Abstract
Anxiety occurs across ontogeny, but there is evidence that its etiology may vary across the lifespan. The kappa opioid receptor (KOR) system mediates some of the anxiogenic effects of stress and drug exposure, and is involved in aversive responses to environmental stimuli. However, much of this work has been conducted in adult males. Work assessing the effects of KOR activation in younger males has demonstrated that this system produces an anxiolytic/no response, indicating that that this system may be developmentally regulated. Despite these discrepancies, a direct comparison of KOR-induced anxiety in stress-naïve adolescents and adults has not been done. Additionally, the effects of KOR activation in females are poorly understood. Therefore, we assessed the impact of KOR activation on anxiety-like behavior in adolescent and adult male and female Sprague-Dawley rats. Animals were given an i.p. injection of the KOR agonist U69593 (0.01, 0.1, 1.0 mg/kg or vehicle) and were tested using the elevated plus maze. U69593 decreased open arm time in adult males, indicating increased anxiety-like behavior. Adolescents exhibited decreased stretch attend postures when collapsed across sex, suggesting reduced anxiety-like behavior. Adult females were not affected by U69593 administration. These data support studies that have identified age-dependent changes in the KOR system in males, and provide novel evidence that females may not exhibit this ontogenetic change. Given the prevalence of stress and drug exposure during the adolescent period, differences in how the KOR system may mediate the effects of these exposures across age and sex should be explored.
Keywords: kappa opioid receptor, anxiety, development, adolescent, adult, female
Adolescence is the developmental period between childhood and adulthood during which hormonal, physiological, and behavioral maturation typically occur. Extensive reorganization of the brain also occurs during this period, producing neural and behavioral profiles that are unique to adolescents, and which are eliminated by adulthood [1]. Included among these are increased risk-taking behaviors and focus on peer interactions, and greater likelihood of experimentation with drugs [1]. Although adolescence is also thought to be a highly stressful period of life [2], the adolescent brain may be uniquely resilient to particular stressors. Specifically, the kappa opioid receptor (KOR) system in the brain has historically been implicated in the negative affective states related to stress and drug dependence (for review, see [3]); however, accumulating evidence suggests that adolescents may be relatively insensitive to the aversive effects of KOR activation (for review, see [4]).
Several studies have indicated that KOR function may differ by age in discrete brain regions, including regions associated with anxiety [5–7]. Previous work has shown that excitability and long-term potentiation within the basolateral amygdala (BLA), a brain region critical for anxiety- and fear-related behaviors, is reduced in the presence of a KOR agonist in mid-late adolescent rats [8]. Recent work by our lab has provided evidence that KOR-induced suppression of BLA excitability may be specific to adolescents, as adults do not show any change in this region in response to KOR activation [7]. The presence of age-dependent changes in KOR function in specific brain regions is also reflected in the behavioral effects of KOR manipulation. Studies using adults have demonstrated that KOR activation is anxiogenic (for review, see [3]); however, studies using younger animals (in the adolescent weight-range) have shown a reduction in anxiety-like behavior following systemic KOR activation [9–11]. Studies investigating the appetitive or aversive quality of KOR activation using a conditioning task have demonstrated that KOR activation is appetitive in neonatal rodents [12], contrasting with findings from studies using adult rodents (for review, see [4]). Consistent with age-dependent differences in the effects of KOR activation, our lab recently demonstrated that ontogenetic differences in KOR function begin during the juvenile stage, prior to adolescence, when assessing social anxiety-related behaviors [13]. Despite evidence to support an ontogenetic time-course for the development of the KOR system, a direct comparison of adult and adolescent anxiety-like responses to KOR activation has not been done.
In addition to age-dependent effects of KOR activation, sex may be a determining factor for the response to KOR manipulation. Although only a limited number of studies have included females, those few studies have shown females to be less sensitive or insensitive to the effects of KOR activation/blockade compared to males (for review, see [14]). However, no studies to date have investigated whether age-dependent differences in KOR function exist in females. Based upon these gaps in knowledge regarding the effects of KOR activation in males and females on anxiety-like behavior, and apparent patterns of age-dependent effects in males, the objective of the current study was to systematically test age and sex as factors that moderate responses to KOR activation on the elevated plus maze (EPM) test of anxiety-like behavior.
To pursue this objective, adolescent (postnatal day ((P) 33-38, n = 8 per sex) and adult (P83-88, n = 10 per sex) Sprague-Dawley rats were bred in-house, pair-housed, and allowed food and water ad libitum following weaning (P21) - all animal protocols were approved by the Binghamton University Animal Care and Use Committee. Animals were handled for six days prior to anxiety-like testing on EPM for five minutes as previously described [15]. 20 minutes prior to testing, animals received an intraperitoneal injection of one of either 0.01, 0.1, or 1.0 mg/kg U69593, a KOR agonist, (Sigma-Aldrich; St. Louis, MO) or vehicle (20% propylene glycol). U69593 doses were based off of previous assessment of this compound in presumed adolescent males on anxiety-like behavior using the EPM [11]. Behaviors assessed included open arm time and open arm entries as traditional measures of anxiety-like behavior. Closed arm time, closed arm entries, and total number of transitions were also assessed as indices of overall locomotor behavior. Ethological behaviors were analyzed as well to expand on our measures of anxiety-like behavior; these included head dips over the edge of the maze, an indicator of exploratory behavior that is negatively associated with anxiety-like behavior [16, 17], and stretch-attend postures into the open arms of the maze, a behavior that is positively associated with anxiety-like behavior [17–21]. All behaviors were video-recorded and later analyzed by an experimenter blind to the drug condition.
Analyses were performed using Statistica Version 12 (StatSoft, Inc., Tulsa, OK). All data were initially analyzed as an omnibus ANOVA across age, sex, and dose. Given a significant difference across age on all variables assessed (p < 0.0001), age groups were analyzed separately as a two-factor between-subjects ANOVA across sex and dose as we have previously done [22–24]. Open/Closed arm time and open/closed arm entries are represented as percentages (open time or entries/open + closed time or entries). All post-hoc analyses were performed using Fisher’s LSD. Data are presented as mean ± SEM, with significance set to p ≤ 0.05
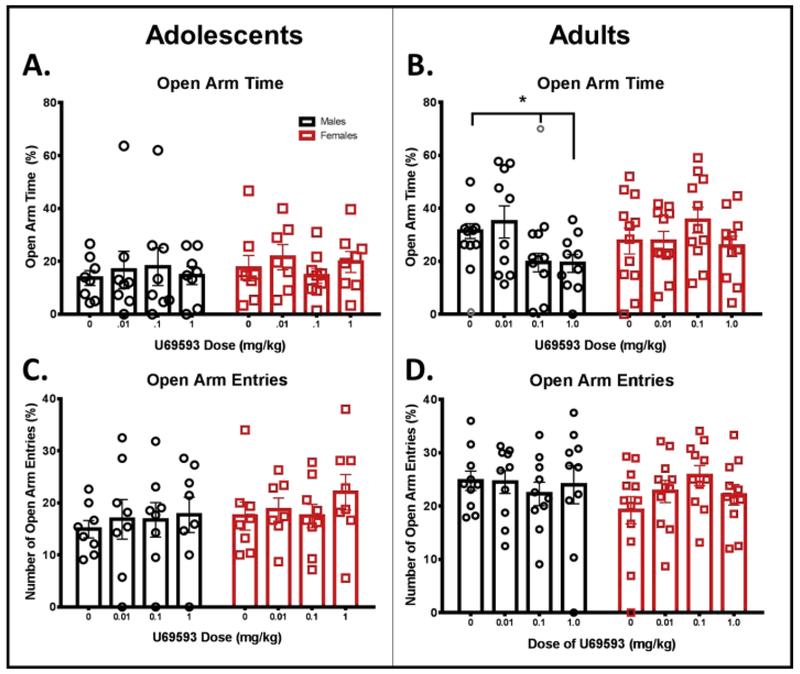
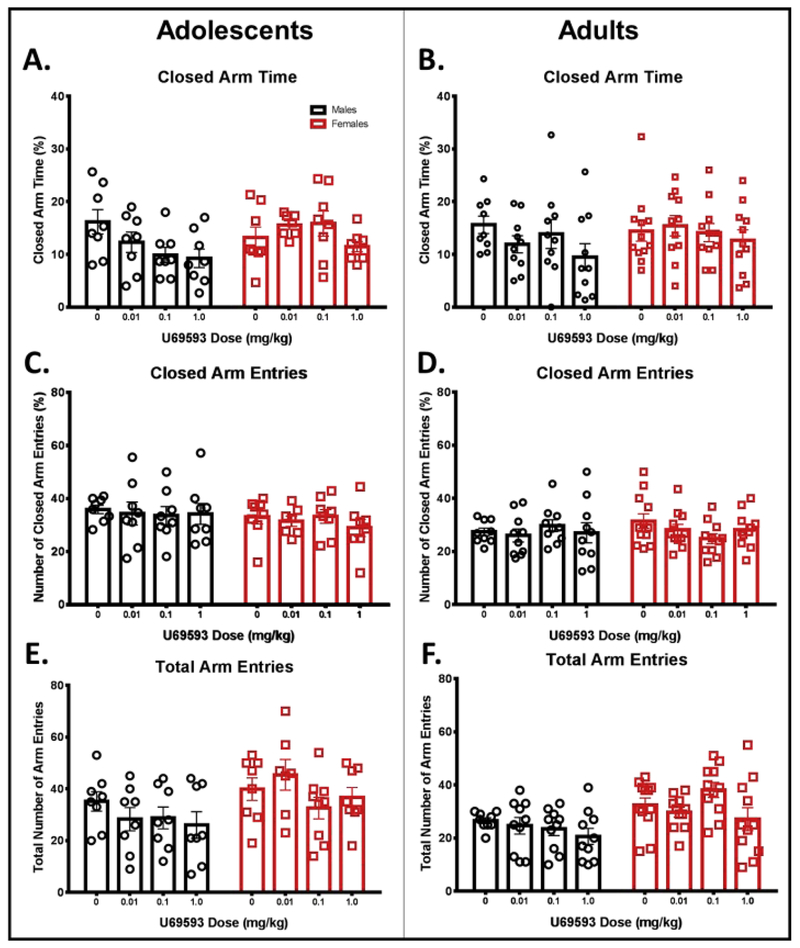
In adolescents, separate two-way ANOVAs of percent open arm time, open arm entries, closed arm time, and closed arm entries revealed no significant main effects (sex or drug dose) or significant interaction (see Fig. 1A, 1C, 2A, 2C). A two-way ANOVA for total arm entries, a measure of general locomotor behavior, revealed a significant main effect of sex, F (1,56) = 8.160, p < 0.05, with adolescent males exhibiting fewer overall transitions between arms compared to adolescent females (see Fig. 2E). No significant main effect of dose or significant sex by dose interaction was found for total arm entries in adolescents.
Figure 1.
Anxiety-like behavior following administration of KOR activation assessed on the EPM in male and female adolescents (left) and adults (right). Animals were given an i.p. injection of KOR agonist U69593 (0.01, 0.1, or 1 mg/kg) or vehicle, and anxiety-like behaviors were tested 20 min following injection. There was a significant overall difference between adults and adolescents (not indicated). (A, B) Percent time spent in the open arms; (C, D) total number of open arm entries. Open gray circles are used to identify statistical outliers that have been removed from analysis. * - p = 0.05 compared to vehicle.
Figure 2.
Measures related to locomotor activity on the EPM following KOR activation in male and female adolescents (left) and adults (right). Animals were given an i.p. injection of KOR agonist U69593 (0.01, 0.1, or 1 mg/kg) or vehicle, and anxiety was tested 20 min following injection. There was a significant difference between adolescents and adults in closed arm time (A, B), closed arm entries (C, D), and total arm entries (E, F; difference not indicated). There was a significant sex difference in both ages on total number of arm entries (E, F; not indicated).
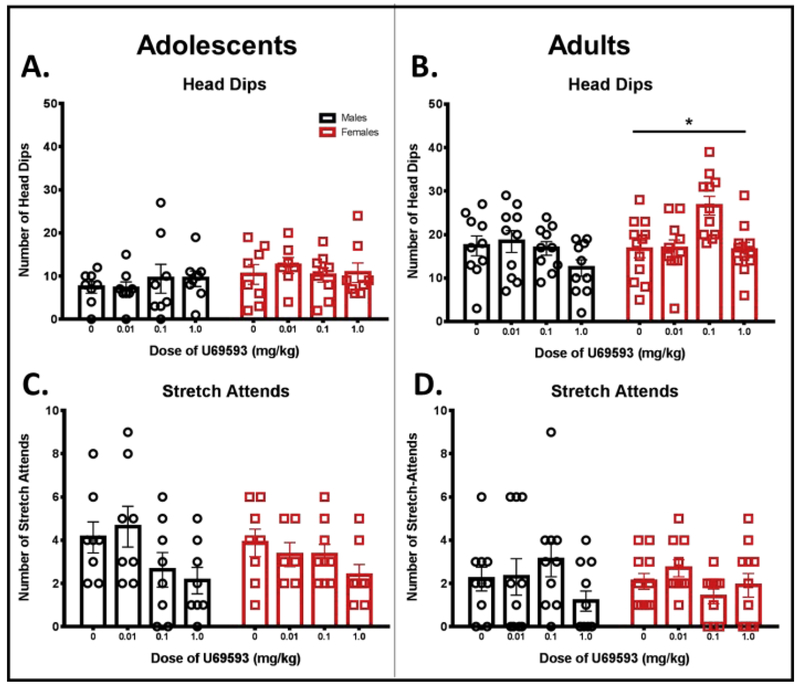
A two-way ANOVA for number of head dips also revealed no significant main effects or significant interaction (Fig. 3A). However, there was a significant main effect of drug dose for number of stretch attends, F (1,56) = 3.913, p < 0.05, without a significant main effect of sex or significant interaction (Fig. 3C). Post-hoc analysis indicated that the highest dose of U69593 significantly suppressed stretch attend postures in adolescent animals when data from male and female adolescents were combined (p < 0.05).
Figure 3.
Ethological variables related to anxiety-like behavior following administration of KOR activation on the EPM in male and female adolescents (left) and adults (right). Animals were given an i.p. injection of KOR agonist U69593 (0.01, 0.1, or 1 mg/kg) or vehicle, and anxiety was tested 20 min following injection. There was a significant overall difference between adults and adolescents (not indicated). (A, B) Total number of unprotected head dips; (C, D) total number of stretch attend postures. * p < 0.05 for main effect of dose in females only.
In adults, Grubb’s outlier test identified a significant outlier in the vehicle and 0.1 mg/kg U69593 groups for open arm time (indicated by an open, gray circle in Fig. 1B). These animals were subsequently removed from all other variables. A two-way ANOVA of percent open arm time revealed a significant sex by dose interaction, F (3, 77) = 3.010, p < 0.05 (Fig. 1B). Fisher’s LSD revealed significant reductions in open arm time compared to vehicle at the two highest doses of U69593 in males (0.1 mg/kg: p = 0.05; 1.0 mg/kg: p = 0.05) with no dose effects in females. Separate two-way ANOVAs of open arm entries, closed arm time, and closed arm entries in adults revealed no significant main effects or significant interactions (see Fig. 1D, 2B, and 2D). A two-way ANOVA of total arm entries revealed a significant main effect of sex, F (1,76) = 15.61, p < 0.05, with adult males showing fewer overall transitions between arms than adult females. No main effect of dose or significant sex by dose interaction was found for total arm entries in adults (see Fig. 2F). A two-way ANOVA for number of head dips revealed no significant sex by dose interaction (see Fig. 3B); however, a significant main effect of sex was found, F (1,77) = 4.110, p < 0.05, as well as a main effect of dose, F (3, 77) = 4.403, p < 0.01. These effects were driven by a significant main effect of U69593 dose in females, F (3, 41) = 6.346, p < 0.01. No significant main effects or significant interaction were observed in our two-way ANOVA of number of stretch attends in adults (see Fig. 3D).
The goal of the present work was to determine whether age or sex act as determining factors in KOR-induced alterations of anxiety-like behavior. Our results show that time in the open arms was dose-dependently decreased only in adult males, suggesting increased anxiety-like behavior. Conversely, U69593 did not alter anxiety-like behavior in either adult females or adolescents of either sex. We also found a dose-dependent increase in frequency of unprotected head dips, a behavior that indicates increased exploratory behavior and potentially reduced anxiety-like behavior [16, 17], only in adult females. Finally, we found a dose dependent decrease in protected stretch attend postures in adolescents, a risk-assessment behavior positively associated with anxiety that has been well validated on multiple behavioral assays, including the EPM [17–21]. These data may indicate a reduction in anxiety-like behavior at one specific dose in adult females and at higher doses of U69593 in all adolescents. It is worth noting that our data indicate that adolescents in the control condition spent less time in the open arms compared to their adult counterparts, raising the possiblity of floor effects in open arm time. However, given that adolescents spent a similar amount of time in the closed arms as adults but exhibited more total arm entries (an index of locomotor activity), it is possible that the low open arm time in adolescents is more indicative of increased exploration in the adolescents, rather than an inability to observe KOR-induced reductions in open arm time in this age group. Additionally, no other measures indicated that KOR activation may be increasing anxiety-like behavior in adolescents; contrary to this, the reduction in stretch-attend postures points toward a potential reduction in anxiety-like behavior [17–21].
The anxiogenic effects of KOR activation presented here in adult males are supported by much of the literature examining the effects of KOR manipulations on anxiety/aversion, the majority of which has focused on adult males (for review, see [3, 4]. In contrast to aversive/anxiogenic effects of KOR activation in adults, studies using adolescent animals (based on either reported ages or weights) report reduced sensitivity to the aversive and anxiogenic properties of KOR agonists [25, 26]. Consistent with this, we found that time spent in the light side of the EPM did not change in adolescents (P33-38) following any of the tested doses of U69593. Interestingly, a handful of studies have reported KOR-induced anxiolytic effects in adolescents [9–11]. A number of factors can contribute to these reported effects, such as the specific KOR agonist used, procedural differences (i.e. timing after injection, handling before test day, etc.), and behavioral task employed/specific variable assessed. A previous exposure to stress could also contribute to anxiolytic effects of KORs given that several studies used animals that were procured from animal suppliers and were therefore exposed to shipping stress [10, 11], an experience shown to impact anxiety-like behavior when it occurs in adolescence [27]. Additionally, we recently examined the effects of restraint stress on KOR-mediated changes in social behavior and found that KOR activation increased social preference in juvenile and adolescent animals that had been exposed to restraint stress, but not adults, suggesting KOR-mediated reductions in social anxiety-like responses in younger, stressed animals [13]. Additionally, previous work examining the effects of developmental exposures to ethanol have demonstrated that these exposures increase prodynorphin and KOR mRNA levels/protein in infant and adolescent rats, which was associated with changes in anxiety-like and avoidance behavior ([28, 29], for review, see [4]). However, given that the animals used in the current study were bred on site and naïve to ethanol, the present findings therefore reflect a relatively stress- and drug-naïve state, and are consistent with other studies using stress-naïve younger animals [6, 25, 26, 30].
Although the underlying neurobiological effects associated with reduced sensitivity to KOR activation in adolescent males are unknown, KOR activation is known to exert unique neurobiological effects in anxiety-related brain structures in adolescents. For example, we and others showed that KOR activation can reduce the excitability of the BLA [8] and increase BLA GABAergic inhibition [7] in adolescent males. This effect on GABA transmission is absent in adults [7], suggesting a possible ontogenetic switch in KOR function in this nucleus. Additionally, the magnitude of KOR-induced outward currents robustly increases between 2 and 4 weeks of age, followed by a reduction by 8 weeks of age in the paraventricular nucleus of thalamus [5]. While these neurophysiological findings likely contribute to the behavioral effects of KOR activation in adolescents, more work is needed to better understand this phenomenon.
Another key component of the present study was to assess whether sex differences exist in KOR activation-induced anxiety, since the majority of studies examining the role of KORs have used males exclusively. Sex differences in KOR function have been reported [14, 31–33], and several studies have identified factors that may contribute to these differences [14, 31–35]. For example, males respond more strongly to KOR activation than females [14], although sex-dependent effects appear to also depend on the behavior being assessed [33]. Consistent with this, we recently reported that females were less sensitive to administration of the KOR agonist, U62066, regardless of age, but only on social interaction [13]. While the mechanisms that contribute to sex differences in KOR function are unclear, sex dependent differences in pDyn gene polymorphisms and KOR binding, in addition to sex hormone interactions with the KOR system (testosterone and estrogen), have been reported and are thoroughly reviewed by Chartoff and Mavrikaki [14]. One possible caveat to our findings is that the EPM has been suggested to be a poor measure of anxiety-like behavior in female rats, given that male and female rodents may express anxiety-like behavior in different ways [36]. However, we have previously done factor analyses to determine the appropriateness of the EPM for different behavioral profiles, and have found that males and females present similar anxiety-related behaviors as both adolescents and adults; this suggests that this assay may be appropriate for assessing female anxiety-like behavior, particularly in both age groups [17]. Regardless of the suitability of the EPM for females, future studies should expand upon our findings by including a battery of behavioral assays to more fully probe the mood-related effects of KOR activation in females. Importantly, the current findings support and extend previous work demonstrating reduced sensitivity of females to KOR activation regardless of age.
Taken together, the findings from the current study provide additional support for age-dependent differences in KOR function as it relates to anxiety-like behaviors. These results also extend our understanding of the KOR system in females. Overall, this provides us with more rationale for continued examination of how the KOR system develops across ontogeny as the interest in targeting the KOR system for therapeutic purposes grows.
Highlights.
Kappa opioid receptors (KOR) are involved in anxiety
Females and adolescent males may be less sensitive to KOR effects than adult males
KOR activation dose-dependently increases anxiety in adult males
KOR activation dose-dependently decreases anxiety in adolescent males
KOR activation does not impact anxiety in females
Age- and sex-dependent KOR function may mediate differences in anxiety disorders
Acknowledgments
Funding: This work was supported by NIAAA grants R03AA024890, P50AA017823 the Center for Development and Behavioral Neuroscience and the Psychology Department at Binghamton University. Declarations of interest: none.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spear LP, The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews, 2000. 24(4): p. 417–463. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MM, Tottenham N, and Casey B, Translational developmental studies of stress on brain and behavior: Implications for adolescent mental health and illness? Neuroscience, 2013. 249: p. 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hang A, et al. , The role of the dynorphin kappa opioid receptor system in anxiety. Acta Pharmacol Sin, 2015. 36(7): p. 783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz MR, Przybysz KR, and Rouzer SK, Age as a factor in stress and alcohol interactions: A critical role for the kappa opioid system. Alcohol, 2018. 72: p. 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, et al. , Dynorphin activation of kappa opioid receptor reduces neuronal excitability in the paraventricular nucleus of mouse thalamus. Neuropharmacology, 2015. 97: p. 259–69. [DOI] [PubMed] [Google Scholar]
- 6.Tejeda HA, et al. , Dysregulation of kappa-opioid receptor systems by chronic nicotine modulate the nicotine withdrawal syndrome in an age-dependent manner. Psychopharmacology (Berl), 2012. 224(2): p. 289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Przybysz KR, Werner DF, and Diaz MR, Age-dependent regulation of GABA transmission by kappa opioid receptors in the basolateral amygdala of Sprague-Dawley rats. Neuropharmacology, 2017. 117: p. 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huge V, et al. , Activation of kappa opioid receptors decreases synaptic transmission and inhibits long-term potentiation in the basolateral amygdala of the mouse. Eur J Pain, 2009. 13(2): p. 124–9. [DOI] [PubMed] [Google Scholar]
- 9.Alexeeva EV, Nazarova GA, and Sudakov SK, Effects of peripheral mu, delta, and Kappa-opioid receptor agonists on the levels of anxiety and motor activity of rats. Bull Exp Biol Med, 2012. 153(5): p. 720–1. [DOI] [PubMed] [Google Scholar]
- 10.Braida D, et al. , Potential anxiolytic- and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br J Pharmacol, 2009. 157(5): p. 844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Privette T and Terrian D, Kappa opioid agonists produce anxiolytic-like behavior on the elevated plus-maze. Psychopharmacology, 1995. 118(4): p. 444–450. [DOI] [PubMed] [Google Scholar]
- 12.Petrov ES, et al. , Dynorphin A (1–13) and responsiveness of the newborn rat to a surrogate nipple: Immediate behavioral consequences and reinforcement effects in conditioning. Behavioural brain research, 2006. 170(1): p. 1–14. [DOI] [PubMed] [Google Scholar]
- 13.Varlinskaya EI, Spear LP, and Diaz MR, Stress alters social behavior and sensitivity to pharmacological activation of kappa opioid receptors in an age-specific manner in Sprague Daw ley rats. Neurobiology of Stress, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chartoff EH and Mavrikaki M, Sex Differences in Kappa Opioid Receptor Function and Their Potential Impact on Addiction. Front Neurosci, 2015. 9: p. 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouzer SK, et al. , Moderate Maternal Alcohol Exposure on Gestational Day 12 Impacts Anxiety-Like Behavior in Offspring. Front Behav Neurosci, 2017. 11: p. 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodgers RJ and Dalvi A, Anxiety, Defence and the Elevated Plus Maze. Neuroscience and Biobehavioral Reviews, 1997. 21(6): p. 801–810. [DOI] [PubMed] [Google Scholar]
- 17.Doremus TL, Varlinskaya EI, and Spear LP, Factor analysis of elevated plus-maze behavior in adolescent and adult rats. Pharmacology Biochemistry and Behavior, 2006. 83(4): p. 570–577. [DOI] [PubMed] [Google Scholar]
- 18.Holly KS, Omdorff CO, and Murray TA, MATSAP: An automated analysis of stretch-attendposture in rodent behavioral experiments. Sci Rep, 2016. 6: p. 31286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molewijk H, Van der Poel A, and Olivier B, The ambivalent behaviour “stretched approach posture” in the rat as a paradigm to characterize anxiolytic drugs. Psychopharmacology, 1995. 121(1): p. 81–90. [DOI] [PubMed] [Google Scholar]
- 20.Kaesermann HP, Stretched attend posture, a non-social form of ambivalence, is sensitive to a conflict-reducing drug action. Psychopharmacology, 1986. 89(1): p. 31–37. [DOI] [PubMed] [Google Scholar]
- 21.Cryan JF and Holmes A, Model organisms: the ascent of mouse: advances in modelling human depression and anxiety. Nature reviews Drug discovery, 2005. 4(9): p. 775. [DOI] [PubMed] [Google Scholar]
- 22.Doremus-Fitzwater TL, Varlinskaya EI, and Spear LP, Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiology & behavior, 2009. 97(3-4): p. 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varlinskaya EI and Spear LP, Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacology Biochemistry and Behavior, 2012. 100(3): p. 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vetter-O’Hagen C, Varlinskaya E, and Spear L, Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol & Alcoholism, 2009. 44(6): p. 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson RI, et al. , Pharmacological activation of kappa opioid receptors: aversive effects in adolescent and adult male rats. Psychopharmacology (Berl), 2014. 231(8): p. 1687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morales M, et al. , Effects of the kappa opioid receptor antagonist, nor-binaltorphimine, on ethanol intake: impact of age and sex. Dev Psychobiol, 2014. 56(4): p. 700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chappell AM, et al. , Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats. Alcohol Clin Exp Res, 2013. 37 Suppl 1: p. E394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wille-Bille A, et al. , Prenatal ethanol induces an anxiety phenotype and alters expression of dynorphin & nociceptin orphanin FO genes. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 2018. 85: p. 77–88. [DOI] [PubMed] [Google Scholar]
- 29.Nizhnikov ME, et al. , Brief prenatal ethanol exposure alters behavioral sensitivity to the kappa opioid receptor agonist (U62,066E) and antagonist (Nor-BNI) and reduces kappa opioid receptor expression. Alcohol Clin Exp Res, 2014. 38(6): p. 1630–8. [DOI] [PubMed] [Google Scholar]
- 30.Anderson RI, et al. , Stress, kappa manipulations, and aversive effects of ethanol in adolescent and adult male rats. Neuroscience, 2013. 249: p. 214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang YJ, et al. , Sex difference in kappa-opioid receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5′-O-(3-[35S]thiotriphosphate) binding in the guinea pig. J Pharmacol Exp Ther, 2011. 339(2): p. 438–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker J and Chartoff E, Sex Differences in Neural Mechanisms Mediating Reward and Addiction. 2018. [DOI] [PMC free article] [PubMed]
- 33.Williams AV and Trainor BC, The impact of sex as a biological variable in the search for novel antidepressants. Front Neuroendocrinol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright EC, et al. , Activation of kappa opioid receptors in the dorsal raphe have sex dependent effects on social behavior in California mice. Behav Brain Res, 2018. 351: p. 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell SE, et al. , Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry, 2014. 76(3): p. 213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scholl JL, et al. , Sex differences in anxiety-like behaviors in rats. Physiol Behav, 2019. 211: p. 112670. [DOI] [PubMed] [Google Scholar]