Abstract
Disseminated infection with the dimorphic pathogenic fungus Penicillium marneffei is increasingly seen among patients with AIDS in southeast Asian countries. Previous studies have demonstrated the presence of humoral immune responses to this fungus in patient sera; we have confirmed this work using sera from P. marneffei-infected patients (n = 21) to develop Western blots of P. marneffei cytoplasmic yeast antigen (CYA). P. marneffei CYA was then partially purified by liquid isoelectric focusing, and fractions were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Immunoenzyme development of the Western blots with pooled sera from patients with P. marneffei infection and with pooled sera from patients with aspergillosis (n = 20), candidiasis (n = 10), cryptococcosis (n = 9), and histoplasmosis (n = 11) revealed three antigens with relative molecular masses of 61, 54, and 50 kDa. These antigens were specifically recognized by the pooled sera from the P. marneffei-infected patients. The 61- and 54-kDa antigens were subsequently purified to homogeneity by preparative gel electrophoresis, and the 50-kDa antigen was partially purified by the same technique. N-terminal amino acid sequencing revealed that the 61-kDa antigen had a strong homology (87% identity) with the antioxidant enzyme catalase. The three antigens were then subjected to SDS-PAGE and Western blotting and to immunoenzyme development with individual patient sera; sera from 86% of P. marneffei-infected patients recognized the 61-kDa antigen, sera from 71% recognized the 54-kDa antigen, and sera from 48% recognized the 50-kDa antigen. These specifically recognized antigens are the first to be purified from P. marneffei and can be used either singly or in combination to detect antibody responses in a large percentage of individuals infected with P. marneffei.
Penicillium marneffei was first described in 1956 after its isolation from a bamboo rat (Rhizomys sinensis) (1), although the organism’s pathogenicity in humans was not established until the infection of a laboratory worker in 1959 (17). It is the only known Penicillium species which displays dimorphism, and it is thought to be free-living in the mycelial form but exists in tissue as yeast or fission arthroconidia which divide by transverse fission (5, 11). Until recently, human infection with P. marneffei was only rarely observed, although it has been suggested that this disease may frequently be misdiagnosed as tuberculosis (19). The majority of cases reported in the literature have occurred in individuals with some form of immunosuppression (6), and there has been an increase in the rate of infection with this fungus as the human immunodeficiency virus (HIV) pandemic has penetrated areas where P. marneffei is endemic. For example, between 1991 and 1994 in Chiang Mai University Hospital in Chiang Mai, which is in northern Thailand, there were 550 cases of infection with P. marneffei (2).
A presumptive diagnosis of P. marneffei infection is made on the basis of clinical symptoms in conjunction with the identification of yeast dividing by transverse fission on microscopic examination of bone marrow aspirate and/or touch smears of skin or lymph node biopsy specimens stained with Wright’s stain (18). Diagnosis is confirmed by direct culture of the organism (21). There is, however, a need for the development of serologically based diagnostic tests which would enable the identification of either those individuals with initial asymptomatic forms of disease or those demonstrating nonspecific symptoms of P. marneffei infection. Until relatively recently little was known of the antigenic composition of P. marneffei, making the development of such diagnostic systems problematic. Several studies have now, however, identified a number of antigenic determinants in secreted antigen preparations from P. marneffei (3, 20). These include antigen with relative molecular masses of 54 and 50 kDa which were recognized by sera from 60.6 and 57.6% of 28 P. marneffei-infected patients, respectively, and more recently a 38-kDa molecule recognized by 45% of sera from a sample of AIDS patients with culture-confirmed P. marneffei infection (3).
However, it is noteworthy that to date there have been no reports of the purification of P. marneffei antigens and their direct use in the detection of specific serological responses in patients with P. marneffei infection. In this report we describe the identification of three specifically recognized P. marneffei antigens, two of which have been purified to homogeneity, and the recognition of these antigens by the sera of individuals with P. marneffei infection.
MATERIALS AND METHODS
Fungal isolates.
The clinical isolate P. marneffei NCPF 4160 was provided by the National Collection of Pathogenic Fungi, Mycological Reference Laboratory, Bristol, United Kingdom. The clinical isolate P. marneffei F1620 was provided by Chiang Mai University, Chiang Mai, Thailand.
Antigen preparation.
P. marneffei NCPF 4160 was cultured for antigen preparation in the yeast phase at 37°C on brain heart infusion medium (BHIM; Difco Laboratories Ltd., Surrey, United Kingdom) agar slopes. For the preculture a 10-μl inoculating loop was used to add yeast cells from a 7-day-old slope to 50 ml of BHIM broth in a 250-ml conical flask, which was then incubated in a shaking incubator at 120 rpm for 48 h at 37°C. Subsequently, 10 ml of the preculture was used to inoculate 200 ml of BHIM broth in a 1-liter conical flask which was incubated under the same conditions for 5 to 7 days (approximately mid-log phase). After treatment with 0.02 g of thimerosal per liter at room temperature for 24 h, the yeasts were harvested by centrifugation at 2,000 × g for 20 min. P. marneffei F1620 was grown at Chiang Mai University under the same conditions except that incubation was achieved with a shaking heated water bath. P. marneffei cytoplasmic yeast antigen (CYA) was prepared by mixing packed yeast cells with an equal volume of 0.5-mm glass Ballotini beads in phosphate-buffered saline (PBS). The mixture was added to the chamber of a Bead Beater homogenizer (Biospec Products, Bartlesville, Okla.), packed in ice, and homogenized 15 times in 1-min bursts with an interval of 10 min between each homogenization. The homogenate was transferred to 40-ml centrifuge tubes, the insoluble debris was removed by centrifugation at 10,000 × g for 15 min, and the cytoplasmic antigen solution was decanted (10). The Coomassie blue method was used to determine the protein content of each preparation (16).
Liquid IEF of CYA.
Approximately 15 to 20 mg of CYA from P. marneffei NCPF 4160 was dialyzed overnight at 4°C with several changes of distilled water to remove the buffer salts present from the PBS used in the antigen preparation protocol and was then made up to 50 ml by the addition of 1 ml of ampholytes (Biolyte; pH range, 3 to 10; Bio-Rad Laboratories Ltd., Hemel Hempstead, United Kingdom) and distilled water. The sample was loaded onto a liquid isoelectric focusing (IEF) system (Rotofor; Bio-Rad), and separation was achieved with a constant power input of 12 W. The 20 fractions were harvested once the voltage reading had stabilized for 30 min or more. The pH of the fractions was measured, and the protein content was determined by the Coomassie blue method (16). IEF fractions were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting, followed by immunoenzyme development with human immune sera.
SDS-PAGE, Western blotting, and immunoenzyme development.
SDS-PAGE was performed with 10% polyacrylamide gels as described previously (8). The gels were stained with either Coomassie brilliant blue (16) or silver stain (14). The relative molecular masses of the protein bands were determined by a standard curve method with kaleidoscope markers (Bio-Rad). For protein transfer onto polyvinylidene fluoride membranes (Millipore UK Ltd., Hertfordshire, United Kingdom), the gels were subjected to Western blotting as described previously (8). The blots that were to be used for the N-terminal amino acid sequencing of purified protein were prepared by using the appropriate modification as described previously (10). The blots that were to be used for immunoenzyme development were incubated overnight at 4°C in 2% casein with sodium azide (0.02%) in PBS and were then air dried and stored at 4°C until required.
Immunoenzyme development of the blots was carried out as described previously (8) with pooled human sera at a dilution of 1:1,000 in 1% casein–PBS–Tween (0.05% Tween 20; Sigma Aldrich Ltd., Dorset, United Kingdom) or individual serum specimens at a dilution of 1:100 in 1% casein–PBS–Tween. Goat anti-human immunoglobulin G (IgG) peroxidase-linked conjugate (Jackson, West Grove, Pa.) was used at a dilution of 1:1,000 in 1% casein–PBS–Tween to recognize bound human IgG, and the blots were developed with tablets of 10 mg of 3,3′-diaminobenzidine tetrahydrochloride (Sigma Aldrich Ltd.) and 30 mg of 4-chloro-1-naphthol (Sigma Aldrich Ltd.). Each tablet was dissolved by mixing in 2 ml of absolute ethanol, and the mixture was added to 100 ml of PBS before the addition of 30 μl of H2O2. After development in this solution, the blots were washed in 0.1 M H2SO4 and, finally, in distilled water before being allowed to air dry.
Human sera.
Sera from patients with a variety of fungal infections, as listed in Table 1, were used in immunoblot assays. Those from the patients with P. marneffei infections were used either as pools of sera or as individual serum samples. Those from the patients with other mycoses were used as pools of sera. Control sera from healthy human controls were taken from an area of low endemicity for P. marneffei (Bangkok, Thailand). Two pools of sera from P. marneffei-infected patients were used for the immunoblot assays: Pool Pm1 was pooled sera from 28 P. marneffei-infected patients taken in 1995 at Chiang Mai University Hospital and had previously been used in a study by Vanittanakom et al. (20). The individual sera from the study by Vanittanakom et al. (20) were no longer available and could not be used in the present study. The second pool of sera (pool Pm2) was a mixture of 14 serum samples taken from P. marneffei-infected patients diagnosed by positive histology and culture at Chiang Mai University Hospital in 1996 plus a further 7 samples from patients diagnosed on the same basis at the Institute of Dermatology, Bangkok, Thailand. All serum samples were taken at the time of diagnosis, when patients had active P. marneffei infection. The individual serum samples which made up pool Pm2 were those used in the single-antigen immunoblot studies.
TABLE 1.
Nature and source of sera used in immunoblot assays
| Patient group | Country of origin | No. of serum samples |
|---|---|---|
| P. marneffei | Thailand | 21 |
| Aspergillosis | United Kingdom | 20 |
| Candidiasis | United Kingdom | 10 |
| Cryptococcosis | United Kingdom | 9 |
| Histoplasmosis | Colombia | 11 |
| Normal human serum | Thailand | 30 |
Purification of antigens by Prep-cell preparative gel electrophoresis.
IEF fractions 10 and 11 were pooled and subjected to separation on the basis of size by using the Prep-cell preparative gel electrophoresis system (Bio-Rad). Samples were reduced with 2-mercaptoethanol (Sigma Aldrich Ltd.) and were loaded onto a gel column consisting of a 1.75-cm stacking gel and an 8-cm, 7.5% resolving gel (acrylamide/bisacrylamide ratio, 35:1). Electrophoresis was performed at a constant power of 10 W, and once the dye front was eluted from the column, 2.5-ml volumes were collected. Fractions were concentrated either by the use of Microsep centrifugal concentrators (Flowgen Instruments Ltd., Kent, United Kingdom) or by acetone precipitation (15) and were analyzed by SDS-PAGE.
RESULTS
Immunoenzyme development of Western blots of P. marneffei CYA with sera from patients with P. marneffei infection.
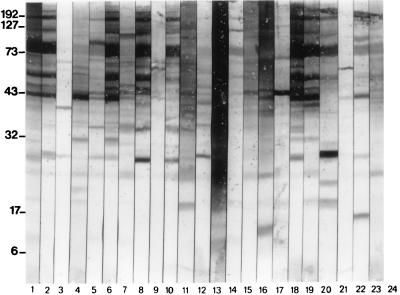
Pooled immune sera from P. marneffei-infected patients reacted with a large number of P. marneffei antigenic determinants in CYA with relative molecular masses of between 10 and 200 kDa. Of particular note were highly reactive determinants at approximately 40, 61, 88, 135, and 190 kDa. Individual human sera demonstrated a wide variety of patterns of reactivity (Fig. 1, lanes 3 to 23) varying from a small degree of recognition (lanes 3, 9, and 21) to the recognition of a large number of determinants (lanes 6, 8, and 18).
FIG. 1.
Reactivities of pooled and individual serum samples from P. marneffei-infected patients to P. marneffei CYA by Western blotting. Lanes 1 and 2, reactivities of sera in pools Pm1 and Pm2, respectively (pooled sera were used at 1:100, and peroxidase-conjugated goat anti-human IgG polyclonal sera were used at 1:1,000); lanes 3 to 23, reactivities of individual sera from P. marneffei-infected patients (sera were used at 1:100, and peroxidase-conjugated goat anti-human IgG polyclonal sera were used at 1:1,000) to P. marneffei CYA; lane 24, negative control (no primary antibody). Molecular masses (on the left) are in kilodaltons.
Separation of P. marneffei CYA by IEF and identification of specific P. marneffei antigens.
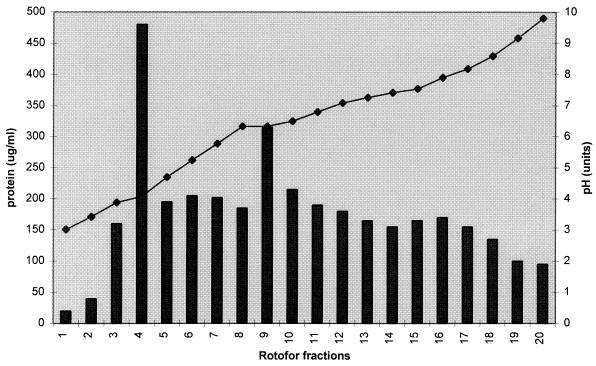
P. marneffei CYA was separated into 20 fractions with the Rotofor liquid IEF system (Fig. 2). Large amounts of precipitated proteins (which could not be measured) were found at the anodic and cathodic ends of the Rotofor system. Of the remaining fractions, fractions 4 and 9 contained the most detectable protein. Individual fractions obtained with the Rotofor system were subjected to SDS-PAGE, Western blotting, and immunoenzyme development with pooled sera from the groups described in Table 1.
FIG. 2.
Separation of P. marneffei CYA by liquid IEF. Bars, protein concentration (in micrograms per milliliter); diamonds, pH units.
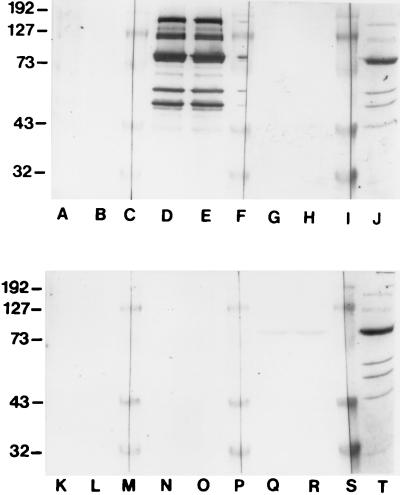
Fractions 10 and 11 obtained with the Rotofor system contained a number of antigens which were strongly reactive with pooled sera from P. marneffei-infected patients (Fig. 3, lane D and E; data are presented for IEF fraction 11, but those for fraction 10 were identical). The Pm2 serum pool recognized bands of 40, 50, 54, 61, 70, 78, 90, 135, and 190 kDa, although not all of these were visualized when the bands were stained with Coomassie blue (Fig. 3). Three of these antigens with relative molecular masses of 61, 54, and 50 kDa demonstrated no cross-reactivity with pooled sera from healthy individuals or with sera from patients with histoplasmosis, candidosis, aspergillosis, and cryptococcosis (Fig. 3). On the basis of the pH of fractions 10 and 11 obtained with the Rotofor system, these antigens would appear to have pIs of between 6.5 and 7.0.
FIG. 3.
Immunoenzyme development of Western blots of fraction 11 obtained by IEF with various pooled sera. Lanes A and B, reactivities of pooled normal human sera (n = 30); lanes D and E, pooled sera from P. marneffei-infected patients (pool Pm2; n = 21); lanes G and H, pooled sera from histoplasmosis patients (n = 11); lanes K and L, pooled sera from candidiasis patients (n = 9); lanes N and O, pooled sera from aspergillosis patients (n = 20); lanes Q and R, pooled sera from cryptococcosis patients (n = 10); lanes C, F, I, M, P, and S, molecular mass markers; lanes J and T, duplicates of Coomassie blue-stained fraction 11 obtained by IEF. Pooled sera were used at a dilution of 1:1,000, and the peroxidase-conjugated goat anti-human IgG peroxidase conjugate was used at 1:1,000. Molecular masses (on the left) are in kilodaltons.
Purification of the 61-, 54-, and 50-kDa antigens.
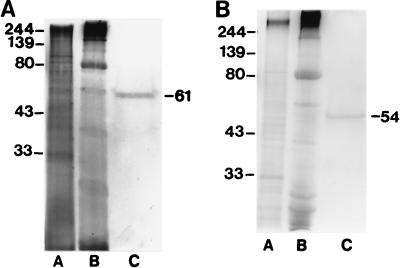
The 61- and 54-kDa antigens were purified to homogeneity from IEF fractions 10 and 11 by Prep-cell fractionation. Purification of each antigen was monitored by Coomassie blue staining (data not shown) and silver staining analysis of SDS-polyacrylamide gels (Fig. 4). A 39-kDa band was sometimes observed to copurify with the 61-kDa molecule after Prep-cell preparative electrophoresis (data not shown). It proved to be impossible to purify the 50-kDa antigen to homogeneity because of the presence of a contaminating antigen at 46 kDa in all Prep-cell fractions containing the 50-kDa antigen (data not shown).
FIG. 4.
Purification of the 61-kDa (A) and 54-kDa (B) P. marneffei antigens as monitored by silver staining. Lane A, CYA; lane B, fractions 10 and 11 (pooled) obtained with the Rotofor system; lane C, purified antigen after preparative SDS-PAGE (61- or 54-kDa antigen as annotated on the right). Molecular masses (on the left) are in kilodaltons.
N-terminal amino acid sequencing of the 61-kDa antigen revealed a sequence with 87% identity with Escherichia coli catalase (Table 2). Despite attempts at N-terminal amino acid analysis for the 54-kDa antigen, we obtained no data suggesting that the N terminus of this protein is blocked.
TABLE 2.
N-terminal amino acid sequence of 61-kDa antigen and its homology with other proteins in the GENEMBL database
| Protein | N-terminal amino acid sequence dataa | % Identity |
|---|---|---|
| Penicillium marneffei 61-kDa antigen | M N D E E S V A L I A | |
| Escherichia coli catalase | M N D E E T V A L I A | 83.7 |
| Bacillus stearothermophilus catalase | M N D E E T V A L I A | 83.7 |
| Salmonella typhimurium catalase | M N D E E T V A L I A | 83.7 |
Underscoring indicates amino acids with same identity as that for the 61-kDa antigen of P. marneffei.
Recognition of the 61-, 54-, and 50-kDa antigens by sera from patients infected with P. marneffei.
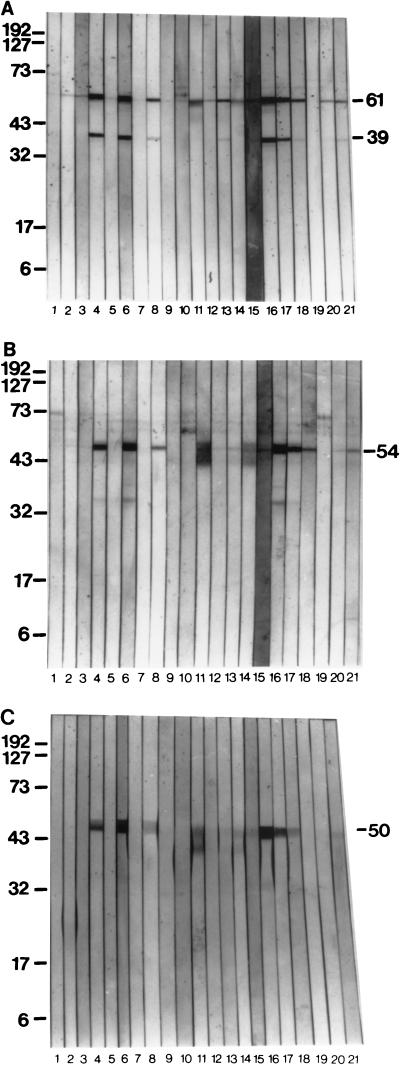
The purified 61-kDa antigen was recognized by 86% (18 of 21) of the serum samples from P. marneffei-infected patients (Fig. 5A) when the samples were used in the immunoenzyme development of Western blots. A 39-kDa molecule, which had been seen to sometimes copurify with the 61-kDa antigen, was reactive with 48% (10 of 21) of the serum samples from P. marneffei-infected patients (Fig. 5A). The 54-kDa antigen was recognized by 71% of the sera from P. marneffei-infected patients (Fig. 5B), while the 50-kDa antigen (Fig. 5C) was reactive with 48% (10 of 21) of the patient sera. In total, 18 of 21 (86%) of all serum samples from P. marneffei-infected patients tested recognized at least one of these antigens. Other faint immunoreactive bands were observed on the immunoblots, although these proteins were not visible by Coomassie blue or silver staining techniques (Fig. 5B).
FIG. 5.
Reactivities of 21 immune serum samples from P. marneffei-infected patients to the 61-kDa (A), 54-kDa (B), and 50-kDa (C) antigens as determined by immunoenzyme development of Western blots. Lanes 1 to 21, individual sera from P. marneffei-infected patients (sera were used at 1:100). Peroxidase-conjugated goat anti-human IgG conjugate was used at 1:1,000. Molecular masses (on the left) are in kilodaltons.
DISCUSSION
Three antigens which may be of use in the diagnosis of asymptomatic P. marneffei infection have been identified and purified either partially or to homogeneity from P. marneffei CYA. The first of these, a 61-kDa antigen, may be the same as that previously reported as being present in P. marneffei culture medium (20). However, there is a disparity between the number of patient sera recognizing this antigen in the earlier study compared to the number recognizing this antigen in the present study. Thus, in our study the purified 61-kDa antigen from P. marneffei CYA was recognized by 86% of 21 serum samples by Western blotting, whereas only 24% of 28 serum samples were positive in the previous study (20). N-terminal amino acid sequencing revealed that the 61-kDa antigen was probably a catalase; similar data would have to be obtained for the previously identified extracellular 61-kDa antigen to establish the relationship between the two antigens. Catalase antigens are known to be produced by a number of pathogenic fungi including Histoplasma capsulatum (7) and Aspergillus fumigatus (13) and may play a role in virulence (12). It is of note that while both H. capsulatum and A. fumigatus possess catalases which induce immune responses (7, 13), sera from patients with these infections do not appear to recognize the putative P. marneffei catalase, indicating antigenic differences between these enzymes.
A 39-kDa molecule was often observed to copurify with the 61-kDa antigen. It is very likely that this is a breakdown product of the 61-kDa antigen since this molecule typically appeared in fractions containing the 61-kDa antigen which had been separated by Prep-cell fractionation. The latter procedure is capable of separating molecules with a size difference of as little as 2 kDa, and therefore, the copurification of two unrelated molecules with relative molecular masses of 61 and 39 kDa by this method is a highly unlikely event. The reactivity of the 39-kDa molecule with sera from patients infected with P. marneffei supports the supposition that this molecule is a breakdown product of the 61-kDa antigen: in this study the 39-kDa molecule was reactive with 48% of the human immune serum samples. This was lower than the percentage of sera which recognized the 61-kDa antigen, but the result could be accounted for by the small amount of this antigen on the blot; the 39-kDa band was often invisible when either gels or blots were stained by conventional techniques. It is also possible that some reactivity is lost in the process of degradation. It is noteworthy that Vanittanakom et al. (20) also mentioned the reactivity of a 39-kDa extracellular protein which gave a positive reaction with 27% of immune sera in their study. Furthermore, recently published data have suggested that a 38-kDa molecule secreted from P. marneffei is specific to this fungus, and antibody to a 38-kDa molecule has been detected in 45% of AIDS patients with culture-confirmed P. marneffei infections (3).
The second antigen identified in this study had a reduced relative molecular mass of 54 kDa and was purified to homogeneity by a method similar to that described for the 61-kDa antigen. When used in a Western blot system with patient sera, this antigen demonstrated 100% specificity for P. marneffei, with no cross-reactivity with pooled sera from patients with other mycoses. It is quite possible that this molecule corresponds to a protein of 54 kDa identified by Vanittanakom et al. (20) in P. marneffei culture filtrate. Thus, of the 21 serum samples from patients used in this study, 71% recognized the 54-kDa molecule, whereas 60% of the serum samples from 28 patients in the culture filtrate antigen study recognized this molecule (20). This similarity in the pattern of recognition by patient sera suggests that the 54-kDa antigens described in the two studies are probably the cytoplasmic and extracellular forms of the same molecule. However, in contrast to the situation with the 61-kDa antigen, we were unable to obtain any N-terminal amino acid data for the 54-kDa antigen (as a result of N-terminal blockage), and as such, the identity of the 54-kDa antigen remains unknown.
Purification of the third antigen, the 50-kDa molecule, was difficult since it was present only in very small amounts in the IEF fractions used. However, this antigen was specific for P. marneffei and it was recognized by sera from 48% of the patients. The latter value equates broadly with the rate of recognition (58%) of a previously described 50-kDa antigen present in P. marneffei culture filtrate, which is again suggestive of a relationship between the two antigens (20). In common with the other two antigens described in this study, the 50-kDa antigen has a pI of approximately 6.5 to 7.0 on the basis of the results of liquid IEF analysis.
The specificity and relatively high sensitivity of the 61-, 54-, 50-, and 39-kDa molecules when used in the detection of humoral immune responses to P. marneffei make these antigens directly applicable to the serological diagnosis of P. marneffei infections. It is highly likely that many patients harbor asymptomatic P. marneffei infection for many months before developing disseminated disease. This hypothesis is supported by the number of individuals who develop this infection many months after leaving an area where the organism is endemic (4, 9). These patients are likely to develop disseminated P. marneffei infection because their immunity is eroded by the progression to full-blown AIDS, and these are the patients who may benefit from targeted antifungal therapy. Antibody reactivity against the 38-kDa molecule described by Chongtrakool et al. (3) was found in 17% of the sera from apparently P. marneffei-negative, HIV-positive patients living in areas where P. marneffei is endemic, whereas it was found in the sera of only 2% of those living in areas where the organism is not endemic, which the authors suggested was due to asymptomatic infection. Since there is evidence to suggest that the 38-kDa molecule of Chongtrakool et al. (3) may be recognized by asymptomatic P. marneffei-infected individuals, it could be of high diagnostic value if tests with this molecule were applied to sera from at-risk populations such as HIV-positive patients living in areas where the organism is endemic. The theory that many individuals may suffer asymptomatic P. marneffei infection for a considerable time before clinical symptoms become obvious is given further support by the results obtained by Vanittanakom et al. (20), since serum from one of the AIDS patients used as a negative control repeatedly gave a positive reaction against the 54- and 50-kDa antigens present in P. marneffei yeast-phase culture filtrate. This patient finally developed clinical symptoms of P. marneffei infection 2 months after initial testing, indicating that antibody responses can be present before clinical symptoms become apparent. The utility of the use of the antigens described in this study in tests with sera from an at-risk population without obvious symptoms of P. marneffei infection merits further investigation. Especially useful in this regard would be the elucidation of any relationship between the cytoplasmic antigens described in this report with the secretory antigens of Vanittanakom et al. (20), of which the 54- and 50-kDa antigens have already been demonstrated to be useful indicators of early P. marneffei infection in at least one patient.
Our initial studies with sera from AIDS patients with disseminated P. marneffei infection demonstrate that these patients do mount detectable humoral immune responses, despite having low CD4 counts. However, any approach involving the detection of antibody responses as a marker of P. marneffei infection in this group of patients should involve a number of antigens in order to identify as many asymptomatic sufferers as possible. The 61-, 54-, 50-, and 39-kDa antigens are prime candidates for this purpose since they appear to be identified only by the antibody responses of individuals with P. marneffei infections and are not recognized by sera from patients with other mycoses, which may, like mycosis caused by H. capsulatum, occur in the same area where P. marneffei is endemic.
As yet, the relationship (if any) of the 61-, 54-, and 50-kDa antigens described in this study to the secretory antigens described by Vanittanakom et al. (20) has not been ascertained because a direct comparison of one group of sera to both secretory and cytoplasmic antigens has not been possible (the individual sera which comprise the Pm1 pool are no longer available). It is hoped that further studies will elucidate any such relationship, and efforts are under way to purify the secretory antigens for such a study.
Future studies will involve an examination of the 39-kDa molecule in an attempt to determine its identity by N-terminal amino acid analysis, which would reveal its precise relationship with the 61-kDa antigen. Work already under way involves the development of an enzyme-linked immunosorbent assay incorporating various combinations of the 61-, 54-, and 50-kDa antigens for the identification of individuals with both symptomatic and nonsymptomatic P. marneffei infection.
ACKNOWLEDGMENT
This work was supported by a grant from the Wellcome Trust.
REFERENCES
- 1.Capponi M, Sureau P, Segretain G. Pénicillose de Rhizomys sinensis. Bull Soc Pathol Exot. 1956;49:418–421. [PubMed] [Google Scholar]
- 2.Chariyalertsak S, Sirisanthana T, Supparatpinyo K, Nelson K E. Seasonal variation of disseminated Penicillium marneffei infections in northern Thailand: a clue to the reservoir? J Infect Dis. 1996;173:1490–1493. doi: 10.1093/infdis/173.6.1490. [DOI] [PubMed] [Google Scholar]
- 3.Chongtrakool P, Chaiyaroj S C, Vithayasai V, Trawatcharegon S, Teanpaisan R, Kalnawakul S, Sirisinha S. Immunoreactivity of a 38-kilodalton Penicillium marneffei antigen with human immunodeficiency virus-positive sera. J Clin Microbiol. 1997;35:2220–2223. doi: 10.1128/jcm.35.9.2220-2223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drouhet E. Penicilliosis due to Penicillium marneffei: a new emerging systemic mycosis in AIDS patients travelling or living in southeast Asia. J Mycol Med. 1993;4:195–224. [Google Scholar]
- 5.Drouhet E, Dupont B. Infection a Penicillium marneffei: mycose systémique a manifestations cutanées associée au SIDA. J Mycol Med. 1995;5:21–34. [Google Scholar]
- 6.Duong T A. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis. 1996;23:125–130. doi: 10.1093/clinids/23.1.125. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton A J, Bartholomew M A, Figueroa J, Fenelon L E, Hay R J. Evidence that the M-antigen of Histoplasma capsulatum var capsulatum is a catalase which exhibits cross-reactivity with other dimorphic fungi. J Med Vet Mycol. 1990;28:479–485. [PubMed] [Google Scholar]
- 8.Hamilton A J, Jeavons L, Hobby P, Hay R J. A 32- to 38-kilodalton Cryptococcus neoformans glycoprotein produced as an exoantigen bearing a glycosylated species-specific epitope. Infect Immun. 1992;60:143–149. doi: 10.1128/iai.60.1.143-149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilmarsdottir I, Meynard J L, Rogeaux O, Guermonprez G, Datry A, Katlama C, Bruker G, Coutellier A, Danis M, Gentilini M. Disseminated Penicillium marneffei infection associated with human immunodeficiency virus: a report of two cases and a review of 35 published cases. J Acquired Immune Defic Syndr. 1993;6:466–471. [PubMed] [Google Scholar]
- 10.Jeavons L, Hunt L, Hamilton A J. Immunochemical studies of heat-shock protein 80 of Histoplasma capsulatum. J Med Vet Mycol. 1994;32:47–57. doi: 10.1080/02681219480000071. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman L, Standard P G, Anderson S A, Jalbert M, Swisher B L. Development of specific fluorescent-antibody test for tissue form of Penicillium marneffei. J Clin Microbiol. 1995;33:2136–2138. doi: 10.1128/jcm.33.8.2136-2138.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latgé J P, Beauvais A, Paris S, Sarfati J, Diaquin M, Debeaupuis J P, Calera J A, Leal F, Monod M. Program and abstracts of the 13th Congress of the International Society for Human and Animal Mycology. 1997. Aspergillus fumigatus, pathogen or saprophyte? abstr. S71; p. 58. [Google Scholar]
- 13.Lopez-Medrano R, Ovejero M C, Calera J A, Puente P, Leale F. An immunodominant 90-kilodalton Aspergillus fumigatus antigen is the subunit of a catalase. Infect Immun. 1995;63:4774–4780. doi: 10.1128/iai.63.12.4774-4780.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merril C R, Goldman D, Sedman S A, Ebert M H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebral spinal fluid proteins. Science. 1981;211:1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz B L, Garcia A M, Restrepo A, McEwen J G. Immunological characterization of a recombinant 27-kilodalton antigenic protein from Paracoccidioides brasiliensis. Clin Diagn Lab Immunol. 1996;3:239–241. doi: 10.1128/cdli.3.2.239-241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Read S M, Northcote D H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981;116:53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- 17.Segretain G. Penicillium marneffei n. sp., agent d’une mycose du système réticulo-endothélial. Mycopathol Mycol Appl. 1959;11:327–353. doi: 10.1007/BF02089507. [DOI] [PubMed] [Google Scholar]
- 18.Supparatpinyo K, Khamwan C, Baosoung V, Nelson K E, Sirisanthana T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344:110–113. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 19.Tsang D N C, Chan J K C, Lau Y T, Lim W, Tse C H, Chan N K. Penicillium marneffei infection: an underdiagnosed disease? Histopathology. 1988;13:311–318. doi: 10.1111/j.1365-2559.1988.tb02041.x. [DOI] [PubMed] [Google Scholar]
- 20.Vanittanakom N, Mekaprateep M, Sittisombut N, Supparatpinyo K, Kanjanasthiti P, Nelson K E, Sirisanthana T. Western immunoblot analysis of protein antigens of Penicillium marneffei. J Med Vet Mycol. 1997;35:123–131. doi: 10.1080/02681219780001011. [DOI] [PubMed] [Google Scholar]
- 21.Yuen K, Wong S S, Tsang D N, Chau P. Serodiagnosis of Penicillium marneffei infection. Lancet. 1994;344:444–445. doi: 10.1016/s0140-6736(94)91771-x. [DOI] [PubMed] [Google Scholar]