Abstract
Background:
Renin-angiotensin system inhibitors improve outcomes in patients with heart failure with reduced ejection fraction (HFrEF). However, less is known about their effectiveness in patients with HFrEF and advanced kidney disease.
Methods:
In the Medicare-linked OPTIMIZE-HF, 1582 patients with HFrEF (ejection fraction, ≤40%) had advanced kidney disease (estimated glomerular filtration rate <30 ml/min/1.73 m2). Of these, 829 were not receiving angiotensin-converting enzyme (ACE) inhibitors or angiotensin-receptor blockers (ARBs) prior to admission, of whom 214 were initiated on these drugs prior to discharge. We calculated propensity scores for receipt of these drugs for each of the 829 patients and assembled a matched cohort of 388 patients, balanced on 41 baseline characteristics (mean age, 78 years; 52% women; 10% African American; 73% receiving beta blockers). Hazard ratios (HR) and 95% CIs were estimated comparing 2-years outcomes in 194 patients initiated on ACE inhibitors or ARBs to 194 patients not initiated on those drugs.
Results:
The combined endpoint of heart failure readmission or all-cause mortality occurred in 79% and 84% of patients initiated and not initiated on ACE inhibitors or ARBs, respectively (HR associated with initiation, 0.79; 95% CI, 0.63–0.98). Respective HRs (95% CIs) for all-cause mortality and heart failure readmission were 0.81 (0.63–1.03) and 0.63 (0.47–0.85).
Conclusions:
The findings from our study add new information to the body of cumulative evidence that suggest that renin-angiotensin system inhibitors may improve clinical outcomes in patients with HFrEF and advanced kidney disease. These hypothesis-generating findings need to be replicated in contemporary patients.
Keywords: Heart failure, renin-angiotensin system inhibitors, advanced kidney disease, mortality, hospitalization
Therapy with renin-angiotensin system (RAS) inhibitors improves clinical outcomes in patients with heart failure with reduced ejection fraction (HFrEF).1 Because major randomized clinical trials of RAS inhibitors in HFrEF excluded those with advanced kidney disease, there is limited evidence about their efficacy and effectiveness in this population.2 Several observational studies suggested potential clinical benefits of RAS inhibition in patients with heart failure and impaired kidney disease,3–7 but less is known about their effectiveness in those with advanced kidney disease.8 National heart failure guidelines recommend that RAS inhibitors be used with caution in patients with HFrEF with advanced kidney disease,1, 2 and these drugs remain underutilized in this high risk subset.9, 10 In the current study, we examined the associations of angiotensin-converting enzyme (ACE) inhibitor or angiotensin-receptor blocker (ARB) use and clinical outcomes in patients with HFrEF and advanced kidney disease, defined as an estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2.
Methods
Data Source and Study Patients
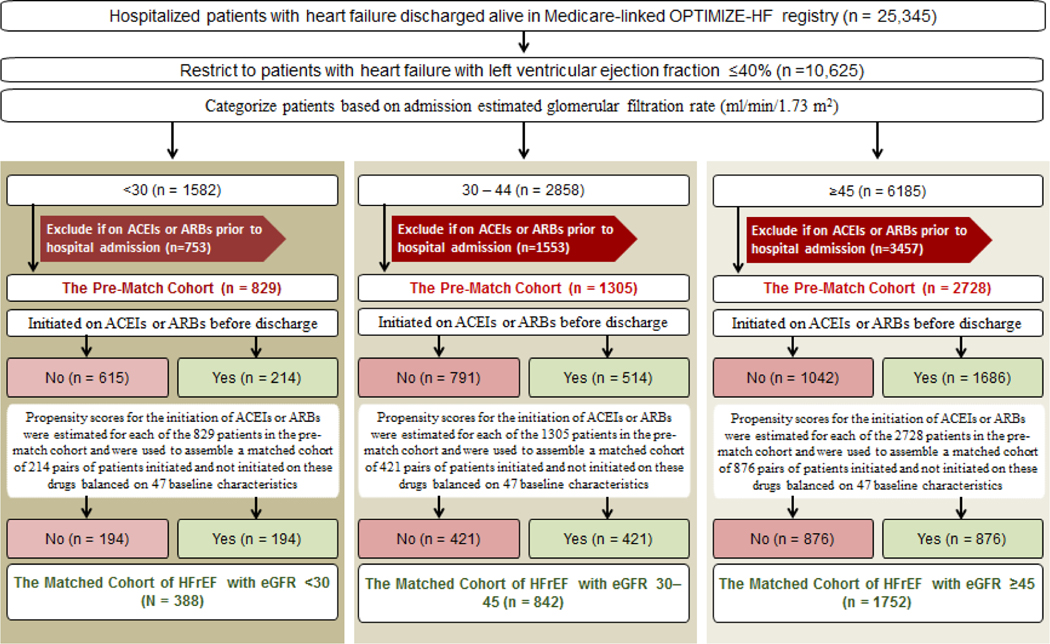
We analyzed data from the Medicare-linked Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) registry, the details of which have been previously described.11–13 Briefly, the OPTIMIZE-HF registry is based on an extensive web-based review of medical record of 48,612 hospitalizations due to heart failure between 2003 and 2004. Of the 25,345 unique patients discharged alive and linked to Medicare for outcomes data,13 10,625 had HFrEF, defined as left ventricular EF ≤40%.14, 15 Of these, 1582 had advanced kidney disease, defined as eGFR <30 ml/min/1.73 m2 (Figure 1, left panel).2 We calculated eGFR using admission serum creatinine and a modified Modification of Diet in Renal Disease (MDRD) formula that excluded race.16 For reading convenience, in the rest of the manuscript, we will use eGFR without mentioning the unit ml/min/1.73 m2. To attenuate prevalent user bias, 753 patients receiving ACE inhibitors or ABRs prior to hospital admission were excluded to assemble inception cohorts eligible for initiation of new therapy with those drugs.17 Of the 829 patients with advanced kidney disease (eGFR <30), 214 were initiated on ACE inhibitors or ARBs before hospital discharge (Figure 1, left panel). We then categorized the 9043 patients without advanced kidney disease to 2858 who had moderate to severely impaired kidney function (eGFR 30 to 44; Figure 1, center panel) and 6185 patients who had normal (eGFR ≥60) or mildly impaired (eGFR 45 to 59) kidney function (Figure 1, right panel).2 We used eGFR 45 as a cutoff as values <45 have been shown to be prognostically important.18, 19 The latter two cohorts without advanced kidney disease were used to examine the consistency of associations across the spectrum of kidney function.
Figure 1.
Flow chart displaying the assembly of a propensity score-matched cohort of patients with HFrEF (≤40%) and advanced kidney disease (eGFR <30) who were not receiving ACE inhibitors or ARBs (left panel). Corresponding data on those with moderate to severely impaired kidney function (eGFR 30–45) and normal to mildly impaired kidney function (eGFR ≥45) are presented in the middle and right panels, respectively. The propensity score model for all three cohorts are based on 47 baseline characteristics, displayed in Figure 2. ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; EF = ejection fraction; HF = heart failure; HFrEF = heart failure and reduced ejection fraction; eGFR = estimated glomerular filtration rate (ml/min/1.73 m2); OPTIMIZE-HF = Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure
Assembly of a Balanced Cohort
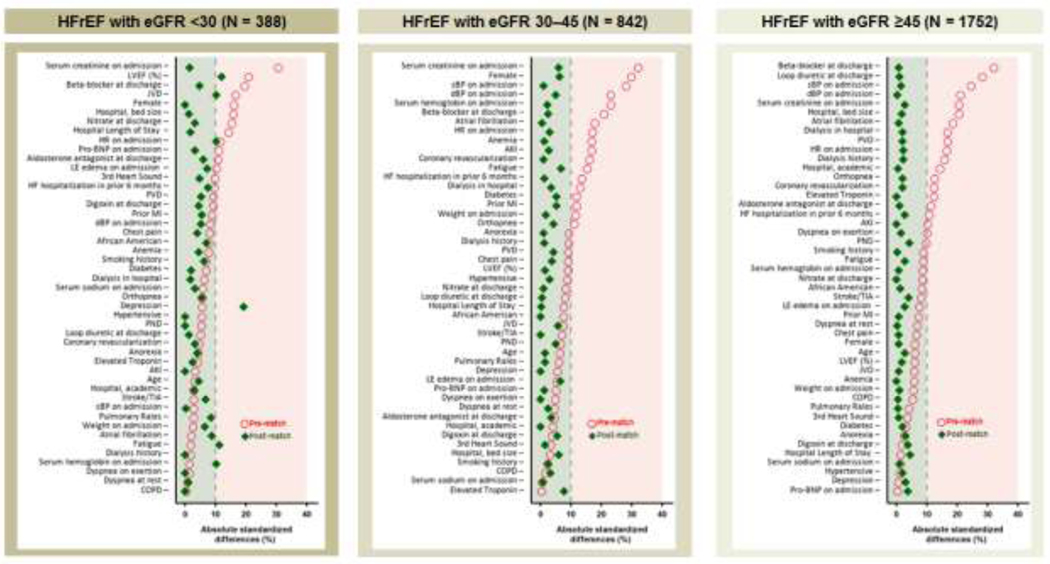
Using a non-parsimonious multivariable logistic regression model, we estimated propensity scores for initiation of ACE inhibitors or ARBs for each of the 829 patients with HFrEF and advanced kidney disease using 47 baseline characteristics as covariates (Figure 2).20–23 Using matching algorithms previously described,5, 6 we matched 194 (91% of 214) patients initiated on ACE inhibitors or ARBs with 194 not initiated on them, thus assembling a matched cohort of 388 patients (Figure 1, left panel). We assessed between-group balance for each of the 47 baseline characteristics by estimating their pre- and post-match absolute standardized differences (Figure 2, left panel). An absolute standardized difference value of 0% indicates no residual bias and bias associated with values <10% are considered inconsequential. We then replicated the above process to assemble two separate matched cohorts of 842 patients with moderate to severely impaired kidney function (Figure 1, center panel), and 1752 patients with normal or mildly impaired kidney function (Figure 1, right panel).
Figure 2.
Love plots displaying absolute standardized differences of 47 baseline characteristics in patients initiated and not initiated on ACE inhibitors or ARBs before discharge in patients with HFrEF (≤40%) and advanced kidney disease (eGFR <30), before and after propensity score matching (left panel). Corresponding data on those with moderate to severely impaired kidney function (eGFR 30–45) and normal to mildly impaired kidney function (eGFR ≥45) are presented in the middle and right panels, respectively. Absolute standardized differences values <10% suggest inconsequential confounding and a 0% values suggest no residual confounding. ACE = angiotensin-converting enzyme; AKI = acute kidney injury; ARB = angiotensin receptor blocker; BNP = B-Type natriuretic peptide; COPD = chronic obstructive pulmonary disease; dBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate (ml/min/1.73 m2); HF = heart failure; HFrEF = heart failure with reduced ejection fraction; HR = heart rate; JVP = jugular venous pressure; LE = lower extremity; LVEF = left ventricular ejection fraction; sBP = systolic blood pressure; PND = paroxysmal nocturnal dyspnea; PVD = peripheral vascular disease; TIA = transient ischemic attack.
Outcomes Data
Our outcomes of interest in the study were all-cause mortality, HF readmission, and the combined endpoint of HF readmission or all-cause mortality. We limited the follow-up duration to 2 years considering the poor outcomes in patients with HFrEF and advanced kidney disease.2 Data on outcome events and time to those events were obtained from Medicare data up to December 31, 2008.13
Statistical Analysis
Descriptive analyses comparing between-group baseline characteristics before and after propensity score matching were conducted using Pearson’s Chi-square test and Student’s t tests. Hazard ratios (HRs) and 95% confidence intervals (CIs) for 2-year outcomes associated with a discharge initiation of ACE inhibitors or ARBs were estimated in the matched cohort of 388 patients with HFrEF and advanced kidney disease using a Cox regression model. We repeated the analysis, adjusting for all baseline characteristics with a post-match absolute standardized difference value of ≥10%. For mortality outcomes, patients who survived were censored at study end and for the readmission outcomes, patients without the event of interest were censored at study end or death, whichever occurred first. We then repeated these analyses in the two other matched cohorts without advanced kidney disease. Kaplan-Meier survival plots were generated to compare the 2-year all-cause mortality across the three eGFR categories. Sensitivity analyses were conducted using Rosenbaum’s approach to assess the impact of a potential unmeasured binary confounder on significant associations in the matched HFrEF and advanced kidney disease cohort.24 All statistical tests were two-tailed with a p-value <0.05 considered significant. SPSS for Windows version 28 (IBM Corp., Armonk, NY) and SAS for Window version 8.2 (Cary, NC) were used for data analyses.
Results
Baseline Characteristics
The 388 matched patients with HFrEF and advanced kidney disease had a mean age (±SD) of 78 (±9) years, 52% were women and 10% were African American (Table 1). Before matching, patients initiated on ACE inhibitors or ARBs had a higher prevalence of women, had a lower mean ejection fraction and serum creatinine, and a higher proportion were discharged on beta-blockers (data not shown in Table 1). These and other clinically important baseline characteristics were balanced after matching (Table 1, Figure 2). Although none of the p values were <0.05, likely due to small sample size, depression had a post-match absolute standardized difference value of 19%, and five others had values between 10% and 12% (Figure 2, left panel). Descriptive data and absolute standardized difference (all <10%) for the two cohorts without advanced kidney disease are displayed in Table 1, and Figure 2, center and right panels.
Table 1.
Baseline characteristics by discharge initiation of ACEI or ARB in a propensity score-matched cohort of patients with HFrEF and advanced kidney disease (eGFR <30; left panel). Corresponding data on those with moderate to severely impaired kidney function (eGFR 30–45) and normal to mildly impaired kidney function (eGFR ≥45) are presented in the middle and right panels, respectively.
| eGFR <30 (N = 388) | eGFR 30 – 45 (N = 842) | eGFR ≥45 (N = 1752) | ||||
|---|---|---|---|---|---|---|
| ACEI or ARB initiated | ACEI or ARB initiated | ACEI or ARB initiated | ||||
| No (n=194) | Yes (n=194) | No (n=421) | Yes (n=421) | No (n=876) | Yes (n=876) | |
| Age (years) | 78 (±9) | 78 (±10) | 79 (±9) | 79 (±10) | 75 (±12) | 75 (±11) |
| Female | 101 (52%) | 101 (52%) | 208 (49%) | 195 (46%) | 342 (39%) | 345 (39%) |
| African American | 21 (11%) | 17 (9%) | 38 (9%) | 38 (9%) | 124 (14%) | 128 (15%) |
| Ejection fraction (%) | 27 (±8) | 26 (±8) | 27 (±8) | 27 (±8) | 28 (±8) | 27 (±9) |
| Past medical history | ||||||
| Smoking history | 26 (13%) | 22 (11%) | 39 (9%) | 42 (10%) | 144 (16%) | 145 (17%) |
| HF hospitalization in prior 6 months | 47 (24%) | 41 (21%) | 76 (18%) | 78 (19%) | 114 (13%) | 122 (14%) |
| Hypertension | 132 (68%) | 132 (68%) | 265 (63%) | 271 (64%) | 522 (60%) | 513 (59%) |
| Myocardial infarction | 68 (35%) | 63 (32%) | 120 (29%) | 130 (31%) | 231 (26%) | 234 (27%) |
| Coronary revascularization | 74 (38%) | 77 (40%) | 162 (38%) | 160 (38%) | 293 (33%) | 285 (33%) |
| Diabetes mellitus | 90 (46%) | 88 (45%) | 164 (39%) | 175 (42%) | 292 (33%) | 300 (34%) |
| Stroke / TIA | 36 (19%) | 31 (16%) | 75 (18%) | 75 (18%) | 130 (15%) | 143 (16%) |
| Peripheral arterial disease | 37 (19%) | 33 (17%) | 82 (19%) | 75 (18%) | 129 (15%) | 123 (14%) |
| Atrial fibrillation | 58 (30%) | 66 (34%) | 131 (31%) | 132 (31%) | 288 (33%) | 285 (33%) |
| Acute kidney injury | 10 (5%) | 10 (5%) | 13 (3%) | 11 (3%) | 17 (2%) | 17 (2%) |
| Dialysis history | 8 (4%) | 8 (4%) | 14 (3%) | 13 (3%) | 20 (2%) | 23 (3%) |
| COPD | 49 (25%) | 49 (25%) | 106 (25%) | 112 (27%) | 236 (27%) | 238 (27%) |
| Anemia | 57 (29%) | 53 (27%) | 78 (19%) | 76 (18%) | 105 (12%) | 105 (12%) |
| Depression | 34 (18%) | 21 (11%) | 38 (9%) | 38 (9%) | 92 (11%) | 84 (10%) |
| Admission symptoms and signs | ||||||
| Dyspnea on exertion | 111 (57%) | 111 (57%) | 267 (63%) | 267 (63%) | 537 (61%) | 531 (61%) |
| Orthopnea | 61 (31%) | 56 (29%) | 103 (24%) | 111 (26%) | 232 (26%) | 224 (26%) |
| Paroxysmal nocturnal dyspnea | 29 (15%) | 29 (15%) | 53 (13%) | 60 (14%) | 140 (16%) | 127 (14%) |
| Dyspnea at rest | 92 (47%) | 91 (47%) | 176 (42%) | 181 (43%) | 373 (43%) | 374 (43%) |
| Chest pain | 33 (17%) | 36 (19%) | 80 (19%) | 74 (18%) | 193 (22%) | 190 (22%) |
| Fatigue | 39 (20%) | 48 (25%) | 88 (21%) | 100 (24%) | 211 (24%) | 201 (23%) |
| Anorexia | 13 (7%) | 15 (8%) | 25 (6%) | 26 (6%) | 44 (5%) | 50 (6%) |
| Jugular venous pressure elevated | 53 (27%) | 62 (32%) | 126 (30%) | 137 (33%) | 257 (29%) | 254 (29%) |
| Third heart Sound | 23 (12%) | 26 (13%) | 42 (10%) | 44 (10%) | 87 (10%) | 89 (10%) |
| Pulmonary rales | 115 (59%) | 123 (63%) | 280 (67%) | 277 (66%) | 554 (63%) | 556 (63%) |
| Lower extremity edema | 111 (57%) | 118 (61%) | 256 (61%) | 269 (64%) | 512 (58%) | 524 (60%) |
| Admission vital signs | ||||||
| Weight (kg) | 75 (±18) | 76 (±18) | 76 (±19) | 76 (±21) | 77 (±20) | 78 (±21) |
| Heart rate (bpm) | 83 (±17) | 85 (±20) | 87 (±22) | 86 (±20) | 90 (±22) | 90 (±21) |
| Systolic blood pressure (mmHg) | 132 (±27) | 132 (±27) | 137 (±29) | 137 (±27) | 136 (±28) | 136 (±26) |
| Diastolic blood pressure (mmHg) | 73 (±14) | 72 (±16) | 74 (±15) | 75 (±15) | 77 (±16) | 77 (±15) |
| Admission laboratory findings | ||||||
| Hemoglobin (g/dL) | 11.5 (±2.0) | 11.8 (±2.1) | 12.2 (±2.0) | 12.1 (±2.0) | 12.7 (±2.1) | 12.6 (±2.3) |
| Serum creatinine (mg/dL) | 2.5 (±0.5) | 2.5 (±0.6) | 1.7 (±0.3) | 1.7 (±0.3) | 1.1 (±0.3) | 1.1 (±0.3) |
| Serum sodium (mEq/L) | 136 (±13) | 136 (±14) | 136 (±14) | 136 (±13) | 136 (±12) | 136 (±12) |
| Serum pro-BNP (pg/ml) | 1951 (±2049) | 1896 (±1359) | 1637 (±1155) | 1624 (±1133) | 1358 (±1085) | 1319 (±945) |
| Serum troponin, elevated | 42 (22%) | 44 (23%) | 111 (26%) | 97 (23%) | 158 (18%) | 158 (18%) |
| Dialysis in hospital | 16 (8%) | 17 (9%) | 21 (5%) | 18 (4%) | 28 (3%) | 31 (4%) |
| Discharge medications | ||||||
| Beta-blocker at discharge | 140 (72%) | 144 (74%) | 291 (69%) | 296 (70%) | 553 (63%) | 556 (63%) |
| Aldosterone antagonist at discharge | 13 (7%) | 16 (8%) | 57 (14%) | 62 (15%) | 132 (15%) | 135 (15%) |
| Loop diuretic at discharge | 154 (79%) | 153 (79%) | 343 (81%) | 342 (81%) | 659 (75%) | 663 (76%) |
| Digoxin at discharge | 54 (28%) | 58 (30%) | 134 (32%) | 145 (34%) | 327 (37%) | 343 (39%) |
| Nitrate at discharge | 60 (31%) | 63 (32%) | 139 (33%) | 137 (33%) | 205 (23%) | 205 (23%) |
| Hospital length of stay (days) | 7.3 (±8.9) | 7.5 (±5.2) | 6.5 (±5.9) | 6.5 (±6.3) | 5.6 (±5.3) | 5.8 (±4.6) |
| Hospital, bed size | 422 (±260) | 425 (±237) | 419 (±255) | 434 (±268) | 386 (±209) | 390 (±217) |
| Hospital, academic | 85 (44%) | 88 (45%) | 201 (48%) | 201 (48%) | 379 (43%) | 381 (43%) |
Values are mean ±SD or n (%). ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BNP = B-Type natriuretic peptide; COPD = chronic obstructive pulmonary disease; eGFR = estimated glomerular filtration rate, presented as ml/min/1.73 m2; HF = heart failure; HFrEF = heart failure with reduced ejection fraction (≤40%); TIA = transient ischemic attack
Combined Endpoint
During 2 years of post-discharge follow-up, the combined endpoint of heart failure readmission or all-cause mortality occurred in 79% and 84% of the patients with HFrEF and advanced kidney disease who were initiated and not initiated on ACE inhibitors or ARBs prior to hospital discharge, respectively (HR associated with initiation, 0.79; 95% CI, 0.63–0.98; Table 2, left panel). This association remained unchanged when adjusted for the 6 baseline characteristics with ≥10% post-match absolute standardized differences (HR, 0.79; 95% CI, 0.63–0.98; Table 2, footnote). Results of sensitivity analysis suggest that a binary unmeasured confounder that is a near-perfect predictor of 2-year combined endpoint could explain away this association if it increased the odds of ACE inhibitor or ARB initiation by 2.4% (Table 2, footnote). HR (95% CI) for the 2-year combined endpoint 0.75 (0.65–0.88) in patients with moderate to severely impaired kidney function (Table 2, center panel) and 0.98 (0.87–1.10) in those with normal to mildly impaired kidney function (Table 2, right panel). These associations were similarly observed during 30-day and 12-month follow-up (Table 2).
Table 2.
Outcomes by discharge initiation of ACEI or ARB in a propensity score-matched cohort of patients with HFrEF and advanced kidney disease (eGFR <30; left panel). Corresponding data on those with moderate to severely impaired kidney function (eGFR 30–45) and normal to mildly impaired kidney function (eGFR ≥45) are presented in the middle and right panels, respectively.
| eGFR <30 ml/min/1.73 m2 (N = 388) | eGFR 30 – 44 ml/min/1.73 m2 (N = 842) | eGFR ≥45 ml/min/1.73 m2 (N = 1752) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ACEIs or ARBs initiated | Hazard ratio (95% CI) | ACEIs or ARBs initiated | Hazard ratio (95% CI) | ACEIs or ARBs initiated | Hazard ratio (95% CI) | ||||
| No (n=194) | Yes (n=194) | No (n=421) | Yes (n=421) | No (n=876) | Yes (n=876) | ||||
| HF readmission or all-cause mortality | |||||||||
| 30 days | 57 (29%) | 35 (18%) | 0.56 (0.37–0.85) | 85 (20%) | 62 (15%) | 0.72 (0.52–0.99) | 129 (15%) | 103 (12%) | 0.78 (0.60–1.01) |
| 1 year | 139 (72%) | 127 (66%) | 0.78 (0.61–0.98) | 284 (68%) | 248 (59%) | 0.77 (0.65–0.91) | 451 (52%) | 435 (50%) | 0.92 (0.81–1.05) |
| 2 years | 163 (84%) | 154 (79%) | 0.79 (0.63–0.98)*,§ | 336 (80%) | 295 (70%) | 0.75 (0.65–0.88) | 568 (65%) | 580 (66%) | 0.98 (0.87–1.10) |
| All-cause mortality | |||||||||
| 30 days | 23 (12%) | 16 (8%) | 0.67 (0.36–1.27) | 48 (11%) | 25 (6%) | 0.51 (0.31–0.83) | 45 (5%) | 34 (4%) | 0.75 (0.48–1.17) |
| 1 year | 102 (53%) | 90 (46%) | 0.82 (0.62–1.08) | 181 (43%) | 150 (36%) | 0.77 (0.62–0.95) | 278 (32%) | 230 (26%) | 0.78 (0.66–0.93) |
| 2 years | 133 (69%) | 119 (61%) | 0.81 (0.63–1.03)‡,§ | 249 (59%) | 212 (50%) | 0.77 (0.64–0.93) | 410 (47%) | 356 (41%) | 0.81 (0.70–0.93) |
| HF readmission | |||||||||
| 30 days | 36 (19%) | 20 (10%) | 0.51 (0.29–0.87) | 42 (10%) | 40 (10%) | 0.93 (0.61–1.44) | 88 (10%) | 71 (8%) | 0.78 (0.57–1.07) |
| 1 year | 83 (43%) | 67 (35%) | 0.68 (0.50–0.94) | 179 (43%) | 151 (36%) | 0.74 (0.60–0.92) | 277 (32%) | 300 (34%) | 1.04 (0.88–1.22) |
| 2 years | 100 (52%) | 76 (39%) | 0.63 (0.47–0.85)†,§ | 206 (49%) | 177 (42%) | 0.74 (0.60–0.90) | 340 (39%) | 382 (44%) | 1.08 (0.93–1.25) |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BNP = B-Type natriuretic peptide; COPD = chronic obstructive pulmonary disease; eGFR = and estimated glomerular filtration rate, presented as ml/min/1.73 m2; HF = heart failure; HFrEF = heart failure with reduced ejection fraction (≤40%)
Results of the formal sensitivity analyses for the 2-year associations in the matched cohort with HFrEF with eGFR <30 are presented below (performed only for associations that were significant):
For the combined endpoint of 2-year HF readmission or death, in 96% (187/194) of the matched pairs, we were able to determine which patients within a pair clearly had longer time to HF readmission or death during 2 years of follow-up, and in 58% (108/187) of those pairs, these patients belonged to the group initiated on ACEIs or ARBs before discharge (sign-score test P=0.034). A hidden confounder that is a near-perfect predictor of this combined endpoint could explain away this association if it could increase the odds of ACEI or ARB initiation by 2.38%.
For 2-year HF readmission, in 53% (103/194) of the matched pairs, we were able to determine which patients within a pair clearly had longer time to HF readmission during 2 years of follow-up, and in 61% (63/103) of those pairs, these patients belonged to the group initiated on ACEIs or ARBs before discharge (sign-score test P=0.023). A hidden confounder that is a near-perfect predictor of this combined endpoint could explain away this association if it could increase the odds of ACEI or ARB initiation by 6.24%.
For 2-year all-cause mortality, in 87% (168/194) of the matched pairs, we were able to determine which patients within a pair clearly had longer survival during 2 years of follow-up, and in 57% (96/168) of those pairs, these patients belonged to the group initiated on ACEIs or ARBs before discharge (sign-score test P=0.064). However, since this association was not significant, there was no formal sensitivity analysis for this outcome.
The 2-year associations remained unchanged when adjusted for the 6 baseline characteristics with ≥10% post-match absolute standardized differences (fatigue, depression, pulse, jugular venous pressure elevation, hemoglobin, and ejection fraction) with HRs (95% CIs) of 0.79 (0.63–0.98), 0.80 (0.62–1.02) and 0.65 (0.48–0.88), respectively for the combined endpoint, death and HF readmission.
All-Cause Mortality
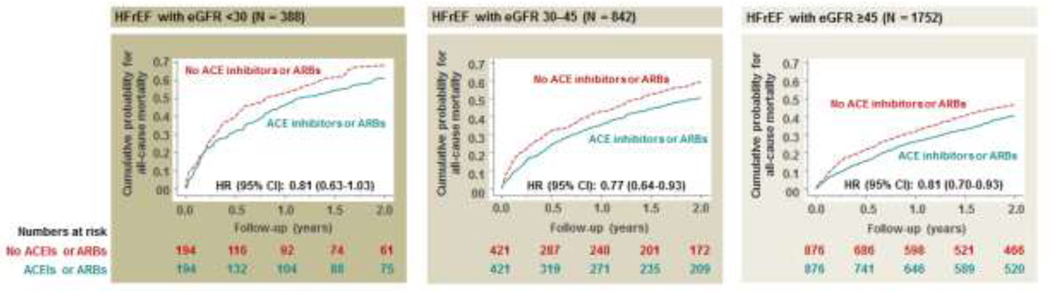
During 2 years of follow-up, all-cause mortality occurred in 61% and 69% of the patients with HFrEF and advanced kidney disease initiated and not initiated on ACE inhibitors or ARBs, respectively (HR associated with initiation, 0.81; 95% CI, 0.63–1.03; Table 2, left panel; Figure 3, left panel). Respective HRs (95% CIs) were 0.77 (0.64–0.93) in patients with moderate to severely impaired kidney function (Table 2, center panel; Figure 3, center panel) and 0.81 (0.70–0.93) in those with normal to mildly impaired kidney function (Table 2, right panel; Figure 3, right panel). Associations with 30-day and 12-month all-cause mortality are displayed in Table 2.
Figure 3.
Kaplan Meier plots displaying all-cause mortality among patients initiated and not initiated on ACE inhibitors or ARBs before discharge in a propensity score-matched cohort of patients with HFrEF and advanced kidney disease (eGFR <30; left panel), who were not receiving these drugs before hospital admission. Corresponding data on those with moderate to severely impaired kidney function (eGFR 30–45) and normal to mildly impaired kidney function (eGFR ≥45) are presented in the middle and right panels, respectively. ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; eGFR = estimated glomerular filtration rate, presented as ml/min/1.73 m2; HFrEF = heart failure with reduced ejection fraction (≤40%).
Heart Failure Readmission
During 2 years of follow-up, HF readmission occurred in 39% and 52% of the patients with HFrEF and advanced kidney disease initiated and not initiated on ACE inhibitors or ARBs, respectively (HR associated with initiation, 0.63; 95% CI, 0.47–0.85; Table 2, left panel). Respective HRs (95% CIs) were 0.74 (0.60–0.90) in those with moderate to severely impaired kidney function (Table 2, center panel) and 1.08 (0.93–1.25) in those with normal to mildly impaired kidney function (Table 2, right panel). Associations with 30-day and 12-month HF readmission are displayed in Table 2. RAS inhibitor use had no association with all-cause readmission.
Discussion
The findings from our study demonstrate that in hospitalized older patients with HFrEF and advanced kidney disease not receiving therapy with ACE inhibitors or ARBs before hospital admission, the initiation of these drugs prior to hospital discharge was associated with a significantly lower risk of the composite endpoint of heart failure readmission or all-cause mortality that became apparent during the first 30 days of follow-up and continued for up to 2 years. Although the associated lower risk of mortality did not reach statistical significance, likely due to inadequate statistical power, the associated risk of heart failure readmission was significantly lower. To the best of our knowledge, this is the first study to use propensity score-matching and a new-user design to provide evidence of associated improved clinical outcomes including hospitalization in patients with HFrEF and advanced kidney disease initiated on RAS inhibitors.
Because patients with moderate to severely impaired kidney function were excluded from randomized controlled trials of ACE inhibitors and ARBs in HFrEF, the evidence of their efficacy in these patients has been considered weak, especially for those with advanced kidney disease.2 In the Studies of Left Ventricular Dysfunction (SOLVD) Treatment trial, the first major randomized trial of a RAS inhibitor in patients with chronic HFrEF, those with a serum creatinine of >2 mg/dL were excluded (41% had eGFR <60, and 11% had eGFR <45).25, 26 This would exclude typical heart failure patients such as a 65-year-old female patient with a serum creatinine of 2.1 mg/dL (eGFR, 26). However, the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) Alternative and Added trials enrolled patients with HFrEF with serum creatinine up to 3.0 mg/dL,27–29 which would include a 65-year-old male patient with a serum creatinine of 2.9 mg/dL (eGFR, 23). The inclusion of patients with more advanced kidney disease in CHARM and other later trials, and findings from subgroup analysis of the SOLVD trial,26 and other observational studies,3–6 suggest a potential clinical benefit of RAS inhibition in patients with HFrEF with impaired kidney function. However, patients with eGFR <30 continue to be excluded from the randomized trials of RAS inhibitors in HFrEF,30 and RAS inhibitors continue to be underutilized in patients with HFrEF with eGFR <30.9, 10
Despite a perceived concern of renal harm from RAS inhibitors, these drugs have been shown to improve renal outcomes in patients with renal insufficiency.31–33 Although RAS inhibitors are more likely to increase serum creatinine and/or potassium in patients with heart failure than without,34 these increases are often minimal and considered natural extensions of their hemodynamic and neurohormonal effects.26, 35 In the SOLVD trial, among patients randomized to enalapril, the rise of serum creatinine during the first 12 months was greater in those with eGFR ≥60 than <60 (by 0.09 vs. 0.04 mg/dL, respectively; p=0.003).26 When compared with the 12-month rise in serum creatinine in the placebo group, the rise in the enalapril group were by 0.04 and 0.06 mg/dL for those with eGFR ≥60 and <60, respectively.26 Although similar data is not available in patients with advanced kidney disease, the consistency of the RAS inhibitor-associated lower mortality across the spectrum of kidney function in our study suggests that baseline kidney function or a potential worsening of kidney function during therapy did not confound the association in those with advanced kidney disease.
Although we had no data on baseline serum potassium, the between-group imbalance in baseline serum potassium would be expected to be minimum considering that major predictors of hyperkalemia, such as eGFR, prevalence of diabetes mellitus and use of loop diuretics and mineralocorticoid receptor antagonists, were balanced in our study. In the SOLVD trial, among patients randomized to enalapril, the rise of serum potassium during the first 12 months was similar in those with eGFR ≥60 and <60 (by 0.18 vs. 0.20 mEq/L, respectively; p=0.632).26 When compared with the 12-month rise in serum potassium in the placebo group, the rise in the enalapril group were by 0.22 and 0.26 mEq/L for those with eGFR ≥60 and <60, respectively.26
In patients with eGFR <30, we observed that the RAS inhibition-associated risk reduction for 2-year HF readmission (37%) was greater than it was for death (19%), which is consistent with the findings from the SOLVD trial.25 However, unlike in the SOLVD trial, there was no association with heart failure readmission in patients with eGFR ≥45 despite their kidney function being similar to that of SOLVD participants.25, 26 While this is intriguing, the effect of RAS inhibitors has been known to be more pronounced in those with impaired kidney function.7, 36 In the Heart Outcomes and Prevention Evaluation (HOPE) trial that excluded patients with heart failure, ramipril, an ACE inhibitor, significantly reduced the risks of death and heart failure hospitalization in 980 patients with renal insufficiency, but not in 8307 patients without.36 One potential explanation is the lower baseline risk (in the non-RAS inhibitor group) in patients with better kidney function (eGFR >30). For example, these patients had a 30-day heart failure readmission rate of 10%, while it was 19% for those with eGFR <30, likely attributable in part to congestion and fluid retention that occur with advanced kidney disease.37 Diuretic effects are known to be more pronounced in patients with greater congestion.39–41 It is possible that the RAS inhibitor-associated reduction in heart failure hospitalization observed in our study was in part mediated by the natriuretic properties of these drugs.38
Prior studies of RAS inhibitors in HFrEF with advanced kidney disease are mostly limited to subgroup analyses of RCTs and registries.2 In one study, in a propensity score-matched cohort of HFrEF with advanced kidney disease, RAS inhibitor use was associated with a lower risk of death.8 Our study is distinguished by our use of a new user design to avoid prevalent user bias and the examination of non-death outcomes.17, 42 Furthermore, we examined the association of RAS inhibitors with outcomes in two separately assembled propensity score-matched balanced cohorts of patients with eGFR 30–44 and ≥45, which allowed us to examine consistency of the observed associations across the kidney function spectrum within the same OPTIMIZE-HF population. The findings from the current study provide evidence to support the use of RAS inhibitors in HFrEF with advanced kidney disease. However, the risk of RAS inhibitor-associated hyperkalemia is higher in these patients and serum potassium needs to be monitored. Emerging evidence suggests that newer potassium binders are effective in reducing the risk of hyperkalemia in patients with kidney disease treated with RAS inhibitors.43, 44 Future studies need to examine the effectiveness of these drugs in improving clinical outcomes.45
Although it would be ideal to test and confirm the hypothesis-generating findings of our study in adequately powered randomized trials, it is unlikely to occur due to ethical and financial constraints. It may be unethical to recruit symptomatic patients with heart failure and advanced kidney disease into long-term randomized placebo-controlled trials considering the collective evidence of the efficacy of RAS inhibitor in heart failure from multiple trials, and evidence of effectiveness from subsequent observational studies, including in patients with heart failure with advanced kidney disease.7 Findings of the current study, taken together with the collective randomized and observational evidence, now suggest that the clinical benefits of RAS inhibitors may be extended to this high-risk subset of the HFrEF population with advanced kidney disease. Future studies need to replicate these findings in contemporary HFrEF populations with advanced kidney disease receiving mineralocorticoid receptor antagonists.46
Limitations
As in any observational study, bias due to unmeasured confounders is possible. We had no data on the dosages of RAS inhibitors used. Although dose of RAS inhibitor is less relevant in patients with HFrEF,47, 48 below-target doses have been shown to be more effective in HFrEF with CKD.26 We had no data on incident end-stage kidney disease. Lack of data on serum potassium levels at baseline or during follow-up is another limitation. Finally, results of our study based on fee-for-service Medicare beneficiaries may limit generalizability to other populations.
Conclusions
Among hospitalized older patients with HFrEF and advanced kidney disease, the initiation of therapy with ACE inhibitors or ARBs was associated with significantly improved clinical outcomes, which is similar to those observed in patients without advanced kidney disease. These findings add new information to the body of cumulative evidence that suggest that the clinical benefit of RAS inhibition in HFrEF may be extended to those with advanced kidney disease. These hypothesis-generating findings need to be replicated in larger and more contemporary cohorts of HFrEF and advanced kidney disease.
Clinical Significance.
Less is known whether renin-angiotensin system inhibitors would improve clinical outcomes in patients with heart failure with reduced ejection fraction with advanced kidney disease.
Findings from our study suggest that initiation of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers before hospital discharge is associated with improved clinical outcomes in HFrEF patients with advanced kidney disease.
Clinical benefits are notable across the spectrum of renal impairment.
Sources of Funding
Dr. Ahmed was in part supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) (R01-HL085561 and R01-HL097047) and the Department of Veterans Affairs Office of Research and Development (I01HX002422). OPTIMIZE-HF was sponsored by GlaxoSmithKline, but played no role in the design, conduct, analyses or interpretation of the current study.
Footnotes
All authors had access to the data and a role in writing the manuscript.
Disclosures
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs. Dr. Fonarow reports consulting with Abbott, Amgen, Astra Zeneca, Bayer, Cytokinetics, Janssen, Medtronic, Merck, and Novartis, and was the Principal Investigator of OPTIMIZE-HF. None of the other authors report any conflicts of interest related to this manuscript. Dr Rossignol reports consulting for Bayer, CinCor, G3P, Idorsia, and KBP; honoraria from Ablative Solutions, AstraZeneca, Bayer, Boehringer-Ingelheim, Corvidia, CVRx, Fresenius, Novartis, Novo Nordisk, Relypsa Inc., a Vifor Pharma Group Company, Roche, Sanofi, Sequana Medical, Servier, and Vifor Fresenius Medical Care Renal Pharma; Cofounder: CardioRenal, a company developing potassium and creatinine sensors for home monitoring. Dr Pitt has received personal fees (consulting) from Bayer, Boehringer Ingelheim/Lilly, KBP pharmaceuticals, AstraZeneca, Relypsa/Vifor, Sanofi/Lexicon, scPharmaceuticals, Sarfez Pharmaceuticals, Cereno Scientific, SQinnovations, G3 Pharmaceuticals, Phasebio, and Tricida; has received stock options from KBP Pharmaceuticals, scPharmaceuticals, Sarfez Pharmaceuticals, Relypsa, Cereno Scientific, SQinnovations, G3 Pharmaceuticals, and Tricida; holds US patent 9931412 for site specific delivery of eplerenone to the myocardium; and has pending US patent 63/045,783 for histone–acetylation–modulating agents for the treatment and prevention of organ injury. All other authors have nothing to disclose.
Declaration of Competing Interest
None of the other authors report any conflicts of interest related to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 2.Hein AM, Scialla JJ, Edmonston D, Cooper LB, DeVore AD, Mentz RJ. Medical Management of Heart Failure With Reduced Ejection Fraction in Patients With Advanced Renal Disease. JACC Heart Fail. 2019;7:371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed A, Kiefe CI, Allman RM, Sims RV, DeLong JF. Survival benefits of angiotensin-converting enzyme inhibitors in older heart failure patients with perceived contraindications. J Am Geriatr Soc. 2002;50:1659–1666. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A, Love TE, Sui X, Rich MW. Effects of angiotensin-converting enzyme inhibitors in systolic heart failure patients with chronic kidney disease: a propensity score analysis. J Card Fail. 2006;12:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed A, Fonarow GC, Zhang Y, et al. Renin-angiotensin inhibition in systolic heart failure and chronic kidney disease. Am J Med. 2012;125:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed A, Rich MW, Zile M, et al. Renin-angiotensin inhibition in diastolic heart failure and chronic kidney disease. Am J Med. 2013;126:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickstein K. Is substantial renal dysfunction in patients with heart failure no longer a contraindication for RAS inhibition? The power of a large, high-quality registry to illuminate major clinical issues. Eur Heart J. 2015;36:2279–2280. [DOI] [PubMed] [Google Scholar]
- 8.Edner M, Benson L, Dahlstrom U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insufficiency: a prospective propensity score-matched cohort study. Eur Heart J. 2015;36:2318–2326. [DOI] [PubMed] [Google Scholar]
- 9.Patel RB, Fonarow GC, Greene SJ, et al. Kidney Function and Outcomes in Patients Hospitalized With Heart Failure. J Am Coll Cardiol. 2021;78:330–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossignol P, Pitt B. Heart Failure and Chronic Kidney Disease Patients: First It Is Necessary to Act. J Am Coll Cardiol. 2021;78:344–347. [DOI] [PubMed] [Google Scholar]
- 11.Fonarow GC, Abraham WT, Albert NM, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148:43–51. [DOI] [PubMed] [Google Scholar]
- 12.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Kilgore ML, Arora T, et al. Design and rationale of studies of neurohormonal blockade and outcomes in diastolic heart failure using OPTIMIZE-HF registry linked to Medicare data. Int J Cardiol. 2013;166:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arundel C, Lam PH, Gill GS, et al. Systolic Blood Pressure and Outcomes in Patients With Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol. 2019;73:3054–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam PH, Aronow WS, Tsimploulis A, et al. Initiation of Anti-Hypertensive Drugs and Outcomes in Patients with Heart Failure with Reduced Ejection Fraction. Am J Med. 2021. [DOI] [PubMed]
- 16.Norris KC, Eneanya ND, Boulware LE. Removal of Race From Estimates of Kidney Function: First, Do No Harm. JAMA. 2021;325:135–137. [DOI] [PubMed] [Google Scholar]
- 17.Danaei G, Tavakkoli M, Hernan MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 2012;175:250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowling CB, Feller MA, Mujib M, et al. Relationship between stage of kidney disease and incident heart failure in older adults. Am J Nephrol. 2011;34:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell RC, Sui X, Filippatos G, et al. Association of chronic kidney disease with outcomes in chronic heart failure: a propensity-matched study. Nephrol Dial Transplant. 2009;24:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenbaum PR RD. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 21.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001; 2:169–188. [Google Scholar]
- 22.Austin PC. Primer on statistical interpretation or methods report card on propensity-score matching in the cardiology literature from 2004 to 2006: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;1:62–67. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, ed. Observational Studies. Vol 1. New York: Springer-Verlag; 2002:105–170. [Google Scholar]
- 25.Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 26.Bowling CB, Sanders PW, Allman RM, et al. Effects of enalapril in systolic heart failure patients with and without chronic kidney disease: insights from the SOLVD Treatment trial. Int J Cardiol. 2013;167:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. [DOI] [PubMed] [Google Scholar]
- 28.McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. [DOI] [PubMed] [Google Scholar]
- 29.Young JB, Dunlap ME, Pfeffer MA, et al. Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation. 2004;110:2618–2626. [DOI] [PubMed] [Google Scholar]
- 30.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 31.Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354:131–140. [DOI] [PubMed] [Google Scholar]
- 32.Ihle BU, Whitworth JA, Shahinfar S, Cnaan A, Kincaid-Smith PS, Becker GJ. Angiotensin-converting enzyme inhibition in nondiabetic progressive renal insufficiency: a controlled double-blind trial. Am J Kidney Dis. 1996;27:489–495. [DOI] [PubMed] [Google Scholar]
- 33.The GISEN Group Investigators. Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- 34.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–693. [DOI] [PubMed] [Google Scholar]
- 35.The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429–1435. [DOI] [PubMed] [Google Scholar]
- 36.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. [DOI] [PubMed] [Google Scholar]
- 37.Vasavada N, Agarwal R. Role of excess volume in the pathophysiology of hypertension in chronic kidney disease. Kidney Int. 2003;64:1772–1779. [DOI] [PubMed] [Google Scholar]
- 38.Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97:1411–1420. [DOI] [PubMed] [Google Scholar]
- 39.Faselis C, Arundel C, Patel S, et al. Loop Diuretic Prescription and 30-Day Outcomes in Older Patients With Heart Failure. J Am Coll Cardiol. 2020;76:669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faselis C, Lam PH, Patel S, et al. Loop Diuretic Prescription and Long-Term Outcomes in Heart Failure: Association Modification by Congestion. Am J Med. 2021;134:797–804. [DOI] [PubMed] [Google Scholar]
- 41.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–186. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed A, Fonarow GC. The prevalent-user bias in observational studies and the importance of new-user design (letter to the editor). Eur Heart J. 2019. Access date: February 19, 2020. 10.1093/eurheartj/ehz395/5520008#usercomments. [DOI]
- 43.Weir MR, Bushinsky DA, Benton WW, et al. Effect of Patiromer on Hyperkalemia Recurrence in Older Chronic Kidney Disease Patients Taking RAAS Inhibitors. Am J Med. 2018;131:555–564 e553. [DOI] [PubMed] [Google Scholar]
- 44.Pitt B, Bakris GL, Bushinsky DA, et al. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail. 2015;17:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ameri P, Bertero E, Maack C, Teerlink JR, Rosano G, Metra M. Medical treatment of heart failure with reduced ejection fraction: the dawn of a new era of personalized treatment? Eur Heart J Cardiovasc Pharmacother. 2021;7:539–546. [DOI] [PubMed] [Google Scholar]
- 46.Pitt B, Pedro Ferreira J, Zannad F. Mineralocorticoid receptor antagonists in patients with heart failure: current experience and future perspectives. Eur Heart J Cardiovasc Pharmacother. 2017;3:48–57. [DOI] [PubMed] [Google Scholar]
- 47.Lam PH, Dooley DJ, Fonarow GC, et al. Similar clinical benefits from below-target and target dose enalapril in patients with heart failure in the SOLVD Treatment trial. Eur J Heart Fail. 2018;20:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam PH, Packer M, Fonarow GC, et al. Early Effects of Starting Doses of Enalapril in Patients with Chronic Heart Failure in the SOLVD Treatment Trial. Am J Med. 2020;133:e25–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]