Abstract
Purpose
To report 5-year landmark analysis efficacy and safety outcomes in patients with BRAF V600–mutant metastatic melanoma (MM) who received BRAF inhibitor dabrafenib (D) and MEK inhibitor trametinib (T) combination therapy versus D monotherapy in the randomized phase II BRF113220 study part C.
Patients and Methods
BRAF inhibitor–naive patients with BRAF V600–mutant MM were randomly assigned 1:1:1 to receive D 150 mg twice a day, D 150 mg twice a day plus T 1 mg once daily, or D 150 mg twice a day plus T 2 mg once daily (D + T 150/2). Patients who received D monotherapy could cross over to D + T 150/2 postprogression. Efficacy and safety were analyzed 4 and 5 years after initiation in patients with ≥ 5 years of follow-up.
Results
As of October 13, 2016, 18 patients who received D + T 150/2 remained in the study (13 [24%] of 54 enrolled at this dose plus five [11%] of 45 initially administered D who crossed over to D + T). With D + T 150/2, overall survival (OS; 4 years, 30%; 5 years, 28%) and progression-free survival (4 and 5 years, both 13%) appeared to stabilize with extended follow-up. Increased OS was observed in patients who received D + T with baseline normal lactate dehydrogenase (5 years, 45%) and normal lactate dehydrogenase with fewer than three organ sites with metastasis (5 years, 51%). With extended follow-up, one additional patient who received D + T 150/2 improved from a partial to a complete response. No new safety signals were observed.
Conclusion
This 5-year analysis represents the longest follow-up to date with BRAF + MEK inhibitor combination therapy in BRAF V600–mutant MM. Consistent with trends observed in landmark analyses with shorter follow-up, this therapy elicits durable plateaus of long-term OS and progression-free survival that last ≥ 5 years in some patients with MM.
INTRODUCTION
Before the recent advances in the treatment of metastatic melanoma (ie, mitogen-activated protein kinase [MAPK] pathway inhibitors, checkpoint inhibitor immunotherapies), disease outcomes were poor, with a median overall survival (OS) of approximately 7.5 months and a 5-year survival rate of approximately 6%.1,2 The anti–cytotoxic T-lymphocyte–associated protein-4 therapy ipilimumab was the first treatment to demonstrate durable clinical benefit that lasts ≥ 5 years in molecularly unselected patients with advanced melanoma.3 BRAF inhibitor (BRAFi) therapy with or without MEK inhibitor (MEKi) and anti–programmed death-1 (anti–PD-1) immune checkpoint inhibitor–based regimens also have significantly improved clinical outcomes in patients with metastatic melanoma4-14; however, extended follow-up analyses of randomized studies of these therapies typically have been limited to ≤ 3 years.15-25 A general misconception exists that durable responses with targeted therapies are uncommon, but evidence from long-term randomized studies with survival follow-up > 3 years is lacking. With multiple therapies currently available to treat BRAF V600–mutant melanoma, a complete understanding of treatment impact on durable outcomes with extended use is needed to optimize individualized patient treatment strategies.
In a previously reported landmark analysis for the clinical trial BRF113220,17 BRAFi-naive patients with metastatic melanoma randomly assigned to treatment with the combination of dabrafenib (D) and trametinib (T) at the US Food and Drug Administration–approved dose (n = 54) showed a median progression-free survival (PFS) of 9.4 months, and 41%, 25%, and 21% were progression free after 1, 2, and 3 years, respectively. Median OS in these patients was 25 months, with 1-, 2-, and 3-year OS rates of 80%, 51%, and 38%, respectively. In pooled analyses of data from randomized trials of D + T in previously untreated patients with BRAF-mutant metastatic melanoma, median PFS was approximately 11 months and median OS approximately 26 months.26,27 These findings were consistent with reports from individual randomized phase II and III trials that evaluated these agents in patients with BRAF V600E/K–mutant metastatic melanoma.4,7,8,28-30 In the current study, we report a 5-year landmark efficacy and safety analysis for BRAFi-naive patients with metastatic melanoma treated with D + T.
PATIENTS AND METHODS
Study Design and Participants
This open-label phase I and II study of patients with histologically confirmed unresectable stage IIIC or IV BRAF V600E/K–mutant melanoma had four parts (A, B, C, and D) conducted internationally at 16 centers. Patients were age ≥ 18 years and had measurable disease, an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate organ function. A detailed study description, including full eligibility criteria, has been previously published.4,17 The analysis described here includes only patients enrolled in part C because parts A, B, and D did not include patient random assignment to the Food and Drug Administration–approved dose of D 150 mg twice a day plus T 2 mg once daily (D + T 150/2).
All patients in part C were BRAFi and MEKi naive at initial study enrollment. Patients were randomly assigned 1:1:1 to receive D monotherapy twice a day, D 150 mg twice a day plus T 1 mg once daily (D + T 150/1), or D + T 150/2. Patients who progressed in the D monotherapy arm were allowed to cross over to D + T 150/2 treatment.
The protocol was approved by the institutional review board at each study site, and it complied with country-specific regulatory requirements. All patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Study Assessments
Treatment response, duration of response, PFS, and OS were determined by using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 as previously described.4 Adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Statistical Analyses
This 5-year landmark analysis is based on data available as of October 13, 2016. Median PFS and OS were determined by using the Kaplan-Meier method, with 95% CIs estimated using the Brookmeyer-Crowley method. Landmark survival rates were calculated by Kaplan-Meier method. Crossover to D + T 150/2 was permitted for patients in the D monotherapy arm after progression according to the intention-to-treat principle, where any crossover benefit was applied to the randomized therapy arm estimates. The potential influence of previously identified predictive factors for outcomes with D + T (ie, lactate dehydrogenase [LDH] level, number of organ sites that contained metastasis)17,26 on patient-derived benefit were explored with descriptive subgroup stratification. AEs were summarized by preferred terms.
RESULTS
One hundred sixty-two patients were enrolled in part C, with three groups of 54 patients each randomly assigned to receive D monotherapy, D + T 150/1, or D + T 150/2 (Appendix Fig A1, online only). Patient baseline characteristics were well balanced across treatment groups (Table 1). As of the October 13, 2016, data cutoff, patients still alive had a minimum follow-up of 60 months from time of random assignment (median follow-up, 66.5 months). Thirty-eight patients (70%) who were initiated on D + T 150/2 and 43 (80%) initiated on D monotherapy died (Table 2), with two patients (4%) in the D + T 150/2 arm and one (2%) in the D monotherapy arm still receiving their original study treatment.
Table 1.
Baseline Characteristics (N = 162)
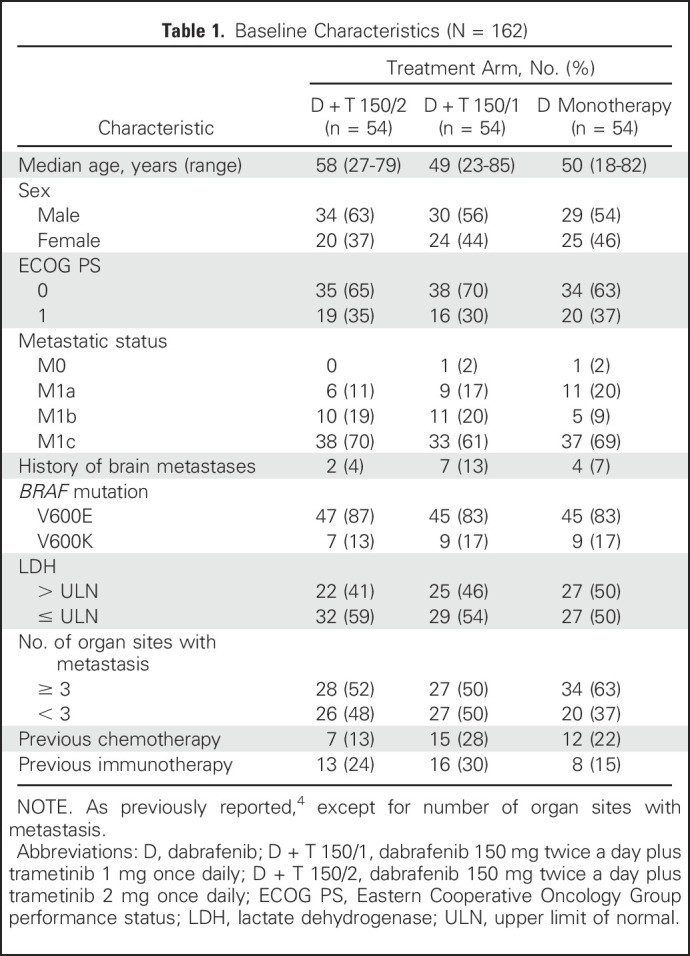
Table 2.
Study Disposition
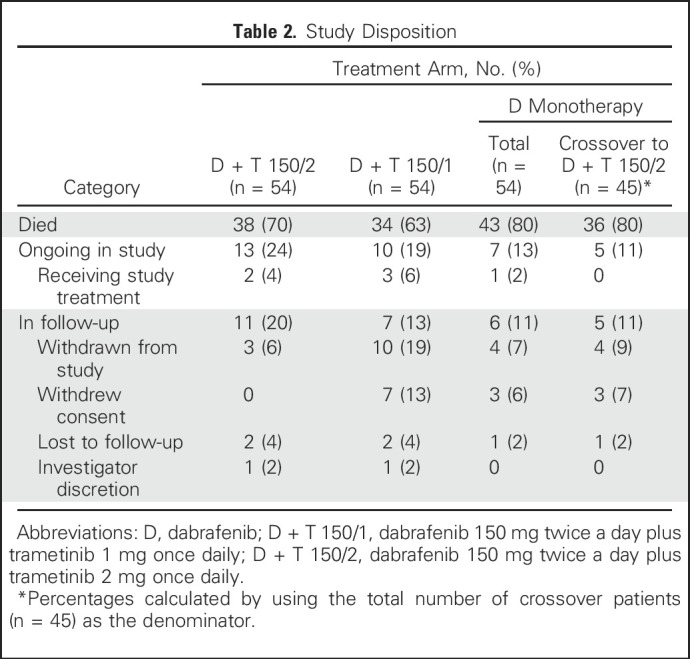
At the time of analysis, 43 patients (80%) in the D + T 150/2 arm and 49 (91%) in the D monotherapy arm had experienced progression or died, with stable 4- and 5-year PFS rates of 13% (95% CI, 5% to 25%) with D + T 150/2 (hazard ratio, 0.44; 95% CI, 0.28 to 0.67) and 3% (95% CI, 0% to 11%) with D monotherapy (Fig 1A). Thirty-eight patients (70%) in the D + T 150/2 arm and 43 (80%) in the D monotherapy arm died, which yielded 4- and 5-year OS rates of 30% (95% CI, 18% to 43%) and 28% (95% CI, 17% to 41%), respectively, with D + T 150/2 (hazard ratio, 0.76; 95% CI, 0.49 to 1.18) and 23% (95% CI, 13% to 35%) and 21% (95% CI, 11% to 33%), respectively, with D monotherapy (Fig 1B). Of note, 45 (83%) of the 54 patients in the D monotherapy arm included in this updated analysis had crossed over to D + T 150/2 (Table 2); the survival outcomes in these crossover patients continued to be followed for the D monotherapy arm.
Fig 1.
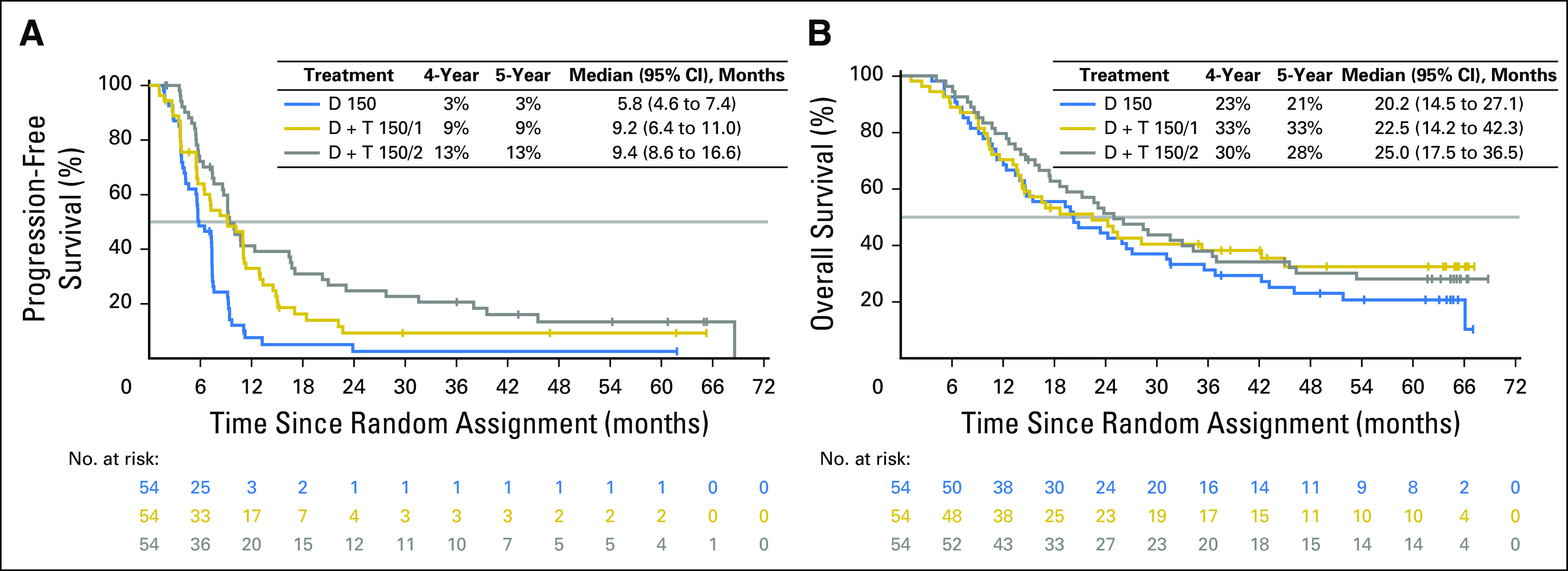
(A) Progression-free survival and (B) overall survival in the intention-to-treat population. D, dabrafenib; D + T 150/1, dabrafenib 150 mg twice a day plus trametinib 1 mg once daily; D + T 150/2, dabrafenib 150 mg twice a day plus trametinib 2 mg once daily.
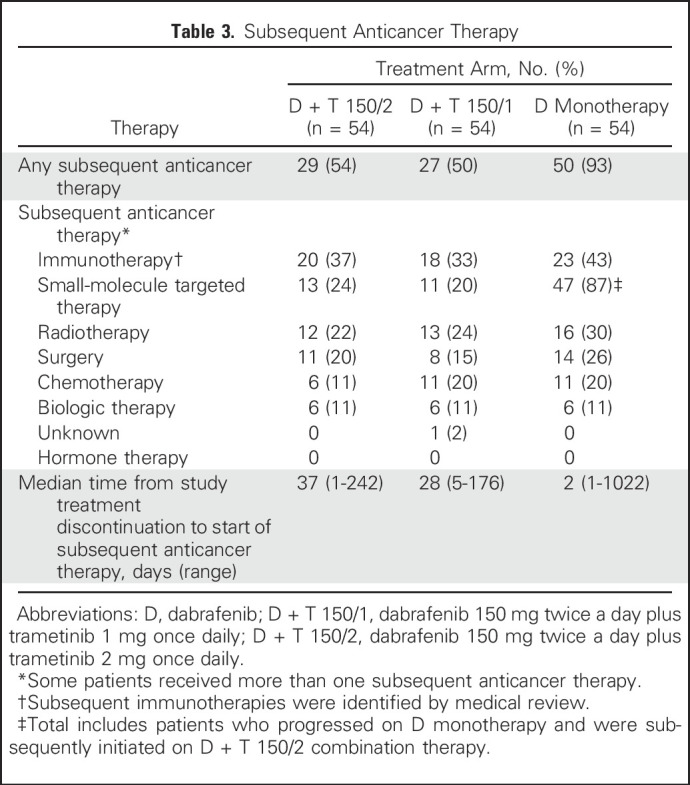
In line with the observed frequency of disease progression in each treatment arm, more patients who were initiated on D monotherapy than on D + T 150/2 received subsequent anticancer therapy (50 [93%] of 54 v 29 [54%] of 54, respectively; Table 3). If patients with disease progression who crossed over from D monotherapy to D + T 150/2 were excluded, 33 (61%) of 54 patients who were initiated on D had subsequent anticancer therapy. Immunotherapy (37% v 43% for D + T 150/2 v D monotherapy, respectively) and small-molecule targeted therapy (24% v 87%, respectively) were the most common subsequent anticancer therapies. The high percentage of patients who received D monotherapy and subsequent targeted therapy reflected the high number of patients who crossed over to the D + T 150/2 treatment arm. Median times from initial study treatment discontinuation to initiation of an alternate anticancer therapy were 37 days (range, 1 to 242 days) in the D + T 150/2 arm and 2 days (range, 1 to 1,022 days [all patients] or 1 to 25 days [which excludes crossover patients]) in the D monotherapy arm.
Table 3.
Subsequent Anticancer Therapy
A RECIST response was confirmed in 41 patients (76%) in the D + T 150/2 arm and 29 (54%) in the D monotherapy arm, with a complete response (CR) observed in nine (17%) and two (4%), respectively (Table 4). Among patients in the D + T 150/2 arm with a best response of CR, PFS was 67% at 3 years and 40% at both 4 and 5 years, and the median PFS was 39.6 months. Among patients with a best response of partial response (PR), 13%, 9%, and 9% remained progression free after 3, 4, and 5 years, respectively, and median PFS was 10.0 months. Among patients in the D + T 150/2 arm with a best response of CR, 3-, 4-, and 5-year OS rates were 67%, 56%, and 44%, respectively, and median OS was 53.4 months. Among patients with a best response of PR, 3-, 4-, and 5-year OS was 31%, 22%, and 22%, respectively, and median OS was 22.9 months. Of note, since the 3-year landmark analysis,17 best response in one patient in the D + T 150/2 arm improved from a PR to a CR with additional follow-up (PR first documented in May 2011 and continued until CR in October 2015). The median duration of response was 10.5 months (95% CI, 7.4 to 19.2 months) in the D + T 150/2 arm and 5.6 months (95% CI, 4.1 to 7.4 months) in the D monotherapy arm (Table 4). Among patients across all arms who achieved a PR or CR, 50% achieved each response within 1.8 months.
Table 4.
Confirmed Response Rates and Duration
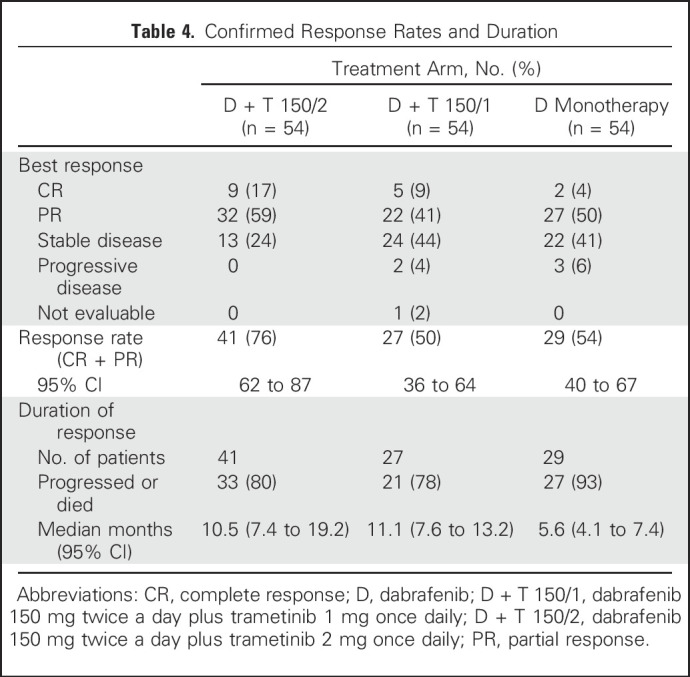
Outcomes also were analyzed in subgroups on the basis of baseline patient characteristics previously associated with clinical benefit.17,26 Within each treatment arm, patients with more-favorable baseline prognostic factors generally had improved outcomes versus those in poorer prognostic subgroups. Within prognostic subgroups, most comparisons showed longer PFS and OS in patients who received D + T 150/2 than those who received D monotherapy. Among patients with normal baseline serum LDH concentrations (less than or equal to the upper limit of normal [ULN]), PFS remained constant, with rates of 23% (95% CI, 9% to 41%) in the D + T 150/2 arm and 6% (95% CI, 2% to 14%) in the D arm after both 4 and 5 years. Four- and 5-year OS rates in these subgroups in the D + T 150/2 arm were 48% (95% CI, 30% to 64%) and 45% (95% CI, 27% to 61%), respectively, and 31% (95% CI, 15% to 50%) and 26% (95% CI, 11% to 45%) in the D monotherapy arm, respectively (Fig 2). D + T also showed the greatest benefit in patients with serum LDH less than or equal to ULN and fewer than three organ sites with metastasis at baseline, with both 4- and 5-year PFS rates of 25% (95% CI, 7% to 49%) in the D + T 150/2 arm and 8% (95% CI, 1% to 31%) in the D monotherapy arm. Four- and 5-year OS rates were 57% (95% CI, 32% to 76%) and 51% (95% CI, 27% to 71%) with D + T 150/2, respectively, and 42% (95% CI, 15% to 67%) and 31% (95% CI, 8% to 58%) with D monotherapy, respectively (Fig 2). No patients with the previously reported poor prognostic indicator of baseline serum LDH greater than ULN17,26 were progression free at 5 years, and both 4- and 5-year OS rates in the D + T 150/2 and D monotherapy arms were low at 5% (95% CI, 0% to 19%) and 15% (95% CI, 5% to 30%), respectively (Fig 2). These findings were based on very-low patient numbers and may have been influenced by the type of subsequent anticancer therapy. Five-year OS rates were similar after treatment with D + T 150/1 versus D + T 150/2 (33% v 28%, respectively), although the response rate (50% v 76%), CR rate (9% v 17%), and PFS rate (9% v 13%) after extended follow-up were all markedly higher with the D + T 150/2 dose (Table 4; Fig 1).
Fig 2.
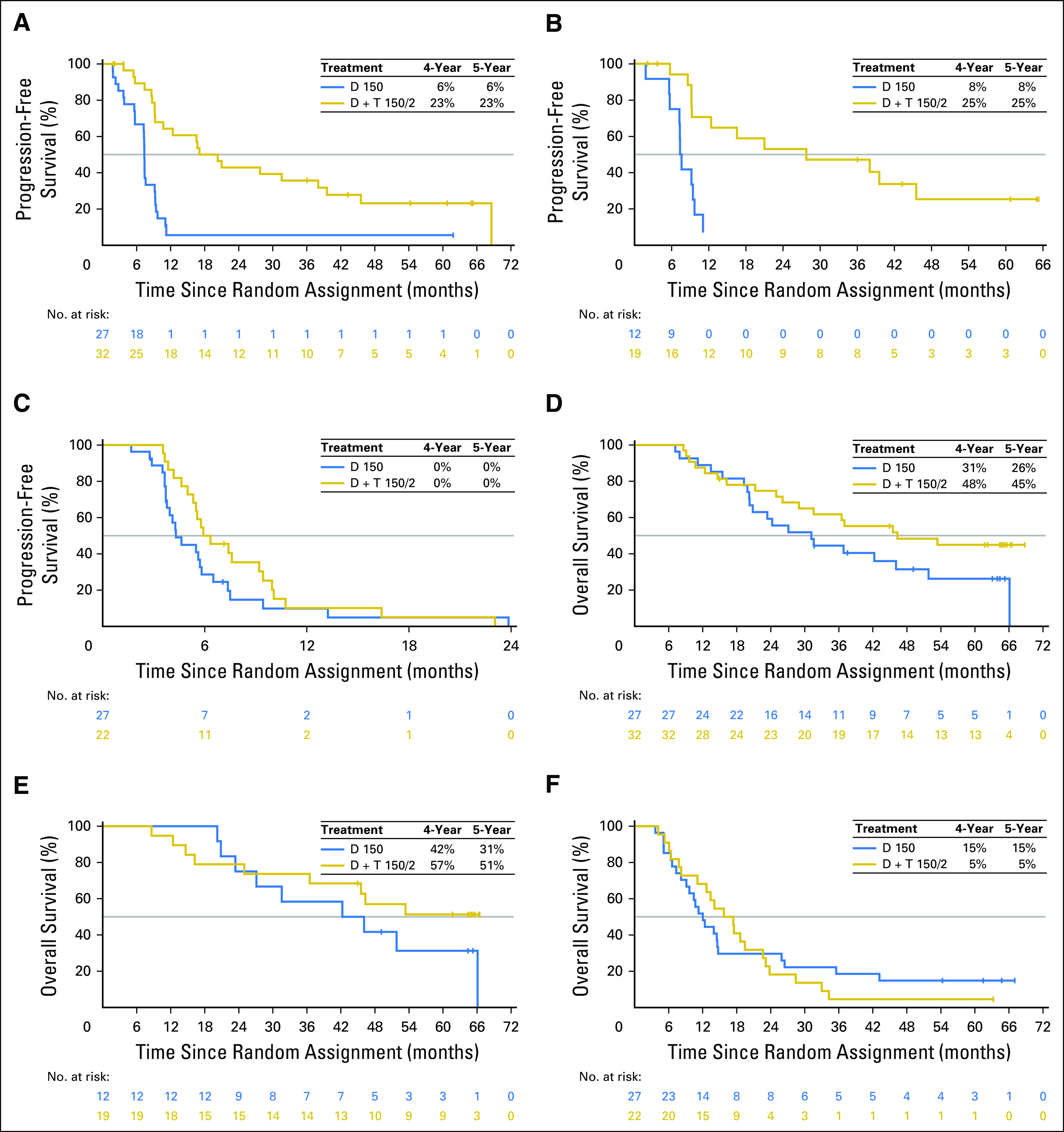
Association of selected patient baseline characteristics on progression-free survival and overall survival in the dabrafenib plus trametinib (D + T) and D monotherapy arms in the intention-to-treat population. (A and D) Patients with normal baseline lactate dehydrogenase (LDH) levels (less than or equal to the upper limit of normal [ULN]). (B and E) Patients with normal baseline LDH levels and fewer than three organ sites with metastasis. (C and F) Patients with elevated baseline LDH (greater than ULN). Patients who progressed on D monotherapy and crossed over to the D 150 mg twice a day plus T 2 mg once daily (D + T 150/2) arm were included and not censored at crossover. D + T 150/1, dabrafenib 150 mg twice a day plus trametinib 1 mg once daily.
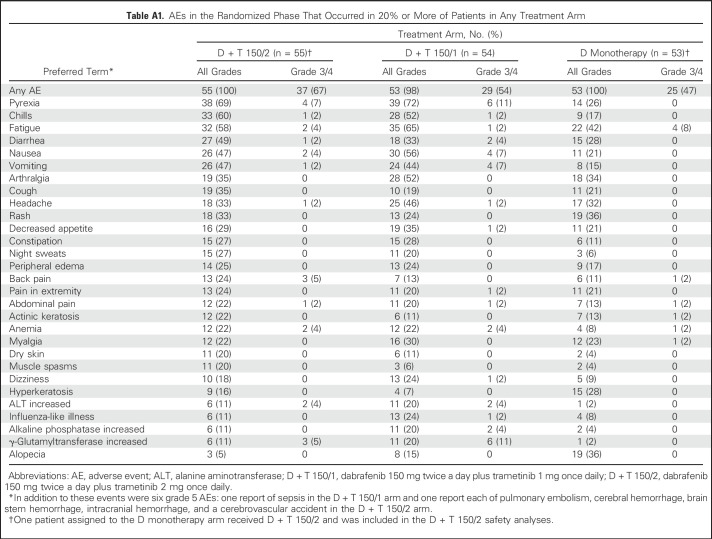
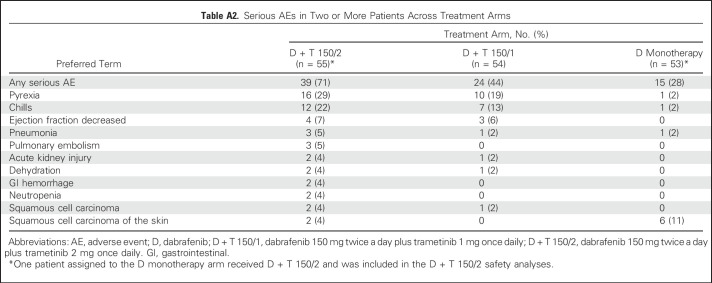
At the time of analysis, median duration of treatment was 10.9 months (range, 1.9 to 68.7 months) for the D + T 150/2 arm and 6.1 months (range, 1.8 to 64.5 months) in the D arm, with 24 (44%) and four (8%) patients in each arm receiving D for > 12 months, respectively, and 22 (40%) in the D + T 150/2 arm receiving T for > 12 months, respectively. All patients in the D + T 150/2 and D monotherapy arms experienced an AE of any grade, with results similar to those previously reported for D + T 150/24,17,31,32 and between the D + T 150/1 and D + T 150/2 treatment doses (Appendix Table A1, online only). Serious AEs were more common with the combined therapy regimens than with D monotherapy (Appendix Table A2, online only).
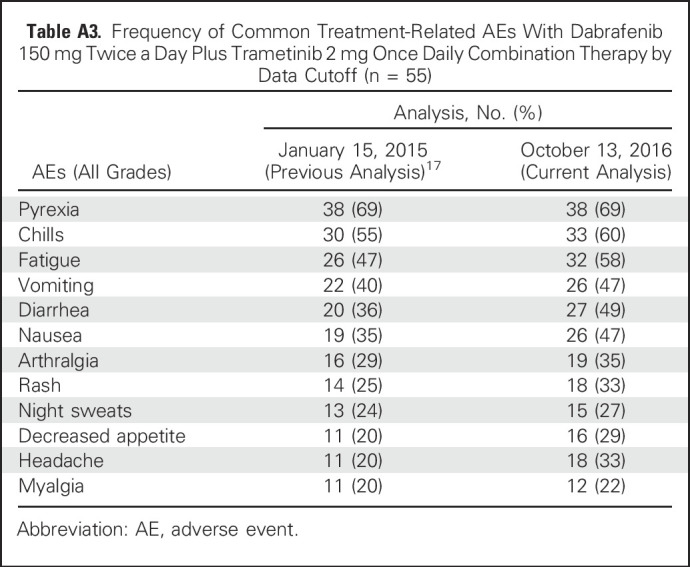
With the additional 21 months of follow-up since the last analysis,17 the frequency of pyrexia, the most commonly reported AE, in the D + T 150/2 arm remained stable at 69%. Reports of some commonly reported AEs (all grades), such as pyrexia, remained the same, whereas others, such as myalgia (2% increase) and headache (13% increase), changed slightly (Appendix Table A3, online only). After 5 years, nine patients (16%) who received D + T 150/2 discontinued study treatment because of an AE, with pyrexia the most frequent cause (two patients [4%]). Furthermore, with an additional approximately 52.5 months of follow-up since the primary analysis,4 the number of AEs that led to dose interruptions (40 patients [73%]) or reductions (33 patients [60%]) in the D + T 150/2 arm increased by 6% and 2%, respectively, beyond the rates reported in the original 14-month analysis.
DISCUSSION
To our knowledge, this 5-year landmark analysis represents the longest follow-up for any randomized trial that has evaluated BRAFi plus MEKi combination therapy and provides evidence that long-term clinical benefit and tolerability are achievable with first-line D + T in some patients with BRAF V600E/K–mutant metastatic melanoma. These results counter the unsupported notion that melanoma treatment with targeted therapies is associated with a rapid initial patient response that is inevitably followed by rapid deterioration as a result of the development of secondary tumor resistance.
The current analysis demonstrates long-term benefit and durable treatment responses in some patients. Although this trend must be confirmed in larger phase III studies, these findings suggest that the survival pattern for patients treated with BRAFi plus MEKi combination therapy that begins at approximately 2 years may mirror the plateau pattern of survival that has been observed with ipilimumab.3 Individual cases of patients who achieve long-term survival when treated with MAPK inhibitors have been previously associated with unusual autoimmune toxicities, which raises the possibility that immune activation may contribute to long-term disease control in isolated patients who receive these drugs.33
Follow-up for anti–PD-1 immune checkpoint inhibitor regimens has generally lagged behind targeted therapy, and 5-year landmark OS results, as reported in this study, are currently available only for a phase I study of nivolumab monotherapy. In patients with melanoma previously treated with one to five lines of prior systemic therapy (n = 107), treatment with nivolumab has resulted in 4- and 5-year OS rates of 35% and 34%, respectively.34 BRAF V600 mutation status and the percentage of patients with stage M1c disease were not reported, but at baseline, 97% of this cohort had an Eastern Cooperative Oncology Group performance status ≤ 1, and 36% showed elevated serum LDH levels. Although cross-trial comparisons are confounded by study differences, such as patient baseline characteristics and the time frame during which studies were conducted, available 5-year landmark data suggest that trends in long-term outcomes are similar between anti–PD-1 and BRAFi plus MEKi combination therapies, although poststudy crossover to the other respective therapy can affect OS.
Because multiple anticancer agents demonstrate significant activity in metastatic melanoma,35 OS results in clinical trials may be confounded by the availability of these various treatments. Because less than one half of patients received subsequent immunotherapy (37%) or small-molecule targeted therapy (24%), one could infer that the effects of D + T largely accounted for the observed 5-year OS. In addition, the study was not powered to compare efficacy between the D + T 150/1 and D + T 150/2 arms, although the landmark PFS and OS were consistently higher in the D + T 150/2 arm to 4 years. At 5 years, the landmark OS was slightly numerically higher in the D + T 150/1 arm; however, the difference was negligible, and the patient numbers were small.
The efficacy of currently available treatments for metastatic melanoma depends on baseline patient characteristics, such as LDH levels and number of organ sites with metastasis, which are key predictive factors for outcomes with D + T.17,26 In the current analysis, although interpretation is limited by small patient numbers in these subsets, the highest 5-year OS was observed among patients with normal baseline LDH levels and three or fewer organ sites with metastasis, and patients with favorable baseline markers who were initiated on first-line D + T 150/2 therapy were most likely to derive long-term benefit. Of note, with an additional 21 months of follow-up from the previous 3-year landmark analysis,17 the RECIST response of one patient who received D + T improved from a PR to a CR, indicating that increased benefit is achievable even after long-term treatment with the combination.
The most commonly reported AE associated with D + T is pyrexia, with fewer instances of the toxicities that are believed to be related to paradoxical MAPK pathway activation with BRAFi monotherapy.4,7,8,17,28,29 Although extended follow-up can result in a more biased study population that consists of patients who remained on and continued to benefit from treatment, the results of the current analysis of 5-year long-term outcomes indicate that long-term D + T treatment is well tolerated in the subgroup of patients who benefit, with no new AEs associated with long-term use.
Overall, this analysis of BRAFi plus MEKi combination therapy demonstrates that long-term survival is achievable with D + T in a proportion of patients with BRAF V600–mutant metastatic melanoma, particularly those with favorable baseline prognostic features. Furthermore, long-term treatment with D + T is tolerable, with no new safety signals. Additional evidence is needed to support optimal treatment strategies for patients with unfavorable prognostic features for melanoma, which remains a great unmet clinical need across the range of current effective melanoma drug therapies.
ACKNOWLEDGMENT
Medical writing in the form of collating author comments, copyediting, and editorial assistance was provided by Christopher J. Barnes and Amanda L. Kauffman of Articulate Science. Their work was funded by Novartis. Additional critical manuscript review comments and editorial assistance were provided by Jorge Moreno-Cantu of Novartis.
Appendix
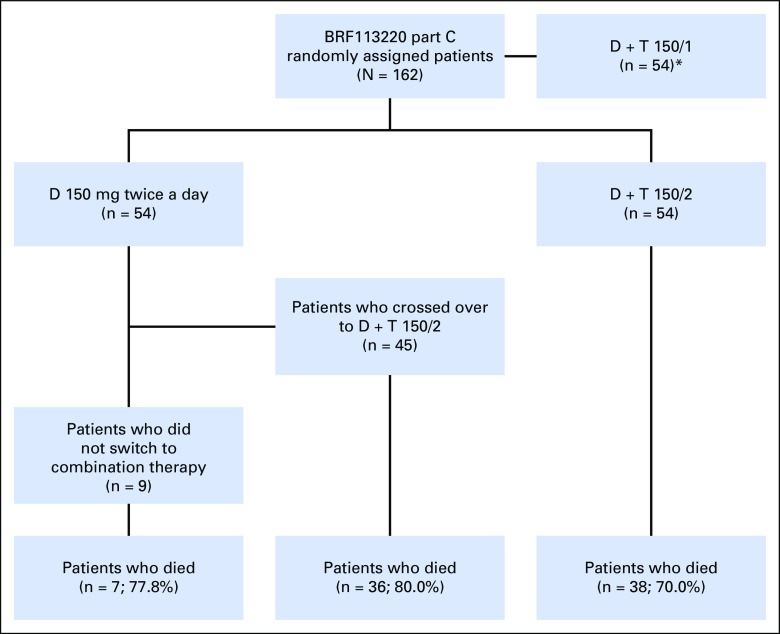
Fig A1.
Study schema for part C of the BRF113220 trial. *Randomized arm not included in this analysis. D, dabrafenib; D + T 150/1, dabrafenib 150 mg twice a day plus trametinib 1 mg once daily; D + T 150/2, dabrafenib 150 mg twice a day plus trametinib 2 mg once daily.
Table A1.
AEs in the Randomized Phase That Occurred in 20% or More of Patients in Any Treatment Arm
Table A2.
Serious AEs in Two or More Patients Across Treatment Arms
Table A3.
Frequency of Common Treatment-Related AEs With Dabrafenib 150 mg Twice a Day Plus Trametinib 2 mg Once Daily Combination Therapy by Data Cutoff (n = 55)
Footnotes
Supported by GlaxoSmithKline. Dabrafenib and trametinib are assets of Novartis as of March 2, 2015.
Presented in part at the European Society for Medical Oncology Congress, Vienna, Austria, September 28-October 2, 2012, and the Annual Meetings of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2011; June 1-5, 2012; May 31-June 4, 2013; May 30-June 3, 2014; and May 29-June 2, 2015.
Clinical trial information: NCT01072175.
AUTHOR CONTRIBUTIONS
Conception and design: Georgina V. Long, Jeffrey Infante, Keith T. Flaherty
Provision of study materials or patients: Georgina V. Long, Adil Daud, Richard Kefford, William Sharfman, Keith T. Flaherty
Collection and assembly of data: Georgina V. Long, Zeynep Eroglu, Jeffrey Infante, Sapna Patel, Adil Daud, Douglas B. Johnson, Rene Gonzalez, Richard Kefford, Omid Hamid, Lynn Schuchter, Jonathan Cebon, William Sharfman, Mario Sznol, Jeffrey Weber, Keith T. Flaherty
Data analysis and interpretation: Georgina V. Long, Jeffrey Infante, Douglas B. Johnson, Omid Hamid, Lynn Schuchter, Jonathan Cebon, William Sharfman, Robert McWilliams, Mario Sznol, Suman Redhu, Eduard Gasal, Bijoyesh Mookerjee, Jeffrey Weber, Keith T. Flaherty
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Outcomes in Patients With BRAF V600–Mutant Metastatic Melanoma Who Received Dabrafenib Combined With Trametinib
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Georgina V. Long
Consulting or Advisory Role: Bristol-Myers Squibb, Roche, Roche (Inst), Genentech, Amgen, Merck MSD, Merck MSD (Inst), Novartis, Novartis (Inst), Array BioPharma, Pierre Fabre
Zeynep Eroglu
Consulting or Advisory Role: Compugen, Sun Pharma
Jeffrey Infante
Research Funding: Celldex Therapeutics (Inst)
Sapna Patel
Consulting or Advisory Role: Castle Biosciences
Speakers’ Bureau: Merck
Research Funding: Bristol-Myers Squibb (Inst), Novartis (Inst), Deciphera Pharmaceuticals (Inst), Reata Pharmaceuticals (Inst)
Adil Daud
Stock or Other Ownership: OncoSec Medical
Consulting or Advisory Role: Pfizer, Merck, Novartis, Genentech
Research Funding: Pfizer, Merck, Bristol-Myers Squibb, Genentech
Douglas B. Johnson
Consulting or Advisory Role: Bristol-Myers Squibb, Genoptix, Merck, Incyte
Research Funding: Incyte
Patents, Royalties, Other Intellectual Property: Intellectual property for OncoSec Medical
Rene Gonzalez
Honoraria: Roche, Novartis, Amgen, Castle Biosciences
Consulting or Advisory Role: Novartis, Amgen, Bristol-Myers Squibb, Castle Biosciences
Research Funding: Novartis (Inst), Amgen (Inst), Bristol-Myers Squibb (Inst), Merck (Inst), Roche (Inst), Incyte (Inst), Syndax (Inst), Array BioPharma (Inst), Boston Biomedical (Inst), Takeda Pharmaceuticals (Inst), Checkmate Pharmaceuticals (Inst), Prometheus (Inst)
Travel, Accommodations, Expenses: Novartis, Bristol-Myers Squibb, Amgen, Castle Biosciences
Richard Kefford
Consulting or Advisory Role: Novartis (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), Amgen (Inst), Teva Neuroscience (Inst)
Speakers’ Bureau: Merck, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Omid Hamid
Honoraria: Bristol-Myers Squibb, Genentech, Novartis, Amgen
Consulting or Advisory Role: Amgen, Novartis, Roche, Bristol-Myers Squibb, Merck
Speakers’ Bureau: Bristol-Myers Squibb, Genentech, Novartis, Amgen
Research Funding: AstraZeneca (Inst), Bristol-Myers Squibb (Inst), Celldex Therapeutics (Inst), Genentech (Inst), Immunocore (Inst), Incyte (Inst), Merck (Inst), Merck Serono (Inst), MedImmune (Inst), Novartis (Inst), Pfizer (Inst), Rinat Neuroscience (Inst), Roche (Inst)
Lynn Schuchter
Research Funding: GlaxoSmithKline (Inst), Merck (Inst), Bristol-Myers Squibb (Inst)
Jonathan Cebon
Honoraria: GlaxoSmithKline, Bristol-Myers Squibb, Novartis, Merck Sharp & Dohme, Amgen, Merck
Consulting or Advisory Role: Amgen (Inst), Bionomics (Inst), Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Bionomics, Novartis, Novartis (Inst)
Research Funding: GlaxoSmithKline (Inst), CSL Behring (Inst)
Patents, Royalties, Other Intellectual Property: GlaxoSmithKline
Expert Testimony: Bristol-Myers Squibb
William Sharfman
Honoraria: Merck, Bristol-Myers Squibb, Castle Biosciences
Consulting or Advisory Role: Merck, Bristol-Myers Squibb, Novartis, Castle Biosciences
Research Funding: Bristol-Myers Squibb, Merck, Novartis
Robert McWilliams
Consulting or Advisory Role: Merrimack (Inst), Bristol-Myers Squibb (Inst), Ipsen (Inst)
Mario Sznol
Stock or Other Ownership: Amphivena, Intensity Therapeutics, Adaptive Biotechnologies
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech, Roche, Amgen, AstraZeneca, MedImmune, Symphogen, Merus, Lion Biotechnologies, Nektar, Eli Lilly, Pfizer, Merck, Adaptimmune, Lycera, Theravance Biopharma, Modulate Therapeutics, Omniox, Seattle Genetics, Inovio Pharmaceuticals, AgonOx, Baxalta-Shire, Arbutus, Agonox, Modulate, Ignyta, Pierre-Fabre, Pieris
Suman Redhu
Employment: Novartis
Stock or Other Ownership: Novartis
Eduard Gasal
Employment: Amgen, Novartis
Stock or Other Ownership: Amgen, Novartis
Travel, Accommodations, Expenses: Amgen, Novartis
Bijoyesh Mookerjee
Employment: Novartis, GlaxoSmithKline
Stock or Other Ownership: Novartis, GlaxoSmithKline, Incyte, AstraZeneca
Jeffrey Weber
Stock or Other Ownership: Altor BioScience, Celldex Therapeutics, CytomX Therapeutics
Honoraria: Bristol-Myers Squibb, Merck, Genentech, AstraZeneca, EMD Sorono, GlaxoSmithKline, Celldex Therapeutics
Consulting or Advisory Role: Celldex Therapeutics, Bristol-Myers Squibb, Merck, Genentech, AstraZeneca, GlaxoSmithKline, EMD Sorono
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), GlaxoSmithKline (Inst), Genentech (Inst)
Patents, Royalties, Other Intellectual Property: Named on a patent submitted by Moffitt Cancer Center for an ipilimumab biomarker, named on a patent from Biodesix for a PD-1 antibody biomarker
Travel, Accommodations, Expenses: Bristol-Myers Squibb, GlaxoSmithKline, Merck, AstraZeneca, Genentech, EMD Sorono
Keith T. Flaherty
Consulting or Advisory Role: Novartis
Research Funding: Novartis
REFERENCES
- 1.Barth A, Wanek LA, Morton DL: Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg 181:193-201, 1995 [PubMed] [Google Scholar]
- 2.Balch CM, Buzaid AC, Soong SJ, et al. : Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 19:3635-3648, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Schadendorf D, Hodi FS, Robert C, et al. : Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 33:1889-1894, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty KT, Infante JR, Daud A, et al. : Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367:1694-1703, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman PB, Hauschild A, Robert C, et al. : Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364:2507-2516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauschild A, Grob JJ, Demidov LV, et al. : Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 380:358-365, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Long GV, Stroyakovskiy D, Gogas H, et al. : Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 386:444-451, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Robert C, Karaszewska B, Schachter J, et al. : Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 372:30-39, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Larkin J, Ascierto PA, Dréno B, et al. : Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 371:1867-1876, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Dummer R, Ascierto PA, Gogas HJ, et al: Results of COLUMBUS part 1: A phase 3 trial of encorafenib (ENCO) plus binimetinib (BINI) versus vemurafenib (VEM) or ENCO in BRAF-mutant melanoma. Presented at the 2016 Society for Melanoma Research Congress, Boston, MA, November 6-9, 2016 [Google Scholar]
- 11. Schachter J, Ribas A, Long GV, et al: Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival analysis of KEYNOTE-006. J Clin Oncol 34, 2016 (suppl; abstr 9504)
- 12.Weber JS, D’Angelo SP, Minor D, et al. : Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16:375-384, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Robert C, Long GV, Brady B, et al. : Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372:320-330, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23-34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McArthur GA, Chapman PB, Robert C, et al. : Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 15:323-332, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chapman PB, Ascierto PA, Schadendorf D, et al: Updated 5-y landmark analyses of phase 2 (BREAK-2) and phase 3 (BREAK-3) studies evaluating dabrafenib monotherapy in patients with BRAF V600–mutant melanoma. J Clin Oncol 35, 2017 (suppl; abstr 9526)
- 17.Long GV, Weber JS, Infante JR, et al. : Overall survival and durable responses in patients with BRAF V600–mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clin Oncol 34:871-878, 2016 [DOI] [PubMed] [Google Scholar]
- 18. Robert C, Karaszewska B, Schachter J, et al: Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/k–mutant cutaneous melanoma. Ann Oncol 27, 2016 (abstr LBA40)
- 19.Ascierto PA, McArthur GA, Dréno B, et al. : Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 17:1248-1260, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Atkinson V, Ascierto PA, Long GV, et al: Two-year survival and safety update in patients with treatment-naive advanced melanoma (MEL) receiving nivolumab or dacarbazine in CheckMate 066. Presented at the 2015 Society for Melanoma Research Congress, San Francisco, CA, November 18-21, 2015 [Google Scholar]
- 21. doi: 10.1200/JCO.2016.71.8023. Larkin J, Minor D, D’Angelo S, et al: Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: A randomized, controlled, open-label phase III trial. J Clin Oncol 36:383-390, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Larkin J, Chiarion-Sileni V, Gonzalez R, et al: Overall survival (OS) results from a phase III trial of nivolumab (NIVO) combined with ipilimumab (IPI) in treatment-naive patients with advanced melanoma (CheckMate 067). Clin Cancer Res 77, 2017 (suppl 13; abstr CT075)
- 23.Long GV, Flaherty KT, Stroyakovskiy D, et al. : Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann Oncol 28:1631-1639, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McArthur GA, Dréno B, Atkinson V, et al: Efficacy of long-term cobimetinib combined with vemurafenib in advanced BRAF V600-mutated melanoma: 3-year follow-up of the phase 3 coBRIM study and 4-year follow-up of the phase 1b BRIM7 study. Presented at the 2016 Society for Melanoma Research Congress, Boston, MA, November 6-9, 2016 [Google Scholar]
- 25. Robert C, Long GV, Schachter J, et al: Long-term outcomes in patients (pts) with ipilimumab (ipi)-naive advanced melanoma in the phase 3 KEYNOTE-006 study who completed pembrolizumab (pembro) treatment. J Clin Oncol 35, 2017 (suppl; abstr 9504)
- 26.Long GV, Grob JJ, Nathan P, et al. : Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: A pooled analysis of individual patient data from randomised trials. Lancet Oncol 17:1743-1754, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Schadendorf D, Long GV, Stroiakovski D, et al. : Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer 82:45-55, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Long GV, Stroyakovskiy D, Gogas H, et al. : Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 371:1877-1888, 2014 [DOI] [PubMed] [Google Scholar]
- 29. Robert C, Karaszewska B, Schachter J, et al: Two year estimate of overall survival in COMBI-v, a randomized, open-label, phase III study comparing the combination of dabrafenib (D) and trametinib (T) with vemurafenib (vem) as first-line therapy in patients (pts) with unresectable or metastatic BRAF V600E/K mutation-positive cutaneous melanoma. Eur J Cancer 51, 2015 (suppl 3; abstr 3301)
- 30. Robert C, Ribas A, Hamid O, et al: Three-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol 34, 2016 (suppl; abstr 9503)
- 31. Grob JJ, Flaherty K, Long GV, et al: Pooled analysis of safety over time and link between adverse events and efficacy across combination dabrafenib and trametinib (D+T) registration trials. J Clin Oncol 34, 2016 (suppl; abstr 9534)
- 32.Grob J, Flaherty KT, Long GV, et al: Pooled analysis of safety with extended 3-year follow-up across combination dabrafenib and trametinib phase 3 trials. Presented at the 2016 Society for Melanoma Research Congress, Boston, MA, November 6-9, 2016 [Google Scholar]
- 33.Klein O, Ribas A, Chmielowski B, et al. : Facial palsy as a side effect of vemurafenib treatment in patients with metastatic melanoma. J Clin Oncol 31:e215-e217, 2013 [DOI] [PubMed] [Google Scholar]
- 34. Hodi FS, Kluger HM, Sznol M, et al: Durable, long-term survival in previously treated patients with advanced melanoma (MEL) who received nivolumab (NIVO) monotherapy in a phase I trial. Cancer Res 76, 2016 (suppl; abstr CT001)
- 35.Devji T, Levine O, Neupane B, et al. : Systemic therapy for previously untreated advanced BRAF-mutated melanoma: A systematic review and network meta-analysis of randomized clinical trials. JAMA Oncol 3:366-373, 2017 [DOI] [PubMed] [Google Scholar]