ABSTRACT
Premetastatic niche (PMN) concept, introduced by David Lyden and colleagues, is an area that can support cancer cells to nurture in it, but the area itself being bereft of cancerous cells. It provides a microenvironment that is congenial for tumor invasion, endurance, and or proliferation of malignant cells to develop into metastasis. These are noncancerous variations in a tumor-free organ and are the most primitive indications of metastasis. These may have a potential to serve as a diagnostic aid, prognostic biomarkers, and therapeutic target. Nevertheless, there is still no clear elucidation on diverse trails of tumor metastasis via lymphatic or hematogenous route, especially in relationship with the PMN. In this review, contemporary knowledge associated with nodal premetastatic niche formation with forthcoming directions on translational and clinical research is deliberated.
KEYWORDS: Biomarker, circulating tumor cells, lymph node, metastasis, premetastatic niche
INTRODUCTION
It has been suggested that the lymph node offers a fertile soil for cancer cell seeding, proliferation, and an alleyway for metastasis.[1-3] These acts as the key metastasis spot and is a decisive prognostic parameter in diverse tumor types.[3,4] Erstwhile to metastatic spread, the primary tumor initiates the sentinel lymph node remodeling by releasing extracellular vesicles, soluble factors, variety of cytokines, and growth factors.[5-8] These imperative noncancerous changes are branded as a pre-metastatic niche which may determine subsequent survival and growth of metastatic cells.[8]
DISCUSSION
A premetastatic niche (PMN) is devoid of tumor cells but has gained cancer-allied properties that can support cancer cell from distant tissue to foster within.[9-10]
Though the PMN, a new terminology, Steven Paget first anticipated it in his “Seed and Soil Hypothesis”.[1] He proposed that there occurs a cross-talk among the cancerous cell and the microenvironment. This seed and soil theory has been later reinforced by emerging evidences that primary tumor may induce systemic modifications by cellular factors to rheostat and initiate microenvironment in the secondary distant organ sites for facilitating and orchestrating circulating tumor cell (CTC) colonization which has also been termed as PMN.[11] Premetastatic niches are now generally presumed to be a true biological process promoting metastatic growth, though there is a difference of opinion onto whether their formation is obligatory for metastases formation.[10-12] Mounting evidences show that metastasis cascades ideally necessitate two events, that is, PMN formation and metastasis formation.[13-15] It is apparent that tumor-promoting PMN formation in secondary organs may bring in an unrecognized degree of complexity in curing metastatic disease.[13-16]
Metastatic environment
Metastasis is a multifaceted process which encompasses the tumor cell dissemination from the primary site to distant organs.[14,17] A favorable environment, pooled of nutrients, extracellular matrix, and immune cells dictate successful seeding of tumor cells in a distant organ.[16,18] This permissive metabolic nutrient network enables cancer cells to survive and seed in a specific site. Metastatic seeding is augmented by certain nutrients and might be controlled in PMN. The scaffold in extracellular matrix attaches and reactivates the cancer cell survival. Though distinct outlines of metastasis can be distinguished varying in different tumor types, lymph node seems to be the most common area where metastatic environment prevails. Lymph node has a bridgehead in the metastatic dissemination of tumors.
Structure and function of lymphatics
Lymph node is structurally demarcated as the cortex, paracortex, and medulla. Dendritic cells reach at the lymph nodes through afferent vessels and then drift into cortex. In germinal follicles, B lymphocytes interrelate with follicular dendritic cells and in paracortex T lymphocytes intermingle with dendritic cells and followed by interactions on reticular fibers. Migration of reticular fibers to the high endothelial venules (HEVs) occurs where they relate with native lymphocytes.
The lymphatic organization is an incessant unidirectional network consisting lymphatic capillaries, vessels, and lymphoid organs (like LNs) which play that a major role remains vivacious for upholding fluid homeostasis. It facilitates lipid absorption, transportation of immune cells, soluble antigens, and so on from peripheral tissues to lymph nodes and vascular system.[14]
Establishment of lymph node PMN
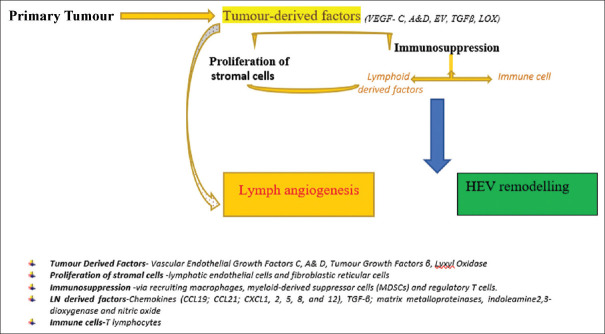
Lymph angiogenesis and HEV remodeling are decisive vascular events in the premetastatic niche formation. Integrin, erythropoietin, VEGF-A, and VEGF-C may be associated with lymph angiogenesis which surges systemic metastatic potential.[14,18] The primary tumor releases tumor-derived factors (like VEGF-C) leading to proliferation of stromal cells. The proliferation and migration of lymphatic endothelial cell induce budding and widening of lymphatic vessels; thus, the probable lymphatic contact surface with tumor cells is expanded. The largening of collecting lymphatics occurs, resulting in an enhanced flow rate, thus increasing sentinel LN metastases.
The primary tumor secretes soluble factors leading to lymphatic remodeling in draining LNs even before the arrival of tumor cells in it.[19] The expanded lymphatic system in LNs leads to formation of the premetastatic niche which later fosters metastasis.[14,15] The establishment of lymph node PMN is depicted in a simple flowchart below [Figure 1].
Figure 1.
Schematic representation on Establishment of PMN in Lymph nodes
Effects of tumor on lymph nodes
LN is exposed to molecular, cellular, and histological remodeling under pathological conditions. In malignant conditions, it is proposed that an antitumoral response is induced by tumor antigens that initially arrest metastasis.[14] As tumor progress further, immunomodulatory factors drained from primary tumor create an immunosuppressive environment which accelerates and supports metastatic outgrowth.[19] Tumor-associated macrophages, Tregs (regulatory T cells), myeloid-derived suppressor cells, and so on are the key immune cells in tumor growth and metastasis. The gestures of an immunosuppressive microenvironment and a chronic inflammatory milieu support the metastatic outgrowth. These may later inhibit antitumoral immune events of CD4, CD8 T cells, and natural killer cells. Immune cells that actively support premetastatic niche formation and LN metastatic cell colonization ultimately depart from LN into the circulatory system.[16,19]
PMN formation and the sequelae
PMN is a combined effect of secretory factors and extracellular vesicles shed by the primary tumor. Exosomes are macrovesicles with diameter less than 100 nm carrying a variety of consignments like DNA, miRNAs, mRNAs, and proteins. Exosomes fuse with target cells and regulate cell behavior. The first in the sequence of events that occur in PMN is vascular leakiness followed by local milieu changes that attract CTCs.[19] The migratory tumor cells found in peripheral blood are known to be CTCs but seen in bone marrow are generally called disseminated tumor cells (DTCs). B Costa-Silva et al.[20] suggested pancreatic cancer-derived exosomes enriched by macrophage migration inhibitory factor persuade PMN formation in the liver, ensuing an augmented metastatic burden in liver. After tumor basement membrane degradation, cells invade and advance toward the vasculature forming CTCs.CTCs home toward niche microenvironments at a target organ by absconding the vasculature by a process called extravasation and are classified as a DTC. DTCs may be capable of adhering and colonizing at the site only if a permissible niche exists. The team from Tel Aviv University reported molecular markers of PMN found to be expressed in cervical lymph nodes in oral cancer, suggesting the better permissive pathway remotely tiled by the primary oral tumor for receiving the metastatic cells.[21,22]
Tumor-derived exosomes have been found to drain to lymph nodes to modify the microenvironment, thus helping a premetastatic niche formation. Recently, studies on melanoma revealed exosomes seen to adhere to regional lymph nodes. They supposed to organize a variety of premetastatic changes like remodeling, angiogenesis, and immune suppression, resulting in consequent recruitment of disseminating tumor cells to exosome-rich sites in the lymph node.[23]
PMN concept is unique which propositions that the primary tumor preconditions definite organ sites for future metastasis via tumor-derived factors. Targeting the PMN may have a potential to prevent metastasis.[15] These elements in premetastatic–metastatic cascades like CTCs and exosomes add diagnostic value to the PMN concept.[24] CTCs, circulating tumor DNA, and exosomes are noninvasive liquid biopsy technique which aid in diagnosis, treatment, and subsequent follow-ups for disease monitoring.[20,24,25]
CONCLUSION
Oral cancer is the most common head and neck malignancies with tumor fatality rates in its peak. Cancer research has engrossed excessive on metastasis with relatively little therapeutically or diagnostically effective on metastasis prevention. PMN has attracted researches inward toward a newer concept in metastasis or relapse. Better insights into the molecular, biological characterization and function allow us in developing ideal biomarkers for early diagnosis and have therapeutic inferences. PMN can reverse immune suppression induced by tumor with a broader perception of molecular signals. PMN lymph nodes could provide therapeutic targets to arrest metastasis. Also, impending researchers can develop biomarkers to recognize patients at peril of lymph node metastases before it progressing toward an incurable stage. These also have greater diagnostic implications in monitoring oral cancer evolution, relapse, and metastasis.
Financial support and sponsorship
The manuscript was supported partly by DHR(GoI) research grant (1101-2021).
Conflict of interest
The authors declare no conflict of interest.
Acknowledgment
This manuscript was a part of the research grant from ICMR–DHR (1101-2021) HRD Scheme which is being availed by Rajalakshmi G.
CTCs = Circulating Tumor Cells
DTCs = Disseminated Tumor Cells
HEVs = High Endothelial Venules.
REFERENCES
- 1.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–3. [PubMed] [Google Scholar]
- 2.Psaila B, Lyden D. The metastatic niche:Adapting the foreign soil. Nat Rev Cancer. 2009;9:285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr I. Lymphatic metastasis. Cancer Metastasis Rev. 1983;2:307–17. doi: 10.1007/BF00048483. [DOI] [PubMed] [Google Scholar]
- 4.Fidler IJ. Critical determinants of metastasis. Semin Cancer Biol. 2002;12:89–96. doi: 10.1006/scbi.2001.0416. [DOI] [PubMed] [Google Scholar]
- 5.Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches:Organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302–17. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- 6.Fidler IJ. Modulation of the organ microenvironment for the treatment of cancer metastasis (editorial) J Natl Cancer Inst. 1995;87:1588–92. doi: 10.1093/jnci/87.21.1588. [DOI] [PubMed] [Google Scholar]
- 7.Sleeman JP. The lymph node pre-metastatic niche. J Mol Med (Berl) 2015;93:1173–84. doi: 10.1007/s00109-015-1351-6. [DOI] [PubMed] [Google Scholar]
- 8.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation:Old sayings and new thoughts. Semin Cancer Biol. 2011;21:139–46. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the premetastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. DOI:10.1038/natur.e04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chin AR, Wang SE. Cancer tills the premetastatic field:Mechanistic basis and clinical implications. Clin Cancer Res. 2016;22:3725–33. doi: 10.1158/1078-0432.CCR-16-0028. DOI:10.1158-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sleeman JP. The metastatic niche and stromal progression. Cancer Metastasis Rev. 2012;31:429–40. doi: 10.1007/s10555-012-9373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakisaka N, Hasegawa Y, Yoshimoto S, Miura K, Shiotani A, Yokoyama J, et al. Primary tumour-secreted lymphangiogenic factors induce pre-metastatic lymph vascular niche formation at sentinel lymph nodes in oral squamous cell carcinoma. PLoS ONE. 2005;10:e0144056. doi: 10.1371/journal.pone.0144056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Xiang J. Remodeling the microenvironment before occurrence and metastasis of cancer. Int. J. Biol. Sci. 2019;15:105–13. doi: 10.7150/ijbs.28669. https://doi.org/10.7150/ijbs.28669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillot L, Baudin L, Rouaud L, Kridelka F, Noël A. The pre-metastatic niche in lymph nodes:Formation and characteristics. Cell Mol Life Sci. 2021;78:5987–6002. doi: 10.1007/s00018-021-03873-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duda DG, Jain RK. Premetastatic lung “niche”:Is vascular endothelial growth factor receptor 1 activation required? Cancer Res. 2010;70:5670–3. doi: 10.1158/0008-5472.CAN-10-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss L. The pathobiology of metastasis within the lymphatic system. Surg Oncol Clin N Am. 1996;5:15–24. [PubMed] [Google Scholar]
- 17.Wang H, Pan J, Barsky L, Jacob JC, Zheng Y, Gao C, et al. Characteristics of pre-metastatic niche:The landscape of molecular and cellular pathways. Mol Biomed. 2021;2:3. doi: 10.1186/s43556-020-00022-z. https://doi.org/10.1186/s43556-020-00022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erdogan B, Webb DJ. Cancer-associated fibroblasts modulate growth factor signalling and extracellular matrix remodelling to regulate tumour metastasis. Biochem Soc Trans. 2017;45:229–36. doi: 10.1042/BST20160387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu YL, Huang MS, Hung JY, Chang WA, Tsai YM, Pan YC, et al. Bone-marrow-derived cell-released extracellular vesicle miR92a regulates hepatic pre-metastatic niche in lung cancer. Oncogene. 2020;39:739–53. doi: 10.1038/s41388-019-1024-y. [DOI] [PubMed] [Google Scholar]
- 20.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–26. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vered M, Schiby G, Schnaiderman-Shapiro A, Novikov I, Bello IO, Salo T, et al. Key architectural changes in tumor-negative lymph nodes from metastatic-free oral cancer patients are valuable prognostic factors. Clin Exp Metastasis. 2014;31:327–38. doi: 10.1007/s10585-013-9631-4. [DOI] [PubMed] [Google Scholar]
- 22.Vered M, Shnaiderman-Shapiroa A, Schiby G, Zlotogorski-Hurvitz A, Salo T, Yahalom R. Markers of the pre-metastatic niche “knock on the door”of metastasis-free cervical lymph nodes in patients with oral cancer. Acta Histoche. 2019;121:151447. doi: 10.1016/j.acthis.2019.151447. [DOI] [PubMed] [Google Scholar]
- 23.Nathanson SD, Haas GP, Mead MJ, Lee M. Spontaneous regional lymph node metastases of three variants of the B16 melanoma:Relationship to primary tumor size and pulmonary metastases. J Surg Oncol. 1986;33:41–5. doi: 10.1002/jso.2930330112. [DOI] [PubMed] [Google Scholar]
- 24.Augustine D, Patil S. “Circulating tumor cells in oral cancer.”. J Int Oral Health. 2015;7:i–ii. [PMC free article] [PubMed] [Google Scholar]
- 25.Masuda T, Haysahi N, Iguchi T, Ito S, Eguchi H, Mimori K. Clinical and biological significance of circulatory tumour cells in cancer. Mol Oncol. 2016;10:408–17. doi: 10.1016/j.molonc.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]