Abstract
Background & objectives:
The diagnosis of scrub typhus (ST) is usually done using enzyme-linked immunosorbent assay (ELISA) due to its ease of performance and reading objectivity. The cut-off value for ELISA needs to be calculated for each geographical location as it depends on zonal endemicity of the disease. This study was, therefore, undertaken to calculate the pan-India cut-off for anti-Orientia tsutsugamushi (OT) immunoglobulin M (IgM) by ELISA.
Methods:
Samples from cases (cases of ST) and controls (voluntary, consenting, healthy adults) were collected by a network of 29 laboratories across India and tested for anti-OT IgM by immunofluorescence assay (IFA), the considered gold standard test. These samples were retested by ELISA for anti-OT IgM and their optical densities (ODs) were used for cut-off estimation by receiver operating characteristic (ROC) curve.
Results:
Anti-OT IgM ELISA ODs from 273 controls and 136 cases were used for the cut-off estimation. The ODs of the anti-OT IgM ELISA on healthy individuals and those of confirmed ST cases ranged from 0.1 to 0.75 and 0.5 to 4.718, respectively. ROC curve-based cut-off for ELISA was calculated as 0.554 at a sensitivity of 95.2 per cent and specificity of 95.1 per cent. A value of >1 was noted to have a specificity of 100 per cent in diagnosing ST.
Interpretation & conclusions:
The cut-off calculated for India was similar to the previous cut-off that was used until now.
Keywords: Enzyme-linked immunosorbent assay cut-off, indirect immunofluorescence assay, Orientia tsutsugamushi, immunoglobulin M - scrub typhus
In India, scrub typhus (ST) is being considered as a re-emerging disease owing to several reports of its outbreak from different parts of the country1-6. ST is an acute febrile illness caused by the bacterium Orientia tsutsugamushi (OT) belonging to the family Rickettsiaceae2. The clinical manifestations of ST vary from mild illness to life threatening disease, with a mortality rate as high as 50 per cent in the absence of proper treatment. It has been found to be an important cause of acute encephalitis syndrome3, 4, acute febrile illness4,5, acute respiratory distress syndrome6, acute kidney injury6 both from rural3 and urban areas4 and from different ecological niches such as plains, mountains and coastal areas1-3. The usual clinical symptoms of abrupt high-grade fever, myalgia, rash, lymphadenopathy and severe headache overlap considerably with other common prevalent causes in India such as Dengue fever and Chikungunya, thereby making accurate laboratory diagnosis mandatory.
Diagnosis of ST relies on laboratory tests, the most commonly used method being enzyme-linked immunosorbent assay (ELISA). Immunofluorescence assay (IFA) is considered to be the gold standard; however, since it is an expensive and cumbersome method of diagnosis, the most commonly used method for the diagnosis in most laboratories in India is by detection of anti-OT immunoglobulin (Ig) M by ELISA6,7. ELISA offers an economical and technically simple solution for efficiently screening a large number of serum samples, while also enabling the detection of antibody levels8. One of the major challenges in diagnosing scrub typhus by ELISA is the unavailability of a standardized cut-off value. The cut-off for ELISA needs to be calculated for each geographical location as it depends on zonal endemicity of the disease. This study was therefore undertaken to calculate the cut-off for anti-OT IgM by ELISA, for whole of India.
Material & Methods
The study was conducted in the Virus Diagnostic and Research Laboratory (VRDL) at the department of Microbiology, King George’s Medical University (KGMU), Lucknow. The study was approved by the Institutional Ethics Committee of the respective participating centres.
Inclusion & exclusion criteria for study participants: A total of 136 cases and 273 controls were included in the study. Cases were defined as individuals with clinical suspicion of ST presenting as either acute encephalitis syndrome (AES) or fever rash syndrome (FRS) and testing positive for anti-OT IgM by IFA. AES was defined as an individual of any age presenting with acute onset of fever at any time throughout the year and having at least one of the following symptoms: altered sensorium (disorientation, confusion, coma or inability to talk) and/or new onset of seizures (exclusive of simple febrile seizures) as defined by the WHO9. FRS was defined as an individual with a history of febrile illness and rash of <15 days duration. Individuals with a prior history of head injury, hypertension, poisoning; with any other confirmed diagnosis or unwilling to participate were excluded from the study.
Controls were defined as voluntary, consenting, healthy adults testing negative for anti-OT IgM by IFA. After obtaining a written informed consent, approximately 3-5 ml of blood was drawn from both cases and controls. The blood sample was allowed to clot and later centrifuged to separate the serum. The samples were collected over a period of one year from March 2019 to March 2020. Since it was a multi-centric study, the samples were also obtained from 29 VRDLs established across different states of India.
Immunoglobulin M (IgM) indirect immunofluorescence assay (IFA): The serum samples from all participants including cases and controls were subjected to anti-OT IgM estimation by immunofluorescence assay (IFA) (Fuller Laboratories, CA, USA) as per the manufacturer’s instructions. Briefly, serum samples of cases and controls were added to antigen pre-coated IFA slides. The dilution of 1:64 was taken as the screening dilution and any sample testing positive was subjected to further doubling dilutions (up to 1:512). The highest dilution showing fluorescence above the background was considered the endpoint titre.
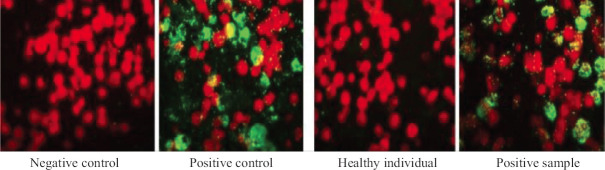
The antigen–antibody reaction was viewed using a fluorescent microscope (Eclipse 90i, Nikon Healthcare, USA) at ×400 magnification. Small green fluorescent dots against counterstained red spherical shaped cells were indicative of a positive reaction and a negative reaction showed no green immunofluorescence (Fig. 1).
Fig. 1.
Images of immunoglobulin M indirect immunofluorescence assay (x 400 magnification).
Immunoglobulin (Ig) M ELISA: Commercial ST Detect IgM ELISA Kit (InBios International Inc., USA) was used for the detection of anti-OT IgM as per the manufacturer’s instructions. OD value of each sample was recorded.
Statistical analysis: Receiver operating characteristic (ROC) curve was generated using SPSS 25 statistical software (v 25.0; SPSS Inc., Chicago, IL, USA). The area under the curve was determined with its 95 per cent confidence interval. The coordinates of the curve were used to determine the appropriate cut-off for the test OD values at a meaningful (≥95%, for either or both) level of sensitivity and specificity.
Results
A set of 290 serum samples collected from healthy volunteers from 29 laboratories located at different sites of India (10 per centre) were used to establish a pan-India cut-off for anti-OT IgM ELISA. Of these 290 samples, 17 tested positive by IFA and hence were excluded. Of these 17 samples, six were obtained from Shimla (IFA titres ≥1:512), three from Kangra (IFA titres ranging between 1:256 and ≥1:512), two from Jaipur (IFA titres 1:64), two from Port Blair (IFA titres ranging between 1:128 and 1:256), two from Srinagar (IFA titres 1:128), one each from Thiruvananthapuram and Raipur (IFA titre 1:64). Only samples from Shimla and Kangra were positive with a high titre (≥1:512). Hence, only 273 sera from healthy controls were used for cut-off estimation. Furthermore, 14 centres could provide a total of 152 samples from positive cases, of which 16 tested negative by IFA. Hence, only 136 IFA-positive samples from cases could be enrolled.
Anti-OT IgM ELISA was performed on all the selected serum samples from both cases and controls. ODs of anti-OT IgM ELISA on serum from healthy individuals and those of confirmed ST cases ranged from 0.1 to 0.75 and 0.5 to 4.718, respectively. Among the healthy individuals, five had ODs between 0.5 and 0.75 and they belonged to Thiruvananthapuram, Srinagar, Raipur, Jaipur and Kangra. With the ROC curve prepared from the OD values of serum samples from cases and controls, the cut-off for ST IgM by ELISA was calculated as 0.554 at a sensitivity of 95.2 per cent and specificity of 95.1 per cent. The area under the curve was calculated as 0.994, which implies a good test. Since the 95% confidence interval (0.990, 0.998), does not contain 0.500 hence we can conclude that our AUC is significantly better. The test was also significant (P<0.05; Fig. 2). The coordinates of the curve obtained are given in the Supplementary Table. A cut-off value of >1 was noted to have a specificity of 100 per cent in diagnosing ST by ELISA.
Fig. 2.
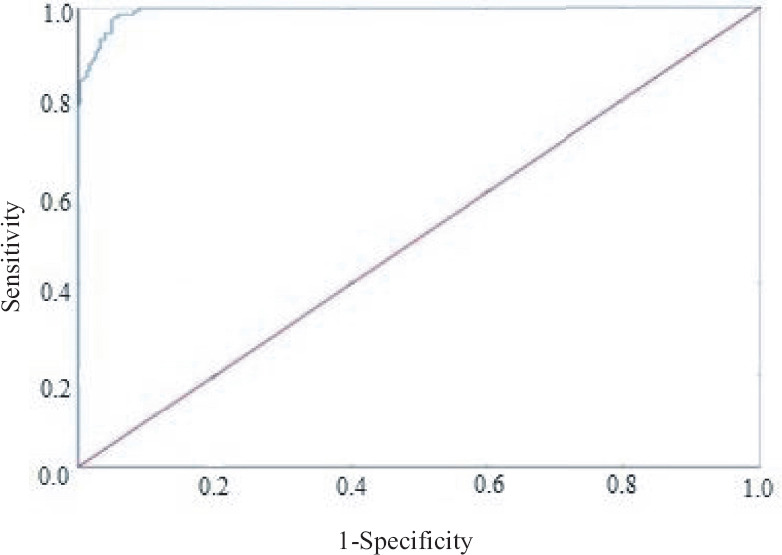
ROC curve analysis for estimation of cut-off. ROC, receiver operating characteristic; ELISA, enzyme-linked immunosorbent assay; OD, optical density.
Supplementary Table.
Coordinates of the curve
| Test result variable (s): Test | ||
|---|---|---|
|
| ||
| Positive if greater than or equal toa | Sensitivity | 1-Specificity |
| −0.946000 | 1.000 | 1.000 |
| 0.056000 | 1.000 | 0.997 |
| 0.058500 | 1.000 | 0.993 |
| 0.064000 | 1.000 | 0.990 |
| 0.070500 | 1.000 | 0.987 |
| 0.072500 | 1.000 | 0.984 |
| 0.073500 | 1.000 | 0.980 |
| 0.075000 | 1.000 | 0.974 |
| 0.076500 | 1.000 | 0.970 |
| 0.078500 | 1.000 | 0.967 |
| 0.080500 | 1.000 | 0.964 |
| 0.082000 | 1.000 | 0.961 |
| 0.084000 | 1.000 | 0.957 |
| 0.086000 | 1.000 | 0.948 |
| 0.088500 | 1.000 | 0.944 |
| 0.090500 | 1.000 | 0.941 |
| 0.091500 | 1.000 | 0.938 |
| 0.092500 | 1.000 | 0.931 |
| 0.094500 | 1.000 | 0.921 |
| 0.097000 | 1.000 | 0.911 |
| 0.098500 | 1.000 | 0.908 |
| 0.099500 | 1.000 | 0.905 |
| 0.100500 | 1.000 | 0.898 |
| 0.101500 | 1.000 | 0.895 |
| 0.102500 | 1.000 | 0.892 |
| 0.103500 | 1.000 | 0.889 |
| 0.104500 | 1.000 | 0.879 |
| 0.105500 | 1.000 | 0.869 |
| 0.106500 | 1.000 | 0.862 |
| 0.107500 | 1.000 | 0.859 |
| 0.108500 | 1.000 | 0.846 |
| 0.109500 | 1.000 | 0.839 |
| 0.110500 | 1.000 | 0.836 |
| 0.111500 | 1.000 | 0.826 |
| 0.113500 | 1.000 | 0.823 |
| 0.116000 | 1.000 | 0.813 |
| 0.117500 | 1.000 | 0.807 |
| 0.120000 | 1.000 | 0.800 |
| 0.122500 | 1.000 | 0.793 |
| 0.123500 | 1.000 | 0.784 |
| 0.124500 | 1.000 | 0.777 |
| 0.126000 | 1.000 | 0.774 |
| 0.127500 | 1.000 | 0.767 |
| 0.129000 | 1.000 | 0.761 |
| 0.130500 | 1.000 | 0.757 |
| 0.131500 | 1.000 | 0.754 |
| 0.132500 | 1.000 | 0.748 |
| 0.133500 | 1.000 | 0.738 |
| 0.134500 | 1.000 | 0.731 |
| 0.135500 | 1.000 | 0.728 |
| 0.137000 | 1.000 | 0.725 |
| 0.138500 | 1.000 | 0.715 |
| 0.139500 | 1.000 | 0.708 |
| 0.141000 | 1.000 | 0.705 |
| 0.143000 | 1.000 | 0.702 |
| 0.144500 | 1.000 | 0.692 |
| 0.146000 | 1.000 | 0.682 |
| 0.147500 | 1.000 | 0.679 |
| 0.149000 | 1.000 | 0.675 |
| 0.150500 | 1.000 | 0.672 |
| 0.152000 | 1.000 | 0.669 |
| 0.153500 | 1.000 | 0.666 |
| 0.154500 | 1.000 | 0.656 |
| 0.155500 | 1.000 | 0.649 |
| 0.157500 | 1.000 | 0.643 |
| 0.160000 | 1.000 | 0.630 |
| 0.161500 | 1.000 | 0.623 |
| 0.164000 | 1.000 | 0.616 |
| 0.166500 | 1.000 | 0.613 |
| 0.168000 | 1.000 | 0.610 |
| 0.169500 | 1.000 | 0.593 |
| 0.171000 | 1.000 | 0.587 |
| 0.173000 | 1.000 | 0.580 |
| 0.174500 | 1.000 | 0.574 |
| 0.176000 | 1.000 | 0.570 |
| 0.177500 | 1.000 | 0.567 |
| 0.178500 | 1.000 | 0.557 |
| 0.180500 | 1.000 | 0.548 |
| 0.182500 | 1.000 | 0.541 |
| 0.183500 | 1.000 | 0.538 |
| 0.184500 | 1.000 | 0.534 |
| 0.185500 | 1.000 | 0.531 |
| 0.186500 | 1.000 | 0.525 |
| 0.187500 | 1.000 | 0.515 |
| 0.188500 | 1.000 | 0.511 |
| 0.189500 | 1.000 | 0.508 |
| 0.191000 | 1.000 | 0.489 |
| 0.192500 | 1.000 | 0.485 |
| 0.193500 | 1.000 | 0.479 |
| 0.194500 | 1.000 | 0.475 |
| 0.196500 | 1.000 | 0.469 |
| 0.198500 | 1.000 | 0.449 |
| 0.199500 | 1.000 | 0.443 |
| 0.201500 | 1.000 | 0.439 |
| 0.203500 | 1.000 | 0.430 |
| 0.204500 | 1.000 | 0.426 |
| 0.205500 | 1.000 | 0.420 |
| 0.207000 | 1.000 | 0.416 |
| 0.208500 | 1.000 | 0.407 |
| 0.209500 | 1.000 | 0.393 |
| 0.210500 | 1.000 | 0.390 |
| 0.211500 | 1.000 | 0.387 |
| 0.212500 | 1.000 | 0.380 |
| 0.213500 | 1.000 | 0.370 |
| 0.214500 | 1.000 | 0.367 |
| 0.217000 | 1.000 | 0.364 |
| 0.219500 | 1.000 | 0.357 |
| 0.220500 | 1.000 | 0.354 |
| 0.222000 | 1.000 | 0.351 |
| 0.227000 | 1.000 | 0.344 |
| 0.231500 | 1.000 | 0.341 |
| 0.233000 | 1.000 | 0.331 |
| 0.234500 | 1.000 | 0.321 |
| 0.238000 | 1.000 | 0.315 |
| 0.241500 | 1.000 | 0.311 |
| 0.243000 | 1.000 | 0.308 |
| 0.244500 | 1.000 | 0.298 |
| 0.247500 | 1.000 | 0.295 |
| 0.250500 | 1.000 | 0.292 |
| 0.252500 | 1.000 | 0.289 |
| 0.257500 | 1.000 | 0.282 |
| 0.263000 | 1.000 | 0.279 |
| 0.266000 | 1.000 | 0.275 |
| 0.267500 | 1.000 | 0.272 |
| 0.268500 | 1.000 | 0.269 |
| 0.269500 | 1.000 | 0.259 |
| 0.271500 | 1.000 | 0.256 |
| 0.273500 | 1.000 | 0.252 |
| 0.275500 | 1.000 | 0.249 |
| 0.278000 | 1.000 | 0.246 |
| 0.279500 | 1.000 | 0.243 |
| 0.281500 | 1.000 | 0.239 |
| 0.283500 | 1.000 | 0.233 |
| 0.285000 | 1.000 | 0.230 |
| 0.287000 | 1.000 | 0.226 |
| 0.288500 | 1.000 | 0.223 |
| 0.289500 | 1.000 | 0.220 |
| 0.293000 | 1.000 | 0.213 |
| 0.299500 | 1.000 | 0.210 |
| 0.307000 | 1.000 | 0.203 |
| 0.314500 | 1.000 | 0.200 |
| 0.318500 | 1.000 | 0.197 |
| 0.319500 | 1.000 | 0.193 |
| 0.321000 | 1.000 | 0.190 |
| 0.323500 | 1.000 | 0.187 |
| 0.326000 | 1.000 | 0.184 |
| 0.328000 | 1.000 | 0.180 |
| 0.330500 | 1.000 | 0.174 |
| 0.337000 | 1.000 | 0.167 |
| 0.343500 | 1.000 | 0.164 |
| 0.350000 | 1.000 | 0.161 |
| 0.355500 | 1.000 | 0.157 |
| 0.357000 | 1.000 | 0.148 |
| 0.359500 | 1.000 | 0.144 |
| 0.361500 | 1.000 | 0.141 |
| 0.364000 | 1.000 | 0.138 |
| 0.371000 | 1.000 | 0.134 |
| 0.380000 | 1.000 | 0.131 |
| 0.388500 | 1.000 | 0.128 |
| 0.398500 | 1.000 | 0.125 |
| 0.406500 | 1.000 | 0.118 |
| 0.409500 | 1.000 | 0.111 |
| 0.411500 | 1.000 | 0.108 |
| 0.419500 | 1.000 | 0.105 |
| 0.426500 | 1.000 | 0.098 |
| 0.430000 | 1.000 | 0.095 |
| 0.438500 | 1.000 | 0.092 |
| 0.445500 | 1.000 | 0.089 |
| 0.447500 | 0.993 | 0.089 |
| 0.450500 | 0.993 | 0.085 |
| 0.454500 | 0.993 | 0.082 |
| 0.456500 | 0.986 | 0.082 |
| 0.467500 | 0.986 | 0.079 |
| 0.485000 | 0.986 | 0.075 |
| 0.502000 | 0.986 | 0.069 |
| 0.516000 | 0.986 | 0.066 |
| 0.520500 | 0.986 | 0.059 |
| 0.522000 | 0.980 | 0.059 |
| 0.526500 | 0.980 | 0.056 |
| 0.536000 | 0.980 | 0.052 |
| 0.542500 | 0.973 | 0.049 |
| 0.543500 | 0.966 | 0.049 |
| 0.547500 | 0.959 | 0.049 |
| 0.554000 | 0.952 (95.2%) | 0.049 (95.1%) |
| 0.562000 | 0.946 | 0.049 |
| 0.577500 | 0.946 | 0.046 |
| 0.598500 | 0.946 | 0.043 |
| 0.610500 | 0.946 | 0.039 |
| 0.619500 | 0.932 | 0.039 |
| 0.628500 | 0.932 | 0.036 |
| 0.632500 | 0.932 | 0.033 |
| 0.639000 | 0.918 | 0.033 |
| 0.649000 | 0.912 | 0.033 |
| 0.655500 | 0.912 | 0.030 |
| 0.661000 | 0.905 | 0.030 |
| 0.669000 | 0.905 | 0.026 |
| 0.674500 | 0.891 | 0.026 |
| 0.677500 | 0.891 | 0.023 |
| 0.678500 | 0.884 | 0.023 |
| 0.686000 | 0.884 | 0.020 |
| 0.713000 | 0.878 | 0.020 |
| 0.737000 | 0.878 | 0.016 |
| 0.757000 | 0.871 | 0.016 |
| 0.774500 | 0.864 | 0.016 |
| 0.782500 | 0.864 | 0.013 |
| 0.797500 | 0.857 | 0.013 |
| 0.808500 | 0.850 | 0.013 |
| 0.830500 | 0.850 | 0.010 |
| 0.868500 | 0.844 | 0.007 |
| 0.893500 | 0.844 | 0.003 |
| 0.909500 | 0.837 | 0.003 |
| 0.920000 | 0.830 | 0.003 |
| 0.950500 | 0.816 | 0.003 |
| 0.982500 | 0.810 | 0.003 |
| 0.986000 | 0.803 | 0.003 |
| 0.988000 | 0.796 | 0.003 |
| 1.006000 | 0.782 | 0.000 |
| 1.029000 | 0.769 | 0.000 |
| 1.039500 | 0.762 | 0.000 |
| 1.046500 | 0.755 | 0.000 |
| 1.051500 | 0.748 | 0.000 |
| 1.054500 | 0.741 | 0.000 |
| 1.062500 | 0.735 | 0.000 |
| 1.073500 | 0.728 | 0.000 |
| 1.083500 | 0.721 | 0.000 |
| 1.090500 | 0.714 | 0.000 |
| 1.091500 | 0.707 | 0.000 |
| 1.101000 | 0.701 | 0.000 |
| 1.118500 | 0.694 | 0.000 |
| 1.136000 | 0.687 | 0.000 |
| 1.188500 | 0.680 | 0.000 |
| 1.244500 | 0.673 | 0.000 |
| 1.265000 | 0.667 | 0.000 |
| 1.281500 | 0.660 | 0.000 |
| 1.302000 | 0.653 | 0.000 |
| 1.316500 | 0.646 | 0.000 |
| 1.319500 | 0.639 | 0.000 |
| 1.335500 | 0.633 | 0.000 |
| 1.355500 | 0.626 | 0.000 |
| 1.369000 | 0.619 | 0.000 |
| 1.401500 | 0.612 | 0.000 |
| 1.484000 | 0.605 | 0.000 |
| 1.594000 | 0.599 | 0.000 |
| 1.656500 | 0.592 | 0.000 |
| 1.673000 | 0.585 | 0.000 |
| 1.695000 | 0.578 | 0.000 |
| 1.740000 | 0.571 | 0.000 |
| 1.777350 | 0.565 | 0.000 |
| 1.821350 | 0.558 | 0.000 |
| 1.868000 | 0.551 | 0.000 |
| 1.884000 | 0.544 | 0.000 |
| 1.893000 | 0.537 | 0.000 |
| 1.903000 | 0.531 | 0.000 |
| 1.933500 | 0.524 | 0.000 |
| 1.970500 | 0.517 | 0.000 |
| 1.984500 | 0.503 | 0.000 |
| 1.988000 | 0.490 | 0.000 |
| 1.989500 | 0.476 | 0.000 |
| 1.994500 | 0.469 | 0.000 |
| 2.025000 | 0.463 | 0.000 |
| 2.075500 | 0.456 | 0.000 |
| 2.100500 | 0.449 | 0.000 |
| 2.101500 | 0.442 | 0.000 |
| 2.106000 | 0.435 | 0.000 |
| 2.111000 | 0.429 | 0.000 |
| 2.116000 | 0.415 | 0.000 |
| 2.120500 | 0.408 | 0.000 |
| 2.122000 | 0.401 | 0.000 |
| 2.123500 | 0.388 | 0.000 |
| 2.129000 | 0.381 | 0.000 |
| 2.158500 | 0.374 | 0.000 |
| 2.203000 | 0.367 | 0.000 |
| 2.233500 | 0.361 | 0.000 |
| 2.262500 | 0.354 | 0.000 |
| 2.297000 | 0.340 | 0.000 |
| 2.360500 | 0.333 | 0.000 |
| 2.410000 | 0.327 | 0.000 |
| 2.415500 | 0.320 | 0.000 |
| 2.602000 | 0.313 | 0.000 |
| 2.871500 | 0.306 | 0.000 |
| 2.980000 | 0.299 | 0.000 |
| 3.051000 | 0.293 | 0.000 |
| 3.105000 | 0.286 | 0.000 |
| 3.116500 | 0.279 | 0.000 |
| 3.160500 | 0.272 | 0.000 |
| 3.205000 | 0.265 | 0.000 |
| 3.212500 | 0.259 | 0.000 |
| 3.217000 | 0.252 | 0.000 |
| 3.296000 | 0.245 | 0.000 |
| 3.441000 | 0.231 | 0.000 |
| 3.512000 | 0.218 | 0.000 |
| 3.618000 | 0.211 | 0.000 |
| 3.795500 | 0.204 | 0.000 |
| 3.885000 | 0.197 | 0.000 |
| 3.922500 | 0.184 | 0.000 |
| 3.961000 | 0.177 | 0.000 |
| 3.981500 | 0.170 | 0.000 |
| 3.985000 | 0.156 | 0.000 |
| 4.004000 | 0.150 | 0.000 |
| 4.059000 | 0.143 | 0.000 |
| 4.098000 | 0.136 | 0.000 |
| 4.105500 | 0.129 | 0.000 |
| 4.117000 | 0.122 | 0.000 |
| 4.148500 | 0.116 | 0.000 |
| 4.175000 | 0.109 | 0.000 |
| 4.181000 | 0.102 | 0.000 |
| 4.198500 | 0.095 | 0.000 |
| 4.257500 | 0.082 | 0.000 |
| 4.309000 | 0.075 | 0.000 |
| 4.318000 | 0.068 | 0.000 |
| 4.330500 | 0.061 | 0.000 |
| 4.342500 | 0.054 | 0.000 |
| 4.347500 | 0.048 | 0.000 |
| 4.373500 | 0.041 | 0.000 |
| 4.418000 | 0.034 | 0.000 |
| 4.469000 | 0.027 | 0.000 |
| 4.514500 | 0.020 | 0.000 |
| 4.623500 | 0.014 | 0.000 |
| 4.885500 | 0.007 | 0.000 |
| 6.053000 | 0.000 | 0.000 |
apositive actual state
Discussion
Appropriate diagnostic cut-offs are important for correct diagnosis and management of infectious diseases including scrub typhus. Lower cut-offs often result in false-positive results leading to the risk of unnecessary treatment. Higher cut-offs on the other hand would result in false-negative results leading to the risk of missing the true positives. In the present study, we have shown a range of possible cut-offs for anti-OT IgM ELISA that can be used for diagnosis of ST infection throughout India (Supplementary Table). The most appropriate cut-off was estimated to be an OD value of 0.554. Similar recommendation was also made in the Indian Council of Medical Research (ICMR) guidelines for managing rickettsial disease10. However, commercial kits recommend estimation of cut-off for each geographical area. So far only a few studies are available from India that have calculated region - specific cut-offs7,11, using different ways of analysis. Using ROC curve analysis, Gupta et al7 and Patricia et al12 determined the cut-off as 0.89 and 0.406, respectively, for regions in and around Delhi and Puducherry, respectively. Using mean OD+3SD analysis, the cut-offs were determined as 0.89 for Delhi region13 and 0.56 for Puducherry14. Therefore, it can be seen that even for the same region, different observers estimated different cut-off values by using various methodologies. A study conducted in Laos estimated the cut-off for an in-house developed anti-ST IgM ELISA to be between 0.8 and 1.0 by plotting an ROC curve15. In contrast, in Thailand, this cut-off was reported around 2.0 for an in-house developed ELISA by ROC curve estimation16. A meta-analysis17 showed that of the total studies considered, 47.5 per cent (n=22) either did not explain or explained insufficiently about the methods for calculation of cut-off and 15.2 per cent (n=7) did not even mention the cut-off. They concluded that based on the available literature, no consensus cut-off OD could be determined17. The available studies, in addition to a lack of standardized ELISA, also differ in what is considered as the gold standard reference assay to determine diagnostic cut-offs. Therefore, a more elaborate study was required to estimate ELISA cut-off. In the present study, sites for sample collection were chosen such that almost all the States of India were represented to derive a common cut-off.
The accurate diagnosis of ST is essential for patient management using appropriate antibiotic therapy and to prevent complications leading to significant detrimental effects. ST is known to be endemic in India2,4, still its diagnosis is often missed because it shares the seasonal prevalence (post monsoon period) and clinical manifestations with other febrile diseases like dengue or chikungunya fever. Weil Felix test is still widely used in India10 for establishing its serological diagnosis but ELISA is preferred because of its better sensitivity and specificity without significantly adding to the cost or requirement of technical expertise. In the present study, IFA was considered as the gold standard for diagnosis. The positive samples that were chosen for our study were also tested for other co-infections using tests such as dengue IgM, chikungunya IgM and Japanese encephalitis IgM so as to reduce the chances of cross-reactivity.
In the present study, serum samples from five healthy individuals had ELISA OD values above the cut-off (range 0.5-0.75) but gave negative results when tested on the gold standard IFA. Such an ELISA positivity may indicate the chances of false positivity due to the cross-reactivity with other pathogens. It is therefore, recommended that the results of IgM detection should always be clinically correlated before starting specific treatment.
Serum samples from 17 healthy individuals tested positive on IFA, which were excluded while calculating the cut-off. Majority of these samples with high IFA titres (≥1:256) were from the State of Himachal Pradesh (HP) which is an endemic region where subclinical infections and reinfections commonly occur13. Antibodies may also persist after recovery from previous ST or other rickettsial infections. Therefore, the cut-off of 0.554 OD may not be applicable for such areas. We recommend estimating separate cut-offs for highly endemic areas.
The limitation of this study was that since the number of samples from each State was low, a separate OD could not be established for each area. However, since the samples were obtained from healthy controls and diseased individuals from various geographical areas across the country, the cut-off OD can be considered as representative of the whole country.
The present study suggests a cut-off OD value of 0.554 for anti-OT IgM estimation, a value slightly higher than published ICMR guidelines10. This value is suggested for use in India for the detection of anti-ST IgM antibodies using ELISA kits from InBios International Inc. USA or any other commercial ELISA kit using the same recombinant antigens of Gilliam, Karp, Kato and Boryong strains of OT in the same proportion. This value may not be applicable for kits using other antigens or different combinations of these antigens and for the highly-endemic areas.
Financial support and sponsorship
The study was financially supported by the Indian Council of Medical Research, Department of Health Research, New Delhi (grant number: 15013/03/2019-HR-VRDL).
Conflicts of interest
None.
Acknowledgment:
The authors acknowledge Prof H.S. Malhotra, Department of Neurology, King George’s Medical University, Lucknow, for his guidance and support for calculation of cut-off by ROC.
References
- 1.Gurung S, Pradhan J, Bhutia PY. Outbreak of scrub typhus in the North East Himalayan region-Sikkim: An emerging threat. Indian J Med Microbiol. 2013;31:72–4. doi: 10.4103/0255-0857.108729. [DOI] [PubMed] [Google Scholar]
- 2.Narvencar KP, Rodrigues S, Nevrekar RP, Dias L, Dias A, Vaz M, et al. Scrub typhus in patients reporting with acute febrile illness at a tertiary health care institution in Goa. Indian J Med Res. 2012;136:1020–4. [PMC free article] [PubMed] [Google Scholar]
- 3.Takhar RP, Bunkar ML, Arya S, Mirdha N, Mohd A. Scrub typhus: A prospective, observational study during an outbreak in Rajasthan, India. Natl Med J India. 2017;30:69–72. [PubMed] [Google Scholar]
- 4.Premraj SS, Mayilananthi K, Krishnan D, Padmanabhan K, Rajasekaran D. Clinical profile and risk factors associated with severe scrub typhus infection among non-ICU patients in semi-urban south India. J Vector Borne Dis. 2018;55:47–51. doi: 10.4103/0972-9062.234626. [DOI] [PubMed] [Google Scholar]
- 5.Pathania M, Amisha, Malik P, Rathaur VK. Scrub typhus: Overview of demographic variables, clinical profile, and diagnostic issues in the sub-Himalayan region of India and its comparison to other Indian and Asian studies. J Family Med Prim Care. 2019;8:1189–95. doi: 10.4103/jfmpc.jfmpc_124_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain D, Nand N, Giri K, Bhutani J. Scrub typhus infection, not a benign disease: An experience from a tertiary care center in Northern India. Med Pharm Rep. 2019;92:36–42. doi: 10.15386/cjmed-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta N, Chaudhry R, Thakur CK. Determination of Cutoff of ELISA and immunofluorescence assay for scrub typhus. J Glob Infect Dis. 2016;8:97–9. doi: 10.4103/0974-777X.188584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan M, Chan KH, Peiris JS, Kwan SW, Lam SY, Pang CM, et al. Evaluation and validation of an enzyme-linked immunosorbent assay and an immunochromatographic test for serological diagnosis of severe acute respiratory syndrome. Clin Diagn Lab Immunol. 2004;11:699–703. doi: 10.1128/CDLI.11.4.699-703.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.PATH. Navigation vaccine introduction: a guide for decision makers Japanese Encephalitis (JE). Module 1: Does my country need JE vaccine? [accessed on January 26, 2017]. Available from: http://www.path.org/files/WHO_surveillance_standards_JE.pdf .
- 10.Rahi M, Gupte MD, Bhargava A, Varghese GM, Arora R. DHR-ICMR Guidelines for diagnosis &management of Rickettsial diseases in India. Indian J Med Res. 2015;141:417–22. doi: 10.4103/0971-5916.159279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pote K, Narang R, Deshmukh P. Diagnostic performance of serological tests to detect antibodies against acute scrub typhus infection in central India. Indian J Med Microbiol. 2018;36:108–12. doi: 10.4103/ijmm.IJMM_17_405. [DOI] [PubMed] [Google Scholar]
- 12.Patricia KA, Hoti SL, Kanungo R, Jambulingam P, Shashikala N, Naik AC. Improving the diagnosis of scrub typhus by combining groEL based polymerase chain reaction and IgM ELISA. J Clin Diagn Res. 2017;11:DC27–31. doi: 10.7860/JCDR/2017/26523.10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta N, Chaudhry R, Kabra SK, Lodha R, Mirdha BR, Das BK, et al. Comparative evaluation of serological and molecular methods for the diagnosis of scrub typhus in Indian settings. Jpn J Infect Dis. 2017;70:221–2. doi: 10.7883/yoken.JJID.2016.139. [DOI] [PubMed] [Google Scholar]
- 14.Anitharaj V, Stephen S, Pradeep J, Park S, Kim SH, Kim YJ, et al. Serological diagnosis of acute scrub typhus in Southern India: Evaluation of InBios Scrub Typhus Detect IgM Rapid Test and Comparison with other Serological Tests. J Clin Diagn Res. 2016;10:DC07–10. doi: 10.7860/JCDR/2016/24051.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elders PND, Dhawan S, Tanganuchitcharnchai A, Phommasone K, Chansamouth V, Day NPJ, et al. Diagnostic accuracy of an in-house Scrub Typhus enzyme linked immunoassay for the detection of IgM and IgG antibodies in Laos. PLoS Negl Trop Dis. 2020;14:e0008858. doi: 10.1371/journal.pntd.0008858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phanichkrivalkosil M, Tanganuchitcharnchai A, Jintaworn S, Kantipong P, Laongnualpanich A, Chierakul W, et al. Determination of optimal diagnostic cut-offs for the Naval Medical Research Center Scrub Typhus IgM ELISA in Chiang Rai, Thailand. Am J Trop Med Hyg. 2019;100:1134–40. doi: 10.4269/ajtmh.18-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saraswati K, Phanichkrivalkosil M, Day NPJ, Blacksell SD. The validity of diagnostic cut-offs for commercial and in-house scrub typhus IgM and IgG ELISAs: A review of the evidence. PLoS Negl Trop Dis. 2019;13:e0007158. doi: 10.1371/journal.pntd.0007158. [DOI] [PMC free article] [PubMed] [Google Scholar]