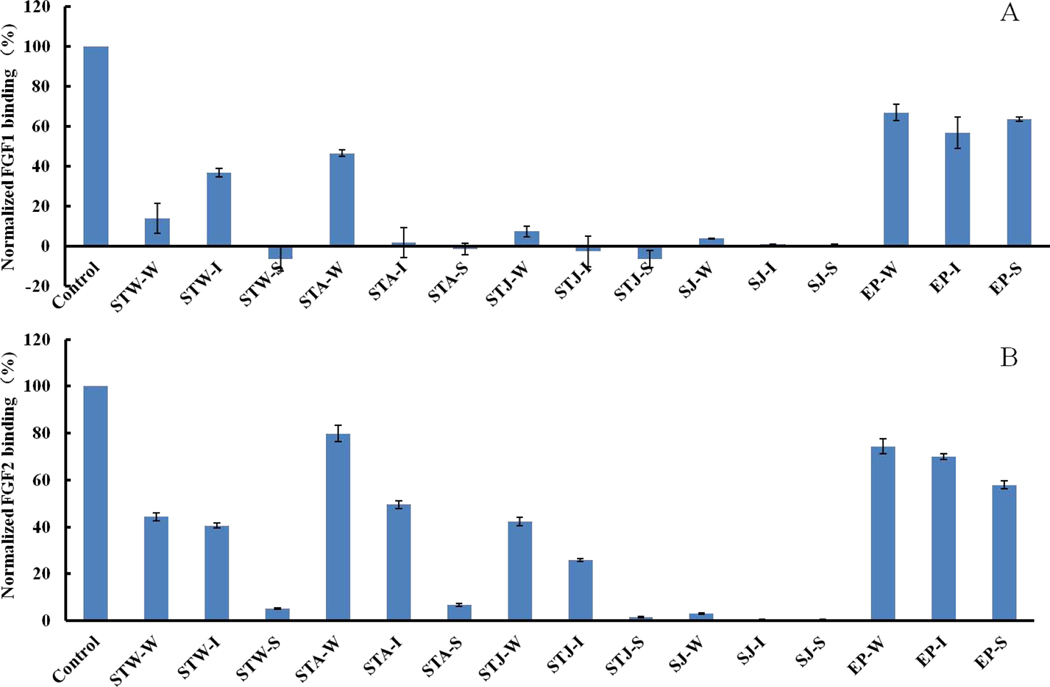
Anion exchange chromatography was next performed. All crude polysaccharides were fractionated into three fractions: fraction W was eluted with 0.5 M NaCl, fraction I was eluted with 1 M NaCl, and fraction S was eluted with 2 M NaCl. Polysaccharide fractions, EP-W, EP-I and EP-S, showed relatively low affinities towards FGF1 (36~43%) and FGF2 (26~42%) in Fig 3. STW-W and STJ-W displayed very strong affinities towards FGF1, while STA-W only displayed 53% inhibitory activity. STW-W and STJ-W showed modest affinities (>40%) towards FGF2 while STA-W only displayed 20% affinity towards FGF2. Strong affinities (>60%) towards FGF2 were observed for STJ-I while STW-I and STJ-I has only modest affinity. However, STA-I and STJ-I had very strong affinity towards FGF1 while STW-I had 63% inhibitory activities. In addition, it is noteworthy that very strong affinities towards FGF1 and FGF2 for STW-S, STA-S and STJ-S were observed. Moreover, all fractions (SJ-W, SJ-I and SJ-S) from SJ exhibited very strong affinities towards FGF1 and FGF2.
Figure 3.
Bar graphs of normalized different growth factors binding preference to surface heparin by competing with different polysaccharides in solution. (A) FGF1 and (B) FGF2. Concentrations were 100 and 33 nM for FGF1 and FGF2, respectively, and concentrations of different polysaccharides were 1000 nM. All bar graphs based on triplicate experiments. Std. dev. is displayed.
3.4. SPR Solution competition study of low molecular weight polysaccharide (SJ-D) and its fractions
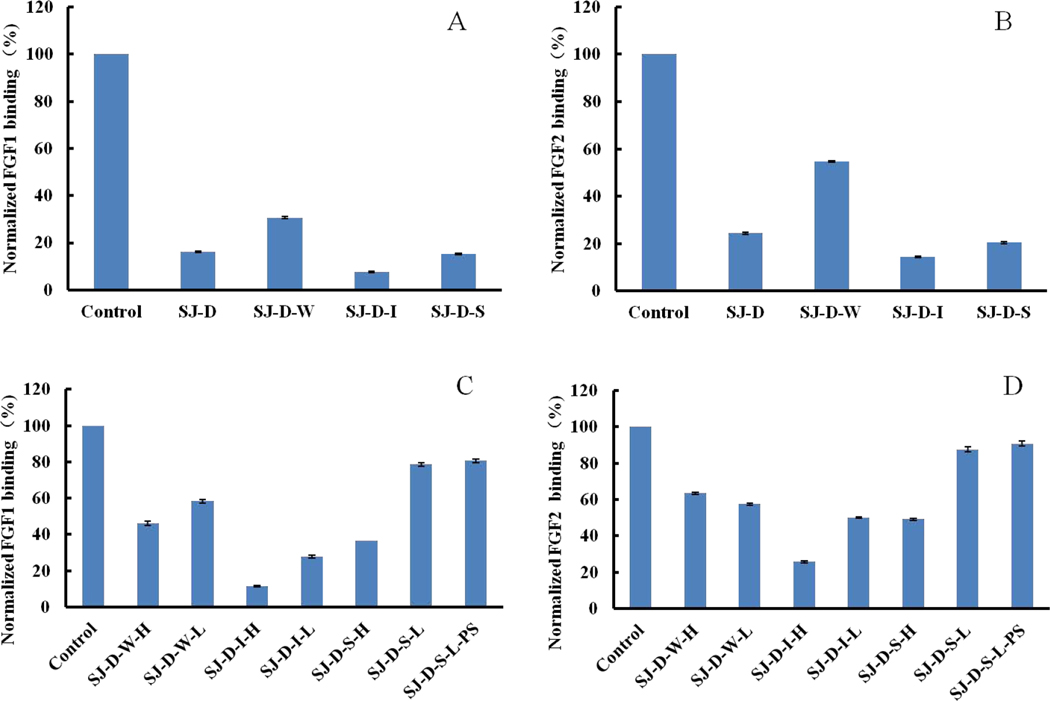
A partially depolymerized polysaccharide with 11.5 kDa molecular weight (SJ-D) was prepared using H2O2 and ascorbic acid. SJ-D had strong affinity (~80%) for FGF1 and FGF2 in Fig 4A and 4B. In addition, SJ-D was also fractionated by anion exchange chromatography into three fractions, SJ-D-W, SJ-D-I and SJ-D-S. SJ-D-I and SJ-D-S exhibited stronger affinity for FGF1 and FGF2 than SJ-D-W. Previous studies [34–36] showed that both SJ-D-I and SJ-D-S were mainly sulfated galactofucan and suggest that sulfated galactofucan might be the major active component of fucoidan polysaccharides.
Figure 4.
Bar graphs of normalized different growth factors binding preference to surface heparin by competing with different polysaccharides in solution. (A and C) FGF1 and (B and D) FGF2. Concentrations were 100 and 33 nM for FGF1 and FGF2, respectively, and concentrations of different polysaccharides were 1000 nM. All bar graphs based on triplicate experiments. Std. dev. is shown.
3.5. SPR Solution competition study on modified polysaccharides
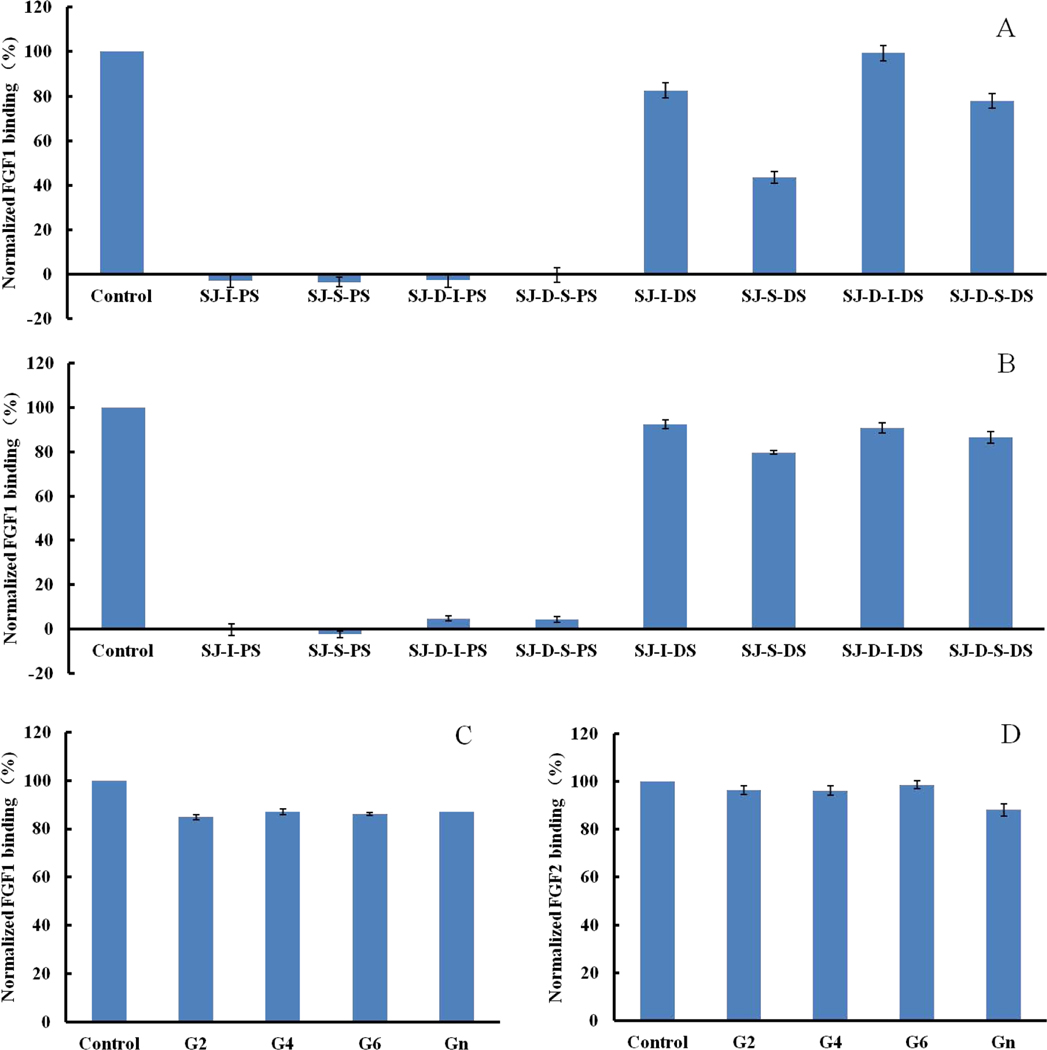
Autohydrolysis was performed, and two fractions, the higher molecular weight H-type fraction and the lower molecular weight L-type fraction were obtained. For SJ-D-W, both SJ-D-W-H and SJ-D-W-L in Fig 4C and 4D showed relatively low affinities towards FGF1 and FGF2. However, for SJ-D-I and SJ-D-S, the H-type fractions, SJ-D-I-H and SJ-D-S-H, displayed higher FGF affinity than the L-type fractions, SJ-D-I-L and SJ-D-S-L, suggesting that molecular weight had an impact on the inhibitory activity. SJ-D-S-L-S, obtained by oversulfation of SJ-D-S-L showed no enhancement in FGF binding.
Desulfated polysaccharides, SJ-I-DS, SJ-S-DS, SJ-D-I-DS and SJ-D-S-DS, and oversulfated polysaccharides SJ-I-PS, SJ-S-PS, SJ-D-I-PS and SJ-D-S-PS, were prepared to elucidate the impact of sulfate content on FGF binding affinity. It was apparent to see in Fig 5 that Oversulfated polysaccharides enhanced binding affinities (>80%) towards both FGF1 and FGF2 while desulfated polysaccharides showed only weak binding affinities. SJ-S-DS was an exception with only modest inhibitory activity towards to FGF1, suggesting that sulfate content play an important role in FGF binding affinity. In addition, the inhibitory activities of glucuronomannan and its oligomers were next determined. Unfortunately, all of these showed extremely low affinity for FGF.
Figure 5.
Bar graphs of normalized different growth factors binding preference to surface heparin by competing with different polysaccharides in solution. (A and C) FGF1 and (B and D) FGF2. Concentrations were 100 and 33 nM for FGF1 and FGF2, respectively, and concentrations of different polysaccharides were 1000 nM. All bar graphs based on triplicate experiments. Std. dev. is shown.
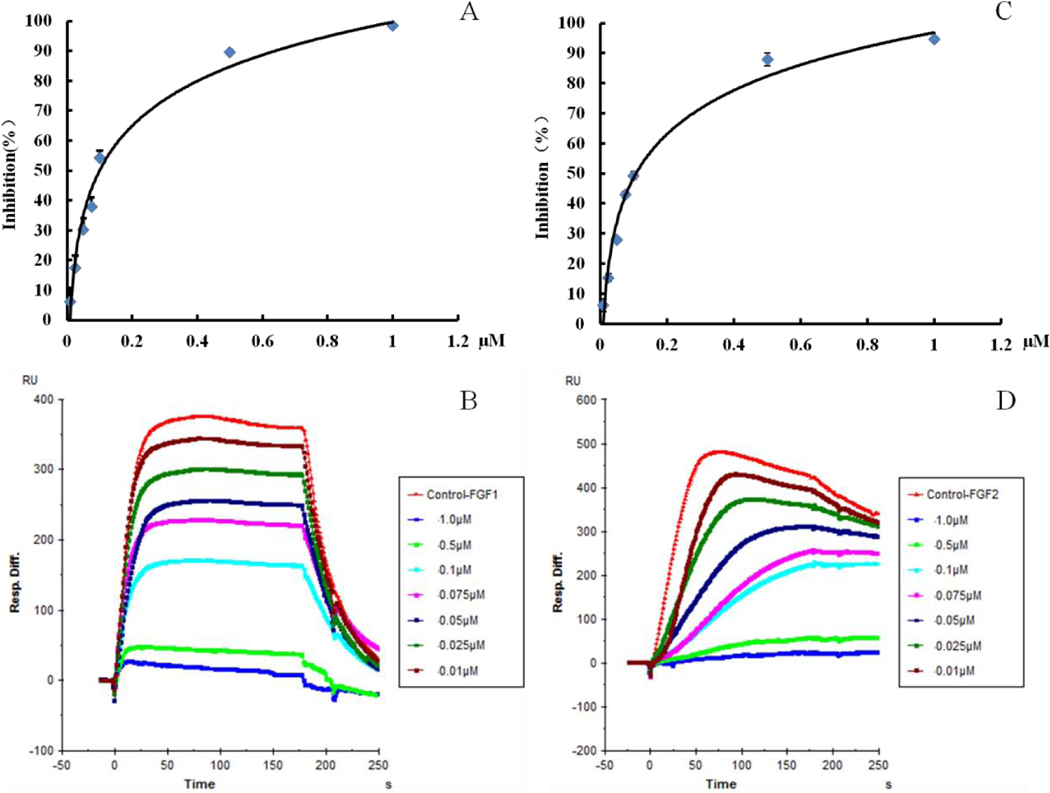
3.6. Kinetics measurements of growth factors interaction with SJ-D-2
The FGFs are well known heparin-binding proteins [5, 42–45]. Kinetic measurements of the interaction of SJ-D-I with FGF1 and FGF2 were determined by solution-based affinities (Ki), which was calculated from IC50 values measured from SPR competition experiments. The IC50 values of SJ-D-I binding to FGF1 and FGF2 are shown in Fig 6. Using the equation of Ki is Ki=IC50/(1+[C]/KD), where [C] is the concentration of FGF1 (100 nM) and FGF2 (33 nM) used in the competition SPR, and Kd for FGF1 (KD=220 nM) and FGF2 (KD=1.2 nM) binding affinity (KD) for heparin we calculated in a previous study [5, 46]. The Ki of SJ-D-I binding to FGF1 and FGF2 were calculated to be 69 nM and 3.9 nM, respectively. SJ-D-I showed preferential binding to FGF1 when compared with heparin.
Figure 6.
SJ-D-2 competes with heparin for FGF1 (A and B) binding with a IC50 of 100 nM and SJ-D-I competes with heparin for FGF2 (C and D) binding with an IC50 of 110 nM. Concentrations were 100 and 33 nM for FGF1 and FGF2, respectively. All bar graphs based on triplicate experiments. Std. dev. is shown.
3.7. Structure features of SJ-D-I
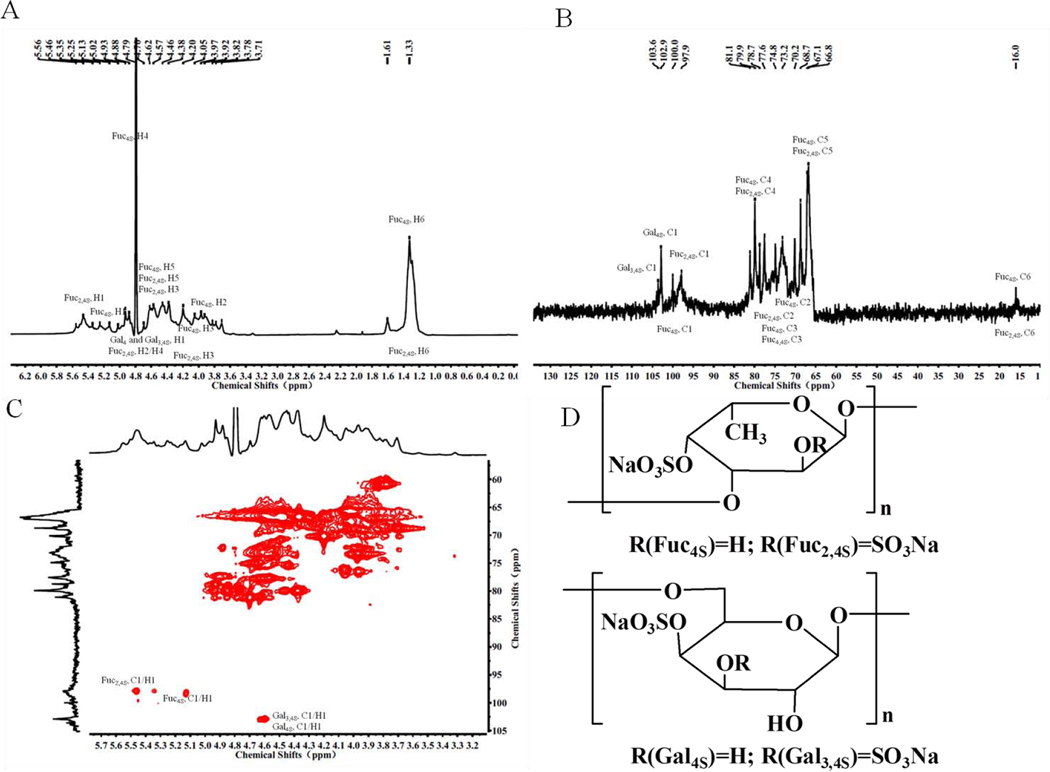
The molar ratio of the monosaccharides comprising SJ-D-I are 0.26: 1 (galactose (Gal): fucose (Fuc)), demonstrating that SJ-D-I is a sulfated galactofucan with a fucose content of 41.63 % and a sulfate content of 48.26%. The galactose content was calculated from the equation: 0.26*(Fuc content/164)*180, was 11.88%. The molar ratio of sulfate to Fuc was calculated from the equation: (sulfate content/96)/(Fuc content/164)) and was 1.98, while the molar ratio of sulfate to (Fuc+Gal), calculated from the equation: (sulfate content/96)/(Fuc content/164+Gal content/180)), was 1.57. DEPT-135, 1H – NMR and two-dimensional NMR was performed on SJ-D-I to elucidate its structure and the results are shown in Fig 7. Based on previous studies [37, 47–53], the spectra of SJ-D-I showed resonances with chemical shifts 97.9–100.0 (C-1) / 5.13–5.56 (H-1) ppm that are characteristic of the 1, 3-linked α-L-fucopyranose sulfated at C4 or C2/C4. Resonances with chemical shifts of 102.9–103.6 (C-1) / 4.62 (H-1) were assigned to 1, 6-linked β-D-galactopyranose sulfated at C4 or C3/C4. Therefore, we concluded that SJ-D-I contained two units, one unit was 1, 6-linked β-D-galactopyranose (β-D-Gal)n, sulfated at C4 and/or C3/C4, and the second unit was 1,3-linked α-L-fucopyranose residues (α-L-Fuc)n, sulfated mainly at C4 and/or C2/C4.
Figure 7.
1H-NMR spectrum (A), The DEPT-135 spectrum (B), HSQC spectrum (C) and primary structure (D) of SJ-D-I.
4. Discussion and Conclusion
The FGF family has eighteen secreted protein members that interact with four signaling tyrosine kinase FGF receptors (FGFRs) [10]. At the cellular level, secreted FGFs control fundamental cellular processes including positive and negative regulation of proliferation, survival, migration, differentiation and metabolism [10]. Heparin could regulate the biological activity through directly binding the FGF-FGFR complex [10]. Marine seaweeds contain large amounts of sulfated polysaccharides that are structurally similar to heparin. Thus, this source of polysaccharides has attracted much attention for their array of biological activities, including as anti-cancer, anticoagulant, antithrombotic, antiviral, immune-inflammatory and neuroprotective agents [32, 54–66].
Five types of crude polysaccharides were prepared to perform the SPR solution competition study to screen for their ability to compete with heparin for binding to FGF1 and FGF2. Some of the crude polysaccharides exhibit very strong FGF binding affinity similar to heparin. However, the affinities of these seaweed-derived polysaccharides are influenced by the extraction methods as well as the type of seaweed from which they are derived. Because the molecular weights of crude polysaccharides are very large, polysaccharide fractions of different molecular weights were prepared by ultrafiltration. SPR results showed that polysaccharide fractions with certain molecular weight exhibited very strong inhibitory activity. The anti-complement activity of sulfated fucan in the classical pathway reportedly increases with increasing molecular weight and reached a plateau at 40 kDa, indicating that molecular weight can be an important positive factor on the activity [67]. All FGFs, except the FGF19 subfamily (FGF15, 19, 21 and 23), show a strong affinity for heparin and require 1–1.5 M NaCl for elution from a heparin-Sepharose column [5]. Therefore, the crude seaweed-derived polysaccharides were fractionated by anion exchange chromatography. Fractions eluting at 1 M and 2 M NaCl showed strong FGF binding affinities. Polysaccharide fractions from S. japonica eluting with 1 M and 2 M NaCl were mainly sulfated galactofucans [34]. NMR studies showed that SJ-D-I, a typical sulfated galactofucan from Saccharina japonica, was composed of (1, 3-linked 4-sulfated α-L-fucose (Fuc)) n1, (1, 3-linked 2, 4-disulfated α-L-Fuc) n2, (1, 6-linked 4-sulfated β-D-Gal) n3 and/or (1, 6-linked 3, 4-sulfated β-D-Gal) n4. Modification by autohydrolysis and desulfation decreased the FGF binding affinity while oversulfation increased FGF binding affinity. The IC50 of SJ-D-I towards to FGF1 and FGF2 were 100 nM and 110 nM, respectively, suggesting that solution-based affinities (Ki) of SJ-D-I to FGF1 and FGF2 were 69 nM and 3.9 nM. SJ-D-I shows more preferential binding to FGF1 over FGF2 than does heparin.
In conclusion, sulfated galactofucans from Saccharina japonica might represent a good regulator of FGF1.
Supplementary Material
Acknowledgements
This study was supported by the Open Fund of Key Laboratory of Experimental Marine Biology, Chinese Academy of Sciences (NO. KF2018NO2); the National Natural Science Foundation of China (No. 41906095 and 41506165); the Zhejiang Provincial Natural Science Foundation of China (No. LY19D060006); China Scholarship Council (W.J.); the National Institutes of Health (DK111958 and CA231074).
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Cross M, Dexter TM, Growth factors in development, transformation, and tumorigenesis, Cell, 64 (1991) 271–80. [DOI] [PubMed] [Google Scholar]
- [2].Aaronson SA, Growth factors and cancer, Science, 254 (1991) 1146. [DOI] [PubMed] [Google Scholar]
- [3].Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, Moe OW, Liang G, Li X, Mohammadi M, alpha-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling, Nature, 553 (2018) 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li Y, Guo XB, Wang JS, Wang HC, Li LP, Function of fibroblast growth factor 2 in gastric cancer occurrence and prognosis, Mol Med Rep, (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang F, Zheng L, Cheng S, Peng Y, Fu L, Zhang X, Linhardt RJ, Comparison of the Interactions of Different Growth Factors and Glycosaminoglycans, Molecules, 24 (2019) 3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kennon JC, Awad ME, Chutkan N, DeVine J, Fulzele S, Current insights on use of growth factors as therapy for Intervertebral Disc Degeneration, Biomol Concepts, 9 (2018) 43–52. [DOI] [PubMed] [Google Scholar]
- [7].Lam NT, Tandon I, Balachandran K, The role of fibroblast growth factor 1 and 2 on the pathological behavior of valve interstitial cells in a three-dimensional mechanically-conditioned model, J Biol Eng, 13 (2019) 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ornitz DM, Itoh N, The Fibroblast Growth Factor signaling pathway, Wiley Interdiscip Rev Dev Biol, 4 (2015) 215–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gallagher J, Fell-Muir Lecture: Heparan sulphate and the art of cell regulation: a polymer chain conducts the protein orchestra, Int J Exp Pathol, 96 (2015) 203–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ornitz DM, Itoh N, Fibroblast growth factors, Genome biology, 2 (2001) REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yamada K, Miyamoto Y, Tsujii A, Moriyama T, Ikuno Y, Shiromizu T, Serada S, Fujimoto M, Tomonaga T, Naka T, Yoneda Y, Oka M, Cell surface localization of importin alpha1/KPNA2 affects cancer cell proliferation by regulating FGF1 signalling, Sci Rep, 6 (2016) 21410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu D, Esko JD, Demystifying heparan sulfate-protein interactions, Annu Rev Biochem, 83 (2014) 129–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dimassi S, Tabary N, Chai F, Blanchemain N, Martel B, Sulfonated and sulfated chitosan derivatives for biomedical applications: A review, Carbohydr Polym, 202 (2018) 382–396. [DOI] [PubMed] [Google Scholar]
- [14].Pomin VH, Marine non-glycosaminoglycan sulfated glycans as a potential pharmaceuticals, Pharmaceuticals (Basel), 8 (2015) 848–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jin W, Zhang W, Liang H, Zhang Q, The Structure-Activity Relationship between Marine Algae Polysaccharides and Anti-Complement Activity, Mar Drugs, 14 (2015) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jin W, Liu G, Zhong W, Sun C, Zhang Q, Polysaccharides from Sargassum thunbergii: Monthly variations and anti-complement and anti-tumour activities, Int J Biol Macromol, 105 (2017) 1526–1531. [DOI] [PubMed] [Google Scholar]
- [17].Jin W, Zhang W, Liu G, Yao J, Shan T, Sun C, Zhang Q, The structure-activity relationship between polysaccharides from Sargassum thunbergii and anti-tumor activity, Int J Biol Macromol, 105 (2017) 686–692. [DOI] [PubMed] [Google Scholar]
- [18].Chevolot L, Foucault A, Chaubet F, Kervarec N, Sinquin C, Fisher A-M, Boisson-Vidal C, Further data on the structure of brown seaweed fucans: relationships with anticoagulant activity, Carbohyd Res, 319 (1999) 154–165. [DOI] [PubMed] [Google Scholar]
- [19].Chevolot L, Mulloy B, Ratiskol J, Foucault A, Colliec-Jouault S, A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae, Carbohyd Res, 330 (2001) 529–535. [DOI] [PubMed] [Google Scholar]
- [20].Daniel R, Berteau O, Chevolot L, Varenne A, Gareil P, Goasdoue N, Regioselective desulfation of sulfated L-fucopyranoside by a new sulfoesterase from the marine mollusk Pecten maximus: application to the structural study of algal fucoidan (Ascophyllum nodosum), Eur J Biochem, 268 (2001) 5617–26. [DOI] [PubMed] [Google Scholar]
- [21].Mourao PA, Pereira MS, Searching for alternatives to heparin: sulfated fucans from marine invertebrates, Trends Cardiovasc Med, 9 (1999) 225–32. [DOI] [PubMed] [Google Scholar]
- [22].Ale MT, Mikkelsen JD, Meyer AS, Important determinants for fucoidan bioactivity: a critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds, Mar Drugs, 9 (2011) 2106–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ferreira SS, Passos CP, Madureira P, Vilanova M, Coimbra MA, Structure-function relationships of immunostimulatory polysaccharides: A review, Carbohydr Polym, 132 (2015) 378–96. [DOI] [PubMed] [Google Scholar]
- [24].Hayashi T, Studies on evaluation of natural products for antiviral effects and their applications, Yakugaku Zasshi, 128 (2008) 61–79. [DOI] [PubMed] [Google Scholar]
- [25].Holtkamp AD, Kelly S, Ulber R, Lang S, Fucoidans and fucoidanases--focus on techniques for molecular structure elucidation and modification of marine polysaccharides, Appl Microbiol Biotechnol, 82 (2009) 1–11. [DOI] [PubMed] [Google Scholar]
- [26].Kusaykin M, Bakunina I, Sova V, Ermakova S, Kuznetsova T, Besednova N, Zaporozhets T, Zvyagintseva T, Structure, biological activity, and enzymatic transformation of fucoidans from the brown seaweeds, Biotechnol J, 3 (2008) 904–15. [DOI] [PubMed] [Google Scholar]
- [27].Li B, Lu F, Wei X, Zhao R, Fucoidan: structure and bioactivity, Molecules, 13 (2008) 1671–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mourao PA, Use of sulfated fucans as anticoagulant and antithrombotic agents: future perspectives, Curr Pharm Des, 10 (2004) 967–81. [DOI] [PubMed] [Google Scholar]
- [29].Mulloy B, The specificity of interactions between proteins and sulfated polysaccharides, An Acad Bras Cienc, 77 (2005) 651–64. [DOI] [PubMed] [Google Scholar]
- [30].Pomin VH, Fucanomics and galactanomics: current status in drug discovery, mechanisms of action and role of the well-defined structures, Biochim Biophys Acta, 1820 (2012) 1971–9. [DOI] [PubMed] [Google Scholar]
- [31].Pomin VH, Mourao PA, Structure, biology, evolution, and medical importance of sulfated fucans and galactans, Glycobiology, 18 (2008) 1016–27. [DOI] [PubMed] [Google Scholar]
- [32].Senthilkumar K, Manivasagan P, Venkatesan J, Kim SK, Brown seaweed fucoidan: biological activity and apoptosis, growth signaling mechanism in cancer, Int J Biol Macromol, 60 (2013) 366–74. [DOI] [PubMed] [Google Scholar]
- [33].Soares PA, Queiroz IN, Pomin VH, NMR structural biology of sulfated glycans, J Biomol Struct Dyn, 35 (2017) 1069–1084. [DOI] [PubMed] [Google Scholar]
- [34].Jin W, Wang J, Jiang H, Song N, Zhang W, Zhang Q, The neuroprotective activities of heteropolysaccharides extracted from Saccharina japonica, Carbohydr Polym, 97 (2013) 116–20. [DOI] [PubMed] [Google Scholar]
- [35].Wang J, Zhang Q, Zhang Z, Li Z, Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica, Int J Biol Macromol, 42 (2008) 127–32. [DOI] [PubMed] [Google Scholar]
- [36].Wang J, Zhang Q, Zhang Z, Song H, Li P, Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica, Int J Biol Macromol, 46 (2010) 6–12. [DOI] [PubMed] [Google Scholar]
- [37].Jin W, Tang H, Zhang J, Wei B, Sun J, Zhang W, Zhang F, Wang H, Linhardt RJ, Zhong W, Structural analysis of a novel sulfated galacto-fuco-xylo-glucurono-mannan from Sargassum fusiforme and its anti-lung cancer activity, Int J Biol Macromol, 149 (2020) 450–458. [DOI] [PubMed] [Google Scholar]
- [38].Jin W, Wang J, Ren S, Song N, Zhang Q, Structural analysis of a heteropolysaccharide from Saccharina japonica by electrospray mass spectrometry in tandem with collision-induced dissociation tandem mass spectrometry (ESI-CID-MS/MS), Mar Drugs, 10 (2012) 2138–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jin W, Ren L, Liu B, Zhang Q, Zhong W, Structural Features of Sulfated Glucuronomannan Oligosaccharides and Their Antioxidant Activity, Mar Drugs, 16 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang J, Zhang Q, Wang J, Shi X, Zhang Z, Analysis of the Monosaccharide Composition of Fucoidan by Precolumn Derivation HPLC, Chin. J. Oceanol. Limnol. 27 (2009) 1–5. [Google Scholar]
- [41].Zhang W, Biotechnology of Glycoconjugated, 2nd ed.; Zhejiang University Press: Hangzhou, (1999) 91–92. [Google Scholar]
- [42].Ibrahimi OA, Zhang F, Hrstka SC, Mohammadi M, Linhardt RJ, Kinetic model for FGF, FGFR, and proteoglycan signal transduction complex assembly, Biochemistry, 43 (2004) 4724–30. [DOI] [PubMed] [Google Scholar]
- [43].Xu R, Ori A, Rudd TR, Uniewicz KA, Ahmed YA, Guimond SE, Skidmore MA, Siligardi G, Yates EA, Fernig DG, Diversification of the structural determinants of fibroblast growth factor-heparin interactions: implications for binding specificity, J Biol Chem, 287 (2012) 40061–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Olsen SK, Ibrahimi OA, Raucci A, Zhang F, Eliseenkova AV, Yayon A, Basilico C, Linhardt RJ, Schlessinger J, Mohammadi M, Insights into the molecular basis for fibroblast growth factor receptor autoinhibition and ligand-binding promiscuity, Proc Natl Acad Sci U S A, 101 (2004) 935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kan M, Wu X, Wang F, McKeehan WL, Specificity for fibroblast growth factors determined by heparan sulfate in a binary complex with the receptor kinase, J Biol Chem, 274 (1999) 15947–52. [DOI] [PubMed] [Google Scholar]
- [46].Zhao J, Liu X, Kao C, Zhang E, Li Q, Zhang F, Linhardt RJ, Kinetic and Structural Studies of Interactions between Glycosaminoglycans and Langerin, Biochemistry, 55 (2016) 4552–9. [DOI] [PubMed] [Google Scholar]
- [47].Wang J, Zhang Q, Zhang Z, Zhang H, Niu X, Structural studies on a novel fucogalactan sulfate extracted from the brown seaweed Laminaria japonica, Int J Biol Macromol, 47 (2010) 126–31. [DOI] [PubMed] [Google Scholar]
- [48].Menshova RV, Anastyuk SD, Ermakova SP, Shevchenko NM, Isakov VI, Zvyagintseva TN, Structure and anticancer activity in vitro of sulfated galactofucan from brown alga Alaria angusta, Carbohydr Polym, 132 (2015) 118–25. [DOI] [PubMed] [Google Scholar]
- [49].Vinnitskiy DZ, Krylov VB, Ustyuzhanina NE, Dmitrenok AS, Nifantiev NE, The synthesis of heterosaccharides related to the fucoidan from Chordaria flagelliformis bearing an alpha-L-fucofuranosyl unit, Org Biomol Chem, 14 (2016) 598–611. [DOI] [PubMed] [Google Scholar]
- [50].Zhou J, Yang L, Hu W, Stereoselective Synthesis of a Sulfated Tetrasaccharide Corresponding to a Rare Sequence in the Galactofucan Isolated from Sargassum polycystum, J Org Chem, 79 (2014) 4718–4726. [DOI] [PubMed] [Google Scholar]
- [51].Kasai A, Arafuka S, Koshiba N, Takahashi D, Toshima K, Systematic synthesis of low-molecular weight fucoidan derivatives and their effect on cancer cells, Org Biomol Chem, 13 (2015) 10556–68. [DOI] [PubMed] [Google Scholar]
- [52].Zong C, Li Z, Sun T, Wang P, Ding N, Li Y, Convenient synthesis of sulfated oligofucosides, Carbohydr Res, 345 (2010) 1522–1532. [DOI] [PubMed] [Google Scholar]
- [53].Bilan MI, Grachev AA, Shashkov AS, Kelly M, Sanderson CJ, Nifantiev NE, Usov AI, Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima, Carbohydr Res, 345 (2010) 2038–47. [DOI] [PubMed] [Google Scholar]
- [54].Jiao G, Yu G, Zhang J, Ewart HS, Chemical structures and bioactivities of sulfated polysaccharides from marine algae, Mar Drugs, 9 (2011) 196–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cumashi A, Ushakova NA, Preobrazhenskaya ME, D’Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE, Berman AE, Bilan MI, Usov AI, Ustyuzhanina NE, Grachev AA, Sanderson CJ, Kelly M, Rabinovich GA, Iacobelli S, Nifantiev NE, A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds, Glycobiology, 17 (2007) 541–552. [DOI] [PubMed] [Google Scholar]
- [56].Pomin VH, An Overview About the Structure-Function Relationship of Marine Sulfated Homopolysaccharides with Regular Chemical Structures, Biopolymers 91 (2009) 601–609. [DOI] [PubMed] [Google Scholar]
- [57].Usov AI, Bilan MI, Fucoidans-sulfatedd polysaccharides of brown algae, Russ Chem Rev, 78 (2009) 785–799. [Google Scholar]
- [58].Mestechkina NM, Shcherbukhin VD, Sulfated Polysaccharides and Their Anticoagulant Activity:A Review, Appl Biochem Micro, 46 (2010) 291–298. [PubMed] [Google Scholar]
- [59].Fitton JH, Therapies from Fucoidan; Multifunctional Marine Polymers, Mar Drugs, 9 (2011) 1731–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pangestuti R,Kim SK, Neuroprotective Effects of Marine Algae, Marine Drugs, 9 (2011) 803–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wijesekara I, Pangestuti R, Kim S-K, Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae, Carbohyd Polym, 84 (2011) 14–21. [Google Scholar]
- [62].Wang W, Wang S-X, Guan H-S, The Antiviral Activities and Mechanisms of Marine Polysaccharides: An Overview, Mar Drugs, 10 (2012) 2795–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wijesinghe WAJP, Jeon Y-J, Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review, Carbohyd Polym, 88 (2012) 13–20. [Google Scholar]
- [64].Senthilkumar K, Manivasagan P, Venkatesan J, Kim SK, Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer, Int J Biol Macromol, 60 (2013) 366–374. [DOI] [PubMed] [Google Scholar]
- [65].Moussavou G, Kwak DH, Obiang-Obonou BW, Maranguy CAO, Dinzouna-Boutamba SD, Lee DH, Pissibanganga OGM, Ko K, Seo JI,Choo YK, Anticancer Effects of Different Seaweeds on Human Colon and Breast Cancers, Mar Drugs, 12 (2014) 4898–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fitton JH, Stringer DN, Karpiniec SS, Therapies from Fucoidan: An Update, Mar Drugs, 13 (2015) 5920–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Blondin C, Chaubet F, Nardella A, Sinquin C, Jozefonvicz J, Relationships between chemical characteristics and anticomplementary activity of fucans, Biomaterials, 17 (1996) 597–603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.