Abstract
The aim of the study was to determine the effect of in ovo administration of zinc glycine chelate (Zn-Gly), and a multistrain probiotic on the hatchability and selected parameters of the cellular and humoral immune response of chickens. The study was conducted on 1,400 fertilized eggs from commercial broiler breeders (Ross x Ross 708). Material for the study consisted of peripheral blood and spleens of chicks taken 12 h and 7 d after hatching. The results showed that both combined and single in ovo administration of the multistrain probiotic and zinc glycine chelate significantly reduced hatchability of chicks. The flow cytometry study showed that the highest percentage of CD4+ T cells, CD4+CD25+, and high expression of KUL01 in the serum were obtained in the group supplemented with probiotic and Zn-Gly both 12 h and 7 d after hatching. In birds supplemented with probiotic and zinc chelate, a high percentage of TCRγδ+ cells was found in serum and spleen 12 h after hatching and in serum after 7 d. The percentage of Bu-1A+ lymphocytes in serum and spleen 12 h and 7 d after hatching was the highest in the group supplemented with probiotic and Zn-Gly. The highest expression of CD79A was observed in the group supplemented only with zinc chelate. There were no significant differences in the percentage of CD4+ cells in the spleens of birds in the groups receiving the multistrain probiotic at 12 h after hatching, and after 7 d, the percentage of CD4+ T cells was lower in the experimental groups than in the control group. The percentage of CD8+ cells in the serum of birds after hatching was lower in the group supplemented with multistrain probiotic and Zn-Gly than in the control group, but reached the highest value on d 7 after hatching. The obtained results confirm the strong effect of the combined administration of a multistrain probiotic and Zn-Gly chelate on lymphocyte proliferation and stimulation of cellular immune mechanisms in birds.
Key words: chicken, in-ovo, multistrain probiotic, zinc glycine chelate, flow cytometry
INTRODUCTION
Modern poultry rearing and breeding systems are aimed at genetic improvement of production traits, manifested as shorter broiler fattening time, higher weight gains, improved meat quality, and increased profitability of farms through a reduced feed conversion ratio (FCR) (Lukić et al., 2022; Olejnik et al., 2022). Obtaining the desired traits in practice often entails immune disorders and increased susceptibility of birds to disease (Yegani and Korver, 2008; Dal Bosco et al., 2021). These unfavorable phenomena can be counteracted at many stages of the production cycle, including through improvement of the birds’ diet (Dal Bosco et al., 2021). One of the critical periods affecting hatching parameters and chick quality is the hatching process (Elibol and Brake, 2004). An overly prolonged hatching period, lasting more than 24–36 h, results in delayed access to water and feed in the chicks hatched earlier (Willemsen et al., 2010; Jha et al., 2019). In addition, technological procedures such as sexing carried out during this period induce stress, which adversely affects the condition of the birds and their potential immunity (Willemsen et al., 2010; Hedlund et al., 2021). Bar Shira et al. (2005) and Taha-Abdelaziz et al. (2018) showed that delayed feeding of chicks inhibits the development of the lymphoid organs, which affects the size of subpopulations of peripheral blood lymphocytes and disturbs the humoral immune response following vaccine administration. The substantial weight loss in chicks during this period, in combination with disturbances in intestinal and muscle development and immune system dysfunction, increases susceptibility to infection by environmental pathogens and thus mortality rates, which affects the farm's economic outcomes (Jha et al., 2019; Wickramasuriya et al., 2023 ). These negative effects can be limited by obtaining chicks with high health parameters. In this context, particular importance is ascribed to the period of embryonic development. This period has been shown to affect not only the development of the embryo, hatching results, and performance after hatching, but also modulation of the GIT (gastrointestinal tract) microbiota in the early stage of development, mechanisms regulating gene expression, and the development of lymphoid organs and the immune system (Yin et al., 2010, Roto et al., 2016; Dai et al., 2020).
The use of in ovo technology to administer bioactive substances during embryonic development, that is, on d 12, 17, or 18 of incubation, alters the embryonic environment and leads to early microbial programming and to in ovo feeding (Roto et al., 2016). This affects the development of chicks and their adaptation to environmental conditions in the posthatching period, including the development and maturation of the immune system. In this way, it is possible to administer vaccines, medicines, hormones, or competitive exclusion cultures, such as probiotics, prebiotics, synbiotics, and nutrients, including proteins, amino acids, peptides, carbohydrates, nucleotides, electrolytes, micro- and macroelements, vitamins, L-carnitine, creatine, and plant extracts (Kadam et al., 2013; Hou et al., 2018; Lu et al., 2022). Among these substances, increasing importance is ascribed to probiotics and essential trace elements such as zinc, which stimulate immunity in birds and improve productivity parameters (Kadam et al., 2013; Dunisławska et al., 2017; Hou et al., 2018; Alizadeh et al., 2020).
In ovo administration of probiotics accelerates their interaction with the gut microbiome of the embryo and colonization of the gut with useful bacteria modifying the microbiome and limiting colonization of the gastrointestinal tract by pathogens during the posthatching period (Wilson et al., 2019). Stimulation of the gut microbiome through in ovo administration of probiotic microbes plays an important role in the development and regulation of innate and acquired immunity (Clavijo and Flórez, 2018), not only during embryonic development, but also after hatching, when immature gut-associated lymphoid tissue (GALT) undergoes antigenic stimulation by environmental microbes. Reprogramming of the gut microbiome of chickens during early embryonic development can increase their resistance owing to targeted modulation of cellular and/or humoral immune mechanisms (Shehata et al., 2021). Evidence supporting this hypothesis is provided by the results of experiments showing widely targeted immunomodulatory activity of probiotic microbes in relation to local and systemic lymphoid organs in birds (Alizadeh et al., 2020). Similar studies have also shown that in ovo administration of probiotics based on lactic acid bacteria stimulate antibody synthesis in the posthatching period, which suggests beneficial effects of these bacteria on acquired immunity and stimulation of the humoral immune response (Alizadeh et al., 2020).
In addition to probiotics, the normal development, growth, and other vital functions of organisms also depend on intake of macro- and microelements (Savarinoet al., 2021). Zinc is an essential trace element that plays an important role in the metabolism of carbohydrates, proteins, lipids and nucleic acids, and also functions as a cofactor of many enzymes (Jarosz et al., 2017). Zinc is needed for cell proliferation and differentiation as well as the growth and development of the body, including the nervous, reproductive, and skeletal systems (Jarosz et al., 2017). The effects of zinc on the immune system of birds are important as well (Hidayat et al., 2020). Zinc is involved in the development of the lymphoid organs, the maturation and differentiation of T lymphocytes, and the functioning of heterophils and mononuclear phagocytes (Jarosz et al., 2017a). In order to reduce zinc deficiencies caused by poor bioavailability this microelement from plant components of feed (Ellis et al., 1982; Fordyce et al., 1987), as well as to reduce environmental pollution associated with excessive excretion of zinc, organic forms of zinc are currently used as an additive to poultry feed (Carter and Kim, 2013). These forms include zinc glycine chelate. This compound is more bioavailable than inorganic forms and also promotes cellular immune mechanisms by regulating the Th1/Th2 immune response and production of pro- and anti-inflammatory cytokines (Jarosz et al., 2017a). Zinc glycine chelates also increase synthesis of class A, M and Y immunoglobulins in poultry, indicating promotion of humoral immunity (Feng et al., 2010) and demonstrating the role of chelates in modulation and regulation of the immune response. In current poultry production systems, stimulation of the growth and development of embryos and chicks as well as enhancement of immunity can be achieved through in ovo administration of Zn (Tako et al., 2005). The efficiency of this method of supplying essential trace elements has been confirmed in several experimental studies (Goel et al. 2012; Olatunbosun et al., 2023 ; Kim and Kang 2022), in which administration of organic and inorganic forms of zinc induced stimulation of cellular immunity, expressed as proliferation and differentiation of lymphocytes in organs, and the humoral response, expressed as increased immunoglobulin concentrations.
Despite the beneficial effect of multistrain probiotics and zinc glycine chelates on immunity in poultry, demonstrated in numerous studies, the mechanisms responsible for the effects of these preparations on immune functions in poultry are not fully known. Moreover, the effect of these compounds on immune system development in terms of programming of chicken embryos has not been determined. The multifaceted impact of multistrain probiotics and zinc glycine chelates led us to adopt the hypothesis that in ovo administration of a probiotic and chelate in the early embryonic period has immunomodulatory effects and enhances cellular and humoral immune mechanisms, protecting birds against infection in the initial period of life after hatching.
The aim of the study was to determine the effect of combined in ovo administration of zinc glycine chelate and a multistrain probiotic on selected parameters of the cellular and humoral immune response of chicken by assessing the peripheral blood and spleen mononuclear cell in particular the percentage of cells with expression of CD3+/CD4+, CD3+/CD8α+, CD4+/CD25+, CD25+, MHC Class II+, Bu-1a+, CD79A+, TCRγδ+, and monocyte/macrophage (KUL01+) molecules.
MATERIAL AND METHODS
Incubation Period
Eggs were collected from a commercial broiler breeder flock (Ross x Ross 708) at 36 wk of age and transported to the Experimental Station of the Poznan University of Life Sciences, Gorzyń 4, Międzychód commune. The eggs were stored at 21°C under commercial conditions for 24 h before weighing and setting. Prior to being placed in the incubator, all eggs were individually weighed and marked, and the eggshells were disinfected with Viron FF (glutaraldehyde, didecyldimethylammonium chloride, quaternary ammonium compounds and benzyl-C12-C16-alkyldimethyl; DDD-1, Bielsko Biała, Poland) in 1 ml/L solution.
A total of 1,500 eggs were randomly distributed in JARSON Model JD-18 incubators (Gostyń, Poland). Eggs were incubated under standard commercial conditions (T 37.8°C, RH 55%–60%) from 1 to 18 d. On the 19th day of incubation (DOI), the eggs were transferred to a JARSON Model ATLAS-180 hatching chamber. During the last 3 d of incubation (19–21 DOI), the relative humidity was maintained at 60% to 65%. At 7 and 17 d of incubation, all eggs were candled, and those with cracked hells, infertile eggs, and those containing dead embryos were removed (Ernst et al., 2004).
On embryonic d 17, a total of 1,400 fertilized eggs of similar weight were randomly distributed into 4 treatment groups, with 10 replicates per group and 35 eggs per replicate (350 eggs per group). The following 4 treatment groups were established: eggs injected with sterile 0.9% physiological saline (control group: I); eggs injected with a multistrain probiotic: group II (1 × 105 CFU/egg Saccharomyces cerevisiae; 1 × 107 CFU/egg Lactobacillus casei; 1 × 107 CFU/egg Lactobacillus plantarum); eggs injected with a multistrain probiotic and zinc glycine chelate (Zn-Gly): group III (1 × 105 CFU/egg S. cerevisiae; 1 × 107 CFU/egg L. casei; 1 × 107 CFU/egg L. plantarum; 100 µg/egg Zn-Gly); and eggs injected with zinc glycine chelate (Zn-Gly): group IV (100 µg/egg Zn-Gly) (Table 1).
Table 1.
In ovo supplementation scheme.
| In ovo injection—(17 DOI) |
||||
|---|---|---|---|---|
| Group | Replicates/number of eggs per replicate N = | Amount of multistrain probiotic in 100 µL | Amount of zinc glycine chelate (Zn-Gly) in 100 µL | Volume and solution of bioactive compounds injected in ovo |
| I—control group | 10/35 350 |
non-injected | non-injected | 500 µL 0.9% NaCl |
| II | 10/35 350 |
1 × 105 CFU/eggs S. cerevisiae 1 × 107 CFU/eggs L. casei 1 × 107 CFU/eggs L. plantarum |
- | 100 µL multistrain probiotic + 400 µL MQ water |
| III | 10/35 350 |
1 × 105 CFU/ eggs S. cerevisiae 1 × 107 CFU/eggs L. casei 1 × 107 CFU/eggs L. plantarum |
100 µg Zn-Gly/100 µL MQ water/eggs | 100 µL multistrain probiotic + 100 µL Zn-Gly + 300 µL MQ water |
| IV | 10/35 350 |
- | 100 µg Zn-Gly/100 µL MQ water/eggs | 100 µL Zn-Gly + 400 µL MQ water |
Abbreviations: DOI, days of incubation; MQ, milli Q water; N, number of eggs in all replicates.
The multistrain probiotic EM Provet used in the experiment is manufactured by Greenland Technologia EM (Janowiec, Poland). EM Provet was provided by Greenland Technologia EM in powder form. It contains a mixture of live microorganisms, as shown in Table 2. Then EM Provet was diluted in Buffered Saline Solution (PBS) to obtain a solution containing 1 × 105 CFU S. cerevisiae, 1 × 107 CFU L. casei, and 1 × 107 CFU L. plantarum in 100 µL of solution. The viability of the probiotic bacterial cells and their content per gram of the product (CFU/g) was tested as described by Weese and Martin (2011). Furthermore, the company manufacturing the EM Provet preparation evaluated the viability of the probiotic bacterial cells and their content per gram of the product in their laboratory, thus guaranteeing that the product used in the experiment meets all quality criteria for products containing live bacterial cultures.
Table 2.
EM Provet composition.
| Microbial composition | Strain number | Content per gram of product | |
|---|---|---|---|
| 1. | Saccharomyces cerevisiae | Y200007 | 5 × 106 CFU/g |
| 2. | Lactobacillus casei | ATCC 7469 | 5 × 108 CFU/g |
| 3. | Lactobacillus plantarum | ATCC 8014 | 5 × 108 CFU/g |
The Zn glycine chelate used in the experiment is manufactured by ARKOP Sp. z o.o. The Zn-Gly powder contains 250 mg Zn-Gly per g of product. The powder was dissolved in deionized water to obtain solutions containing 100 µg Zn-Gly/100 µL MQ water.
In Ovo Inoculations
At 17 DOI, the eggs in each group received an equal volume (500 μL) of 0.9% physiological saline or a bioactive compound, injected in ovo into the amniotic sac (Table 1). Before injection, all eggs were sterilized by spraying with 75% ethanol. Additionally, the instruments were autoclaved before use. Embryonated eggs were injected through the air cell with a blunt tip injector needle (1.27 mm bore width) to target the amnion. Next, 0.5 mL of the solution was injected into each amniotic cavity using a 23-gauge 2.5-cm needle. Following injection, the pinhole sites in the eggs were immediately sealed with sterile paraffin, after which the eggs were returned to the incubator. The in ovo injection procedure was completed within 30 min. All methods are described in detail by Alizadeh et al. (2020).
Birds and Housing
After hatching, all birds in each group were sexed, and 150 one-day-old female chickens from each group were selected at random for analyses. Each treatment group had 10 replicates with 15 birds per replicate. The rearing period was 7 d. The birds were fed a basal diet with no bioactive compounds, coccidiostats, or antibiotics. Birds received compound feed appropriate for the initial rearing period, that is, starter feed, S (d 1–21), which was provided to the chickens in crumble form. Food and water were available ad libitum. The basal diet was formulated to meet the dietary recommendations for Ross 308 broiler chickens (Aviagen, Broiler Ross Nutrition Supplement). The nutrient content of the diets was calculated on the basis of the chemical composition of the raw feedstuffs and metabolizable energy value. The composition of the basal diet is presented in Table 3.
Table 3.
Composition and nutrient value of basal diet (%).
| Percentage % | |
|---|---|
| Component | Starter (d 1–21) |
| Wheat (group I, VI) | 35.02 |
| Soybean meal | 34.02 |
| Maize | 22.54 |
| Rapeseed oil | 2.01 |
| Lard | 2.00 |
| Rapeseed meal | 1.00 |
| Premix without coccidiostat1 | 1.00 |
| Monocalcium phosphate | 0.72 |
| Calcium carbonate | 0.62 |
| L-Methionine | 0.30 |
| L-Lysine | 0.22 |
| Sodium chloride | 0.20 |
| NaHCO3 | 0.12 |
| Threonine | 0.12 |
| L-valine | 0.12 |
| Optiphos (0.01%)2 | 0.01 |
| AMEN | 12.47 |
| Crude protein | 22.7 |
| Crude fat | 6.02 |
| P available | 0.48 |
| Ca | 0.96 |
| Na | 0.16 |
| Cl | 0.16 |
| Lys dig. | 1.25 |
| Met dig. | 0.6 |
| Thr dig. | 0.84 |
| Val dig | 0.94 |
Vitamin–mineral premix provided per kg diet: Mn, 55 mg; Zn, 50 mg; Fe, 80 mg; Cu, 5 mg; Se, 0.1 mg; I, 0.36 mg; Na, 1.6 g, retinol, 2.48 mg; cholecalciferol, 25 µg; DL-ɑ-tocopherol, 60 mg; cyanocobalamin, 0.012 mg; menadione sodium bisulfite, 1.1 mg; niacin, 53 mg; choline chloride, 1020 mg; folic acid, 0.75 mg; biotin, 0.25 mg; riboflavin, 5.5 mg; xylanase (Econase HCP 4000; AB Vista, Marlborough, UK), 4mg.
Optiphos - 6-phytase derived from Escherichia coli.
The experimental birds were housed under uniform, controlled environmental conditions, and in accordance with the recommendations for this line. Chickens were reared in pens on wood shavings in a room with controlled temperature and humidity. The pens were equipped with feeding lines and nipple drinkers. The lighting regime was adjusted to the age and diurnal rhythm of the birds. The light intensity was 30 to 40 lux up to d 7, the temperature was maintained at 31°C to 33°C up to d 7, and relative humidity was 60% ± 10%.
Growth Performance in Chickens
On hatch day, the hatchability percentage was recorded using the following formula: hatchability percentage = (number of chicks hatched on d 21/number of eggs fed in ovo) × 100 (Table 4).
Table 4.
Effects of in ovo injection on hatchability.
| Group | Hatchability, % (n) |
|---|---|
| I—control group | 95.4% (334)a |
| II | 90.8% (318)b |
| III | 83.4% (292)c |
| IV | 86.2% (302)b |
n: number of hatching chicks; I: control group—eggs injected with sterile 0.9% physiological saline; group II: eggs injected with a multistrain probiotic; group III: eggs injected with a multistrain probiotic and zinc glycine chelate (Zn-Gly); group IV: eggs injected with zinc glycine chelate (Zn-Gly). a, b, c, d - statistical differences (p ≤ 0.05).
During the experiment, the birds were weighed before feeding on d 0 (12 h after hatch) and 7. Average body weight gains (BWG) were calculated for the initial rearing period: 0 to 7 d of life. In addition, feed intake (FI) and FCR were recorded on a per-pen basis on d 0 and 7 and calculated for the initial rearing period (d 0–7). Feed conversion ratio was calculated using BWG and FI and adjusted for mortality. Chicken mortality was recorded daily during morning and afternoon inspection throughout the trial. Feed intake and FCR were corrected for mortality accordingly (Table 5).
Table 5.
Growth performance in chickens.
| Group | BWG 0–7 | FI 0–7 | FCR 0–7 |
|---|---|---|---|
| I | 130ab | 161a | 1.24a |
| II | 127b | 146c | 1.15bc |
| III | 135a | 152ab | 1.13c |
| IV | 131ab | 156ab | 1.19ab |
| SEM | 0.892 | 1.269 | 0.01 |
| P value | 0.0058 | <0.001 | <0.001 |
Abbreviations: BWG, body weight gain; FCR, feed conversion ratio; FI, feed intake; SEM, standard error of the mean.
: statistical differences; 0–7: days of life; I: control group—eggs injected with sterile 0.9% physiological saline; group II: eggs injected with a multistrain probiotic; group III: eggs injected with a multistrain probiotic and zinc glycine chelate (Zn-Gly); group IV: eggs injected with zinc glycine chelate (Zn-Gly).
Blood Samples and Separation of Peripheral Blood Mononuclear Cells
The materials for analysis by flow cytometry were samples of peripheral blood collected 12 h after hatching from chicks killed by decapitation and from the wing vein on 7 d after hatching. The blood samples were collected in sterile vacuum tubes containing heparin as an anticoagulant (Vacuette, Medlab Products, Raszyn, Poland). Blood samples were collected 12 h after hatching, before the chicks were given feed and water, and at 7 d of age. Blood was sampled from 3 birds from each replicate (30 samples in total) in each experimental group. Blood samples were not pooled. The samples were transported to the laboratory at +4°C to 8°C within 1 h.
Peripheral blood mononuclear cells (PBMC) were separated from the blood using Histopaque-1077 (Sigma-Aldrich Sp. z.o.o., Poznań, Poland) according to the manufacturer's instructions. Briefly, at room temperature, 5 mL aliquots of Histopaque-1077 were overlaid with the blood and centrifuged for 30 min at 400 × g. Following centrifugation, the opaque interface was collected and washed twice at 4°C with phosphate-buffered saline containing 0.2% Bovine Serum Albumin and 0.2% sodium azide before centrifuging for 10 min at 250 × g at 4°C. Cell numbers were calculated and the cell concentrations adjusted to 1 × 106 cells/mL for indirect fluorescent antibody labeling. Cell viability was consistently greater than 95% according to the trypan blue dye exclusion method.
Determination of Cellular and Humoral Immune Response Parameters
Flow cytometry was used to determine selected cellular and humoral immune response parameters. Samples of blood and spleen from chickens were tested in a BD FACSVerse flow cytometer (Becton Dickinson, Franklin Lakes, Brea, NJ). From 10,000 to 30,000 events were collected in each measurement by flow cytometer. Electronic compensation was used to eliminate residual spectral overlaps between individual fluorochromes. Flow cytometry was repeated 3 times for each sample and compared for repeatability. The results were analyzed using XL SYSTEM II v.3.0 software and FCS 2.0 format to obtain data in the form of histograms.
Antibodies
Fluorochrome-labeled monoclonal antibodies for the surface molecules of chicken lymphocytes were used for the cytometric tests (Table 6). One-step cell labeling was used for the following antibodies: CD3:FITC, CD25:FITC, CD4:RPE or CD4:FITC, CD8α:RPE, CD79A:FITC, MHC Class II Monomorphic:FITC, and Bu-1a:RPE. For flow cytometric analysis using monoclonal antibodies, 100 µL of the separated PBMC from each sample was used. First, 10 μL of the monoclonal antibody was added to 100 μL of the separated PBMC. After thorough mixing with a vortex mixer, the samples were incubated for 30 min in the dark at room temperature (25°C). Next, the cell suspension was rinsed 3 times with PBS solution, of which 500 μL was added to each sample. The sample was centrifuged after each washing at 1,500 rpm for 5 min. Following the final centrifuging and decanting of the supernatant, 500 µL of PBS was added to the resulting cell pellet, and after thorough mixing, the mixture was left for 10 min at room temperature. The prepared samples were used for flow cytometric analysis. A 2-step cell labeling method was used for TCRγδ+ and monocyte/macrophage (KUL01+). First, 10 μL of the antibody was added to 100 μL of the separated PBMC. After thorough mixing with a vortex mixer, the samples were incubated for 30 min in the dark at room temperature (25°C). Next, the cell suspension was rinsed 3 times with PBS solution, of which 500 μL was added to each sample. The sample was centrifuged after each washing at 1,500 rpm for 5 min. Then 10 μL of polyclonal secondary antibodies (Rabbit F(ab')2 anti-Mouse IgG:FITC or Goat anti-Mouse IgG (H/L):FITC) were added to the samples, and after thorough mixing, the samples were incubated at room temperature (18°C–25°C) for 30 min. The final procedure for preparing the samples for cytometric tests was similar to the procedure for one-step cell labeling. An analogous procedure was used for control samples prepared using isotype antibodies. In each blood sample, CD3+/CD4+, CD3+/CD8α+, CD4+/CD25+, CD25+, MHC Class II+, Bu-1a+, CD79A+, TCRγδ+, and monocyte/macrophage (KUL01+) were determined separately. Immunophenotyping was described in detail by Fair et al. (2008) and Jarosz et al. (2017a).
Table 6.
Antibodies used for flow cytometry in the study.
| Marker | Clone | Isotype | Conjugate | Target cells |
|---|---|---|---|---|
| CD3 | CD3-12 | Rat IgG1 | FITC | T cells |
| CD4 | 2-35 | Mouse IgG2b | FITC and RPE | CD4+ T cells |
| CD8α | 11-39 | Mouse IgG1 | RPE | CD8+ T cells |
| CD25 | AbD13504 | HuCAL Fab-dHLX-MH bivalent | FITC | activated T-lymphocytes |
| MHC Class II | 21-1A6 | Mouse IgG1 | FITC | Antigen-presenting cells (dendritic cells, macrophages, and B cells) |
| TCRγδ | TCR-1 | Mouse IgG1 | Secondary antibodies - Rabbit F(ab')2 anti-Mouse IgG:FITC | γδ T cells |
| Bu-1a | L22 | Mouse IgG1 | FITC | B cells |
| Human CD79A (cross-reactivity) | HM57 | Mouse IgG1 | FITC | B lymphocytes during differentiation |
| Monocyte/macrophage | KUL01 | Mouse IgG1 | Secondary antibodies - Goat anti-Mouse IgG (H/L):FITC | Monocytes and macrophages |
Abbreviations: FITC, fluorescein isothiocyanate; RPE, phycoerythrin.
Spleen Mononuclear Cell Preparation and Flow Cytometry Analysis
At 12 h after hatching, 3 spleen samples were taken from each replicate, both in the experimental and control groups. A total of 120 spleen samples were collected for immunological tests 12 h after hatching (30 spleen samples: group I, 30 spleen samples: group II, 30 spleen samples: group III, and 30 spleen samples: group IV). The same number of spleen samples was collected on the seventh day after hatching. Spleen samples were not pooled. Spleen samples were rinsed 3 times in Hank's Balanced Salt Solution and filtered through a 40-μm nylon cell strainer using the flat end of a 1 mL syringe plunger. Cells were resuspended in 5 mL RPMI (Invitrogen, Burlington, Canada) containing 10% fetal bovine serum, 2.5% HEPES (Sigma Aldrich, St Louis, MO), 1% Penicillin-Streptomycin (Gibco, Grand Island, NY), 0.5% Gentamicin (Gibco, Grand Island, NY), and 0.05% 2-Mercaptoethanol (Sigma Aldrich, St Louis, MO), and overlaid on 4 mL Histopaque-1077 (Sigma, Oakville, Canada) for density gradient separation. Mononuclear cells at the interface were harvested and washed twice in RPMI (Gibco, Grand Island, NY) media. Cell numbers were calculated and the cell concentrations adjusted to 1 × 106 cells/mL for fluorescent antibody labeling. Cell viability was consistently greater than 95% according to the trypan blue dye exclusion method. The analysis was described in detail by Alizadeh et al. (2020). In each spleen mononuclear cell sample, CD3+/CD4+, CD3+/CD8α+, CD4+/CD25+, CD25+, MHC Class II+, Bu-1a+, CD79A+, TCRγδ+, and monocyte/macrophage (KUL01+) were determined separately using a BD FACSVerse flow cytometer.
Statistical Analysis
Statistical analysis of the results was performed using Statistica 13.2 PL software (StatSoft, Krakow, Poland). The distribution of data was analyzed by the Shapiro–Wilk test, and the results were expressed as arithmetic mean and standard deviation. Due to the lack of normal distribution, nonparametric tests of significance of differences were used for P < 0.05. Kruskal nonparametric analysis of variance (ANOVA) on ranks and post-hoc tests for independent samples were used to evaluate the cellular and humoral parameters of the immune response between the control group (I) and experimental groups (II–IV) on individual days (12 h, 7 d). The effect of sampling period (grouping variable) on the immune response was analyzed using the Mann–Whitney U test. The results are presented in graphic form (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6), with the same lowercase letters indicating a lack of statistically significant differences for groups I to IV, and capital letters indicating comparisons in time. The following designations were used for individual variables (experimental samples): I: control group, II: +multistrain probiotic, III: +multistrain probiotic + zinc glycine chelate (Zn-Gly), and IV: + zinc glycine chelate (Zn-Gly).
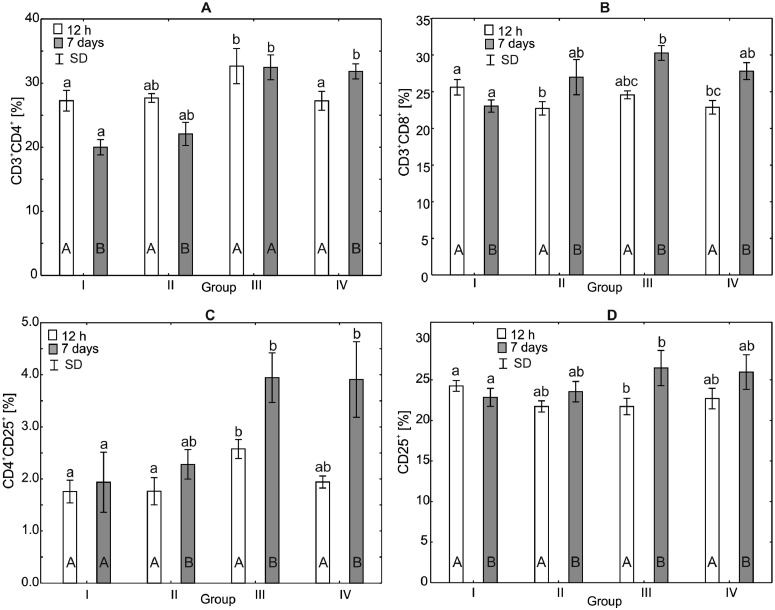
Figure 1.
One-way ANOVA and Mann–Whitney U test for CD3+CD4+, CD3+CD8+, CD4+CD25+, CD25+ percentage in the peripheral blood of broiler chickens at 12 h after hatching and at 7 d of age. Statistical differences (P ≤ 0.05) are marked with different letters. I: control group, II–IV: experimental groups. Abbreviation: SD, standard deviation.
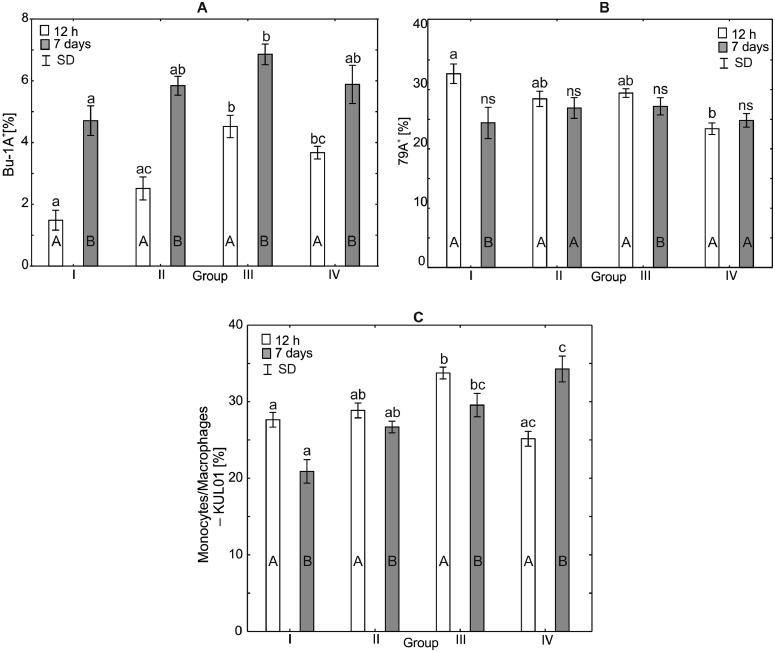
Figure 2.
One-way ANOVA and Mann–Whitney U test for Bu-1a+, CD79A+, monocyte/macrophage (KUL01+) percentage in the peripheral blood of broiler chickens at 12 h after hatching and at 7 d of age. Statistical differences (P ≤ 0.05) are marked with different letters. I: control group, II–IV: experimental groups. Abbreviation: SD, standard deviation.
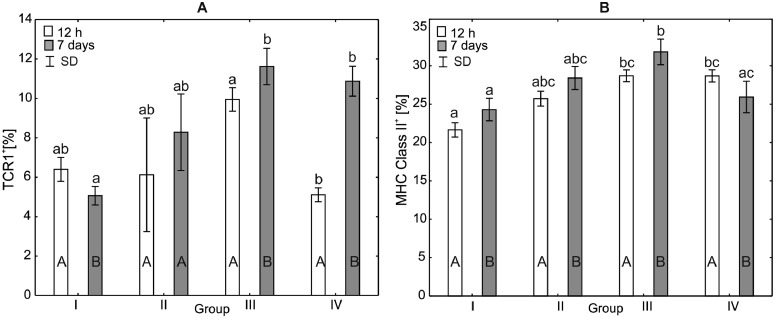
Figure 3.
One-way ANOVA and Mann–Whitney U test for TCRγδ+ and MHC Class II+ percentage in the peripheral blood of broiler chickens at 12 h after hatching and at 7 d of age. Statistical differences (P ≤ 0.05) are marked with different letters. I: control group, II–IV: experimental groups. Abbreviation: SD, standard deviation.
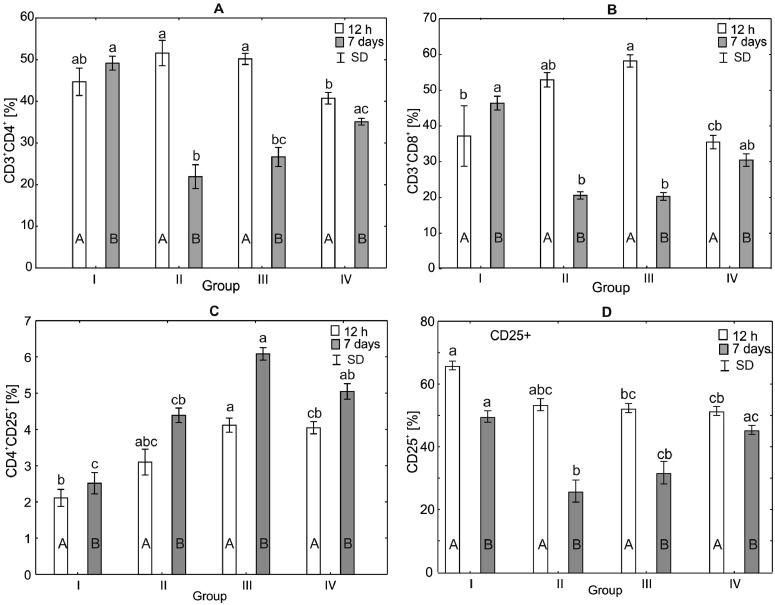
Figure 4.
One-way ANOVA and Mann–Whitney U test for CD3+CD4+, CD3+CD8+, CD4+CD25+, CD25+ percentage in the spleen mononuclear cell of broiler chickens at 12 h after hatching and at 7 d of age. Statistical differences (P ≤ 0.05) are marked with different letters. I: control group, II–IV: experimental groups. Abbreviation: SD, standard deviation.
Figure 5.
One-way ANOVA and Mann–Whitney U test for Bu-1a+, CD79A+, monocyte/macrophage (KUL01+) percentage in the spleen mononuclear cell of broiler chickens at 12 h after hatching and at 7 d of age. Statistical differences (P ≤ 0.05) are marked with different letters. I: control group, II–IV: experimental groups. Abbreviation: SD, standard deviation.
Figure 6.
One-way ANOVA and Mann–Whitney U test for TCRγδ+ and MHC Class II+ percentage in the spleen mononuclear cell of broiler chickens at 12 h after hatching and at 7 d of age. Statistical differences (P ≤ 0.05) are marked with different letters. I: control group, II–IV: experimental groups. Abbreviation: SD, standard deviation.
The statistical evaluation of performance results was carried out using SAS v. 9.4 statistics software (SAS, 2011). All data were presented as mean values with pooled standard error of the mean (SE). Two-way ANOVA was used to determine the effect of the experimental factors. For all characteristics, the significance of differences between group mean values was verified by Tukey's test.
RESULTS
Effect of In Ovo Injection on Hatchability of Broiler Chickens
In all experimental groups, the hatchability percentage was lower than that observed in the control group. The lowest hatchability percentage was observed in group III (83.4%). The smallest difference in hatchability percentage compared to the control group was observed in group II (90.8%, Table 4).
Growth Performance of Broiler Chickens
No statistically significant differences in BWG were observed between the control group and the supplemented group during the first 7 d of the experiment. A statistically significant difference in BWG was noted between groups II and III of experimental chickens. Statistically significant differences in FI were observed between the control group and group II, and between group II and other experimental groups. Statistically significant differences in FCR were observed between the control group and experimental groups II and III, and between group IV and group III. There was no statistically significant difference in FCR between the control group and group IV (Table 5).
Analysis of the Percentage of CD3+CD4+, CD3+CD8+, CD4+CD25+, and CD25+ in the Serum of Broiler Chickens
After 12 h of the experiment, the percentage of CD3+CD4+ in group III (H = 13.58, P < 0.0035) was statistically significantly different from that observed in the experimental group IV and in the control group. After 7 d of the experiment, the percentages of CD3+CD4+ in experimental groups III and IV were statistically significantly higher than in the control group (H = 18.30, P < 0.0004). Within-group comparisons with the Mann–Whitney U test indicated that the time of the experiment was a factor influencing the results, with statistically significant differences demonstrated in all study groups except group III (Figure 1).
Analysis of the percentage of CD3+CD8+ after 12 h of the experiment showed statistically significant differences (H = 17.16, P < 0.0007) between the control group, and groups II and IV. There were no statistically significant differences in the percentages of CD3+CD8+ between the control group and group III. After 7 d of the study, statistically significant difference was observed in the percentage of CD3+CD8+ between the control group and group III, but there were no statistically significant differences observed in groups II and IV and the control group (Figure 1).
The percentage of CD4+CD25+ after 12 h of the experiment in the control group (H = 15.21, P < 0.0016) was statistically significantly lower than in group III, but not statistically different from the percentages obtained in groups II and IV. After 7 d of the study, statistically significantly higher percentages of CD4+CD25+ (H = 17.97, P < 0.0004) were observed in groups III and IV compared to the control group. There were no statistically significant differences in CD4+CD25+ percentages between group II and the control group. Within-group comparisons over time showed statistically significant differences in the percentages of CD4+CD25+ in all experimental groups except the control group (Figure 1).
After 12 h of the experiment, statistically significantly difference in the percentage of CD25+ was observed between the control group and group III (H = 13.80, P < 0.0032). There were no statistically significant differences in CD25+ percentages between the control group and the other experimental groups. Similarly, after 7 d of the experiment, a statistically significantly difference in the percentage of CD25+ was observed between the control group and group III. Within-group comparisons over time showed statistically significant differences in all study groups (Figure 1).
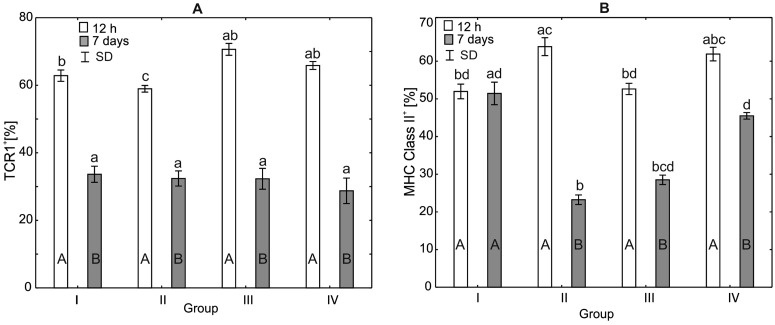
Analysis of the Percentage of TCRγδ+ and MHC Class II+ in the Serum of Broiler Chickens
After 12 h of the experiment, statistically significant differences (H = 15.62, P < 0.014) in the percentages of TCRγδ+ were observed between groups III and IV. The TCRγδ+ percentages in the control group and group II did not differ statistically significantly from those observed in the other groups. After 7 d of the experiment, it was observed that the percentage of TCRγδ+ in the control group was statistically significantly different compared to groups III and IV (H = 18.12, P < 0.004). Within-group comparisons over time showed statistically significant differences in all study groups except group II (Figure 2).
After 12 h of the experiment, statistically significant differences in the percentage of MHC Class II+ (H = 19.45, P < 0.0002) were observed in groups III and IV compared to the control group. There were no statistically significant differences in the percentage of MHC Class II+ between group II and the control group. After 7 d of the experiment, a statistically significant difference in the percentage of MHC class II+ was observed between the control group and group III. There was also a statistically significant difference in the percentage of MHC class II+ between groups III and IV. Within-group comparisons over time showed statistically significant differences in all experimental groups and in the control group (Figure 2).
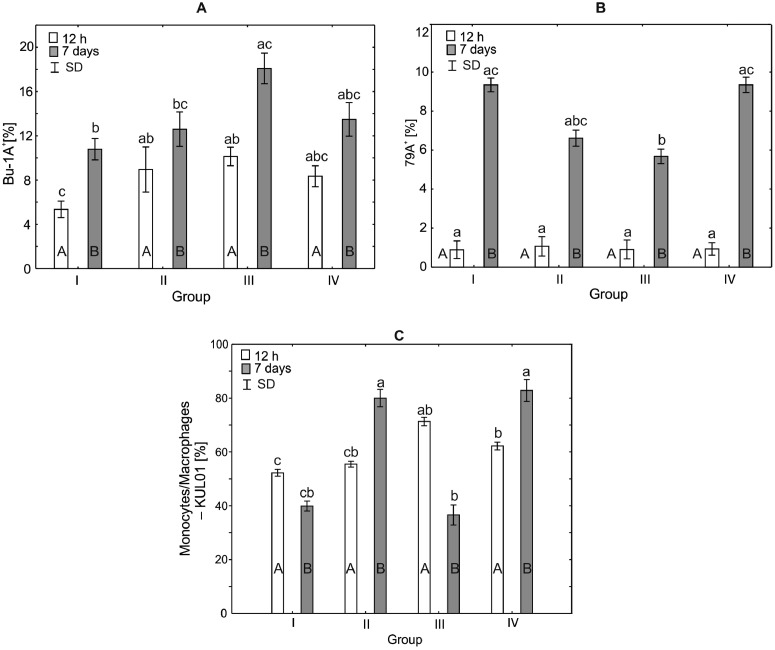
Analysis of the Percentage of Bu-1A+, CD79A+, and Monocytes/Monophages—KUL01+ in the Serum of Broiler Chickens
After 12 h of the experiment, statistically significant differences were observed in the mean percentage of Bu-1A+ between the control group and groups III and IV (P < 0.0001), but there were no statistically significant differences between the percentages of Bu-1A+ observed in the control group and group II. After 7 d of the experiment, a statistically significant difference in the percentage of Bu-1A+ was observed between the control group and group III (H = 18.41, P < 0.0004). Within-group comparison over time showed statistically significant differences in all groups (Figure 3).
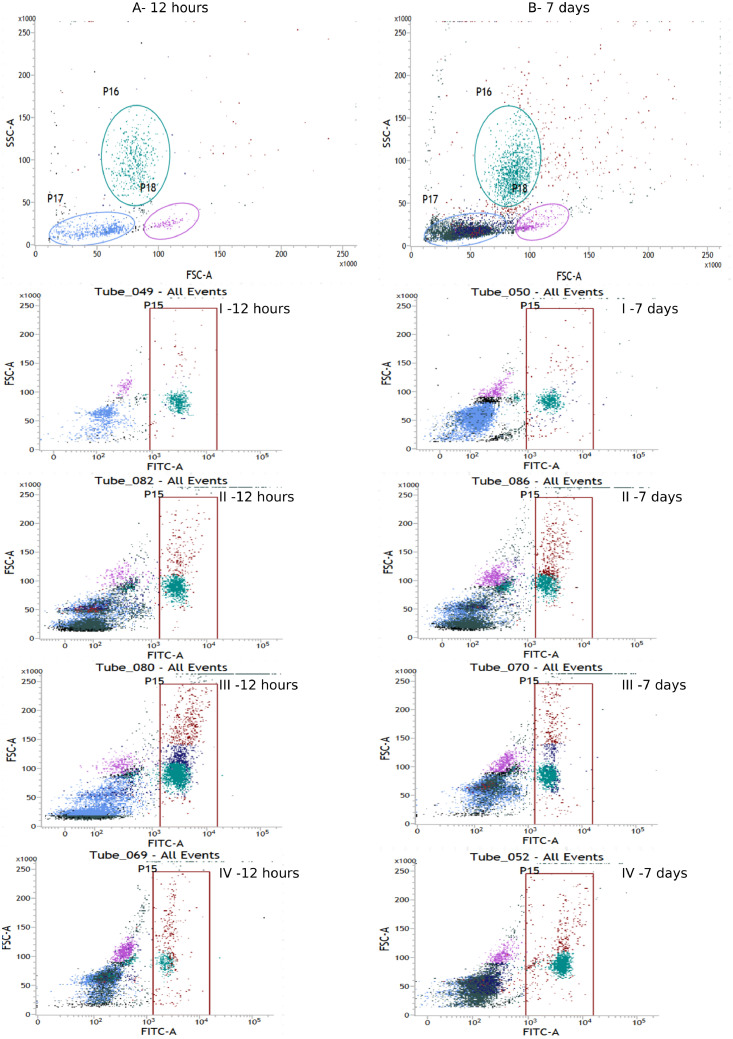
After 12 h of the study, a statistically significant difference in the percentage of CD79A+ was observed between the control group and the group IV (H = 19.57, P < 0.0002). There were no statistically significant differences in the percentages of CD79A+ between the control group and the other experimental groups. After 7 d of the experiment, the percentages of CD79A+ did not differ statistically significantly (H = 8.40, P < 0.038) in any of the experimental groups compared to the control group. Within-group comparison over time showed statistically significant differences in groups I and III, and no differences in groups II and IV (Figures 3 and 7).
Figure 7.
Flow cytometric analysis of CD79A+ in blood of chickens (dot plots). (A) Chicken white blood cells 12 h after hatching, (B) chicken white blood cells at 7 d of age, lymphocytes (P17), monocytes (P18), heterophils (P16). I: control group—blood from chickens that hatched from eggs injected with sterile 0.9% physiological saline; group II—blood from chickens that hatched from eggs injected with a multistrain probiotic; group III—blood from chickens that hatched from eggs injected with a multistrain probiotic and zinc glycine chelate (Zn-Gly); group IV—blood from chickens that hatched from eggs injected with zinc glycine chelate (Zn-Gly), P15—gate containing cells with expression CD79A+ labeled with FITC (fluorescein isothiocyanate). Abbreviation: FSC, forward scatter.
After 12 h of the experiment, the average percentage of KUL01+ monocytes/macrophages in group III (33.73%) was statistically significantly different from that observed in the control group (H = 20.22, P < 0.0002). A statistically significant difference in the percentage of monocytes/macrophages-KUL01+ was also observed between groups III and IV. After 7 d of the study, the percentages of monocytes/macrophages-KUL01+ in groups III and IV (29.55% and 25.16%, respectively) were statistically significantly different (H = 21.13, P < 0.0001) from that observed in the control group. Within-group comparisons over time showed statistically significant differences across all study groups (Figure 3).
Analysis of CD3+CD4+, CD3+CD8+, CD4+CD25+, and CD25+ Percentages in the Spleen of Broiler Chickens
After 12 h of the experiment, no statistically significant differences in CD3+CD4+ values were observed between the control group and the experimental groups. However, the value of CD3+CD4+ in group IV (H = 17.55, P < 0.0005) after 12 h of the experiment was statistically significantly different compared to the values observed in groups II and III. After 7 d of the study, the CD3+CD4+ values in groups II and III showed statistically significant differences compared to the value observed in the control group (H = 20.57, P < 0.0001). No statistically significant differences in CD3+CD4+ values were observed between the control group and the group IV. Comparison of the experimental groups by the Mann–Whitney U test indicated that the time of the experiment was an important factor influencing the results. Statistically significant differences in CD3+CD4+ values were observed in all groups (Figure 4).
Analysis of the level of CD3+CD8+ showed statistically significant differences after 12 h of the experiment between group III, (H = 15.26, P < 0.0016), and groups IV and I (control). CD3+CD8+ values in the control group (I) did not differ statistically significantly from the values obtained in groups II and IV. After 7 d of the experiment, the value of CD3+CD8+ in group I was statistically significantly different (H = 20.51, P < 0.0001) compared to groups II and III. No statistically significant differences in CD3+CD8+ values were observed between groups I and IV (Figure 4).
The analysis of the impact of CD4+CD25+ on the spleen after 12 h of the experiment showed that the value in the control group was statistically significantly different (H = 19.55, P < 0.0002) from the value observed in group III and did not differ statistically significantly from the observed in groups II and IV. After 7 d of the experiment, statistically significant differences in CD4+CD25+ values were observed between the control group and groups III and IV (Figure 4).
The level of CD25+ after 12 h of the experiment in the control group (I) was statistically significantly different from the levels in groups III and IV (H = 15.26, P < 0.0016). There were no statistically significant differences in the level of CD25+ in the control group compared to group II. After 7 d of the experiment, statistically significant differences (H = 20.51, P < 0.001) in CD25+ levels were observed between the control group (I), and groups II and III (Figure 4).
Analysis of the Percentage of TCRγδ+ and MHC Class II+ in the Spleen of Broiler Chickens
After 12 h of the experiment, statistically significant differences (H = 21.05, P < 0.001) in the percentage of TCRγδ+ were observed between groups I and II. The percentage of TCRγδ+ after 7 d of the experiment in all supplemented groups remained at the same level and did not show statistically significant differences compared to the control group (H = 6.45, P < 0.09, Figure 5).
After 12 h of the experiment, statistically significant differences (H = 17.99, P < 0.0004) was observed in the percentages of MHC Class II+ in group II compared to the control group. In groups III and IV, there were no statistically significant differences in the percentage of MHC Class II+ compared to the control group. Similar differences were observed after 7 d of the experiment (H = 21.60, P < 0.0001, Figure 5).
Analysis of the Percentage of Bu-1A+, CD79A+, and Monocytes/Monophages—KUL01+ in the Spleen of Broiler Chickens
After 12 h of the experiment, statistically significant differences were observed in the mean percentage of Bu-1A+ between the control group (H = 15.71, P < 0.0013) and experimental groups II and III (8.95% and 10.13%, respectively). After 7 d of the experiment, a statistically significant difference in the percentage of Bu-1A+ was observed between the control group (H = 17.55, P < 0.0005) and group III. The percentage of Bu-1A+ in the remaining experimental groups was not statistically significantly different from the control group (Figure 6).
After 12 h of the experiment, the mean percentages of CD79A+ in the experimental groups did not differ statistically significantly (H = 0.33, P < 0.95) from the control group. After 7 d of the study, the percentage of CD79A+ in the control group was statistically significantly different (H = 20.51, P < 0.0001), compared to group III (5.67%; Figure 6).
After 12 h of the experiment, the percentages of monocytes/macrophages-KUL01+ in groups III and IV (71.33% and 62.20%, respectively), differed statistically significantly from the percentage in the control group (H = 21.60, P < 0.0001). After 7 d of the experiment, the percentage of monocytes/macrophages-KUL01+ in groups II and IV (88.0% and 82.85%, respectively), differed statistically significantly (H = 18.69, P < 0.0003), from the values observed in the groups I and III (Figure 6).
DISCUSSION
In commercial broiler production, the period from the 18th day of incubation to 4 to 7 d after hatching is critical for the survival and further development of chicks (Ferket, 2006). In ovo administration of probiotics and microelements during this period stimulates the development of individual systems and organs, especially the immune system, thereby reducing morbidity and mortality rates among chicks (Cox and Dalloul, 2015). The results of the study, aimed at evaluating the immune status of chicks in the initial period after hatching following in ovo administration of a multistrain probiotic and zinc glycine chelate, shed new light on mechanisms of immune system modulation induced by the use of feed additives, indicating that they can be used to reduce prophylactic administration of medicine to chicks in the initial rearing period.
Adequate hatchability of chicken eggs is crucial for the economic functioning of the poultry farm, particularly its production results (de Oliveira et al., 2014). The experiment showed that the combined in ovo administration of a multistrain probiotic and zinc glycine chelate (group III) significantly reduced hatching rates. Similarly, in ovo administration of the individual supplements, that is, the multistrain probiotic (group II) or zinc glycine chelate (group IV), also reduced hatchability, but no statistically significant differences were shown between groups. The results are consistent with those published by Meijerhof and Hulet (1997), Hashemzadeh et al. (2010), Hosseini-Mansoub et al. (2011), and Yamawaki et al. (2013), who reported that in ovo application of certain probiotic bacteria reduces hatchability. Contrasting results were published by Pender et al. (2017), who observed no changes in chick hatching rates between the experimental and control groups following in ovo administration of Primalac W/S, containing Lactobacillus acidophilus, L. casei, Enterococcus faecium, and Bifidobacterium bifidum. This effect of the formulation seems to be due in part to the types of probiotic bacteria included in it and the level of the supplement applied.
Similar observations pertain to zinc, which is commonly considered to be a nontoxic element for people and animals, although its exact mechanism of action on the body is not fully known (Roohani et al., 2013). Although our study showed lower hatching rates following in ovo administration of zinc glycine chelate alone or in combination with the multistrain probiotic, previously published results showed no negative influence of in ovo administration of Zn on the developing chicken embryo (Tako et al., 2005; Goel et al., 2012; Oliveira et al., 2015). It is likely that the effect of zinc on the embryo was in this case dependent on the dose of zinc as well as its chemical form and bioavailability. Higher levels of zinc have been shown to increase embryo mortality and reduce hatching rates (Oliveira et al., 2015, Sun et al., 2018). Similarly, zinc in organic form, with its greater bioavailability, may have toxic effects, causing damage to embryos (Star et al., 2012; Swain et al., 2016). The reduction of hatchability to the level of 86% was shown by Yair et al. (2013), after in ovo administration of 0.6 mg zinc/egg in the form of bioplex zinc along with other minerals and vitamins. Also Joshua et al. (2016), after in ovo administration of various doses nano zinc (20–80 μg/egg), showed a reduction in hatchability compared to the control group, which was at the level of 96% to 88%. Despite lower hatchability, Joshua, et al. (2016) did not observe differences in body weight of hatched chicks. Similar results were obtained in the presented study (Table 5). Tako et al. (2005) observed that in ovo administration of 0.5 mg zinc chelates on 17 d of embryo incubation may result in increased biochemical activity of the brush-border enzymes and transporters, and increased the jejunal villus surface area, thereby enhancing the intestinal development. It can be assumed that the lower hatchability rates will be compensated by the beneficial effects of zinc compounds on the chicks after hatching. It should be emphasized that despite the obtained reduction in hatchability after the combined administration of in ovo multistrain probiotics and zinc glycine chelate (group III), and in ovo supplementation with zinc glycine chelate (group IV), we showed an increase in the process of proliferation and differentiation of TCD3+CD4+ and TCD3+CD8+ lymphocytes, and stimulation of cellular immune response mechanisms. This is extremely important in the first period of chicks’ life because they are exposed to numerous environmental bacterial and viral infections. The functional mobilization of the immune system demonstrated in the study will have a positive (upregulated) effect on the antigen presentation during the induction of the immune response. On the one hand, this will protect the chick against infection and death, and on the other hand, it will modulate the pro- and anti-inflammatory response and maintain the balance between Th1 and Th2. The organic form of zinc used in the present study, that is, glycine chelate, is better absorbed and assimilated than inorganic forms, which may have increased the zinc concentration in the yolk sac and reduced hatching rates. The initial content of zinc in the yolks of hatching eggs has been shown to vary depending on the breeding flocks, in which different feed ingredients and diet supplements are used (Wilson, 1997). Additional administration of bioavailable Zn in the form of glycine chelate during embryonic development may cause mineral imbalances (Oliveira et al., 2015), with excessive accumulation of zinc and its harmful effects on the embryo. Moreover, the biomass of probiotic bacteria includes microelements such as zinc, facilitating its absorption, which can lead to an increase in its concentrations in the tissues and organs and to toxic effects (Hussain et al., 2022).
Probiotic bacterial strains and zinc positively influence intestinal development and function, which translates to improved digestion, absorption and production results (Shah et al., 2019). In our experiment, the highest body weight in the first 7 d of life was noted in birds receiving the multistrain probiotic and zinc glycine chelate in ovo, although the value of this parameter did not differ statistically from the values obtained in the other groups. Similar observations were reported by Jose et al. (2018), who found that in ovo injection of Zn at 0.25 and 0.50 mg/egg in the form of sulfate, methionate, and nano-oxide did not increase the growth of broilers posthatch, but weight gains in this group of birds were higher in these groups than in the control. Joshua et al. (2016) also showed that in ovo administration of 40 μg nano-zinc significantly increased BWG and FCR. Similarly, higher body weight and weight gains were noted in chicks posthatch in the case of in ovo administration of probiotic strains (Edens et al., 1997; Teague et al., 2017; Duan et al., 2021). Contrasting results were obtained by Goel et al. (2012), Yair et al. (2013), and Kim and Kang (2022) who showed that in ovo administration of zinc did not improve the growth performance of posthatch chickens. The same applies to in ovo administration of probiotic strains (Majidi-Mosleh et al., 2017; Oladokun et al., 2021), which did not significantly increase production parameters in chicks 42 d after hatching. The data indicate that administration of probiotics in the late embryonic stage can have positive effects on the gastrointestinal tract of birds. Interestingly, the additives used in our study significantly reduced the FCR relative to the control group, with the lowest FCR noted in the group receiving the multistrain probiotic and zinc glycine chelate in ovo. It should be stressed that in ovo administration of probiotics leads to early colonization of the digestive tract by useful microbes and to changes in the intestinal microbiome (Shehata et al., 2021). These processes influence the physiological development of the gastrointestinal tract of the chick in the first 21 d after hatching, including the length of the intestines and gut pH (Shehata et al., 2021). Previous research using rats (Tesán et al., 2011) indicates that the bioavailability of zinc increases when used in combination with probiotics, and it acts synergistically with the probiotic to increase villus height and the total number of goblet cells (Shah et al., 2019). Therefore, combined administration of zinc and probiotics increases the absorption of zinc, which improves production parameters by stimulating metabolism of proteins, sugars and fats (Liu et al., 2015). The discrepancies in data on the effects of in ovo application of multistrain probiotics and zinc glycine chelate on production parameters suggest the need for further research to determine the optimal probiotic strains, dose of the probiotic, and period of embryonic development when it should be administered to achieve beneficial effects throughout the growth period.
Optimal physiological functioning of poultry in the posthatch period, when birds are exposed to numerous endogenous and exogenous factors, requires interactions between specific immunocompetent cells, including T and B cells, natural killer cells, antigen-presenting cells, and others (Kaiser, 2010; Sławińska et al., 2014a). Early interference in immune mechanisms through in ovo administration of a multistrain probiotic and zinc glycine chelate leads to modulation of innate immune processes, owing to which newly hatched chicks are already able to respond to antigens, thereby reducing the risk of infection after hatching (Sławińska et al., 2014, Alizadeh et al., 2020). In this context, the results of the complete immunophenotypic assessment of cells taking part in the humoral and cellular response can be considered pioneering, enabling reliable determination of the usefulness of in ovo administration of a multistrain probiotic together with zinc glycine chelate.
The highest percentage of CD4+ T helper cells in the serum was noted in group III, 12 h after hatching and at 7 d of age. These findings confirm the strong effect of combined administration of a multistrain probiotic and Zn-Gly chelate on lymphocyte differentiation and proliferation and are evidence of stimulation of cellular immune mechanisms in birds. CD4+ T cells are involved in various immune response processes, including activation of B lymphocytes, macrophages, and cytotoxic CD8+ T lymphocytes (Luckheeram et al., 2012). This is confirmed by the high percentage of these cells in the serum of birds from group III together with the immunophenotype of immunocompetent cells, especially the high expression of KUL01 on monocytes/macrophages. CD4+ T cells also take part in antigen presentation by interacting with major histocompatibility complex (MHC) class II molecules (Vignali, 1994). The high percentage of this subpopulation of cells shown in the study in conjunction with the high expression of MHC II molecules in this group on both sampling days is evidence of enhancement of antigen presentation processes. The results suggest that a multistrain probiotic administered in ovo, containing numerous bacterial antigens, activates an immune response to stimulation with these antigens, whereas T lymphocytes in the serum are a population of functional cells involved in the early immune response to antigens. These cells most likely appear in the serum as a result of selective colonization of the intestinal epithelium by probiotic bacteria and activation of intraepithelial lymphocytes (IELs), which play a critical role in the protective immune response to intestinal pathogens (Wickramasuriya et al., 2022).
Contrasting results were obtained from the analysis of splenic mononuclear cells, which showed that 12 h after hatching there were no significant differences in the percentage of CD4+ cells in the groups receiving a multistrain probiotic. In addition, in group IV, following in ovo administration of Zn-Gly chelate, this expression was lower than in the other experimental groups and the control group. At 7 d as well, the percentage of CD4+ T cells was lower in the experimental groups than in the control group. Similar observations were made by Alizadeh et al. (2020), who following in ovo inoculation of multistrain lactobacilli found no changes in the percentage of splenocytes with CD4+ expression up to 10 d posthatch. The low percentage of CD4+ cells in the spleen in the experimental groups indicates that no inflammatory processes were taking place in response to the antigens administered in ovo. The high percentage of these cells noted in the control group indicates colonization of the body by environmental antigens stimulating a systemic immune response, manifested by the induction and proliferation of lymphocytes in the lymphoid organs. The differentiation of T cells and regulation of the immune response were additionally confirmed on both sampling days by the high percentage of B cells with Bu-1A expression in the serum and spleen in group III, in which the multistrain probiotic and zinc glycine chelate were administered in ovo. This costimulation of B cells through antigen presentation by T cells improves the immunoglobulin synthesis process and the body's humoral response (Vazquez et al., 2015).
Divergent results were obtained for CD8+ lymphocytes. The percentage of these cells in the serum immediately after hatching was lower in group III, which received the multistrain probiotic and zinc glycine chelate in ovo, than in the control group, after which it rose and attained the highest value on d 7. A different pattern was shown for CD8+ cells in the spleen, with the highest percentage noted in group III at 12 h after hatching, whereas at 7 d posthatch, it was the lowest in this group. The increase in the percentage of these cells after hatching in the absence of disease symptoms in this group is indicative of immunomodulation of T cells by compounds used in the in ovo supplementation. Performing the role of antigens, the multistrain probiotic and zinc glycine chelate probably stimulate CD8+ lymphocytes by enhancing the proliferation and functions of T cells. Contrasting results were obtained by Alizadeh et al. (2020), who observed no differences in the percentage of CD8+ cells up to 10 d posthatch following in ovo inoculation of multistrain lactobacilli, whereas administration of lactobacilli increased the expression of cytokines in the spleen, including IFN-α, IFN-β, IFN-γ, IL-8, and IL-12. Sławińska et al. (2014) reported that in ovo administration of synbiotics increased mRNA expression of IL-4, IL-6, IFN-β, and IL-18 in the spleen during rearing of chickens. Together with the results of our experiment, these findings indicate that one of the effects of a multistrain probiotic and zinc glycine chelate in poultry is the induction of pro-inflammatory signals activating the cytotoxicity of cells taking part in protection against infection, as well as modulation of the immune response and its Th1/Th2 polarization. This effect maintains the pro- and anti-inflammatory balance in response to the action of numerous antigens in the form of microbial strains and zinc. The posthatch increase in the activity of Th1 lymphocytes, mainly with CD8+ expression, is indicative of promotion of cellular immune mechanisms and does not rule out a developing inflammatory process in response to in ovo application of antigens and environmental factors.
One of the lymphocyte subpopulations analyzed in the experiment was cells with CD4+CD25+ expression, which have immunosuppressive and functional properties in chickens similar to those of CD4+CD25+Foxp3+ Treg cells in mammals (Selvaraj, 2013; Calefi et al., 2016). They have also been shown to function as Treg cells in chickens, suppressing excessive immune reactions and inhibiting excessive development of T lymphocytes (Shanmugasundaram and Selvaraj, 2011). The high percentage of these cells in the serum of group III chickens at 12 h and 7 d after hatching following in ovo administration of the multistrain probiotic and Zn-Gly chelate suggests that some of these cells play an immunosuppressive role as regulator cells. These cells probably inhibit the inflammatory response caused by excessive intake of the multistrain probiotic acting as antigen on the embryo. The simultaneous high percentage of TCD4+ and TCD8+ lymphocytes in this group additionally confirms the immunoregulatory role of these cells in response to the antigens, that is, the bacterial strains and Zn-Gly chelate. Similarly, the high expression of CD4+CD25+ cells in the spleen at 12 h and 7 d posthatch, especially in group III, is due to migration of these cells from the peripheral pool and indicates activation of immunoregulatory mechanisms in response to the antigens introduced in ovo.
It should be stressed that it cannot be inferred from the CD4+CD25+ cell immunophenotype itself that these cells belong to the population of immunosuppressive lymphocytes. For this reason, it seems that they should be included among cells of numerous subsets of lymphocytes with various types of activity, including immunosuppressive, regulatory, or memory, as evidenced by CD25+ and TCR expression (Izcue et al., 2006; Teng et al., 2006). The increase in TCR and CD25+ expression on lymphocytes at 12 h posthatch in the spleen and at 7 d in the serum suggests that T memory cells produced following in ovo supplementation, tasked with suppressing the immune response, are dominant during this period. At 7 d of age, the percentage of CD25+ and TCR cells in the spleen of the chicks was low, which suggests the functioning of CD4+CD25+ Treg lymphocytes maintaining homeostasis between the pro- and anti-inflammatory response. CD4+CD25+ cells also migrate between the thymus, bone marrow, and other lymphoid organs in chickens, and mostly likely the surface of the intestinal mucosa is enriched with Treg cells, as in mammals (Shanmugasundaram and Selvaraj, 2011). These phenomena explain why the concentrations of these cells in the experiment are varied. It should be noted that changes of this type are observed especially in consequence of strong antigen stimulation, which in the experiment was induced by the in ovo supplementation.
Similar patterns as for CD3+CD8+ expression were also noted for expression of the CD25+ molecule. The higher percentage of T lymphocytes with the CD25+ phenotype in group III after hatching is evidence of activation of a specific immune response through stimulation of Th1 lymphocytes. The high percentage of these cells in the serum of the same group on d 7 of the experiment indicates proliferation of T lymphocytes and enhancement of the immune response induced by foreign antigens introduced in ovo. The high percentage of CD25+ cells in the serum of the birds in group III in combination with the increase in the percentage of CD4 and CD8 cells confirms that the multistrain probiotic and zinc glycine chelate administered in ovo stimulate cellular mechanisms responsible for recognition and elimination of antigens. T lymphocytes activated in this way will also effectively prevent environmental infections in poultry, which may significantly improve the health of the flock.
The high percentage of MHC II cells shown in groups II and III indicates activation of antigen presentation to effector cells, especially Th lymphocytes. MHC II molecules are known to be strongly expressed on macrophages, dendritic cells, B lymphocytes, and activated T lymphocytes (Silva and Gallardo, 2020). These data together with the results obtained for the immunophenotype of T lymphocytes indicate that in ovo administration of a multistrain probiotic, as well as a probiotic in combination with Zn-Gly chelate, through the MHC II complex effectively activates mechanisms of cellular surveillance in the response to the antigens, which is particularly evident in the early posthatching period.
Another cell population analyzed in the experiment was TCRγδ+ T cells, which perform numerous effector and cytotoxic functions in poultry, taking part in the early stages of the immune response (Smith and Hayday, 2000). γδ T cells are believed to play an important role in immune processes in the initial period of life and are the first T cells to develop in the thymus and migrate to the peripheral tissues and organs (Bucy et al., 1991). These cells have been shown to be involved in the response to pathogens and to protect the body from tumor development and inflammation (Chien et al., 2014). In chickens, γδ T cells are the dominant population and can produce various cytokines and interferons. They may also exhibit cytotoxic properties, taking part in the immune response to pathogens and vaccine antigens (Pieper et al., 2008; Kjærup et al., 2014; Fenzl et al., 2017). In our experiment, the birds in group III, receiving in ovo supplementation of a multistrain probiotic and zinc glycine chelate, had a statistically significant high percentage of TCRγδ+ cells in the serum and spleen at 12 h after hatching and in the serum at 7 d. These findings are partially in agreement with those reported by Szczypka et al. (2021), who following in ovo stimulation with probiotics and synbiotics noted a high percentage of TCRγδ+ cells in the spleen of chickens up to 35 d of life. Our results indicate that a multistrain probiotic administered in ovo stimulates and promotes mechanisms of the cellular immune response to antigens of the microbiome. The lack of differences in the percentages of these cells in the spleen at 7 d of age between the experimental and control groups is surprising. It can probably be explained by the migration of these cells to the mucosa, mainly of the gastrointestinal tract, and their role in the recognition and elimination of antigens. Huang et al. (2013), however, showed no differences in the percentage of γδ+ TCR T cells in the intestinal mucosa of poultry in the period between 7 and 14 d of age between a group receiving a probiotic and the control group. At the same time, they noted an increase in the percentage of CD8+ cells, which indicates that γδ T cells stimulate specific expansion of cytotoxic T effector cells, enhancing intestinal immunity and protecting against infection. However, a full understanding of the mechanisms of action of this subpopulation of lymphocytes requires further research.
The percentage of Bu-1A+ lymphocytes in the serum and spleen was high in all experimental groups at 12 h and 7 d after hatching, and was highest in group III, in which the birds received a multistrain probiotic and Zn-Gly chelate in ovo. This indicates that a multistrain probiotic and Zn-Gly chelate stimulate humoral immune mechanisms through active B cell production and antibody synthesis. Contrasting results were obtained by Alizadeh et al. (2021, 2022), who found no changes in the percentage of Bu-1A+ cells following in ovo supplementation with multistrain lactobacilli. The high expression of Bu-1A on B lymphocytes in the serum and spleen of poultry can be assumed to indicate that the multistrain probiotic and zinc glycine chelate stimulate the humoral response, which is responsible for eliminating antigens entering the body. It is worth noting that Alizadeh et al. (2020), following in ovo inoculation of multistrain lactobacilli, showed an increase in the concentrations of class M and G antibodies in the serum of poultry as a result of stimulation of keyhole limpet hemocyanin (KLH). In addition, in the groups of birds receiving probiotics in ovo, the experiment showed stimulation of a specific immune response mediated by antibodies against the highly immunogenic T-cell-dependent antigen (KLH), which suggests that probiotic bacteria influence acquired immunity. It should be emphasized that stimulation of the immune system with the use of a multistrain probiotic has a positive effect on selection of lymphocytes and their maturation in the lymph nodes and contributes to the production of plasma cells and B memory cells. In addition, previous research has shown that the use of chelates of various organic forms of Zn stimulates B lymphocyte proliferation and differentiation in broilers through cytokine activation and causes changes in the lymphoid organs—the thymus, spleen, and bursa of Fabricius, suggesting stimulation of humoral and cellular immune mechanisms (Bartlett and Smith 2003; Jasim and Al-Qaisy, 2019). Similarly, Chitithoti et al. (2012) and Ezzati et al. (2012) showed that zinc methionine promotes the immune response by enhancing T cell maturation and secondary activation of B cells by T helper cells. These findings together with the results of our experiment indicate that the combined in ovo application of a multistrain probiotic and zinc glycine chelate, through activation of Th1 cells and their release of cytokines, stimulates the type Th2 response, thereby reducing the severity of inflammatory processes and supporting the humoral immune response, while inhibiting the Th1 response.
One of the markers of B cells is the CD79a molecule, an integral membrane protein expressed in the early stage of development of B lymphocytes, preceding expression of CD19, and in the late stage of B cell differentiation, that is, in plasma cells (Mason et al., 1995). Expression of this marker is specific for B lymphocytes and plays an important role in the development, survival and activation of this cell subpopulation (Lai et al., 2000). In the present study, significant differences in the expression of this molecule in the spleen were not observed until 7 d after hatching. The high expression of CD79a in group IV, which received Zn-Gly chelate in ovo, may be explained by the effect of zinc accumulated in the organs of birds, influencing the development of B cells. Zinc deficiencies and disturbances of zinc homeostasis have been shown to impair the differentiation of B cells so that they remain at the pre-B stage (Anzilotti et al., 2019). Administration of highly bioavailable zinc chelate in ovo may therefore increase proliferation of B lymphocytes and humoral immune mechanisms. This is confirmed by the expression of the Bu-1A molecule, which was higher in this group at 7 d posthatch, in both the spleen and the serum. Contrasting results were obtained for CD79a expression in groups II and III, in which the birds received a multistrain probiotic and a multistrain probiotic with zinc glycine chelate, respectively, in ovo. At 7 d of age expression of this molecule in the spleen was lower in both experimental groups, whereas in the serum no differences in this parameter were shown between groups. It may be that in the conditions of the experiment, biosorption of Zn ions by the biomass of microbes supplied in the probiotic formulation led to the gradual release of zinc and its absorption in the intestine. On the other hand, it may indicate limitation of B cell differentiation by the various microbial strains contained in the probiotic administered in ovo. These results in conjunction with the expression of Bu-1A, which was highest in group III in the serum and spleen at 12 h and 7 d posthatch, suggest that probiotic microbes cause strong antigenic stimulation and promotion of humoral immune mechanisms, that is, differentiation of B cells, their activation, and antibody production.
Among innate elements of the body's system of defense against pathogens, particular importance is ascribed to macrophages (Qureshi et al., 2000). Apart from functioning as antigen-presenting cells, they eliminate pathogens through phagocytosis and indirectly by regulating the response of other immunocompetent cells (Qureshi et al., 2000). Tissue macrophages, including those present in the spleen, also take part in immunosurveillance by regulating the response of CD4+ T cells (Kurotaki et al., 2011) and in suppression of the immune response to antigens, by stimulating apoptosis (Miyake et al., 2007). In the present study, a statistically significant high percentage of KUL01+ cells was noted in the serum and spleen at 12 h posthatch in the group receiving the multistrain probiotic and zinc glycine chelate in ovo. These results confirm previous findings reported by Alizadeh et al. (2021), who showed that in ovo administration of lactobacilli increases the pool of KUL01+ cells in the spleen. The components of probiotic bacterial cells, including exopolysaccharides, peptidoglycans, lipoteichoic acid and their metabolites, such as short-chain fatty acids and amino acids, stimulate the immune system by inducing the proliferation of phagocytic cells and phagocytic activity (Taverniti and Guglielmetti, 2011; Xiu et al., 2018). They also induce production of nitric oxide (NO) and cytokines in macrophages, including TNF-α and IFN-γ, thus taking part in antigen presentation to Th cells and enhancing immunoregulatory processes (Taverniti and Guglielmetti, 2011; Xiu et al., 2018). The high expression of KUL01 and Bu-1A at 12 h posthatch in the group receiving a multistrain probiotic and zinc glycine chelate in ovo demonstrates that these supplements trigger the phagocytic activity of macrophages, take part in presentation of the antigens contained in the probiotic to other immunocompetent cells, and stimulate antibody production. Activated phagocytes, acting in a nonspecific manner, also protect against invasive factors through antigen presentation to T cells and stimulation of cellular defense mechanisms. This translates to increased resistance to infection and improved health parameters in the first few days after hatching. The experiment also showed that combined supplementation with zinc glycine chelate and a multistrain probiotic decreases the percentage of KUL01+ cells in the spleen at 7 d after hatching in comparison to the groups receiving the multistrain probiotic or zinc glycine chelate alone, which suggests that zinc and the multistrain probiotic have an immunoregulatory function when administered together. Different results were obtained for the serum, in which the highest percentage of KUL01+ cells on d 7 was noted in the group receiving zinc glycine chelate. Zinc acts on a variety of immune cells, affecting the number and functions of phagocytes, neutrophils, monocytes, and macrophages taking part in phagocytosis. Earlier research (Gao et al., 2018) showed that supplementation with zinc increases the percentage of phagocytic monocytes and heterophils, which indicates stimulation of nonspecific defense mechanisms and confirms the results of the present study using zinc glycine chelate in ovo.
CONCLUSION
Combined in ovo administration of multistrain probiotics and zinc glycine chelate has a multidirectional stimulating effect on the immune system of the embryo and chicks in the first period after hatching. The high expression of CD4+ and CD8+ molecules in the serum and spleen of chicks after in ovo supplementation with multistrain probiotics and Zn-Gly chelate proves the promotion of cellular mechanisms of the immune response, expressed in an increase in the population of antigen-presenting Th1 lymphocytes. This phenomenon should contribute to more effective elimination of microorganisms in the first period of chicks' life. At the same time, the high percentage of Treg CD4+CD25+ cells in the serum and spleen of chicks proves the immunoregulatory effect of multistrain probiotics and Zn-Gly chelate administered in ovo on Th1 cells and ensures the maintenance of a balance in the body between the Th1/Th2 response. Stimulation of the cellular response is accompanied by stimulation of the humoral response through activation of B lymphocytes. The weakness of the combined in ovo administration of multistrain probiotics and zinc glycine chelate is the reduction of hatchability of chicks; therefore, further research is needed on the effects of these preparations on the mechanisms of toxicity, embryo mortality, and the regulation of the immune response at the cellular level within the developing embryo.
ACKNOWLEDGMENTS
The authors wish to thank the Greenland Technologia EM, Janowiec, Poland, for supporting research.
This work was financed by the University of Life Sciences in Lublin, Poland (project no. SD/43/WET/2022).
Ethical Statement: All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. The experiment was conducted at the Experimental Station of the Poznan University of Life Sciences, Gorzyń 4, Międzychód commune. Consent for all research procedures was obtained from the Local Ethics Committee for Animal Testing at the University of Life Sciences in Lublin, Poland (approval no. 106/2022 of October 17, 2022).
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article, and are available on request from the corresponding author.
DISCLOSURES
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria, educational grants, participation in speakers’ bureaus, membership, employment, consultancies, stock ownership, or other equity interest, expert testimony or patent-licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
REFERENCES
- Alizadeh M., Astill J., Alqazlan N., Shojadoost B., Taha-Abdelaziz K., Bavananthasivam J., Doost J.S., Sedeghiisfahani N., Sharif S. In ovo co-administration of vitamins (A and D) and probiotic lactobacilli modulates immune responses in broiler chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh M., Bavananthasivam J., Shojadoost B., Astill J., Taha-Abdelaziz K., Alqazlan N., Boodhoo N., Doost J.S., Sharif S. In ovo and oral administration of probiotic Lactobacilli modulate cell- and antibody-mediated immune responses in newly hatched chicks. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.664387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh M., Shojadoost B., Astill J., Taha-Abdelaziz K., Karimi S.H., Bavananthasivam J., Kulkarni R.R., Sharif S. Effects of in ovo inoculation of multi-strain Lactobacilli on cytokine gene expression and antibody-mediated immune responses in chickens. Front. Vet. Sci. 2020;7:105. doi: 10.3389/fvets.2020.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzilotti C., Swan D.J., Boisson B., Deobagkar-Lele M., Oliveira C., Chabosseau P., Engelhardt K.R., Xu X., Chen R., Alvarez L., Berlinguer-Palmini R., Bull K.R., Cawthorne E., Cribbs A.P., Crockford T.L., Dang T.S., Fearn A., Fenech E.J., de Jong S.J., Lagerholm B.C., Ma C.S., Sims D., van den Berg B., Xu Y., Cant A.J., Kleiner G., Leahy T.R., de la Morena M.T., Puck J.M., Shapiro R.S., van der Burg M., Chapman J.R., Christianson J.C., Davies B., McGrath J.A., Przyborski S., Santibanez Koref M., Tangye S.G, Werner A., Rutter G.A., Padilla-Parra S., Casanova J.L., Cornall R.J., Conley M.E., Hambleton S. An essential role for the Zn2+ transporter ZIP7 in B cell development. Nat Immunol. 2019;20:350–361. doi: 10.1038/s41590-018-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar Shira E., Sklan D., Friedman A. Impaired immune response in broiler hatchling hindgut following delayed access to feed. Vet. Immunol. Immunopathol. 2005;105:33–45. doi: 10.1016/j.vetimm.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Bartlett J.R., Smith M.O. Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult. Sci. 2003;82:1580–1588. doi: 10.1093/ps/82.10.1580. [DOI] [PubMed] [Google Scholar]
- Bucy R.P., Chen C.H., Cooper M.D. Analysis of gamma delta T cells in the chicken. Semin. Immunol. 1991;3:109–117. [PubMed] [Google Scholar]
- Calefi A.S., Quinteiro-Filho W.M., Fukushima A.R., da Cruz D.S.G., de Siqueira A., Salvagni F.A., Namazu L.B., Gomes C.O.M.S., Ferreira A.J.P., Palermo Neto J. Dexamethasone regulates macrophage and Cd4+Cd25+ cell numbers in the chicken spleen. Rev. Bras. Cienc. Avic. 2016;18:93–100. [Google Scholar]
- Carter S.D., Kim HJ. Technologies to reduce environmental impact of animal wastes associated with feeding for maximum productivity. Anim. Front. 2013;3:42–47. [Google Scholar]
- Chien Y., Meyer C., Bonneville M. γδ T cells: first line of defense and beyond. Annu. Rev. Immunol. 2014;32:121–155. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
- Chitithoti A.K., Venkata R.J., Jwalapu R.P., Devanesan S.S., Reddy S. Immuno stimulatory effect of dietary supplementation of zinc sulphate and zinc-methionine on immune response in broilers. Adv. Appl. Sci. Res. 2012;3:2785–2788. [Google Scholar]
- Clavijo V., Flórez M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 2018;97:1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.M., Dalloul R.A. Immunomodulatory role of probiotics in poultry and potential in ovo application. Benef. Microbes. 2015;6:45–52. doi: 10.3920/BM2014.0062. [DOI] [PubMed] [Google Scholar]
- Dai D., Wu S.G., Zhang H.J., Qi G.H., Wang J. Dynamic alterations in early intestinal development, microbiota and metabolome induced by in ovo feeding of L-arginine in a layer chick model. J. Anim. Sci. Biotechnol. 2020;11:19. doi: 10.1186/s40104-020-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Bosco A., Mattioli S., Cartoni Mancinelli A., Cotozzolo E., Castellini C. Extensive rearing systems in poultry production: the right chicken for the right farming system. A review of twenty years of scientific research in Perugia University, Italy. Animals (Basel) 2021;11:1281. doi: 10.3390/ani11051281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira J.E., van der Hoeven-Hangoor E., van de Linde I.B., Montjin R.C., van der Vossen J.M. In ovo inoculation of chicken embryos with probiotic bacteria and its effect on posthatch Salmonella susceptibility. Poult. Sci. 2014;93:818–829. doi: 10.3382/ps.2013-03409. [DOI] [PubMed] [Google Scholar]
- Duan A.Y., Ju A.Q., Zhang Y.N., Qin Y.J., Xue L.G., Ma X., Luan W.M., Yang S.B. The effects of in ovo injection of synbiotics on the early growth performance and intestinal health of chicks. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.658301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunisławska A., Sławińska A., Stadnicka K., Bednarczyk M., Gulewicz P., Jozefiak D D., Siwek M. Synbiotics for broiler chickens—in vitro design and evaluation of the influence on host and selected microbiota populations following in ovo delivery. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens F.W., Parkhurst C.R., Casas I.A., Dobrogosz W.J. Principles of ex ovo competitive exclusion and in ovo administration of Lactobacillus reuteri. Poult. Sci. 1997;76:179–196. doi: 10.1093/ps/76.1.179. [DOI] [PubMed] [Google Scholar]
- Elibol O., Brake J. Identification of critical periods for turning broiler hatching eggs during incubation. Br. Poult. Sci. 2004;45:631–637. doi: 10.1080/00071660400006271. [DOI] [PubMed] [Google Scholar]
- Ellis R., Morris E.R., Hill A.D. Bioavailability to rats of iron and zinc in calcium-iron-phytate and calcium-zinc-phytate complexes. Nutr. Res. 1982;2:319–322. [Google Scholar]
- Ernst R.A., Bradley F.A., Abbott U.K., Craig R.M. ANR Publication; Oakland, CA: 2004. Egg Candling and Breakout Analysis; p. 8134.http://anrcatalog.ucdavis.edu/pdf/8134.pdf Accessed Mar. 2007. [Google Scholar]
- Ezzati M.S., Bozorgmehrifard M.H., Bijanzad P., Rasoulinezhad S., Moomivand H., Faramarzi S., Ghaedi A., Ghabel H., Stabraghi E. Effects of different levels of zinc supplementation on broilers performance and immunity response to Newcastle disease vaccine. Eur. J. Exp. Biol. 2012;3:497–501. [Google Scholar]
- Fair J.M., Taylor-McCabe K.J., Shou Y., Marrone B.L. Immunophenotyping of chicken peripheral blood lymphocyte subpopulations: individual variability and repeatability. Vet. Immunol. Immunopathol. 2008;125:268–273. doi: 10.1016/j.vetimm.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Feng J., Ma W.Q., Niu H.H., Wu X., Wang Y. Effects of zinc glycine chelate on growth, hematological, and immunological characteristics in broilers. Biol. Trace. Elem. Res. 2010;133:203–211. doi: 10.1007/s12011-009-8431-9. [DOI] [PubMed] [Google Scholar]
- Fenzl L., Göbel T.W., Neulen M.L. γδ T cells represent a major spontaneously cytotoxic cell population in the chicken. Dev. Comp. Immunol. 2017;73:175–183. doi: 10.1016/j.dci.2017.03.028. [DOI] [PubMed] [Google Scholar]
- Ferket P.R. Incubation and in ovo nutrition affects neonatal development. Pages 18–30 in 33rd Annual Carolina Poultry Nutrition Conference; New York, NY; 2006. [Google Scholar]
- Fordyce E.J., Forbes R.M., Robins K.R., Erdman J.W., Jr Phytate × calcium/zinc molar ratios: are they predictive of zinc bioavailability? J. Food Sci. 1987;52:440–444. [Google Scholar]
- Gao H., Dai W., Zhao L., Min J., Wang F. The role of zinc and zinc homeostasis in macrophage function. J. Immunol. Res. 2018;2018 doi: 10.1155/2018/6872621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A., Bhanja S.K., Mehra M., Pande V. World Poultry Congress; Salvador, Bahia, Brazil: 2012. Does in ovo Administration of Zinc or Iodine Modulate Differential Expression of Growth and Immune Related Genes in Broiler Chickens. [Google Scholar]
- Hashemzadeh Z., Karimi Torshizi M.A., Rahimi S., Razban V., Zahraei Salehi T. Prevention of Salmonella colonization in neonatal broiler chicks by using different routes of probiotic administration in hatchery evaluated by culture and PCR techniques. J. Agric. Sci. Tech. 2010;12:425–432. [Google Scholar]
- Hedlund L., Palazon T., Jensen P. Stress during commercial hatchery processing induces long-time negative cognitive judgement bias in chickens. Animals. 2021;11:1083. doi: 10.3390/ani11041083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidayat C., Sumiati A.Jayanegara, Wina E. Effect of zinc addition on the immune response and production performance of broilers: a meta-analysis. Asian-Australas. J. Anim. Sci. 2020;33:465–479. doi: 10.5713/ajas.19.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini-Mansoub N., Vahdatpour T., Arjomandi M., Vahdatpour S. Comparison of different methods of probiotic prescription against Salmonella infection in hatchery broiler chicken. Adv. Environ. Biol. 2011;5:1857–1860. [Google Scholar]
- Hou T., Tako E. The in ovo feeding administration (Gallus Gallus)—an emerging in vivo approach to assess bioactive compounds with potential nutritional benefits. Nutrients. 2018;10:418. doi: 10.3390/nu10040418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A., Shibata E., Nishimura H., Igarashi Y., Isobe N., Yoshimura Y. Effects of probiotics on the localization of T cell subsets in the intestine of broiler chicks. J. Poult. Sci. 2013;50:275–281. [Google Scholar]
- Hussain S., Khan M., Sheikh T.M.M., Mumtaz M.Z., Chohan T.A., Shamim S., Liu Y. Zinc essentiality, toxicity, and its bacterial bioremediation: a comprehensive insight. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.900740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A., Coombes J.L., Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- Jarosz Ł., Marek A., Grądzki Z., Kwiecień M., Kalinowski M. The effect of feed supplementation with zinc chelate and zinc sulphate on selected humoral and cell-mediated immune parameters and cytokine concentration in broiler chickens. Res. Vet. Sci. 2017;112:59–65. doi: 10.1016/j.rvsc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Jarosz M., Olbert M., Wyszogrodzka G., Młyniec K., Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacol. 2017;25:11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasim M.S., Al-Qaisy N.E. Effect of in ovo injection of zinc methonine on hatching traits, production performance and immunity response of broiler chickens. Plant Arch. 2019;19:1235–1240. [Google Scholar]
- Jha R., Singh A.K., Yadav S., Berrocoso J.F.D., Mishra B. Early nutrition programming (in ovo and post-hatch feeding) as a strategy to modulate gut health of poultry. Front. Vet. Sci. 2019;6:82. doi: 10.3389/fvets.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose N., Elangovan A.V., Awachat V.B., Shet D., Ghosh J., David C.G. Response of in ovo administration of zinc on egg hatchability and immune response of commercial broiler chicken. J. Anim. Physiol. Anim. Nutr. 2018;102:591–595. doi: 10.1111/jpn.12777. [DOI] [PubMed] [Google Scholar]
- Joshua P.P., Valli C., Balakrishnan V. Effect of in ovo supplementation of nano forms of zinc, copper, and selenium on post-hatch performance of broiler chicken. Vet. World. 2016;9:287–294. doi: 10.14202/vetworld.2016.287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam M.M., Barekatain M.R., Bhanja S.K., Iji P.A. Prospects of in ovo feeding and nutrient supplementation for poultry: the science and commercial applications–a review. J. Sci. Food Agric. 2013;93:3654–3661. doi: 10.1002/jsfa.6301. [DOI] [PubMed] [Google Scholar]
- Kaiser P. Advances in avian immunology—prospects for disease control: a review. Avian Pathol. 2010;39:309–324. doi: 10.1080/03079457.2010.508777. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Kang H.K. Effects of in ovo injection of zinc or diet supplementation of zinc on performance, serum biochemical profiles, and meat quality in broilers. Animals. 2022;12:630. doi: 10.3390/ani12050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjærup R.M., Dalgaard T.S., Norup L.R., Hamzić E., Sørensen P., Juul-Madsen H.R. Characterization of cellular and humoral immune responses after IBV infection in chicken lines differing in MBL serum concentration. Viral Immunol. 2014;27:529–542. doi: 10.1089/vim.2014.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurotaki D., Kon S., Bae K., Ito K., Matsui Y., Nakayama Y., Kanayama M., Kimura C., Narita Y., Nishimura T., Iwabuchi K., Mack M., van Rooijen N., Sakaguchi S., Uede T., Morimoto J. CSF-1-dependent red pulp macrophages regulate CD4 T cell responses. J. Immunol. 2011;186:2229–2237. doi: 10.4049/jimmunol.1001345. [DOI] [PubMed] [Google Scholar]
- Lai R., Juco J., Lee S.F., Nahirniak S., Etches W.S. Flow cytometric detection of CD79a expression in T-cell acute lymphoblastic leukemias. Am. J. Clin. Pathol. 2000;113:823–830. doi: 10.1309/391R-93YF-DB4D-1L35. [DOI] [PubMed] [Google Scholar]
- Liu Z.H., Lu L., Wang R.L., Lei H.L., Li S.F., Zhang L.Y., Luo X.G. Effects of supplemental zinc source and level on antioxidant ability and fat metabolism-related enzymes of broilers. Poult. Sci. 2015;94:2686–2694. doi: 10.3382/ps/pev251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Morawong T., Molee A., Molee W. Influences of L-Arginine in ovo feeding on the hatchability, growth performance, antioxidant capacity, and meat quality of slow-growing chickens. Animals (Basel) 2022;12:392. doi: 10.3390/ani12030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckheeram R.V., Zhou R., Verma A.D., Xia B. CD4⁺T cells: differentiation and functions. Clin. Dev. Immunol. 2012;2012 doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukić M., Petričević V., Delić N., Tolimir N., Dosković V., Rakonjac S., Škrbić Z. How does the choice of genotype and feed in the local market affect broiler performance and the farm economy? A case study in Serbia. Agriculture. 2022;12:843. [Google Scholar]
- Majidi-Mosleh A., Sadeghi A.A., Mousavi S.N., Chamani M., Zarei A. Effects of in ovo infusion of probiotic strains on performance parameters, jejunal bacterial population and mucin gene expression in broiler chicken. Rev. Bras. Cienc. Avic. 2017;19:97–102. [Google Scholar]
- Mason D.Y., Cordell J.L., Brown M.H., Borst J., Jones M., Pulford K., Jaffe E., Ralfkiaer E., Dallenbach F., Stein H. CD79a: a novel marker for B-cell neoplasms in routinely processed tissue samples. Blood. 1995;86:1453–1459. [PubMed] [Google Scholar]
- Meijerhof R., Hulet R.M. In ovo injection of competitive exclusion culture in broiler hatching eggs. J. Appl. Poult. Res. 1997;6:260–266. [Google Scholar]
- Miyake Y., Asano K., Kaise H., Uemura M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell – associated antigens. J. Clin. Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladokun S., Koehler A., MacIsaac J., Ibeagha-Awemu E.M., Adewole D.I. Bacillus subtilis delivery route: effect on growth performance, intestinal morphology, cecal short-chain fatty acid concentration, and cecal microbiota in broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunbosun O.B., Sogunle O.M., Adetola O.O., Odutayo O.J., Ayodeji T.M., Ayoola A.A., Abiona J.A. In ovo feeding of organic salts of zinc and copper: effects on growth performance and health status of two strains of broiler chickens. Trop. Agric. (Trinidad) 2023;99:146–165. [Google Scholar]
- Olejnik K., Popiela E., Opaliński S. Emerging precision management methods in poultry sector. Agriculture. 2022;12:718. [Google Scholar]
- Oliveira T.F.B., Bertechini A.G., Bricka R.M., Kim E.J., Gerard P.D., Peebles E.D. Effects of in ovo injection of organic zinc, manganese, and copper on the hatchability and bone parameters of broiler hatchlings. Poult. Sci. 2015;94:2488–2494. doi: 10.3382/ps/pev248. [DOI] [PubMed] [Google Scholar]
- Pender C.M., Kim S., Potter T.D., Ritzi M.M., Young M., Dalloul R.A. In ovo supplementation of probiotics and its effects on performance and immune-related gene expression in broiler chicks. Poult. Sci. 2017;96:1052–1062. doi: 10.3382/ps/pew381. [DOI] [PubMed] [Google Scholar]
- Pieper J., Methner U., Berndt A. Heterogeneity of avian γδ T cells. Vet. Immunol. Immunopathol. 2008;124:241–252. doi: 10.1016/j.vetimm.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Qureshi M.A., Heggen C.L., Hussain I. Avian macrophage: effector functions in health and disease. Dev. Comp. Immunol. 2000;24:103–119. doi: 10.1016/s0145-305x(99)00067-1. [DOI] [PubMed] [Google Scholar]
- Roohani N., Hurrell R., Kelishadi R., Schulin R. Zinc and its importance for human health: an integrative review. J. Res. Med. Sci. 2013;18:144–157. [PMC free article] [PubMed] [Google Scholar]
- Roto S.M., Kwon Y.M., Ricke S.C. Applications of in ovo technique for the optimal development of the gastrointestinal tract and the potential influence on the establishment of its microbiome in poultry. Front. Vet. Sci. 2016;3:63. doi: 10.3389/fvets.2016.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino G., Corsello A., Corsello G. Macronutrient balance and micronutrient amounts through growth and development. Ital. J. Pediatr. 2021;47:109. doi: 10.1186/s13052-021-01061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS (Statistical Analysis System). 2011. SAS Version 9.3. Procedure Guide. SAS Inc., Cary, North Carolina, USA. https://support.sas.com/documentation/onlinedoc/base/procstat93m1.pdf.
- Selvaraj R.K. Avian CD4(+)CD25(+) regulatory T cells: properties and therapeutic applications. Dev. Comp. Immunol. 2013;41:397–402. doi: 10.1016/j.dci.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Shah M., Zaneb H., Masood S., Khan R.U., Ashraf S., Sikandar A., Rehman H.F.U., Rehman H.U. Effect of dietary supplementation of zinc and multi-microbe probiotic on growth traits and alteration of intestinal architecture in broiler. Probiotics Antimicrob. Proteins. 2019;11:931–937. doi: 10.1007/s12602-018-9424-9. [DOI] [PubMed] [Google Scholar]
- Shanmugasundaram R., Selvaraj R.K. Regulatory T cell properties of chicken CD4+CD25+ cells. J. Immunol. 2011;186:1997–2002. doi: 10.4049/jimmunol.1002040. [DOI] [PubMed] [Google Scholar]
- Shehata A.M., Paswan V.K., Attia Y.A., Abdel-Moneim A.E., Abougabal M.S., Sharaf M., Elmazoudy R., Alghafari W.T., Osman M.A., Farag M.R., Alagawany M. Managing gut microbiota through in ovo nutrition influences early-life programming in broiler chickens. Animals (Basel) 2021;11:3491. doi: 10.3390/ani11123491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A.P.D., Gallardo R.A. The chicken MHC: insights into genetic resistance, immunity, and inflammation following infectious bronchitis virus infections. Vaccines (Basel) 2020;8:637. doi: 10.3390/vaccines8040637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sławińska A., Siwek M., Żylińska J., Bardowski J., Brzezińska J., Gulewicz K.A., Nowak M., Urbanowski M., Płowiec A., Bednarczyk M. Influence of synbiotics delivered in ovo on immune organs development and structure. Folia Biol. (Kraków) 2014;62:277–285. doi: 10.3409/fb62_3.277. [DOI] [PubMed] [Google Scholar]
- Sławińska A., Siwek M.Z., Bednarczyk M.F. Effects of synbiotics injected in ovo on regulation of immune-related gene expression in adult chickens. Am. J. Vet. Res. 2014;75:997–1003. doi: 10.2460/ajvr.75.11.997. [DOI] [PubMed] [Google Scholar]
- Smith A.L., Hayday A.C. An αβ T-cell-independent immunoprotective response towards gut coccidia is supported by γδ cells. Immunology. 2000;101:325–332. doi: 10.1046/j.1365-2567.2000.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Star L., van der Klis J.D., Rapp C., Ward T.L. Bioavailability of organic and inorganic zinc sources in male broilers. Poult. Sci. 2012;91:3115–3120. doi: 10.3382/ps.2012-02314. [DOI] [PubMed] [Google Scholar]
- Sun X., Lu L., Liao X., Zhang L., Lin X., Luo X., Ma Q. Effect of in ovo zinc injection on the embryonic development and epigenetics-related indices of zinc-deprived broiler breeder eggs. Biol. Trace Elem. Res. 2018;185:456–464. doi: 10.1007/s12011-018-1260-y. [DOI] [PubMed] [Google Scholar]
- Swain P.S., Rao S.B.N., Rajendran D., Dominic and G., Selvaraju S. Nano zinc, an alternative to conventional zinc as animal feed supplement: a review. Anim. Nutr. 2016;2:134–141. doi: 10.1016/j.aninu.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczypka M., Suszko-Pawłowska A., Kuczkowski M., Gorczykowski M., Lis M., Kowalczyk A., Łukaszewicz E., Poradowski D., Zbyryt I., Bednarczyk M., Stefaniak T. Effects of selected prebiotics or synbiotics administered in ovo on lymphocyte subsets in bursa of the fabricius, thymus, and spleen in non-immunized and immunized chicken broilers. Animals (Basel) 2021;11:476. doi: 10.3390/ani11020476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha-Abdelaziz K., Hodgins D.C., Lammers A., Alkie T.N., Sharif S. Effects of early feeding and dietary interventions on development of lymphoid organs and immune competence in neonatal chickens: a review. Vet. Immunol. Immunopathol. 2018;201:1–11. doi: 10.1016/j.vetimm.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Tako E., Ferket P.R., Uni Z. Changes in chicken intestinal zinc exporter mRNA expression and small intestinal functionality following intra-amniotic zinc-methionine administration. J. Nutr. Biochem. 2005;16:339–346. doi: 10.1016/j.jnutbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Taverniti V., Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept) Genes Nutr. 2011;6:261–274. doi: 10.1007/s12263-011-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague K.D., Graham L.E., Dunn J.R., Cheng H.H., Anthony N., Latorre J.D., Menconi A., Wolfenden R.E., Wolfenden A.D., Mahaffey B.D., Baxter M., Hernandez-Velasco X., Merino-Guzman R., Bielke L.R., Hargis B.M., Tellez G. In ovo evaluation of FloraMax®-B11 on Marek's disease HVT vaccine protective efficacy, hatchability, microbiota composition, morphometric analysis, and Salmonella enteritidis infection in broiler chickens. Poult. Sci. 2017;96:2074–2082. doi: 10.3382/ps/pew494. [DOI] [PubMed] [Google Scholar]
- Teng Q.Y., Zhou J.Y., Wu J.J., Guo J.Q., Shen H.G. Characterization of chicken interleukin 2 receptor alpha chain, a homolog to mammalian CD25. FEBS Lett. 2006;580:4274–4281. doi: 10.1016/j.febslet.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Tesán F., Hernández F., Torti H., Massot F., Huarte M., de Celis E.R., Barreiro M., Weill R., Cremaschi G., Boccio J. Glycine-stabilized zinc gluconate has similar bioavailability than zinc sulfate in a zinc fortified probiotic food. Open Nutraceut. J. 2011;4:136–143. [Google Scholar]
- Vazquez M.I., Catalan-Dibene J., Zlotnik A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine. 2015;74:318–326. doi: 10.1016/j.cyto.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali D.A. The interaction between CD4 and MHC class II molecules and its effect on T cell function. Behring Inst. Mitt. 1994;94:133–147. [PubMed] [Google Scholar]
- Weese J.S., Martin H. Assessment of commercial probiotic bacterial contents and label accuracy. Can. Vet. J. 2011;52:43–46. [PMC free article] [PubMed] [Google Scholar]
- Wickramasuriya S.S., Park I., Lee K., Lee Y., Kim W.H., Nam H., Lillehoj H.S. Role of physiology, immunity, microbiota, and infectious diseases in the gut health of poultry. Vaccines (Basel) 2023;10:172. doi: 10.3390/vaccines10020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen H., Debonne M., Swennen Q., Everaert N., Careghi C., Han H., Bruggeman V., Tona K., Decuypere E. Delay in feed access and spread of hatch: importance of early nutrition. Worlds Poult. Sci. J. 2010;66:177–188. [Google Scholar]
- Wilson H.R. Effects of maternal nutrition on hatchability. Poult. Sci. 1997;76:134–143. doi: 10.1093/ps/76.1.134. [DOI] [PubMed] [Google Scholar]
- Wilson K.M., Rodrigues D.R., Briggs W.N., Duff A.F., Chasser K.M., Bielke L.R. Evaluation of the impact of in ovo administered bacteria on microbiome of chicks through 10 days of age. Poult. Sci. 2019;98:5949–5960. doi: 10.3382/ps/pez388. [DOI] [PubMed] [Google Scholar]
- Xiu L., Zhang H., Hu Z., Liang Y., Guo S., Yang M., Du R., Wang X. Immunostimulatory activity of exopolysaccharides from probiotic Lactobacillus casei WXD030 strain as a novel adjuvant in vitro and in vivo. Food Agric. Immunol. 2018;29:1086–1105. [Google Scholar]
- Yair R., Shahar R., Uni Z. Prenatal nutritional manipulation by enrichment influences bone structure, composition, and mechanical properties. J. Anim. Sci. 2013;91:2784–2793. doi: 10.2527/jas.2012-5548. [DOI] [PubMed] [Google Scholar]
- Yamawaki R.A., Milbradt E.L., Coppola M.P., Rodrigues J.C.Z., Andreatti Filho R.L., Padovani C.R., Okamoto A.S. Effect of immersion and enoculation in ovo of Lactobacillus spp. in embryonated chicken eggs in the prevention of Salmonella enteritidis after hatch. Poult. Sci. 2013;92:1560–1563. doi: 10.3382/ps.2012-02936. [DOI] [PubMed] [Google Scholar]
- Yegani M., Korver D.R. Factors affecting intestinal health in poultry. Poult. Sci. 2008;87:2052–2063. doi: 10.3382/ps.2008-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Lei F., Zhu L., Li S., Wu Z., Zhang R., Gao G.F., Zhu B., Wang X. Exposure of different bacterial inocula to newborn chicken affects gut microbiota development and ileum gene expression. ISME J. 2010;4:367–376. doi: 10.1038/ismej.2009.128. [DOI] [PubMed] [Google Scholar]