Abstract
Background
Despite the high sensitivity of cerebrospinal fluid (CSF) amyloid beta (Aβ)42 to detect amyloid pathology, the Aβ42/Aβ40 ratio (amyR) better estimates amyloid load, with higher specificity for Alzheimer’s disease (AD). However, whether Aβ42 and amyR have different meanings and whether Aβ40 represents more than an Aβ42-corrective factor remain to be clarified. Our study aimed to compare the ability of Aβ42 and amyR to detect AD pathology in terms of p-tau/Aβ42 ratio and brain glucose metabolic patterns using fluorodeoxyglucose-positron emission tomography (FDG-PET).
Methods
CSF biomarkers were analyzed with EUROIMMUN ELISA. We included 163 patients showing pathological CSF Aβ42 and normal p-tau (A + T − = 98) or pathological p-tau levels (A + T + = 65) and 36 control subjects (A − T −). A + T − patients were further stratified into those with normal (CSFAβ42 + /amyR − = 46) and pathological amyR (CSFAβ42 + /amyR + = 52). We used two distinct cut-offs to determine pathological values of p-tau/Aβ42: (1) ≥ 0.086 and (2) ≥ 0.122. FDG-PET patterns were evaluated in a subsample of patients (n = 46) and compared to 24 controls.
Results
CSF Aβ40 levels were the lowest in A − T − and in CSFAβ42 + /amyR − , higher in CSFAβ42 + /amyR + and highest in A + T + (F = 50.75; p < 0.001), resembling CSF levels of p-tau (F = 192; p < 0.001). We found a positive association between Aβ40 and p-tau in A − T − (β = 0.58; p < 0.001), CSFAβ42 + /amyR − (β = 0.47; p < 0.001), and CSFAβ42 + /amyR + patients (β = 0.48; p < 0.001) but not in A + T + . Investigating biomarker changes as a function of amyR, we observed a weak variation in CSF p-tau (+ 2 z-scores) and Aβ40 (+ 0.8 z-scores) in the normal amyR range, becoming steeper over the pathological threshold of amyR (p-tau: + 5 z-scores, Aβ40: + 4.5 z-score). CSFAβ42 + /amyR + patients showed a significantly higher probability of having pathological p-tau/Aβ42 than CSFAβ42 + /amyR − (cut-off ≥ 0.086: OR 23.3; cut-off ≥ 0.122: OR 8.8), which however still showed pathological values of p-tau/Aβ42 in some cases (cut-off ≥ 0.086: 35.7%; cut-off ≥ 0.122: 17.3%) unlike A − T − . Accordingly, we found reduced FDG metabolism in the temporoparietal regions of CSFAβ42 + /amyR − compared to controls, and further reduction in frontal areas in CSFAβ42 + /amyR + , like in A + T + .
Conclusions
Pathological p-tau/Aβ42 and FDG hypometabolism typical of AD can be found in patients with decreased CSF Aβ42 levels alone. AmyR positivity, associated with higher Aβ40 levels, is accompanied by higher CSF p-tau and widespread FDG hypometabolism.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-023-01291-w.
Keywords: Alzheimer’s disease, Cerebrospinal fluid biomarkers, Amyloid beta 40, Amyloid beta 42, Amyloid beta 42/40 ratio, Phosphorylated-tau, Fluorodeoxyglucose-positron emission tomography
Background
According to the amyloid cascade hypothesis, Alzheimer’s disease (AD) develops due to amyloid peptides (Aβ) deposition in senile plaques, followed by the accumulation of hyperphosphorylated tau proteins (p-tau) in tangles, ultimately leading to neuronal degeneration and cognitive decline. In 2018, the National Institute on Aging-Alzheimer’s Association (NIA-AA) reported that the highest probability of identifying AD pathology in vivo is achieved by combining markers of Aβ (A) and p-tau (T) pathology [1]. Indeed, it has been demonstrated that the co-presence of A + and T + status, as well as combining them in the CSF p-tau/Aβ42 ratio, provides excellent predictive power for the presence of AD pathology at autopsy [2, 3]. However, when there is no concordance between A and T, and notably when the CSF levels of Aβ42 are abnormal (A +) without concomitant abnormal values of p-tau (T −), we encounter an ambiguous biological profile, labeled “AD pathological changes,” which is still considered part of the Alzheimer’s continuum. Given the crucial role of the “A” status in this biological condition, inconsistencies among different amyloid biomarkers, from cerebrospinal fluid (CSF) or imaging, can represent a significant limitation in supporting a possible diagnosis of AD, thus fueling the need to improve their accuracy.
In this regard, it has been demonstrated that the addition of CSF Aβ40 to CSF Aβ42 levels in the Aβ42/Aβ40 ratio (amyR) could account for inter-individual variations in Aβ production, thereby enhancing the diagnostic performance of this biomarker [4–8]. Furthermore, amyR seems to better reflect the total amount of amyloid brain deposition [9–12]. Nevertheless, Vromen and colleagues also found that the decrease of CSF Aβ42 alone shows excellent accuracy in detecting autopsy-confirmed AD [13]. Together, these data support the idea that amyR and Aβ42 may provide different kinds of information on the underlying AD pathophysiology.
In the present study, we aimed to determine whether CSF Aβ40 has a specific meaning, which enables amyR to better estimate the AD-related burden, and whether pathological CSF Aβ42 in the presence of normal amyR can still identify AD pathology. To address this issue, we focused on the A + T − biological condition, stratifying patients with decreased CSF Aβ42 into those with normal (CSFAβ42 + /amyR −) or pathological amyR (CSFAβ42 + /amyR +). We assessed differences and overlaps with healthy control subjects (A − T −) and full-blown AD patients (A + T +) in terms of disease burden measured as CSF p-tau/Aβ42 [2, 14] and cerebral glucose hypometabolism, evaluated with fluorodeoxyglucose-positron emission tomography (FDG-PET), whose specific topographical patterns have achieved an increasingly supportive role in the diagnostic algorithm of AD [15–17].
Materials and methods
Subjects’ enrolment
Between 2017 and 2021, we evaluated 250 patients at the Memory Clinic of the “Policlinico Tor Vergata” in Rome. The criteria for retrospective inclusion were as follows: (1) a complete diagnostic workup, including standardized neurological examination, laboratory testing, MRI imaging, FDG-PET scan, neuropsychological assessment, APOE genotyping, and CSF analysis, and (2) fulfillment of the diagnostic criteria for dementia [18] or mild cognitive impairment due to AD [19]. Exclusion criteria were as follows: (1) presence of other neurological or psychiatric diseases or medical conditions potentially associated with cognitive deficits; (2) major comorbidities such as oncological history, systemic inflammatory conditions, and organ failure; (3) prominent cortical or subcortical infarcts; (4) history of drug or alcohol abuse; (5) use of antipsychotics, antidepressants, or serotonergic drugs.
Eventually, we selected 163 patients belonging to the Alzheimer’s continuum (ADc) according to their biomarker profile [1]. Further stratification into AT groups was performed according to the presence of decreased CSF levels of Aβ42 (A) and increased CSF p-tau (T) (see Additional file 1).
Furthermore, we identified 36 controls among inpatients from the Neurology Unit of Policlinico Tor Vergata who had undergone a complete neurological evaluation, brain CT, and lumbar puncture for diagnostic purposes and for which the presence of any primary neurological disease had been excluded. All CSF analyses showed normal cell counts and biomarker profiles.
CSF sampling/analysis and APOE genotyping
All lumbar punctures were performed between 8 and 10 am. An 8 ml CSF sample was collected for each patient in polypropylene tubes, 2 ml were used for routine biochemical analysis, 6 ml were centrifuged at 2000 g at + 4 °C for 10 min, and frozen at – 80 °C. All samples were processed according to the manufacturer’s instructions and to laboratory standard operating procedures.
The levels of CSF Aβ42 and Aβ40, t-tau, and p-tau phosphorylated at Thr181 (p-tau181) were determined using a sandwich enzyme-linked immunosorbent assay (EUROIMMUN ELISA©). The cut-off values for CSF Aβ42, CSF p-tau, and CSF t-tau were determined following EUROIMMUN guidelines: CSF Aβ42 > 600 pg/ml, CSF amyR > 0.06, CSF p-tau < 65 pg/ml, CSF t-tau < 400 pg/ml. Given the absence of manufacturer’s guidelines for the p-tau/Aβ42 cut-off and of neuropathological/amyloid PET imaging validation in literature, we performed our analyses on AD-related burden (p-tau/Aβ42) considering two separate thresholds to determine pathological values: (1) ≥ 0.086, determined with a similar ELISA technique (INNOTEST©) and validated on neuropathological data [2], and (2) ≥ 0.122, determined on EUROIMMUN assays with a Youden Index analysis to discriminate between biologically defined AD and non-AD patients [20].
APOE genotyping was performed using allelic discrimination technology with real-time PCR (TaqMan; Applied Biosystems). Patients were classified as APOE4 when carrying either one (APOE ε3/ε4) or two (APOE ε4/ε4) ε4 alleles. All the remaining patients were identified as APOE3 (ε3/ε3).
FDG-PET substudy
Study population
The study was conducted at the Nuclear Medicine Unit of Policlinico Tor Vergata in Rome (General Electric VCT PET/CT scanner). Among the enrolled patients, 46 had undergone FDG-PET at our site (CSFAβ42 + /amyR − , n = 9; CSFAβ42 + /amyR + , n = 17; A + T + , n = 20).
Moreover, 24 subjects (male, 10; female, 14; mean age, 66.56 ± 10.44 years) undergoing FDG-PET/CT for other reasons were enrolled as part of a control group (CG) upon showing no signs of hypometabolism suggestive of neurodegenerative disorders nor other cerebral abnormalities, as assessed by visual reads (A.C). All subjects had an MRI performed within 14 ± 4 days before PET/CT examination, showing absence of brain alterations. An experienced neurologist (A.M.) evaluated all participants to assess the absence of clinical signs of cognitive decline. Patient and control selection strategies for the sub-study are summarized in Additional file 2.
FDG-PET/CT scanning and acquisition
The same scanning and acquisition protocol was used for both patients and CG. All subjects were injected with intravenous FDG (dose range 185–295 megabecquerels) and hydrated with 500 ml of saline (0.9% sodium chloride). PET/CT acquisition started 30 ± 5 min after FDG injection and lasted for 10 min in all subjects. The reconstruction parameters were as follows: ordered subset expectation maximization, four subsets and 12 iterations; matrix 256 × 256; full width at half maximum (FWHM): 5 mm.
Data management and statistical analysis
CSF biomarkers analysis
All continuous variables were expressed as mean ± standard deviation. The Kruskal–Wallis test and Dunn’s post hoc analysis were used for multiple comparisons. Categorical variables were analyzed using Pearson’s chi-square test. We computed a linear regression model with age and sex as covariates to study the association between CSF amyloid biomarkers and CSF p-tau levels. We then performed a robust locally weighted regression analysis [21] and plotted CSF biomarkers levels as a function of amyR, using CSF biomarkers values converted to z-scores by subtracting the mean and dividing by the standard deviation of the control group (A − T −). Eventually, logistic multivariate regressions were performed to evaluate the odds of pathological p-tau/Aβ42 values according to amyR status, accounting for clinical and demographic factors (age, sex, APOE, diabetes mellitus, hypertension, dyslipidemia).
All statistical analyses were performed using StataCorp© (Stata Statistical Software: Release 13. College Station, TX: StataCorp) and GraphPad Prism version 9.3.1 (GraphPad Software, San Diego, California, USA). All results were computed with two-tailed significance; p < 0.05 were considered significant.
FDG-PET analysis
First, all FDG-PET scans were visually evaluated in standardized transaxial, coronal, and sagittal plans by an experienced nuclear medicine physician (A.C) and interpreted according to the latest EANM guidelines for FDG-PET imaging [22]. We used Statistical Parametric Mapping 12 (SPM12) in MATLAB 2018a (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) to perform statistical analysis. FDG-PET data were converted from DICOM to Nifti format using Mricron software (https://www.nitrc.org/projects/mricron) and then subjected to a normalization process. A bias regularization was applied (0.0001) to limit biases due to smoothness, spatially varying artifacts that modulate the intensity of the image and that can impede automating processing of images. FWHM of Gaussian smoothness of bias (to prevent the algorithm from trying to model out intensity variation due to different tissue types) was set at 60 mm cut-off; tissue probability map implemented in SPM12 was used (TPM.nii). A mutual information affine registration with the tissue probability maps [23] was used to achieve approximate alignment to ICBM space template—European brains [24, 25]. Warping regularization was set with the following 1 by 5 array (0, 0.001, 0.5, 0.05, 0.2); smoothness (to cope with functional anatomical variability that is not compensated by spatial normalization and to improve the signal-to-noise ratio) was set at 5 mm; sampling distance (that encodes the approximate distance between sampled points when estimating the model parameters) was set at 3. We applied an 8-mm isotropic Gaussian filter to blur the individual variations (especially gyral variations) and to increase the signal-to-noise ratio. We used the following parameters and post-processing tools before regression analysis was applied: global normalization (that scales images to a global value) = 50 (using proportional scaling); masking threshold (that helps to identify voxels with an acceptable signal in them) was set to 0.8; transformation tool of statistical parametric maps into normal distribution; correction of SPM coordinates to match the Talairach coordinates, subroutine implemented by Matthew Brett (http://www.mrc-cbu.cam.ac.uk/Imaging). Brodmann areas (BA) were identified at a range from 0 to 3 mm from the corrected Talairach coordinates of the SPM output isocenter, using a Talairach client available at http://www.talairach.org/index.html. As proposed by Bennett et al. [26], SPM t-maps were corrected for multiple comparisons using the false discovery rate (p ≤ 0.05) and corrected for multiple comparisons at the cluster level (p ≤ 0.001). The level of significance was set at 100 contiguous voxels (5 × 5 × 5 voxels, i.e., 11 × 11 × 11 mm). The following voxel-based comparisons were assessed: CG vs. CSFAβ42 + /amyR − , CSFAβ42 + /amyR − vs. CSFAβ42 + /amyR + , and CSFAβ42 + /amyR + vs. A + T + . We used a full factorial design implemented in SPM12 to test the hypothesis that differences among groups exist overall. Comparisons between groups were performed using the two-sample t-test model of SPM12. For both analyses, age and sex were used as covariates, and the threshold was set at p < 0.001 (p < 0.05 FWE corrected at the cluster level).
Results
Participants’ selection and characteristics
From 250 subjects with suspected AD-related cognitive impairment, we enrolled 163 patients with decreased CSF levels of Aβ42 and normal CSF p-tau (A + T − = 98) or pathological CSF p-tau (A + T + = 65), as well as 36 sex-/age-matched healthy controls (A − T −) (see Additional file 1). We further stratified A + T − patients according to the presence of either pathological (CSFAβ42 + /amyR + , n = 46) or normal amyR (CSFAβ42 + /amyR − , n = 52). Table 1 shows the clinical and demographic characteristics of the patients according to AT status.
Table 1.
Demographic and clinical characteristics across AT groups
| A − T − (n = 36) | CSFAβ42 + /amyR − (n = 52) | CSFAβ42 + /amyR + (n = 46) | A + T + (n = 65) | p | |
|---|---|---|---|---|---|
| Age (years) | 70.72 ± 3.81 | 68.90 ± 9.55 | 71.34 ± 8.91 | 72.31 ± 6.56 | 0.15 |
| Male (%) | 33.3% | 55.7% | 60.8% | 33.8% | < 0.01 |
| CSF Aβ42 (pg/ml) | 1270.91 ± 196.67 | 394.45 ± 85.80 | 341.92 ± 81.18 | 389.17 ± 105.41 | < 0.001 |
| CSF Aβ40 (pg/ml) | 5870.36 ± 1283.07 | 4740.74 ± 1457.22 | 8110.55 ± 2490.97 | 10092.15 ± 3352.14 | < 0.001 |
| CSF p-tau (pg/ml) | 25.27 ± 6.65 | 29.12 ± 13.63 | 44.84 ± 13.28 | 101.37 ± 30.89 | < 0.001 |
| CSF t-tau (pg/ml) | 165.82 ± 60.60 | 149.30 ± 77.60 | 233.84 ± 72.53 | 646.54 ± 261.38 | < 0.001 |
| CSF p-tau/Aβ42 | 0.02 ± 0.01 | 0.07 ± 0.04 | 0.14 ± 0.05 | 0.28 ± 0.11 | < 0.001 |
| Qalb | 5.62 ± 3.40 | 8.75 ± 6.33 | 7.46 ± 4.11 | 7.44 ± 6.58 | < 0.01 |
| MMSE | 29.19 ± 1.97 | 21.31 ± 4.82 | 21.50 ± 5.15 | 20.25 ± 4.95 | < 0.001 |
| MCI/Dementia (n) | 0/0 | 21/31 | 18/28 | 15/50 | 0.08 |
| Diabetes (%) | 38.9% | 55.8% | 56.5% | 58.5% | 0.26 |
| Hypertension (%) | 47.2% | 21.2% | 19.6% | 15.4% | < 0.01 |
| Dyslipidemia (%) | 27.8% | 19.2% | 26.1% | 35.4% | 0.28 |
| APOE4 (%) | n.a | 28.8% | 32.6% | 55.4% | < 0.01 |
Data are presented as mean ± standard deviation or percentages, when applicable. CSF cerebrospinal fluid, Qalb albumin quotient, MMSE Mini-Mental State Examination, p p-value. MMSE score of ≥ 24 was used as a cut-off to separate MCI from dementia patients. Percentages indicate subjects showing the presence of each variable within the group. Bold values represent p-value < 0.05
CSF AD biomarkers and disease burden
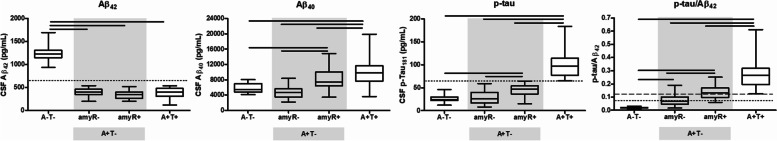
CSF levels of Aβ42 were significantly lower in CSFAβ42 + /amyR − , CSFAβ42 + /amyR + , and A + T + patients than in A − T − subjects (F = 560.9; p < 0.001), but no differences were found among the patient groups (p > 0.05). Conversely, CSF Aβ40 levels were similarly lower in A − T − and CSFAβ42 + /amyR − , significantly higher in CSFAβ42 + /amyR + , and even higher in A + T + patients (F = 50.75; p < 0.001), suggesting that pathological amyR in CSFAβ42 + /amyR + and A + T + could be sustained by the concomitant presence of increased Aβ40 and decreased Aβ42 in the CSF. Similarly, the ANOVA showed lower CSF p-tau levels in A − T − and CSFAβ42 + /amyR − , higher in CSFAβ42 + /amyR + , and highest in A + T + (F = 192; p < 0.001).
Finally, p-tau/Aβ42 levels were the lowest in A − T − and progressively increased in CSFAβ42 + /amyR − , CSFAβ42 + /amyR + , and A + T + patients (F = 131.9; p < 0.001) (Fig. 1).
Fig. 1.
Intergroup differences of cerebrospinal fluid (CSF) biomarkers. The gray zone includes patients classified as A + T − . Dotted lines represent cut-off values used for Aβ42, p-tau, and the p-tau/Aβ42 cut-off 0.086; the dashed line represents p-tau/Aβ42 cut-off 0.122. Bold lines represent comparisons with p-values < 0.05 at the Kruskal–Wallis
Association between amyloid biomarkers and p-tau CSF levels
To explore the association between different CSF amyloid biomarkers (Aβ42, Aβ40, and amyR) and CSF p-tau levels, we performed correlation (see Additional file 3) and linear regression analyses adjusting for age and sex (see Table 2). Aβ42 was associated with p-tau levels only in A − T − subjects (β = 0.48; p = 0.003), but not in any other AT subgroup. In contrast, a positive association between Aβ40 and CSF p-tau levels was found in A − T − (β = 0.58; p < 0.001), CSFAβ42 + /amyR − (β = 0.47; p < 0.001), and CSFAβ42 + /amyR + patients (β = 0.48; p < 0.001), but not in A + T + patients. Similarly, amyR was associated with CSF p-tau levels in A − T − (β = − 0.33; p = 0.047), CSFAβ42 + /amyR − (β = − 0.49; p < 0.001), and CSFAβ42 + /amyR + patients (β = − 0.45; p < 0.001), but not in A + T + patients.
Table 2.
Linear regression analyses, adjusted for age and sex, assessing the association between CSF amyloid biomarkers and CSF levels of p-tau
| A − T − | CSFAβ42 + /amyR − | CSFAβ42 + /amyR + | A + T + | |||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| Aβ42 | .48 | .003 | .10 | .44 | .12 | .33 | .16 | .20 |
| Aβ40 | .58 | < .001 | .47 | < .001 | .48 | < .001 | − 0.07 | .58 |
| amyR | − .33 | .047 | − .49 | < .001 | − .45 | < .001 | .23 | .08 |
Bold values represent p-value < 0.05
amyR Aβ42/Aβ40, β standardized coefficient, p p-value
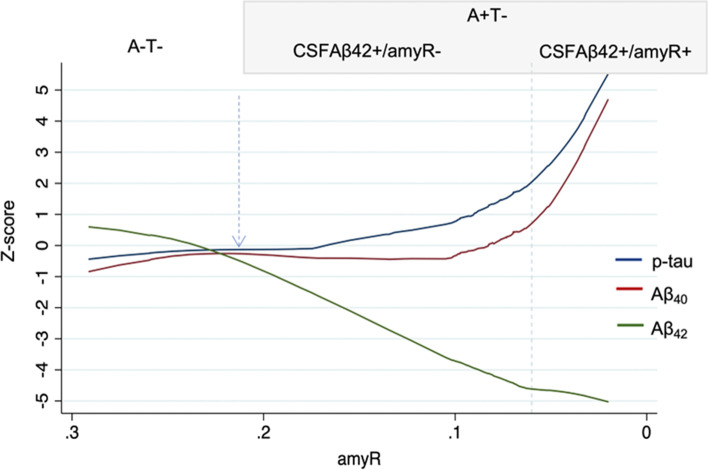
Given the previous association between amyloid and p-tau markers in the A − T − and A + T − subgroups, we applied a robust locally weighted regression model to trace the trajectories of standardized (z-scores) CSF biomarkers as a function of amyR. We observed that CSF Aβ42 dramatically declined, reaching − 4.5 z-scores, when amyR levels were still normal, and plateaued when amyR became pathological (− 5 z-scores). Remarkably, CSF Aβ40 and p-tau also started to increase before the amyR cut-off was reached (2 z-scores for CSF p-tau and 0.8 z-scores for Aβ40), but steeply increased above the pathological threshold of amyR, eventually reaching the highest z-scores (5 z-scores for CSF p-tau and 4.5 z-scores for Aβ40) (Fig. 2).
Fig. 2.
Results of the robust locally weighted regression analysis showing changes of CSF biomarkers as a function of amyR. Values of p-tau, Aβ42, and Aβ40 are expressed as z-score standardized on the average and standard deviation of the A − T − group. The dotted line represents threshold value of pathological amyR (0.06), the dotted arrow indicates the point of change of Aβ42 from normal to pathological values. CSF, cerebrospinal fluid; amyR, Aβ42/Aβ40; A, amyloid status; T, p-tau status)
Pathological amyR increases odds of pathological p-tau/Aβ42 in A + T −
To evaluate the effect of amyR on the AD-related burden, in terms of p-tau/Aβ42 ratio, we applied Pearson’s chi-squared test to A + T − patients and found a different distribution of pathological p-tau/Aβ42 levels between CSFAβ42 + /amyR − and CSFAβ42 + /amyR + subgroups [cut-off ≥ 0.086: 35.7% vs 78%, χ2 (1, n = 105) = 27.018, p < 0.001; cut-off ≥ 0.122: 17.3% vs 63%, χ2 (1, n = 105) = 21.506, p < 0.001].
In the logistic multivariate analyses, considering the variables age, sex, APOE E4 allele, hypertension, diabetes, and dyslipidemia, pathological amyR was significantly associated with a higher likelihood of having pathological p-tau/Aβ42 [cut-off ≥ 0.086: OR 23.3 with a 95% CI of 4.0980–28.4627, p < 0.001; cut-off ≥ 0.122: OR 8.8 with a 95% CI of 3.28–23.68, p < 0.001] (Table 3). Nevertheless, some patients with normal amyR in the CSFAβ42 + /amyR − group still showed pathological p-tau/Aβ42 levels (cut-off ≥ 0.086: 35.7%; cut-off ≥ 0.122: 17.3%).
Table 3.
Results of multivariate regression analysis exploring the odds of having pathological values of p-tau/Aβ42 in A + T − patients
| p-tau/Aβ42 cut-off ≥ 0.086 | p-tau/Aβ42 cut-off ≥ 0.122 | |||
|---|---|---|---|---|
| Odds ratio (CI) | p | Odds ratio (CI) | p | |
| amyR | 23.27 (6.47–83.60) | < 0.001 | 8.82 (3.28–23.68) | < 0.001 |
| Diabetes | 0.38 (0.11–1.32) | 0.13 | 0.51 (0.19–1.40) | 0.19 |
| Hypertension | 0.99 (0.26–3.72) | 0.99 | 1.25 (0.39–4.03) | 0.71 |
| Dyslipidemia | 2.79 (0.71–10.95) | 0.14 | 1.09 (0.34–3.47) | 0.88 |
| APOE4 | 5.93 (1.61–21.79) | 0.007 | 1.27 (0.45–3.58) | 0.65 |
| Age | 1.07 (0.99–1.14) | 0.06 | 1.02 (0.96–1.07) | 0.48 |
| Sex | 1.31 (0.43–4.03) | 0.63 | 1.82 (0.68–4.91) | 0.23 |
Bold values represent p-value < 0.05
amyR Aβ42/Aβ40, CI 95% confidence interval, p p-value
FDG-PET substudy
Demographics of the participants to the substudy are reported in Additional file 4. Two expert raters (A.M. and A.C.) separately evaluated FDG-PET scans from the 46 patients in the substudy and identified 37 typical AD patterns, four possible AD patterns, and five normal scans, showing good inter-rater agreement (Cohen’s k = 0.9). Holding scans with both typical and possible patterns as AD-positive, Cohen’s k analysis demonstrated a significant agreement between abnormal FDG-PET and pathological CSF p-tau/Aβ42 (Cohen’s k = 0.73), with a mismatch of 6% between them.
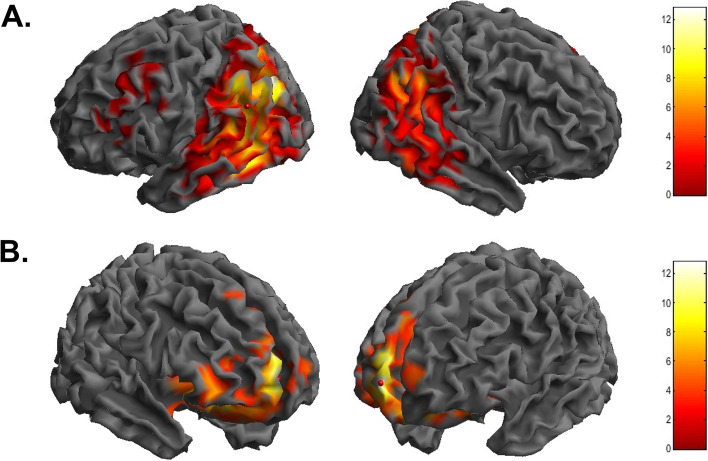
Comparisons between FDG-uptake patterns of each patient group versus CG showed a significant reduction in the temporo-parietal and frontal regions (see Additional file 5). The full factorial design, accounting for inter-group differences, resulted in a significant main effect of groups, and results from the post hoc analysis are shown in Table 4. Compared to the control group (CG), CSFAβ42 + /amyR − patients showed a significant reduction in brain glucose consumption in a wide cluster encompassing the left parietal (BAs 19 and 40), temporal (BA 37), and frontal lobes (BAs 6, 8, and 46) (Fig. 3A). With respect to CSFAβ42 + /amyR − , CSFAβ42 + /amyR + showed an additional reduction in FDG uptake in the anterior regions, namely the anterior cingulate and frontomedial areas (BAs 32 and 10) (Fig. 3B). No differences were found between the hypometabolic patterns of CSFAβ42 + /amyR + and A + T + patients (p > 0.05).
Table 4.
Numerical results of SPM comparisons of FDG uptake in CG vs. CSFAβ42 + /amyR − , CSFAβ42 + /amyR − vs. CSFAβ42 + /amyR + , and CSFAβ42 + /amyR + vs. A + T +
| Analysis | Cluster level | Voxel level | ||||||
|---|---|---|---|---|---|---|---|---|
| Cluster p (FWE-corr) | Cluster p (FDR-corr) | Cluster extent | Cortical region | Z score of maximum | Talairach coordinates | Cortical region | BA | |
| CG vs CSFAβ42 + /amyR − | 0.000 | 0.000 | 31,175 | L parietal lobe | Inf | − 36, − 74, 38 | Precuneus | 19 |
| L temporal lobe | Inf | − 52, − 56, − 10 | Middle temporal gyrus | 37 | ||||
| L parietal lobe | 7.63 | − 48, − 48, 44 | Inferior parietal lobule | 40 | ||||
| 0.001 | 0.000 | 1795 | L frontal lobe | 3.81 | − 26, 10, 58 | Middle frontal gyrus | 6 | |
| L frontal lobe | 3.61 | − 30, 26, 46 | Middle frontal gyrus | 8 | ||||
| L frontal Lobe | 3.43 | − 48, 30, 16 | Inferior frontal gyrus | 46 | ||||
| CSFAβ42 + /amyR − vs CSFAβ42 + /amyR + | 0.000 | 0.000 | 6851 | R frontal lobe | 5.06 | 14, 62, 0 | Medial frontal gyrus | 10 |
| R frontal lobe | 4.63 | 8, 60, 14 | Medial frontal gyrus | 10 | ||||
| R limbic lobe | 4.58 | 16, 32, 18 | Anterior cingulate | 32 | ||||
| CSFAβ42 + /amyR + vs A + T + | n.s | n.a | n.a | n.a | n.a | n.a | ||
In the “cluster level” section (left), the number of voxels, corrected p-value of significance, and cortical region where the voxel is found are all reported for each significant cluster. In the “voxel level” section, all the coordinates of the correlation sites (with the Z-score of the maximum correlation point), the corresponding cortical region and BA are reported for each significant cluster (CG, control group; Inf., infinite; L, left; R, right; BA, Brodmann area). When the maximum correlation is achieved outside the gray matter, the nearest gray matter (within a range of 5 mm) is indicated by the corresponding BA
Fig. 3.
3D brain rendering showing significant clusters obtained in SPM when comparing A − T − vs. CSFAβ42 + /amyR − (A) and CSFAβ42 + /amyR − vs. CSFAβ42 + /amyR + (B). Color scale represents t-statistics values
Discussion
In the complex AD framework, the discordance between biomarkers, especially those accounting for amyloid status, might encourage both clinicians and researchers to prefer biomarkers with higher specificity, despite the risk of losing sensitivity and likely missing the problem of the different meanings associated with those biomarkers.
In the present study, we investigated whether Aβ42 and amyR have different associations with AD-related burden, measured by CSF p-tau/Aβ42 [2, 14, 20] and FDG-PET brain hypometabolism.
Main findings in CSF AD biomarkers and disease burden
CSF Aβ42 levels did not significantly change across the AD continuum. Conversely, we identified a stepwise increase in CSF Aβ40, with lower levels in controls as in CSFAβ42 + /amyR − , higher levels in CSFAβ42 + /amyR + , and the highest in A + T + , resembling the CSF variations of p-tau and p-tau/Aβ42.
Decreased CSF Aβ42 levels are a well-established finding in AD and could reflect the aggregation of Aβ42 in brain tissue [27, 28], the formation of semisoluble Aβ42 oligomers [29], or even the binding of peptides in complexes that mask epitopes targeted by analytical assays [30]. Moreover, the hypothetical temporal changes of AD biomarkers presume that the CSF Aβ42 reduction begins in the pre-clinical stage [31]. However, repeated CSF measurements from symptomatic patients showed longitudinal stability of CSF Aβ42 levels, making this biomarker unsuitable for reflecting the underlying dynamics of amyloid metabolism over time [32]. On the other hand, amyR is a better predictor of abnormal cortical amyloid plaque burden in AD [11, 12], possibly because the addition of CSF Aβ40 in the ratio can correct for interindividual variability of amyloid production [33, 34]. However, another reasonable explanation may lie in a specific role of Aβ40 in AD. Biochemical studies have shown that Aβ42 has faster aggregation properties than other Aβ species and that Aβ40 monomers inhibit its aggregation [35–37], by preferentially binding to protofibrillar Aβ42 [38, 39]. Moreover, different ratios of Aβ42 to Aβ40 can result in different kinetics of amyloid fibrillization and aggregation. Chang and colleagues demonstrated that an equimolar Aβ40/Aβ42 sample generates oligomers with the highest neurotoxic effects on neuritic length, while an increase in the proportion of Aβ40 stabilizes the fibrillization pathway [40]. However, an Aβ40-dominant ratio causes changes in calcium dynamics, resulting in a higher elevation of intracellular calcium levels and neuronal apoptosis [41]. Thus, we may speculate that CSF levels of Aβ40 initially increase to quench the fibrillization pathway triggered by Aβ42 oligomerization; however, beyond a certain threshold, they may have toxic effects on neurons.
When we plotted the changes in CSF biomarkers as a function of amyR, we noticed different trends in CSF biomarkers variations. We observed that Aβ42 had the steepest decrease before the amyR-positivity cut-off and then encountered a plateau phase. In contrast, CSF p-tau and Aβ40 started increasing before the pathological threshold of amyR, but this increase was significantly more pronounced when amyR became pathological. This finding is consistent with the notion that cortical Aβ deposition precedes neocortical tau aggregation [34] and with previous studies reporting that the decrease in CSF Aβ42/Aβ40 ratio is followed by a large increase in CSF p-tau also in preclinical AD patients [42]. Indeed, we observed that a decrease of Aβ42 in the CSF is accompanied by an increase of tau phosphorylation but also that the increase of Aβ40 levels—which significantly affects amyR positivity—is accompanied by a steeper increase in CSF p-tau levels, which has recently been described to boost the spread of tau pathology in the brain [43].
In our cohort, we found that among A + T − patients, CSFAβ42 + /amyR + showed a higher probability of having pathological levels of p-tau/Aβ42, a well-known marker of AD-related burden, than CSFAβ42 + /amyR − . Considering the differences in CSF levels of Aβ40, but not Aβ42, we might assume that the higher levels of Aβ40 in CSFAβ42 + /amyR + could concur with these results.
Unexpectedly, although the CSFAβ42 + /amyR − condition might be misinterpreted as an A − status if we relied on amyR more than CSF Aβ42, in our study, we found that pathological values of p-tau/Aβ42 can be present also in these patients. This highlights that amyR, commonly held as the best biomarker for detecting amyloid pathology [35], also shows suboptimal negative predictive power, suggesting that the added value of Aβ40 could lie beyond its Aβ42-corrective function, since correcting for inter-individual amyloid production variability should improve not only amyR specificity but also sensitivity.
The specific meaning of the altered CSF Aβ40 levels could be rooted in its significant correlation with the CSF levels of p-tau, which is also present under physiological conditions [44]. In our results, we observed a positive linear relationship between Aβ40 and p-tau in A − T − , CSFAβ42 + /amyR − , and CSFAβ42 + /amyR + , but not in A + T + . We cannot exclude that, alongside pathological Aβ42 levels, there may be a concomitant increase in CSF Aβ40 (as in CSFAβ42 + /amyR + patients) associated with a parallel increase in tau phosphorylation. Conversely, in our A + T + group tau pathology seems to proceed independently from amyloid pathology as no association was found between amyloid and tau CSF biomarkers. In line with our results, it has been recently demonstrated that the Aβ-induced increase in CSF p-tau levels might play a key role in initiating tau aggregation and spreading in early AD, while local tau seeding and auto-replication predominate once soluble p-tau concentrations reach a plateau in AD dementia [43].
Main findings in FDG-PET metabolic pattern
To support our hypothesis that CSFAβ42 + and amyR + hold different meanings in AD pathophysiology, we considered patterns of brain glucose metabolism. Indeed, specific FDG-PET hypometabolic patterns have been demonstrated to predict AD dementia [15–17] and may detect additional differences between CSFAβ42 + /amyR − and CSFAβ42 + /amyR + .
First, when comparing FDG-PET scans from CSFAβ42 + /amyR − patients and controls, we found a pattern of cortical hypometabolism encompassing the parietal and frontotemporal regions. Thus, the decrease in CSF Aβ42 alone may be associated with synaptic dysfunction and reduced brain glucose uptake in areas typically involved in AD [45, 46]. However, other crucial factors (i.e., such as endothelial dysfunction, neuroinflammation, and astrocytic impairment) could also have influenced synaptic functioning, especially in the very early stages of the disease, before overt amyloid pathology occurs [47–49].
In contrast, patients with CSFAβ42 + /amyR + showed further involvement of the frontal regions, configuring an FDG-PET pattern indistinguishable from A + T + . Previous studies have shown that soluble Aβ40, but not Aβ42, extracted from the frontal cortex of patients with AD gradually increases along with the progression of Braak scores, suggesting a specific link between Aβ40 levels and the progression of tau pathology [50]. Moreover, a positive correlation between soluble tau levels and hypometabolism in the frontal regions has also been reported [51]. Our findings suggest that amyR positivity, which in our cohort is determined by the co-presence of decreased Aβ42 and increased Aβ40 levels, is associated with widespread brain hypometabolism, and probably with the spread of tau pathology, even in the absence of elevated tau proteins in the CSF [52].
The lack of detection of elevated levels of CSF p-tau181 in our cohort does not exclude a possible increase of other p-tau isoforms (e.g., p-tau217 or p-tau231). Moreover, a threshold-based evaluation of CSF p-tau, as for other fluid biomarkers, can have limitations tied to the risk of false negative results, whose occurrence also encompasses pre-analytical (e.g., collection techniques, handling of samples, storage conditions) and analytical variables (e.g., accuracy in sample processing, assay sensitivity) [53]. On the other hand, there may be other yet-to-be elucidated pathophysiological processes underlying a possible mismatch between parenchymal and CSF evidence of tau pathology. Considering all these limitations, a multimodal approach combining imaging and CSF biomarkers could be advisable.
Our results show that among patients classified as A + T − , there is a group with reduced Aβ42 and low Aβ40 (CSFAβ42 + /amyR −) that differ from healthy controls for showing typical AD patterns of cerebral hypometabolism, and a second group with reduced Aβ42 and high Aβ40 (CSFAβ42 + /amyR +) showing widespread reduction of brain glucose uptake, undistinguishable from biologically defined AD patients (A + T +).
Limitations
To our knowledge, this study is the first to directly compare CSFAβ42 + /amyR − and CSFAβ42 + /amyR + patients, as well as the first to examine differences and overlap between them, healthy controls, and A + T + in terms of CSF p-tau, AD-related burden, and brain FDG-PET metabolic patterns. However, because of its cross-sectional design, we could not investigate temporal connection between different biomarkers alteration or evaluate whether higher CSF Aβ40 levels, along with pathological CSF Aβ42, may affect the rapidity of cognitive decline.
Furthermore, fluid biomarkers, represented on a continuous scale, are commonly used in a dichotomous way, applying cut-offs whose thresholds become crucial in clinical practice. Such thresholds are not always available for every biomarker measure, and a global effort of standardization is needed to increase accuracy and reproducibility of these findings. Further investigations using amyloid-PET or tau-PET would be useful to verify our results on the prevalence of pathological AD-related burden across patient groups—which we evaluated indirectly via the CSF p-tau/Aβ42 ratio—and to assess any causal connections between the alteration of Aβ42, Aβ40, and p-tau in the CSF and parenchymal amyloid and tau deposition. Finally, extending the analysis by comparing blood-based and CSF biomarkers could be useful to confirm these relationships and to assess their extensibility for diagnostic and prognostic purposes.
Conclusions
Fluid biomarkers are nowadays being introduced into clinical routine practice, positing challenges on their correct use for either supporting or excluding AD diagnosis.
Patients with cognitive symptoms and decreased CSF Aβ42 without pathological amyR nor increased p-tau levels could, under certain circumstances, be essentially considered non-AD, but this conclusion can be hard to reach when clinical presentation and FDG-PET pattern are highly suggestive of AD. Our results suggest that decreased CSF Aβ42 alone could detect Alzheimer’s pathology, since these patients are distinguished from controls by reduced FDG uptake in brain regions typical of AD, and some of them also show pathological measures of AD-related burden.
On the other hand, the increase in CSF Aβ40 levels, traced by pathological amyR, associates with higher levels of soluble hyperphosphorylated tau, higher AD-related burden, and a widespread pattern of FDG hypometabolism.
Considering the many experimental novel drugs that see Aβ and tau proteins as therapeutic targets, the specific meaning of different amyloid CSF biomarkers should be considered to support a more accurate selection of patients and search for the best time window for treatment.
Supplementary Information
Additional file 1. Flowcharts summarizing patients’ enrolment (A) and control group selection (B) procedures for the CSF study.
Additional file 2. Flowcharts summarizing patients’ enrolment (A) and control group selection (B) procedures for the FDG-PET substudy.
Additional file 3. Scatter Plots showing correlations (Spearman’s rho) between CSF p-tau and different amyloid biomarkers (Aβ42, Aβ40, amyR) in A-T-, CSFAβ42+/amyR-, CSFAβ42+/amyR+ and A+T+.
Additional file 4. Demographical, clinical and biomarkers data from FDG-PET substudy.
Additional file 5. Numerical results of SPM comparisons of FDG uptake in CG vs. CSFAβ42+/amyR-, CG vs. CSFAβ42+/amyR+ and CG vs. A+T+.
Acknowledgements
None.
Abbreviations
- AD
Alzheimer’s disease
- Aβ
Amyloid-β
- amyR
Amyloid ratio
- BA
Brodmann area
- CSF
Cerebrospinal fluid
- CT
Computed tomography
- FDG
Fludeoxyglucose
- MRI
Magnetic resonance imaging
- MMSE
Mini-Mental State Examination
- PET
Positron emission tomography
- PCR
Polymerase chain reaction
- SPM
Statistical parametric mapping
Authors’ contributions
CM and AM conceptualized the study and revised the manuscript. CM, MDD, CGB and AC analyzed and interpreted the data, drafted and revised the manuscript, did the statistical analysis, and prepared the figures. MA, NBM and GK participated in the interpretation of the data and revision of the manuscript.
Funding
This study was not supported by any grant.
Availability of data and materials
The dataset analyzed in this study is available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All participants or legal representatives signed a written informed consent for the anonymization, storage, and analysis of all clinical and biological data. The local ethics committee approved this protocol as an observational retrospective study, which was conducted according to the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jack CR, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66(3):382–389. doi: 10.1001/ARCHNEUROL.2008.596. [DOI] [PubMed] [Google Scholar]
- 3.Long JM, Coble DW, Xiong C, et al. Preclinical Alzheimer’s disease biomarkers accurately predict cognitive and neuropathological outcomes. 2022. 10.1093/brain/awac250. [DOI] [PMC free article] [PubMed]
- 4.Baldeiras I, Santana I, Leitão MJ, et al. Addition of the Aβ42/40 ratio to the cerebrospinal fluid biomarker profile increases the predictive value for underlying Alzheimer’s disease dementia in mild cognitive impairment. Alzheimers Res Ther. 2018;10(1). 10.1186/S13195-018-0362-2. [DOI] [PMC free article] [PubMed]
- 5.Slaets S, Le Bastard N, Martin JJ, et al. Cerebrospinal fluid Aβ1-40 improves differential dementia diagnosis in patients with intermediate P-tau181P levels. J Alzheimers Dis. 2013;36(4):759–767. doi: 10.3233/JAD-130107. [DOI] [PubMed] [Google Scholar]
- 6.Sauvée M, DidierLaurent G, Latarche C, Escanyé M-C, Olivier J-L, Malaplate-Armand C. Additional use of Aβ 42/Aβ 40 ratio with cerebrospinal fluid biomarkers P-Tau and Aβ 42 increases the level of evidence of Alzheimer’s disease pathophysiological process in routine practice. J Alzheimer’s Dis. 2014;41(2):377–386. doi: 10.3233/JAD-131838. [DOI] [PubMed] [Google Scholar]
- 7.Dorey A, Perret-Liaudet A, Tholance Y, Fourier A, Quadrio I. Cerebrospinal fluid Aβ40 improves the interpretation of Aβ42 concentration for diagnosing Alzheimer’s disease. Front Neurol. 2015;6(NOV). 10.3389/FNEUR.2015.00247. [DOI] [PMC free article] [PubMed]
- 8.Dumurgier J, Schraen S, Gabelle A, et al. Cerebrospinal fluid amyloid-β 42/40 ratio in clinical setting of memory centers: a multicentric study. Alzheimers Res Ther. 2015;7(1). 10.1186/S13195-015-0114-5. [DOI] [PMC free article] [PubMed]
- 9.Niemantsverdriet E, Ottoy J, Somers C, et al. The cerebrospinal fluid Aβ1-42/Aβ1-40 ratio improves concordance with amyloid-PET for diagnosing Alzheimer’s disease in a clinical setting. J Alzheimers Dis. 2017;60(2):561–576. doi: 10.3233/JAD-170327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amft M, Ortner M, Eichenlaub U, et al. The cerebrospinal fluid biomarker ratio Aβ42/40 identifies amyloid positron emission tomography positivity better than Aβ42 alone in a heterogeneous memory clinic cohort. Alzheimer’s Res Ther. 2022;14(1):1–9. doi: 10.1186/S13195-022-01003-W/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janelidze S, Pannee J, Mikulskis A, et al. Concordance between different amyloid immunoassays and visual amyloid positron emission tomographic assessment. JAMA Neurol. 2017;74(12):1492–1501. doi: 10.1001/JAMANEUROL.2017.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewczuk P, Mroczko B, Fagan A, Kornhuber J. Biomarkers of Alzheimer’s disease and mild cognitive impairment: a current perspective. Adv Med Sci. 2015;60(1):76–82. doi: 10.1016/J.ADVMS.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Vromen EM, de Boer SCM, Teunissen CE, et al. Biomarker A+T-: is this Alzheimer’s disease or not? A combined CSF and pathology study. Brain. 2022. 10.1093/BRAIN/AWAC158. [DOI] [PMC free article] [PubMed]
- 14.Maddalena A, Papassotiropoulos A, Müller-Tillmanns B, et al. Biochemical diagnosis of Alzheimer disease by measuring the cerebrospinal fluid ratio of phosphorylated tau protein to beta-amyloid peptide42. Arch Neurol. 2003;60(9):1202–1206. doi: 10.1001/ARCHNEUR.60.9.1202. [DOI] [PubMed] [Google Scholar]
- 15.Perani D, Cerami C, Caminiti SP, et al. Cross-validation of biomarkers for the early differential diagnosis and prognosis of dementia in a clinical setting. Eur J Nucl Med Mol Imaging. 2016;43(3):499–508. doi: 10.1007/S00259-015-3170-Y. [DOI] [PubMed] [Google Scholar]
- 16.Caminiti SP, Ballarini T, Sala A, et al. FDG-PET and CSF biomarker accuracy in prediction of conversion to different dementias in a large multicentre MCI cohort. NeuroImage Clin. 2018;18:167–177. doi: 10.1016/J.NICL.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inui Y, Ito K, Kato T. Longer-term investigation of the value of 18F-FDG-PET and magnetic resonance imaging for predicting the conversion of mild cognitive impairment to Alzheimer’s disease: a multicenter study. J Alzheimers Dis. 2017;60(3):877–887. doi: 10.3233/JAD-170395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Constantinides VC, Paraskevas GP, Boufidou F, et al. CSF Aβ42 and Aβ42/Aβ40 ratio in Alzheimer’s disease and frontotemporal dementias. Diagnostics. 2023;13(4):783. 10.3390/DIAGNOSTICS13040783. [DOI] [PMC free article] [PubMed]
- 21.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829–836. doi: 10.1080/01621459.1979.10481038. [DOI] [Google Scholar]
- 22.Guedj E, Varrone A, Boellaard R, et al. EANM procedure guidelines for brain PET imaging using [18F]FDG, version 3. Eur J Nucl Med Mol Imaging. 2022;49(2):632–651. doi: 10.1007/S00259-021-05603-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Agostino E, Maes F, Vandermeulen D, Suetens P. Atlas-to-image non-rigid registration by minimization of conditional local entropy. Lect Notes Comput Sci (including Subser Lect Notes Artif Intell Lect Notes Bioinformatics). 2007;4584 LNCS:320–332. 10.1007/978-3-540-73273-0_27/COVER. [DOI] [PubMed]
- 24.Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. Neuroimage. 1995;2(2):89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- 25.Mazziotta J, Toga A, Evans A, et al. A four-dimensional probabilistic atlas of the human brain. J Am Med Inform Assoc. 2001;8(5):401. doi: 10.1136/JAMIA.2001.0080401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett CM, Wolford GL, Miller MB. The principled control of false positives in neuroimaging. Soc Cogn Affect Neurosci. 2009;4(4):417–422. doi: 10.1093/SCAN/NSP053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strozyk D, Blennow K, White LR, Launer LJ. CSF Aß 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60(4):652–656. doi: 10.1212/01.WNL.0000046581.81650.D0. [DOI] [PubMed] [Google Scholar]
- 28.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59(3):512–519. doi: 10.1002/ANA.20730. [DOI] [PubMed] [Google Scholar]
- 29.Walsh DM, Klyubin I, Shankar GM, et al. The role of cell-derived oligomers of Aβ in Alzheimer’s disease and avenues for therapeutic intervention. Biochem Soc Trans. 2005;33(5):1087–1090. doi: 10.1042/BST20051087. [DOI] [PubMed] [Google Scholar]
- 30.Bjerke M, Portelius E, Minthon L, et al. Confounding factors influencing amyloid beta concentration in cerebrospinal fluid. Int J Alzheimers Dis Published online. 2010 doi: 10.4061/2010/986310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stomrud E, Minthon L, Zetterberg H, Blennow K, Hansson O. Longitudinal cerebrospinal fluid biomarker measurements in preclinical sporadic Alzheimer’s disease: a prospective 9-year study. Alzheimer’s Dement Diagnosis, Assess Dis Monit. 2015;1(4):403–411. doi: 10.1016/j.dadm.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Bastard N, Aerts L, Sleegers K, et al. Longitudinal stability of cerebrospinal fluid biomarker levels: fulfilled requirement for pharmacodynamic markers in Alzheimer’s disease. J Alzheimers Dis. 2013;33(3):807–822. doi: 10.3233/JAD-2012-110029. [DOI] [PubMed] [Google Scholar]
- 33.Biscetti L, Salvadori N, Farotti L, et al. The added value of Aβ42/Aβ40 in the CSF signature for routine diagnostics of Alzheimer’s disease. Clin Chim Acta. 2019;494:71–73. doi: 10.1016/J.CCA.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s disease. Alzheimers Res Ther. 2019;11(1):1–15. doi: 10.1186/S13195-019-0485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray MM, Bernstein SL, Nyugen V, Condron MM, Teplow DB, Bowers MT. Amyloid protein: A40 inhibits A42 oligomerization. 2009;131(18):6136–6137. 10.1021/ja8092604. [DOI] [PMC free article] [PubMed]
- 36.Jan A, Gokce O, Luthi-Carter R, Lashuel HA. The ratio of monomeric to aggregated forms of Abeta40 and Abeta42 is an important determinant of amyloid-beta aggregation, fibrillogenesis, and toxicity. J Biol Chem. 2008;283(42):28176–28189. doi: 10.1074/JBC.M803159200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasegawa K, Yamaguchi I, Omata S, Gejyo F, Naiki H. Interaction between A beta(1–42) and A beta(1–40) in Alzheimer’s beta-amyloid fibril formation in vitro. Biochemistry. 1999;38(47):15514–15521. doi: 10.1021/BI991161M. [DOI] [PubMed] [Google Scholar]
- 38.Yan Y, Wang C. Abeta40 protects non-toxic Abeta42 monomer from aggregation. J Mol Biol. 2007;369(4):909–916. doi: 10.1016/J.JMB.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Pauwels K, Williams TL, Morris KL, et al. Structural basis for increased toxicity of pathological aβ42:aβ40 ratios in Alzheimer disease. J Biol Chem. 2012;287(8):5650–5660. doi: 10.1074/JBC.M111.264473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuperstein I, Broersen K, Benilova I, et al. Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010;29(19):3408–3420. doi: 10.1038/EMBOJ.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang YJ, Chen YR. The coexistence of an equal amount of Alzheimer’s amyloid-β 40 and 42 forms structurally stable and toxic oligomers through a distinct pathway. FEBS J. 2014;281(11):2674–2687. doi: 10.1111/FEBS.12813. [DOI] [PubMed] [Google Scholar]
- 42.Milà-Alomà M, Salvadó G, Gispert JD, et al. Amyloid beta, tau, synaptic, neurodegeneration, and glial biomarkers in the preclinical stage of the Alzheimer’s continuum. Alzheimers Dement. 2020;16(10):1358–1371. doi: 10.1002/ALZ.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pichet Binette A, Franzmeier N, Spotorno N, et al. Amyloid-associated increases in soluble tau relate to tau aggregation rates and cognitive decline in early Alzheimer’s disease. Nat Commun. 2022;13(1):6635. doi: 10.1038/S41467-022-34129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lehmann S, Dumurgier J, Ayrignac X, et al. Cerebrospinal fluid A beta 1–40 peptides increase in Alzheimer’s disease and are highly correlated with phospho-tau in control individuals. Alzheimers Res Ther. 2020;12(1). 10.1186/S13195-020-00696-1. [DOI] [PMC free article] [PubMed]
- 45.Nobili F, Arbizu J, Bouwman F, et al. European Association of Nuclear Medicine and European Academy of Neurology recommendations for the use of brain 18 F-fluorodeoxyglucose positron emission tomography in neurodegenerative cognitive impairment and dementia: Delphi consensus. Eur J Neurol. 2018;25(10):1201–1217. doi: 10.1111/ENE.13728. [DOI] [PubMed] [Google Scholar]
- 46.Minoshima S, Cross D, Thientunyakit T, Foster NL, Drzezga A. 18F-FDG PET imaging in neurodegenerative dementing disorders: insights into subtype classification, emerging disease categories, and mixed dementia with copathologies. J Nucl Med. 2022;63(Suppl 1):2S–12S. doi: 10.2967/JNUMED.121.263194. [DOI] [PubMed] [Google Scholar]
- 47.Salvadó G, Shekari M, Falcon C, et al. Brain alterations in the early Alzheimer’s continuum with amyloid-β, tau, glial and neurodegeneration CSF markers. Brain Commun. 2022;4(3). 10.1093/BRAINCOMMS/FCAC134. [DOI] [PMC free article] [PubMed]
- 48.Tubi MA, Kothapalli D, Hapenney M, et al. Regional relationships between CSF VEGF levels and Alzheimer’s disease brain biomarkers and cognition. Neurobiol Aging. 2021;105:241–251. doi: 10.1016/J.NEUROBIOLAGING.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonomi CG, Chiaravalloti A, Camedda R, et al. Functional correlates of microglial and astrocytic activity in symptomatic sporadic Alzheimer’s disease: a CSF/18F-FDG-PET study. Biomed. 2023;11(3):725. 10.3390/BIOMEDICINES11030725. [DOI] [PMC free article] [PubMed]
- 50.Thorwald MA, Silva J, Head E, Finch CE. Amyloid futures in the expanding pathology of brain aging and dementia. Alzheimer’s Dement. 2023;19(6):2605–2617. doi: 10.1002/ALZ.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiaravalloti A, Barbagallo G, Ricci M, et al. Brain metabolic correlates of CSF Tau protein in a large cohort of Alzheimer’s disease patients: A CSF and FDG PET study. Brain Res. 2018;1678:116–122. doi: 10.1016/J.BRAINRES.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 52.Tijms BM, Gobom J, Reus L, et al. Pathophysiological subtypes of Alzheimer’s disease based on cerebrospinal fluid proteomics. Brain. 2020;143(12):3776–3792. doi: 10.1093/BRAIN/AWAA325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fourier A, Portelius E, Zetterberg H, Blennow K, Quadrio I, Perret-Liaudet A. Pre-analytical and analytical factors influencing Alzheimer’s disease cerebrospinal fluid biomarker variability. Clin Chim Acta. 2015;449:9–15. doi: 10.1016/J.CCA.2015.05.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Flowcharts summarizing patients’ enrolment (A) and control group selection (B) procedures for the CSF study.
Additional file 2. Flowcharts summarizing patients’ enrolment (A) and control group selection (B) procedures for the FDG-PET substudy.
Additional file 3. Scatter Plots showing correlations (Spearman’s rho) between CSF p-tau and different amyloid biomarkers (Aβ42, Aβ40, amyR) in A-T-, CSFAβ42+/amyR-, CSFAβ42+/amyR+ and A+T+.
Additional file 4. Demographical, clinical and biomarkers data from FDG-PET substudy.
Additional file 5. Numerical results of SPM comparisons of FDG uptake in CG vs. CSFAβ42+/amyR-, CG vs. CSFAβ42+/amyR+ and CG vs. A+T+.
Data Availability Statement
The dataset analyzed in this study is available from the corresponding author upon reasonable request.