Abstract
Background
Malaria remains a major global health challenge and a serious cause of morbidity and mortality in sub-Saharan Africa. In Uganda, limited access to medical facilities has perpetuated the reliance of indigenous communities on herbal medicine for the prevention and management of malaria. This study was undertaken to document ethnobotanical knowledge on medicinal plants prescribed for managing malaria in Rukungiri District, a meso-endemic malaria region of Western Uganda.
Methods
An ethnobotanical survey was carried out between May 2022 and December 2022 in Bwambara Sub-County, Rukungiri District, Western Uganda using semi-structured questionnaire. A total of 125 respondents (81 females and 44 males) were randomly selected and seven (7) key informants were engaged in open interviews. In all cases, awareness of herbalists on malaria, treatment-seeking behaviour and herbal treatment practices were obtained. The ethnobotanical data were analyzed using descriptive statistics, informant consensus factor and preference ranking.
Results
The study identified 48 medicinal plants belonging to 47 genera and 23 families used in the treatment of malaria and its symptoms in the study area. The most frequently cited species were Vernonia amygdalina, Aloe vera and Azadirachta indica. Leaves (74%) was the most used plant organ, mostly for preparation of decoctions (41.8%) and infusions (23.6%) which are administered orally (89.6%) or used for bathing (10.4%).
Conclusions
Indigenous knowledge of medicinal plants used as prophylaxis and for treatment of malaria still exist among the local communities of Bwambara Sub-County. However, there is a need to investigate the antimalarial efficacy, phytochemical composition and safety of species (such as Digitaria abyssinica and Berkheya barbata) with high percentage use values to validate their use.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41182-023-00541-9.
Keywords: African traditional medicine, Antimalarial resistance, Ethnobotanical knowledge, Malaria, Medicinal plants
Background
Malaria has afflicted humanity for millennia [1]. It is one of the most fatal, preventable and curable parasitic diseases globally, with about 619,000 deaths and 247 million new cases reported in 2021 [2]. In the WHO African Region, 593,000 malaria deaths were reported in 2021 [2]. Recent estimates indicate that almost half of the global population live in 82 malaria-endemic countries [3]. The majority of malaria deaths are common in the tropical and subtropical regions, particularly Central, Western and East Africa, where there is limited healthcare and/or vector (female Anopheles mosquito) control [4].
According to World Malaria Report 2022 [2], about 96% of the global malaria-related deaths were reported in 29 countries. Uganda has one of the highest recorded malaria transmission rates on the African continent, with over 90% of the population at risk [5]. Malaria has also remained the major cause of morbidity and mortality in Uganda, as evidenced by 30–50% of outpatient visits and 15–20% of hospital admissions. The average economic loss in Uganda due to malaria is more than US$ 500 million per year [6]. In 2021, the World Health Organization (WHO) estimated that there were 12.4 million malaria cases and over 31,350 malaria deaths in Uganda [2]. In 2022, the Ugandan Ministry of Health indicated that 68 districts recorded malaria upsurge [7]. Rukungiri District recorded 196,070 malaria cases and some deaths between 25th April and 1st May 2022, where Bwambara Sub-County (the focal area of this study) accounted for all the malaria deaths within the whole district [7]. This could be due to the poor public healthcare system in the Sub-County [8].
Inefficient malaria treatment has promoted antimalarial drug resistance and this has been cited to be of considerable concern in East Africa [2]. In Uganda, artemisinin resistance by Plasmodium falciparum was identified, where two pathogenic mutations were found in more than 15% of the collected samples [9]. The WHO calls for response to the emergence of antimalarial drug resistance by ensuring that efficacious treatments remain available. This calls for alternative interventions for malaria treatment which include use and exploration of medicinal plants [2].
Medicinal plants contain phytochemicals with intriguing biological activities which provide prospects for new drug discoveries [10]. Plants have remained the focus of many studies aiming at discovering antimalarials, because the current conventional antimalarial drugs such as artemisinin and quinine were discovered from Artemisia annua and Cinchona species [11]. In this context, documentation of medicinal plants used in the treatment of malaria constitutes a vital task in the preservation of indigenous knowledge and biodiversity, as well as improvement of malaria treatment interventions amongst communities. It also contributes to bioprospecting, which lays a foundation for further research on the safety and efficacy of medicinal plants, and identification of bioactive compounds that could act as druggable hit molecules for the development of new antimalarial therapies.
Previous studies in Rukungiri District have documented the ethnomedicinal plants used for managing pediatric diseases [12] and "African" diseases [13]. Based on the latter report, we found that indigenous communities of Bwambara Sub-County (Rukungiri District) cherish medicinal plants and knowledge of their utilization, management and conservation. We identified one of the most used species in this study area (Gouania longispicata Engl.) and reported on its antimicrobial activity [14]. Despite the floral biodiversity and rich ethnocultural heritage of Bwambara Sub-County [15] and Rukungiri District as a whole, there is no report on medicinal plants used in malaria prevention and treatment. Coupled with the rapid loss of vegetation and adoption of foreign cultures by the local societies [16], there is a pressing need to record such indigenous knowledge before it is lost forever. Thus, this study documented the ethnobotanical knowledge on medicinal plants that are used for treatment of malaria in Bwambara Sub-County, Rukungiri District, Western Uganda.
Materials and methods
Study area
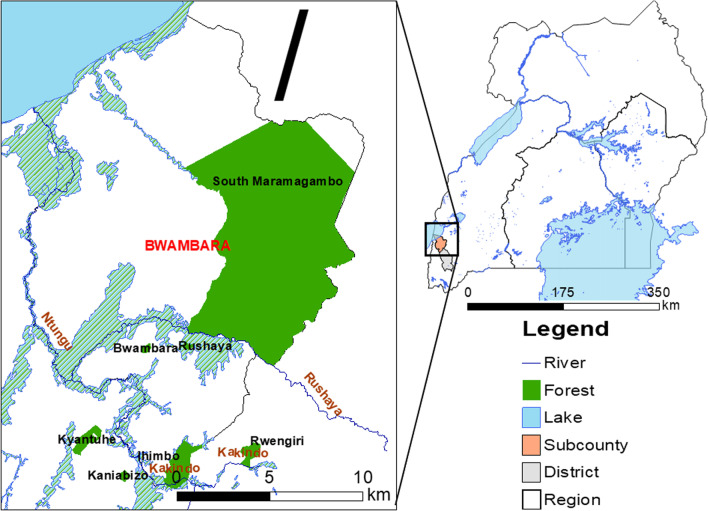
This study was conducted in Bwambara Sub-County (latitudes: − 0.33778 and − 0.67119, and longitudes: 29.69882 and 29.86939), Rukungiri District, Western Uganda (Fig. 1). The area is dominated by the Bakiga ethnic group, but other ethnic groups such as the Bahororo and Banyabutumbi are also represented. The main occupation is subsistence farming, except in Rwenshama Parish, where fishing is the main economic activity [13]. The major crops grown are coffee, matooke, grapes, pears and peaches, while cattle are majorly kept for milk production [17]. Bwambara Sub-County is bordered by Lake Edward, Queen Elizabeth National Park and Kigezi Game Reserve [18].
Fig. 1.
Map of Rukungiri district, Western Uganda showing the location of the study area (Bwambara Sub-County)
The area has forests, forest/savannah mosaic and savannah type of vegetation [19]. It experiences a bimodal rainfall, with a short rainy season from March to May and a long rainy season between September and December. Thus, malaria transmission is perennial with maximum prevalence shortly after the rainy seasons [8, 20] and is, therefore, meso-endemic for malaria with moderate-to-high transmission [21]. The public healthcare system in the Sub-County has four health centres that serve its 79 villages with an estimated population of 28,900 people [8]. Two are Public Health Centre II but only limited to outpatient services. The remaining two are Public Health Centre III facilities, which in addition to outpatient services, offer maternity services, has an inpatient ward and microscopy for diagnosing malaria. However, these health centres have inadequate facilities, are poorly stocked and have few health workers [18]. Majority of the population are unable to access good services at the health centres due to long distances, unavailability of medicine, poverty and neglect by medical staff.
Sample size determination
The calculated sample size (S) was 125 respondents, obtained using the formula suggested by Krejcie and Morgan [22], that is
where S is the required sample size, X2 = the table value of chi-square for 1 degree of freedom at the desired confidence level (3.841), N = the population size, P = the population proportion (assumed to be 0.50, since this would provide the maximum sample size) and d = the degree of accuracy expressed as a proportion (0.05).
Ethnobotanical survey
A cross-sectional survey using semi-structured questionnaires was undertaken between May 2022 and December 2022. With the help of local council representatives, seven specialized herbalists known to treat patients using herbal medicine were purposively sampled and interviewed as key informants. The study was conducted in Rukiga/Runyankole, the commonly used local languages in the area. The questions mainly focused on how informants prevented and diagnosed malaria, and how medicinal plants were prepared and administered for treatment of malaria.
Voucher specimens were prepared for all the collected plant species and deposited at the Department of Biology, Mbarara University of Science and Technology (Uganda), where they were authenticated by a botanist. The scientific plant names were given according to The World Flora Online (http://www.worldfloraonline.org) and International Plant Name Index (www.ipni.org). The family names of the plant species were cross-checked with the Angiosperm Phylogeny Group (www.gbif.org).
Data analysis
Numerical data were captured in Microsoft Excel for Windows (Microsoft Corporation, USA) and thereafter exported to SPSS software (v26, SPSS Inc., USA) for analysis. Qualitative data on malaria prevention, ethnobotanical knowledge and respondents’ socio-demographic characteristics were analyzed using descriptive statistics, such as percentages and frequencies.
Quantitative ethnobotanical data were used to calculate frequency of citation (FC) and informant consensus factor (ICF). The FC was used to assess the number of informants who were familiar with usage of a particular medicinal plant to treat malaria. Use report (UR) was recorded whenever any informant mentioned use of a medicinal plant in a particular way. The ICF was used to determine the homogeneity of the collected ethnobotanical data using the formula [23]:
where Nur = total number of use reports for each disease category, and Nt = total number of species in each use category. The ICF value ranges from 0 to 1. A high ICF value (close to 1) signifies that a high proportion of informants uses a few plant species to treat a particular disease, and this suggests that the community possesses a well-defined mechanism for exchange of indigenous knowledge. Conversely, low ICF values (close to 0) indicate that many plant species are reported to be used by a high proportion of informants to treat a particular disease, implying that there is lack of information exchange within the community or the medicinal plants are randomly selected [24].
Results
Socio-demographic characteristics
The study involved 125 informants, of which there were 64.8% females and 35.2% males (Table 1). The largest number of respondents were 31–40 years (27.8%). In general, 53.5% were above 40 years, while only 18.5% were ≤ 30 years. The findings revealed that most of the informants had attended primary school (55.6%), while 30.4% did not attain any formal education. The main religion in the area is Anglican (45.6%) and Catholic (31.6%).
Table 1.
Socio-demographic characteristics of informants from Bwambara Sub-County, Rukungiri District, Uganda
| Variable | Characteristic | Percentage (%) |
|---|---|---|
| Gender | Male | 35.2 |
| Female | 64.8 | |
| Age group (years) | 18–19 | 0.8 |
| 20–30 | 17.7 | |
| 31–40 | 27.8 | |
| 41–50 | 21.5 | |
| 51–60 | 15.2 | |
| > 60 | 17 | |
| Educational status | None | 30.4 |
| Primary | 55.6 | |
| Secondary | 13.2 | |
| College | 0.8 | |
| Religious affiliation | Anglican | 45.6 |
| Catholic | 31.6 | |
| Pentecostal | 16.5 | |
| Moslem | 6.3 | |
| Ethnic group | Bakiga | 83.5 |
| Bahororo | 5.1 | |
| Banyabutumbi | 3.8 | |
| Banyankole | 2.5 | |
| Banyarwanda | 0.8 | |
| Congolese | 0.8 | |
| Occupation | Peasant farmer | 82.3 |
| Business | 10.1 | |
| Teacher | 0.8 | |
| Income (UgX) | None | 31.6 |
| < 50,000 | 27.8 | |
| 50,001–80,000 | 19 | |
| > 80,000 | 12.7 |
UgX Ugandan shillings, and 1 US$ = 3827 UgX
Approximately 31.6% of the respondents declared a lack of monthly (source of) income, while 27.8% others earned less than 50,000 Uganda shillings (UgX) (US$ 13.07) per month. With the exception of a formally employed primary school teacher who receive monthly salary, the rest got their earnings from harvesting crops. The monthly earnings were got from the sum of the block money earned after selling the harvests at the end seasons divided by 12 months. Therefore, the locals hardly had any income outside harvesting periods. Moreover, most locals carried out subsistence farming, where food is simply grown for consumption. The highest monthly income was UgX 560,000 (US$ 146.33), earned by the primary school teacher.
Source of knowledge of medicinal plants
Majority of the respondents (62%) acquired knowledge on ethnomedicinal plants for malaria treatment from their parents, while 16.5% got knowledge from older family members, usually grandparents (Fig. 2). Traditional healers contributed 5.1% to the transfer of indigenous knowledge. However, a very small portion of the respondents acquired ethnobotanical knowledge through other means, such as spiritual revelations, radio and television programs, and reading ethnobotanical books.
Fig. 2.
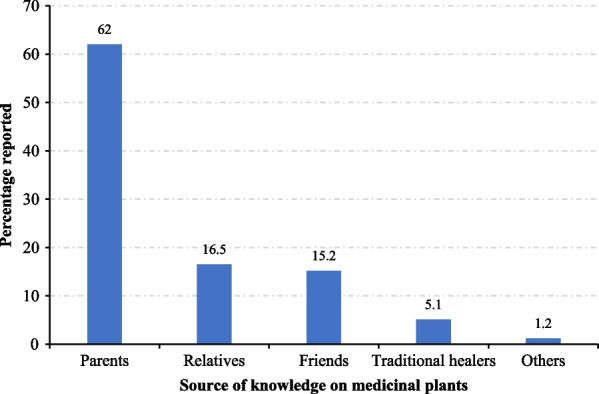
Source of knowledge on plants used for malaria treatment in Bwambara Sub-County, Rukungiri District, Uganda
Symptoms of malaria and treatment-seeking behaviour of patients
In the study area, malaria is locally known as Omushweija gw’ensiri (Omushweija means fever, ensiri means mosquito). Thus, this is directly translated to mean “fever of mosquitoes”. For this, all informants were aware that malaria is caused by mosquito bites. Some informants reported that drinking unboiled water, exposure to morning dew and rain may cause malaria. Other indirect causes of malaria such as having grown bushes around homesteads, leaving the house doors and windows open in the evening hours and having stagnant water were also cited.
The most important symptoms of malaria were feeling cold (having goosebumps), high body temperature, headache, shivering, vomiting and joint pains (Table 2). It should be noted that all or a combination of the listed symptoms may be present in a malaria patient. Thus, the informants diagnose a certain disease as malaria if the patient presented with at least three of the listed symptoms. The participants were aware that not all patients recover from malaria after taking herbal remedies, which necessitated referral to hospitals. Participants reported that referrals are made basing on severity of the symptoms (i.e., extremely high body temperature and confusion) (Table 3).
Table 2.
Malaria symptoms mentioned by respondents in Bwambara, Rukungiri District, Uganda (n = 125)
| General malaria symptoms | Local language (Runyankore/Rukiga) | Percentage |
|---|---|---|
| Feeling cold/goosebumps | Okupiipa | 82 |
| High temperature | Omuriro | 79 |
| Headache | Okuterwa omutwe | 77 |
| Shivering | Okutetema | 75 |
| Vomiting | Okutanaka | 72 |
| Joint pains | Okushaash engyingo | 70 |
| Loss of appetite | Okuremwa kurya | 58 |
| General body weakness | Okubura amaani | 42 |
| Abdominal pain | Okushaasha omunda | 37 |
| Wound or burns | Ebironda/amasya | 26 |
| Sweating | Okututuuka | 23 |
| Sticky or red eyes | Okutukura amaisho | 17 |
| Bitter or sour mouth | Okushariira akanwa | 15 |
| Anemia-very pale palms, fingernails | Okuhwamu eshagama–okwera engaro n’enono | 13 |
| False smell | Okunukirwa ebitariho | 12 |
| Flue | Sanyiga | 11 |
| Diarrhea | Okwirukana | 10 |
| Frequent thirst | Okugyira eiriho buri kanya | 9 |
| Teary eyes | Okurira amaisho | 8 |
| Severe dehydration indicated by pale eyes | Okuhwamu amaizi–amaisho garikwera | 7 |
| Yellow eyes | Amaisho g’akinekye | 7 |
| Difficulty in breathing | Okwisya kubi | 2 |
| Painful swelling or lumps in the skin | Okuzimba | 2 |
Table 3.
Severe malaria symptoms that warrant hospitalization among indigenous communities in Bwambara Sub-County, Rukungiri District, Western Uganda
| Severe symptoms | Informants | Total | Ranking | ||||||
|---|---|---|---|---|---|---|---|---|---|
| I1 | I2 | I3 | I4 | I5 | I6 | I7 | |||
| Coma/loss of consciousness | 5 | 4 | 3 | 5 | 4 | 4 | 5 | 30 | 3rd |
| Confusion | 3 | 7 | 5 | 6 | 6 | 5 | 6 | 38 | 2nd |
| Convulsions | 6 | 3 | 7 | 3 | 3 | 6 | 1 | 29 | 4th |
| Diarrhea | 1 | 2 | 1 | 4 | 1 | 1 | 2 | 12 | 7th |
| Extremely high body temperature | 7 | 6 | 6 | 7 | 7 | 7 | 7 | 47 | 1st |
| Fits in children | 4 | 5 | 4 | 3 | 5 | 3 | 4 | 28 | 5th |
| Vomiting—cannot keep any food or drink | 2 | 1 | 2 | 1 | 2 | 2 | 3 | 13 | 6th |
I Informant
Prevention of malaria
All the informants reported that sleeping under treated mosquito net prevents contracting malaria. Other preventive measures included destruction of mosquito breeding areas such aas stagnant waters and elimination of unwanted vegetation within and around homesteads (Fig. 3).
Fig. 3.

Measures used for malaria prevention in Bwambara Sub-County, Rukungiri District, Western Uganda
Diversity of ethnomedicinal plants used for malaria treatment
A total of 55 plant species were mentioned to be used for treatment of malaria (Table 4). However, only 48 of the voucher specimens collected were taxonomically identified. The other seven species could not be found within the study area during field visits, and only their local names were recorded (Table 4). The identified species belonged to 47 genera and 23 families. Most plant species belonged to family Asteraceae (22.9%), Fabaceae (10.4%), Lamiaceae (8.3%) and Poaceae (8.3%). The calculated ICF value for malaria was 0.81. Of the medicinal plants reported for malaria treatment, a paired comparison indicates that the highest rank was given for Vernonia amygdalina (FC = 76; UV = 0.608), followed by Aloe vera (FC = 35; UV = 0.28) and then Azadirachta indica (FC = 15; UV = 0.12).
Table 4.
Medicinal plants used in the management of malaria in Bwambara Subcounty, Rukungiri District, Uganda
| No. | Family Botanical name |
Voucher number | Part used | Life form | FC | UV |
|---|---|---|---|---|---|---|
| Acanthaceae | ||||||
| 1 | Justicia anselliana (Nees) T. Anderson | H22-013 | L | Shrub | 1 | 0.008 |
| Apocynaceae | ||||||
| 2 | Mondia whitei (Hook.f.) Skeels | H22-030 | L | Vine | 1 | 0.008 |
| 3 | Cascabela thevetia (L.) Lippod | H22-049 | L | Tree | 1 | 0.008 |
| Asphodelaceae | ||||||
| 4 | Aloe vera (L.) Burm.f | H22-022 | L | Herb | 35 | 0.28 |
| Asteraceae | ||||||
| 5 | Baccharoides lasiopus (O.Hoffm) H.Rob | H22-008 | L, RB | Shrub | 6 | 0.048 |
| 6 | Berkheya barbata (L.f.) Hutch | H22-040 | L | Herb | 1 | 0.008 |
| 7 | Bidens pilosa L | H22-035 | Fl | Herb | 3 | 0.024 |
| 8 |
Bothriocline longipes (Oliv. & Hiern) N.E.Br |
H22-046 | L | Herb | 1 | 0.008 |
| 9 | Crassocephalum vitellinum (Benth.) S. Moore | H22-016 | L | Herb | 1 | 0.008 |
| 10 | Vernonia amygdalina Delile | H22-002 | L, RB | Shrub | 76 | 0.608 |
| 11 | Hoffmannanthus abbotianus (O.Hoffm.) H.Rob., S.C.Keeley & Skvarla | H22-019 | L | Herb | 4 | 0.032 |
| 12 | Laggera alata (D.Don) Sch.Bip. ex Oliv | H22-020 | L | Herb | 1 | 0.008 |
| 13 | Solanecio mannii (Hook.f.) C. Jeffrey | H22-005 | L | Shrub | 2 | 0.016 |
| 14 | Sonchus oleraceus L | H22-011 | St | Herb | 5 | 0.04 |
| 15 | Tithonia diversifolia (Hemsl.) A.Gray | H22-050 | L | Shrub | 5 | 0.04 |
| Bignoniaceae | ||||||
| 16 | Kigelia africana (Lam.) Benth | H22-036 | L | Tree | 1 | 0.008 |
| 17 | Markhamia lutea (Benth.) K.Schum | H22-031 | RB | Tree | 1 | 0.008 |
| Canellaceae | ||||||
| 18 | Warburgia ugandensis Sprague | H22-014 | SB | Tree | 3 | 0.024 |
| Caricaceae | ||||||
| 19 | Carica papaya L | H22-047 | SB, L, Fr | Tree | 6 | 0.048 |
| Cleomaceae | ||||||
| 20 | Cleome gynandra L | H22-001 | L | Herb | 1 | 0.008 |
| Cucurbitaceae | ||||||
| 21 | Momordica foetida Schumach | H22-025 | L, SB | Vine | 2 | 0.016 |
| Euphorbiaceae | ||||||
| 22 | Manihot esculenta Crantz | H22-033 | L | Shrub | 1 | 0.008 |
| 23 | Shirakiopsis elliptica (Hochst.) Esser | H22-009 | L | Tree | 1 | 0.008 |
| Fabaceae | ||||||
| 24 | Albizia coriaria Welw. ex Oliv | H22-012 | SB | Tree | 2 | 0.016 |
| 25 | Erythrina abyssinica Lam | H22-010 | L | Tree | 3 | 0.024 |
| 26 | Indigofera arrecta Hochst. ex A.Rich | H22-006 | L, RB | Shrub | 1 | 0.008 |
| 27 | Senna didymobotrya (Fresen.) H.S. Irwin & Barneby | H22-037 | L | Shrub | 2 | 0.016 |
| 28 | Vachellia hockii (De Wild.) Seigler & Ebinger | H22-041 | SB | Tree | 1 | 0.008 |
| Lamiaceae | ||||||
| 29 | Clerodendrum capitatum (Wild.) Schumach | H22-021 | L | Shrub | 5 | 0.032 |
| 30 | Ocimum gratissimum | H22-003 | L | Shrub | 1 | 0.008 |
| 31 | Ocimum kilimandscharicum Gürke | H22-018 | L | Shrub | 2 | 0.016 |
| 32 | Plectranthus barbatus Andrews | H22-007 | L | Shrub | 3 | 0.024 |
| Malvaceae | ||||||
| 33 | Sida alba L | H22-044 | L | Shrub | 3 | 0.024 |
| Meliaceae | ||||||
| 34 | Azadirachta indica A. Juss | H22-048 | L | Tree | 15 | 0.12 |
| Moringaceae | ||||||
| 35 | Moringa oleifera Lam | H22-027 | Seed, L | Tree | 4 | 0.032 |
| Musaceae | ||||||
| 36 | Musa acuminata Colla | H22-023 | L | Herb | 1 | 0.008 |
| Myrtaceae | ||||||
| 37 | Eucalyptus grandis W. Hill ex Maiden | H22-039 | L | Tree | 2 | 0.016 |
| Poaceae | ||||||
| 38 | Digitaria abyssinica (Hochst. ex A.Rich.) Stapf | H22-026 | L | Grass | 6 | 0.048 |
| 39 | Imperata cylindrica (L.) P.Beauv | H22-042 | L | Grass | 1 | 0.008 |
| 40 | Pennisetum purpureum Schumach | H22-029 | St | Grass | 2 | 0.016 |
| 41 | Saccharum officinarum L | H22-004 | St | Grass | 1 | 0.008 |
| Rhamnaceae | ||||||
| 42 | Gouania longispicata Engl | H22-028 | L | Liana | 1 | 0.008 |
| Rutaceae | ||||||
| 43 | Citrus limon (L.) Burm.fil | H22-043 | L, SB | Tree | 2 | 0.016 |
| 44 | Clausena anisata (Willd.) Hook.f | H22-032 | L | Shrub | 1 | 0.008 |
| Solanaceae | ||||||
| 45 | Nicotiana tabacum L | H22-015 | L | Herb | 1 | 0.008 |
| 46 | Physalis peruviana L | H22-034 | L, RB | Herb | 3 | 0.024 |
| Verbenaceae | ||||||
| 47 | Lantana trifolia L | H22-024 | L | Shrub | 3 | 0.024 |
| Vitaceae | ||||||
| 48 | Cyphostemma adenocaule (Steud. ex A.Rich.) Desc | H22-017 | L | Liana | 1 | 0.008 |
| 49 | Unidentified | NA | L | NA | 6 | 0.048 |
| 50 | Unidentified | NA | L | NA | 3 | 0.024 |
| 51 | Unidentified | NA | L | NA | 1 | 0.008 |
| 52 | Unidentified | NA | L | NA | 2 | 0.016 |
| 53 | Unidentified | NA | L | NA | 4 | 0.032 |
| 54 | Unidentified | NA | L | NA | 1 | 0.008 |
| 55 | Unidentified | NA | L | NA | 1 | 0.008 |
NA not applicable, FC frequency of citation, UV use value, plant parts: Bk bark, Fl flowers, Fr fruits, L leaf, RB root bark, St stem, SB stem bark
Life forms, plant parts, preparation and administration of herbal remedies for malaria treatment
The identified plant species consisted of mainly shrubs (31.3%), trees (27.1%) and herbs (25.0%) (Fig. 4; Additional file 1). On the other hand, the most used plant organs were leaves (74%), followed by stem bark (9%) and root bark (8%) (Fig. 5). Most of the plants were used in combination with each other (65.5%). All the plant materials were used immediately after collection (while still fresh). Only the stem bark of Warburgia ugandensis and leaves of Azadirachta indica were reported to be used either when fresh or in powder form.
Fig. 4.

Life forms of medicinal plants used in the management of malaria in Bwambara Sub-County, Rukungiri District, Western Uganda
Fig. 5.
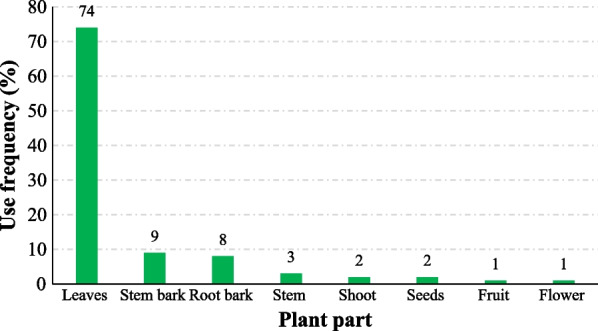
Plant parts used in preparation of remedies for malaria treatment in Bwambara Sub-County, Rukungiri District, Western Uganda
The most common preparation methods were decoctions (41.8%) and cold infusion (23.6%) (Additional file 1). Other methods included bathing (3.6%) and steaming (3.6%). Some herbalists cited continuous use of some medicinal plants such as Azadirachta indica as tea or in tea as a prophylaxis for malaria.
Discussion
This study showed that most of the respondents were females (Table 1). This observation is contrary to a recent report by Tabuti et al. [24] in Eastern Uganda. Previous studies in Uganda have indicated higher participation of females than males in ethnobotanical surveys, since they are the ones who take healthcare needs of their families [25]. Moreover, more females could have participated because of their indigenous knowledge of nutri-medicinal plants as home managers and disease managers amongst children [26, 27]. Most respondents were the Bakiga, because they were the first group of Bantu ethnic group to migrate into this area as early as the 1950s, where the original inhabitants were the minority Banyabutumbi [28]. There were low-income levels among the respondents indicating that most of them depended on subsistence farming. This is typical in Uganda, and may also explain their preference of medicinal plants over conventional antimalarial drugs.
Regarding medicinal plant knowledge dynamics, most respondents hinted that they inherited the indigenous knowledge about medicinal plants for malaria treatment from their parents or grandparents (Fig. 2). This is not surprising, because African traditional medicine recipes are usually a guarded family secret, so that they are only passed orally to children from parents or through apprenticeships from relatives [16]. It was interesting to note that traditional healers contributed 5.1% to the transfer of indigenous knowledge (Fig. 2). This may be attributed to the cultural acceptability and strong belief in herbal medicines which has driven commercialization of herbal products. Therefore, the impetus behind acquiring herbal medicine knowledge observed in this study may be related to the need to establish a source of income through sale of herbal products [29]. Absence of a respondent who had acquired folk knowledge through formal training is because herbal medicine training centres are rare in Uganda.
The indigenous communities surveyed were well-informed that malaria is caused by mosquito bites, though some believed that taking unboiled water, exposure to morning dew as well as rain are other potential causes. Other indirect causes of malaria such as having grown bushes around homesteads, leaving the house doors and windows open in the evening hours and having stagnant water were also cited. A previous study in Uganda by Adia et al. [25] reported that 96% of the respondents understood that malaria was caused by infected mosquito bites. In similar study in Zimbabwe, all respondents recognized mosquito bites as the main cause of malaria [30] but traditional healers still held it that exposure to morning dew and drinking water from unprotected wells caused malaria. In Kenya, it was cited that malaria is believed to be a direct cause of mosquito bites, exposure to cold weather [31] or consumption of fresh unboiled milk (Cheko che makiyo in the Tugen dialect), dirty water, ikwek (i.e., vegetables, such as Gynadropis gynadra and Solanum nigrum) [32], witchcraft (sorcery attacks) and supernatural forces [33, 34]. In Eastern Uganda, some people believe that keeping a dirty homestead with dense bushes or pools of stagnant water caused malaria [35]. Though bushes and stagnant water may not be the direct causes of malaria, they are breeding sites for mosquitoes which transmit malaria. In this case, some communities would close windows and doors in the evening to guard against mosquito bites [35].
The most important symptoms of malaria were feeling cold/goosebumps, high body temperature, headache, shivering, vomiting and joint pains. These findings are consistent with Stangeland et al. [36] who reported high body temperature, shivering and headache as the most frequent symptoms of malaria in Nyakayojo Sub-County, Western Uganda. Another study in Central Uganda mentioned high body temperature, red eyes and vomiting as the most common malaria symptoms [25]. In the malaria-endemic Eastern Uganda, fever was mentioned as the major malaria symptom, along with vomiting, body weakness, headache, diarrhea, convulsions, inappetence and body chills [24]. Outside Uganda, malaria has been associated with periodic fever, sweating, backache, chills, headache, vomiting, diarrhea and joint pains [37, 38], coldness, goose pimples and headaches [30], which aligns well with our findings.
As indicated by the herbalists, some severe malaria symptoms such as very high body temperatures and confusion (probably due to cerebral malaria) were indicative that the patient needed to be referred for further treatment using conventional medicine (Table 3). This suggests that most people seek for conventional medical treatment when malaria is in severe stages. The listed symptoms relate with those of cerebral malaria, which may signify its prevalence in the study area. A study by Stangeland et al. [36] in Western Uganda found that hospital referrals were being recommended only in cases, where herbal remedies did not relieve malaria symptoms within 2–3 days of treatment or if the patient presented severe symptoms. The current findings agree with previous studies among traditional healers which reported few hospital referrals in exceptional cases when patients showed severe malaria symptoms or herbal remedies failed completely [24, 30, 37]. In a study by Ngarivhume et al. [30], lack of a patient’s improvement was attributed to evil spirits, where the patient needed cleansing/placating the spirits and then repeat or be given a different prescription thereafter. Such practices have been cited in Kenya, where malaria which is thought to be caused by supernatural forces required the intervention of diviners (such as Oloiboni among the Maasai and Orgoiyon among the Tugen) [33]. Such practices and beliefs, coupled with improper diagnosis and wrong choice of appropriate herbal remedies may result in death. This requires sensitization of the population about proper diagnosis of malaria and the dangers associated with late treatment.
All the informants reported that sleeping under treated mosquito net prevents contracting malaria (Fig. 3). This could be because since November 2018, Uganda as part of the eleven (high burden to high impact) countries distributed insecticide-treated mosquito nets (ITN) across the country [39]. This countrywide campaign could be the reason as to why each informant knew about ITN. Other preventive measures included the destruction of mosquito breeding areas, such as stagnant waters, and the reduction of unwanted vegetation within and around the homesteads. In Uganda, Tabuti [35] and Adia et al. [25] reported similar preventive measures used to guard against malaria. These findings are comparable to that in Zimbabwe where the use of ITN, destruction of mosquito breeding and resting areas, and reduction of unwanted undergrowth and puddles within and around homesteads, avoiding dew and residual spraying as malaria preventive measures are used to prevent malaria [30].
We identified 48 species, out of the 55 species mentioned by the respondents. The other species which could not be found during the field visits suggest that some plants are rare in the study area, or may have become extinct due to over exploitation or habitat destruction [18]. These raises need for instituting conservation measures. The identified plant species were majorly members of family Asteraceae, Fabaceae, Lamiaceae and Poaceae, which is in agreement with studies in Uganda [24, 25, 36, 40] and elsewhere [37, 41]. The prevalence of these families may be attributed to the frequent distribution in the area, diverse habitat, availability and presence of diverse secondary metabolites which increase the effectiveness of herbal remedies from them [42].
Of the 48 plants mentioned by herbalists, most species were listed for use in the management of malaria in Uganda and other countries. For instance, Carica papaya, Albizia coriaria, Warburgia ugandensis, Azadirachta indica, Aloe vera and Vernonia amygdalina in Uganda [24, 25, 40, 43–45], Zimbabwe [30], Kenya [38], Cameroon [46], Indonesia [47] and Ethiopia [48]. The calculated ICF for malaria was 0.81, implying that there is substantial agreement amongst the local population about the medicinal plants used in malaria treatment.
Leaves of shrubs, trees and herbs are the commonly used parts for preparing malaria remedies, corroborating some ethnobotanical reports in other parts the country [24, 25, 35, 36]. This could translate into a more sustainable use of the plants, as compared to roots. Leaves regenerate under favorable conditions to preserve the occurrence of the plants but their high use frequency in this study may plausibly be related to their year-round availability [49]. The observed use of different plants or plant parts could be to exploit their synergistic therapeutic effects [36, 50, 51], mask toxicity of efficacious plants or as a trick of keeping the secrecy of herbal formulae [52].
Oral administration (89.6%) was the major route used, but use of prepared decoctions for bathing was also practiced (10.4%). This is expected, because oral dosage forms are easy to prepare and administer, especially because malaria is an internal disease [38, 53]. One striking feature was that posology of the remedies were based on cups or glasses, a phenomenon that has been cited among herbalists throughout the East African botanical plate [24, 38, 40].
An in-depth search of literature indicated that 22 species recorded in this ethnobotanical study have been evaluated for their antimalarial/antiplasmodial activities (Table 5). Most species possess acceptable preclinical efficacy and safety, with the exception of Vernonia amygdalina [54] and Senna Didymobotrya [55] which showed high toxicity. It is worth noting that Azadirachta indica and Vernonia amygdalina has been subjected to clinical trials. A homeopathic Azadirachta indica preparation was shown to be effective for reducing malaria attacks in a highly endemic area for Plasmodium falciparum [56]. Similarly, Challand and Willcox [57] performed a clinical trial utilizing a remedy of Vernonia amygdalina leaves for the treatment of uncomplicated malaria. They found that there was an adequate clinical response at day 14 in 67% of cases. Total parasite clearance was observed only in 32% of those with adequate clinical response, where recrudescence occurred in 71% and this hampers complete remission of the parasite from the body [57]. In conclusion, these reports support that the inventoried medicinal plants possess antimalarial properties which can be further investigated for development of new antimalarial drug candidates. Specifically, clinical trials should be done on some of the species that have exhibited promising antimalarial efficacy. However, these maybe impeded by the strict regulatory requirements for clinical studies, as well as the financial muscle required [38]. In addition, formulations rich in known bioactive compounds from these species should be specifically targeted.
Table 5.
Antiplasmodial, antimalarial and toxicity studies of extracts and compounds isolated from plants reported in malaria phytotherapy of Bwambara Sub-County, Rukungiri District, Western Uganda
| Medicinal plant | Plant organ | Solvent1 | Antiplasmodial/antimalarial (Plasmodium spp) efficacy2 | Reported bioactive phytochemicals | Toxicity profile | References |
|---|---|---|---|---|---|---|
| Aloe vera | Leaves | Water | EC50 values for extract: 0.289 to 1056 μg/mL (MRC-2), 169.76 (3D7) for aloin | Aloin | Considered to be safe | [58, 59] |
| Albizia coriaria | Stem bark | MeOH | 15.2 (D6); 16.8 (W2) | Triterpenoids, lupeol, lupenone | Cytotoxic to the human glioblastoma cell line U87 CD4 CXCR4 (CC50 = 6.4 and 14.9 μg/ml for ethanol and DMSO extracts) | [60–63] |
| Azadirachta indica | Leaves | Water, MeOH | 17.9 (D6); 43.7 (W2) | Terpenoids, isoprenoids, gedunin, limonoids: khayanthone, meldenin, nimbinin | Cytotoxicity LD50 of 101.26 and 61.43 µg/ml for water and methanol extracts | [64–68] |
| Bidens pilosa | Leaves | DCM, water, MeOH | 8.5, 5, 11, 70 (D10) | No reports | Hydro and ethanolic extracts are not toxic in mice (LD50 = 12.3 and 6.2 g/kg bw, respectively). Safe in humans | [69–71] |
| Carica papaya | Leaves | Ethyl acetate | 2.96 (D10), 3.98 (DD2), 0.2 µM (carpaine) | Carpaine | Carpaine has high selectivity (106), nontoxic to normal red blood cells and rat skeletal myoblast (L6) cells | [72–74] |
| Clausena anisata | Twigs, Leaves | DCM/MeOH, water | 18 (D10), > 100 (D10); 55, > 100 (D10) | No reports | Potentially toxic | [69, 75] |
| Cleome gynandra | Whole plant | Hexane, ethyl acetate, MeOH | Schizonticidal activity in vitro (NK65) |
Apigenin, caffeic, Ferulic, hexadecanoic acids, kaempferol, taraxasterol, pheophytin A, sitosterol, stigmasterol; 5,7,13,15-eicosatetraen-9, 12-diol and 9-hydroxy-5,7,13,15-eicosatetraen-12-one |
No report | [76] |
| Clerodendrum capitatum | Leaves | Ethanol | 65.3% chemosuppression at 400 mg/kg | Tannins, flavonoids, alkaloids, triterpenes | LD50 > 5000 mg/kg | [77] |
| Erythrina abyssinica | Stem bark | Ethyl acetate | 83.6% inhibition of P. falciparum at 10 μg/ml | Chalcones (5-prenylbutein, homobutein), flavanones such as 5-deoxyabyssinin II, abyssinin III and abyssinone IV | Minimal toxicity reported in animal studies | [54, 78] |
| Kigelia africana | Bark, fruit | Chloroform/ethyl acetate, MeOH | 59.9 (K39), 83.8 (V1/S); fruits had 165.9 (K39) | None reported | Aqueous fruit extract elicited hepatorenal toxic effects in rats | [79, 80] |
| Markhamia lutea | Leaves | Ethyl acetate | 71% inhibition of P. falciparum at 10 μg/ml | Phenylpropanoid glycosides, cycloartane triterpenoids, musambins A-C, Candmusambiosides A-C | Aqueous leaf and ethanolic extracts with curative anti-inflammatory activity attenuated paclitaxel toxicity in rat’s intestine | [54, 81, 82] |
| Momordica foetida | Shoot | Water | 6.16 (NF54); 0.35 (FCR3) | Saponins, alkaloids, cardiac glycosides | No toxicity against human hepatocellular (HepG2) and urinary bladder carcinoma (ECV-304, derivative of T-24) cells | [83–85] |
| Mondia whitei | Whole root | MeOH | 2% parasitemia suppression (ANKA) | Chlorogenic acid | Low toxicity on mice exposed to extract for 90 days | [86, 87] |
| Moringa oleifera | Flower, leaves | Water, MeOH, acetone, hexane | Parasitemia suppression upto 99.48% | Flavonoids | Not toxic | [88] |
| Ocimum gratissimum | Leaves/twigs | DCM | 8.6 (W2) | Flavonoids | LD50 of the butanolic and ethyl acetate fraction of MeOH leaf extract were 2154.1 and 3807.9 mg/kg | [64, 89, 90] |
| Ocimum kilimandscharicum | Leaves, twigs | DCM | 0.843 (D6); 1.547 (W2) | No reports | No reports | [89] |
| Plectranthus barbatus | Leaves | DCM | No activity | No toxicity recorded | No reports | [89, 91] |
| Rootbark | Water (hot), chloroform/MeOH | 100 mg/kg/day of extracts had 55.23% and 78.69% parasite chemosuppression | No report | No reports | ||
| Senna didymobotrya | Leaves twigs | MeOH, DCM/MeOH (1:1) | > 100 (K39), 9.5 (D10) | Quinones | Root extracts were toxic (LD50 = 1927 mg/kg) | [55, 69, 92] |
| Solanecio mannii | Leaves | MeOH | 21.6 (3D7), 26.2 (W2) | Phytosterols, n-alkanes and N-hexacosanol | No reports | [36, 93] |
| Tithonia diversifolia | Aerial parts, leaves | MeOH, ether | 1.2 (3D7); 1.5 (W2), MeOH extract had 74% parasitemia suppression | Tagitinin C, sesquiterpene lactones | Aerial parts reportedly cytotoxic against cells from human foetal lung fibroblast cell line | [94–97] |
| Vernonia amygdalina | Leaves | MeOH/DCM, ethanol | 2.7 (K1), 9.83. In vivo parasite suppression of between 57.2–72.7% in combination with chloroquine | Vernolepin, vernolin, vernolide, vernodalin and hydroxy vernodalin, steroid glucosides | Petroleum ether extract elicited strong cytotoxicity | [36, 54, 57, 85, 95, 98] |
| Warburgia ugandensis | Stem bark | MeOH, water, DCM |
6.4 (D6); 6.9 (W2), 12.9 (D6); 15.6 (W2) 69% parasite suppression |
Coloratane sesquiterpenes, e.g., muzigadiolide | Cytotoxic to the human glioblastoma cell line U87 CD4 CXCR4 (CC50 = 7.2 and 2.0 μg/ml for ethanol and DMSO extracts | [60, 61, 95, 99–101] |
1Solvents used: DCM = dichloromethane, MeOH = methanol. 2Antiplasmodial activity are reported as IC50 (μg/mL). Plasmodium falciparum isolates: chloroquine sensitive strains are 3D7, MRC-2, D6, D10, FCR3, and NF54; chloroquine resistant strains include DD2, K1 and W2
Conclusion
The local communities in Bwambara sub-county of Rukungiri District possess rich and diverse indigenous knowledge of plant-based medicine used to manage malaria. This could indicate high malaria cases, and/or accessibility, availability and potency of herbal remedies. More than 30% of the plants recorded in this study have been reported elsewhere for treatment of malaria and have experimentally recorded antimalarial and antiplasmodial activities. No study has reported any pharmacological properties, safety and efficacy of Digitaria abyssinica and Berkheya barbata. There is need to validate the toxicity and efficacy of other unstudied species to foster the discovery of efficacious, safe and effective standardized antimalarial drugs.
Supplementary Information
Additional file 1. Details of medicinal plants used in the management of malaria in Bwambara Subcounty, Rukungiri District, Uganda.
Acknowledgements
The authors acknowledge the local communities and key informants for sharing this valuable indigenous knowledge. Miss Racheal Akatuhebwa is highly appreciated for proofreading this work.
Abbreviations
- DCM
Dichloromethane
- ICF
Informant consensus factor
- FC
Frequency of citation
- ITN
Insecticide-treated mosquito nets
- MeOH
Methanol
- WHO
World Health Organization
Author contributions
HG, EAO and PM conceived and designed the study. HG collected ethnobotanical data. HG, JBL and TO analyzed and interpreted the collected data. HG and TO wrote the first draft of the manuscript. HG, EAO, PM, JBL and TO revised, read and approved the final manuscript.
Funding
This research was supported by the Faculty of Science, Mbarara University of Science and Technology, Mbarara, Uganda (No. PF/Conf-2022/1).
Availability of data and materials
The raw data supporting the conclusions of this study are available within this article and its supplementary files.
Declarations
Ethics approval and consent to participate
This study was approved by the Uganda National Council for Science and Technology (Approval no. NS34ES). The Resident District Commissioner (RDC) of Rukungiri District and the Local Council I (LC I) were consulted and subsequently approved the study to be done in the respective villages. The respondents were first informed about the study objectives and those who accepted to participate gave a written informed consent at the beginning of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yi B, Zhang L, Yin J, Zhou S, Xia Z. 1-3-7 surveillance and response approach in malaria elimination: China’s practice and global adaptions. Malar J. 2023;22:152. doi: 10.1186/s12936-023-04580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. World Malaria Report 2022. World Health Organization 2022. https://apps.who.int/iris/rest/bitstreams/1484818/retrieve . Accessed 06 May 2023.
- 3.Global Health Policy. The President’s Malaria Initiative and Other U.S. Government Global Malaria Efforts. https://www.kff.org/global-health-policy/fact-sheet/the-presidents-malaria-initiative-and-other-u-s-government-global-malaria-efforts/. Accessed 20 Apr 2023.
- 4.WHO. Malaria. https://www.who.int/news-room/fact-sheets/detail/malaria. Accessed 10 Jun 2023.
- 5.Nuwa A, Oola J, Obonyo SO, Feldman M, Karungi S, Kertho E, Odong DS, Kimera I, Magumba G, Beinomugisha G, Chitty A, Tibenderana J, Opigo J, Abwaimo F. District-led malaria surveillance and response as an effective way to manage malaria upsurges following the withdrawal of indoor residual spraying: a case study from Nwoya District, Northern Uganda. Malar J. 2022;21:55. doi: 10.1186/s12936-022-04066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zalwango JF, Nankabirwa JI, Kitutu FE, Akunzirwe R, Buhuguru R, Rokani JB, Ssendikwanawa E, et al. Malaria diagnostic and treatment practices for febrile children under 5 years at two general hospitals in Karamoja, a high transmission setting in Uganda. Malar J. 2022;21:312. doi: 10.1186/s12936-022-04329-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MOH. Weekly Malaria Report. Ministry of Health-Uganda. http://library.health.go.ug/sites/default/files/resources/Malaria%20Bulletin-Epi%20week%2026.pdf. Accessed 20 Jun 2023.
- 8.Lal S, Ndyomugenyi R, Alexander ND, Lagarde M, Paintain L, Magnussen P, et al. Health facility utilisation changes during the introduction of community case management of malaria in South Western Uganda: an interrupted time series approach. PLoS ONE. 2015;10:e0137448. doi: 10.1371/journal.pone.0137448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asua V, Conrad MD, Aydemir O, Duvalsaint M, Legac J, Duarte E, Tumwebaze P, Chin DM, Cooper RA, Yeka A, Kamya MR, Dorsey G, Nsobya SL, Bailey J, Rosenthal PJ. Changing prevalence of potential mediators of aminoquinoline, antifolate, and artemisinin resistance across Uganda. J Infect Dis. 2021;223:985–994. doi: 10.1093/infdis/jiaa687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasim N, Sandeep IS, Mohanty S. Plant-derived natural products for drug discovery: current approaches and prospects. Nucleus. 2022;65:399–411. doi: 10.1007/s13237-022-00405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muangphrom P, Seki H, Fukushima EO, Muranaka T. Artemisinin-based antimalarial research: application of biotechnology to the production of artemisinin, its mode of action, and the mechanism of resistance of Plasmodium parasites. J Nat Med. 2016;70:318–334. doi: 10.1007/s11418-016-1008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tugume P, Nyakoojo C. Ethno-pharmacological survey of herbal remedies used in the treatment of paediatric diseases in Buhunga parish, Rukungiri District, Uganda. BMC Complement Altern Med. 2019;19:353. doi: 10.1186/s12906-019-2763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gumisiriza H, Sesaazi CD, Olet EA, Kembabazi O, Birungi G. Medicinal plants used to treat "African" diseases by the local communities of Bwambara sub-county in Rukungiri District, Western Uganda. J Ethnopharmacol. 2021;268:113578. doi: 10.1016/j.jep.2020.113578. [DOI] [PubMed] [Google Scholar]
- 14.Gumisiriza H, Birungi G, Lejju JB, Olet EA, Kembabazi O, Duncan C, Sesaazi CD. Ethnobotany and antimicrobial activity of Gouania longispicata Engl. J Complement Med Res. 2020;11:86–94. doi: 10.5455/jcmr.2020.11.01.10. [DOI] [Google Scholar]
- 15.Hartter J, Stampone MD, Ryan SJ, Kirner K, Chapman CA, Goldman A. Patterns and perceptions of climate change in a biodiversity conservation hotspot. PLoS ONE. 2012;7:e32408. doi: 10.1371/journal.pone.0032408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omara T. Plants used in antivenom therapy in rural Kenya: ethnobotany and future perspectives. J Toxicol. 2020;2020:1828521. doi: 10.1155/2020/1828521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Common wealth network. Uganda. Rukungiri. 2020. https://www.commonwealthofnations.org/info/regions-uganda/western-region/rukungiri/#:~:text=The%20majority%20of%20the%20workforce. Accessed on 02 Aug 2023.
- 18.Gumisiriza H, Birungi G, Olet EA, Sesaazi CD. Medicinal plant species used by local communities around Queen Elizabeth National Park, Maramagambo Central Forest Reserve and Ihimbo Central Forest Reserve, South western Uganda. J Ethnopharmacol. 2019;239:111926. doi: 10.1016/j.jep.2019.111926. [DOI] [PubMed] [Google Scholar]
- 19.African Development Bank. Road Sector Support Project 5 (RSSP V)—upgrading of Rukungiri–Kihihi–Ishasha/Kanungu and Mbale-BumbobiManafwa-Lwakhakha Roads. 2013. https://www.afdb.org/en/documents/document/uganda-road-sector-support-project-5-rssp-v-upgrading-of-rukungiri-kihihi-ishasha-kanungu-and-mbale-bumbobi-manafwa-lwakhakha-roads-esia-rap-summary-44822. Accessed 16 Aug 2023.
- 20.Monitor. Residents turn mosquito nets into building materials. 2021. https://www.monitor.co.ug/uganda/news/national/residents-turn-mosquito-nets-into-building-materials-3461638. Accessed 02 Aug 2023.
- 21.Kigozi SP, Kigozi RN, Sebuguzi CM, Cano J, Rutazaana D, Opigo J, et al. Spatial-temporal patterns of malaria incidence in Uganda using HMIS data from 2015 to 2019. BMC Public Health. 2020;20:1913. doi: 10.1186/s12889-020-10007-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krejcie RV, Morgan DW. Determining sample size for research activities. Educ Psychol Meas. 1970;30:607–610. doi: 10.1177/001316447003000308. [DOI] [Google Scholar]
- 23.Trotter RJ, Logan MH. Informant consensus. A new approach for identifying potentially effective medicinal plants. In: Etkin NL, editor. Plants in indigenous medicine and diet. Bedford Hills: Newyork: Redgrave; 1986, pp. 22.
- 24.Tabuti JRS, Obakiro SB, Nabatanzi A, Anywar G, Nambejja C, Mutyaba MR, Omara T, Waako P. Medicinal plants used for treatment of malaria by indigenous communities of Tororo District, Eastern Uganda. Trop Med Health. 2023;51:34. doi: 10.1186/s41182-023-00526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adia MM, Anywar G, Byamukama R, Kamatenesi-Mugisha M, Sekagya Y, Kakudidi EK, Kiremire BT. Medicinal plants used in malaria treatment by Prometra herbalists in Uganda. J Ethnopharmacol. 2014;155:580–588. doi: 10.1016/j.jep.2014.05.060. [DOI] [PubMed] [Google Scholar]
- 26.Asiimwe S, Namutebi A, Borg-Karlsson A, Kamatenesi-Mugisha M, Oryem-Origa H. Documentation and consensus of indigenous knowledge on medicinal plants used by the local communities of western Uganda. J Nat Prod Plant Resour. 2014;4:34–42. [Google Scholar]
- 27.Nalumansi P, Kamatenesi-Mugisha M, Anywar G. Medicinal plants used in paediatric health care in namungalwe sub county, Iganga district, Uganda. Nova J Med Biol Sci. 2014;2:1–14. doi: 10.20286/nova-jmbs-030234. [DOI] [Google Scholar]
- 28.Linden I. The History of Kigezi—a history of Kigezi in South-West Uganda. Edited by Donald Denoon. The National Trust. Adult Education Centre, Kampala, Uganda, 1972. pp. 302. J Afr Hist. 1972; 13: 694–695.
- 29.Walusansa A, Asiimwe S, Ssenku JE, Anywar G, Namara M, Nakavuma JL, Kakudidi EK. Herbal medicine used for the treatment of diarrhea and cough in Kampala city, Uganda. Trop Med Health. 2022;50:5. doi: 10.1186/s41182-021-00389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ngarivhume T, Van't Klooster CI, de Jong JT, Van der Westhuizen JH. Medicinal plants used by traditional healers for the treatment of malaria in the Chipinge district in Zimbabwe. J Ethnopharmacol. 2015;159:224–237. doi: 10.1016/j.jep.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Olala CN. Identification of plants used for treatment of malaria and factors influencing their use in Boro division, Siaya county, Kenya. MSc Thesis, Kenyatta University, Nairobi, Kenya. 2014.
- 32.Kaendi JM. Coping with Malaria and Visceral Leishmaniasis (Kala-azar) in Baringo District, Kenya: Implications for Disease Control. PhD Thesis, University of California, Los Angeles, CA, USA. 1994.
- 33.Sindiga I. Indigenous medical knowledge of the Maasai. Indig Knowl Dev Monit. 1994;2:16–18. [Google Scholar]
- 34.Kiringe JW. A survey of traditional health remedies used by the Maasai of Southern Kaijiado district, Kenya. Ethnobot Res Appl. 2006;4:61–73. doi: 10.17348/era.4.0.61-74. [DOI] [Google Scholar]
- 35.Tabuti J. Herbal medicines used in the treatment of malaria in Budiope county, Uganda. J Ethnopharmacol. 2008;116:33–42. doi: 10.1016/j.jep.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 36.Stangeland T, Alele PE, Katuura E, Lye KA. Plants used to treat malaria in Nyakayojo sub-county, western Uganda. J Ethnopharmacol. 2011;137:154–166. doi: 10.1016/j.jep.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Mukungu N, Abuga K, Okalebo F, Ingwela R, Mwangi J. Medicinal plants used for management of malaria among the Luhya community of Kakamega East sub-County, Kenya. J Ethnopharmacol. 2016;194:98–107. doi: 10.1016/j.jep.2016.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omara T. Antimalarial plants used across Kenyan communities. Evid Based Complement Altern Med. 2020;2020:4538602. doi: 10.1155/2020/4538602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO. World Malaria Report 2021. World Health Organization 2021. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021. Accessed 17 Jun 2023.
- 40.Opio D, Andama E, Kureh GT. Ethnobotanical survey of antimalarial plants in areas of: Abukamola, Angeta, Oculokori and Omarari of Alebtong District in Northern Uganda. EJMP. 2018;21:1–14. doi: 10.9734/EJMP/2017/38043. [DOI] [Google Scholar]
- 41.Suleman S, Beyene Tufa T, Kebebe D, Belew S, Mekonnen Y, Gashe F, Mussa S, Wynendaele E, Duchateau L, De Spiegeleer B. Treatment of malaria and related symptoms using traditional herbal medicine in Ethiopia. J Ethnopharmacol. 2018;213:262–279. doi: 10.1016/j.jep.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 42.Obakiro SB, Kiprop A, Kowino I, Kigondu E, Odero MP, Omara T, Bunalema L. Ethnobotany, ethnopharmacology, and phytochemistry of traditional medicinal plants used in the management of symptoms of tuberculosis in East Africa: a systematic review. Trop Med Health. 2020;48:68. doi: 10.1186/s41182-020-00256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okello D, Kang Y. Exploring antimalarial herbal plants across communities in Uganda based on electronic data. Evid Based Complement Alternat Med. 2019;2019:3057180. doi: 10.1155/2019/3057180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee YJ, Adusumilli G, Kazungu R, Anywar G, Kyakulaga F, Katuura E, Parikh S, Willcox M. Treatment-seeking behavior and practices among caregivers of children aged ≤5 y with presumed malaria in rural Uganda. Trans R Soc Trop Med Hyg. 2019;131:525–533. doi: 10.1093/trstmh/trz039. [DOI] [PubMed] [Google Scholar]
- 45.Anywar G, Byamukama R, vant Klooster CIEA, Wilcox M, Nalumansi P, de Jong J, Rwaburindori P, Kiremire BT. Medicinal plants used in the treatment and prevention of malaria in Cegere sub-county, Northern Uganda. J Ethnobot Appl Res. 2016;14:505–516. doi: 10.17348/era.14.0.505-516. [DOI] [Google Scholar]
- 46.Pierre S, Toua V, Tchobsala, Tchuenguem FF, Alexandre-Michel NN, Jean M. Medicinal plants used in traditional treatment of malaria in Cameroon. J Ecol Nat Environ. 2011; 3: 104–117.
- 47.Taek MM, Bambang PEW, Mangestuti A. Plants used in traditional medicine for treatment of malaria by Tetun ethnic people in West Timor Indonesia. Asian Pac J Trop Med. 2018;11:630–637. doi: 10.4103/1995-7645.246339. [DOI] [Google Scholar]
- 48.Alebie G, Urga B, Worku A. Systematic review on traditional medicinal plants used for the treatment of malaria in Ethiopia: trends and perspectives. Malaria J. 2017;16:307. doi: 10.1186/s12936-017-1953-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ssenku JE, Okurut SA, Namuli A, Kudamba A, Tugume P, Matovu P, Wasige G, Kafeero HM, Walusansa A. Medicinal plant use, conservation, and the associated traditional knowledge in rural communities in Eastern Uganda. Trop Med Health. 2022;50:39. doi: 10.1186/s41182-022-00428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Namukobe J, Kasenene JM, Kiremire BT, Byamukama R, Kamatenesi-Mugisha M, Krief S, Dumontet V, Kabasa JD. Traditional plants used for medicinal purposes by local communities around the Northern sector of Kibale National Park, Uganda. J Ethnopharmacol. 2011;136:236–245. doi: 10.1016/j.jep.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 51.Dossou AJ, Fandohan AB, Omara T, Gbenou J. Traditional knowledge and phytochemical screening of plants used in snakebite prevention in Benin. Bull Natl Res Cent. 2022;46:160. doi: 10.1186/s42269-022-00851-8. [DOI] [Google Scholar]
- 52.Kuria KA, De Coster S, Muriuki G, Masengo W, Kibwage I, Hoogmartens J, Laekeman GM. Antimalarial activity of Ajuga remota Benth (Labiatae) and Caesalpinia volkensii Harms (Caesalpiniaceae): in vitro confirmation of ethnopharmacological use. J Ethnopharmacol. 2001;74:141–148. doi: 10.1016/S0378-8741(00)00367-6. [DOI] [PubMed] [Google Scholar]
- 53.Murphy MW, Dunton RF, Perich MJ, Rowley WA. Attraction of Anopheles (Diptera: culicidae) to volatile chemicals in Western Kenya. J Med Entomol. 2001;38:242–244. doi: 10.1603/0022-2585-38.2.242. [DOI] [PubMed] [Google Scholar]
- 54.Lacroix D, Prado S, Kamoga D, Kasenene J, Namukobe J, Krief S, Dumontet V, Mouray E, Bodo B, Brunois F. Antiplasmodial and cytotoxic activities of medicinal plants traditionally used in the village of Kiohima, Uganda. J Ethnopharmacol. 2011;133:850–855. doi: 10.1016/j.jep.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 55.Nyamwamu LB, Ngeiywa M, Mulaa M, Lelo AE, Ingonga J, Kimutai A. Acute toxicity of Senna didymobotrya Fresen Irwin roots used as a traditional medicinal plant in Kenya. Am Int J Contemp Res. 2015;5:74–77. [Google Scholar]
- 56.Barlow-Benschop NM, Gamba C, Barlow SP, Blasco TM. The effect of a homeopathic neem preparation for the prophylaxis of malaria. An exploratory trial in an at-home setting in Tanzania, pp. 1–12. https://pdfs.semanticscholar.org/177a/390381a2a7c7a19566a20f57330f50751101.pdf.
- 57.Challand S, Willcox M. A clinical trial of the traditional medicine Vernonia amygdalina in the treatment of uncomplicated malaria. J Alternat Compl Med. 2009;15:1231–1237. doi: 10.1089/acm.2009.0098. [DOI] [PubMed] [Google Scholar]
- 58.Kumar S, Yadav M, Yadav A, Rohilla P, Yadav JP. Antiplasmodial potential and quantification of aloin and aloe-emodin in Aloe vera collected from different climatic regions of India. BMC Complement Altern Med. 2017;17:369. doi: 10.1186/s12906-017-1883-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Zyl R, Viljoen A. In vitro activity of Aloe extracts against Plasmodium falciparum. South Afr J Bot. 2002;68:106–110. doi: 10.1016/S0254-6299(15)30451-8. [DOI] [Google Scholar]
- 60.Muthaura CN, Keriko JM, Mutai C, Yenesew A, Gathirwa JW, Irungu BN, Nyangacha R, Mungai GM, Derese S. Antiplasmodial potential of traditional antimalarial phytotherapy remedies used by the Kwale community of the Kenyan Coast. J Ethnopharmacol. 2015;170:148–157. doi: 10.1016/j.jep.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 61.Anywar GU, Kakudidi E, Oryem-Origa H, Schubert A, Jassoy C. Cytotoxicity of medicinal plant species used by traditional healers in treating people suffering from HIV/AIDS in Uganda. Front Toxicol. 2022;4:832780. doi: 10.3389/ftox.2022.832780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Omara T, Kiprop AK, Kosgei VJ. Isolation and characterization of compounds in ethanolic extract of Albizia coriaria (Welw ex. Oliver) leaves: a further evidence of its ethnomedicinal diversity. Bull Natl Res Cent. 2022;46:30. doi: 10.1186/s42269-022-00716-0. [DOI] [Google Scholar]
- 63.Omara T, Kiprop AK, Kosgei VJ. Two new pentacyclic triterpenoids, an alkaloid and a long-chain fatty acid from Albizia coriaria (Welw ex. Oliver) Fr-Uk J Chem. 2022;10:128. doi: 10.17721/fujcV10I1P128-141. [DOI] [Google Scholar]
- 64.Asase A, Akwetey GA, Achel DG. Ethnopharmacological use of herbal remedies for the treatment of malaria in the Dangme West District of Ghana. J Ethnopharmacol. 2010;129:367–376. doi: 10.1016/j.jep.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 65.Bray DH, Warhurst DC, Connolly JD, O’neill MJ, Phillipson JD. Plants as sources of antimalarial drugs. Part 7. Activity of some species of Meliaceae plants and their constituent limonoids. Phytother Res. 1990;4:29–35. doi: 10.1002/ptr.2650040108. [DOI] [Google Scholar]
- 66.Khalid SA. Isolation and characterization of antimalarial agents of the neem tree Azadirachta indica. J Nat Prod. 1989;52:922–927. doi: 10.1021/np50065a002. [DOI] [PubMed] [Google Scholar]
- 67.Kirira PG, Rukunga GM, Wanyonyi AW, Muregi FM, Gathirwa JW, Muthaura CN, Omar SA, Tolo F, Mungai GM, Ndiege IO. Anti-plasmodial activity and toxicity of extracts of plants used in traditional malaria therapy in Meru and Kilifi Districts of Kenya. J Ethnopharmacol. 2006;106:403–407. doi: 10.1016/j.jep.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 68.Nanyingi MO, Kipsengeret KB, Wagate CG, Langat BK, Asaava LL, Midiwo JO. In vitro and in vivo antiplasmodial activity of Kenyan medicinal plants. In: J. O. Midiwo and J. Clough, editors. Aspects of African biodiversity: proceedings of the Pan-Africa chemistry network, RCS Publishing, Cambridge, UK; 2010. pp. 20–28.
- 69.Clarkson C, Maharaj VJ, Crouch NR, Grace OM, Pillay P, Matsabisa MG, Bhagwandin N, Smith PJ, Folb PI. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J Ethnopharmacol. 2004;92:177–191. doi: 10.1016/j.jep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 70.Frida L, Rakotonirina S, Rakotonirina A, Savineau JP. In vivo and in vitro effects of Bidens pilosa L. (Asteraceae) leaf aqueous and ethanol extracts on primed-oestrogenized rat uterine muscle. Afr J Tradit Complement Altern Med. 2007;5:79–91. [PMC free article] [PubMed] [Google Scholar]
- 71.Lai BY, Chen TY, Huang SH, Kuo TF, Chang TH, Chiang CK, Yang MT, Chang CL. Bidens pilosa formulation improves blood homeostasis and β-cell function in men: a pilot study. Evid Based Complement Alternat Med. 2015;2015:832314. doi: 10.1155/2015/832314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teng WC, Chan R, Suwanarusk W, Ong A, Ho HK, Russell B, Rénia L, Koh HL. In vitro antimalarial evaluations and cytotoxicity investigations of Carica papaya leaves and carpaine. Nat Prod Comm. 2019;14:33–36. [Google Scholar]
- 73.Melariri P, Campbell W, Etusim P, Smith P. Antiplasmodial properties and bioassay-guided fractionation of ethyl acetate extracts from Carica papaya Leaves. J Parasitol Res. 2011;2011:104954. doi: 10.1155/2011/104954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Julianti T, De Mieri M, Zimmermann S, Ebrahimi SN, Kaiser M, Neuburger M, Raith M, Brun R, Hamburger M. HPLC-based activity profiling for antiplasmodial compounds in the traditional Indonesian medicinal plant Carica papaya L. J Ethnopharmacol. 2014;155:426–434. doi: 10.1016/j.jep.2014.05.050. [DOI] [PubMed] [Google Scholar]
- 75.Omara T, Kiprop AK, Kosgei VJ, Kagoya S. Clausena anisata (Willd.) Hook.f. ex. Benth. (Rutaceae): ethnomedicinal uses, phytochemistry, pharmacological activities, toxicity, and clinical application. Tradit Med Res. 2022;7:51. doi: 10.53388/TMR20220417001. [DOI] [Google Scholar]
- 76.Igoli JO, Masao CA, Orkpeh U, Nakamatte E, Yakumbur DT, Nnadozie T, et al. In vivo antimalarial activity of Cleome gynandra extracts. J Nat Prod Res Updates. 2016;2:19–29. [Google Scholar]
- 77.Busola OA, Iwuji IG, Nasir HA, Abdulrahman A. Antimalarial activity of Clerodendrum capitatum (Willd) ethanol leaf extract in Plasmodium berghei infected mice. Afr J Pharmaceut Res Dev. 2020;12:248–256. [Google Scholar]
- 78.Obakiro SB, Kiprop A, Kigondu E, K’Owino I, Odero MP, Manyim S, Omara T, Namukobe J, Owor RO, Gavamukulya Y, Bunalema L. Traditional medicinal uses, phytoconstituents, bioactivities, and toxicities of Erythrina abyssinica Lam. ex DC. (Fabaceae): a systematic review. Evid Based Complement Alternat Med. 2021;2021:5513484. doi: 10.1155/2021/5513484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oketch-Rabah HA, Dossaji SF, Mberu EK. Antimalarial activity of some Kenyan medicinal plants. Pharmaceut Biol. 1999;37:329–334. doi: 10.1076/phbi.37.5.329.6053. [DOI] [Google Scholar]
- 80.Farah H, El Hussein MA, Khalid EH, Osman HM. Toxicity of Kigelia africana fruit in rats. Adv Res. 2018;12:1–9. doi: 10.9734/AIR/2017/38539. [DOI] [Google Scholar]
- 81.Lacroix D, Prado S, Deville A, Krief S, Dumontet V, Kasenene J, Mouray E, Bories C, Bodo B. Hydroperoxy-cycloartane triterpenoids from the leaves of Markhamia lutea, a plant ingested by wild chimpanzees. Phytochemistry. 2009;70:1239–1245. doi: 10.1016/j.phytochem.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 82.Azanze NE, Mbiantcha M, Madjo KYK, Yousseu NW, Fagni Njoya ZL, Adjouzem CF, Marthe VM, Ateufack G. Markhamia lutea leaves aqueous and ethanolic extract with curative anti-inflammatory activity attenuates paclitaxel toxicity in rat's intestine. J Complement Integr Med. 2023 doi: 10.1515/jcim-2023-0017. [DOI] [PubMed] [Google Scholar]
- 83.Adia MM, Emami SN, Byamukama R, Faye I, Borg-Karlson AK. Antiplasmodial activity and phytochemical analysis of extracts from selected Ugandan medicinal plants. J Ethnopharmacol. 2016;186:14–19. doi: 10.1016/j.jep.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 84.Froelich S, Onegi B, Kakooko A, Siems K, Schubert C, Jenett-Siems K. Plants traditionally used against malaria: phytochemical and pharmacological investigation of Momordica foetida. Rev Bras Farmacogn. 2007;17:1–17. doi: 10.1590/S0102-695X2007000100002. [DOI] [Google Scholar]
- 85.Obbo CJD, Kariuki ST, Gathirwa JW, Olaho-Mukani W, Cheplogoi PK, Mwangi EM. In vitro antiplasmodial, antitrypanosomal and antileishmanial activities of selected medicinal plants from Ugandan flora: refocusing into multi-component potentials. J Ethnopharmacol. 2019;229:127–136. doi: 10.1016/j.jep.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 86.Olanlokun JO, Bodede O, Prinsloo G, Olorunsogo OO. Comparative antimalarial, toxicity and mito-protective effects of Diospyros mespiliformis Hochst. ex A. DC. and Mondia whitei (Hook.f.) Skeels on Plasmodium berghei infection in mice. J Ethnopharmacol. 2021;268:113585. doi: 10.1016/j.jep.2020.113585. [DOI] [PubMed] [Google Scholar]
- 87.Oloro J, Tanayen J, Barbra K, Lawrence I, Waako P, Francis B, Amon A. Toxicity of four herbs used in erectile dysfunction: Mondia whiteii, Cola acuminata, Urtica massaica, and Tarenna graveolensin male rats. Afr J Pharm Pharmacol. 2015;9:756–763. doi: 10.5897/AJPP2015.4299. [DOI] [Google Scholar]
- 88.Bezerra JJL, Pinheiro AAV, Dourado D. Antimalarial potential of Moringa oleifera Lam. (Moringaceae): a review of the ethnomedicinal, pharmacological, toxicological, and phytochemical evidence. J Venom Anim Toxins Incl Trop Dis. 2023;29:e20220079. doi: 10.1590/1678-9199-jvatitd-2022-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Owuor BO, Ochanda JO, Kokwaro JO, Cheruiyot AC, Yeda RA, Okudo CA, Akala HM. In vitro antiplasmodial activity of selected Luo and Kuria medicinal plants. J Ethnopharmacol. 2012;144:779–781. doi: 10.1016/j.jep.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 90.Njan AA, Olaoye SO, Afolabi SO, Ejimkonye BC, Soje A, Olorundare OE, Iwalewa EO. Safety effect of fractions from methanolic leaf extract of Ocimum gratissimum on reproduction in male Wistar rats. Toxicol Rep. 2019;6:496–504. doi: 10.1016/j.toxrep.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kiraithe MN, Nguta JM, Mbaria JM, Kiama SG. Evaluation of the use of Ocimum suave Willd (Lamiaceae), Plectranthus barbatus Andrews (Lamiaceae) and Zanthoxylum chalybeum Engl (Rutaceae) as antimalarial remedies in Kenyan folk medicine. J Ethnopharmacol. 2016;178:266–271. doi: 10.1016/j.jep.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 92.Muregi FW, Chhabra SC, Njagi EN, Lang'at-Thoruwa CC, Njue WM, Orago AS, Omar SA, Ndiege IO. In vitro antiplasmodial activity of some plants used in Kisii, Kenya against malaria and their chloroquine potentiation effects. J Ethnopharmacol. 2003;84:235–239. doi: 10.1016/S0378-8741(02)00327-6. [DOI] [PubMed] [Google Scholar]
- 93.Kigondu EV, Rukunga GM, Keriko JM, Tonui WK, Gathirwa JW, Kirira PG, Irungu B, Ingonga JM, Ndiege IO. Anti-parasitic activity and cytotoxicity of selected medicinal plants from Kenya. J Ethnopharmacol. 2009;123:504–509. doi: 10.1016/j.jep.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 94.Muganga R, Angenot L, Tits M, Frédérich M. Antiplasmodial and cytotoxic activities of Rwandan medicinal plants used in the treatment of malaria. J Ethnopharmacol. 2010;128:52–57. doi: 10.1016/j.jep.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 95.Onguéné P, Ntie-Kang F, Lifongo LL, Ndom JC, Sippl W, Mbaze LM. The potential of anti-malarial compounds derived from African medicinal plants, part I: a pharmacological evaluation of alkaloids and terpenoids. Malar J. 2013;12:449. doi: 10.1186/1475-2875-12-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goffin E, Ziemons E, De Mol P, de Madureira MC, Martins AP, da Cunha AP, Philippe G, Tits M, Angenot L, Frederich M. In vitro antiplasmodial activity of Tithonia diversifolia and identification of its main active constituent: tagitinin C. Planta Med. 2002;68:543–545. doi: 10.1055/s-2002-32552. [DOI] [PubMed] [Google Scholar]
- 97.Oyewole IO, Ibidapo CA, Moronkola DO, Oduola AO, Adeoye GO, Anyasor GN, Obansa JA. Anti-malarial and repellent activities of Tithonia diversifolia (Hemsl.) leaf extracts. J Med Plants Res. 2008;2:171–175. [Google Scholar]
- 98.Omoregie ES, Pal A, Sisodia B. In vitro antimalarial and cytotoxic activities of leaf extracts of Vernonia amygdalina (Del.) Niger J Basic Appl Sci. 2011;19:121–126. [Google Scholar]
- 99.Wube A, Bucar F, Gibbons S, Asres K, Rattray L, Croft SL. Anti-protozoal activity of sesquiterpenes from Warburgia ugandensis towards Trypanosoma bruceirhodesiense and Plasmodium falciparum in vitro. Planta Med. 2008;74:PA222. doi: 10.1055/s-0028-1084220. [DOI] [Google Scholar]
- 100.Were PS, Kinyanjui P, Gicheru MM, Mwangi E, Ozwara HS. Prophylactic and curative activities of extracts from Warburgia ugandensis Sprague (Canellaceae) and Zanthoxylum usambarense (Engl) Kokwaro (Rutaceae) against Plasmodium knowlesi and Plasmodium berghei. J Ethnopharmacol. 2010;130:158–162. doi: 10.1016/j.jep.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 101.Okello D, Komakech R, Matsabisa MG, Kang YM. A review on the botanical aspects, phytochemical contents and pharmacological activities of Warburgia ugandensis. J Med Plants Res. 2018;12:448–455. doi: 10.5897/JMPR2018.6626. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Details of medicinal plants used in the management of malaria in Bwambara Subcounty, Rukungiri District, Uganda.
Data Availability Statement
The raw data supporting the conclusions of this study are available within this article and its supplementary files.