Abstract
Structural modifications in the dendritic morphology of neurons occur following many forms of experience, including exposure to drugs, complex housing, and training in specific behavioral tasks. The present study examined morphological changes in orbitofrontal (OFC) and medial prefrontal cortex (mPFC) neurons of female rats following experience with a variety of social partners or non-social olfactory stimuli. We reasoned that experience with various social partners or olfactory stimuli, and the associated behavioral adaptations, would drive structural modifications in prefrontal cortex neurons engaged by these stimuli. Social experience was manipulated by providing rats with a novel cage-mate or housing the animal with the same cage-mate throughout the study. Similarly, olfactory experience was manipulated by introducing novel, non-social odors in the home-cage or exposing the animals to the same home-cage odor throughout the study. Both forms of experience resulted in altered dendritic morphology in OFC neurons, whereas morphological changes in mPFC were comparatively small and limited to changes in spine density. These observations indicate that OFC and mPFC neurons respond differently to social and non-social olfactory stimulation in adulthood and join the growing body of data illustrating differential effects of experience on structural plasticity in OFC and mPFC.
Keywords: prefrontal cortex, olfaction, social behavior, Golgi analysis
The ability to recognize individual conspecifics is fundamental to mammalian social behavior. Among rodents a primary source of recognition is olfaction, which can convey information about individual identity including sex, social status, and reproductive state (e.g., Brown, 1979). Social recognition memory has been linked to the amygdala and hippocampus (Zinn et al., 2016), however, the role of frontal cortex regions in in olfactory recognition is not well understood. One likely region is the agranular insular (AI) region of the orbitofrontal cortex (OFC). This region receives olfactory input via the piriform cortex as well as through the dorsomedial thalamic nucleus (e.g., Cinelli et al., 1987; Price et al., 1991; Uylings et al, 2003). Neuronal recordings in primates and rodents have shown evidence of olfactory processing in the OFC (e.g., Rolls, 2014; Schoenbaum & Eichenbaum, 1995), and olfactory discrimination ability is correlated with the volume of OFC in humans (Seubert et al., 2013). Lesions to OFC including the AI region are also known to disrupt olfactory learning (e.g., Schoenbaum et al., 2003) and social behavior in rats, cats, and humans (e.g., de Bruin et al., 1983; Kolb, 1974; Nonneman & Kolb, 1974; Pellis et al., 2006; Rolls et al., 1994). For example, increased social aggression has been observed in rats with lesions of the OFC but not the medial prefrontal cortex (mPFC; Kolb, 1974; Rudebeck et al., 2007). Further, alterations in social investigation (anogenital sniffing) and wrestling behavior associated with prenatal alcohol exposure in the rat have been linked to reductions in expression of the immediate early gene Arc in AI during social encounters (Hamilton et al., 2010), and selective blockade of GluN2BRs in AI decreased wrestling behavior observed in these animals (Bird et al., 2017).
The structure of neurons in the OFC is modified by a wide range of experiences including social play (e.g., Bell et al., 2010; Burelson et al., 2016; Himmler et al., 2013) and sexual behavior (Fiorino, 2003) so we hypothesized that social interaction, and perhaps non-social olfactory experience, could modify neuronal morphology in OFC. Although the medial prefrontal cortex (mPFC) does not have a known role in olfactory behavior, it does play a role in certain aspects of play and social behavior (Bell et al., 2009; Hamilton et al., 2010; Himmler et al., 2014; Kolb, 1974) and object recognition (Barker et al., 2007; Chao, et al., 2016; Decoteau, et al., 2009; Spanswick and Dyck, 2012; but see Ennaceur et al., 1997). Thus, repeated experiences with conspecifics or non-social odors could involve recognition processes that engage mPFC neurons. Thus, we also examined neuronal structure in mPFC for purposes of comparison.
The current study included experiments designed to assess the effects of varied experience in domains that engage frontal cortex neurons. In Experiment 1 study female rats were paired with new cage-mates every 2 days for 40 days and were compared to rats that were paired with the same animals over that time. In Experiment 2 the effects of varied experience with non-social olfactory experience were evaluated. Novel, non-social odors were placed in cages with pairs of female rats for 48 hours and changed over 40 days as in Experiment 1. The results of this manipulation were compared to a condition in which the same odor was present in the cage over the course of the experiment. At the end of the experiments the brains were extracted and processed for Golgi-Cox staining and subsequent analysis of dendritic morphology. The hypothesis that varied experience within the social (Experiment 1) and non-social olfactory (Experiment 2) domains would alter dendritic morphology of pyramidal neurons in OFC and mPFC was tested.
Procedures
Subjects
A total of 24 female Long–Evans (hooded) rats bred at the University of Lethbridge vivarium (originally from Charles River stock, St. Constant, Quebec) were used in this study. All rats weighed between 200–250 g at the beginning of the study. Prior to experimentation, all rats were housed in groups of 3 in plastic hanging cages. The animal colony rooms were maintained on a 12–12 hr light–dark cycle with the light cycle beginning at 0700. All experimental procedures and housing conditions were approved by the University of Lethbridge Institutional Animal Care and Use Committee.
Behavioral Methods
Experiment 1: Social Experience
At postnatal day 90 female rats were housed in pairs in standard plastic hanging cages. All rats were of comparable body weight. At 48 h intervals over a period of 40 days rats were removed from their cages and moved to clean cages with fresh bedding, food, and water. For rats in the Control condition the cage-mate remained the same. For rats in the Social condition the cage-mate was changed at the time of the cage change. All cage changes occurred approximately 2-3 h after the onset of the light cycle. The sequence of cage-mates for rats in the Social condition was determined randomly without replacement such that each animal was housed with each of the 5 possible cage-mates at least once before being housed with a particular cage-mate again. Over the course of the 40 days, each rat in the Social condition was paired with each possible cage-mate 4 times. Interactions were recorded during the first 10 minutes after animals were first placed together in the home cage (i.e., for each of the 20 cage changes). The number of times rats engaged anogenital sniffing and wrestling was quantified. For analysis purposes wrestling was defined as any interaction during which the partners were engaged in tumbling/rolling or pinning the partner down, and anogenital sniffing was defined as any instance of one animal making snout contact with the anogenital region of the partner. Videos illustrating these behaviors are available in (Hamilton, Magcalas, et al., 2014). Average behavioral measures during the final cage change were analyzed in separate analyses of variance (ANOVAs) with group as a between-subjects factor.
Experiment 2: Non-social olfactory experience
At postnatal day 90 female rats were housed in pairs in standard plastic hanging cages with a cage-mate of comparable weight. At 48 h intervals over a period of 40 days rats were removed from their cages and moved to clean cages with fresh bedding, food, and water. Olfactory stimuli were included by adding 2 drops of scented oil to a cotton swab placed inside a 50 ml conical tube with a small hole in one end. The tube was attached to the outside of the wire top and remained in place for the entire 48 h period (i.e., the odors remained for a total of 2 days). Three odors were utilized (vanilla, patchouli, lavender). For rats in the Control condition the odor remained the same throughout the 40-day period. For rats in the Olfactory experience condition the odor was changed at the time of the cage change. All cage changes occurred approximately 2-3 h after the onset of the light cycle. The sequence of odors for rats in the Olfactory condition was determined randomly without replacement such that each pair of animals experienced each of the 3 possible odors at least once before being experiencing a particular odor again. Over the course of the 40 days, each pair of rats in the Olfactory condition experienced each odor 6-7 times.
Anatomical analyses
At the completion of behavioral testing the rats received an overdose of sodium pentobarbital solution (i.p.) and were intracardially perfused with a 0.9% saline solution. The brains were extracted and placed in bottles containing a Golgi-Cox solution. The brains remained in this solution, in total darkness, for 14 days. Following removal of the brains from the Golgi-Cox solution, they were placed into a 30% sucrose solution, where they remained for at least 3 days. The brains were then sectioned at 200 µm on a Vibratome and mounted on gelatin-coated slides for staining, as outlined by Gibb and Kolb (1998).
Analysis consisted of layer II/III neurons selected from the mPFC, specifically Zilles Cg3 region, which corresponds to prelimbic cortex, and the orbital frontal cortex, defined as the dorsal agranular insular cortex (AID, layer II/III), as described by Zilles (1985). A microscope mounted Camera Lucida was used to trace individual Golgi-Cox stained neurons at 250X, and terminal segments for spine density estimation traced at 1500X. All tracings were done by an individual who was blind to experimental conditions.
In order to be included in the data analysis, the dendritic trees of pyramidal cells had to be well impregnated and not obscured with blood vessels or astrocytes, and the neuron had to appear to be largely intact and completely visible in the plane of section. Measures of neuron morphology included quantification of total dendritic branching and total length estimated separately for basilar and apical dendritic fields. Total branch counts were estimated using the procedure of Coleman and Riesen (1968). Branch order was determined for the basilar dendrites such that branches originating at the cell body were first order; after one bifurcation, second order; and so on. For apical dendrites the primary apical branch (from the apex of the soma to the major apical branch prior to the apical tuft) was designated order zero (not counted) and all branches emerging from the primary apical were designated as first order. Dendritic length was estimated using a Sholl analysis (Sholl, 1981) of ring intersections. The number of intersections of dendrites with a series of concentric spheres at 25 μm intervals from the center of the cell body was counted for the apical (inclusive of the primary apical dendrite) and basilar fields of each cell. Total dendritic length (in micrometers) for each field was estimated by multiplying the number of intersections by 25 and converted to mm. For each individual brain 10 neurons were drawn in each region, with 5 tracings per hemisphere. Statistical analyses with experimental group as a single between-subjects factor were conducted on the mean data for each region averaged across hemispheres.
Spine density was estimated by tracing a third-order dendritic branch spanning from the branch point to the terminal tip of at least 50 µm in length (> 80 µm on average) and marking spine locations at a final magnification of 1500X. A total of five segments per hemisphere were sampled from both the apical and basilar dendrites. Spine density was calculated using the number of spines per 10 µm following the determination of the exact length of the dendritic segment. Total spine numbers were estimated by multiplying the number of total third order branches by the number of spines on the sampled third order branches. Statistical analyses comparing the Control and Social groups were conducted on spine density and spine number in each dendritic field after averaging across hemispheres. Spine density and number were not measured in the olfactory experiment (2) because the brains from this experiment were left for about 2 years after the dendrites were drawn and there was significant fading of the spines over that time making the measurement unreliable.
Results
Behavioral observations
Each time cage-mates were changed there was intense social interaction, whereas animals placed back in the same cage with the same partner showed much less social interaction. Measurement of anogenital sniffing and wrestling showed a significant increase in the Social change group as shown in Figure 1. An ANOVA on anogenital sniffing showed a main effect [Social > Control; F(1,10)=16.9, p=.002, ] as did the effect of wrestling [Social > Control; F(1,10)=5.66, p=.039, ]. There were no instances of fighting observed.
Figure 1.
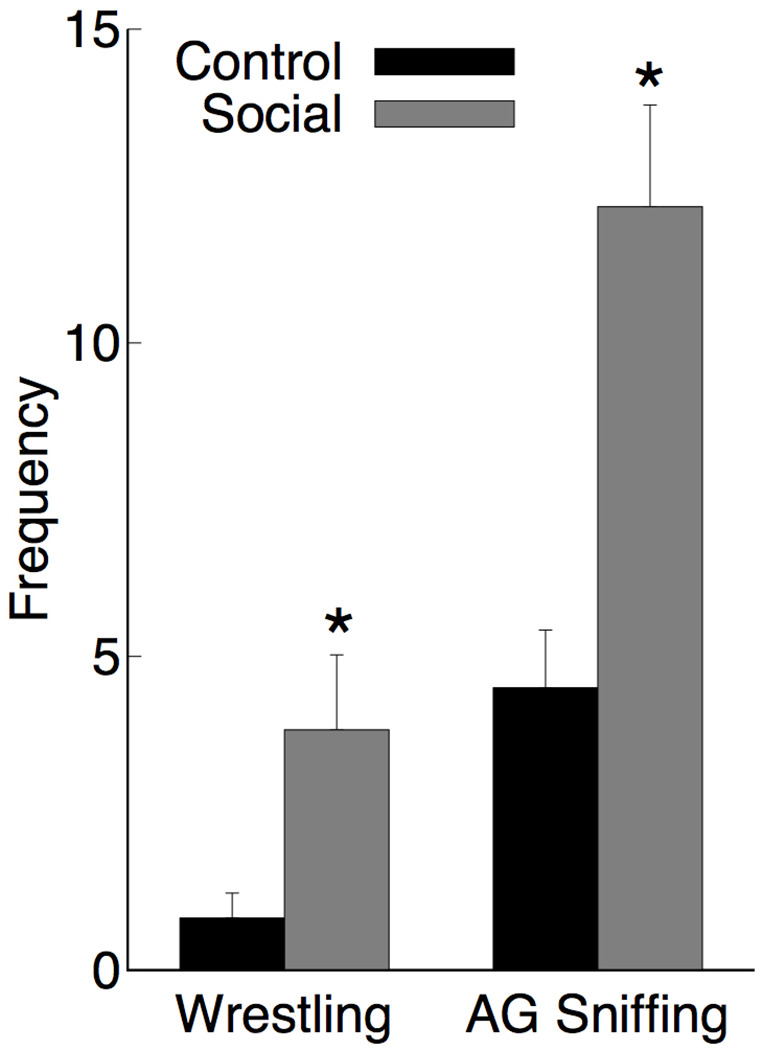
Mean frequency + SEM rats in the control and social experience groups engaged in wrestling and anogenital (AG) sniffing during the first 10 minutes following a change in cage-mate (social) or the reintroduction of the regular cage-mate (control). N = 6 for each group, * p < 0.05.
Anatomical analyses
The Golgi-Cox staining was of high quality and similar to what we have reported and illustrated in previous studies (e.g., Kolb et al., 2003b). Representative camera lucida drawings of layer II/III pyramidal neurons are shown in Figure 2.
Figure 2.
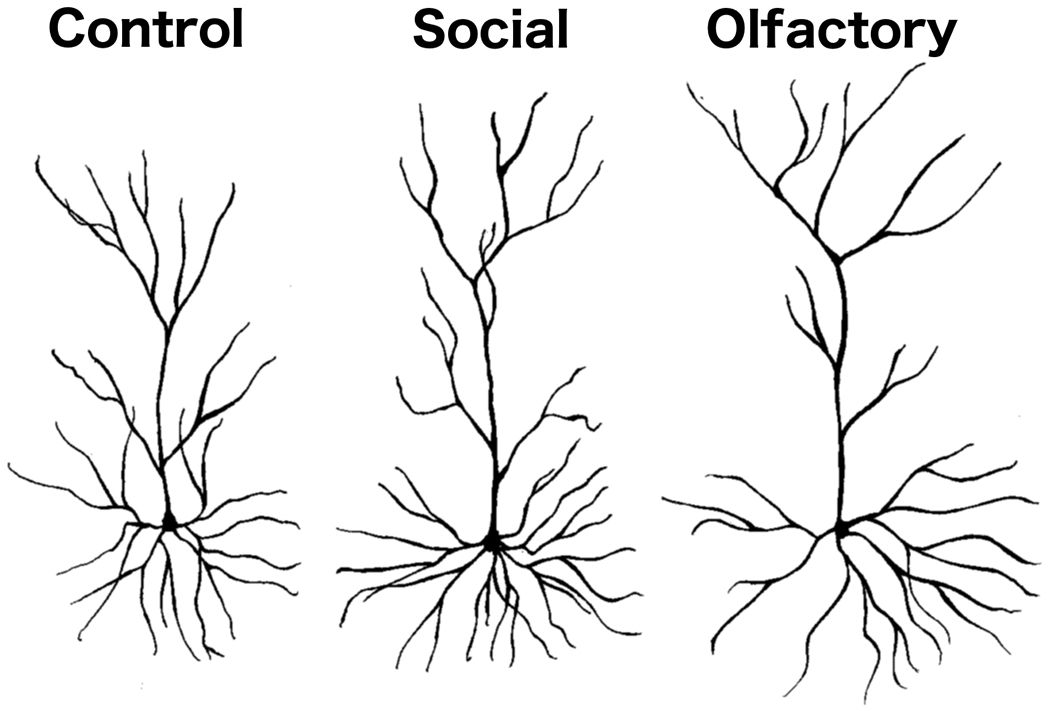
Representative camera lucida drawings of layer II/III OFC pyramidal neurons from rats in the control and social experience groups of Experiment 1 and the non-social olfactory experience group of Experiment 2.
Experiment 1
The results and statistics are summarized in Table 1. The social manipulation increased spine number in the apical dendrites of OFC neurons, but did not alter length, branching, or spine density in the apical field. Social experience also increased dendritic length and branching in the basilar dendrites in OFC, but decreased spine density. The combined increase in dendritic length and decreased spine density resulted in no overall modification in basilar spine number in OFC. In contrast, in mPFC there were no effects on the basilar dendrites and an increase only in spine density within the apical field.
Table 1.
Summary of Social Interaction Anatomy
| Control | Social | F-ratio, p-value, Effect Size | |
|---|---|---|---|
| OFC Apical | |||
| Branches | 22.72±.84 | 25.48±1.5 | F(1,10)=2.59, p=0.14, |
| Length | 61.33±1.66 | 66.66±4.42 | F(1,10)=1.27, p=0.29, |
| Spine Density | 9.28±.047 | 8.85±0.28 | F(1,10)=0.62, p=0.47, |
| Spine Number | 391.02±21.72 | 470.46±26.91 | F(1,10)=5.28, p=0.04+, |
| OFC Basilar | |||
| Branches | 36.87±0.45 | 42.35±1.06 | F(1,10)=22.92, p=0.001*, |
| Length | 86.32±1.80 | 96.78±2.53 | F(1,10)=11.33, p=0.007*, |
| Spine Density | 10.94±0.30 | 9.80±0.24 | F(1,10)= 8.88, p=0.014+, |
| Spine Number | 1112±39.06 | 1122±49.16 | F(1,10)= 0.23, p=0.88, |
| mPFC Apical | |||
| Branches | 25.29±1.74 | 25.79±0.30 | F(1,10)=0.08, p=0.78, |
| Length | 115.98±7.82 | 117.84±3.16 | F(1,10)=0.05, p=0.83, |
| Spine Density | 4.39±0.22 | 5.57±0.48 | F(1,10)=4.99, p=0.05*, |
| Spine Number | 339.16±39.52 | 372.56±30.26 | F(1,10)=0.45, p=0.52, |
| mPFC Basilar | |||
| Branches | 41.85±0.76 | 42.69±1.12 | F(1,10)=0.38, p=0.55, |
| Length | 130.63±8.19 | 145.30±4.0 | F(1,10)=2.59, p=0.14, |
| Spine Density | 6.45±0.26 | 6.91±0.33 | F(1,10)=1.18, p=0.30, |
| Spine Number | 785.75±49.29 | 724.07±41.19 | F(1,10)=0.92, p=0.36, |
Values represent mean±SEM for measures of total branch counts (Branches), total dendritic length (Length mm), spine density per 10μm , and total spine number estimated on third order branches in the Apical and Basilar dendritic fields for each brain Control (n=6) and Social (n=6) groups of Experiment 1. Values are means of 10 observations per brain (5 per hemisphere). Test statistics, p values, and effect sizes were obtained from ANOVA with group as a single factor.
Social>Control;
Control>Social
Additional analyses were performed to further address modifications to OFC dendritic fields and spines in the social group. Mean counts of individual branch orders (1-6+) and the outcome of statistical tests are shown in Table 2. Compared to controls, there were significantly more third order branches in the apical and basilar dendritic fields of AID neurons, and significantly more fourth order branches in the basilar dendritic field in the social group. Measurements of the length of individual third order branches on which spines counts were performed revealed no significant group differences for apical [M(SEM)CONTROL = 83.52(2.84)μm; M(SEM)SOCIAL = 84.38(1.99)μm] or basilar [M(SEM)CONTROL = 90.01(2.22)μm; M(SEM)SOCIAL = 88.58(1.56)μm] dendritic fields [both ps > 0.61]. These observations suggest that the 12.1% increase in overall basilar dendritic length in the social group was primarily due to the 14.9% increase the number of branches.
Table 2.
Summary of Branch Counts by Branch Order for Social Experience
| Control | Social | F-ratio, p-value, and Effect Size | |
|---|---|---|---|
| OFC | |||
| Apical Branch 1 | 5.52±.17 | 5.78±.36 | F(1,10)=0.46, p=0.52, |
| Apical Branch 2 | 7.43±.31 | 8.07±.56 | F(1,10)=1.00, p=0.34, |
| Apical Branch 3 | 5.08±.26 | 6.33±.40 | F(1,10)=6.85, p=0.03*, |
| Apical Branch 4 | 2.88±.24 | 3.15±.44 | F(1,10)=0.29, p=0.60, |
| Apical Branch 5 | 1.27±.16 | 1.52±.15 | F(1,10)=1.30, p=0.28, |
| Apical Branch 6+ | 0.53±.11 | 0.63±.14 | F(1,10)=0.31, p=0.59, |
| Basilar Branch 1 | 5.74±.15 | 5.87±.25 | F(1,10)=0.21, p=0.66, |
| Basilar Branch 2 | 9.90±.32 | 10.63±.18 | F(1,10)=3.90, p=0.08, |
| Basilar Branch 3 | 11.33±.36 | 12.93±.43 | F(1,10)=8.01, p=0.02*, |
| Basilar Branch 4 | 6.55±.48 | 8.42±.61 | F(1,10)=5.83, p=0.04*, |
| Basilar Branch 5 | 2.45±.44 | 3.60±.44 | F(1,10)=4.58, p=0.06, |
| Basilar Branch 6+ | 0.90±.30 | 0.90±.22 | F(1,10)=0.00, p=1.00, |
| mPFC | |||
| Apical Branch 1 | 5.13±.25 | 4.79±.12 | F(1,10)=1.43, p=0.26, |
| Apical Branch 2 | 7.79±.48 | 7.42±.17 | F(1,10)=0.54, p=0.48, |
| Apical Branch 3 | 6.54±.55 | 6.46±.15 | F(1,10)=0.02, p=0.89, |
| Apical Branch 4 | 4.33±.87 | 5.29±.34 | F(1,10)=1.05, p=0.33, |
| Apical Branch 5 | 1.00±.37 | 1.96±.39 | F(1,10)=3.18, p=0.11, |
| Apical Branch 6+ | 0.08±.08 | 0.29±.15 | F(1,10)=1.47, p=0.25, |
| Basilar Branch 1 | 6.06±.27 | 5.48±.08 | F(1,10)=4.18, p=0.07, |
| Basilar Branch 2 | 9.96±.47 | 8.96±.15 | F(1,10)=4.08, p=0.07, |
| Basilar Branch 3 | 13.04±.39 | 12.79±.22 | F(1,10)=0.31, p=0.59, |
| Basilar Branch 4 | 9.25±.72 | 9.96±.28 | F(1,10)=0.83, p=0.38, |
| Basilar Branch 5 | 3.38±.33 | 3.92±.57 | F(1,10)=0.67, p=0.43, |
| Basilar Branch 6+ | 0.88±.36 | 0.88±.25 | F(1,10)=0.00, p=1.00, |
Values represent mean±SEM for measures of total branch counts (Branches) for each branch order analyzed (1-6+) in the apical and basilar dendritic fields of OFC and mPFC for Control (n=6) and Social (n=6) groups of Experiment 1. Values are means of 10 observations per brain (5 per hemisphere). Test statistics, p values, and effect sizes were obtained from ANOVA with group as a single factor.
Social>Control.
The increase in overall basilar length coupled with the decrease in spine density complicates the estimation of the effect of social experience on total spine number. Spine density is lowest at the terminal tip and increases more proximal to the branch point. The dendritic branches sampled for the summary measures of spine density (Table 1) were > 80 μm in length and spanned from the terminal tip back to the branch point, thus, it is possible that spine density changes following social experience varied as a function of location on the dendritic branch. Because this, coupled with the observation of increased dendritic length, could complicate interpretation of the summary spine density measures To further characterize the effects of social experience on spines at various distances from the tip, the spine density within 10μm of the terminal tip were counted, along with spine density within two segments more distal from the tip and more proximal to the branch point (30-40μm and 60-70μm from the terminal tip; see Figure 3). We note that all these dendritic segments were calculated directly from the same drawings used for spine density summary measurement for which total reduced spine density measures were observed. Mean spine densities were lower in the terminal tip segment of both apical and basilar branches and group differences (Social < Control) were only observed in the terminal segment 0-10μm from the terminal tip [F(1,10)=13.57, p=0.004, ]. No other significant group differences were observed [all ps > 0.16]. It is noted that basilar field spine density for the social group was numerically lower in the 30-40μm segment, but comparable to that of controls in the 60-70μm segment. Coupled with the observation that social experience significantly increased third order branches, these observations prompted estimations of total spine number calculated as the product of the number of third order branches and spines density per segment (see Figure 3). In the apical field there was a group effect (Social > Control) for the 30-40μm [F(1,10)=9.23, p=0.012, ] and 60-70μm segments [F(1,10)=7.87, p=0.019, ]. In the basilar field there was a group effect (Social > Control) for the 60-70μm segments [F(1,10)=7.12, p=0.023, ]. These outcomes suggest an overall increase in spine number in the social group that was masked by decreases in spine density at the most distal segment of the terminal tip.
Figure 3.
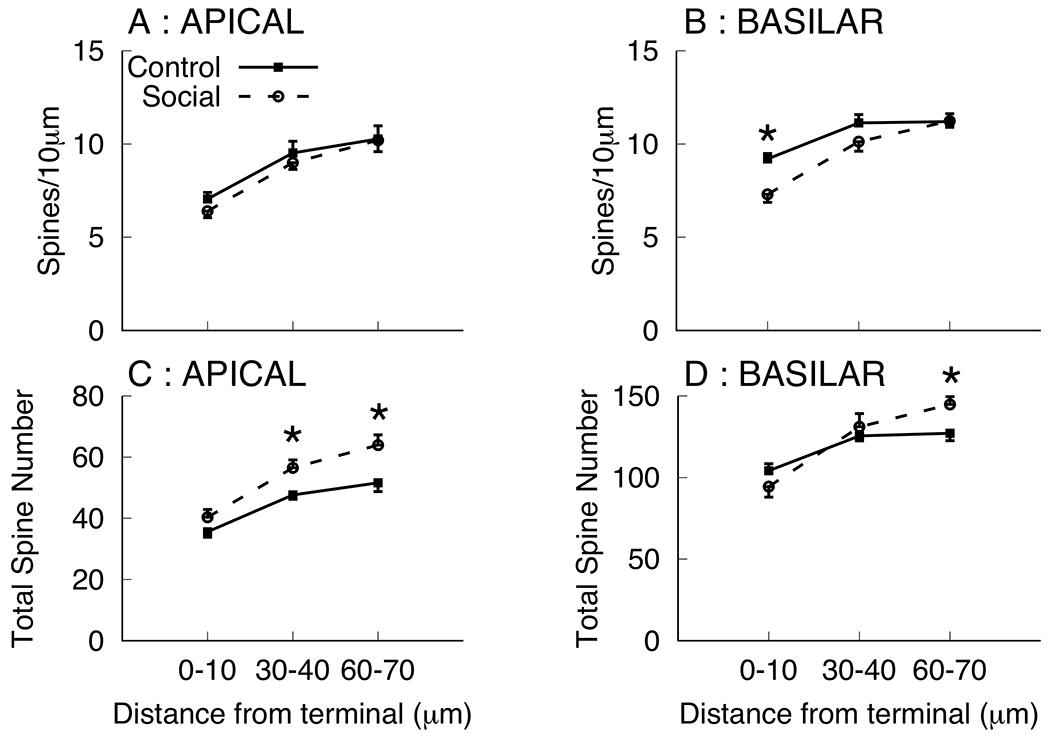
Mean spine density per 10μm (A, B) and total spine number (C, D) in dendritic segments 0-10, 30-40, and 60-70 μm from the terminal tip on third order branches in apical (A, C) and basilar (B, D) dendritic fields for the control and social groups of Experiment 1. Total spine numbers were calculated as the product of the total number of third order branches and mean spine number per segment. * p < 0.05.
Experiment 2
The results and statistics are summarized in Table 3. The olfactory manipulation resulted in an increase in dendritic length in both the apical and basilar fields in OFC, but had no effect on branching in either area. Thus, the effects were similar to those of Experiment 1 in that dendritic morphology changes were larger in OFC than mPFC, however, the pattern of effects within OFC observed in the two experiments differed; the effects of olfactory experience affected only dendritic length and not branching in OFC. Further, the sole effect in mPFC was a significant reduction in dendritic branching in the apical dendrites, which was not observed in Experiment 1.
Table 3.
Summary of Olfactory Stimulation Anatomy
| Control | Olfactory | Test Statistic, p-value, and Effect Size | |
|---|---|---|---|
| OFC | |||
| Apical Branches | 18.45±.080 | 17.98±0.52 | F(1,10)=0.25, p=0.63, |
| Apical Length | 66.49±1.28 | 71.69±1.00 | F(1,10)=10.28, p=0.009*, |
| Basilar Branches | 31.68±0.42 | 32.68±0.59 | F(1,10)=1.92, p=0.20, |
| Basilar Length | 86.47±3.24 | 96.67±3.33 | F(1,10)=4.80, p=0.053*, |
| mPFC | |||
| Apical Branches | 27.91±1.31 | 23.74±1.30 | F(1,10)=5.05, p=0.05+, |
| Apical Length | 124.47±3.72 | 128.12±2.59 | F(1,10)=0.65, p=0.44, |
| Basilar Branches | 45.10±1.29 | 44.46±1.14 | F(1,10)=0.14, p=0.72, |
| Basilar Length | 159.12±6.00 | 169.84±6.0 | F(1,10)=2.12, p=0.18, |
Values represent mean±SEM for measures of total branch counts (Branches) and total dendritic length (Length) in the Apical and Basilar dendritic fields for each brain Control (n=6) and Olfactory (n=6) groups of Experiment 2. Values are means of 10 observations per brain (5 per hemisphere). Test statistics, p values, and effect sizes were obtained from ANOVA with group as a single factor.
Olfactory>Control;
Control>Olfactory
Discussion
Overall the results showed that both social and non-social olfactory stimulation altered the morphology of the dendritic fields in agranular insular region of OFC, but had little effect on the neurons in the prelimbic component of mPFC. The effects in the non-social olfactory experiment were smaller but in the same general direction as the social stimulation. One explanation for these effects is that the social experience likely had an effect on general activity, but we do not believe this explains the neuronal changes because extensive wheel running (Robinson & Kolb, 1999), nose poking for sucrose pellets (Crombag et al., 2005), or complex housing (Kolb et al., 2003a) have no significant effect on neuronal morphology in the mPFC or OFC of adult rats. Furthermore, Marks et al. (2017) have shown that social play alters socio-cognitive function in ground squirrels, but locomotor play does not, suggesting that it is the social aspect of experience responsible for changes in prefrontal regions rather than activity per se.
Social experience with varied cage-mates was associated with robust behavioral changes including increased wrestling and investigation in the form of anogenital sniffing. These outcomes corresponded closely to the morphological changes in OFC neurons. An exploratory analysis of correlations between dendritic morphology and social behavior (not shown) indicated that the majority of the relationships between behavior and dendritic morphology were driven by variability related to group membership (animals in the Social condition engaged in more instances of these behaviors and showed changes in OFC dendritic morphology). This was confirmed in separate stepwise regression models for which behavioral outcomes (anogenital sniffing or wrestling) were predicted using group and the dendritic morphology measures as predictors. In both cases, group emerged as the only significant predictor of behavior. Thus, it seems most meaningful to inquire about relationships between behavior and morphology within groups. Using data from the 6 animals in each group we detected one significant effect between OFC basilar spines and wrestling in the Social group (r = 0.82, p = 0.046), while none of the other relationships were significant (in all cases p > 0.06). The values, however, were in the expected direction and ranged from r = −.63 (anogenital sniffing and basilar spines in OFC) to r = 0.79 (wrestling and apical length). Considering the small sample sizes within groups future studies with larger numbers could more thoroughly address these relationships.
In view of the known effects of OFC lesions on both social and olfactory behavior in rats (de Bruin et al., 1983; Kolb, 1974; Nonneman & Kolb, 1974; Pellis et al., 2006; Rolls et al., 1994; Schoenbaum et al., 2003) it is likely that the morphological changes are a direct result of social and olfactory experiences, although we do note that it is difficult to ascribe the changes in the social experiment to social interaction independent of the concurrent social olfactory stimulation. Importantly, the goal of Experiment 2 was not to serve as a control for social olfactory experience in Experiment 1, but to independently evaluate the effects of varied olfactory experience on neuron morphology in frontal cortex. Future experiments utilizing social odors in a similar manner to that utilized with non-social odors in Experiment 2 are needed to evaluate the possibility that the results of Experiment 1 are attributable to the olfactory experiences associated with the social experience manipulation. At present, however, several studies lead us to suggest that the social rather than olfactory component is central to the neuronal changes in OFC. First, OFC lesions do not interfere with olfactory discriminations, odor-guided navigation, or the discrimination of individuals or sex (e.g., Petrulis et al., 1998; Wallace et al., 2003). Second, the patterns of morphological changes are different in the two experiences: the social experience affected all measures in the basilar fields, the number of apical third order branches, and spine density in the apical field whereas the non-social olfactory experience altered dendritic length, but not branching, in both the apical and basilar fields. We also note that the increase in basilar dendritic length in OFC neurons in Experiment 1 is largely driven by the increase in number of branches. Although we were not able to provide a measurement of spines in Experiment 2, the significant modifications in dendritic length in OFC neurons suggest modification of morphology in response to varied experience in the olfactory domain. Collectively, the differential observations in the two regions suggest that the neural networks involving the OFC were modified differently in response to the two types of experience.
One somewhat unusual result is that the social experience increased dendritic length and branching in the basilar field of OFC neurons but decreased spine density, which resulted in no overall change in spine number within this dendritic field. Although differential effects of experience on dendritic length and branching versus spine density are not typical, the present observations join an increasing number of reports showing increased length and decreased spine density. We have found similar results for the effects of complex housing in young rats (Kolb et al., 2003b) and with manipulations of the number of cage mates (Bell et al., 2010; Himmler et al., 2018). Kolb, Cioe, and Comeau (2008) observed that rats given training in the Morris water task displayed increased dendritic length and decreased spine density in occipital cortex neurons, whereas motor training resulted in increased length and decreased spine density in motor cortex. The pattern of increased length and decreased spine density was also observed in frontal cortex neurons in rats housed in complex environments during development (Kolb, Gibb, and Gorny, 2003). Fetoni et al. (2013) observed increased dendritic length and decreased spine density in auditory cortex neurons following noise induced hearing loss, and Mychasiuk et al. (2013) observed this pattern in medium spiny neurons of the nucleus accumbens following prenatal nicotine exposure. Thus, the direction of dendritic length and spine density modifications in relation to experience do not always correspond, and the pattern of increased length and decreased spine density appears not to be limited to any particular type of experience or brain region, or type of neuron.,
The detection of opposite changes in length and spine density suggests that the cells under observation are modified in relation to experience, however, at present the reasons underlying this pattern of changes is not understood. One possibility is that the combination of reduced spine density and increased length results in no net change in synaptic connections, or alternatively, that some synapses are gained and others are lost in response to experience. Indeed, within the basilar field of OFC neurons there were no overall changes in spine number estimated on third order branches. Another possibility is that the new growth in the terminal segments of dendritic arbors could be occupied by fewer synaptic connections that are selective to a restricted set of afferents and/or more specialized for particular functions. Investigation of the distribution of spines in Experiment 1 revealed that the overall decrease in spine density on third order basilar branches in OFC observed in the social group was primarily due to reductions in spine number only at the most distal 10 μm of the terminal tips on branches that were sampled, whereas numbers in segments of the branch more proximal to the branch point were comparable for each group. Because there were significantly more third order branches in the social group, the estimate of total spine numbers were significantly higher in the segments more proximal to the branch point and comparable for the terminal tips, suggesting that most dendritic segments actually underwent an increase in spine number. Further, there were significant increases in apical spine numbers on third order branches, which resulted from numerical (non-significant) overall increases in branches, length, and spine density. Overall, these observations suggest that social experience actually increased overall spine numbers, and for the basilar field where length and spine density were changed in opposite direction these increases may be masked by the effects localized to most distal portion of the terminal tip. An important cautionary note regarding interpretation of decreases in estimates of spine density is also apparent. Although the summary measures of spine density taken here have been utilized in a large number of publications, the present observations indicate that sampling of any restricted set of dendritic segments likely does not provide an accurate measure of total spine numbers reflective of changes in the whole cell. Perhaps the only way to definitively achieve such estimates would be to quantify all spines for each neuron, however, this would require counting of thousands of spines per neuron and would still necessarily be an underestimate as all spines cannot be visualized. In cases where length and density do not covary in the same direction the present findings suggest the need for additional assessment of spine density as a function of location on the branch rather in addition to more global assessments of spine number.
The experience-dependent changes observed in the current study can be viewed the context of the broader field of experience-dependent changes in mPFC and OFC. A form of experience that generally has the largest effect across the cerebral hemispheres is complex housing (e.g., Sirevaag & Greenough, 1988). Curiously, as noted earlier, complex housing has very little chronic effect on either mPFC or OFC of adult rats (Kolb et al., 2003a), although there appears to be a transient effect on these regions (Comeau et al., 2010). In contrast, however, pyramidal cells in prefrontal regions are significantly altered by a range of specific experiences that have very little effect on other cortical regions including psychoactive drugs (Robinson & Kolb, 2004), stress (Kolb & Gibb, 2015; McEwen & Morrison, 2013), play (Bell et al., 2010; Himmler et al., 2013) and gonadal hormones (e.g., Garrett and Wellman, 2009; Kolb & Stewart, 1991; Koss et al., 2015; Markham & Juraska, 2002; Willing & Juraska, 2015). One characteristic of these experiences is that the changes in the mPFC and OFC are generally in the opposite direction. For example, psychomotor stimulants increase dendritic arbor and spine density in mPFC and decrease these outcomes in OFC (e.g., Crombag et al., 2005), whereas stress or opiate exposure has the opposite effect (Liston et al., 2006; Robinson et al., 2002). Although the effects of experience in the current study are not opposite, they are largely localized to just the OFC. These area-dependent differences in prefrontal regions are not surprising given that the afferents and efferents of these regions are strikingly different (e.g., Uylings et al., 2003).
The observations that stress alters prefrontal cortex dendritic morphology (Liston et al., 2006) and that social instability is a stressor for female rats (Haller et al., 1999; Herzog et al., 2009) prompts consideration of whether the present results with social experience could be attributed to chronic stress. In the present study we did not obtain direct measures of stress, and therefore cannot rule out the potential impact of stress on the behavioral and morphological outcomes reported here. We note, however, that the effects observed by Liston et al. (2006) with restraint stress yielded increased dendritic morphology in OFC neurons, while robust decreases were observed in mPFC. In the present study we observed robust effects only in OFC, and a single modest increase in apical spine density in mPFC. Further, although social partners were routinely changed in the present experiment it is not clear that this induced chronic stress, as we observed no instances of fighting nor injuries during social interaction. Studies that have demonstrated stress associated with social instability (e.g., Herzog et al., 2009) have utilized unpredictable isolation or overcrowding, whereas in the manipulations in the present study only involved varying the identity of a familiar cage-mate or non-social odor. Collectively, these considerations suggest that if stress was a factor in the outcomes it was likely less significant than the stressors utilized in previous studies. Future studies examining the direct impact of the social housing manipulation utilized here on stress responses are needed to address this issue.
Finally, we would be remiss if we did not suggest a mechanism for the neuronal changes related to social and olfactory stimulation. Both experience (stress) and psychomotor stimulants alter gene expression differently in mPFC and OFC (Mychasiuk et al., 2013; 2016). Olfactory and social experience would alter (increase or decrease) neuronal activity and this in turn likely alters gene expression differentially in the mPFC and OFC. Hamilton et al. (2010) reported increased expression of c-fos mRNA in both mPFC and OFC following social interaction, however, increased expression of mRNA for activity-related cytoskeletal protein (Arc) was only observed in the agranular insular component of OFC. Considering that c-fos provides an index of general neuronal activity and Arc is linked to synaptic plasticity, these findings suggest that multiple regions of prefrontal cortex are engaged by social interaction, but plastic changes in response to social experience may primarily involve modifications to OFC. To our knowledge, the effects of olfactory experience on differential gene expression in mPFC and OFC have not been explored, however, we would expect to observe a comparable pattern of outcomes.
References
- Barker GRI, Bird F, Alexander V, & Warburton EC (2007). Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. Journal of Neuroscience, 27, 2948–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell HC, Pellis SM, & Kolb B (2010). Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortex. Behavioural Brain Research, 207, 7–13. [DOI] [PubMed] [Google Scholar]
- Bell HC, McCaffrey D, Forgie ML, Kolb B, & Pellis SM (2009). The role of the medial prefrontal cortex in the play fighting in rats. Behavioral Neuroscience, 123, 1158–1168. [DOI] [PubMed] [Google Scholar]
- Bird CW, Barto D, Magcalas CM, Rodriguez CI, Donaldson T, Davies S, . . . Hamilton DA (2017). Ifenprodil infusion in agranular insular cortex alters social behavior and vocalizations in rats exposed to moderate levels of ethanol during prenatal development. Behavioural Brain Research, 320, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE (1979). Mammalian Social Odors: A Critical Review. In Rosenblatt JS, Hinde RA, Beer C, & Busnel M-C (Eds.), Advances in the Study of Behavior (Vol. 10, pp. 103–162): Academic Press. [Google Scholar]
- Burleson CA, Pedersen RW, Seddighi S, DeBusk LE, Burghardt GM, & Cooper MA (2016). Social play in juvenile hamsters alters dendritic morphology in the medial prefrontal cortex and attenuates effects of social stress in adulthood. Behavioral Neuroscience, 130, 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao OY, Huston JP, Li JS, Wang AL, & Silva MAD (2016). The medial prefrontal cortex-lateral entorhinal cortex circuit is essential for episodic-like memory and associative object-recognition. Hippocampus, 26, 633–645. [DOI] [PubMed] [Google Scholar]
- Cinelli AR, Ferreyra-Moyano H, & Barragan E (1987). Reciprocal functional connections of the olfactory bulbs and other olfactory treated areas with the prefrontal cortex. Brain Research Bulletin, 19, 651–661. [DOI] [PubMed] [Google Scholar]
- Coleman PD, & Riesen AH (1968). Environmental effects on cortical dendritic fields. I. Rearing in the dark. Journal of Anatomy, 102, 363–374. [PMC free article] [PubMed] [Google Scholar]
- Comeau W, McDonald R, & Kolb B (2010). Learning-induced structural changes in the prefrontal cortex. Behavioural Brain Research, 214, 91–101. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Gorny G, Li Y, Kolb B, & Robinson TE (2005) Opposite Effects of Amphetamine Self-administration Experience on Dendritic Spines in the Medial and Orbital Prefrontal Cortex. Cerebral Cortex, 15, 341–348. [DOI] [PubMed] [Google Scholar]
- Debruin JPC, Vanoyen HGM, & Vandepoll N (1983). Behavioral changes following lesions of the orbital prefrontal cortex in male rats. Behavioural Brain Research, 10, 209–232. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, McElvaine D, Smolentzov L, & Kesner RP (2009). Effects of rodent prefrontal lesions on object-based, visual scene memory. Neurobiology of Learning and Memory, 92, 552–558. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, & Aggleton JP (1997). Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental Brain Research, 113, 509–519. [DOI] [PubMed] [Google Scholar]
- Fetoni AR, De Bartolo P, Eramo SL, Rolesi R, Paciello F, Bergamini C, Fato R, Paludetti G, Petrosini L, & Troiani D (2013). Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: cochlear and cortical responses after an increase in antioxidant defense. Journal of Neuroscience 33, 4011–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorino D & Kolb B (2003). Sexual experience leads to long-lasting morphological changes in male rat prefrontal cortex, parietal cortex, and nucleus accumbens neurons. Society for Neuroscience Abstracts, 29. 402.3. [Google Scholar]
- Garrett JE, and Wellman CL (2009). Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience 162, 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb R & Kolb B (1998) A method for vibratome sectioning of Golgi-Cox stained whole rat brain. Journal of Neuroscience Methods, 79, 1–4. [DOI] [PubMed] [Google Scholar]
- Haller J, Fuchs E, Halász J, & Makara GB (1999). Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Research Bulletin, 50, 33–39. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Candelaria-Cook FT, Akers KG, Rice JP, Maes LI, Rosenberg M, Valenzuela CF, and Savage DD (2010). Patterns of social-experience-related c-fos and Arc expression in the frontal cortices of rats exposed to saccharin or moderate levels of ethanol during prenatal brain development. Behavioural Brain Research, 214, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Magcalas CM, Barto D, Bird CW, Rodriguez CI, Fink BC, Pellis S Davies SM, and Savage DD (2014). Moderate Prenatal Alcohol Exposure and Quantification of Social Behavior in Adult Rats. Jove-Journal of Visualized Experiments, 94, e52407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmler BT, Pellis SM, & Kolb B (2013). Juvenile play experience primes neurons in the medial prefrontal cortex to be more responsive to later experiences. Neuroscience Letters, 556, 42–45. [DOI] [PubMed] [Google Scholar]
- Himmler BT, Mychasiuk R, Nakahashi A, Himmler SM, Pellis SM & Kolb B (2018). Juvenile social experience and differential age-related changes in the dendritic morphologies of subareas of the prefrontal cortex in rats. Synapse, 72 (e22022), 1–9. [DOI] [PubMed] [Google Scholar]
- Himmler BT, Bell HC, Horwood L, Harker A, Kolb B, & Pellis SM (2014). The role of the medial prefrontal cortex in regulating inter-animal coordination of movements. Behavioral Neuroscience, 128, 603–613. [DOI] [PubMed] [Google Scholar]
- Herzog CJ, Czéh B, Corbach S, Wuttke W, Schulte-Herbrüggen O, Hellweg R, Flügge G, & Fuchs E (2009). Chronic social instability stress in female rats: a potential animal model for female depression. Neuroscience, 159, 982–992. [DOI] [PubMed] [Google Scholar]
- Kolb B (1974). The social behavior of rats with chronic prefrontal lesions. Journal of Comparative and Physiological Psychology, 87, 466–474. [DOI] [PubMed] [Google Scholar]
- Kolb B, Cioe J, & Comeau W (2008). Contrasting effects of motor and visual spatial learning tasks on dendritic arborization and spine density in rats. Neurobiology of Learning and Memory, 90, 295–300. [DOI] [PubMed] [Google Scholar]
- Kolb B, & Gibb R (2015) Plasticity in the prefrontal cortex of adult rats. Frontiers in Cellular Neuroscience, 9:15. doi: 10.3389/fncel.2015.00015. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gibb R & Gorny G (2003a) Experience-dependent changes in dendritic arbor and spine density in neocortex vary with age and sex. Neurobiology of Learning and Memory, 791, 1–10. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Sonderpalm A, & Robinson TE (2003b) Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse, 48, 149–153. [DOI] [PubMed] [Google Scholar]
- Kolb B, & Stewart J (1991). Sex-related differences in dendritic branching of cells in the prefrontal cortex of rats. Journal of Neuroendocrinology, 3, 95–99. [DOI] [PubMed] [Google Scholar]
- Koss WA, Lloyd MM, Sadowski RN, Wise LM, & Juraska JM (2015). Gonadectomy Before Puberty Increases the Number of Neurons and Glia in the Medial Prefrontal Cortex of Female, but Not Male, Rats. Developmental Psychobiology, 57, 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. (2006). Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. Journal of Neuroscience, 26, 7870–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, and Juraska JM (2002). Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiology of Aging, 23, 579–588. [DOI] [PubMed] [Google Scholar]
- Marks KA, Vizconde DL, Gibson ES, Rodriguez JR, & Nunes S (2017). Behavior and responses to novel situations in juvenile ground squirrels. Mammalogy, 98, 1202–1210. [Google Scholar]
- McEwen BS, and Morrison JH (2013). The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron, 79, 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Muhammad A, Gibb R, & Kolb B (2013). Long-term alterations to dendritic morphology and spine density associated with prenatal exposure to nicotine. Brain Research, 1499, 53–60. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Muhammad A, Ilnytsky S, & Kolb B (2013). Persistent Gene Expression Changes in NAc, mPFC, and OFC Associated with Previous Nicotine or Amphetamine Exposure. Behavioural Brain Research, 256, 655–651. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Muhammad A, & Kolb B (2016). Chronic Stress Induces Persistent Changes in Global DNA Methylation and Gene Expression in the Medial Prefrontal Cortex, Orbitofrontal Cortex, and Hippocampus. Neuroscience, 322, 4898–499. [DOI] [PubMed] [Google Scholar]
- Nonneman AJ, & Kolb B (1974). Lesions of hippocampus or prefrontal cortex alter species typical behavior in the cat. Behavioral Biology, 12, 41–54. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Hastings E, Takeshi T, Kamitakahara H, Komorowska J, Forgie ML, & Kolb B (2006) The effects of orbital frontal cortex damage on the modulation of defensive responses by rats in playful and non-playful social contexts. Behavioral Neuroscience, 120, 72–84. [DOI] [PubMed] [Google Scholar]
- Petrulis A, DeSouza I, Schiller M, Johnston RE (1998). Role of frontal cortex in social odor discrimination and scent-marking in female golden hamsters (Mesocricetus auratus). Behavioral Neuroscience, 112, 199–212. [DOI] [PubMed] [Google Scholar]
- Price JL, Carmichael ST, Carnes KM, Clugnet M, Kuroda M, & Ray JP (1991). Olfactory input to the prefrontal cortex. In Davies J and Eichenbaum H (Eds). Olfaction: A model system for Computational Neuroscience, p 101–120. Cambridge, MA: MIT Press. [Google Scholar]
- Robinson TE, Gorny G, Savage V, & Kolb B (2002) Widespread but regionally-specific effects of self-administered versus experimenter-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, sensory cortex, and prefrontal cortex of the rat. Synapse, 46, 271–279. [DOI] [PubMed] [Google Scholar]
- Robinson TE & Kolb B (1999). Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. European Journal of Neuroscience, 11:1598–1604. [DOI] [PubMed] [Google Scholar]
- Robinson TE & Kolb B (2004). Structural plasticity associated with drugs of abuse. Neuropharmacology, 47 Suppl 1, 33–46. [DOI] [PubMed] [Google Scholar]
- Rolls ET (2014) taste, olfactory, and food reward value processing in the brain. Progress in Neurobiology, 127-128, 64–90. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. (1994). Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry, 57, 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Millette BHP, Shirley E, Rushworth MFS, & Bannerman DM (2007). Distinct contributions of frontal areas to emotion and social behaviour in the rat. European Journal of Neuroscience, 26(8), 2315–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, & Eichenbaum H (1995). Information coding in the rodent prefrontal cortex. I. single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. Journal of Neurophysiology, 74, 733–750. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. (2003). Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learning and Memory, 10:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert J, Freiherr J, Frasnelli J, Hummel T, & Lundstrom JN (2013). Orbitofrontal cortex and olfactory bulb volume predict distinct aspects of olfactory performance in healthy subjects. Cerebral Cortex, 23, 2448–2456. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, and Greenough WT (1988). A multivariate statistical summary of synaptic plasticity measures in rats exposed to complex, social and individual environments. Brain Research, 471, 299–304. [DOI] [PubMed] [Google Scholar]
- Sholl DA (1981). The organization of the cerebral cortex. London: Methuen. [Google Scholar]
- Spanswick SC, & Dyck RH (2012). Object/Context specific memory deficits following medial frontal cortex damage in mice. Plos One, 7(8), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings H, Groenewegen H, & Kolb B (2003). Does the rat have a prefrontal cortex? Behavioural Brain Research, 146, 3–17. [DOI] [PubMed] [Google Scholar]
- Wallace D, Kolb B & Whishaw IQ (2003). Odor tracking in rats with orbital frontal lesions. Behavioral Neuroscience, 117, 616–620. [DOI] [PubMed] [Google Scholar]
- Willing J, & Juraska JM (2015). The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience, 301, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K (1985). The cortex of the rat: A stereotaxic atlas. Berlin. [Google Scholar]
- Zinn CG, Clairis N, Cavalcante LES, Furini CRG, Myskiw JD, & Izquierdo I (2016). Major neurotransmitter systems in dorsal hippocampus and basolateral amygdala control social recognition memory. Proceedings of the National Academy of Sciences of the United States of America, 113, E4914–E4919. [DOI] [PMC free article] [PubMed] [Google Scholar]


