Abstract
A flow cytometric assay has been developed for the measurement of susceptibilities to ganciclovir of laboratory strains and clinical isolates of human cytomegalovirus (HCMV). The assay uses fluorochrome-labeled monoclonal antibodies to HCMV immediate-early and late antigens to identify HCMV-infected cells and flow cytometry to detect and quantitate the number of antigen-positive cells. By this assay, the 50 and 90% inhibitory concentrations (IC50 and IC90, respectively) of ganciclovir for the AD169 strain of HCMV were 1.7 and 9.2 μM, respectively, and the IC50 for the ganciclovir-resistant D6/3/1 derivative of the AD169 strain was greater than 12 μM. The ganciclovir susceptibilities of 17 HCMV clinical isolates were also determined by flow cytometric analysis of the effect of ganciclovir on late-antigen synthesis in HCMV-infected cells. The average IC50 of ganciclovir for drug-sensitive HCMV clinical isolates was 3.79 μM (±2.60). The plaque-reduction assay for these clinical isolates yielded an average IC50 of 2.80 μM (±1.46). Comparison of the results of the flow cytometry assays with those obtained from the plaque-reduction assays demonstrated acceptable bias and precision. Flow cytometric and plaque-reduction analysis of cells infected with ganciclovir-resistant clinical isolates failed to show a reduction in the percentage of late-antigen-positive cells or PFU, even at 96 μM ganciclovir. The flow cytometric assay for determining ganciclovir susceptibility of HCMV is quantitative, and objective, and potentially automatable, and its results are reproducible among laboratories.
Human cytomegalovirus (HCMV) is a major cause of morbidity and mortality among immunocompromised patients (6, 7, 11). Three drugs, ganciclovir, cidofovir, and foscarnet, are available for treatment of retinitis caused by HCMV (2, 5, 18, 20). With long-term administration of these antiviral compounds, drug-resistant HCMV mutants may emerge, potentially nullifying the usefulness of these therapies (1, 8). Drug susceptibilities of HCMV clinical isolates are usually determined by a quantitative plaque-reduction assay (12, 22). DNA hybridization and fluorochrome-labeled-antibody techniques are also used (3, 10). These assays are very time-consuming and labor-intensive and are often subjective even when they are performed by highly skilled technicians. More reliable, less intensive techniques are needed for determining antiviral susceptibility.
Fluorochrome-labeled monoclonal antibodies to immediate-early, early, or late HCMV antigen have been used in conjunction with flow cytometry to detect and quantitate HCMV-infected cells (9, 15, 16, 21). We used this procedure and our understanding of the mode of action of ganciclovir to develop a quantitative procedure for determining the susceptibilities of laboratory strains of HCMV to ganciclovir. Modifications of this procedure that involve a low multiplicity of infection (MOI) and the ability of ganciclovir to block the spread of infection from the input virus-infected cells to uninfected cells were used for a determination of the susceptibilities of HCMV clinical isolates to ganciclovir. This assay alleviates much of the labor and subjectivity associated with quantitating the plaque-reduction assay and may be an asset to those laboratories involved in drug susceptibility assays for HCMV.
MATERIALS AND METHODS
Cell cultures, viruses, and virus-infected cells.
Human embryo fibroblast (MRC-5) cells were obtained from the American Type Culture Collection (CCL 171), human foreskin fibroblasts (HFF) were obtained from ViroMed, Inc. (Minneapolis, Minn.), and human embryonic lung fibroblasts (HELF) were prepared in the laboratory of one of the investigators. Cells were propagated in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), penicillin, streptomycin, and amphotericin B (Life Technologies, Inc., Grand Island, N.Y.) in 75- or 25-cm2 tissue culture flasks (Corning, Inc., Corning, N.Y.) at 37°C and passaged weekly.
The ganciclovir-sensitive AD169 laboratory strain of HCMV was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. The ganciclovir-resistant D6/3/1 derivative of the AD169 strain of HCMV was obtained from Nell Lurain (14). Ganciclovir-sensitive HCMV clinical isolates K8313 and V379354 and ganciclovir-resistant HCMV clinical isolates V917401 and MR11979 were obtained from W. Lawrence Drew and Alejo Erice and provided to us by the DAIDS-sponsored Virology Quality Assurance Laboratory. Additional clinical isolates were obtained from the Clinical Microbiology Laboratories at the Albany Medical Center, Albany, N.Y. Stocks of cell-free AD169 and D6/3/1 strains of HCMV were prepared in HFF cells by standard procedures and stored at −70°C (14).
Cell-associated HCMV clinical isolates were propagated by inoculating cell monolayers with virus-infected cells in MEM supplemented with 10% FBS. When 50 to 100% of the monolayer exhibited cytopathic effects, the HCMV-infected cells were removed from the monolayer, counted, and immediately used in ganciclovir susceptibility experiments or resuspended in 10% FBS–10% dimethyl sulfoxide and frozen at −70°C.
Ganciclovir.
A stock of 5 mM ganciclovir in sterile water was provided to all participating laboratories by the Virology Quality Assurance Laboratory.
Plaque-reduction assay.
A standard plaque-reduction assay was used to determine the 50% inhibitory concentrations (IC50) of HCMV laboratory strains (12, 22). The IC50 were calculated from averages of the numbers of PFU in four wells for each drug concentration by fitting an inhibitory sigmoid Emax effect model to the data. Point estimates of parameter values were obtained with the ADAPT II package of programs (4).
Monoclonal antibodies.
Appropriately labeled isotype control murine monoclonal antibodies (MAB821), fluorescein isothiocyanate (FITC)-labeled murine monoclonal antibody to the HCMV late antigen (MAB8127), and a combination of FITC-labeled murine monoclonal antibody to the immediate-early antigen and phycoerythrin (PE)-labeled murine monoclonal antibodies to the late antigen (CMV Flow Reagent) were obtained from Chemicon International, Inc., Temecula, Calif.). Individual monoclonal antibodies were diluted to the appropriate concentration in diluent (1% bovine serum albumin in phosphate-buffered saline), whereas the CMV Flow Reagent was used at the concentrations supplied by the manufacturer.
Flow cytometric analysis and determination of ganciclovir susceptibility.
A modified procedure for detection and quantitation of HCMV-infected cells by flow cytometry (9, 15, 16) was used.
(i) Infection.
Cell monolayers were infected with the AD169 or D6/3/1 laboratory strain at an MOI of 1 to 10 PFU/cell. After a 2-h adsorption period, the infected cells were incubated at 37°C for various periods in the presence of various concentrations of ganciclovir in MEM supplemented with 10% FBS. For clinical isolates, 105 HCMV-infected cells were added directly to the media containing various concentrations of ganciclovir and incubated at 37°C for 144 h.
(ii) Fixation, permeabilization, and antibody treatment.
At the end of the incubation period, the cells were removed from the flask, permeabilized, and stored at approximately 500,000 cells/ml at −70°C. For three-color analysis, the cells were resuspended in 0.2 ml of CMV Flow Reagent. For two-color analysis, the cells were resuspended in 0.2 ml of FITC-labeled monoclonal antibody to a late HCMV antigen. The antibody-treated cells were incubated for 60 min at 37°C, washed three times in wash buffer (phosphate-buffered saline–Tween 20), and resuspended in 0.5 ml of RNase (1 μg/ml) and 0.5 ml of 7-amino actinomycin D (7-AAD) (10 μg/ml).
(iii) Flow cytometric analysis.
A FACScan flow cytometer, a FACSCalibur flow cytometer (both from Becton Dickinson Immunocytometry Systems, San Jose, Calif.), or CytoronAbsolute flow cytometer (Ortho Diagnostic Systems, Inc., Raritan, N.J.) were used for analyses. The instruments were aligned with FITC- and PE-labeled beads (Flow Cytometry Standards Corporation, Research Triangle Park, N.C.) or Calibrite beads (Becton Dickinson Immunocytometry Systems). FITC-labeled monoclonal antibodies or a combination of FITC- and PE-labeled monoclonal antibodies of irrelevant specificities were used as isotype controls. For three-color analysis, the cells were initially analyzed for 7-AAD content versus forward-angle light scatter to identify intact cells with a 2 N or greater DNA content and to separate cells from debris. Events corresponding to intact cells were gated. Ten thousand events were collected and analyzed for FITC fluorescence intensity versus PE fluorescence intensity to determine the percentage of cells expressing both the immediate-early and the late antigens. For two-color analysis, the cells were analyzed for the amount of 7-AAD versus that of FITC to identify both uninfected and HCMV-infected cells, 10,000 events were collected, and the percentage of HCMV-infected cells was determined by analyzing the number of cells expressing the late antigen above the background as determined with fluorochrome-labeled isotype control antibodies.
(iv) Data analysis.
The ImmunoCount II program of the Ortho Diagnostic Systems, Inc., CytoronAbsolute flow cytometer, the Lysis II software with the FACScan flow cytometer, and the Cell Quest software with the FACSCalibur flow cytometer were used to analyze and plot the data. The IC50 and IC90 of ganciclovir for HCMV laboratory strains and the IC50 for clinical isolates were calculated as described above for the plaque-reduction assay (4). The two assays were compared, with determinations of the bias and precision of the flow cytometry assay relative to the bias and precision of the plaque-reduction assay. Bias was calculated as mean percent error with the formula (flow cytometry assay IC50 − plaque-reduction assay IC50) × 100/plaque-reduction assay IC50. Precision was calculated as mean absolute percent error with the formula | flow cytometry assay IC50 − plaque-reduction assay IC50 | × 100/plaque-reduction assay IC50.
RESULTS
Detection and quantitation of cells infected with laboratory strains of HCMV.
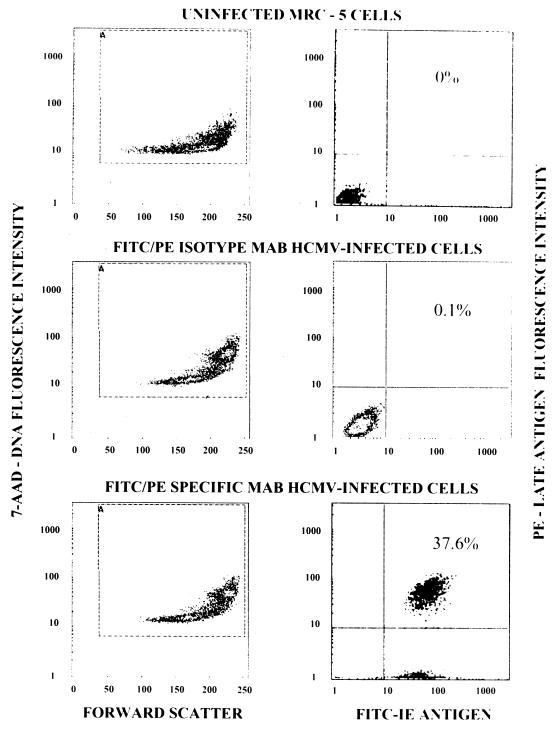
Figure 1 illustrates the three-color flow cytometric analysis of uninfected and HCMV-infected MRC-5 cells. Uninfected MRC-5 cells treated with FITC- and PE-labeled monoclonal antibodies to HCMV-specific antigens and 7-AAD exhibited a relatively low level of binding of 7-AAD to cellular DNA (Fig. 1, upper left-hand panel) and no antigen-positive cells (upper right-hand panel). HCMV-infected MRC-5 cells treated with FITC- and PE-labeled isotype control monoclonal antibodies and 7-AAD exhibited one population of cells that bound 7-AAD at a relatively low level and a second population of larger cells containing replicated viral DNA that bound increased amounts of 7-AAD (Fig. 1, middle left-hand panel) and only 0.1% antigen-positive cells (middle right-hand panel). In contrast, HCMV-infected cells treated with FITC- and PE-labeled monoclonal antibodies to HCMV-specific antigens and 7-AAD showed two 7-AAD-binding populations (lower left-hand panel) of which essentially all of the cells were immediate-early-antigen positive and 37.6% of the cells were positive for both the immediate-early and the late antigens (lower right-hand panel). These results showed that after infection of MRC-5 cells with the AD169 strain of HCMV at an MOI of 10 followed by 96 h of incubation, essentially all of the cells were infected on the basis of the expression of the immediate-early antigen and a considerable percentage of the cells expressed both the immediate-early and late antigens. Similar results were observed with uninfected and HCMV-infected HELF and HFF cells (data not shown).
FIG. 1.
Three-color flow cytometric analysis of uninfected and HCMV-infected cells. Left-hand panels display results with uninfected and HCMV-infected MRC-5 cells analyzed for DNA content (7-AAD) versus cell size (forward scatter). Right-hand panels display results with uninfected and HCMV-infected cells analyzed for late-antigen-positive cells versus immediate-early (IE)-antigen-positive cells. MAB, monoclonal antibody.
The effect of ganciclovir on the expression of immediate-early and late HCMV antigens in cells infected with laboratory strains.
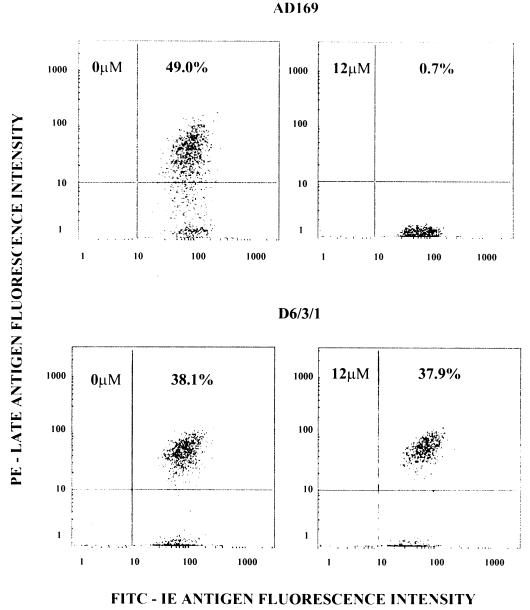
Since ganciclovir inhibits viral DNA synthesis in cells infected with ganciclovir-sensitive strains of HCMV and late-antigen synthesis is dependent on viral DNA synthesis, the percentage of cells expressing late antigen should be reduced in the presence of inhibitory concentrations of ganciclovir (17). Ganciclovir does not inhibit HCMV DNA and late-antigen synthesis in cells infected with ganciclovir-resistant strains of HCMV. At a high MOI with ganciclovir-sensitive or -resistant HCMV, where only a single cycle of virus replication can occur, ganciclovir should have no effect on the synthesis of the immediate-early antigen because it is not dependent on viral DNA synthesis (17). To test this hypothesis, cell monolayers were infected at an MOI of 10 with the ganciclovir-sensitive AD169 or the ganciclovir-resistant D6/3/1 strain of HCMV in the absence or presence of 12 μM ganciclovir, a concentration known to inhibit the replication of the AD169 strain. Figure 2 illustrates the effect of ganciclovir on the syntheses of HCMV immediate-early and late antigens in virus-infected cells. In the absence of ganciclovir, essentially all of the AD169-infected cells expressed the immediate-early antigen and 49.0% of cells expressed both the immediate-early and late antigens. In the presence of 12 μM ganciclovir, essentially all of the AD169-infected cells expressed the immediate-early antigen, but only 0.7% of cells expressed both the immediate-early and late antigens. For cells infected with the D6/3/1 strain, there was no difference in the percentages of cells synthesizing the immediate-early and late antigens in the absence, 38.1%, or in the presence, 37.9%, of 12 μM ganciclovir. These results confirmed that the AD169 strain of HCMV was sensitive and that the D6/3/1 strain was resistant to ganciclovir. Furthermore, 12 μM ganciclovir inhibited late-antigen synthesis without affecting the synthesis of the immediate-early antigen in cells infected with the AD169 strain. These results showed that the flow cytometry technique could be used to determine the effect of ganciclovir on the expression of the late antigen in HCMV-infected cells and that this technique could be used to distinguish between ganciclovir-sensitive and -resistant laboratory strains of HCMV, the basis of any antiviral susceptibility assay.
FIG. 2.
Effect of ganciclovir on the synthesis of immediate-early and late antigens in MRC-5 cells infected with the AD169 and D6/3/1 strains of HCMV. Three-color flow cytometric analysis of AD169- or D6.3/1-infected cells in the absence (left-hand panels) and presence (right-hand panels) of 12 μM ganciclovir. IE, immediate-early.
Determination of the IC50 and IC90 of ganciclovir for laboratory strains of HCMV.
The IC50 and IC90 of ganciclovir for the ganciclovir-sensitive AD169 strain and the ganciclovir-resistant D6/3/1 strain were determined by flow cytometry after infection at an MOI of 10 PFU/cell. The data in Table 1 show that increasing concentrations of ganciclovir had little effect on the percentage of AD169-infected cells expressing the immediate-early antigen but decreased the percentage of cells expressing the late antigen. For cells infected with the D6/3/1 strain, increasing concentrations of ganciclovir did not reduce the percentage of cells synthesizing the immediate-early or late antigen. The IC50 and IC90 determined by the flow cytometry assay are similar to those derived from the plaque-reduction assay for these strains of HCMV (12, 14).
TABLE 1.
IC50 and IC90 of ganciclovir-sensitive (AD169) and -resistant (D6/3/1) strains of HCMV
| Virus | GCVa concn (μM) | % IE Ag+b cells | % Late Ag+ cells | IC50 (μM) | IC90 (μM) |
|---|---|---|---|---|---|
| AD169 | 0 | 98.9 | 40.8 | ||
| 1.5 | 96.2 | 19.1 | 1.7 | ||
| 3.0 | 93.4 | 14.5 | |||
| 12.0 | 96.4 | 2.2 | 9.2 | ||
| D6/3/1 | 0 | 97.3 | 41.6 | ||
| 1.5 | 97.0 | 38.8 | |||
| 3.0 | 96.2 | 38.8 | |||
| 12.0 | 97.4 | 41.7 | >12 | >12 |
GCV, ganciclovir.
IE Ag+, immediate-early antigen-positive.
Intralaboratory and interlaboratory reproducibility of the assay.
The reproducibility of the flow cytometry assay for measuring the inhibitory concentration of ganciclovir for the AD169 strain of HCMV was assessed by five different laboratories. The data in Table 2 show that the concentration of ganciclovir that reduced the percentage of cells synthesizing the late antigen by 50% (IC50) was between 1.5 and 3.0 μM. The IC50 was independent of the cell type used for the assay and the time of harvest, indicating the broad utility of the assay. Two of the five laboratories reported independent replicas of the analysis with excellent within-laboratory, between-day agreement on the IC50 for the AD169 strain (labs 2 and 5 [Table 2]).
TABLE 2.
Inter- and intralaboratory variation for determination of the IC50 of ganciclovir for the ganciclovir-sensitive AD169 strain of HCMV
| Lab | GCV IC50 (μM)a | Cells | h p.i.b | MOI (PFU/cell) |
|---|---|---|---|---|
| 1 | 1.5 | HFF | 96 | 2 |
| 2 | 1.5 | HFF | 72 | 1 |
| 1.5 | HFF | 96 | 1 | |
| 3.0 | MRC-5 | 72 | 1 | |
| 1.5 | MRC-5 | 72 | 1 | |
| 3 | 3.0 | MRC-5 | 72 | 5 |
| 4 | 3.0 | HELF | 48 | 1 |
| 5 | 1.5 | MRC-5 | 72 | 10 |
| 1.5 | MRC-5 | 72 | 10 | |
| 3.0 | MRC-5 | 72 | 10 | |
| 1.5 | MRC-5 | 72 | 10 |
Concentration of ganciclovir (GCV) that reduced the number of antigen-positive cells by 50% (IC50).
Hours postinfection.
Ganciclovir sensitivity of clinical isolates of HCMV.
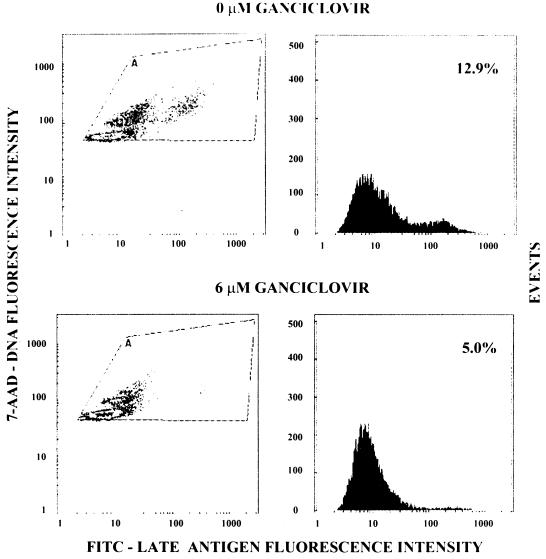
The data presented above demonstrated that the three-color flow cytometry system could be used to determine the IC50 and IC90 of laboratory strains of HCMV when the cells were infected at high MOI. However, HCMV clinical isolates remain cell associated when they are cultured and large numbers of HCMV-infected cells are usually not available to perform studies at high MOI. To simplify the assay and make it more practical for use with clinical isolates, a two-color flow cytometry assay system with FITC-labeled monoclonal antibody to an HCMV late antigen and 7-AAD was developed. Figure 3 illustrates the flow cytometric analysis of the effect of ganciclovir on the percentage of cells synthesizing the late antigen at 144 h postinfection at an MOI of 0.1 infected cell per uninfected cell with a ganciclovir-sensitive clinical isolate. These results showed that when cells were infected at low MOI with a ganciclovir-sensitive clinical isolate, the percentage of cells synthesizing the late antigen was significantly reduced at 6 μM ganciclovir.
FIG. 3.
Effect of ganciclovir on the synthesis of the late antigen in cells infected with a drug-sensitive clinical isolate. Two-color flow cytometric analysis of late-antigen synthesis in the absence and presence of 6 μM ganciclovir. Left-hand panels show DNA content versus the presence of late-antigen-positive cells; right-hand panels show events versus the presence of late-antigen-positive cells.
Determination of the IC50 of HCMV clinical isolates.
Cell monolayers were infected with HCMV clinical isolates at an MOI of 0.1 in the presence of various concentrations of ganciclovir ranging from 0 to 96 μM and analyzed for the percentage of cells synthesizing the late antigen at 144 h postinfection. The data in Table 3 show that for cells infected with the ganciclovir-sensitive clinical isolates, as determined by plaque-reduction assay, the percentage of cells synthesizing the late antigen was reduced by 50% at ganciclovir concentrations ranging between 1.24 and 9.69 μM. For cells infected with ganciclovir-resistant clinical isolates, the percentage of cells synthesizing the late antigen was not reduced by 50% even at 96 μM ganciclovir. With three exceptions, the IC50 of ganciclovir for HCMV clinical isolates as determined by the flow cytometry assay were similar to those determined by the plaque-reduction assay (Table 3). The bias and precision were 78 and 107%, respectively, indicating that, on average, the flow cytometry assay tends to produce values about twofold higher than those produced by the plaque-reduction assay. These results suggest that the flow cytometric analysis of the effect of ganciclovir on the synthesis of late antigen can be used to determine the IC50 of HCMV clinical isolates.
TABLE 3.
IC50 of ganciclovir for HCMV clinical isolates
| Clinical isolate | Flow cytometry late-antigen IC50a | Plaque-reduction assay IC50a | Ganciclovir phenotype |
|---|---|---|---|
| V917401 | >96 | >96 | Resistant |
| MR11979 | >96 | >96 | Resistant |
| V379354 | 5.54 | 5.52 | Sensitive |
| K8313 | 4.40 | 5.84 | Sensitive |
| CS 1 | 2.75 | 3.49 | Sensitive |
| CS 2 | 8.04 | 2.18 | Sensitive |
| CS 3 | 3.41 | 4.10 | Sensitive |
| CS 4 | 1.89 | 3.11 | Sensitive |
| CS 5 | 1.71 | 2.27 | Sensitive |
| CS 6 | 5.10 | 2.30 | Sensitive |
| CS 7 | 2.43 | 2.81 | Sensitive |
| CS 8 | 2.30 | 4.03 | Sensitive |
| CS 9 | 3.19 | 3.28 | Sensitive |
| CS 10 | 2.40 | 0.93 | Sensitive |
| CS 11 | 7.75 | 1.31 | Sensitive |
| CS 12 | 1.35 | 1.00 | Sensitive |
| CS 13 | 1.24 | 2.45 | Sensitive |
| CS 14 | 1.24 | 1.42 | Sensitive |
| CS 15 | 9.69 | 1.64 | Sensitive |
The IC50 are averages of results of triplicate flow cytometry and plaque-reduction assays.
DISCUSSION
We have developed an assay for measuring the susceptibilities of HCMV laboratory strains and clinical isolates to ganciclovir that uses flow cytometric analysis of fluorochrome-labeled HCMV-infected cells to determine the effect of ganciclovir on viral antigen synthesis. Infection at an MOI of 1 to 10 with the AD169 strain in the presence of inhibitory concentrations of ganciclovir reduced the percentage of cells synthesizing the late antigen without any effect on the percentage of cells synthesizing the immediate-early antigen. This result is consistent with the mode of action of ganciclovir, which inhibits viral DNA synthesis required for late-antigen synthesis (17). Ganciclovir had no effect on the synthesis of HCMV antigens in cells infected with D6/3/1, a ganciclovir-resistant derivative of AD169. The IC50 and IC90 for the ganciclovir-sensitive AD169 laboratory strain were 1.7 and 9.2 μM, respectively, and the IC50 for the ganciclovir-resistant D6/3/1 laboratory strain was greater than 12 μM. The IC50 for the AD169 and D6/3/1 strains by the plaque-reduction assay were 3.50 and greater than 96 μM ganciclovir, respectively (data not shown). These results are similar to those obtained from the plaque-reduction assays for AD169 and D6/3/1 strains of HCMV (12, 14). Under the conditions of these experiments, uninfected cells treated with fluorochrome-labeled HCMV-specific monoclonal antibodies and HCMV-infected cells treated with isotype control monoclonal antibodies gave less than 1% antigen-positive cells, indicating the specificity of the assay. The IC50 for ganciclovir determined by the flow cytometry assay for the AD169 strain was independently assessed by five different laboratories, and each laboratory observed an IC50 between 1.5 and 3 μM ganciclovir. These results suggest that the results of this assay are reproducible between laboratories. Furthermore, when individual laboratories performed replicas on the AD169 laboratory strain, they obtained essentially the same IC50, indicating the within-laboratory, between-day reproducibility of the assay.
The susceptibilities of HCMV clinical isolates to ganciclovir were also measured with this assay. After infection at an MOI of 0.1 infected cell per uninfected cell with clinical isolates, 10 to 40% of the cells were positive for the late antigen by 144 h postinfection in the absence of ganciclovir. When cells were infected with ganciclovir-sensitive clinical isolates in the presence of various concentrations of ganciclovir, the average IC50 were between 1.24 and 9.69 μM ganciclovir. In most cases, these IC50 reflected the IC50 obtained from the plaque-reduction assay, which ranged from 0.93 to 5.84 μM ganciclovir. Three clinical isolates, considered to be sensitive on the basis of the plaque-reduction assay, had IC50 greater than 6 μM ganciclovir. Further experiments are required to determine the source of the disparity in the IC50 obtained between the two assays for these clinical isolates and to determine how frequently a disparity exists.
Occasionally, when ganciclovir-sensitive HCMV clinical isolates were assayed by flow cytometry, a 50% reduction in the percent of cells synthesizing the late antigen did not occur even in the presence of 96 μM ganciclovir. However, susceptible clinical isolates showed reductions in the percent of antigen-positive cells of at least 40% at 3 and 6 μM, whereas resistant clinical isolates usually showed no reduction and often showed in increase in the percent of antigen-positive cells with increasing concentrations of ganciclovir. Thus, the flow cytometry assay can be used for an accurate and quantitative determination of the susceptibility of HCMV clinical isolates to ganciclovir even when a 50% reduction in the percent of late-antigen-positive cells is not achieved.
In a recent report, flow cytometry was used to determine the susceptibilities to ganciclovir of cell-associated AD169 and 759D100 HCMV laboratory strains and five HCMV clinical isolates (13). The IC50 determined by flow cytometry and plaque-reduction assays for ganciclovir-sensitive clinical isolates were approximately two to five times higher than those reported here, and the IC50 for ganciclovir-resistant isolates were about twofold less than those reported here. These discrepancies may be due to differences in the monoclonal antibodies used in the two papers (immediate-early versus late), the use of indirect immunofluorescence versus direct immunofluorescence, and differences in the numbers of infected cells used to initiate the infections (102 versus 105). Although there are differences in the IC50 reported between these two papers, it is clear that flow cytometry can be used to determine ganciclovir susceptibility of HCMV clinical isolates. Further support for the use of flow cytometry for antiviral susceptibility assays for herpesviruses was provided by two recent publications that determined antiviral susceptibilities of herpes simplex viruses (16, 19).
The advantages of the flow cytometry assay for measuring the ganciclovir susceptibilities of HCMV clinical isolates include the ability to analyze a large number of virus-infected cells in a short time, the objectivity of the assay, and the potential for automation. By contrast, the plaque-reduction assay is more time-consuming and is not easily automated, and the enumeration of plaques is labor-intensive and subjective even when performed by a skilled technician. Despite the expense of monoclonal antibodies, the ability to use flow cytometry to perform antiviral susceptibility assays will be practical for diagnostic laboratories at large medical centers, pharmaceutical companies, and commercial testing laboratories, institutions that already have the required flow cytometers and are interested in saving labor costs and retaining their skilled personnel. Therefore, the flow cytometry assay should be more useful than the plaque-reduction assay for determining the susceptibilities of herpes simplex viruses and HCMV to antiviral compounds that block DNA synthesis.
ACKNOWLEDGMENTS
We thank Mary Ann Czerniewski, Ann Ogden-McDonough, JoAnna Paolilli, and Betty A. Olson for technical assistance.
This work was supported in part by grants AI30883 and AI32367 and contracts N01-AI35172 (Virology ATL) and N01-AI15104-015 (Pharmacology ATL) from the National Institutes of Health.
REFERENCES
- 1.Crumpacker, C. S. 1996. Drug resistance in cytomegalovirus: current knowledge and implications for patient management. J. Acquired Immune Defic. Syndr. 12(Suppl. 1):S–S18. [PubMed]
- 2.Crumpacker C S. Ganciclovir. N Engl J Med. 1996;335:721–729. doi: 10.1056/NEJM199609053351007. [DOI] [PubMed] [Google Scholar]
- 3.Danker W M, Scholl S, Stanat S C, Martin M, Sonke R L, Spector S A. Rapid antiviral DNA-DNA hybridization assay for human cytomegalovirus. J Virol Methods. 1990;28:293–298. doi: 10.1016/0166-0934(90)90122-v. [DOI] [PubMed] [Google Scholar]
- 4.D’Argenio D Z, Schumitzky A. ADAPT II user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Los Angeles, Calif: Biomedical Simulations Resources; 1997. [Google Scholar]
- 5.Dieterich D T, Polis M A, Lew E A, Mendez P E, Murphy R, Addessi A, Holbrook J T, Naughton K, Friedberg D N. Concurrent use of ganciclovir and foscarnet to treat cytomegalovirus infection in AIDS patients. J Infect Dis. 1993;167:1184–1188. doi: 10.1093/infdis/167.5.1184. [DOI] [PubMed] [Google Scholar]
- 6.Drew W L. Cytomegalovirus infection in patients with AIDS. J Infect Dis. 1989;158:449–456. doi: 10.1093/infdis/158.2.449. [DOI] [PubMed] [Google Scholar]
- 7.Drew W L. Nonpulmonary manifestations of cytomegalovirus infection in immunocompromised patients. Clin Microbiol Rev. 1992;5:204–210. doi: 10.1128/cmr.5.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drew W L, Miner R C, Busch D F, Follansbee S E, Gullett J, Mehalko S G, Gordon S M, Owen W F, Jr, Mathews T R, Buhles W C, DeArmond B. Prevalence of resistance in patients receiving ganciclovir for serious cytomegalovirus infection. J Infect Dis. 1991;163:716–719. doi: 10.1093/infdis/163.4.716. [DOI] [PubMed] [Google Scholar]
- 9.Elmendorf S, McSharry J J, Laffin J, Fogleman D, Lehman J M. Detection of an early cytomegalovirus antigen with two color quantitative flow cytometry. Cytometry. 1988;9:254–260. doi: 10.1002/cyto.990090311. [DOI] [PubMed] [Google Scholar]
- 10.Gerna F, Sarasini A, Percivalle E, Zavattoni M, Baldanti F, Revello M G. Rapid screening for resistance to ganciclovir and foscarnet resistance of primary isolates of human cytomegalovirus from culture-positive blood samples. J Clin Microbiol. 1995;33:738–741. doi: 10.1128/jcm.33.3.738-741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson M A, Mills J. Serious cytomegalovirus disease in the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1988;108:588–594. doi: 10.7326/0003-4819-108-4-585. [DOI] [PubMed] [Google Scholar]
- 12.Jokela J, Erice A, Stanat S, Drew W L, Spector S, Weinberg A, Gilliam B, Yen-Lieberman B, Manischewitz J, Pollard R, Landry M, Chou S, Biron K K, Reichelderfer P, Britt W, Crumpacker C. Abstracts of the 2nd National Conference on Human Retroviruses. 1995. A standardized plaque reduction assay for CMV antiviral susceptibility, abstr. 283. [Google Scholar]
- 13.Lipson S M, Soni M, Biondo F X, Shepp D H, Kaplan M H, Sun T. Antiviral susceptibility testing-flow cytometric analysis (AST-FCA) for the detection of cytomegalovirus drug resistance. Diagn Microbiol Infect Dis. 1997;28:123–129. doi: 10.1016/s0732-8893(97)00040-0. [DOI] [PubMed] [Google Scholar]
- 14.Lurain N S, Thompson K D, Holmes E W, Read G S. Point mutations in the DNA polymerase gene of human cytomegalovirus that result in resistance to antiviral agents. J Virol. 1992;66:7146–7152. doi: 10.1128/jvi.66.12.7146-7152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McSharry J J. Uses of flow cytometry in virology. Clin Microbiol Rev. 1994;7:576–604. doi: 10.1128/cmr.7.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McSharry J J. Flow cytometry-based antiviral resistance assays. Clin Immunol Newsl. 1995;15:113–119. [Google Scholar]
- 17.Mocarski E S., Jr . Cytomegaloviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2447–2492. [Google Scholar]
- 18.Oberg B. Antiviral effects of phosphonoformate. Pharmacol Ther. 1983;19:387–415. doi: 10.1016/0163-7258(82)90074-2. [DOI] [PubMed] [Google Scholar]
- 19.Pavić I, Hartmann A, Zimmermann A, Michel D, Hampl W, Schleyer I, Mertens T. Flow cytometric analysis of herpes simplex virus type 1 susceptibility to acyclovir, ganciclovir, and foscarnet. Antimicrob Agents Chemother. 1997;41:2686–2692. doi: 10.1128/aac.41.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polis M A, Spooner K M, Baird B F, Manischewitz J, Jaffe H S, Fisher P E, Falloon J, Davey R T, Jr, Kovacs J A, Walker R E, Whitcup S M, Nussenblatt R B, Lane H C, Masur H. Anticytomegalovirus activity and safety of cidofovir in patients with human immunodeficiency virus infection and cytomegalovirus viruria. Antimicrob Agents Chemother. 1995;39:882–886. doi: 10.1128/aac.39.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schols D, Snoeck R, Neyts J, DeClercq E. Detection of immediate early, early and late antigens of human cytomegalovirus by flow cytometry. J Virol Methods. 1989;26:247–254. doi: 10.1016/0166-0934(89)90107-9. [DOI] [PubMed] [Google Scholar]
- 22.Wentworth B B, French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970;135:253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]