Abstract
Serology has been used worldwide to detect Helicobacter pylori infection. Using an immunoblot assay with an antigen from strain ATCC 43579, we sought to determine the antibodies which were good markers of colonization and the antibody patterns associated with ulcers or atrophy. Out of 98 dyspeptic patients, 41 were colonized by H. pylori, based on a positive culture or on positive results of both a urease test and direct examination. These 41 patients were seropositive by an enzyme immunoassay, and 12 of them had ulcers and 29 had evidence of atrophy. Fifty-seven of the 98 patients were noncolonized. Twenty-five of the 57 had evidence of gastric atrophy, and 10 were seropositive; 5 of these 10 had ulcers. By Western blot analysis, 12 antibodies were significantly more frequent in sera from colonized patients, and they produced immunoreactive bands at 125, 87, 74, 66, 54, 48, 46, 42, 35, 30, 16 and 14 kDa. The presence of at least one band at 54, 35, or 42 kDa was the best marker of infection (sensitivity, 95%; specificity, 82%). In the group of colonized patients, none of the antibody patterns were correlated to gastric atrophy. Conversely, the presence of a band at 125, 87, or 35 kDa was statistically associated with the presence of an ulcer. The simultaneous presence of bands at 87 and 35 kDa predicted the risk of ulcers with 83% sensitivity and 69% specificity. By using CagA-positive and VacA-positive strains and CagA-negative and VacA-negative isogenic mutants, the antigens corresponding to the bands at 125 and 87 kDa were shown to be CagA and VacA, respectively. On the other hand, the 35-kDa antigen is a novel uncharacterized component of H. pylori. These results may help to optimize the composition of antigenic preparations for serologic detection of H. pylori colonization. Immunoblot assay would be useful for screening patients at high risk of ulcers.
Helicobacter pylori is an important etiologic factor for chronic gastritis and peptic ulcers. It is also associated with gastric atrophy, which can lead to adenocarcinoma, and with gastric lymphoma (3–5, 17, 18, 23, 24, 29, 31, 39). The diagnosis of H. pylori gastric infection can be conducted by using direct (invasive) or indirect (noninvasive) methods (28). Among the indirect methods, serology is a valuable tool for seroepidemiological studies (43, 45) or for posttreatment follow-up (46). The serological assays are, essentially, enzyme immunoassays (EIAs) with a variety of antigenic preparations. The performances of the EIAs are hampered by cross-reactions (33) and there is no consensus as to the best antigenic preparation to use for H. pylori serology (47). Consequently, it would be of interest to know which antigens of H. pylori should be included in an ideal preparation designed for serodiagnosis of H. pylori infection.
Although all H. pylori-infected subjects have gastritis, a considerable number remain asymptomatic whereas others develop severe diseases, such as ulcers, gastric lymphomas, atrophic gastritis, or adenocarcinomas (9). It would be valuable to have predictive markers for severe diseases at our disposal. However, the factors influencing the evolution of H. pylori gastritis remain poorly understood: they could be related to the infecting strain or to the host response (5, 31). The ability of certain strains to produce a vacuolating cytotoxin encoded by the vacA gene has been associated with more severe illnesses (26, 40, 44). Most, but not all, of the cytotoxic strains express the CagA antigen, which has been associated with a more severe inflammatory response (4). The vacuolating toxin and the CagA antigen elicit specific antibodies during infection (4, 6, 7, 32, 49), but the value of these antibodies as predictive factors for the severity of the disease remains controversial (10, 23, 24, 44).
Because it depends on both the characteristics of the strain and the host response, the serum antibody response to H. pylori could provide clues in predicting the severity of H. pylori-associated diseases. Several studies have demonstrated a strong correlation between the levels of total anti-H. pylori immunoglobulin G (IgG) and the colonization of the gastric mucosa by the bacteria (1, 27, 37). However, the anti-H. pylori antibody patterns have been reported to show a high degree of polymorphism (2, 16, 30, 33). This antibody polymorphism could be related to the pathological status and thus may serve as a biological predictor of the type of disease associated with the H. pylori infection. In 1993, Xiang and colleagues described an EIA with a recombinant antigen including a fragment of the CagA protein (48). They demonstrated a positive correlation between the EIA and the Western blotting methods used to detect the anti-CagA antibodies. There was also a strong correlation between the anti-CagA antibody level and the presence of an ulcer. Nevertheless, other antibodies or combinations of antibodies may also be good markers of the severity of the disease.
In this work, we studied the frequencies of the antibodies to 12 major antigens of H. pylori in the sera of 98 patients clinically and histologically documented. We sought to determine the antibodies which are the best markers of colonization and the antibody patterns associated with the presence of an ulcer or a gastric atrophy.
MATERIALS AND METHODS
Patients.
A total of 98 consecutive patients (54 males and 44 females) examined in the Hepato-Gastro-Enterology Department of the University Hospital Center of Poitiers, France, were included in the study between 1995 and 1996. The median ages were 51.4 years (range, 12 to 85 years) and 44.3 years (range, 15 to 79 years) for males and females, respectively. The patients presented with dyspeptic syndrome and underwent an upper gastroduodenal endoscopy with multiple antral and fundic biopsies. They had received neither antimicrobial nor antiacid therapies during the previous 3 months. The biopsies were processed for culture of H. pylori and for histology. Sera were collected the day of the endoscopy; they were aliquoted and frozen at −80°C until they were used.
Bacteriology.
Gastric biopsy specimens were placed into sterile 0.15 M NaCl solution and transported to the laboratory within 30 min. A part of each specimen was ground and inoculated into a nonselective Columbia blood agar (bioMérieux, Marcy l’Etoile, France). The plates were incubated at 37°C under microaerobic conditions for 10 days. The isolates were identified as H. pylori by Gram staining and urease, oxidase, and catalase activities. A part of the ground specimen was smeared and Gram stained for direct search for spiral bacteria. A second part of each specimen was placed into 0.2 ml of 20 mM urea, containing phenol red as a pH indicator, for detection of urease activity. Urease reactions were recorded after 1 h of incubation at 37°C.
Histology.
Gastric specimens were placed into 10% formalin, and multiple sections of each specimen were hematoxylin-eosin or Giemsa stained. Chronic and active chronic gastritis scores were assigned to each biopsy specimen, and these scores were used for classifying the patients into the following categories: (i) normal, (ii) with gastritis, and (iii) with atrophic gastritis. A gastritis score of 0 indicated that no mononuclear cells were present, a score of 1 indicated that mononuclear cells were present in a patchy distribution, a score of 3 indicated a very dense infiltration of mononuclear cells throughout the entire section, and a score of 2 was intermediate between 1 and 3. Similar criteria for polymorphonuclear leukocytes were used for grading acute inflammation (36). In this work, the patients with a gastritis score of ≥1 were considered gastritic patients. Acute and nonacute gastritis were classified into the same group. The gastric atrophy was scored from 1 to 4 on the basis of the degree of atrophy of the glands and the density of mucus-secreting cells. The patients with a score of ≥1 were considered to have gastric atrophy. The presence of spiral bacteria was noted on the Giemsa-stained smear. The presence of an ulcer was noted during the endoscopic examination. Histological, bacteriological, and serological statuses of the patients were established blindly by three independent investigators.
H. pylori strains and antigenic extracts.
Hydrosoluble antigens from H. pylori ATCC 43579 were extracted by a method previously described (2, 13). The strain was CagA positive and produced a VacA vacuolating toxin. H. pylori was cultured on chocolate agar plates and incubated at 37°C under 5% O2 for 48 h. Bacterial cells from each plate were harvested and suspended in 2 ml of sterile 0.15 M NaCl at 4°C. The bacterial suspension was gently vortexed for 60 s, and then the cells were sedimented by centrifugation (10,000 × g for 20 min at 4°C). The supernatant was dialyzed overnight at 4°C against 0.15 M NaCl, the protein concentration of the resulting saline extract was determined by the bicinchoninic acid method (Pierce Chemicals, Rockford, Ill.), and the extract was frozen at −80°C until it was used in immunoassays.
Whole-cell extracts were prepared from the H. pylori wild-type strain 84-183 (ATCC 53726) and from the CagA-negative isogenic mutant 84-183:M21 (42). These extracts were obtained by the sonication of bacterial cells cultured for 48 h on chocolate agar at 37°C under 5% O2. Bacterial cells from each plate were harvested and suspended in 2 ml of sterile 0.15 M NaCl at 4°C. The cells were broken by ultrasonic treatment in a Sonifier 450 (Branson, Osi, Paris, France). After centrifugation at 15,000 × g for 15 min, the supernatant was dialyzed against 0.15 M NaCl for 48 h.
Vacuolating toxin (VacA)-enriched preparations were obtained as previously described (8). Briefly, the H. pylori 60190 (ATCC 49503) wild-type strain and 60190:v1, a mutant strain that does not produce VacA, were cultured for 3 days at 37°C under 5% O2 in brucella broth supplemented with 10% fetal bovine serum and 5% β-cyclodextrin (34). The bacterial cells were pelleted by centrifugation at 4,000 × g for 10 min, and the supernatant was dialyzed against 0.15 M NaCl for 48 h and frozen at −80°C until use.
The strains H. pylori 60190 (ATCC 49503), 60190:v1, 84-183 (ATCC 53726) and 84-183:M21 were kindly donated by T. Cover, Vanderbilt University, Nashville, Tenn.
Determination of antibody levels by ELISA.
To assess IgG antibodies to H. pylori in the human sera, we used an enzyme-linked immunosorbent assay (ELISA) with saline extract from strain ATCC 43579 as the antigen (14). Briefly, 98-well microtiter plates were coated with 250 ng of antigenic protein per well, and the sera were diluted 1:100. The assay was calibrated by using a reference serum included on each plate. The serum specimen and the reference serum were assayed in triplicate. For each serum, results were expressed as an ELISA index obtained by calculation of the ratio of the mean optical density of the serum specimen to the mean optical density of the reference serum. The cutoff value was determined by the construction of a receiver operating characteristic curve (14).
Immunoblot assays.
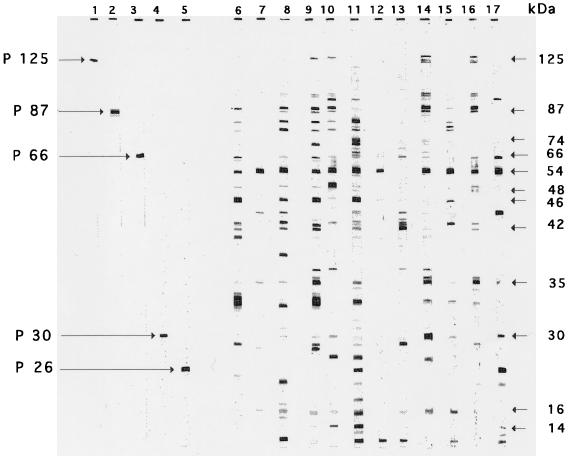
Using a Maxi-Gel apparatus (Bio-Rad, Richmond, Calif.), we carried out sodium dodecyl sulfate-polyacrylamide gel electrophoresis of bacterial extracts as described by Laemmli (25), with a 4% stacking gel and a linear gradient (8 to 16% acrylamide) separating gel (16 by 18 cm). Prior to electrophoresis the antigenic extracts were heated at 100°C for 5 min in Tris-HCl buffer (pH 6.8) containing 1% sodium dodecyl sulfate and 10% β-mercaptoethanol. Preparative slab gels were loaded with samples containing 1 mg of protein. A sample of molecular mass markers (Bio-Rad) was loaded on each gel. Migrations were performed under a constant current of 35 mA until the bromophenol blue dye migrated out of the gel. The proteins were then transferred for 1 h onto prewetted nitrocellulose membranes (Bio-Rad) by using an electrophoretic transfer cell (Trans-Blot; Bio-Rad) under a constant current of 200 V. Following the protein transfer, the nitrocellulose sheets were cut into strips. The strip corresponding to the molecular mass markers was stained with Ponceau red (Sigma) and kept for calibration purposes. The blot strips were incubated for 1 h with the patients’ sera diluted 1:250 or with calibrating monospecific sera (see below) appropriately diluted. The strips were then rinsed three times in Tris-saline blotting buffer (pH 8) and incubated for 1 h in alkaline phosphatase-conjugated anti-human IgG (Dakopatts, Copenhagen, Denmark). After being washed, they were developed with 5-bromo-4-chloro-3-indolylphosphate as the substrate and with nitroblue tetrazolium as the chromogenic indicator. The reactions were stopped after 15 min by washing the strips thoroughly with distilled water. In order to normalize the positions of the immunoreactive bands detected by the patients’ sera we used, as internal references, five monospecific polyclonal rabbit sera raised against the following five high-performance liquid chromatography-purified antigens of H. pylori: (i) an antigen of 26-kDa purified from H. pylori ATCC 43579, (ii) UreA (30 kDa) purified from H. pylori ATCC 43579, (iii) UreB (66 kDa) purified from H. pylori ATCC 43579, (iv) the vacuolating toxin (87 kDa) purified from H. pylori 60190 (ATCC 49503) (7), and (v) the CagA antigen (125 kDa) purified from H. pylori 84-183 (42). The first three sera were generously supplied by Pasteur Mérieux Connought (Marcy l’Etoile, France), and the others were kindly donated by T. Cover. When tested by Western blotting with a saline extract from H. pylori ATCC 43579, these calibrating sera reacted with antigens having the expected apparent molecular masses (Fig. 1, lanes 1 to 5). The positions of the immunoreactive bands revealed by the patients’ sera were assessed with a calibrating curve constructed by plotting the distances of migration (in millimeters) of the immunoreactive bands obtained with the calibrating sera against the molecular masses (in kilodaltons) of the corresponding antigens. The polynomial regression showed a R2 coefficient of 0.995. The calibrating sera and the patients’ sera were studied simultaneously in the same gels. To assess the reproducibility of the migration distances, experiments were carried out four times with the monospecific sera. The coefficients of variation (i.e., the ratios of standard deviations (SD) to means) of the migration distances were <10%.
FIG. 1.
Immunoblot patterns obtained with a saline extract from H. pylori ATCC 43579 with five rabbit sera raised against purified antigens from H. pylori (lanes 1 to 5) and with 12 selected sera from patients infected with H. pylori (lanes 6 to 17). Molecular masses are indicated on the right. The arrows indicate immunoreactive bands corresponding to the antigens p125, p87, p66, p30, and p26. The figure shows a scan of the original nitrocellulose strips.
RESULTS
Status of patients.
Colonized patients were defined as patients who were positive by culture of H. pylori or patients who were positive for spiral bacteria by direct examination of biopsy specimens and who also had positive urease tests. Noncolonized patients were defined as patients who were negative by culture, direct examination, and urease tests. The serum antibody levels, expressed as the ELISA index (see Materials and Methods), ranged from 0.02 to 1.08 for the 98 patients. To define a patient’s serologic status, a cutoff value of 0.15 was determined by the receiver operating characteristic curve method (14), using the direct methods of diagnosis (i.e., culture, direct examination, and urease test) as a “gold standard.” Under these conditions, the EIA showed a sensitivity of 100% and a specificity of 83% for predicting H. pylori colonization.
Of the 98 patients, 41 (41.8%) were colonized by H. pylori and were seropositive. Among these 41, 12 had ulcers and 29 had evidence of gastric atrophy (i.e., scores from 1 to 3; median = 1.8). Fifty-seven (58.2%) of the 98 patients were noncolonized. Among the 57, 25 had evidence of gastric atrophy (i.e., scores from 1 to 3; median = 1.6) and 10 were seropositive (5 of these had ulcers). Colonized and noncolonized patients were similar in age (mean = 45.9 years [SD = 18.3 years] and mean = 48.5 years [SD = 21.4 years], respectively).
Antibody patterns.
Sera from seropositive patients tested by Western blotting revealed from 1 to 15 bands (average 8.1 bands), while sera from seronegative patients revealed from 0 to 5 bands (average, 1.4 bands). A set of blots with sera from seropositive patients and with calibrating sera is depicted in Fig. 1.
The blots were analyzed by three independent investigators. Seventeen different bands were distinguished on the 98 blots. Of the 17, four bands (62, 25, 23, and 13 kDa) were disregarded because their frequencies were not significantly different in the colonized and the noncolonized patients (chi-square test; P > 0.05). An additional band at 19 kDa was disregarded because its frequency was low. The abilities of the 12 remaining immunoreactive bands to predict colonization were evaluated by comparing the frequency of each band in the 41 colonized patients with that in the 57 noncolonized patients (Table 1). The best performance indexes were obtained with bands at 54, 42, and 35 kDa (the performance index is the percentage of patients correctly classified as colonized or noncolonized by the presence or the absence of the specific band). The presence of at least one of these three bands predicts colonization with 95% sensitivity and 82% specificity. These performances could not be improved by considering the presence of any other bands.
TABLE 1.
Frequencies of 12 antibodies to H. pylori in 98 human sera and their abilities to predict H. pylori infection
| Immunoreactive band (kDa) | No. (%) of reacting sera from:
|
Performance index (%)b | |
|---|---|---|---|
| Colonized patients (n = 41)a | Noncolonized patients (n = 57)a | ||
| 125 | 29 (70.7) | 11 (19.3) | 77 |
| 87 | 25 (60.9) | 11 (19.3) | 72 |
| 74 | 14 (34.1) | 6 (10.5) | 66 |
| 66 | 19 (46.3) | 9 (15.8) | 68 |
| 54 | 34 (82.9) | 10 (17.5) | 83 |
| 48 | 14 (34.1) | 4 (7.0) | 68 |
| 46 | 22 (53.7) | 8 (14.0) | 72 |
| 42 | 24 (58.5) | 4 (7.0) | 79 |
| 35 | 26 (63.4) | 6 (10.5) | 79 |
| 30 | 26 (63.4) | 17 (29.8) | 67 |
| 16 | 20 (48.8) | 1 (1.8) | 78 |
| 14 | 15 (36.6) | 7 (12.3) | 66 |
Based on urease assay, direct examination, or culture. The antibodies to the 12 antigens listed were significantly more frequent in the patients infected by H. pylori than in the noninfected patients (chi-square test; P < 0.05).
Percentage of patients correctly classified [i.e., (number of true positive + number of true negative)/number of patients].
Correlations between antibody patterns and pathological status of colonized patients.
We next focused on the 41 colonized patients to see whether the presence of ulcers or atrophy could be correlated with the presence of certain antibody patterns. We compared the frequencies of the 12 antibodies in the populations of patients showing and not showing evidence of ulcers by endoscopy or evidence of gastric atrophy by histology. We failed to demonstrate significant relationships between the presence of gastric atrophy and the antibody pattern. Conversely, a significant relationship (chi-square test; P ≤ 0.05) was established between the presence of a band at 125, 87, or 35 kDa and the presence of ulcers (Table 2). Next we tested different combinations of antibodies for their abilities to predict the presence of ulcers. The best performance index was obtained by the combination of antibodies to both the 87- and the 35-kDa antigens. The simultaneous presence of these two antibodies predicted a risk of ulcer with 83% sensitivity and 69% specificity.
TABLE 2.
Frequencies of three antibodies to H. pylori in 41 sera from H. pylori-colonized patients,a with or without gastric ulcers,b and abilities of the antibodies to predict ulcers
| Immunoreactive band (kDa) | Prevalence (%) of antibodies in patientsc
|
Performance index (%)d | |
|---|---|---|---|
| Ulcer (n = 12) | No ulcer (n = 29) | ||
| 125 | 10 (83.3) | 18 (62.1) | 51 |
| 87 | 10 (83.3) | 14 (48.3) | 61 |
| 35 | 12 (100.0) | 14 (48.3) | 66 |
Based on urease assay, direct examination, or culture.
Based on endoscopy.
The antibodies to the three antigens listed were significantly more frequent in patients with ulcers than in nonulcerous patients (chi-square test; P ≤ 0.05).
Percentage of patients correctly classified [i.e., (number of true positive + number of true negative)/number of patients].
Partial characterization of p125, p87, and p35.
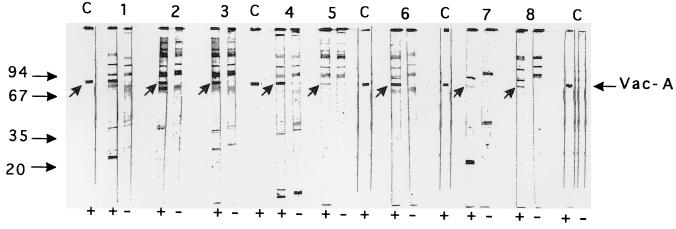
To demonstrate that antigens corresponding to the immunoreactive bands at 125 (p125) and 87 kDa (p87) were actually CagA and VacA, respectively, we prepared bacterial extracts with strains known to express these antigens along with extracts from isogenic mutants which no longer express the specified antigens. We carried out immunoblot assays with eight selected sera showing immunoreactive bands at 125 kDa and eight sera showing immunoreactive bands at 87 kDa. All the sera selected for having antibodies to p125 showed an immunoreactive band at 125 kDa with the extract from the CagA-positive strain 84-183, as did the anti-CagA rabbit serum. Conversely, the 125-kDa band was absent when these sera were tested against an extract from the CagA-negative isogenic mutant 84-183:M21. Similarly, we used a VacA-enriched preparation from the VacA-positive strain 60190 and from the Vac-A negative isogenic mutant 60190:v1 to test eight sera found to have antibodies to p87. All these sera and the anti-VacA control serum showed immunoreactive bands at 85 to 87 kDa when tested with the antigenic preparation from the VacA-positive strain, whereas this band was absent when an extract from the VacA-negative mutant was used as the antigen (Fig. 2). These results suggest that p125 and p87 represent the CagA and the VacA antigen, respectively. Because VacA is known to include a 37-kDa subunit (24, 35), we hypothesized that the 35-kDa antigen (p35) represents this subunit. As shown in Fig. 2, the anti-VacA control serum does not react in the 30- to 37-kDa area (lanes C). Moreover, only one of the eight sera tested exhibited an immunoreactive band at 35 kDa when tested against the extract from the VacA-positive strain. This band remained (although slightly shifted) when the extract was prepared from the VacA-negative mutant (Fig. 2, lanes 3+ and 3−). Furthermore, of the 51 seropositive patients, 9 had antibodies to p87 but no antibody to p35 whereas 8 had antibodies to p35 but no anti-p87. Thus, 17 patients (33.3%) had antibodies to only one of the two antigens. These results suggest that p35 is different from VacA and that it is a novel uncharacterized antigen.
FIG. 2.
Immunoblot patterns obtained with vacuolating toxin-enriched preparations from the VacA-positive strain 60190 (+) and the isogenic VacA-negative mutant 60190:v1 (−) with eight selected patients’ sera which reacted with p87 from H. pylori ATCC 43579 (lanes 1 to 8). Lanes C, serum from a rabbit immunized to purified VacA. The arrows indicate immunoreactive bands corresponding to VacA. Molecular mass markers are on the left. The figure shows a scan of the original nitrocellulose strips.
DISCUSSION
The choice of a single method for the diagnosis of H. pylori infection remains controversial, and at present, two different methods are needed to determine whether a patient is infected (28). The bacteriological method (culture) is unquestionably the most specific, but it is subject to sampling errors and is not very sensitive, giving false-negative results (28). The so-called “global methods” (i.e., breath test and serology) are more sensitive, but they may be less specific. Serology is now widely used as a global method of diagnosis, and a variety of commercially available kits (27) or homemade methods (14) have been described. Most of these methods provide satisfactory results (1, 15, 20, 27, 37, 43); however, significant improvements must be made before they can be considered as reference methods. Improvement of the antigenic preparations is the best way to improve the performances of the serological methods, but to date, there is no consensus as to the most appropriate composition of the preparations. Our data provide indications of antigens that must be included in an “ideal” antigenic preparation for H. pylori serology. The immunoblot assay we used was performed under very stringent conditions, and it was normalized to optimize its reproducibility. We used a mixture of components from a single strain as the antigenic preparation. This kind of preparation must contain the major antigens of H. pylori, particularly the CagA antigen, the vacuolating toxin (6, 7), the heat shock proteins, the urease complex (11, 12), and certain adhesins (13). The use of an antigenic mixture instead of single purified antigens is consistent with a previous study where we demonstrated that a preparation of extracted antigens is more efficient for serology than single purified recombinant antigens (47). Among the most relevant antigenic fractions, we demonstrated that p125 is the CagA antigen and p87 is the VacA antigen. p54 should be HspB, while p35 and p42 have not yet been characterized. The discrepancies between the results of serology and the results of the direct methods may be due to the lack of sensitivity of the direct methods. The 10 patients who were seropositive and noncolonized, on the basis of the direct method, might have been falsely classified as noncolonized. Actually, five of them had ulcers and all of their sera gave more than five bands when tested by Western blotting. Moreover, it should be noted that no patients were seronegative and colonized by the direct methods.
Some, but not all, of these data are in agreement with previous work (16, 19, 33). Nilsson et al. found strong correlations between H. pylori infection and the presence of antibodies to 110- to 120 (presumably CagA)-, 26-, 29-, 30-, 31-, and 33 (presumably our p35)-kDa antigens (33). Faulde et al. found four antigens of 130, 93, 75, and 67 kDa to be the most immunogenic during H. pylori infection (16). Thus, the correlations among the immunoblot analysis findings of different authors are poor. This fact may be due to both the diversity of the technical conditions and the use of different strains as the sources of antigens. It must be emphasized that the use of immunoblotting as a diagnostic tool implies a good standardization of the method. A well-defined strain representative of the clinical strains should be used to obtain the antigenic preparation, and the assay must be calibrated with well-defined antisera. In an unpublished study, we found that a population of clinically isolated strains could be clustered into three groups according to their antigenic profiles. The major group included the strain ATCC 43579 used in the present work. The strains in this group exhibited a richer antigenic composition than those in the other groups, including antigens of 120 to 125 kDa, 80 to 90 kDa, and 54, 42, and 35 kDa (25a). Thus, the strain chosen in this work as the source of antigens appears to be representative of the strains isolated most frequently in our patients. This strain is commercially available.
H. pylori infection can lead to a variety of diseases. Presently, the only reliable way to identify the illness associated with a H. pylori infection remains an endoscopic examination coupled with histologic examination of the gastric mucosa. Attempts have been made before to correlate the severity of the H. pylori-associated diseases to the antibody level, the specificity of the serum antibodies, or the isotypes of these antibodies (4, 6, 10, 17, 21, 22). The polymorphism of the antibody response to H. pylori has been suspected to reflect either an evolution of the immune response or an antigenic shift of the infecting strain (2, 30). Nevertheless, it has also been suspected to be correlated to a predisposition for severe diseases. The presence of anti-CagA has been associated with the presence of ulcers; however, the relevance of this correlation remains controversial (10, 23, 24, 41, 48, 49). In any case, other serum antibodies may be better markers for predicting severe diseases. In the present work, we found that three single antigens (CagA, VacA, and p35) elicited antibodies more frequently in patients suffering from ulcers. The anti-VacA antibody is a more powerful marker of ulcers than anti-CagA. This is not surprising, because the vacuolating toxin has been suspected to be involved in the mechanism of ulcerous lesions of the mucosa (35, 38, 40, 41, 44). The anti-p35 antibody appears to be the best marker of ulcers, and the simultaneous presence of anti-VacA and anti-p35 antibodies predicts, with good sensitivity, a predisposition to ulcers. The poor specificity observed could be due to the fact that peptic ulcers may be intermittently present and certain patients may be nonulcerous at the time of endoscopic examination but may later evolve to an ulcerous state. On the other hand, none of the serologic markers tested have been able to predict atrophic gastritis. It may be necessary to look for other antibodies, or the atrophy could be unrelated to serologic status. Other authors have attempted to establish correlations between certain antibody patterns and gastric cancer (21).
In conclusion, the antigenic preparations designed for H. pylori serology must include CagA, VacA, HspB, and also the uncharacterized antigens p42 and p35. Immunoblot assay would be useful for screening patients at high risk of ulcers. The follow-up of a cohort of H. pylori-infected patients may be of interest to confirm the value of these findings.
ACKNOWLEDGMENTS
We are grateful to T. Cover for supplying isogenic mutants and specific sera and to L. Lissolo and M. Leheur for supplying specific sera. We also thank G. Agius for constructive discussions on the paper and J. Johnson for helping to correct the English.
This work was supported by the Université de Poitiers, by Pasteur Mérieux Connaught, and by Institut de Recherche sur les Maladies Digestives (IRMAD).
REFERENCES
- 1.Andersen L P. The antibody response to Helicobacter pylori infection, and the value of serologic tests to detect H. pylori and for post-treatment monitoring. In: Goodwin C S, Worsley B W, editors. Helicobacter pylori: biology and clinical practice. Boca Raton, Fla: CRC Press; 1993. pp. 285–306. [Google Scholar]
- 2.Bazillou M, Fendri C, Castel O, Ingrand P, Fauchère J L. Serum antibody response to the superficial and released components of Helicobacter pylori. Clin Diagn Lab Immunol. 1994;1:310–317. doi: 10.1128/cdli.1.3.310-317.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser M J, Parsonnet J. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Invest. 1994;94:4–8. doi: 10.1172/JCI117336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser M J, Perez-Perez G, Kleanthous H, Cover T L, Peek R M, Chyou P H, Stemmermann G N, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 5.Calam J. The pathogenesis of Helicobacter pylori infection and duodenal ulcer: the role of gastrin and other soluble factors. In: Goodwin C S, Worsley B W, editors. Helicobacter pylori: biology and clinical practice. Boca Raton, Fla: CRC Press; 1993. pp. 239–256. [Google Scholar]
- 6.Covacci A, Censini S S, Bugnoli M, Petracca R, Burroni D, Machia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cover T L, Blaser M J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 8.Cover T L, Tummuru M K R, Cao P, Thompson S A, Blaser M J. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem. 1994;269:10566–10573. [PubMed] [Google Scholar]
- 9.Cover T L, Blaser M J. Helicobacter pylori: a bacterial cause of gastritis, peptic ulcer disease, and gastric cancer. ASM News. 1995;61:21–26. [Google Scholar]
- 10.Cover T L, Glupczynski Y, Lage A P, Burette A, Tummuru M K R, Perez-Perez G I, Blaser M J. Serologic detection of infection with cagA+Helicobacter pylori strains. J Clin Microbiol. 1995;33:1496–1500. doi: 10.1128/jcm.33.6.1496-1500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn B, Campbell G P, Perez-Perez G I, Blaser M J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 12.Dunn B E, Roop II R M, Sung C C, Sharma S A, Perez-Perez G I, Blaser M J. Identification and purification of a cpn60 heat shock protein homolog from Helicobacter pylori. Infect Immun. 1992;60:1946–1951. doi: 10.1128/iai.60.5.1946-1951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fauchère J L, Blaser M J. Adherence of Helicobacter pylori cells and their surface components to HeLa cell membranes. Microb Pathog. 1990;9:427–439. doi: 10.1016/0882-4010(90)90061-t. [DOI] [PubMed] [Google Scholar]
- 14.Fauchère J L. Evaluation of the anti-Helicobacter pylori serum antibody response. In: Lee A, Mégraud F, editors. Helicobacter pylori: techniques for clinical diagnosis and basic research. W. B. Philadelphia, Pa: Saunders Co.; 1996. pp. 50–74. [Google Scholar]
- 15.Fauchère J L. Réponse immunitaire contre Helicobacter pylori. In: Mégraud F, Lamouliatte H, editors. Helicobacter pylori. Paris, France: Elsevier; 1996. pp. 219–234. [Google Scholar]
- 16.Faulde M, Schröder J P, Sobe D. Serodiagnosis of Helicobacter pylori infections by detection of immunoglobulin G antibodies using an immunoblot technique and enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 1992;11:589–594. doi: 10.1007/BF01961664. [DOI] [PubMed] [Google Scholar]
- 17.Forman, D. 1996. Helicobacter pylori and gastric cancer. Scand. J. Gastroenterol. 31(Suppl. 215):48–51. [PubMed]
- 18.Gormally S, Sherman P, Drumm B. Clinical syndromes of Helicobacter pylori infection in children. In: Goodwin C S, Worsley B W, editors. Helicobacter pylori: biology and clinical practice. Boca Raton, Fla: CRC Press; 1993. pp. 85–94. [Google Scholar]
- 19.Guruge J L, Schalen C, Nilsson I, Ljungh A, Tyzkiewic T, Wikander M, Wadström T. Detection of antibodies to Helicobacter pylori cell surface antigens. Scand J Infect Dis. 1990;22:457. doi: 10.3109/00365549009027078. [DOI] [PubMed] [Google Scholar]
- 20.Jensen A K V, Andersen L P, Wachman C H. Evaluation of eight commercial kits for Helicobacter pylori IgG antibody detection. APMIS. 1993;101:795–801. [PubMed] [Google Scholar]
- 21.Klaamas K, Held M, Wadström T, Lipping A, Kurtenkov O. IgG immune response to Helicobacter pylori antigens in patients with gastric cancer as defined by ELISA and immunoblotting. Int J Cancer. 1996;67:1–5. doi: 10.1002/(SICI)1097-0215(19960703)67:1<1::AID-IJC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Kreuning J, Lindeman J, Biemond I, Lamers C B H. Relation between IgG and IgA antibody titers against Helicobacter pylori in serum and severity of gastritis in asymptomatic subjects. J Clin Pathol. 1994;47:227–231. doi: 10.1136/jcp.47.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labigne A. Pouvoir pathogène de Helicobacter pylori. Ann Inst Pasteur/Actualités. 1995;6:167–178. [Google Scholar]
- 24.Labigne A. Pouvoir pathogène de Helicobacter pylori. In: Mégraud F, Lamouliatte H, editors. Helicobacter pylori. Paris, France: Elsevier; 1996. pp. 119–140. [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25a.Langar, H., P. Aucher, and J. L. Fauchère. Unpublished data.
- 26.Leunk R D, Johnson P T, David B C, Kraft W G, Morgan D R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- 27.Lozniewski A, Aucher P, de Korwin J D, Fauchère J L. Méthodes sérologiques pour l’infection àHelicobacter pylori. In: Mégraud F, Lamouliatte H, editors. Helicobacter pylori. Paris, France: Elsevier; 1996. pp. 349–366. [Google Scholar]
- 28.Mégraud F. Stratégie d’utilisation des tests diagnostiques dans l’infection àHelicobacter pylori. In: Mégraud F, Lamouliatte H, editors. Helicobacter pylori. Paris, France: Elsevier; 1996. pp. 367–383. [Google Scholar]
- 29.Mitchell H M. The epidemiology of Helicobacter pylori infection and its relation to gastric cancer. In: Goodwin C S, Worsley B W, editors. Helicobacter pylori: biology and clinical practice. Boca Raton, Fla: CRC Press; 1993. pp. 95–114. [Google Scholar]
- 30.Mitchell H M, Hazell L S L, Kolesnikow T, Mitchell J, Frommer D. Antigen recognition during progression from acute to chronic infection with a cagA-positive strain of Helicobacter pylori. Infect Immun. 1996;64:1166–1172. doi: 10.1128/iai.64.4.1166-1172.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moran A P. Pathogenic properties of Helicobacter pylori. Scand J Gastroenterol. 1996;31:22–31. [PubMed] [Google Scholar]
- 32.Morgan D R, Leuk R D. Pathogenesis of infection by C. pylori. In: Blaser M J, editor. Campylobacter pylori in gastritis and peptic ulcer disease. New York, N.Y: Igaku-Shoin Publishers, Inc.; 1989. pp. 115–134. [Google Scholar]
- 33.Nilsson I, Ljungh A, Aleljung P, Wadström T. Immunoblot assay for serodiagnosis of Helicobacter pylori infections. J Clin Microbiol. 1997;35:427–432. doi: 10.1128/jcm.35.2.427-432.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olivieri R, Bugnoli M, Armellini D, Bianciardi S, Rappuoli R, Bayeli P F, Abate L, Esposito E, de Gregoria L, Aziz J, Basagni C, Figura N. Growth of Helicobacter pylori in media containing cyclodextrins. J Clin Microbiol. 1993;31:161–162. doi: 10.1128/jcm.31.1.160-162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phadnis S H, Ilver D, Janzon L, Normark S, Westblom T U. Pathological significance and molecular characterization of the vacuolating toxin gene of Helicobacter pylori. Infect Immun. 1994;62:1557–1565. doi: 10.1128/iai.62.5.1557-1565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price A B. The Sydney system: histological division. J Gastroenterol Hepatol. 1991;6:209–222. doi: 10.1111/j.1440-1746.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 37.Pronovost A D, Rose S L, Pawlak J W, Robin H, Schneider R. Evaluation of a new immunodiagnostic assay for Helicobacter pylori antibody detection: correlation with histopathological and microbiological results. J Clin Microbiol. 1994;32:46–50. doi: 10.1128/jcm.32.1.46-50.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ricci V, Ciacci C, Zarrilli R, Sommi P, Tummuru M K R, Del Vecchio Blanco C, Bruni C B, Cover T L, Blaser M J, Romano M. Effect of Helicobacter pylori on gastric epithelial cell migration and proliferation in vitro: role of VacA and CagA. Infect Immun. 1996;64:2829–2833. doi: 10.1128/iai.64.7.2829-2833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talley N J, Noack K B. The worldwide prevalence of Helicobacter pylori: asymptomatic infection and clinical states associated with infection in adults. In: Goodwin C S, Worsley B W, editors. Helicobacter pylori: biology and clinical practice. Boca Raton, Fla: CRC Press; 1993. pp. 63–84. [Google Scholar]
- 40.Tee W, Lambert J R, Dwyer B. Cytotoxin production by Helicobacter pylori from patients with upper gastrointestinal tract diseases. J Clin Microbiol. 1995;33:1203–1205. doi: 10.1128/jcm.33.5.1203-1205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Telford J L, Ghiara P, Dell’Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce M F, Censini S, Covaccin A, Xiang Z, Paini E, Montecucco C, Parent L, Rappuoli R. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tummuru M K R, Cover T L, Blaser M J. Mutation of the cytotoxin-associated cagA gene does not affect the vacuolating cytotoxin activity of Helicobacter pylori. Infect Immun. 1994;62:2609–2613. doi: 10.1128/iai.62.6.2609-2613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van de Wouw B A M, de Boer W A, Jansz A R, Roymans R T J, Staals A P G. Comparison of three commercially available enzyme-linked immunosorbent assays and biopsy-dependent diagnosis for detecting Helicobacter pylori infection. J Clin Microbiol. 1996;34:94–97. doi: 10.1128/jcm.34.1.94-97.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veel J F L, van der Hulst R W M, Gerrits Y, Roorda P, Feller M, Dankert J, Tytgat G N J, van der Ende A. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J Infect Dis. 1996;173:1171–1175. doi: 10.1093/infdis/173.5.1171. [DOI] [PubMed] [Google Scholar]
- 45.Vyas S K, Sharpstone D, Treasure J, Fine D, Hawtin P R. Pre-endoscopy screening using serodiagnosis of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1994;6:783–785. [Google Scholar]
- 46.Wang W M, Chen C Y, Jan C M, Chen L T, Perng D S, Lin S R, Liu C S. Long-term follow-up and serological study after triple therapy of Helicobacter pylori-associated duodenal ulcer. Am J Gastroenterol. 1994;89:1793–1798. [PubMed] [Google Scholar]
- 47.Widmer M, de Korwin J D, Aucher P, Thibergue J M, Labigne A, Fauchère J L. Proceedings of the IX International Workshop on Gastroduodenal Pathology and Helicobacter pylori. (Gut 39(Suppl. 2.) 1996. Performance of native and recombinant antigens for Helicobacter pylori serology, abstr. 4C:47. [Google Scholar]
- 48.Xiang Z, Bugnoli M, Ponzetto A, Morgando A, Figura N, Covacci A, Petracca R, Pennatini C, Censini S, Armellini D, Rappuoli R. Detection in an enzyme immunoassay of an immune response to a recombinant fragment of the 128 kilodalton protein (CagA) of Helicobacter pylori. Eur J Microbiol Infect Dis. 1993;12:739–745. doi: 10.1007/BF02098460. [DOI] [PubMed] [Google Scholar]
- 49.Xiang Z, Censini S, Bayeli P F, Telford J L, Figura N, Rappuoli R, Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995;63:94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]