Abstract
Zn-based aqueous batteries (ZABs) hold great promise for large-scale energy storage applications due to the merits of intrinsic safety and low cost. Nevertheless, the thorny issues of metallic Zn anodes, including dendrite growth and parasitic side reactions, have severely limited the application of ZABs. Despite the encouraging improvements for stabilizing Zn anodes through surface modification, electrolyte optimization, and structural design, fundamentally addressing the inherent thermodynamics and kinetics obstacles of Zn anodes remains crucial in realizing reliable ZABs with ultrahigh efficiency, capacity, and cyclability. The target of this perspective is to elucidate the prominent status of Zn metal anode electrochemistry first from the perspective of zincophilicity and zincophobicity. Recent progress in ZABs is critically appraised for addressing the key issues, with special emphasis on the trade-off between zincophilic and zincophobic electrochemistry. Challenges and prospects for further exploration of a reliable Zn anode are presented, which are expected to boost in-depth research and practical applications of advanced ZABs.
Keywords: aqueous batteries, zincophilicity, zincophobicity, kinetics, thermodynamics, Zn anode
1. Introduction
Exploring reliable electrochemical energy storage systems has been widely considered a promising approach to realizing carbon neutralization.1−5 Of particular interest are rechargeable lithium-ion batteries (LIBs).6 However, a series of endogenous challenges, including safety risks posed by flammable organic electrolytes and potential supply chain shortages caused by the uneven geographical distribution of raw materials, has hindered the further application of LIBs at the grid scale.7−11 Zn-based aqueous batteries (ZABs) based on metallic Zn anodes and water electrolytes have attracted tremendous interest owing to their low potential (−0.76 V in neutral and acidic media or −1.21 V in alkaline media vs standard hydrogen electrode), high theoretical capacity (820 mAh g–1 or 5855 mAh cm–3), low cost, and impressive safety.12,13 Nevertheless, the practical application of ZABs is still harassed by the uncontrolled growth of dendrites with high Young’s modulus during repeated host-less Zn plating/stripping processes, which may ultimately lead to the deterioration of electrochemical performance and trigger the internal short circuit.14,15 Besides, inevitable hydrogen evolution, corrosion, and passivation may lower the redox kinetics and Coulombic efficiency (CE) due to the thermodynamic instability of metallic Zn anodes in weakly acidic electrolytes, ultimately accelerating the degradation of the metallic anode.16,17 Therefore, optimizing nucleation and inducing homogeneous Zn plating/stripping as well as suppressing the parasitic reaction at a fundamental level are urgently required.
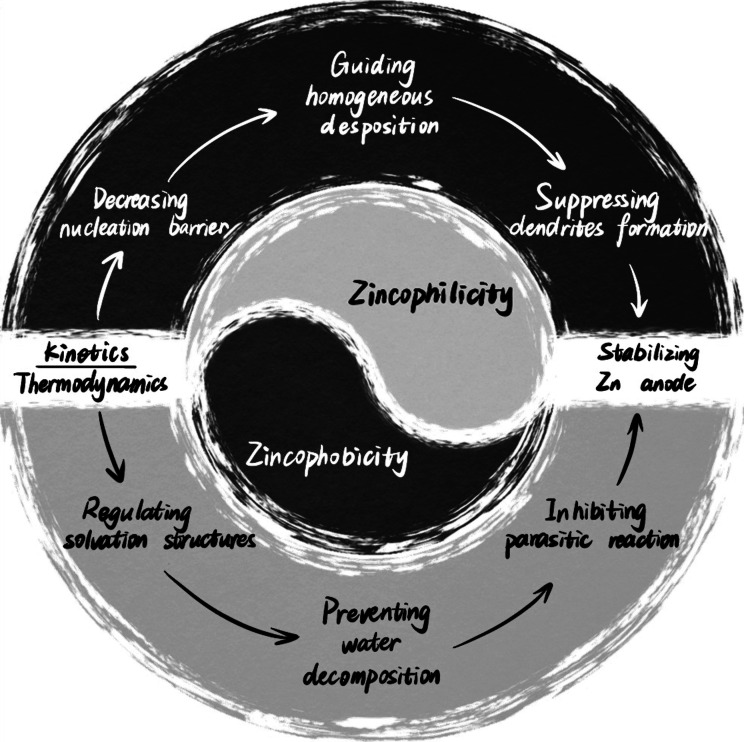
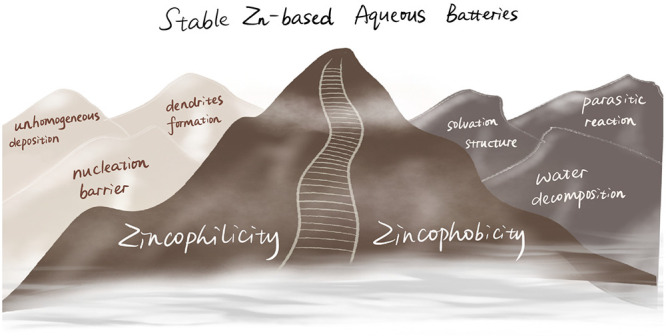
The ability to bond zinc is identified as a decisive factor affecting the initial nucleation and subsequent deposition of Zn metal, playing a critical role in Zn plating/stripping behavior. As shown in Figure 1, generally, strong Zn bonding ability (zincophilicity) could decrease the Zn nucleation energy barrier, guide the initial nucleation, and homogenize the subsequent Zn deposition by forming stable structures with Zn species.18 Meanwhile, weak Zn bonding ability (zincophilicity) can effectively inhibit parasitic reactions by regulating Zn2+ solvation structures and suppressing water decomposition.19 Therefore, choosing between zincophilicity and zincophobicity is a trade-off between dendrites and parasitic reactions. Systematically understanding the correlation between zincophilicity and zincophobicity and establishing the relationship of composition-structure-performance can provide selection criteria for the construction of reliable Zn metal anodes. In particular, a perspective regarding the trade-off between zincophilicity and zincophobicity for stabilizing Zn metal anodes would be timely. Herein, for the first time, the fundamentals, bias, and selection criteria of zincophilic or zincophobic sites are analyzed in-depth. Then critical appraisal is raised regarding how to integratively design Zn metal anodes with concomitant advantages of zincophilicity and zincophobicity. Finally, novel insights are rendered collectively for the future development of stable ZABs.
Figure 1.
Correlation between zincophilicity and zincophobicity in the Zn metal anode.
2. Fundamentals
The quantitative description of the affinity between Zn and the substrate can be theoretically reflected by calculating the adsorption energy (Eads) of Zn atoms on the substrate via density functional theory. The Eads can be obtained according to the following equation:20
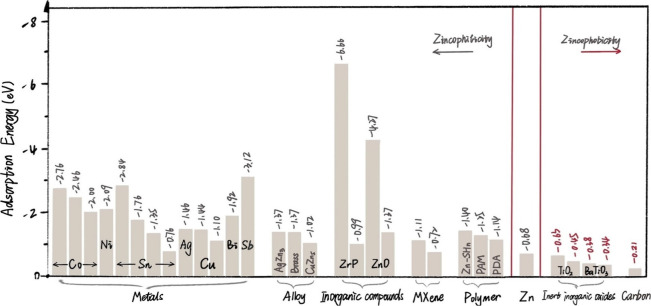
where the Esub-Zn, Esub, and EZn represent the energies of the Zn adsorbed metal surface, the clean metal surface, and Zn atom, respectively. As a key criterion for judging the potential applicable functions (guiding or restricting), a positive correlation appears in the absolute value of Eads along with the affinity between Zn and substrate.21 In other words, if the calculated Eads of the Zn atom on the substrate is substantially higher than that of the Zn atom on Zn metal (−0.68 eV),22 the substrate is considered to be zincophilic. Conversely, if the substrate exhibits more intense repulsion to the Zn atom, it can be considered that the relatively lower Eads leads to zincophobicity.
Until now, various materials with high adsorbing ability for Zn atom, including metals (Co, Ni, Cu, Ag, In, Sn, Au, Bi, Sb),21,23−26 alloy (AgZn3, Brass, ZnZn5),21,23 inorganic metal compounds (ZnO, ZnF2, CuF2, MgF2),21,26 polyanionic hydrogel,27 and MXene28 have been employed to reinforce the interfacial interaction between substrate and Zn. Meanwhile, inert inorganic oxides (CaCO3, TiO2, Kaolin, etc.),26 organic polymers (polybenzimidazole, polyamide, polyanthraquinone, etc.),29,30 and carbon have been reported as zincophobic materials to address the endogenous challenges of Zn anode. A summary of calculated binding energies between different materials and Zn atoms is presented in Figure 2, which can provide materials selection criteria for stabilizing Zn anodes.
Figure 2.
Definition of zincophilicity and zincophobicity. A summary of calculated binding energy between different materials and the Zn atom.
3. To be Zincophilic
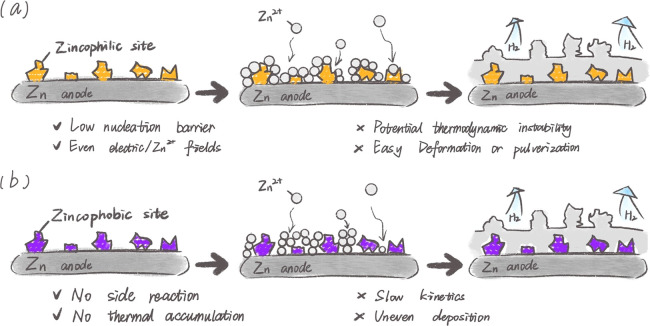
It is the thermodynamic and kinetic factors that control the nucleation processes of Zn at different heterogeneous interfaces. From the perspective of thermodynamics, the nucleation process is determined by the reduction of free energy caused by the phase transition and the increase in surface energy due to the formation of a new interface. The former is the driving force of nucleation, while the latter is the origin of the nucleation barrier.31 According to the classical heterogeneous nucleation theory, tuning the absorb/bonding ability of the interface to Zn2+ to improve the zincophilicity can reduce the nucleation barrier and induce more compact and uniform Zn deposition (Figure 3a),18,23,26 thereby boosting electrochemical performance.
Figure 3.
Schematic diagram of Zn plating behavior on the (a) zincophilic and (b) zincophobic scaffold.
Introducing zincophilic species has been proposed to reinforce the interfacial interaction between the substrate and Zn2+ for suppressing Zn dendrite formation. Although extensive efforts have been demonstrated, the inhomogeneous distribution and agglomeration of zincophilic species usually result in an uneven Zn2+ flux and Zn deposition. Meanwhile, coverage of these zincophilic sites after compounding with Zn or pulverization caused by infinite relative volume change would undoubtedly cause the loss of seeding function, resulting in ineluctable Zn dendrite growth and failure of Zn-based batteries, especially after repeated plating/stripping cycles. In addition, the fact that zincophilic sites are directly exposed to electrolytes may exacerbate the risk of irreversible self-instability, water decomposition, as well as irreversible depletion of Zn2+ from the cation sources.32 Consequently, the zincophilic components must be thermodynamically inert or spatially confined most appropriately through both physical and/or chemical confinement effects.
4. To be Zincophobic
Apart from inducing Zn metal deposition by using zincophilic species to decorate the current collectors, it is also essential to construct an artificial interface with low metal affinity (zincophobicity) to prevent the side reactions by means of shielding the Zn anode from the electrolyte, and inhibit Zn dendrites by redistributing Zn2+ at the anode/electrolyte interface (Figure 3b).19,33 By enabling the electrolyte to penetrate and enrich within a narrow range adjacent to the anode surface, the zincophobic artificial interface limits the reverse Zn2+ transmission, shields hydrated Zn2+ in the electrolyte, and confines the smooth deposition of metallic Zn.
Although the zincophobic interface protected Zn anodes usually exhibit improved stability and reversibility, the inherent problem of low depth of discharge has not been addressed yet due to the limited Zn2+ guiding effect of the zincophobic layer at the electrode–electrolyte interface. Moreover, the sluggish deposition kinetics of Zn2+ caused by insulating zincophobic artificial coating barriers may lead to higher voltage hysteresis and unsatisfactory durability.
5. Zincophilicity and/or Zincophobicity?
More practically, the most significant challenge and inevitable barrier of Zn-based batteries lie in the instability of metallic Zn anodes. Although extensive efforts in interface engineering have been demonstrated, two major issues, namely deteriorated dendrite growth and parasitic side reactions, have plagued further practical development. Therefore, fundamentally addressing the intrinsic thermodynamics and kinetics problems remains crucial in realizing reliable Zn anodes with ultrahigh efficiency, capacity, and cyclability.
5.1. Surface Engineering with Artificial Zincophilicity and/or Zincophobicity
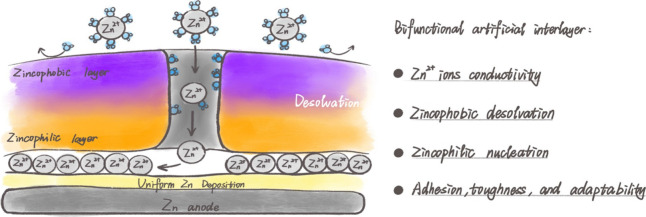
Surface engineering has been widely used to stabilize the Zn–electrolyte interface by adjusting the ion transmission flux, homogenizing the electric field distribution on the electrode surface, and affecting the growth direction of metallic Zn. Generally, the artificial layer makes sense in two ways (Figure 4): 1) as a hindrance that can keep the Zn anode away from the active water in an aqueous electrolyte, and 2) as a guiding layer that can guide the Zn to deposit uniformly.34−36 To ensure superior functionality, the artificial interfaces should fulfill several requisite characteristics of 1) chemical and electrochemical inertia to get rid of the electrolyte-related side-reactions; 2) high Zn2+ conductivity, which facilitates ions to diffuse at high speed and deposit in favorable kinetics; 3) appropriate zincophobic desolvation process, which suppresses free water molecule and anion to react with inner Zn metal, guides and confines the Zn2+ plating and stripping process underneath; 4) abundant zincophilic nucleation sites per unit area to attract Zn2+, which homogenizes the electric field to avoid concentration polarization as well as enhances the following controllability of Zn nucleation and homogeneity of crystal growth; and 5) superadhesion to the metallic Zn surface, along with high mechanical stability and high dynamic adaptability, which allows Zn to withstand the sharp changes in volume effectively during Zn plating/stripping process.
Figure 4.
Schematics of surface engineering via a zincophobic–zincophilic bifunctional artificial interlayer.
To stabilize the Zn–electrolyte interface, a zincophobic–zincophilic bifunctional artificial interlayer can be introduced as a protective layer to effectively avoid the corrosion and dendrite formation of metal anodes. As demonstrated by Zhi and co-workers,37 a spatial gradient 3D heterostructure coating layer consisting of fluorinated alloy nanoparticles was in situ constructed. The inner section of the 3D heterostructure is occupied by a CuZn alloy, while the outermost section is fluorinated compound ZnF2. The electrically insulated ZnF2 prevents direct electron transfer, along with induced H2O decomposition. Moreover, the CuZn biphasic alloy accelerates the kinetics of Zn2+ with a dendrite-free morphology to realize a high cycling stability.
Additionally, Chen et al. put forward an “all-in-one” strategy by combining zincophilicity and zincophobicity to stabilize Zn anodes for high-performance ZABs.38 A bifunctional layer of polyzwitterionic ionic liquid (PZIL) with zwitterionic functional groups can not only guide the Zn2+ distribution to manipulate Zn plating/stripping behavior under the artificial layer but also establish a water-poor interlayer on the surface of the Zn anode to avoid the occurrence of side reactions. Moreover, those oxygen-containing functional groups in the polymer chain lead to high-degree adhesion to Zn metal in the period of electrochemical cycles. Thanks to these peculiar characteristics, PZIL-Zn was able to cycle as long as 2600 h at 1 mA cm–2, with an ultrahigh average CE of 99.65% for 1000 cycles. To modulate Zn plating at high capacities, a Sb/Sb2Zn3 heterostructured interface has been developed.25 Benefiting from the ultralow onset hydrogen evolution reaction (HER) overpotential and homogeneous electric field distribution of the Sb/Sb2Zn3-heterostructured interface throughout the dynamic Zn electrodeposition process, the Zn anode enables an ultrahigh areal capacity of 200 mAh cm–2 with a CE of 98.5%. Our group designed a robust Bi@Zn heterometallic interface with metrics of both kinetics zincophilia and thermodynamics inertia for tough ZABs.24 Kinetically, the zincophilic Bi and in situ-formed Zn–Bi alloy solid solution interface promotes fast and uniform hexagonal crystal nucleation and deposition of Zn due to its low adsorption energy and superior wettability. From a thermodynamic standpoint, the metallic Bi with passivated electrocatalytic character is effective in suppressing side reactions owing to the high Gibbs free energy of hydrogen adsorption at 1.11 eV. The elaborated “kinetics metalphilia and thermodynamics inertia” metrics for the zincophilic–zincophobic interface delivers an ultralong cycling lifespan over more than 4700 cycles with an ultralow overpotential ∼55 mV even at 10 mA cm–2. Despite significant advancements, the long-term operational stability of nondynamic adaptive layers is ultimately constrained by the huge volume change resulting from the hostless nature of the Zn foil anode. To address this limitation and enhance interfacial compatibility, a dual electrolyte/electrode interphase composed of the inner zinc sulfate hydroxide (ZSH) layer and the outer ZSH/CaSO4·2H2O hybrid layer was in situ generated on the surface of the anode.39 This interface could not only inhibit the HER side reaction by presolvating Zn2+ and blocking the electron acquisition of water but also regulate the uniform deposition of Zn2+ by the effect of heterojunction charge aggregation and electric field homogenization.
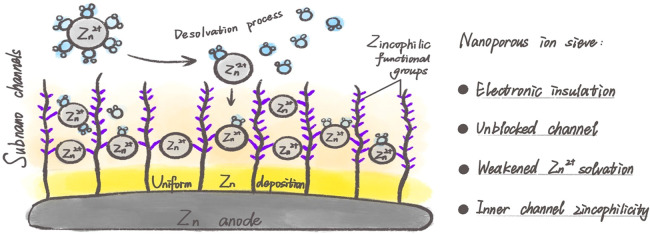
The hydrated [Zn(H2O)x]2+ ion complex, free water outside the Zn2+-solvated sheath, and/or desolvated water molecules can lead to Zn chemical instability, along with aggravation of nonuniform Zn deposition in the aqueous environment.40,41 In addition to constructing a dehydrated environment at the interface between the electrolyte and Zn anode, recently, from the perspective of regulating the solvation structure of Zn2+, well-ordered nanoporous structural materials, including metal–organic frameworks (MOFs),42−45 covalent organic frameworks,46−48 and zeolite molecular,49 have been employed to alleviate the concentration gradient at the interface via ion sieving effect for highly reversible Zn-metal anodes (Figure 5). Generally, the supersaturated electrolyte layer is capable of inhibiting dendrites formation and parasitic reactions via weakening Zn2+ solvation effect and promoting desolvation kinetics.50,51 For instance, ZIF-7 with narrow channels (2.94 Å in size window) was employed as a host to reduce the reactivity of water molecules and confine the association of solvated Zn2+-H2O ions for a homogeneous Zn deposition.42 The design shows multiple merits: 1) rejecting the large fraction of active water via a partial desolvation process inside MOF channels in advance and repelling anions via electrostatic repulsion under an electric field; 2) bonding with the detrimental H2O molecules by the hydrogen bond network existing in the porous MOF layer and ensuring a minor amount of water in neighboring electrode surface. Benefiting from the fewer side reactions, dramatic improvement occurred in the lifespan and electrodeposit morphology for the plating/stripping behavior of the Zn anode. Apart from the aforementioned advantages, the electrokinetic effect induced by the zincophilic sites on the channel surface of well-ordered nanoporous structural materials benefits uniform shock electrodeposition.52 Typically, countless zincophilic nitrogen-containing functional groups on the inner channel wall of ZIF-11 would intensely attract Zn2+, resulting in a uniform interfacial electric double layer. It is conducive to smooth electrokinetic surface conduction as well as stable deionization shock in the channel, which renders dendrite-free and fast Zn deposition.
Figure 5.
Schematics of nanoporous ion sieve-induced zincophilicity or zincophobicity design.
Manipulating planar Zn electrodeposition with preferred orientation along the (002) plane is also desirable to simultaneously boost Zn plating/stripping efficiency and Zn anode stability.47,53 Aiming at achieving uniformly planar and dendrite-free Zn deposition, Guo and co-workers developed a porous fluorinated COF (FCOF) film as a multifunctional platform to protect the Zn anode.47 The porous F-containing nanochannels exert a favorable confinement effect over the film, which contributes to the desolvation and rapid transportation of hydrated Zn2+, and hinders the aqueous electrolyte from Zn corrosion. At the same time, the surface energy of the Zn (002) crystal plane could be reduced by the strong interaction between Zn and F in the FCOF, which guarantees the metallic Zn growth along preferred (002) planes. Consequently, prolonged cycle life can be achieved for the FCOF@Zn anodes under a wide range of current densities of 5–80 mA cm–2. Moreover, oriented-attachment-regulated Zn stacking with a high zinc utilization rate, facilitated by well-spaced Prussian blue analog (PBA) nanocubes acting as Zn2+ tunnels, was also achieved through a trapping-then-planting process.54 The Zn2+ trapped in the isometric nanocavities of PBA is well-spaced at approximately 5 Å, which initiates the oriented-attached Zn (002) preferential orientation and promotes the lateral stacking of Zn nucleates. As a result, the PBA-decorated anode delivers stable zinc plating/stripping for 6600 cycles at 5 mA cm–2 with a rigorous 100% zinc utilization rate protocol.
It can be seen that surface engineering with artificial zincophilicity or zincophobicity strategies can deal with the problematic issues for Zn anodes, but it will increase the resistance and weaken the mass transfer. Meanwhile, Zn2+ channels within the bifunctional protective layers are easily blocked by deposited metallic Zn, resulting in sluggish electrochemical kinetics and restricted Zn2+ migration in the interfacial layer. There is still a lack of sufficient understanding of the effects of basic parameters such as thickness, density, uniformity, and adhesion of the zincophobic–zincophilic bifunctional artificial interlayer on the long-term stable service of Zn anodes.
5.2. Electrolyte In Situ Induced Zincophilicity and/or Zincophobicity
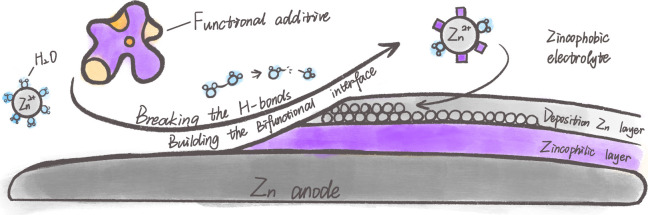
During the metallic Zn plating process, a large number of active H2O molecules are generated on the Zn surface by hydrolysis [Zn(H2O)6]2+. The hydrolysis of Zn2+ occurs as Zn2+ + 6H2O ↔ Zn[(H2O)6]2+ ↔ Zn[(H2O)5OH]+ + H+. As a result, the H2 evolution, metal corrosion, along with aggravation of nonuniform Zn deposition are inevitable by the OH– and/or H+ ions from the active water.55,56 From the thermodynamic aspect, decreasing the content/activity of water molecules belonging to Zn2+ solvation structures in electrolytes, and restricting the free water in the interface of electrode and electrolyte, have been demonstrated effective in suppressing the parasitic reactions and dendrite growth.57−59 Although a dual zincophobic–zincophilic bifunctional artificial interlayer can effectively achieve highly reversible Zn metal anode chemistry, the fabrication and implementation of such a functional layer are relatively difficult and complex. More realistically, the bifunctional characteristic can be achieved by optimizing the composition of the electrolyte to weaken the bonding strength between solvated H2O and Zn2+ (i.e., thermodynamically inert electrolyte with zincophobic water), and in situ construct a uniform Zn-ion conductive solid–electrolyte interface (SEI) on the surface of Zn anode (zincophilic interface with high kinetics) (Figure 6).
Figure 6.
Schematics of electrolyte-induced zincophilicity and/or zincophobicity design.
To this end, Cao et al. constructed a zincophilic/zincophobic bilayer interphase for a highly reversible Zn-metal anode by using a ZnCl2 aqueous electrolyte with DMSO or SnCl2 additive.19,60 The strong interactions between H2O and the additive together with the replacement of H2O in the Zn2+ solvation sheath by DMSO or concentrated Cl– would effectively decrease the water activity. At the same time, a self-repairable and zincophilic SEI with high Zn2+ conductivity can be in situ formed, which can inhibit the decomposition of solvated H2O and suppress Zn dendrite growth. The combination of reducing H2O activity with zincophobic electrolyte and accelerating reaction kinetics with zincophilic interface enables long-term Zn stripping/plating with high CE. Similarly, Luo and co-workers proposed a reasonable bifunctional SEI-design principle for Zn anodes by optimizing the composition of electrolytes.61 The composite SEI composed mainly of Zn3(PO4)2 and ZnF2 was constructed in situ on the Zn surface through a KPF6 additive-induced chemical strategy. Zn3(PO4)2 possesses a high interface energy with Zn metal, which offers great potential to suppress dendrite growth. ZnF2 exhibits unique advantages in terms of accelerating Zn2+ transfer and deposition kinetics. Thanks to the synergistic effect, the versatile SEI could not only circumvent the intractable electrochemical route but also improve the thermo-kinetic and deposition behaviors of Zn anode. Besides ionic additives, nonionic additives, including inorganics and organics, can also inhibit Zn dendrites and reduce side reactions. Silk fibroin (SF) has been proposed as an effective electrolyte additive for dendrite-free Zn anode.62 Specifically, SF could preferentially adsorb on the Zn surface. The abundant polar groups endow SF with high zincophilicity, which effectively guides the homogeneous redistribution of Zn2+ flux and promises a dendrite-free Zn anode. At the same time, SF could easily coordinate with Zn2+ to reduce coordinated H2O, thus alleviating the water-induced side reactions. In addition to manipulating Zn2+ solvation structure, the synergetic coupling of zincophobic repulsion effect and facilitating Zn2+ flux have also been employed. Wang and coauthors proposed a zincophobic electrolyte by introducing succinenitrile molecules.63 The intrinsically zincophobic electrolyte could reduce the affinity of Zn metal to the electrolyte and prevent the occurrence of corrosion and HER caused by the interface water. Moreover, the zincophobic electrolyte exhibits a stronger affinity for the SEI formed by the horizontal stacking of zinc hydroxide sulfate, which ensures rapid Zn2+ flux while inhibiting the growth of dendrites. To further enhance the durability and reliability of aqueous Zn chemistry, Li et al. designed a zincophilicity and hydrophobicity self-assembled multilayer (SAM) using l-cysteine as a molecular building block.64 This SAM is capable of reconfiguring in response to environmental conditions and triggering a dynamic replenishment interphase to confront the change in electrode morphology. The structure of the electrical double layer is modulated by the molecular multilayer, leading to accelerated electrode dynamics and the mitigation of the concentration polarization at the interface. The hydrophobic properties effectively inhibit the occurrence of water-mediated parasitic side reactions. Furthermore, the topological structure offers ample deposition space, enabling the realization of the area-induced deposition effect, which significantly contributes to the formation of a dense and well-organized zinc deposition morphology.
The in situ-induced zincophilicity and/or zincophobicity by means of electrolyte optimization has attracted much attention due to the features of facile preparation and cost-effectiveness. Despite these advances, it should be noted that SEI-stabilized electrolyte additives are easily consumed during the cycling process. Moreover, the problems of the aqueous environment, especially the low chemical/electrochemical/thermal stability of the electrolyte, are still severe challenges.
5.3. Electrode Structural Design toward Zincophilicity and/or Zincophobicity
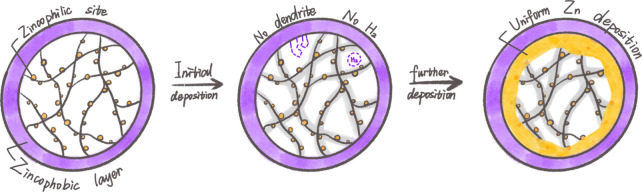
From the perspective of electrode structural design, fabricating 3D porous conductive host to anchor or confine Zn is considered an effective method to minimize dendrite formation by means of decreasing local current density and uniformizing Zn deposition.65 Meanwhile, embedding Zn-metal into the 3D host can support massive Zn deposition.66 In addition to guiding dense Zn accumulation, the ability to inhibit side reactions is also a requisite for the host. The essence of hydrogen evolution can be ascribed to the direct contact between active water in the electrolyte and the metallic anode. From the view of thermodynamics, the key to minimizing hydrogen evolution lies in the development of anticatalysts (catalytic passivation) with increased HER overpotential.21,67 Consequently, the synergistic combination of 3D conductive frameworks with zincophilic species and/or anticatalysts would be a feasible approach to achieve stable ZABs with simultaneously ultrahigh Zn stripping/plating rates and superior long-term cycling life (Figure 7).
Figure 7.
Schematics of structural engineering via zincophilicity or zincophobicity design.
To fulfill the multiple requirements mentioned, a 3D porous carbon fiber-based microscaffold decorated with zincophilic Sn nanodots/nanoparticles was fabricated as a host for stabilizing the Zn metal anode.14,68 The highly zincophilic metallic Sn not only favors the nucleation and the inward-growth during Zn plating but also inhibits the hydrogen evolution side reactions. Recently, our group proposed a hierarchical confinement strategy to design spatial traps through a host composed of porous Co-embedded carbon cages (denoted as CoCC) for controlling the electrodeposition behavior of metallic Zn and stabilizing ZABs.69 It is worth noting that the zincophilic Co sites are precisely protected by the zincophobic porous carbon layer, which not only lowers the nucleation energy barrier and guides the preferential internal nucleation of Zn metal but also effectively prevents the zincophilic sites from being directly exposed to the electrolyte, reducing the possibility of water decomposition. The 3D macroporous conductive framework could homogenize the current distribution and redistribute the Zn2+ flux to a wide range. Meanwhile, a 3D all-in-one network with porous hollow carbon cages was used as traps to accommodate and spatially confine the deposition of metallic Zn and suppress dendrite growth. Benefiting from the optimized structure design and composition combination, the developed composite anode shows small voltage hysteresis and prolonged cycling stability, even at high current densities. Apart from improving the HER overpotential, avoiding direct contact of electrolyte with the metal anode by directional encapsulation of Zn metal into a 3D highly zincophilic skeleton with longitudinal sealing and laterally porous structure is also effective in stabilizing the Zn metal anode. Zhou and collaborators constructed a 3D host (denoted as MGA) with zincophilic MXene and graphene for uniform Zn encapsulation.28 The inherent fluorine functional group in MXene could induce an in situ-formed ZnF2-rich SEI at the anode/electrolyte interface, which effectively guides the uniform nucleation. Moreover, passivation and HER during the cycles can be suppressed according to the design of encapsulating the bulk Zn in a 3D microscale pattern. An impressive flat function could be illustrated in a symmetric cell by the MGA@Zn composite anode in a synergistic manner, even at a large current density of 10 mA cm–2 over 1000 cycles.
Reasonable structural design via zincophilicity and/or zincophobicity modifications can homogenize the distribution of Zn2+ flux by reducing the local current density, guiding and regulating uniform Zn deposition within the whole framework, and alleviating partial volume changes during the plating/stripping process. However, the inferior mechanical toughness (both strong and ductile) of the 3D porous host is not sufficient to buffer huge internal stress fluctuations and prevent electrode disintegration, especially under conditions of high depth of discharge and long-term operation. In addition, the increase in the contact area between the electrolyte and metallic Zn will aggravate the corrosion and HER.
6. Conclusions and Perspectives
To conclude, the notable progress in rechargeable ZABs with intrinsic safety and high energy density is encouraging and exciting. Nevertheless, the state-of-the-art ZABs still cannot meet the requirements of practical applications due to the limited cycling stability of Zn metal anodes in aqueous electrolytes. Reasonable zincophilic/zincophobic modification should be able to tune the interfacial properties, thereby suppressing the side reaction and inducing the homogeneous deposition of Zn. More importantly, synergistically integrating and/or balancing the zincophilic and zincophobic sites may enhance the reversibility and stability of Zn to a further extent. Although significant progress and considerable achievements have been achieved in stabilizing the Zn metal anodes, there are still challenges but sufficient scope to meet the practical metrics of ZABs. Therefore, it is suggested that future works may be carried out in the following areas:
-
a)
In-depth understanding of the zincophilic/zincophobic mechanism should be established. At present, there is merely thorough and intensive research on the crucial parameters of zincophilicity or zincophobicity, while a systematic research net, similar to lithium bond chemistry in lithium metal anodes, has yet to be established. Consequently, it is significant and necessary to put forward rules systematically for further understanding of ZABs.
-
b)
The stability of zincophilic/zincophobic sites should be improved. It is possible that the zincophilic/zincophobic sites may undergo chemical action in the electrolyte or go through the electrochemical change in the repeated electric field to defunctionalize or be disabled gradually.
-
c)
Novel forms of zincophilic/zincophobic sites should be developed. Zincophilic sites with self-healing capability after structural damage or zincophobic artificial layers consisting of organic–inorganic composites with excellent mechanical strength/toughness may achieve multiple protection for Zn anodes.
-
d)
The integration of multiple advantages that combine the strategies of structural design, interface modification, electrolyte optimization, and advanced separator in a harmonized manner is expected to achieve synergistically protective and functional strategies.
-
e)
Advanced in situ characterization techniques, including real-time visualization and spectral characterization, can be employed to comprehensively investigate the reaction dynamics, transport kinetics, and thermodynamics of ZABs during the electrochemical reaction process.
-
f)
The comprehensive understanding of Zn metal anodes under practical conditions is still often neglected. Extravagant Zn is employed in current ZABs batteries, leading to high component cost, low Zn utilization rate, and unsatisfactory energy/power densities. Therefore, the cost of the raw material and fabrication process should be considered to ensure competitiveness. In addition, improving the Zn utilization rate while reducing the positive-to-negative electrode ratio is a crucial requirement for the development of ZABs with practicality and commercial feasibility.
Acknowledgments
The authors acknowledge the financial support from the National Natural Science Foundation of China (NSFC Grant Nos. 52103308, 22279023, and 22109029), the Key Basic Research Program of Science and Technology Commission of Shanghai Municipality (23520750400), Natural Science Foundation of Shanghai (22ZR1403600), Natural Science Foundation of Jiangsu Province (Grant No. BK20210826), and Talent Development Funding Project of Shanghai (2021030).
Author Contributions
The manuscript was written through contributions of all authors. CRediT: Hongpeng Li conceptualization, writing-original draft, writing-review & editing; Ruizheng Zhao writing-review & editing; Wanhai Zhou writing-review & editing; Lipeng Wang writing-review & editing; Wei Li writing-review & editing; Dongyuan Zhao writing-review & editing; Dongliang Chao conceptualization, writing-review & editing.
The authors declare no competing financial interest.
References
- Chao D.; Zhou W.; Xie F.; Ye C.; Li H.; Jaroniec M.; Qiao S.-Z. Roadmap for Advanced Aqueous Batteries: From Design of Materials to Applications. Sci. Adv. 2020, 6, eaba4098. 10.1126/sciadv.aba4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Lu X.; Lai F.; Liu T.; Shearing P. R.; Parkin I. P.; He G.; Brett D. J. L. Rechargeable Aqueous Zn-Based Energy Storage Devices. Joule 2021, 5, 2845–2903. 10.1016/j.joule.2021.10.011. [DOI] [Google Scholar]
- Li H.; Liang J. Recent Development of Printed Micro-Supercapacitors: Printable Materials, Printing Technologies, and Perspectives. Adv. Mater. 2020, 32, 1805864. 10.1002/adma.201805864. [DOI] [PubMed] [Google Scholar]
- Zhou W.; Song M.; Liang P.; Li X.; Liu X.; Li H.; Zhang T.; Wang B.; Zhao R.; Zhao Z.; Li W.; Zhao D.; Chao D. High-Energy Sn-Ni and Sn-Air Aqueous Batteries via Stannite-Ion Electrochemistry. J. Am. Chem. Soc. 2023, 145, 10880–10889. 10.1021/jacs.3c03039. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Wang B.; Chen Y.; Zhou W.; Li H.; Zhao R.; Li X.; Zhang T.; Bu F.; Zhao Z.; Li W.; Chao D.; Zhao D. Activating Sulfur Oxidation Reaction via Six-Electron Redox Mesocrystal NiS2 for Sulfur-Based Aqueous Batteries. Natl. Sci. Rev. 2023, 10, nwac268. 10.1093/nsr/nwac268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S. J.; Tian Y. F.; Zhao Y.; Feng X. X.; Zhang J.; Zhang C. H.; Fan M.; Guo J. C.; Yin Y. X.; Wang F.; Xin S.; Guo Y. G. Noncoordinating Flame-Retardant Functional Electrolyte Solvents for Rechargeable Lithium-Ion Batteries. J. Am. Chem. Soc. 2022, 144, 18240–18245. 10.1021/jacs.2c08396. [DOI] [PubMed] [Google Scholar]
- Tan S. J.; Wang W. P.; Tian Y. F.; Xin S.; Guo Y. G. Advanced Electrolytes Enabling Safe and Stable Rechargeable Li-Metal Batteries: Progress and Prospects. Adv. Funct. Mater. 2021, 31, 2105253. 10.1002/adfm.202105253. [DOI] [Google Scholar]
- Liu S.; Kang L.; Jun S. C. Challenges and Strategies toward Cathode Materials for Rechargeable Potassium-Ion Batteries. Adv. Mater. 2021, 33, 2004689. 10.1002/adma.202004689. [DOI] [PubMed] [Google Scholar]
- Liu S.; Kang L.; Hu J.; Jung E.; Zhang J.; Jun S. C.; Yamauchi Y. Unlocking the Potential of Oxygen-Deficient Copper-Doped Co3O4 Nanocrystals Confined in Carbon as an Advanced Electrode for Flexible Solid-State Supercapacitors. ACS Energy Lett. 2021, 6, 3011–3019. 10.1021/acsenergylett.1c01373. [DOI] [Google Scholar]
- Liu S.; Kang L.; Zhang J.; Jung E.; Lee S.; Jun S. C. Structural Engineering and Surface Modification of MOF-Derived Cobalt-Based Hybrid Nanosheets for Flexible Solid-State Supercapacitors. Energy Storage Mater. 2020, 32, 167–177. 10.1016/j.ensm.2020.07.017. [DOI] [Google Scholar]
- Kang L.; Zhang M.; Zhang J.; Liu S.; Zhang N.; Yao W.; Ye Y.; Luo C.; Gong Z.; Wang C.; Zhou X.; Wu X.; Jun S. C. Dual-Defect Surface Engineering of Bimetallic Sulfide Nanotubes Towards Flexible Asymmetric Solid-State Supercapacitors. J. Mater. Chem. A 2020, 8, 24053–24064. 10.1039/D0TA08979F. [DOI] [Google Scholar]
- Liu S.; Zhang R.; Mao J.; Zhao Y.; Cai Q.; Guo Z. From Room Temperature to Harsh Temperature Applications: Fundamentals and Perspectives on Electrolytes in Zinc Metal Batteries. Sci. Adv. 2022, 8, eabn5097. 10.1126/sciadv.abn5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R.; Elzatahry A.; Chao D.; Zhao D. Making MXenes More Energetic in Aqueous Battery. Matter 2022, 5, 8–10. 10.1016/j.matt.2021.12.005. [DOI] [Google Scholar]
- Yu H.; Zeng Y.; Li N. W.; Luan D.; Yu L.; Lou X. W. D. Confining Sn Nanoparticles in Interconnected N-Doped Hollow Carbon Spheres as Hierarchical Zincophilic Fibers for Dendrite-Free Zn Metal Anodes. Sci. Adv. 2022, 8, eabm5766. 10.1126/sciadv.abm5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; Chen A.; Wang D.; Pei Z.; Zhi C. “Soft Shorts” Hidden in Zinc Metal Anode Research. Joule 2022, 6, 273–279. 10.1016/j.joule.2021.12.009. [DOI] [Google Scholar]
- Liu J.; Zhou W.; Zhao R.; Yang Z.; Li W.; Chao D.; Qiao S. Z.; Zhao D. Sulfur-Based Aqueous Batteries: Electrochemistry and Strategies. J. Am. Chem. Soc. 2021, 143, 15475–15489. 10.1021/jacs.1c06923. [DOI] [PubMed] [Google Scholar]
- Hou Z.; Zhang T.; Liu X.; Xu Z.; Liu J.; Zhou W.; Qian Y.; Fan H. J.; Chao D.; Zhao D. A Solid-to-Solid Metallic Conversion Electrochemistry toward 91% Zinc Utilization for Sustainable Aqueous Batteries. Sci. Adv. 2022, 8, eabp8960. 10.1126/sciadv.abp8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue P.; Guo C.; Li L.; Li H.; Luo D.; Tan L.; Chen Z. A MOF-Derivative Decorated Hierarchical Porous Host Enabling Ultrahigh Rates and Superior Long-Term Cycling of Dendrite-Free Zn Metal Anodes. Adv. Mater. 2022, 34, 2110047. 10.1002/adma.202110047. [DOI] [PubMed] [Google Scholar]
- Cao L.; Li D.; Soto F. A.; Ponce V.; Zhang B.; Ma L.; Deng T.; Seminario J. M.; Hu E.; Yang X. Q.; Balbuena P. B.; Wang C. Highly Reversible Aqueous Zinc Batteries Enabled by Zincophilic-Zincophobic Interfacial Layers and Interrupted Hydrogen-Bond Electrolytes. Angew. Chem., Int. Ed. 2021, 60, 18845–18851. 10.1002/anie.202107378. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Hou Y. Comprehensive Analyses of Aqueous Zn Metal Batteries: Characterization Methods, Simulations, and Theoretical Calculations. Adv. Energy Mater. 2021, 11, 2003823. 10.1002/aenm.202003823. [DOI] [Google Scholar]
- Xie C.; Li Y.; Wang Q.; Sun D.; Tang Y.; Wang H. Issues and Solutions toward Zinc Anode in Aqueous Zinc-Ion Batteries: A Mini Review. Carbon Energy 2020, 2, 540–560. 10.1002/cey2.67. [DOI] [Google Scholar]
- Yang Q.; Li Q.; Liu Z.; Wang D.; Guo Y.; Li X.; Tang Y.; Li H.; Dong B.; Zhi C. Dendrites in Zn-Based Batteries. Adv. Mater. 2020, 32, 2001854. 10.1002/adma.202001854. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Luan J.; Tang Y.; Ji X.; Wang H. Interfacial Design of Dendrite-Free Zinc Anodes for Aqueous Zinc-Ion Batteries. Angew. Chem., Int. Ed. 2020, 59, 13180–13191. 10.1002/anie.202000162. [DOI] [PubMed] [Google Scholar]
- Zhao R.; Dong X.; Liang P.; Li H.; Zhang T.; Zhou W.; Wang B.; Yang Z.; Wang X.; Wang L.; Sun Z.; Bu F.; Zhao Z.; Li W.; Zhao D.; Chao D. Prioritizing Hetero-Metallic Interfaces via Thermodynamics Inertia and Kinetics Zincophilia Metrics for Tough Zn-Based Aqueous Batteries. Adv. Mater. 2023, 35, 2209288. 10.1002/adma.202209288. [DOI] [PubMed] [Google Scholar]
- Zheng X.; Liu Z.; Sun J.; Luo R.; Xu K.; Si M.; Kang J.; Yuan Y.; Liu S.; Ahmad T.; Jiang T.; Chen N.; Wang M.; Xu Y.; Chuai M.; Zhu Z.; Peng Q.; Meng Y.; Zhang K.; Wang W.; Chen W. Constructing Robust Heterostructured Interface for Anode-Free Zinc Batteries with Ultrahigh Capacities. Nat. Commun. 2023, 14, 76. 10.1038/s41467-022-35630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin R.; Wang Y.; Yao L.; Yang L.; Zhao Q.; Ding S.; Liu L.; Pan F. Progress in Interface Structure and Modification of Zinc Anode for Aqueous Batteries. Nano Energy 2022, 98, 107333. 10.1016/j.nanoen.2022.107333. [DOI] [Google Scholar]
- Yang J. L.; Li J.; Zhao J. W.; Liu K.; Yang P.; Fan H. J. Stable Zinc Anodes Enabled by a Zincophilic Polyanionic Hydrogel Layer. Adv. Mater. 2022, 34, 2202382. 10.1002/adma.202202382. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Xie M.; Wu F.; Mei Y.; Hao Y.; Li L.; Chen R. Encapsulation of Metallic Zn in a Hybrid MXene/Graphene Aerogel as a Stable Zn Anode for Foldable Zn-Ion Batteries. Adv. Mater. 2022, 34, 2106897. 10.1002/adma.202106897. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Zhao J.; Hu Z.; Li J.; Li J.; Zhang Y.; Wang C.; Cui G. Long-Life and Deeply Rechargeable Aqueous Zn Anodes Enabled by a Multifunctional Brightener-Inspired Interphase. Energy Environ. Sci. 2019, 12, 1938–1949. 10.1039/C9EE00596J. [DOI] [Google Scholar]
- Xie D.; Wang Z. W.; Gu Z. Y.; Diao W. Y.; Tao F. Y.; Liu C.; Sun H. Z.; Wu X. L.; Wang J. W.; Zhang J. P. Polymeric Molecular Design Towards Horizontal Zn Electrodeposits at Constrained 2D Zn2+ Diffusion: Dendrite-Free Zn Anode for Long-Life and High-Rate Aqueous Zinc Metal Battery. Adv. Funct. Mater. 2022, 32, 2204066. 10.1002/adfm.202204066. [DOI] [Google Scholar]
- Chen X.; Chen X.-R.; Hou T.-Z.; Li B.-Q.; Cheng X.-B.; Zhang R.; Zhang Q. Lithiophilicity Chemistry of Heteroatom-Doped Carbon to Guide Uniform Lithium Nucleation in Lithium Metal Anodes. Sci. Adv. 2019, 5, eaau7728. 10.1126/sciadv.aau7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M.; Zhang K.; Du D.; Tang X.; Liu Y.; Wang H.; Zhang M.; Liu S.; Ma Y. SnSb Binary Alloy Induced Heterogeneous Nucleation within the Confined Nanospace: Toward Dendrite-Free, Flexible and Energy/Power Dense Sodium Metal Batteries. Energy Storage Mater. 2021, 42, 219–230. 10.1016/j.ensm.2021.07.032. [DOI] [Google Scholar]
- Xie C.; Zhang Q.; Yang Z.; Ji H.; Li Y.; Li H.; Fu L.; Huang D.; Tang Y.; Wang H. Intrinsically Zincophobic Protective Layer for Dendrite-Free Zinc Metal Anode. Chin. Chem. Lett. 2022, 33, 2653–2657. 10.1016/j.cclet.2021.09.083. [DOI] [Google Scholar]
- Wang S.; Yang Z.; Chen B.; Zhou H.; Wan S.; Hu L.; Qiu M.; Qie L.; Yu Y. A Highly Reversible, Dendrite-Free Zinc Metal Anodes Enabled by a Dual-Layered Interface. Energy Storage Mater. 2022, 47, 491–499. 10.1016/j.ensm.2022.02.024. [DOI] [Google Scholar]
- Zheng J.; Archer L. A. Controlling Electrochemical Growth of Metallic Zinc Electrodes: Toward Affordable Rechargeable Energy Storage Systems. Sci. Adv. 2021, 7, eabe0219. 10.1126/sciadv.abe0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Bi S.; Niu Z.; Zhou W.; Xie S. Design of Zn Anode Protection Materials for Mild Aqueous Zn-Ion Batteries. Energy Mater. 2022, 2, 200012. 10.20517/energymater.2022.08. [DOI] [Google Scholar]
- Liang G.; Zhu J.; Yan B.; Li Q.; Chen A.; Chen Z.; Wang X.; Xiong B.; Fan J.; Xu J.; Zhi C. Gradient Fluorinated Alloy to Enable Highly Reversible Zn-Metal Anode Chemistry. Energy Environ. Sci. 2022, 15, 1086–1096. 10.1039/D1EE03749H. [DOI] [Google Scholar]
- Chen R.; Liu Q.; Xu L.; Zuo X.; Liu F.; Zhang J.; Zhou X.; Mai L. Zwitterionic Bifunctional Layer for Reversible Zn Anode. ACS Energy Lett. 2022, 7, 1719–1727. 10.1021/acsenergylett.2c00124. [DOI] [Google Scholar]
- Guo S.; Qin L.; Hu C.; Li L.; Luo Z.; Fang G.; Liang S. Quasi-Solid Electrolyte Design and In Situ Construction of Dual Electrolyte/Electrode Interphases for High-Stability Zinc Metal Battery. Adv. Energy Mater. 2022, 12, 2200730. 10.1002/aenm.202200730. [DOI] [Google Scholar]
- Li T. C.; Lim Y.; Li X. L.; Luo S.; Lin C.; Fang D.; Xia S.; Wang Y.; Yang H. Y. A Universal Additive Strategy to Reshape Electrolyte Solvation Structure toward Reversible Zn Storage. Adv. Energy Mater. 2022, 12, 2103231. 10.1002/aenm.202103231. [DOI] [Google Scholar]
- Yu H.; Chen D.; Zhang T.; Fu M.; Cai J.; Wei W.; Ji X.; Chen Y.; Chen L. Insight on the Double-Edged Sword Role of Water Molecules in the Anode of Aqueous Zinc-Ion Batteries. Small Struct. 2022, 3, 2200143. 10.1002/sstr.202200143. [DOI] [Google Scholar]
- Yang H.; Chang Z.; Qiao Y.; Deng H.; Mu X.; He P.; Zhou H. Constructing a Super-Saturated Electrolyte Front Surface for Stable Rechargeable Aqueous Zinc Batteries. Angew. Chem., Int. Ed. 2020, 59, 9377–9381. 10.1002/anie.202001844. [DOI] [PubMed] [Google Scholar]
- Liu X.; Yang F.; Xu W.; Zeng Y.; He J.; Lu X. Zeolitic Imidazolate Frameworks as Zn2+ Modulation Layers to Enable Dendrite-Free Zn Anodes. Adv. Sci. 2020, 7, 2002173. 10.1002/advs.202002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C.; Du Y.; Guo Z.; Chen A.; Liu N.; Lu X.; Fan L.; Zhang Y.; Zhang N. Missing-Linker Bifunctional Mil-125(Ti)-Zn Interface Modulation Layer to Simultaneously Suppress Hydrogen Evolution Reaction and Dendrites for Zn Metal Anodes. Energy Storage Mater. 2022, 53, 322–330. 10.1016/j.ensm.2022.09.014. [DOI] [Google Scholar]
- Liu H.; Wang J.-G.; Hua W.; Ren L.; Sun H.; Hou Z.; Huyan Y.; Cao Y.; Wei C.; Kang F. Navigating Fast and Uniform Zinc Deposition via a Versatile Metal–Organic Complex Interphase. Energy Environ. Sci. 2022, 15, 1872–1881. 10.1039/D2EE00209D. [DOI] [Google Scholar]
- Park J. H.; Kwak M. J.; Hwang C.; Kang K. N.; Liu N.; Jang J. H.; Grzybowski B. A. Self-Assembling Films of Covalent Organic Frameworks Enable Long-Term, Efficient Cycling of Zinc-Ion Batteries. Adv. Mater. 2021, 33, 2101726. 10.1002/adma.202101726. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Wang R.; Peng C.; Chen W.; Wu T.; Hu B.; Weng W.; Yao Y.; Zeng J.; Chen Z.; Liu P.; Liu Y.; Li G.; Guo J.; Lu H.; Guo Z. Horizontally Arranged Zinc Platelet Electrodeposits Modulated by Fluorinated Covalent Organic Framework Film for High-Rate and Durable Aqueous Zinc Ion Batteries. Nat. Commun. 2021, 12, 6606. 10.1038/s41467-021-26947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C.; Zhou J.; Chen Y.; Zhuang H.; Li Q.; Li J.; Tian X.; Zhang Y.; Yao X.; Chen Y.; Li S. L.; Lan Y. Q. Synergistic Manipulation of Hydrogen Evolution and Zinc Ion Flux in Metal-Covalent Organic Frameworks for Dendrite-Free Zn-Based Aqueous Batteries. Angew. Chem., Int. Ed. 2022, 61, e202210871. 10.1002/anie.202210871. [DOI] [PubMed] [Google Scholar]
- Yang H.; Qiao Y.; Chang Z.; Deng H.; Zhu X.; Zhu R.; Xiong Z.; He P.; Zhou H. Reducing Water Activity by Zeolite Molecular Sieve Membrane for Long-Life Rechargeable Zinc Battery. Adv. Mater. 2021, 33, 2102415. 10.1002/adma.202102415. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Li J.; Liu D.; Liu M.; Zhou T.; Qi K.; Shi L.; Zhu Y.; Qian Y. Ultra-Long-Life and Highly Reversible Zn Metal Anodes Enabled by a Desolvation and Deanionization Interface Layer. Energy Environ. Sci. 2021, 14, 3120–3129. 10.1039/D0EE03898A. [DOI] [Google Scholar]
- Chang Z.; Yang H.; Qiao Y.; Zhu X.; He P.; Zhou H. Tailoring the Solvation Sheath of Cations by Constructing Electrode Front-Faces for Rechargeable Batteries. Adv. Mater. 2022, 34, 2201339. 10.1002/adma.202201339. [DOI] [PubMed] [Google Scholar]
- He M.; Shu C.; Hu A.; Zheng R.; Li M.; Ran Z.; Long J. Suppressing Dendrite Growth and Side Reactions on Zn Metal Anode via Guiding Interfacial Anion/Cation/H2O Distribution by Artificial Multi-Functional Interface Layer. Energy Storage Mater. 2022, 44, 452–460. 10.1016/j.ensm.2021.11.010. [DOI] [Google Scholar]
- Pu S. D.; Gong C.; Tang Y. T.; Ning Z.; Liu J.; Zhang S.; Yuan Y.; Melvin D.; Yang S.; Pi L.; Marie J. J.; Hu B.; Jenkins M.; Li Z.; Liu B.; Tsang S. C. E.; Marrow T. J.; Reed R. C.; Gao X.; Bruce P. G.; Robertson A. W. Achieving Ultrahigh-Rate Planar and Fendrite-Free Zinc Electroplating for Aqueous Zinc Battery Anodes. Adv. Mater. 2022, 34, 2202552. 10.1002/adma.202202552. [DOI] [PubMed] [Google Scholar]
- Deng D.; Fu K.; Yu R.; Zhu J.; Cai H.; Zhang X.; Wu J.; Luo W.; Mai L. Ion Tunnel Matrix Initiated Oriented Attachment for Highly Utilized Zn Anodes. Adv. Mater. 2023, 10.1002/adma.202302353. [DOI] [PubMed] [Google Scholar]
- Cui Y.; Zhao Q.; Wu X.; Wang Z.; Qin R.; Wang Y.; Liu M.; Song Y.; Qian G.; Song Z.; Yang L.; Pan F. Quasi-Solid Single Zn-Ion Conductor with High Conductivity Enabling Dendrite-Free Zn Metal Anode. Energy Storage Mater. 2020, 27, 1–8. 10.1016/j.ensm.2020.01.003. [DOI] [Google Scholar]
- Chao D.; Qiao S.-Z. Toward High-Voltage Aqueous Batteries: Super- or Low-Concentrated Electrolyte?. Joule 2020, 4, 1846–1851. 10.1016/j.joule.2020.07.023. [DOI] [Google Scholar]
- Yang W.; Yang Y.; Yang H.; Zhou H. Regulating Water Activity for Rechargeable Zinc-Ion Batteries: Progress and Perspective. ACS Energy Lett. 2022, 7, 2515–2530. 10.1021/acsenergylett.2c01152. [DOI] [Google Scholar]
- Cao J.; Zhang D.; Zhang X.; Zeng Z.; Qin J.; Huang Y. Strategies of Regulating Zn2+ Solvation Structures for Dendrite-Free and Side Reaction-Suppressed Zinc-Ion Batteries. Energy Environ. Sci. 2022, 15, 499–528. 10.1039/D1EE03377H. [DOI] [Google Scholar]
- Liu C.; Li Z.; Zhang X.; Xu W.; Chen W.; Zhao K.; Wang Y.; Hong S.; Wu Q.; Li M. C.; Mei C. Synergic Effect of Dendrite-Free and Zinc Gating in Lignin-Containing Cellulose Nanofibers-MXene Layer Enabling Long-Cycle-Life Zinc Metal Batteries. Adv. Sci. 2022, 9, 2202380. 10.1002/advs.202202380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L.; Li D.; Hu E.; Xu J.; Deng T.; Ma L.; Wang Y.; Yang X. Q.; Wang C. Solvation Structure Design for Aqueous Zn Metal Batteries. J. Am. Chem. Soc. 2020, 142, 21404–21409. 10.1021/jacs.0c09794. [DOI] [PubMed] [Google Scholar]
- Chu Y.; Zhang S.; Wu S.; Hu Z.; Cui G.; Luo J. In Situ Built Interphase with High Interface Energy and Fast Kinetics for High Performance Zn Metal Anodes. Energy Environ. Sci. 2021, 14, 3609–3620. 10.1039/D1EE00308A. [DOI] [Google Scholar]
- Zhang L.; Zhang T.; Xin W.; Peng H.; Yan Z.; Zhu Z. Additive Engineering for a Hydrophilic/Zincophilic Polymeric Layer Towards Dendrite-Free Zinc Anode. Mater. Today Energy 2022, 29, 101130. 10.1016/j.mtener.2022.101130. [DOI] [Google Scholar]
- Wang N.; Chen X.; Wan H.; Zhang B.; Guan K.; Yao J.; Ji J.; Li J.; Gan Y.; Lv L.; Tao L.; Ma G.; Wang H.; Zhang J.; Wang H. Zincophobic Electrolyte Achieves Highly Reversible Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2300795. 10.1002/adfm.202300795. [DOI] [Google Scholar]
- Li D.; Tang Y.; Liang S.; Lu B.; Chen G.; Zhou J. Self-Assembled Multilayers Direct a Buffer Interphase for Long-Life Aqueous Zinc-Ion Batteries. Energy Environ. Sci. 2023, 10.1039/D3EE01098H. [DOI] [Google Scholar]
- Blanc L. E.; Kundu D.; Nazar L. F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. 10.1016/j.joule.2020.03.002. [DOI] [Google Scholar]
- Zhang W.; Fan L.; Tong Z.; Miao J.; Shen Z.; Li S.; Chen F.; Qiu Y.; Lu Y. Stable Li-Metal Deposition via a 3D Nanodiamond Matrix with Ultrahigh Young’s Modulus. Small Methods 2019, 3, 1900325. 10.1002/smtd.201900325. [DOI] [Google Scholar]
- Wan F.; Zhou X.; Lu Y.; Niu Z.; Chen J. Energy Storage Chemistry in Aqueous Zinc Metal Batteries. ACS Energy Lett. 2020, 5, 3569–3590. 10.1021/acsenergylett.0c02028. [DOI] [Google Scholar]
- Yang J.-L.; Yang P.; Yan W.; Zhao J.-W.; Fan H. J. 3D Zincophilic Micro-Scaffold Enables Stable Zn Deposition. Energy Storage Mater. 2022, 51, 259–265. 10.1016/j.ensm.2022.06.050. [DOI] [Google Scholar]
- Li H.; Guo C.; Zhang T.; Xue P.; Zhao R.; Zhou W.; Li W.; Elzatahry A.; Zhao D.; Chao D. Hierarchical Confinement Effect with Zincophilic and Spatial Traps Stabilized Zn-Based Aqueous Battery. Nano Lett. 2022, 22, 4223–4231. 10.1021/acs.nanolett.2c01235. [DOI] [PubMed] [Google Scholar]