Abstract
Selenium is one of the necessary micronutrients needed for enhanced gut microbiota and oxidative stress of poultry, so it improves their performance. In this study, Bacillus subtilus DA20 isolate that identified at the gene level by PCR was employed to produce eco-friendly selenium nanoparticles (BSeNPs) and investigate their effects on growth performance, carcass characteristics, blood parameters, and gut microbiota of Indian River (IR) broiler chickens. The obtained selenium nanoparticles were spherical with size of 56 nm and net negative charge of −22.36 mV; the BSeNPs were surrounded with active compounds, which besides the tiny size attributed to antioxidant and antibacterial activity. Forty hundred and eighty unsexed IR broilers, 1-day old, were reared for 35 d. The chicks were weighed separately and distributed into 3 treatment groups; each group contained 4 replicates (40 birds per replicate). Chicks in the first, second, third, fourth groups were fed control diets supplemented with 0, 20, 40, and 60 µg/kg of BSeNPs, respectively; but the fifth group was fed 300 µg/kg bulk selenium. Dietary supplementation with BSeNPs (40 µg/kg diet) significantly increased the body weight of chicks and decreased the feed conversion ratio. Additionally, dietary BSeNPs significantly (P = 0.046) lowered the fat content in broiler by 24% compared to the control; on the other hand, the breast muscle significantly increased (P = 0.035) by 19%. The content of total bacterial count (TBC), total yeast mold count (TYMC), E. coli, and Salmonella counts significantly was decreased with BSeNPs and Se compared to the control. However, lactic acid bacteria (LAB) was significantly increased with BSeNPs (60 μg/kg) when compared to control, showing the beneficial effects of BSeNPs in reducing pathogens and enhancing the beneficial bacteria, which reflects on the broiler performance.
Key words: Bacillus subtilus, selenium nanoparticles, performance, blood chemistry, gut microbiota
INTRODUCTION
Selenium (Se) is one of the necessary micronutrients needed for ideal poultry performance (Elnaggar et al., 2020). It regulates physiological assignments such as growth performance, survivability, meat quality, and antioxidant safety. Twenty-five sorts of selenoproteins are implicated in many bodies' biological and physiological procedures, demanding enough selenium amounts (Sonet et al., 2016). Selenium has an important active function in preventing the oxidation of cells and cell membranes because of selenium prominence in glutathione peroxidase synthesis (Sobolev et al., 2018). The Se chemical shape identifies efficacy in some enzymes in vivo or in vitro (Pouri et al., 2018). Se could be supplemented into poultry diets in 2 forms: organic (L-selenomethionine) and inorganic (selenite). Conversely, the Se organic form is higher absorption and faster transmission into the body than the inorganic form (Selim et al., 2015). The Se content in the poultry diet differs and depends on its content in the plant ingredients and the soil Se percentage, whereas the plants were implanted. The production of organic selenomethionine could be affected by soil selenite, thereby establishing seleno-amino acids (Sharma et al., 2015). Moreover, selenomethionine is the main seleno-compound in Se-enriched yeast, which is adopted as a source of Se commercial poultry rations. Small sizes and active surfaces distinguish bionanotechnology-produced materials, making them unique medicinal and nutritional materials (Þorgeirsdóttir et al., 2023).
Traditionally, chemical and physical methods were used to make nanomaterials. However, both procedures have drawbacks, such as the high cost of using expensive techniques with high pressure and temperature or a poisonous chemical severely influencing the environment (Visha et al., 2015). Since soil is a medium rich in various minerals, it is a favorable environment for microorganisms can reduce these metals, producing nanoparticles. Several investigations focused on the possible fabricating of nanoparticles by soil microbes via biological mechanisms (El-Saadony et al., 2021b; Saad et al., 2022). So, the majority of microorganisms can fabricate biological nanoparticles.
In this investigation, soil Bacillus species are isolated. In general, phenotypic, biochemical, and immunological tests and molecular techniques are utilized for detecting, quantifying, and characterizing bacteria, including those belonging to Bacillus. However, these approaches have low resolution when discriminating between closely related species and frequently result in the misclassification and misidentification of strains (Sangal et al., 2016). Particularly, whereas the relative prevalence of the Bacillus has been recognized using pyrosequencing for numerous varieties of kimchi, few species-level data have been recorded (Kang et al., 2016). 16S rRNA genes have been utilized to characterize diverse microorganisms for many years. This technique primarily determines a sample's bacteria and archaea's relative abundances and diversity. Bacillus species, that is, B. licheniformis, B. subtilis, and B. velezensis, have been assigned taxonomically and phylogenetically using PCR-based molecular analysis based on sequencing the 16S rRNA gene (Dunlap et al., 2016; Fan et al., 2017).
Selenium addition in poultry diets at a specified level is essential to improve antioxidant and immunological responses (Lee et al., 2014). Incorporating SeNPs into the poultry diet significantly improved reproduction, feed efficiency, growth performance, and immunological response (Marković et al., 2018). Furthermore, SeNPs supplementation significantly influenced carcass qualities in broilers without negatively affecting internal organs (Ahmadi et al., 2018; Bhattacharjee et al., 2019). The presence of Se in an ideal dose is required to meet the needs of birds and to enable the safe accumulation of this element in the bodies of birds to provide humans with enough Se for a variety of physiological activities (Taylor and Sunde, 2017; Alagawany et al., 2021c). Previous studies have largely investigated and compared the health impacts of dietary Se on agricultural stock and humans, where dietary Se has shown various benefits, including enhanced growth performance, immune functions, and nutritional quality of meats, with reduced oxidative stress and inflammation, and finally enhanced thyroid health and fertility in humans. The emergence of nanoparticles presents a novel and innovative technology. Notably, Se in the form of nanoparticles (SeNPs) has lower toxicity, higher bioavailability, lower excretion in animals, and is linked to more powerful and superior biological activities (at a comparable Se dose) than traditional chemical forms of dietary Se (Au et al., 2023).
This work investigates the antioxidant and antibacterial activity of bacterial selenium nanoparticles (BSeNPs) synthesized by a Bacillus isolate identified at the gene level of Bacillus subtilus DA20, and then evaluates the effect of BseNPs as a dietary supplement compared to bulk Se as a source of Se on growth performance, carcass characteristics, and blood parameters in IR broiler chickens.
MATERIALS AND METHODS
Materials
From different regions in Rabigh, KSA, the soil samples were taken near the rhizosphere. Luria broth and Muller Hinton agar media were obtained from Oxoid Ltd. (Basingstoke, Hampshire, UK), DPPH and sodium selenite from (Sigma), and Merck supplied plate count agar (PCA) (Eschenstr, Taufkirchen, Germany). The bacterial strains Bacillus cereus, Staphylococcus aureus, Campylobacter jejuni, and Escherichia coli were used in this study to investigate the antibacterial activity of selenium nanoparticles.
Isolating and Screening of Se-Resistant Bacteria
Diverse soil samples were collected from various areas in Rabigh, Saudi Arabia. Ten grams of soil samples were dispersed in a 90 mL peptone buffer solution. One milliliter of soil suspension was dissolved in 9 mL peptone buffer to prepare serial dilutions up to 10−7. Each dilution was distributed on the top of PCA plates complemented with sodium selenite (1, 2, 3, 4, 5, and 6 mM) for screening. The plates were incubated at 30°C for 24 h (Saad et al., 2022).
Identification of Se-Resistant Bacteria
According to Bergey's manual, the obtained bacterial isolate was morphologically, biochemically, and physiologically characterized by special tests (De Vos and Garrity, 2009). Bacterial cells were treated with mutanolysin, then with mutanolysin, achromopeptidase, and lysostaphin before being extracted with a phenol-chloroform solution (Mannerová et al., 2003). The DNA was isolated by agarose gel electrophoresis (1.5%), Tris/borate/EDTA (TBE) was used as a buffer system, the obtained gel was stained with ethidium bromide, and the DNA bands were seen under ultraviolet light. The size of DNA fragments was assessed by comparing them to a 50 bp ladder (Fermentas). The primer sequences utilized for PCR amplification are PFf 5′ AGGGATGTATTTATTAGATAAAAAATCAA 3′ and PFr 5′ GCAGTAGTTTCTTCAGTAAATC 3′. Mannerová et al. (2003) amplified and partly sequenced the 16S rRNA gene using PCR. RNAmmer version 1.2 was used to rebuild gene sequences from whole-genome shotgun (WGS) data (Yoon et al., 2017) compared to other Bacillus species.
Fabrication of Bacterial Selenium Nanoparticles
An aliquot (100 µL) of a bacterial suspension (108 CFU) was homogenized into 100 mL of Luria-Bertani (LB) broth and incubated at 35°C and 200 rpm for 2 d in a shaking incubator. The mixture was centrifuged for 20 min at 8,000 rpm. The supernatant was obtained, then mixed with 100 mL of EM broth supplemented with 6.0 mM Na2SeO3. The combination was cultured for 3 d at 30°C in a 180 rpm shaking incubator. After the incubation, the color change from yellow to red demonstrates the transformation of Na2SeO3 to BSeNPs by the chosen isolate supernatant. Under identical circumstances, the flask containing Na2SeO3 and only bacterial supernatant without sodium selenite (6 mM) was maintained (Saad et al., 2022).
Characterization of Bacterial Selenium Nanoparticles
BSeNPs were characterized. The transmission electron microscopy images were used to evaluate the BSeNPs' form (TEM; JEOL 1010, Tokyo, Japan). The UV-visible spectrophotometer measured the BSeNPs' UV absorption spectra in 200 to 1,000 nm (Jenway 6320D, Nottingham, UK). Zeta potential and Zeta sizer analyzer (Nano Z2, Malvern, UK) were used to determine the surface charge and the precise size of BSeNPs. FTIR spectroscopy (Bruker, Berlin, Germany) at 3,500 to 500 cm−1 was used to identify the active components in BSeNPs (Alagawany et al., 2021c; El-Saadony et al., 2021a; Abdel-Moneim et al., 2022).
Biological Activity of BSeNPs
The Antioxidant Activity
The BSeNPs (10, 20, 30, 40, 50, and 60 µg/mL) were tested for their ability to scavenge DPPH (Abd Elkader et al., 2022). First, 50 µL of BSeNPs was added to 50 µL of DPPH; the mix was loaded in a microtiter plate and left for 30 min; the generating color was read at 517 nm using a microtiter plate reader (BioTek, Vermont), and the obtained values were applied in the equation
| (4) |
The Antibacterial Activity
The BSeNPs (10, 20, 30, 40, 50, and 60 µg/mL) were performed against various bacterial strains. The bacteria strains were cultivated in MHB overnight at 37°C under a shaking incubator until (1 × 108 CFU/mL) concentration. The disk assay assessed antibacterial activity (Saad et al., 2021). Hundred microliters of activated bacteria were spread onto the Petri dishes' surface, then BSeNPs-saturated paper disks (6 mm) were placed on the plates' surface. The plates were incubated for 24 h at 37°C. A ruler estimated the inhibition zones surrounding the disks (cm). The results were examined with the antibiotic, Levofloxacin as a positive control.
Indian River Broiler Experiment
A total of 480 unsexed Indian River (IR) broilers, 1-day old, were reared for 35 d. The chicks were weighed separately and distributed into 3 treatment groups; each group contained 4 replicates (40 birds per replicate). Chicks in the first, second, third, fourth groups were fed control diets supplemented with 0, 20, 40, and 60 µg/kg of BseNPs, respectively; but the fifth group were fed 300 µg/kg bulk selenium as previously described (Prokisch and Zommara, 2011).
Chicks were fed a starter diet during the first 3 wk of age and a finisher diet during the last 2 wk. The chicks were randomly delivered feed and drink in a chained bird feeder and an automatic nipple cup drinker. All diets were designed to provide the required requirements for broilers based on the IR broiler requirements. The experimental building was open with the same environmental conditions throughout the period. Birds were vaccinated at recommended times against common viruses. For the first 3 d, the lighting system was set to 23:1 h light/dark, then 20:4 h light/dark until the end of the study (Khalid et al., 2023).
Growth Performance
The feed conversion ratio (FCR) and beginning and final body weights, body weight gain (BWG), and feed intake (FI) were evaluated weekly. The health status and mortality rates were monitored daily during the study phase (Bień et al., 2023).
| (5) |
| (6) |
| (7) |
where W1 is the initial weight, W2 is the final weight, and T is the acclimation days.
Carcass Characteristics
At the end of the experiment, 5 male birds from each replication (20 birds per treatment) were selected and slaughtered to determine the weight of carcass, liver, gizzard, heart, and abdominal fat (Bień et al., 2023).
Blood Biochemical
Five milliliters of blood were collected at slaughtering, centrifuged at 3,000 rpm for 20 min, and the plasma was kept at 20°C until testing. Following the manufacturer's instructions, the blood collected was subgrouped into 2 tubes; the first type was hired to determine the hematological criteria using blood hematology analyzer (HB 7021). While, the second type was used to obtain the plasma. For extraction of plasma from the blood, blood cells removed by centrifugation (at 2,000 × g for 15 min at 4°C), and then kept (at −20°C) for pending investigation. Blood plasma metabolites, including total protein (TP), albumin (ALB), globulins (GL), A/G ration, alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea, creatinine, total bilirubin (TB), direct bilirubin (DB), glucose, and triglycerides (TG) were assessed by spectrophotometric method conferring to the manufacturer's (Alagawany et al., 2022).
Blood Hematology
The whole heparinized blood was analyzed immediately after being drawn to estimate red blood cells (RBCs), white blood cells (WBCs), packed cell volume (PCV), hemoglobin concentration, and its parameters, mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC) (Alagawany et al., 2021b). Each sample was processed into thin blood smears on 2 clean microscope slides. The slides were dried at room temperature, then stained and coated with a modified Wright's stain. Under 100 lens power, 100 cells were counted, and the number of neutrophils, lymphocytes, monocytes, eosinophils, and basophils was calculated (Alagawany et al., 2021b).
Statistical Analysis
The SAS software (v 9.4, Cary, NC) was used to evaluate the collected data. The LSD test was used to determine the significance of mean differences, and all changes were deemed significant at P < 0.05.
RESULTS AND DISCUSSION
Isolation and Screening of Se-Resistant Bacteria
Table 1 shows the isolation of thirty bacterial isolates from soil samples in Rabigh city on PCA plates supplemented with sodium selenite, proving their potential resistance to selenium. The first region, Petro Rabigh shows 11 isolates (DA1–DA16), the second region, Mastorah, shows 9 isolates (DA20–DA39), and the third region (Thuwal) shows 10 isolates (DA41–DA55). The selected isolates were classified according to the various concentration of sodium selenite (1, 2, 3, 4, 5, and 6 mM) (Table 2). The first screening showed that all bacterial strains were grown at 1 mM of sodium selenite, and 21 isolates were accommodated with sodium selenite (2 mM), and the second screening showed that 18 isolates could survive at sodium selenite (3 mM). The third screening showed thirteen isolates survive at 4 mM. on the other hand, only 6 isolates survive at sodium selenite (5 mM) coded as DA6, DA10, DA20, DA35, DA46, and DA50, and the final screening showed 1 isolate coded as DA20 can resistant to sodium selenite (6 mM). Saad et al. (2022) isolated 34 selenium-resistant isolates from the soil, and they found that AS12 isolate can tolerate sodium selenite at 5 mM. Also, El-Saadony et al. (2021a) found that HM1 isolate can tolerate sodium selenite concentrations. Moreover, Ullah et al. (2020) isolated 50 isolates from Jiuqu block, they found that the D4 strain had the highest enriched Se content, at 452.7 μg/g of dw. The strains A5, C7, and D11 showed relatively low enrichment, while the C7 strain had the lowest Se content of all, at 64.25 μg/g of dw.
Table 1.
Isolation of selenium-resistant bacteria from a different region in Rabigh, Saudi Arabia.
| Isolation places | No of isolates |
|---|---|
| Rabigh–Petro Rabigh | DA1, DA2, DA5, DA6, DA7, DA9, DA10, DA11, DA12, DA15, DA16 |
| Rabigh–Mastorah | DA20, DA22, DA23, DA25,DA27,DA30, D33,DA35, DA39 |
| Rabigh–Thuwal | DA41,DA44, DA45, DA46, DA47,DA48, DA49, DA50, DA52, DA55 |
Table 2.
Screening of selenium-resistant bacteria isolated from a different region in Rabigh, Saudi Arabia.
| Isolates | Sodium selenite concentration (mM) |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| DA1 | + | + | + | − | − | − |
| DA2 | + | − | − | − | − | − |
| DA5 | + | + | + | + | − | − |
| DA6 | + | + | + | + | + | − |
| DA7 | + | − | − | − | − | − |
| DA9 | + | + | − | − | − | − |
| DA10 | + | + | + | + | + | − |
| DA11 | + | − | − | − | − | − |
| DA12 | + | + | + | − | − | − |
| DA15 | + | − | − | − | − | − |
| DA16 | + | + | − | − | − | − |
| DA20 | + | + | + | + | + | + |
| DA22 | + | − | − | − | − | − |
| DA23 | + | + | + | + | − | − |
| DA25 | + | + | + | + | − | − |
| DA27 | + | + | + | − | − | − |
| DA30 | + | − | − | − | − | − |
| DA33 | + | + | − | − | − | − |
| DA35 | + | + | + | + | + | − |
| DA39 | + | − | − | − | − | − |
| DA41 | + | + | + | − | − | − |
| DA44 | + | + | + | + | − | − |
| DA45 | + | − | − | − | − | − |
| DA46 | + | + | + | + | + | − |
| DA47 | + | + | + | − | − | − |
| DA48 | + | + | + | + | − | − |
| DA49 | + | + | + | + | − | − |
| DA50 | + | + | + | + | + | − |
| DA52 | + | − | − | − | − | − |
| DA55 | + | + | + | + | − | − |
Identification of Selected Isolate
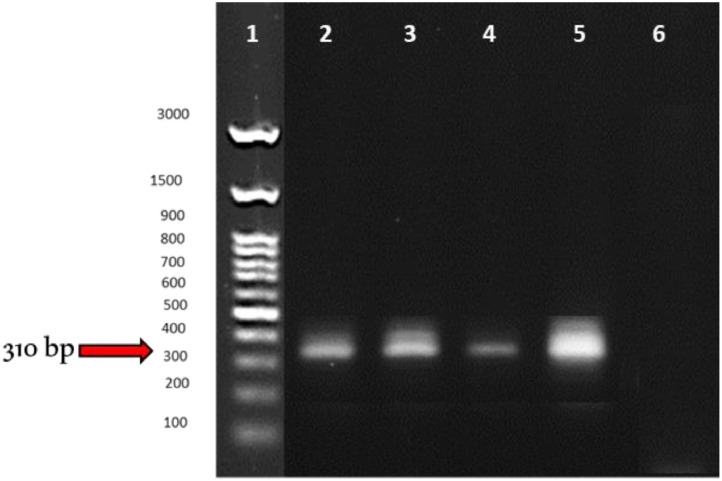
Under a light microscope, the DA20 isolate appeared gram-positive, rod-shaped, motile, and composed of single cells based on biochemical assays. DA20 produced round, flattened, uniform, transparent, off-white colonies with undulating borders on LB agar. It solubilized 55.6 mg/L of Ca3(PO4)2 after 7 d, created 7.8 ppm IAA in the presence of tryptophan, converted 0.6 mol/h/vial of acetylene to ethylene on nitrogen-free malate medium (NFM), used 1-amino cyclopropane-1-carboxylic acid (ACC) as a carbon source, and developed biofilm on the glass surface. The morphological and biochemical tests indicated that our isolate resembles the Bacillus species. Our isolate was a 99% resemblance to the morphology and 16S rDNA gene sequence analysis of Bacillus subtilis ATCC 6633 on Gene Bank, designated as Bacillus subtilis DA20. The appearance of a single band in the PCR results of the isolate (Figure 1) indicates that PCR effectively amplified the 310-bp 16S rRNA gene sequence. This study's 16S ribosomal DNA sequencing examination indicated that the Se-resistant isolate DA20 (band 5) is closely linked to Bacillus strains (bands 2–4). Moreover, quantitative real-time PCR (RT-PCR) indicated that DA20 expresses all genes required for selenium resistance. The findings of this study add a new member to the Bacillus genus, which previously represented selenium resistance only. Ali et al. (2014) isolated the bacterial strain RMB7 from the soil, and based on 16S rRNA gene sequencing, strain RMB7 was identified as Bacillus species. Similar results by Ullah et al. (2020) who isolated 50 bacterial isolates form Jiuqu block, the best resistant Se isolate was identified a gene level by 16rRNA PCR, the authors selected strain B4, as Bacillus subtilis (BSN313), for single-factor optimization in SF and bioreactor. Finally, we optimized conditions, using an orthogonal approach, to maximize the cell density (OD600) and Se enrichment.
Figure 1.
16S rRNA genes of selenium-resistant bacterial isolate, Lane 1, Ladder [(L) 100–1,500 bp]; Lane 2 to 4, Positive controls (P, Bacillus subtitles); Lane 5, identified isolate at 310 bp; Lane 6, Negative control (N, Staphylococcus aureus).
Characterization of Bacterial Selenium Nanoparticles Fabricated by Bacillus Subtitles DA20
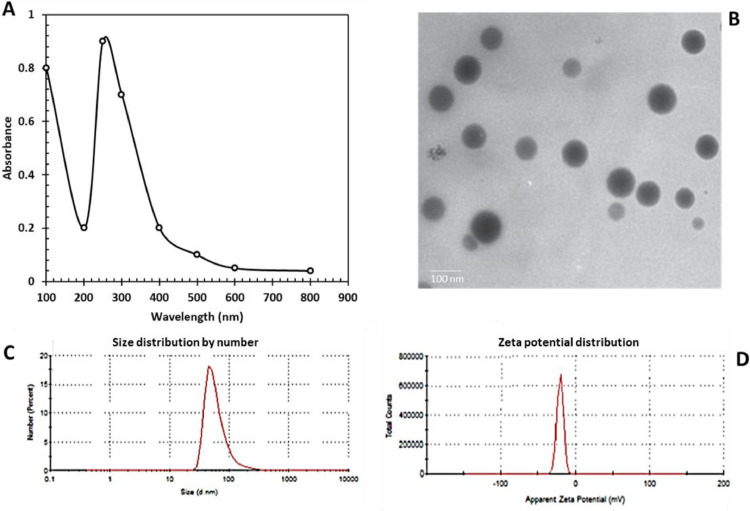
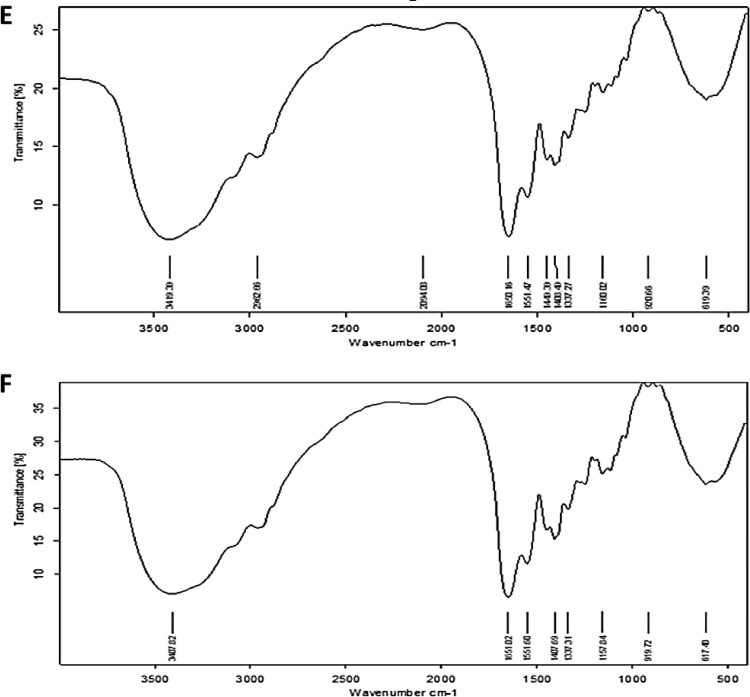
When adding Bacillus subtilis DA20 supernatant to LB supplemented with sodium selenite solution, the color changes from colorless to red, indicating the biotransformation of sodium selenite into BSeNPs. The obtained BSeNPs absorb UV at 270 nm (Figure 2A), confirming the Se transformation. The TEM picture (Figure 2B) of the synthesized BSeNPs exhibited a spherical particle ranging between 20 and 65 nm. The FTIR spectrum of Bacillus subtilis DA20 supernatant revealed 8 bands (Figure 2F), while the FTIR spectrum of BSeNPs revealed 11 bands between 3,500 and 500 cm−1 (Figure 2E). These bands indicate the presence of active groups in the structure of SeNPs, such as alcohol, phenols, amides, and amines. The 3,419.39 cm−1 corresponds to the OH and NH2, whereas the 2,982.66 cm−1 corresponds to the CH group. The band at 2,094 cm−1 corresponds to the alkyne group, whereas the bands between 1,650.09, 1,373, and 920.66 cm−1 correspond to alkanes, alkenes, and aromatics compounds. Esters at 1,150 cm−1 showed the presence of esters, while halide compounds at 617.39 cm−1 agree with (El-Saadony et al., 2021a; Puri and Patil, 2022; Saad et al., 2022). Dynamic light scattering (DLS) is a method used to examine the size and charge of nanoparticles in suspension. Figure 2C and D depicts the DLS analysis of stable BSeNPs, which reveals a precise size of 56 nm (Figure 2C) and a net negative charge of −22.35 mV (Figure 2D). These data show that BSeNPs underwent monodisperse biotransformation. Our results are correlated with Qiao et al. (2020) found that biogenic selenium nanoparticles synthesized by Lactobacillus casei revealed sizes between 50 and 80 nm, 3 to 50 nm (El-Saadony et al., 2021a) and 25 to 85 nm in the study of Saad et al. (2022). It was found that the particle size and zeta potential of SeNPs synthesized by carambola leaf extract were 124.7 nm and −23.20 mV (Ganeshkar et al., 2023), and UV absorbance was at 289 (Puri and Patil, 2022). Generally the characterization of BSeNPs is correlated with Jha et al. (2022); the particle size of synthesized RMLP-SeNPs was around 54.85 nm under optimal conditions by DLS measurement. HR-TEM results showed that RMLP-SeNPs were a uniform spherical shape with an average size of 31.82 nm. UV-vis analysis showed the synthesis of RMLP-SeNPs with a characteristic peak at 265 nm which was further confirmed by FTIR and EDX spectrum.
Figure 2.
Characterization of BSeNPs fabricated by Bacillus subtitles DA20 supernatant (A) UV absorbance at 270 nm, (B) TEM showed spherical nanoparticles, (C) Size of 56 nm by zeta sizer, (D) Zeta potential records nanoparticles charge of −22.35, (E) Active groups surrounded BSeNPs, (F) Active groups in Bacillus subtitles DA20 detected by FTIR.
Biological Activity of BSeNPs
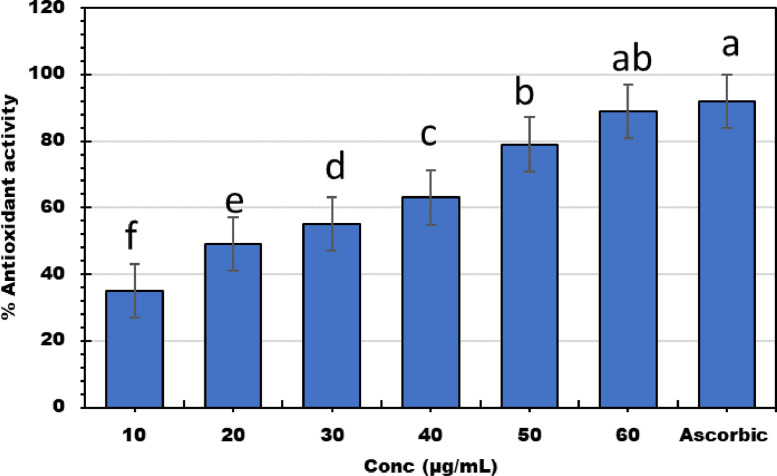
Antioxidant
Figure 3 shows the antioxidant activity of BSeNPs concentrations of 20 to 60 µg/mL. The scavenging activity increased with increasing concentrations, where BSeNPs (60 µg/mL) scavenged 90% of DPPH free radical with no significant differences to ascorbic acid 92%. The SC50 of BSeNPs was 20 µg/mL. The antioxidant activity of BSeNPs was greater than bacterial supernatant because the coat of polyphenols and proteins surrounded nanoparticles. Puri and Patil (2022) found that the antioxidant activity of SeNPs was 76.43% compared to ascorbic acid, 93.15%. The IC50 of SeNPs wad 24.72 µg/mL, selenium comparing ascorbic acid) at 12.51 ± 0.16 µg/mL. The antioxidant capacity of SeNPs was much higher than that of D. montana extract and equivalent to ascorbic acid. Reduction capacity was substantially associated with antioxidant capacity, suggesting it is a crucial antioxidant characteristic. The selenium nanoparticles synthesized by mangrove Rhizophora mucronata have considerable antioxidant activity against DPPH and ABTS, where scavenged 86.9% of DPPH radicals (Jha et al., 2022).
Figure 3.
Antioxidant activity of bacterial selenium nanoparticles (BSeNPs) fabricated by Bacillus subtitles DA20 supernatant. Lowercase letters above columns indicate significant difference at P ≤ 0.05.
Antibacterial
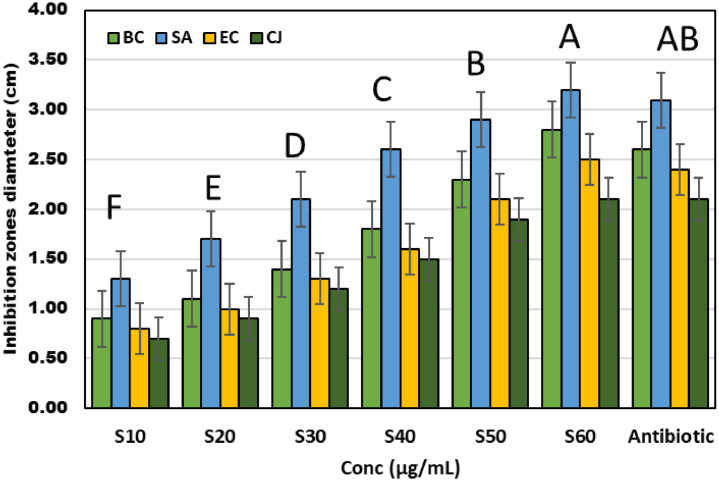
Figure 4 shows that BSeNPs exhibit antibacterial activity against pathogenic bacteria, Bacillus cereus, Staphylococcus aureus, Campylobacter jejuni, and Escherichia coli with inhibition zone diameters (0.7–3.2 cm). Staphylococcus aureus was the most sensitive bacteria to BSeNPs concentration (IZD, 3.2 cm), and Campylobacter jejuni was the most resistant to selenium nanoparticles (IZD, 2.1 cm). The antibacterial activity of BSeNPs excels the antibiotic because the nanoparticles have different mechanisms against bacteria. In addition to their diminutive size, the antibacterial capability of BSeNPs may affect DNA replication, protein synthesis, and metabolism, affecting the plasma membrane's functioning and integrity (Saad et al., 2022). Therefore, it is improbable that negatively charged NPs (−22.35 mV) will interact chemically with negatively charged cell membranes. Therefore, the possible interactions of BSeNPs may be physical rather than chemical. It may initiate with repelling between the negatively negative charges in NPs and their analogs at the cell membrane, leading to Brownian motion in other bacterial cells. This putative interaction between identical NPs with equivalent negative charges might double collisional motions with bacterial cells, boosting cellular damage. Detail the powerful antimicrobial effect of NPs (Saad et al., 2022). Puri and Patil (2022) found that Se nanoparticles synthesized by D. montana bark extract exhibited potent antibacterial activity against pathogenic bacteria, E. coli (48 mm), K. pneumonia (36.20 mm), B. subtilis (44.14 mm), and S. aureus (34.16 mm). The difference in IZDs may depend on the source and fabrication of selenium nanoparticles. Also, Miglani and Tani-Ishii (2021) found that Selenium nanoparticles produced by fresh guava leaves (Psidium guajava) have antibacterial efficacy against E. faecalis was evaluated by agar well diffusion method.
Figure 4.
Antibacterial activity of bacterial selenium nanoparticles (BSeNPs) fabricated by Bacillus subtitles DA20 supernatant against pathogenic bacteria. Lowercase letters above columns indicate significant difference at P ≤ 0.05.
The Impact of BSeNPs on Indian River Broiler Performance, Hematological and Biochemical Serum Incidences
Growth Performance
Table 3 shows the beneficial effect of BSeNPS at 3 levels on the growth performance of IR broiler compared to bulk Selenium. The initial BW of IR chicks was not significantly different from the control, nor was there a significant difference between Bulk Se (300 µg/kg) and control. Dietary supplementation with BSeNPs (40 µg/kg) significantly increased the ultimate body weight of chicks, whereas the control group had the lowest FBW (2,150 vs. 1,950 g, P < 0.05) with a relative increase of 10%. As for FI, the chicks fed the BSeNPs diet (40 µg/kg) had the lowest amount, compared with those fed control and Se diets. As for FCR, chicks fed the BSeNPs diet had the best value, while those fed control diets had the lowest value, compared with those fed Se diets (1.490 and 1.655 vs. 1.569 g/g, P < 0.05, respectively). Chicks fed SeNPs diets significantly recorded the lowest protein and energy intake compared with those fed control and Se diets. Chicks fed control diets had the highest protein and energy efficiency ratios, followed by those fed Se diets, while SeNPs diets had the lowest values. Generally, the BSeNPs (40 µg/kg) have the best performance between control and other groups Khan et al. (2022) demonstrated that the addition of SeNPs-L-COS to the diet of broiler chicken raised (P < 0.05) body weight growth and enhanced (P < 0.05) feed conversion ratio. Similarly, Rehman et al. (2022) discovered that adding SeNP-MOS to the broiler diet enhanced growth performance, feed intake, and feed conversion ratio (FCR); the weekly BWG and ultimate body weight of broilers of market age were dramatically increased.
Table 3.
The effect of dietary BSeNPS and bulk Se on the growth performance of IR broiler from 0 to 35 d of age.
| Parameters | Control | BSeNPs (µg/kg) |
Bulk Se | P value | ||
|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 300 µg/kg | |||
| IBW (g) | 44.33 ± 0.2 | 44.36 ± 0.3 | 44.39 ± 0.6 | 44.44 ± 0.8 | 44.50 ± 0.1 | 0.09 |
| FBW (g) | 1950.1 ± 1.3c | 2080.69 ± 2.2b | 2150.2 ± 1.1a | 2155.6 ± 1.9a | 2050.5 ± 0.9b | 0.03 |
| FI (g) | 3150.2 ± 2.3a | 3090 ± 1.3b | 3025.2 ± 1.6c | 3021 ± 2.0c | 3045.8 ± 1.1c | 0.025 |
| FCR (g/g) | 1.655 ± 0.1a | 1.500 ± 0.2b | 1.490 ± 0.3c | 1.485 ± 0.6c | 1.569 ± 0.7b | 0.015 |
| PI (g) | 650.22 ± 1.2a | 640.66 ± 0.9ab | 635.12 ± 0.9b | 632.65 ± 0.8b | 648.64 ± 1.9a | 0.02 |
| PER | 0.345 ± 0.02a | 0.332 ± 0.02ab | 0.325 ± 0.05 | 0.321 ± 0.03ab | 0.336 ± 0.09a | 0.04 |
| EI (kcal) | 10200 ± 3.6a | 9823.5 ± 3.2b | 9502.3 ± 2.1bc | 9495.22 ± 1.8c | 9752.1 ± 4.2b | 0.025 |
| EFR | 5.136 ± 0.04a | 4.632 ± 0.1c | 4.596 ± 0.02cd | 4.589 ± 0.02cd | 4.725 ± 0.06b | 0.036 |
Initial body weight (IBW), final body weight (FBW), feed intake (FI), feed conversion ratio (FCR), protein intake (PI), protein efficiency ratio (PER), energy intake (EI), energy efficiency ratio (EFR). Data are presented mean ±SD. P value ≤0.05 indicates significant difference. Lowercase letters (a–c) in the same row indicate significant difference at P ≤ 0.05.
Carcass Traits
Table 4 shows the effect of BSeNPS at 3 levels on the carcass properties of IR broiler compared to bulk selenium. The results indicated that there were no significant differences between all treatments in the weights of carcass, liver, spleen, and heart of broilers, while the addition of BSeNPS to broiler diet significantly P = 0.046 lowered the fat content in broiler by 24% compared to the control; on the other hand, the breast muscle significantly increased P = 0.035 by 19%. Ahmadi et al. (2018) found that Breast and drumstick percentages were greater in NS-supplemented Male Ross 308 chicks compared to control birds (P ≤ 0.05), although the fat content reduced in NS-supplemented birds compared to control birds (P ≤ 0.05). The relative weight of testicles varied significantly across treatments (P ≤ 0.05). Compared to the control group, broilers given a feed reinforced with different sources of selenium had a higher dressing and abdomen percentage, but there were no variations (P ≤ 0.05) in internal organs across treatments. Nano-selenium produced the best performance overall (Elkhateeb et al., 2022).
Table 4.
Effect of dietary BSeNPS and bulk Se on carcass traits of IR broiler chickens.
| Parameters (%) | Control | BSeNPs (µg/kg) |
Bulk Se | P value | ||
|---|---|---|---|---|---|---|
| 20 | 40 | 20 | 300 µg/kg | |||
| Carcass | 75.6 ± 0.5c | 75.9 ± 0.3c | 78.9 ± 0.2ab | 79.2 ± 0.6a | 77.6 ± 0.9b | 0.045 |
| Liver | 1.75 ± 0.1b | 1.74 ± 0.2b | 1.76 ± 0.2ab | 1.79 ± 0.2a | 1.77 ± 0.5ab | 0.09 |
| Spleen | 0.088 ± 0.01a | 0.086 ± 0.02ab | 0.081 ± 0.02b | 0.083 ± 0.05b | 0.082 ± 0.04b | 0.15 |
| Heart | 0.569 ± 0.06b | 0.571 ± 0.03b | 0.586 ± 0.05a | 0.579 ± 0.08ab | 0.512 ± 0.09c | 0.26 |
| Abdominal fat | 1.39 ± 0.1a | 1.23 ± 0.2b | 1.12 ± 0.3c | 1.11 ± 0.2c | 1.35 ± 0.6a | 0.046 |
| Breast muscle | 32.96 ± 0.6c | 33.25 ± 0.4bc | 38.99 ± 0.7a | 39.23 ± 0.6b | 33.69 ± 0.4b | 0.035 |
Data are presented mean ± SD. P value ≤0.05 indicates significant difference. Lowercase letters (a–c) in the same row indicate significant difference at P ≤ 0.05.
Serum Biochemical and Hematological Properties
Table 5 displays the blood biochemical properties affected by various treatments in broiler chickens. Dietary sources of selenium did not affect serum total protein, albumin, globulin, A/G ratio, or total lipids. Despite being within the normal range, the selenium-fed chicks showed lower blood AST and ALT enzyme levels than the control group. AST and ALT blood levels are directly proportional to the severity of tissue damage. In addition, Table 5 depicts the impact of dietary interventions on hematological markers. No significant effects of nano or organic selenium on any hematological markers compared to the control group.
Table 5.
Effect of dietary BSeNPs and bulk Se on hematological and serum biochemical parameters of IR broiler at 35 d of age.
| BSeNPs (µg/kg) |
Bulk Se | P value | ||||
|---|---|---|---|---|---|---|
| Parameters | Control | 20 | 40 | 60 | 300 µg/kg | |
| Hematological parameters | ||||||
| RBCs (106/µL) | 2.39 ± 0.2c | 2.41 ± 0.1b | 2.66 ± 0.1a | 2.69 ± 0.3a | 2.45 ± 0.3b | 0.04 |
| HGB (g/dL) | 11.9 ± 0.3c | 12.0 ± 0.3bc | 12.9 ± 0.5b | 13.2 ± 0.5a | 12.4 ± 0.3b | 0.06 |
| HCT (%) | 25.2 ± 0.9c | 25.6 ± 0.9c | 27.12 ± 0.5a | 27.56 ± 0.2a | 26.05 ± 0.9b | 0.069 |
| MCV (µm3/cell) | 107.6 ± 0.9a | 106.9 ± 0.8b | 105.78 ± 1.5c | 105.68 ± 0.6c | 106.8 ± 0.3b | 0.045 |
| MCH (g/dL) | 51.4 ± 0.8a | 50.99 ± 0.2b | 48.98 ± 0.5d | 48.69 ± 0.4d | 49.02 ± 0.6c | 0.045 |
| MCHC (g/dL) | 47.55 ± 0.2a | 47.02 ± 0.3ab | 46.33 ± 0.6b | 46.56 ± 0.3b | 46.9 ± 0.7b | 0.09 |
| PLT (104/µL) | 38.2 ± 1.8a | 36.55 ± 0.4b | 33.5 ± 1.1d | 33.87 ± 0.4d | 34.6 ± 2.0c | 0.05 |
| WBCs (102/µL) | 137.0 ± 1.1a | 130.66 ± 0.9b | 128.6 ± 0.9c | 128.9 ± 1.0c | 129.7 ± 2.0bc | 0.039 |
| Serum biochemical parameters | ||||||
| Total protein (g/dL) | 2.71 ± 0.9c | 2.77 ± 0.1c | 2.89 ± 0.2ab | 2.98 ± 0.2a | 2.79 ± 0.1b | 0.06 |
| Albumin (g/dL) | 1.33 ± 0.5c | 1.39 ± 0.3c | 1.45 ± 0.1a | 1.49 ± 0.3a | 1.47 ± 0.8b | 0.07 |
| Globulin (g/dL) | 1.28 ± 0.2c | 1.32 ± 0.4b | 1.38 ± 0.3ab | 1.41 ± 0.6a | 1.27 ± 0.1c | 0.09 |
| A/G ratio | 1.03 ± 0.2b | 1.05 ± 0.1c | 1.05 ± 0.1c | 1.05 ± 0.1c | 1.15 ± 0.4a | 0.04 |
| Total lipids (mg/ dL) | 420.67 ± 1.6a | 401.2 ± 0.9c | 390.0 ± 2.3d | 385.23 ± 2.5d | 410.67 ± 3.6b | 0.01 |
| ALT (U/L) | 16.19 ± 0.2a | 10.6 ± 0.2c | 9.98 ± 0.9c | 11.88 ± 0.2b | 11.98 ± 0.3b | 0.012 |
| AST (U/L) | 59.33 ± 0.3a | 44.5 ± 0.9b | 39.01 ± 0.9c | 41.35 ± 0.6bc | 41.67 ± 0.8bc | 0.015 |
Data are presented mean ± SD. P value ≤0.05 indicates significant difference. Lowercase letters (a–d) in the same row indicate significant difference at P ≤ 0.05
There were no significant differences in serum blood biochemical when dietary selenium supplementation (SeNPs or Se) was used. This finding is the same trend as that of Ahmadi et al. (2018), who reported that the concentrations of glucose and total protein in the plasma of the experimental groups (nanoselenium at 0, 0.1, 0.2, 0.3, 0.4, and 0.5 mg/kg of feed) were not substantially different. Also, Attia et al. (2010) found that the level and source of Se did not affect plasma albumin and triglyceride concentrations compared to the control diet. Similarly, Naderi et al. (2017) observed that Se or the combination of Se and vitamin E did not affect plasma triglyceride levels.
However, the selenium-fed chickens had lower serum AST and ALT enzymes than the control, although it was in the normal range. The level of AST and ALT in the blood is proportional to the degree of tissue damage. No significant effects of nano or organic selenium on hematological markers compared to the control. In the same trend, Abdul-Majeed and Abdul-Rahman (2022) found that Se and vitamin E injections did not influence PCV, hemoglobin, or WBC levels. Also, Soliman (2015) stated that there were no changes in PCV and RBC levels in Se (as sodium selenite) and vitamin E-supplemented lambs compared to control lambs. Also, Khalphallah et al. (2022) found no significant variations in erythrocyte count, hemoglobin concentration, or PCV value with vitamin E and Se-added calves. In another rat trial, vitamin E and selenium injections did not affect RBC count, hemoglobin concentration, or PCV value (as sodium selenite).
Gut Microbiota
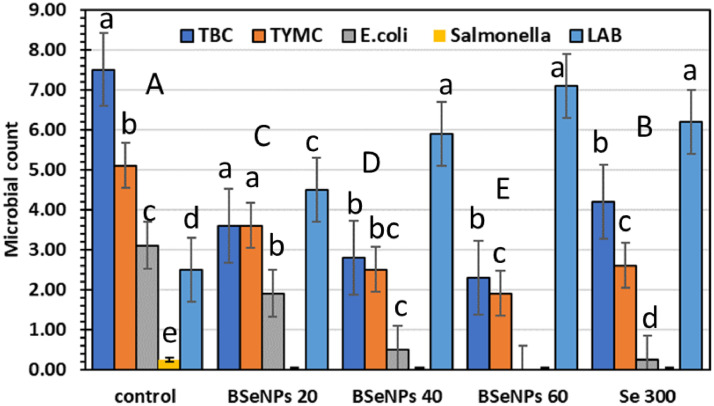
Figure 5 showed that TBC, TYMC, E. coli, and Salmonella counts significantly decreased with BSeNPs and Se compared to control. The decreases were 69 and 62% for TBC and TYMC, respectively, while no detected E. coli and Salmonella in BSeNPs (60 µg/kg) with significant differences over Se and control. However, LAB significantly increased with BSeNPs (60 µg/kg) with a 2-fold increase over control, showing the beneficial effects of BSeNPs in reducing pathogens and enhancing the beneficial bacteria. Comparing the group of SeNPs coupled with chitosan to the chitosan and control groups, the ileal content analysis demonstrated a rise (P ≤ 0.05) in LAB count and a contemporaneous decrease (P ≤ 0.05) in E. coli count (Alagawany et al., 2021a,b; Khan et al., 2022). Also, the supplementation with SeNP-MOS enhanced the gut microbes involved in nutrient absorption. This increased the accessibility of micronutrients for skeletal growth, such as muscle and bone, and increased the avian survival rate (Rehman et al., 2022).
Figure 5.
The effect of dietary BSeNPs on the gut microbiota of IR broiler. Lowercase and uppercase letters above columns indicate significant difference at P ≤ 0.05.
CONCLUSIONS
Bacillus sp. can reduce sodium selenite to BSeNPs with antioxidant and antimicrobial properties because of its tiny size and active groups. Therefore, dietary supplementation with BSeNPs enhances IR broiler performance, intestinal architecture, mucosal protection, and the number of beneficial gut microorganisms. The antioxidant activity of BSeNPs is attributable for its efficiency to reduce, ALT, AST, and raise the total protein; also the considerable antibacterial activity of dietary BSeNPs stimulates the growth of the broiler intestine and optimizes its local environment, reflecting on broiler performance.
DISCLOSURES
There was no conflict of interests.
REFERENCES
- Abd Elkader A.M., Labib S., Taha T.F., Althobaiti F., Aldhahrani A., Salem H.M., Saad A., Ibrahim F.M. Phytogenic compounds from avocado (Persea americana L.) extracts; antioxidant activity, amylase inhibitory activity, therapeutic potential of type 2 diabetes. Saudi J. Biol. Sci. 2022;29:1428–1433. doi: 10.1016/j.sjbs.2021.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M.E., El-Saadony M.T., Shehata A.M., Saad A.M., Aldhumri S.A., Ouda S.M., Mesalam N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022;29:1197–1209. doi: 10.1016/j.sjbs.2021.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Majeed A.F., Abdul-Rahman S.Y. Impact of vitamin e and selenium treatment in-ovo and after hatching of broiler. Iraqi J. Agric. Sci. 2022;53:810–818. [Google Scholar]
- Ahmadi M., Ahmadian A., Seidavi A. Effect of different levels of nano-selenium on performance, blood parameters, immunity and carcass characteristics of broilerchickens. Poult. Sci. J. 2018;6:99–108. [Google Scholar]
- Alagawany M., El-Saadony M., Elnesr S., Farahat M., Attia G., Madkour M., Reda F. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., El-Saadony M.T., El-Rayes T.K., Madkour M., Loschi A.R., Di Cerbo A., Reda F.M. Evaluation of dried tomato pomace as a non-conventional feed: its effect on growth, nutrients digestibility, digestive enzyme, blood chemistry and intestinal microbiota of growing quails. Food Energy Secur. 2022;11:e373. [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276 [Google Scholar]
- Alagawany M., Qattan S.Y., Attia Y.A., El-Saadony M.T., Elnesr S.S., Mahmoud M.A., Madkour M., El-Hack A., Mohamed E., Reda F.M. Use of chemical nano-selenium as an antibacterial and antifungal agent in quail diets and its effect on growth, carcasses, antioxidant, immunity and caecal microbes. Animals. 2021;11:3027. doi: 10.3390/ani11113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Hameed S., Imran A., Iqbal M., Lazarovits G. Genetic, physiological and biochemical characterization of Bacillus sp. strain RMB7 exhibiting plant growth promoting and broad spectrum antifungal activities. Microb. Cell Factories. 2014;13:1–15. doi: 10.1186/s12934-014-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia Y.A., Abdalah A.A., Zeweil H.S., Bovera F., El-Din A.T., Araft M.A. Effect of inorganic or organic selenium supplementation on productive performance, egg quality and some physiological traits of dual-purpose breeding hens. Czech J. Anim. Sci. 2010;55:505–519. [Google Scholar]
- Au A., Mojadadi A., Shao J.Y., Ahmad G., Witting P.K. Physiological benefits of novel selenium delivery via nanoparticles. Int. J. Mol. Sci. 2023;24:6068. doi: 10.3390/ijms24076068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A., Basu A., Bhattacharya S. Selenium nanoparticles are less toxic than inorganic and organic selenium to mice in vivo. Nucleus. 2019;62:259–268. [Google Scholar]
- Bień D., Michalczuk M., Łysek-Gładysińska M., Jóźwik A., Wieczorek A., Matuszewski A., Kinsner M., Konieczka P. Nano-sized selenium maintains performance and improves health status and antioxidant potential while not compromising ultrastructure of breast muscle and liver in chickens. Antioxidants. 2023;12:905. doi: 10.3390/antiox12040905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos P., Garrity G.M. Bergey’s Manual of Systematic Bacteriology: The Firmicutes. Springer; Midtown Manhattan, New York: 2009. [Google Scholar]
- Dunlap C.A., Kim S.-J., Kwon S.-W., Rooney A.P. Bacillus velezensis is not a later heterotypic synonym of Bacillus amyloliquefaciens; Bacillus methylotrophicus, Bacillus amyloliquefaciens subsp. plantarum and ‘Bacillus oryzicola’ are later heterotypic synonyms of Bacillus velezensis based on phylogenomics. Int. J. Syst. Evol. Microbiol. 2016;66:1212–1217. doi: 10.1099/ijsem.0.000858. [DOI] [PubMed] [Google Scholar]
- Elkhateeb F.S., Ghazalah A.A., Abdel-Wareth A.A.A. Effect of selenium sources on growth performance, carcass criteria and physical meat quality of broiler chickens. SVU-Int. J. Agric. Sci. 2022;4:207–217. [Google Scholar]
- Elnaggar A.S., Ghazalah A., Elsayed A.H., Abdelalem A. Impact of selenium sources on productive and physiological performance of broilers. Egypt. Poult. Sci. J. 2020;40:577–597. [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha T.F., Najjar A.A., Zabermawi N.M., Nader M.M., AbuQamar S.F., El-Tarabily K.A., Salama A. Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J. Biol. Sci. 2021;28:6782–6794. doi: 10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Sitohy M.Z., Ramadan M.F., Saad A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innov. Food Sci. Emerg. Technol. 2021;69 [Google Scholar]
- Fan B., Blom J., Klenk H.-P., Borriss R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “operational group B. amyloliquefaciens” within the B. subtilis species complex. Front. Microbiol. 2017;8:22. doi: 10.3389/fmicb.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshkar M.P., Mirjankar M.R., Shivappa P., Gaddigal A.T., Goder P.H., Kamanavalli C.M. Biogenic synthesis of selenium nanoparticles, characterization and screening of therapeutic applications using Averrhoa carambola leaf extract. Part Sci. Technol. 2023;5:1–13. [Google Scholar]
- Jha N., Esakkiraj P., Annamalai A., Lakra A.K., Naik S., Arul V. Synthesis, optimization, and physicochemical characterization of selenium nanoparticles from polysaccharide of mangrove Rhizophora mucronata with potential bioactivities. J. Trace Elem. Min. 2022;2 [Google Scholar]
- Kang B.K., Cho M.S., Park D.S. Red pepper powder is a crucial factor that influences the ontogeny of Weissella cibaria during kimchi fermentation. Sci. Rep. 2016;6:1–8. doi: 10.1038/srep28232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid A., Hussain S.M., Khalid F., Shahzad M.M., Sharif A., Bashir F., Asrar M. Effects of dietary selenium nanoparticles supplementation on growth performance, hematology and body composition of Oreochromis niloticus fingerlings. JAPS: J. Anim. Plant Sci. 2023;33:33–39. [Google Scholar]
- Khalphallah A., Elmeligy E., Zakaria A.M., Ghallab R.S., Abdulkarim A., Mohamed R.H. Comparative study of efficacy of prepartum injection of multivitamins and selenium-vitamin E (ά-tocopherol)-combination on post-partum clinical findings, serum steroids, calf and placental weights, and milk antioxidant biomarkers changes in female dromed. Open Vet. J. 2022;12:657–667. doi: 10.5455/OVJ.2022.v12.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I., Zaneb H., Masood S., Ashraf S., Rehman H.F., Tahir S.K., Rehman H.U., Khan A., Taj R., Rahman S.U., Shah M. Supplementation of selenium nanoparticles-loaded chitosan improves production performance, intestinal morphology, and gut microflora in broiler chickens. J. Poult. Sci. 2022;59:272–281. doi: 10.2141/jpsa.0210026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Lillehoj H., Jang S., Jeong M., Xu S., Kim J., Park H., Kim H., Lillehoj E., Bravo D. Effects of in ovo injection with selenium on immune and antioxidant responses during experimental necrotic enteritis in broiler chickens. Poult. Sci. 2014;93:1113–1121. doi: 10.3382/ps.2013-03770. [DOI] [PubMed] [Google Scholar]
- Mannerová S., Pantucek R., Doškař J.i., Sˇvec P., Snauwaert C., Vancanneyt M., Swings J., Sedlacek I. Macrococcus brunensis sp. nov., Macrococcus hajekii sp. nov. and Macrococcus lamae sp. nov., from the skin of llamas. Int. J. Syst. Evol. Microbiol. 2003;53:1647–1654. doi: 10.1099/ijs.0.02683-0. [DOI] [PubMed] [Google Scholar]
- Marković R., Ćirić J., Drljačić A., Šefer D., Jovanović I., Jovanović D., Milanović S., Trbović D., Radulović S., Baltić M.Ž. The effects of dietary selenium-yeast level on glutathione peroxidase activity, tissue selenium content, growth performance, and carcass and meat quality of broilers. Poult. Sci. 2018;97:2861–2870. doi: 10.3382/ps/pey117. [DOI] [PubMed] [Google Scholar]
- Miglani S., Tani-Ishii N. Biosynthesized selenium nanoparticles: characterization, antimicrobial, and antibiofilm activity against Enterococcus faecalis. PeerJ. 2021;9:e11653. doi: 10.7717/peerj.11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi M., Keyvanshokooh S., Salati A.P., Ghaedi A. Combined or individual effects of dietary vitamin E and selenium nanoparticles on humoral immune status and serum parameters of rainbow trout (Oncorhynchus mykiss) under high stocking density. Aquaculture. 2017;474:40–47. [Google Scholar]
- Prokisch, J., and M. A. Zommara. 2011. Process for producing elemental selenium nanospheres. Google Patents.
- Pouri S., Motamedi H., Honary S., Kazeminezhad I. Biological synthesis of selenium nanoparticles and evaluation of their bioavailability. Braz. Arch. Biol. Technol. 2018;60:e170452. [Google Scholar]
- Puri A., Patil S. Biogenic synthesis of selenium nanoparticles using Diospyros montana bark extract: characterization, antioxidant, antibacterial, and antiproliferative activity. Biosci. Biotechnol. Res. Asia. 2022;19:423–441. [Google Scholar]
- Qiao L., Dou X., Yan S., Zhang B., Xu C. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate diquat-induced intestinal barrier dysfunction in C57BL/6 mice through their antioxidant activity. Food Funct. 2020;11:3020–3031. doi: 10.1039/d0fo00132e. [DOI] [PubMed] [Google Scholar]
- Rehman H.F.U., Zaneb H., Masood S., Yousaf M.S., Hayat K., Majeed K.A., Zeeshan M., Ashraf S., Khan I., Khan A., Rehman H. Effect of selenium nanoparticles and mannan oligosaccharide supplementation on growth performance, stress indicators, and intestinal microarchitecture of broilers reared under high stocking density. Animals. 2022;12:2910. doi: 10.3390/ani12212910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT - Food Sci. Technol. 2021;148 [Google Scholar]
- Saad A.M., Sitohy M.Z., Sultan-Alolama M.I., El-Tarabily K.A., El-Saadony M.T. Green nanotechnology for controlling bacterial load and heavy metal accumulation in Nile tilapia fish using biological selenium nanoparticles biosynthesized by Bacillus subtilis AS12. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.1015613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangal V., Goodfellow M., Jones A.L., Schwalbe E.C., Blom J., Hoskisson P.A., Sutcliffe I.C. Next-generation systematics: an innovative approach to resolve the structure of complex prokaryotic taxa. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep38392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim N., Radwan N., Youssef S., Eldin T.S., Elwafa S.A. Effect of inclusion inorganic, organic or nano selenium forms in broiler diets on: 1-growth performance, carcass and meat characteristics. Int. J. Poult. Sci. 2015;14:135–143. [Google Scholar]
- Sharma V.K., McDonald T.J., Sohn M., Anquandah G.A., Pettine M., Zboril R. Biogeochemistry of selenium: a review. Environ. Chem. Lett. 2015;13:49–58. [Google Scholar]
- Sobolev O., Gutyj B., Petryshak R., Pivtorak J., Kovalskyi Y., Naumyuk A., Petryshak O., Semchuk I., Mateusz V., Shcherbatyy A. Biological role of selenium in the organism of animals and humans. Ukr. J. Ecol. 2018;8:654–665. [Google Scholar]
- Soliman E.B. Dose-response of vitamin E and selenium injection on growth performance, physiological and immune responses of Ossimi lambs. Egypt. J. Sheep Goats Sci. 2015;10:1–14. [Google Scholar]
- Sonet J., Bulteau A.-L., Chavatte L., García-Barrera T., Gómez-Ariza J.L., Callejón-Leblic B., Nischwitz V., Theiner S., Galvez L., Koellensperger G. Metallomics: Analytical Techniques and Speciation Methods. Wiley‐VCH Verlag GmbH; Germany: 2016. Biomedical and pharmaceutical applications; pp. 359–462. [Google Scholar]
- Taylor R.M., Sunde R.A. Selenium requirements based on muscle and kidney selenoprotein enzyme activity and transcript expression in the turkey poult (Meleagris gallopavo) PLoS One. 2017;12 doi: 10.1371/journal.pone.0189001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Þorgeirsdóttir D.Ý., Andersen J.H., Perch-Nielsen M., Møller L.H., Grønbæk-Thorsen F., Kolberg H.G., Gammelgaard B., Kristensen M. Selenomethionine as alternative label to the fluorophore TAMRA when exploiting cell-penetrating peptides as blood-brain barrier shuttles to better mimic the physicochemical properties of the non-labelled peptides. Eur. J. Pharm. Sci. 2023;183 doi: 10.1016/j.ejps.2023.106400. [DOI] [PubMed] [Google Scholar]
- Ullah A., Sun B., Wang F., Yin X., Xu B., Ali N., Mirani Z., Mehmood A., Naveed M. Isolation of selenium-resistant bacteria and advancement under enrichment conditions for selected probiotic Bacillus subtilis (BSN313) J. Food Biochem. 2020;44 doi: 10.1111/jfbc.13227. [DOI] [PubMed] [Google Scholar]
- Visha P., Nanjappan K., Selvaraj P., Jayachandran S., Elango A., Kumaresan G. Biosynthesis and structural characteristics of selenium nanoparticles using Lactobacillus acidophilus bacteria by wet sterilization process. Int. J. Adv. Vet. Sci. Technol. 2015;4:178–183. [Google Scholar]
- Yoon S.-H., Ha S.-M., Kwon S., Lim J., Kim Y., Seo H., Chun J. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017;67:1613. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]