Abstract
Background
Clinical pharmacists have been shown to identify and resolve medication related problems post-discharge, however the impact on patient clinical outcomes is unclear.
Aims
To undertake a systematic review to identify, critically appraise and present the evidence on post-discharge hospital clinics that provide clinical pharmacist medication review; report the patient clinical outcomes measured; and describe the activities of the clinical pharmacist.
Methods
Published studies evaluating a patient clinical outcome following a post-discharge hospital clinic pharmacy service were included. All studies needed a comparative design (intervention vs control or comparator). Pubmed, Embase, CINAHL, PsycnINFO, Web of Science, IPA and APAIS-Health databases were searched to identify studies. The type of clinic and the clinical pharmacist activities were linked to patient clinical outcomes.
Results
Fifty-seven studies were included in the final analysis, 14 randomised controlled trials and 43 non-randomised studies. Three key clinic types were identified: post-discharge pharmacist review alone, inpatient care plus post-discharge review and post-discharge collaborative clinics. The three main outcome metrics identified were hospital readmission and/or representation, adverse events and improved disease state metrics. There was often a mix of these outcomes reported as primary and secondary outcomes. High heterogeneity of interventions and clinical pharmacist activities reported meant it was difficult to link clinical pharmacist activities with the outcomes reported.
Conclusions
A post-discharge clinic pharmacist may improve patient clinical outcomes such as hospital readmission and representation rates. Future research needs to provide a clearer description of the clinical pharmacist activities provided in both arms of comparative studies.
Keywords: Pharmacist, Pharmacy, Pharmaceutical, Medication review, Medication therapy management, Patient discharge, Hospital outpatient clinics, Ambulatory care, Medicine*, Medication*, Review*, Service*, Clinic(s)
Highlights
-
•
Medication errors and adverse drug events are common after discharge from hospital, often leading to readmission.
-
•
Clinical pharmacist activities including medication review and patient education play a key role in transitions of care.
-
•
Clinical outcomes linked to post discharge clinical pharmacist review include hospital readmission and representation rates.
-
•
Optimising medicines use post discharge and providing a comprehensive inpatient clinical service can help improve outcomes.
-
•
A multidisciplinary or collaborative clinic model appeared to be the best method to influence patient clinical outcomes.
1. Introduction
Transitions of care increase the risk of patients experiencing medication related problems (MRPs). This is in part due to patients commonly experiencing multiple medication changes during their hospital admission,1,2 and poor or inaccurate communication about these changes at discharge between healthcare providers and/or the patient.3,4 MRPs are common after discharge from hospital,5,6 which can lead to patients experiencing adverse drug events (ADEs), medication-related harm, and readmission to hospital.7, 8, 9, 10 The risk of readmission due to worsening health or medication-related harm is approximately three times higher in patients with chronic conditions, impaired renal function, previous history of an ADE and those taking multiple medications.4,11, 12, 13., 14
The rate of hospital readmissions due to MRPs ranges from 3 to 64% (median 21%), and up to 64% of these have been considered potentially preventable.15,16 Improving medication management in the post-discharge period is likely to reduce hospital readmissions; a key health service target to improve health outcomes and reduce healthcare costs.11,16, 17., 18, 19, 20 A recent randomised controlled trial (RCT) of a collaborative pharmacist-general practitioner review within 7 days of hospital discharge demonstrated a significant reduction in the rate of hospital readmissions and representations.21 Similarly, a recent meta-analysis by Tomlinson et al demonstrated telephone follow-up after discharge from hospital is associated with reduced hospital readmissions.22 This study however, did not exclusively focus on pharmacist-led interventions, the telephone follow-up services varied, and interventions did not necessarily include clinical pharmacist medication review.
A meta-analysis by Mekonnen et al, demonstrated that pharmacist-led medication reconciliation and/or medication review and/or patient education at hospital transitions of care (admission or discharge to hospital) reduced all-cause readmissions, all-cause representations, and composite ADE-related hospital readmission and representations.23 Likewise, a systematic review exploring the role of the pharmacist in reducing hospital readmissions found medication therapy management (MTM) or pharmacist-led care coordination resulted in patients being less likely to be readmitted.18 However, these two studies focused on clinical pharmacist activities provided predominantly during hospitalisation.
An area of emerging service implementation and research is post-discharge models of pharmacy care. These pharmacy services are offered within a variety of healthcare settings including community pharmacies, primary care, ambulatory care or home-based services, with varying effects on patient clinical outcomes.24, 25, 26, 27, 28 A recent approach to post-discharge clinical pharmacy models is a hospital or healthcare service located post-discharge clinic. However, in the literature there is limited consensus on the patient clinical outcomes reported and the impact of the clinical pharmacist in this post-discharge clinic based setting. In particular, there is a lack of clarity of which clinical pharmacist activities are important when implementing such services. To address this gap in the literature we conducted a systematic review to identify the outcomes reported and the clinical pharmacists’ activities described.
The aim of this systematic review is to identify, critically appraise and present the evidence on post-discharge hospital clinics that provide clinical pharmacist medication review, their reported patient clinical outcomes, and describe the clinical pharmacist activities undertaken during the review.
2. Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.29 The online systematic review platform Covidence was used to manage the screening and review of included studies.30 The protocol was registered with the international prospective register of systematic reviews (PROSPERO; CRD42018086431).31
2.1. Search strategy
The Pubmed, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsychINFO, Web of Science, International Pharmaceutical Abstracts (IPA) and Australian Public Affairs Information Service – Health (APAIS-Health) databases were searched from 01/01/1990 to 20/10/2020. Search terms and keywords were identified in discussion by the authorship team. A librarian assisted with designing the search strategy. The search strategy was designed in Pubmed using the following medical subject heading terms and text words: medication therapy management, pharmacists, pharmacy, patient discharge, outpatients, hospital outpatient clinics, ambulatory care, medicine*, medication*, review*, service*, reconcil*, follow up, clinic(s), and pharmaceutical. Synonymous terms combined with words for pharmacist, follow up, post discharge and clinics were also used (see Appendix 1 for detailed search terms). Search terms were adapted according to the capabilities of each particular database.
After removal of duplicates and screening titles, two reviewers (JC and, NC or HF) independently screened and evaluated the remaining abstracts for full-text review. Disagreement between reviewers was resolved through discussion and if required, by seeking advice from a third reviewer (MB).
2.2. Types of studies, intervention, and outcomes included
Experimental studies, both randomised and non-randomised, that reported a patient clinical outcome (such as hospital readmission or representation) as a primary or secondary outcome were eligible for inclusion in the systematic review. Study characteristics included patients who received a medication review by a clinical pharmacist, delivered in a post-discharge hospital clinic or clinic setting with access to inpatient medical records. This systematic review defines medication review as ‘a structured evaluation of patients’ medicines with the aim of optimising medicines use and improving health outcomes.32 Studies that described medication reconciliation only as the clinical pharmacist intervention were excluded. Likewise, studies that described medication review undertaken by other health care professionals such as medical officers or nurses were also excluded. Patient clinical outcomes were defined as hospital readmissions or representations, adverse events (AEs) and ADEs or disease state metrics. The term ‘clinical pharmacist’ is used to refer to pharmacist activities extending beyond the review of a single medication or the medication supply role of the pharmacist.
Included studies were those that utilised a telephone or virtual review method as well as face to face clinic appointment or a combination of these. Eligible studies included an intervention and control or comparator design, therefore descriptive studies were excluded. Studies published from 01/01/1990 when the concept of pharmaceutical care became widely described, through to 20/10/2020 were included. Only full-length original articles published in English were included. Reference lists of all included reports were reviewed for additional relevant publications. Studies involving paediatrics, cancer care and mental health were excluded.
2.3. Data extraction and synthesis
One author (JC) extracted data from included studies entering the data into Microsoft ExcelTM. The extracted data included general information (first author, year of publication); study design; patient characteristics (sample size, gender, age); method (inclusion and exclusion criteria, control or comparator or usual care, clinical pharmacist activity components, co-involved healthcare provider(s)); study outcomes; and conclusions.
The risk of bias assessment was undertaken by two reviewers (JC and, NC or HF) using the Cochrane tool for assessing risk of bias (RoB), RoB 2.0 for randomised controlled trials (RCTs),33 and the Risk Of Bias In Non-randomised Studies – of Interventions (ROBINS-I) for non-randomised studies.34 Disagreement between reviewers was resolved by seeking advice from a third reviewer (MB).
3. Results
3.1. Study selection
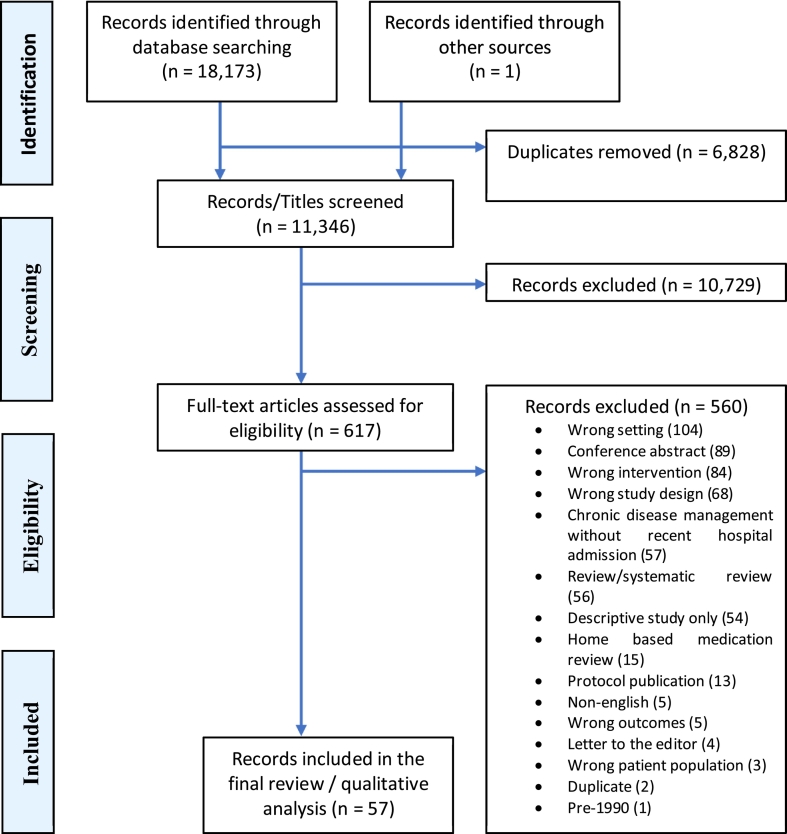
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram (Fig. 1) shows the selection process for eligible studies. A total of 18,173 were identified and after the removal of duplicates, 11,346 citations were screened and 10,729 were excluded. One article was identified through other sources and included for full-text review. Of the 617 full-text articles that were reviewed, 57 were included in the final analysis. Just over half of the studies (31/57) achieved a statistically significant improvement in at least one patient outcome,24,28,35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 most commonly readmission to hospital.
Fig. 1.
PRISMA flow diagram of screening process.
3.2. Study design and characteristics
A summary of study characteristics and outcomes is provided in Table 1a, Table 1b. The studies originated from eight different countries: one each from Brazil,39 Denmark,63 Ireland,50 Northern Ireland,51 and Vietnam64; two studies were from the United Kingdom (UK)65,66; three from China61,62,67; and the remaining 47 were from the United States (US).24,28,35, 36, 37, 38,40, 41, 42, 43, 44, 45, 46, 47, 48,52, 53, 54, 55, 56, 57, 58, 59, 60,68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89 Study sample sizes ranged from 50 to 43,711 (median = 246.5). Study characteristics were tabulated (Table 1a) to describe study inclusion criteria, clinical pharmacist activities constituting intervention and the control or comparator group, as well as outcomes or conclusions with statistical results if available (Table 1b).
Table 1a.
Study characteristics of included studies categorised by clinic type (n = 57).
| Author, Year, Country | Study design (setting) | Method (No. of follow ups) | Sample size n = Total (I) | Inclusion criteria | Control or Comparator | Intervention |
|---|---|---|---|---|---|---|
| Clinic Type: Post-discharge Clinic Pharmacist Review Only (n = 21) | ||||||
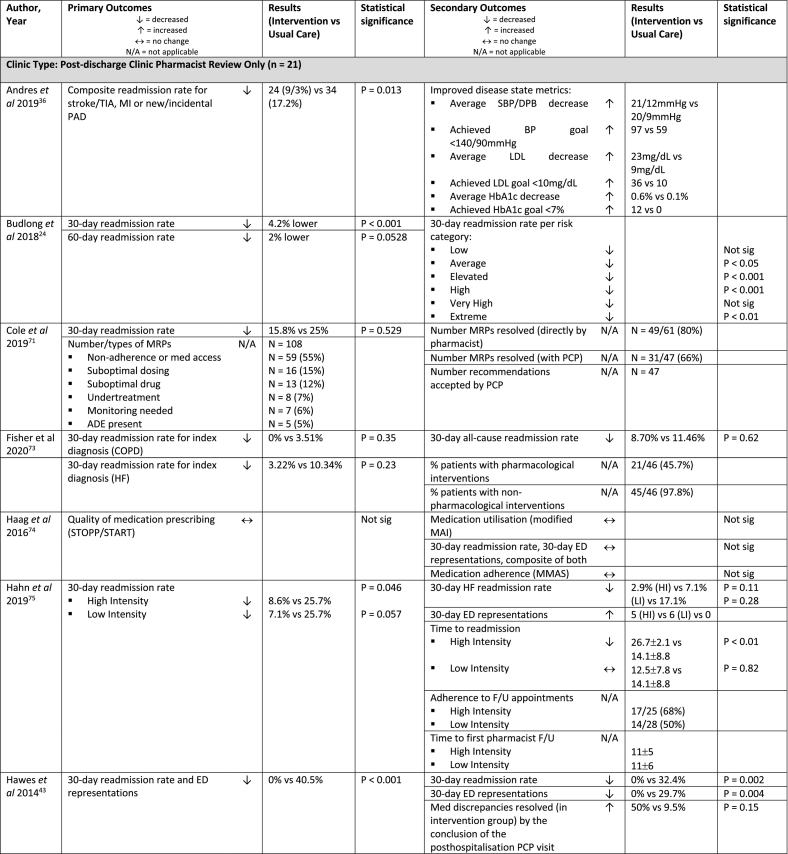
| Andres et al 2019, USA36 | Retrospective cohort study (single centre) | Clinic (2+) | n = 455 (257) | Admitted with stroke (haemorrhagic or ischaemic) or TIA | Scheduled for stroke prevention clinic but not seen or did not attend, received usual care | Pharmacist stroke prevention clinic F/U ≤30 days post-D/C then weekly to annually as needed
|
| Budlong et al 2018, USA24 | Retrospective, complexity-matched control study (multi-centre) | Clinic or phone or virtual (2) | n = 43,711 (1,291) | 18+ years | Inpatient pharmacist service (not defined), no MTM post-D/C | Pharmacist MTM program ± CMM service provided within 30-days of D/C (median 6 days)
|
| Cole et al 2019, USA71 | Prospective pilot study (single centre) | Phone (2) | n = 88 (76) | 18+ years, moderate-high risk for readmissiona, contacted by TOC nurse and referred to TOC pharmacist | Unable to be contacted or declined pharmacist F/U, received usual care | Pharmacist chart review and phone F/U ≤2 days post-D/C
|
| Fisher et al 2020, USA73 | Pre-post, prospective cohort study (multi-centre) | Clinic or phone or virtual (2) | n = 142 (46) | Primary or secondary diagnosis of HF or COPD | Pharmacist med reconciliation and education at D/C, nurse phone F/U at 2 and 7 days post-D/C | Pharmacist phone F/U ≤7 days post-D/C
|
| Haag et al 2016, USA74 | RCT (single centre) | Phone (1) | n = 50 (25) | 60+ years, independent-living elderly, enrolled at the local CTPb |
CTP program included home visit by a nurse practitioner within 3 business days of D/C (review/change meds either directly or via discussion with PCP) +/- follow-up phone calls as needed | Pharmacist MTM program preferably within 3-7 days of D/C
|
| Hahn et al 2019, USA75 | Retrospective cohort study (single centre) | Phone or clinic (1+) | n = 98 (35, High Intensity, 28 Low Intensity) | 18+ years, admission for HF exacerbation | Pharmacist discharge patient education (in hospital) | High Intensity: MTM with CPA clinic F/U within 10 days post-D/C:
|
| Hawes et al 2014, USA43 | RCT, pilot study (single centre) | Clinic (1) | n = 61 (24) | Received primary care at health care system’s outpatient family medicine centre, Year 1: meet 1 of 3 criteria: specific presenting condition, >3 hospitalisations in 5 years, 8+ scheduled meds at D/C; Year 2: 8+ scheduled meds at D/C |
Inpatient clinical pharmacist service (round with medical team daily, review and monitor meds for safety/effectiveness, make recommendations for optimisation, collaborate with medical team to create BPDML for all study patients) | Care transitions clinic visit with pharmacist ∼3 days post-D/C and prior to posthospitalisation PCP visit
|
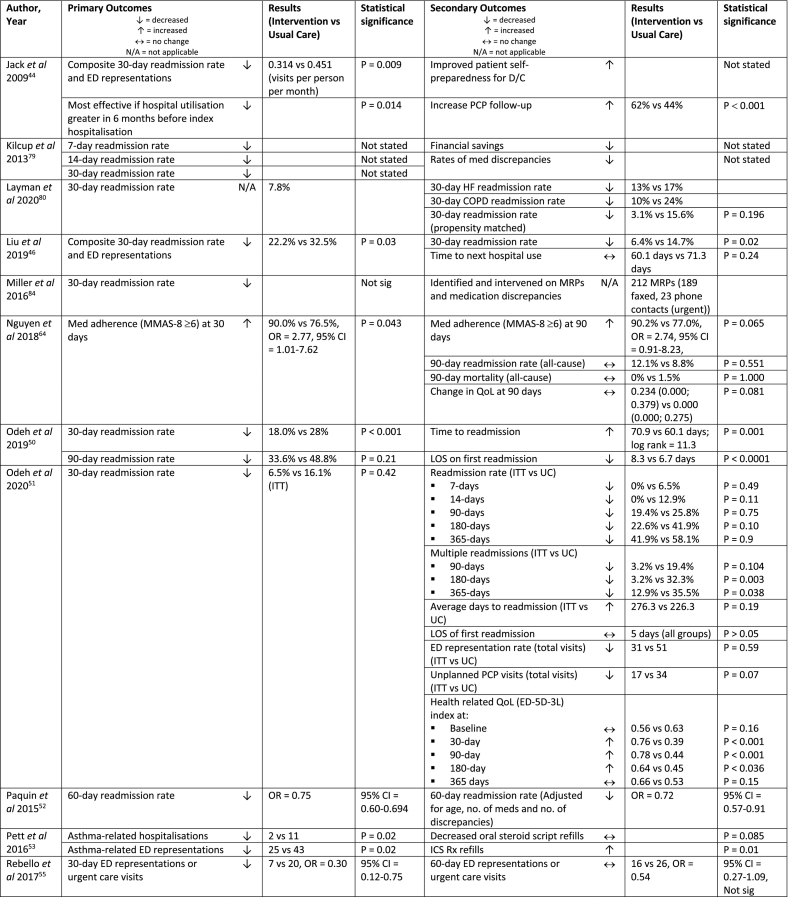
| Jack et al 2009, USA44 | RCT (single centre) | Phone (1) | n = 749 (370) | 18+ years, have a phone, speak English |
No intervention, inpatient care not defined | Nurse DA: arrange follow-up appointments, confirm med reconciliation, patient education with individualised instruction booklet that was sent to PCP; Clinical pharmacist: phoned patients 2-4 days post-D/C
|
| Kilcup et al 2013, USA79 | Ad-hoc, non-randomised, retrospective comparison, observational cohort study (multi-centre) | Phone (1) | n = 494 (243) | High-risk for readmissionc | Care management liaison nurse phoned 1-2 days post-D/C, received usual care | Usual care + pharmacist phone follow-up 3-7 days post-D/C
|
| Layman et al 2020, USA80 | Retrospective review, and retrospective propensity-matched observational analysis (single centre) | Clinic (1) | n = NS (114), n = 61 (32) | Admission diagnosis of HF or COPD exacerbation, required urgent F/U for insulin titration, BP management or lab monitoring, seen in ED and have no assigned PCP | No pharmacist post-D/C F/U, received usual care | CPS clinic F/U within 14 days post-D/C
|
| Liu et al 2019, USA46 | Retrospective chart analysis (single centre) | Phone (1) | n = 833 (166) | All patients D/C home from ED, observation unit or inpatient unit | Phone call not attempted or unable to be reached by phone for any reason, received usual care | Pharmacy student or pharmacist phone follow-up 2-14 days post-D/C
|
| Miller et al 2016, USA84 | Retrospective, segmented time-series, chart analysis (single centre) | Phone (2) | n = NS (314) | 4+ maintenance medications on D/C, discharged home |
Not specified | Pharmacy technician and pharmacist contacted patient by phone within 3 days of D/C
|
| Nguyen et al 2018, Vietnam64 | RCT (single centre) | clinic (2) | n = 166 (79) | Discharge diagnosis of unstable angina or MI | Outpatient F/U every 2-4 weeks to assess health and disease progress and issue prescriptions, received usual care | Pharmacist clinic F/U within 7 days post-D/C
|
| Odeh et al 2019, Ireland50 | Pragmatic, prospective, quasi-experimental study (single centre) | Phone (3) | n = 422 (211) | 18+ years, polypharmacy (≥10 meds) for chronic illness | Not specified | Pharmacist phone F/U within 10 days, at 30 days and 90 days post-D/C
|
| Odeh et al 2020, Northern Ireland51 | RCT (single centre) | Clinic + clinic/phone (2) | n = 62 (31) | 18+ years, admitted to a study ward for an acute/unscheduled medical admission and met at least 1 high-risk criteriad | Standard post-D/C care with no hospital-based pharmacist F/U | Pharmacist clinic F/U within 14 days post-D/C
|
| Paquin et al 2015, USA52 | Retrospective, non-randomised quality improvement initiative secondary data analysis (single centre) | Phone (1) | n = 501 | 65+ years, delirium riske or prescribed a dementia medication | Not specified | Pharmacological Intervention in Late Life (PILL) service, pharmacist telephone follow-up within 5 days of D/C
|
| Pett et al 2016, USA53 | Retrospective, pre-post chart review (single centre) | Clinic (1-6; average = 3.7) | n = 61 | Paediatric and adult Native American, referred by medical provider, diagnosis of asthma, ≥ 1 pharmacy asthma clinic visit in previous 12 months |
Not specified | Pharmacist-provided asthma education and medication management in ambulatory care clinic following an asthma-related hospitalisation or ED visit
|
| Rebello et al 2017, USA55 | Retrospective, non-randomised secondary data analysis, quality improvement initiative, matched controls (multi-centre) | Phone (1+) | n = 200 (100) | 65+ years, in need of medication management supportf, discharged home |
Not specified | Pharmacist phone follow-up 7 days post-D/C
|
| Shaya et al 2015, USA87 | Non-randomised, historical controls, proof of concept study (single centre) | Clinic (4) | n = 101 (28) | 18+ years, attended endocrinology practice clinic, English speaking, T1DM or T2DM, recent transition of care experience (hospitalisation, ED/urgent care/paramedic/acute care visit) |
Returned to endocrinology clinic following urgent or emergent care, minimum 1 HbA1c lab value post-urgent episode, minimum 6 months follow-up data accessible via EMR, received usual care | Pharmacist provided 6 month, 4-visit process (post-D/C, then follow-up at 1 month, 3 months and 6 months)
|
| Westberg et al 2014, USA89 | Prospective, group matched-controlled study (single centre) | Clinic (1) | n = 405 (135) | 65+ years, diagnoses identified as high risk for readmissiong, no previous hospital MTM program; additionally: PCP affiliated with the hospital |
Standard medical care with no CMM, received usual care | Pharmacist CMM visit within 14 days post-D/C and prior to post-hospitalisation PCP visit
|
| Yang et al 2017, UK66 | Case-cohort, pre-post study (multi-centre) | Phone (2) | n = 1,970 (62) | 18+ years, home-dwelling, discharged from ED or general medicine wards, access to a working phone, English-speaking or lives with someone who speaks English |
No phone follow-up, received usual care | Pharmacist phone follow-up within 14 days of D/C at 2-7 days (∼15mins) and after 10 days (∼8 mins) post-D/C
|
| Clinic type: Inpatient Clinical Pharmacist Service with Post-discharge Clinic Pharmacist Follow-up (n = 23) | ||||||
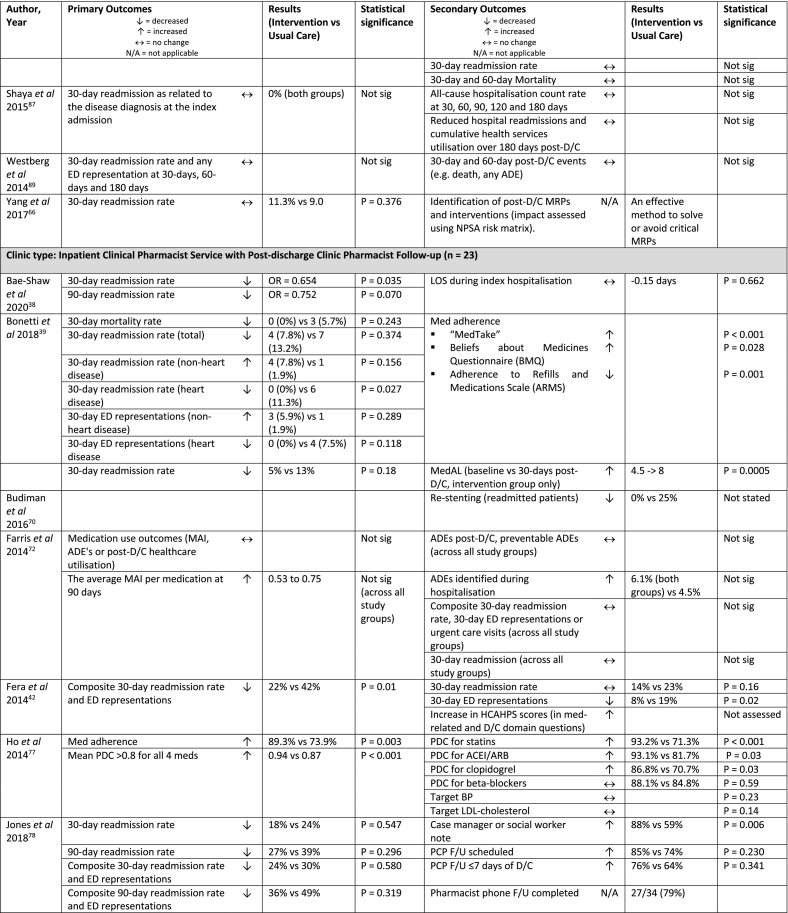
| Bae-Shaw et al 2020, USA38 | Retrospective, pre-post, cohort study with difference-in-difference (DID) approach (single centre) | Phone (1) | n = 4,745 (1,776) | 18+ years, admitted with primary diagnosis of HF, MI, pneumonia or COPD | Not specified | Pharmacist phone F/U to address MRPs Pharmacist inpatient service:
|
| Bonetti et al 2018, Brazil39 | RCT (single centre) | Phone (2) | n = 133 (66) | 18+ years, admitted to cardiology ward with primary diagnosis of stable angina, ACS, HF, valvular disease, arrythmias or HTN | Inpatient med review and recommendations to cardiologist | Pharmacist phone F/U 3 days and 15 days post-D/C to reinforce patient education Pharmacist inpatient service
|
| Budiman et al 2016, USA70 | Non-randomised, historical control study (single centre) | Phone (2) | n = 135 (40) | 18+ years, presenting condition: STEMI who received stents |
No Pharmacist transition of care support, received usual care | Pharmacist phone call 2-3 days and 30-days post-D/C
|
| Farris et al 2014, USA72 | RCT (single centre) | Phone (1) | n = 945 (314 Enhanced/ 315 Minimal) |
18+ years, English or Spanish speaking, cardiovascular-related conditionsh and/or asthma or COPD |
Med reconciliation at admission (according to hospital policy), nurse D/C patient education, med list; D/C summary transcribed and received by mail | Pharmacist Case Manager provided 2 interventions; “Minimal” group:
|
| Fera et al 2014, USA42 | Case study (single centre) | Phone (1) + clinic or home visit as needed | n = 134 (66) | Target diseases: COPD and HF, as well as CTP consultation for polypharmacy | Patients not reached by phone post-D/C, received usual care | Primary Care Resource Centre Pharmacist phone follow-up within 3 days of D/C Pharmacist inpatient service
|
| Ho et al 2014, USA77 | RCT, block randomisation (multi-centre) | Clinic or phone (2) | n = 253 (129) | Presenting complaint: ACS (MI or unstable angina) | Standard hospital D/C instructions (e.g. numbers to call, follow-up appointments, diet and exercise advice), med list, and educational information about cardiac meds | Pharmacist-led med reconciliation and tailoring within 7-10 days of D/C
|
| Jones et al 2018, USA78 | Prospective, case-matched control pilot study (single centre) | Phone (1) | n = 68 (34) | 18+ years, discharged home from a pilot unit, identified as high risk for readmission by integrated electronic health record risk score | D/C from non-pilot unit, received usual care | Pharmacist post-D/C phone F/U ≤3 days of D/C Pharmacist inpatient service
|
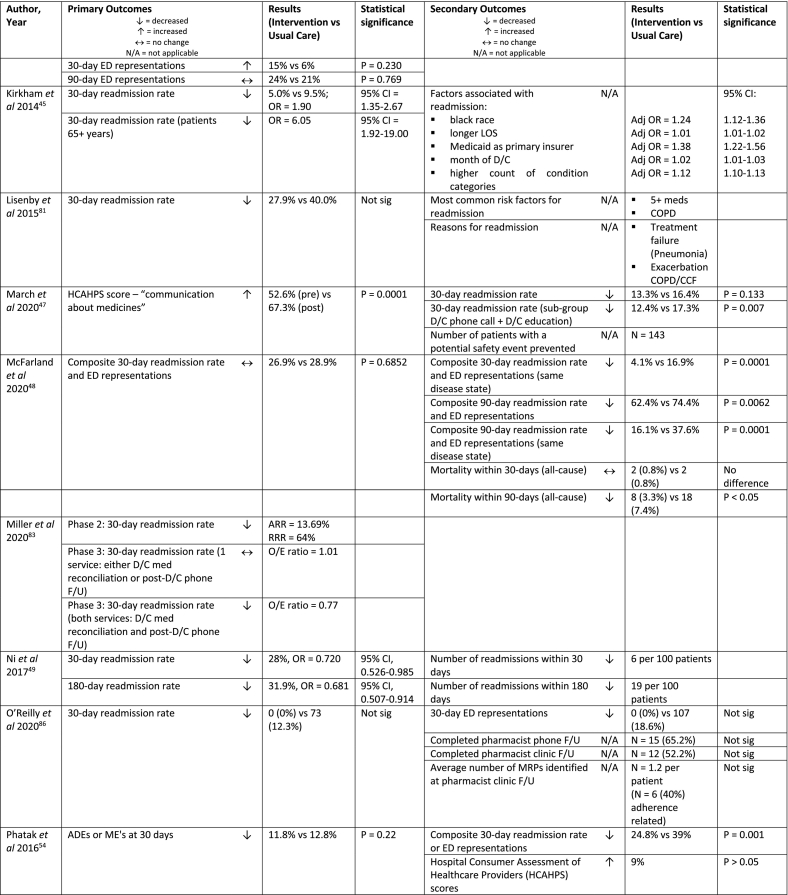
| Kirkham et al 2014, USA45 | Retrospective cohort study (multi-centre) | Phone (1) | n = 19,659 (692) | All patients discharged home | Daily rounds in hospital ward by outpatient pharmacist | Pharmacist phone follow-up 2-3 days post-D/C (opt-in) CTP
|
| Lisenby et al 2015, USA81 | Pilot study, historical control (single centre) | Phone (1) | n = 108 (43) | 19+ years, diagnosis: pneumonia and any of the following: admission within 6 months, 5+ scheduled medications, COPD or HF |
Nurse provided standard education +/- med reconciliation, antibiotic treatment by physician and home health follow-up phone call | Pharmacist post-D/C phone call within 2-4 days of D/C (for HF and Pneumonia)
|
| March et al 2020, USA47 | Retrospective review and pre-post study (single centre) | Phone (1) | n = 1,728 (414) | Patients admitted to general medicine/surgery, cardiology or neurology units with ≥5 meds and/or anticoagulant/ antiplatelet/ insulin/ sulfonylurea and cardiovascular-related condition and/or DM-related condition and/or prior hospital admission within 30 days | No pharmacist input OR D/C education only OR post-D/C phone follow-up only | Post-D/C pharmacist phone follow-up
|
| McFarland et al 2020, USA48 | Quasi-experimental, matched interrupted time series study (multi-centre) | Clinic (1+) | n = 484 (242) | Primary or secondary diagnosis of diabetes, hypertension, COPD or HF), seen in clinic or reached by phone by the patient aligned care team or cardiology clinical pharmacy specialist after discharge | MDT round (provide medication recommendations, education, med reconciliation on D/C) |
CMM pharmacist post-D/C clinic ≤10 days of D/C (instead of physician or NP)
|
| Miller et al 2020, USA83 | Pilot study (multi-centre) | Phone (2) | Phase 2: n = 5,871 (3,711, phase 2); Phase 3: n = NS (9,676 1 service/ 3,881 both) |
Medicare beneficiaries 65+ years, discharged home with HF, COPD, MI, DM or pneumonia (phase 2); Medicare beneficiaries 65+ years (phase 3) | No pharmacist D/C med reconciliation, inpatient case management personnel provide a brief phone call to patients 48 hours post-D/C, patient eligible but not enrolled in the program | Pharmacist phone follow-up 7 days and 21 days post-D/C
|
| Ni et al 2017, USA49 | Non-randomised, matched control study (intervention single centre, control multi-centre) | Phone/clinic (on request) | n = 1,227 (558) | High-risk patientsi, 5+ medications, admitted within last 45 days, Medicaid managed care members from study hospital (intervention group) or neighbouring hospital (control group) | Retrospective matched control, received usual care | Pharmacist ambulatory care transitions of care services (over the 30 days post-D/C)
|
| O’Reilly et al 2020, USA86 | Retrospective pilot study (single centre) | Phone + clinic (2) | n = 574 (23) | 18+ years, admitted to internal medicine team, primary or secondary diagnosis of HF or COPD | Pharmacy technician admission med reconciliation and pharmacist inpatient service (pharmacist audit of med reconciliation, participation in MDT rounds, D/C med list based on physician med reconciliation) |
Post-D/C pharmacist phone F/U ≤3 days of D/C
|
| Phatak et al 2016, USA54 | RCT (single centre) | Phone (3) | n = 278 (137) | Discharged home, on >3 scheduled medications or 1+ high-risk medicationj, willing to participate in minimum 1 post-D/C phone call or experienced an ED visit or readmission within 30-days of D/C |
Pharmacist med reconciliation (from physician's patient history) and med education from physician or nursing staff at D/C; 1 phone call at 30-days by pharmacist to assess study endpoints (ADEs, MEs) | Pharmacist post-D/C phone calls at 3, 14 and 30 days post-D/C
|
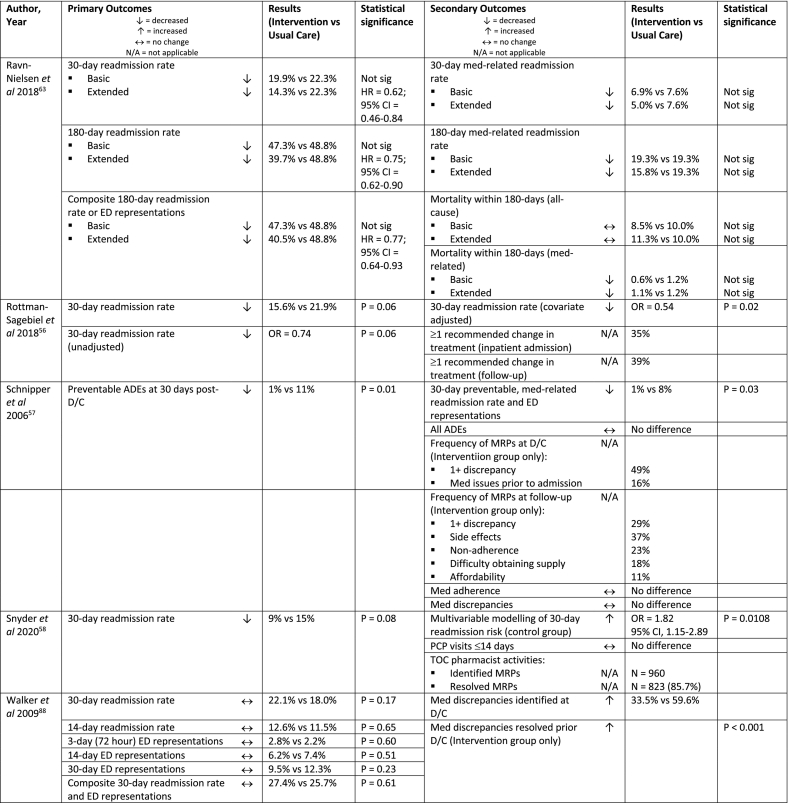
| Ravn-Nielsen et al 2018, Denmark63 | RCT (multi-centre) | Phone (2) | n = 1,498 (498 Basic/497 Extended) | 18+ years, polypharmacy (≥5 prescribed medications daily), Danish speaking, new acute admission | Standard care (i.e. no inpatient medication review, discharge education or follow-up by a clinical pharmacist) | “Extended” intervention group pharmacist post-D/C phone call 7 days and 6 months post-D/C Pharmacist inpatient service
|
| Rottman-Sagebiel et al 2018, USA56 | Prospective, case-matched comparison study (single centre) | Phone (1) | n = 1,577 (388) | ≥70 years and ≥12 meds or ≥65 years and dementia or ≥65 years and meds meeting Beers criteria or ≥65 years and ≥2 hospital admissions within 1 year or ≥65 years and ≥3 ED visits within 1 year | Not specified | Pharmacist phone follow-up post-D/C within 2-3 days of D/C
|
| Schnipper et al 2006, USA57 | RCT (single centre) | Phone (1) | n = 178 (92) | Patients admitted to 1 of 4 teams on general medicine service, discharged home and could be contacted 30 days after D/C, English speaking, cared for by BWH PCP or internal medicine resident, patient or carer provided informed consent |
Routine review of med orders by a ward-based pharmacist and med education by a nurse at D/C | Pharmacist phone follow-up 3-5 days post-D/C Pharmacist inpatient service
|
| Snyder et al 2020, USA58 | Retrospective cohort study (single centre) | Phone (1) | n = 871 (379) | Medicare insurance beneficiaries, discharged home or to an assisted living facility following inpatient or observational stay in the general medical/surgical or intensive care unit, required primary care follow-up, PCP participated in the study | Not specified | Pharmacist phone F/U with patient/carer ≤2 days post-D/C
|
| Walker et al 2009, USA88 | Quasi-experimental design, prospective (single centre) | Phone (2) | n = 724 (358) | 18+ years, discharged home, high-risk for MRPsk |
Interdisciplinary D/C round (attending physician, social worker and D/C coordinator nurse). Nurse provided: D/C instructions and med information, med list, med education and phone follow-up within 3 days to identify, triage and resolve post-D/C problems. |
Pharmacist phone follow-up post-D/C at 3 days and 30-days Pharmacist inpatient service
|
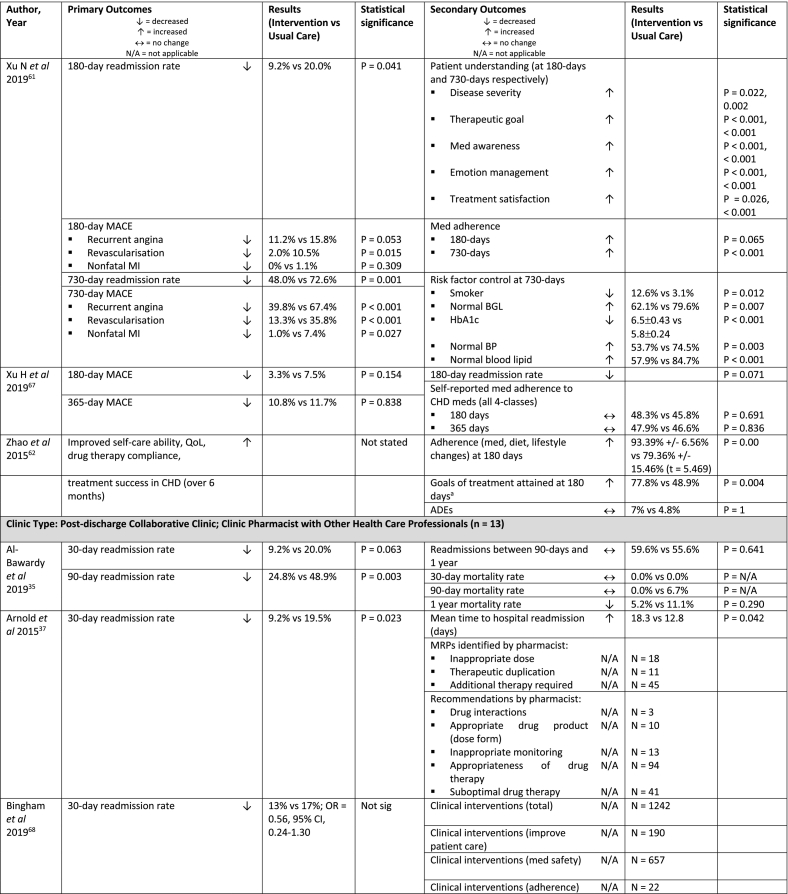
| Xu N et al 2019, China61 | RCT (single centre) | Clinic (24) | n = 193 (98) | 45-75 years, underwent PCI for CHD, able to read and understand the test questionnaire | No pharmacist intervention, received usual care | Pharmacist post-D/C adherence assessment (monthly)
|
| Xu H et al 2019, China67 | RCT (single centre) | Phone (3) | n = 240 (120) | Primary diagnosis of STEMI, NSTEMI or unstable angina with ≥50% occlusion of 1+ major coronary arteries | No pharmacist intervention, dispensing pharmacist care. | Pharmacist post-D/C review with patient at 7 days, 1 and 3 months
|
| Zhao et al 2015, China62 | RCT (single centre) | Phone (6) | n = 85 (43) | 18+ years, diagnosis of CHD by their physician 4+ drugs for heart conditions (e.g. antiplatelet, beta-blockers, ACEI, statin) |
Conventional clinical care without pharmacist support | Pharmacist phone follow-up monthly for 6 months Pharmacist inpatient service
|
| Clinic Type: Post-discharge Collaborative Clinic; Clinic Pharmacist with Other Health Care Professionals (n = 13) | ||||||
| Al-Bawardy et al 2019, USA35 | Prospective, non-randomised study (single centre) | Clinic (1) | n = 154 (109) | Primary D/C diagnosis of HF | Failed to attend clinic appointment, received usual care | Pharmacist post-D/C clinic 7-10 days post-D/C
|
| Arnold et al 2015, USA37 | Prospective study (single centre) | Clinic (1) | n = 236 (98) | >50 years, >5 meds | Hospital follow-up visit by physician alone, received usual care | Post-D/C physician clinic and pharmacist clinic
|
| Bingham et al 2019, USA68 | Retrospective study (single centre) | Phone (2) | n = 456 (340) | 18+ years, Primary D/C diagnosis of asthma or pneumonia or DM or HF or COPD or MI or THR/TKR or CKD or CABG, D/C home or to a N/H |
Phone F/U by TOC nurse 1-3 days post-D/C; opted-out of TOC program or unable to be reached by TOC pharmacist, received usual care | TOC nurse contacted patient or care team within 3 days of D/C to enrol in program and provides care coordination with HP team. TOC pharmacist provided phone F/U ≤7 days post-D/C and 21 days post-D/C
|
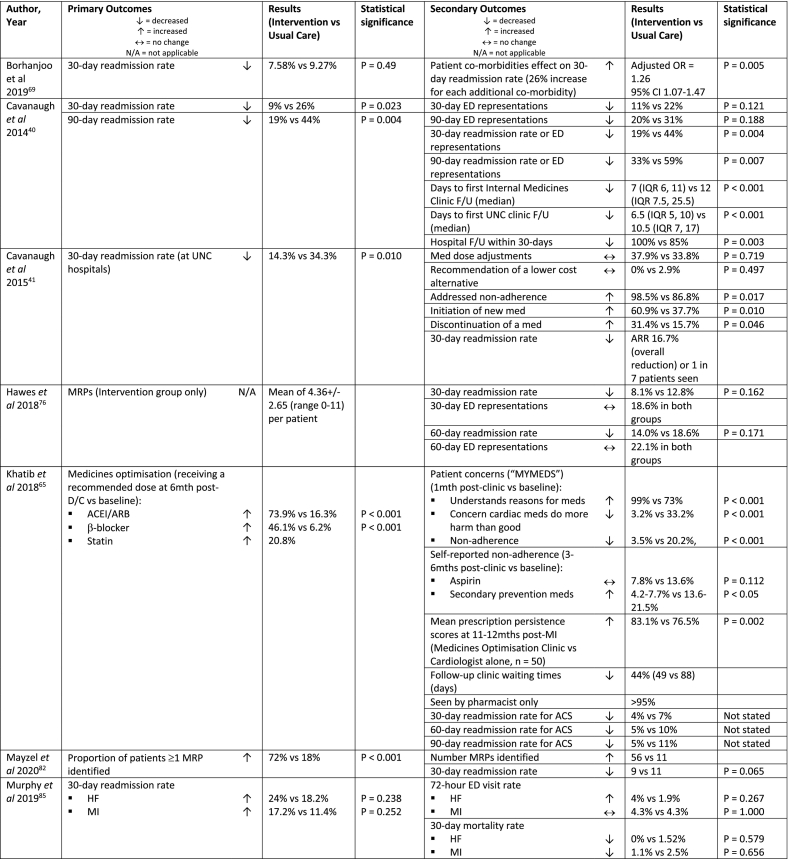
| Borhanjoo et al 2019, USA69 | Retrospective study (single centre) | Clinic (1) | n = 573 (422) | Patients at high risk of re-admissionl, primary D/C diagnosis HF or MI or pneumonia or DM | Post-D/C physician or nurse practitioner clinic F/U only, received usual care | Post-D/C physician or nurse practitioner clinic F/U and pharmacist clinic F/U
|
| Cavanaugh et al 2014, USA40 | Retrospective cohort study (single centre) | Clinic (1) | n = 108 (54) | Patient with established PCP in University of North Carolina (UNC) Internal Medicine Centre | Not referred to the hospital follow-up clinic post-D/C, received usual care | Care manager scheduled appointments and addressed barriers to care (e.g. transportation, obtaining medicines). Attending physician and CPP clinic appointment within 5 days of D/C. Pharmacist CPP service
|
| Cavanaugh et al 2015, USA41 | Retrospective observational sub-analysis study (single centre) | Clinic (1) | n = 140 (70) | Patient enrolled in University of North Carolina (UNC) Internal Medicine follow-up program | Medical resident and attending physician follow-up clinic appointment within 7-days of D/C, received usual care; care manager scheduled appointments and addressed barriers to care (e.g. transportation, obtaining medicines). | Attending physician and CPP clinic appointment within 7 days of D/C Pharmacist CPP service
|
| Hawes et al 2018, USA76 | Retrospective cohort study (single centre) | Clinic (1) | n = 172 (86) | 18+ years, D/C to community dwelling, established primary care with FMC provider and attended hospital follow-up visit within 30 days of D/C |
PCP only visit, received usual care | PCP/CPP hospital follow-up visit within 30 days of D/C (average = 9.5 ± 7.3 days) with pharmacist-enhanced care
|
| Khatib et al 2018, UK65 | Retrospective pre-post study (single centre) | Clinic (1) | n = NS (270) | Admitted to cardiology with MI, attended post-MI medicines optimisation MDT clinic | Cardiologist only follow-up, received usual care | All patients completed “MYMEDS” questionnaire prior to clinic appointment Standard medicines optimisation clinic: consultant cardiology pharmacist and/or cardiologist review
|
| Mayzel et al 2020, USA82 | Pre-post retrospective-prospective study (single centre) | Clinic (1) | n = 100 (50) | Patients discharged from any Cleveland Clinic Foundation Hospital and seen at Hillcrest’s Family Medical/Internal Medicine Clinic | Phone F/U 2 days post-D/C from health practitioner (med reconciliation, address med concerns, confirm med changes, assess adherence, patient education); PCP clinic F/U 7-14 days post-D/C with medical assistant or nurse med reconciliation immediately prior | Usual care and pharmacist clinic F/U within 7-14 days of D/C immediately prior to PCP clinic F/U
|
| Murphy et al 2019, USA85 | Prospective, pre-post study (single centre) | Phone + clinic (2+) | n = 610: HF = 359, MI = 251 (193: HF = 100, MI = 93) | 18+ years, primary admission diagnosis of HF or MI with planned follow-up with a cardiologist | Not specified | Nurse practitioner phone F/U 2-3 days post- D/C, pharmacist phone F/U 4-7 days post-D/C, cardiologist F/U ≤7 days post-D/C, additional MTM F/U ≤28 days post-D/C if needed, dietitian F/U 21 days post-D/C, cardiac rehab session 21 days post-D/C
|
| Thurston et al 2019, USA59 | Retrospective pre-post study (single centre) | Phone (2) | n = 362 (211) | Primary admission diagnosis of HF and at high risk for 30-day readmission based on risk assessment software program | No pharmacist med reconciliation or patient D/C education; readmission risk assessment, patient access to a scale at home, education class on CV disease, HF education pack, nurse post-D/C phone F/U 3 days post-D/C | Usual care and pharmacist inpatient service and pharmacist post-D/C phone F/U 14 and 30 days post-D/C Pharmacist post-D/C service
|
| Trang et al 2015, USA28 | Prospective intervention, retrospective control study (single centre) | Phone + clinic (2) | n = 161 (74) | High-risk patients (minimum 1 criteria: 4+ medications for chronic diseases, oral anticoagulation, COPD, HF, DM, HIV, MI, Pneumonia), discharged from hospital or ED with PCPs at the PCMH | Informal registered nurse phone F/U post-D/C to schedule an appt with PCP, received usual care | Pharmacist Advancement of Transitions of Care to Home (PATCH) service with pharmacist phone follow-up ≤ 2 business days post-D/C and pharmacist clinic within 7-14 days post-D/C Pharmacist phone F/U
|
| Wiegmann et al 2020, USA60 | Retrospective cohort study (single centre) | Clinic (3) | n = 100 (50) | 18+ years, seen by a physician in FMC within 14 days of D/C | Inpatient pharmacist service (clinical recommendations, D/C med rec and patient education) and physician post-D/C follow-up including physical exam, lab test monitoring and med rec | Pharmacist post-D/C clinic and PCP follow-up (ideally on the same day)
|
Legend: ACEI = Angiotensin Converting Enzyme Inhibitor; ACS = Acute Coronary Syndrome; ADE = Adverse Drug Event (or Averse Drug Reaction); BPDML = Best Possible Discharge Medication List; BPMH = Best Possible Medication History; BWH: Brigham and Women’s Hospital; CABG = Coronary Artery Bypass Graft; CHD = Coronary Heart Disease; CKD = Chronic Kidney Disease; CMM = Comprehensive Medication Management; CMR = Comprehensive Medication Review; COPD = Chronic Obstructive Pulmonary Disease; CPA = Collaborative Practice Agreement; CPP = Clinical Pharmacist Practitioner; CPS = Clinical Pharmacy Specialists; CTP = Care Transitions Program; CV = Cardiovascular; DA = Discharge Advocate; DID = Difference-in-difference; DM = Diabetes Mellitus; D/C = Discharge; ED = Emergency Department; EMR = Electronic Medical Record; FMC = Family Medicine Centre; F/U = Follow-up; HF = Heart Failure; HIV = Human Immunodeficiency Virus; HS = Health Service; HTN = Hypertension; I = intervention; MDT = Multi-disciplinary Team; ME = Medication Error; med = medication; MedAL = Medication Adherence and Literacy; MI = Myocardial Infarction; MRP = Medication Related Problem (or Drug Therapy Problem or Drug Related Problem); MTM = Medication Therapy Management; No. = number; NSTEMI = Non-ST Elevated Myocardial Infarction; N/H = Nursing Home; PATCH = Pharmacist Advancement of Transitions of Care to Home; PCI = Percutaneous Intervention; PCMH = Patient-centred Medical Home; PCP = Primary Care Provider; PILL = Pharmaceutical Intervention in Late Life; PIM = Potentially Inappropriate Medication; RCT = Randomised Controlled Trial; STEMI = ST Elevated Myocardial Infarction; THR = Total Hip Replacement; TKR = Total Knee Replacement; TIA = Transient Ischaemic Stroke; TOC = Transition of Care; T1DM = Type 1 Diabetes Mellitus; T2DM = Type 2 Diabetes Mellitus; UK = United Kingdom; UNC = University of North Carolina; USA = United States of America.
Re-admission risk score: low ≤39, moderate = 40-59, high ≥60.
CTP eligibility based on Elders Risk Assessment (ERA) index ≥ 16.
High-risk criteria: current admission = readmission, complex care plans, primary diagnosis of chronic disease, medication changes during hospitalisation, concerns of ability to self-manage.
High-risk criteria: ≥4 regular medications, ≥3 medication changes, concerns of ability to self-manage, ≥1 high-risk medication, ≥2 emergency hospital admissions in prior 6 months.
Delirium risk: cognitive impairment, sensory impairment or dehydration.
Medication management support: polypharmacy, cognitive impairment, CCF and age 75+ years.
High-risk for readmission: CCF, dysrhythmias, genitourinary conditions, IHD and digestive disorders.
Cardiovascular-related conditions: hypertension, hyperlipidaemia, CCF, CAD, MI, Stroke, TIA, receiving oral anticoagulation.
High-risk patients: hospitalisation history, prescription medication utilisation, social history.
High-risk medication: anticoagulant, antiplatelet, hypoglycaemic agents, immunosuppressants, anti-infectives.
High-risk for medication-related problems: 5+ medications, 1 or more high-risk medication, medication requiring monitoring, 2+ medication changes, problems managing medications, dementia or confusion.
Set of specific criteria with high likelihood of readmission.
Table 1b.
Study outcomes of included studies categorised by clinic type (n = 57).
Legend: ACEI = Angiotensin Converting Enzyme Inhibitor; ACS = Acute Coronary Syndrome; ADE = Adverse Drug Event (or Averse Drug Reaction); ARB = Angiotensin Receptor Blocker; BGL = blood glucose level; BP = blood pressure; CHD = Coronary Heart Disease; CI = Confidence Interval; COPD = Chronic Obstructive Pulmonary Disease; DBP = diastolic blood pressure; D/C = Discharge; ED = Emergency Department; F/U = follow-up; HbA1c = Glycated Haemoglobin; HCAHPS = Hospital Consumer Assessment of Healthcare Providers and Systems; HF = Heart Failure; ICS = Inhaled Corticosteroid; ITT = Intention to Treat; LDL = Low-density Lipoprotein; LOS = Length of Stay; MACE = Major Adverse Cardiac Events; MAI = Medication Appropriateness Index; ME = Medication Error; med = medication; MedAL = Medication Adherence and Literacy; MI = Myocardial Infarction; MMAS = Morisky Medication Adherence Scale; MRP = Medication Related Problem (or Drug Therapy Problem or Drug Related Problem); NNT = Number Needed to Treat; Not sig = Not Significant; NPSA = National Patient Safety Agency; OR = Odds Ratio; PAD = Peripheral Artery Disease; PCP = Primary Care Provider; PDC = Proportion of Days Covered; QoL = Quality of Life; RRR = Relative Risk Reduction; SBP = Systolic Blood Pressure; START = Screening Tool to Alert Doctors to the Right Treatment; STOPP = Screening Tool of Older Persons Prescriptions; TIA = Transient Ischaemic Attack; TOC = Transitions of Care; UNC = University of North Carolina.
a Goals of treatment at 6 months: blood pressure, rates of diabetes, dyslipidaemia, average heart rate, body mass index.
3.3. Design
The 57 studies comprised of 14 RCTs,39,43,44,51,54,57,61, 62, 63, 64,67,72,74,77 and 43 non-randomised studies (Table 1a).24,28,35, 36, 37, 38,40, 41, 42,45, 46, 47, 48, 49, 50,52,53,55,56,58, 59, 60,65,66,68, 69, 70, 71,73,75,76,78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89 The non-randomised studies consisted of 11 retrospective pre-post or segmented time-series studies,38,47,53,59,65,66,70,73,81,82,84 9 retrospective cohort,36,40,41,45,58,60,75,76,87 6 prospective observational,35,37,71,78,83,86 6 quasi-experimental prospective,28,48,51,56,83,88 6 retrospective non-randomised,46,52,68,69,79,86 4 retrospective studies with matched controls,24,49,55,80 and 1 case study.42
3.3.1. Clinic type
Study interventions were classified into three clinic types (Table 1a, Table 1b): post-discharge clinic pharmacist review only (21/57),24,36,43,44,46,50, 51, 52, 53,55,64,66,71,73, 74, 75,79,80,84,87,89 inpatient clinical pharmacist service with post-discharge clinic pharmacist follow-up (23/57),38,39,42,45,47, 48, 49,54,56, 57, 58,61, 62, 63,67,70,72,77,78,81,83,86,88 and post-discharge collaborative clinic (a clinic pharmacist with other health care professionals) (13/57).28,35,37,40,41,59,60,65,68,69,76,82,85
3.3.2. Delivery method
Delivery of the clinical pharmacist medication review service varied and included: 29 by phone or predominantly by phone,38,39,44, 45, 46, 47,50,52,54, 55, 56, 57, 58, 59,62,63,66,67,69, 70, 71, 72,74,78,79,81,83,84,88 18 face-to-face in a clinic setting,35, 36, 37,40,41,43,48,53,60,61,64,65,69,76,80,82,87,89 4 by phone and face-to-face clinic visit,28,42,85,86 3 face-to-face clinic or by phone,49,75,77 2 face-to-face clinic, by phone or virtually,24 and 1 face-to-face clinic visit and phone.51
3.3.3. Patient follow up
A single post-discharge follow-up visit was provided in 30 studies,35,37,38,40, 41, 42, 43, 44, 45, 46, 47, 48, 49,52,55, 56, 57, 58,65,69,72,74, 75, 76,78, 79, 80, 81, 82,89 two post-discharge follow-ups in 19 studies,24,28,36,39,51,59,63,64,66,68,70,71,73,77,83, 84, 85, 86,88 and three or more post-discharge follow-up contacts per patient in 8 studies.50,53,54,60, 61, 62,67,87 Time to first contact by the clinical pharmacist after discharge varied from within 7 days in 31 studies,28,39, 40, 41, 42, 43, 44, 45,52,54, 55, 56, 57, 58,63,64,67,68,70, 71, 72, 73, 74,78,79,81,83, 84, 85, 86,88 within 14 days in 12,35,46,48,50,51,59,66,75,77,80,82,89 within 30 days in 7,24,36,49,61,62,76,87 and 7 studies did not provide time to follow-up information.37,38,47,53,60,65,69 Earlier post-discharge follow-up by a clinical pharmacist did not seem to impact the rate of achieving a statistically significant improvement in at least one patient clinical outcome (15,28,39, 40, 41, 42, 43, 44, 45,52,54, 55, 56, 57, 58,63 7,35,46,48,50,51,59,75 4,24,36,49,61 6,37,38,47,53,60,65 respectively).
3.3.4. Clinical Pharmacist activity
The clinical pharmacist activities in the control or comparator groups were either not specified or defined, or described as ‘no pharmacist support (or services)’ or ‘did not attend’ in 46/57 studies (Table 1a).24,28,35, 36, 37, 38,40, 41, 42,44,46,47,49, 50, 51, 52, 53,55,56,58,59,61, 62, 63, 64, 65, 66., 67, 68, 69, 70, 71,73,74,77, 78, 79, 80, 81,83, 84, 85,87, 88, 89, 90 The clinical pharmacist activities in the intervention groups for the post-discharge pharmacist follow-up were not clearly defined in 9/57 studies, all of which were a combined inpatient clinical pharmacist service with post-discharge clinic pharmacist follow-up intervention.38,42,45,49,57,63,72,78,88 The studies which implemented a post-discharge clinic pharmacist review only or collaborative clinic type provided more detail of the post-discharge clinical pharmacist activities.
3.4. Patient clinical outcomes
The most commonly reported outcome (primary or secondary) was 30-day hospital readmissions and/or representations (n = 45/57).24,28,35,37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51,54, 55, 56,58, 59, 60,63,66,68, 69, 70, 71, 72, 73, 74, 75, 76,78, 79, 80, 81, 82, 83, 84,86,88,89 A summary of readmission and representation measures is provided in Table 2.
Table 2.
Summary of readmission and representation outcomes (n = 45).
| Outcome measured | Number of studies |
|---|---|
| 30-day readmission rate only37,39,41,45,47,56,58,59,66,68, 69, 70, 71,73,80, 81, 82, 83, 84,88 | 20 |
| Composite 30-day readmission rate and 30-day representation rate28,44,48,54,89 | 5 |
| 30-day readmission rate and 30-day representation rate and composite of both40,42,43,74,78 | 5 |
| 30-day readmission rate and 30-day representation rate55,75,86 | 3 |
| 30-day readmission rate and composite of both 30-day readmission rate and 30-day representation rate46,72 | 2 |
| 30-day and 60-day readmission rate24,76 | 2 |
| 30-day, 60-day and 90-day readmission rate60 | 1 |
| 30-day and 90-day readmission rate35,37,50 | 3 |
| 30-day and 180-day readmission rate49,63 | 2 |
| 7-day, 14-day and 30-day readmission rate79 | 1 |
| 7-day, 14-day, 30-day, 90-day, 180-day and 365-day readmission rate51 | 1 |
The remaining 12/57 studies that did not report 30-day readmission and/or representation rates reported the following patient outcomes: readmission rate for a specific disease state (n = 4),36,65,85,87 60-day readmission rate (n = 1),52 90-day readmission rate (n = 1),64 180-day readmission rate (n = 1),61 730-day readmission rate (n = 1),61 asthma-related hospitalisation count (n = 1),53 preventable 30-day medication-related readmissions and/or representations (n = 1),57 major adverse cardiac events (n = 2),61,67 ADEs (n = 2),62 or disease state metrics (n = 3).36,62,77
3.4.1. Hospital readmissions and/or representations
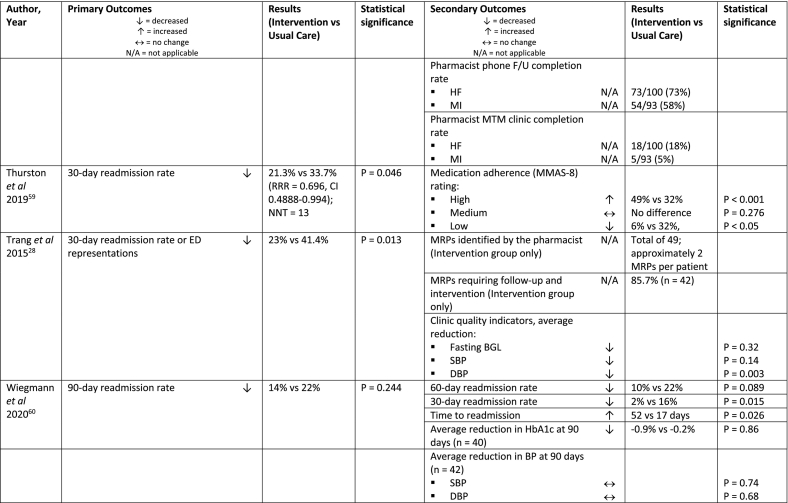
Of the 40 studies that measured 30-day readmission rate, 15 reported a significant reduction in this outcome.24,37,38,40, 41, 42, 43,45,46,49,50,59,60,63,75 An additional 3 studies that didn’t achieve a significant reduction in 30-day readmissions, reported significant changes with secondary sub-group,47 or covariate analysis,56,58 and two studies had significant results in a sub-group analysis for 30-day readmission rate for heart disease or same disease state.39,48
A measure of composite 30-day hospital readmission and representation was significantly reduced in 7/12 studies reporting this outcome,28,40,42, 43, 44,46,54 and a significant reduction in 30-day representation rate was seen in 3/9 studies.42,43,55
One study showed a significant reduction in preventable 30-day medication-related readmissions and/or representations.57 While another study achieved a significant reduction in readmissions at 30-days and 180-days post-discharge for the ‘extended intervention’ arm which included a discharge and post-discharge component, compared to the ‘basic intervention’ arm which was medication reconciliation at admission only.63
Four studies reported on 60-day readmissions,24,52,60,76 two of which were significant24,52; and 8 studies reported on 90-day readmissions,35,38,40,50,51,60,64,78 2 of which were significant.35 Of the 4 studies that reported hospital readmissions up to 180-days,49,51,61,63 three demonstrated a significant reduction.51,61,63 Two studies extended the readmission follow-up to 365 days, of which neither achieved a significant result for this outcome.35,51 Finally, a pre-post study by Pett et al demonstrated a significant reduction in asthma-related hospitalisation count over 12 months.53
3.4.2. Adverse events (AEs) and adverse drug events (ADEs)
The AEs reported in studies included mortality at 30-days,35,39,48,55,85 90-days,35,48,64 180-days, 63 and 365-days.35 A significant reduction in mortality was only seen in one study measured at 90-days post-discharge.48 One study reported a combined measure of mortality and any ADE at 30-days and 60-days which was non-significant.89
Two studies which examined a patient population who underwent stenting for myocardial infarction reported a reduction in re-stenting at 30-days (non-significant),70 and at 180-days or 730-days (significant).61 Major adverse cardiac events which included recurrent angina, re-stenting and nonfatal myocardial infarction were reported in 2 studies, with a significant reduction demonstrated at 180-days and 730-days post discharge,61 and a non-significant reduction demonstrated at 180-days and 365-days in the second study.67
Five studies reported all ADEs as a patient clinical outcome, none of which resulted in a significant decrease in ADEs.54,57,62,72,89 Two of these studies also measured preventable ADEs,57,72 with 1 study reporting a significant reduction in preventable ADEs.57 Other measures reported included potentially prevented AEs,47 3-day representations (non-significant),85,88 and unplanned Primary Care Physician (PCP) visits over 365-days post-discharge (significant).51
3.4.3. Disease state metrics
Six studies reported improved disease state metrics which included blood pressure, cholesterol levels, blood sugar level or glycated haemoglobin (HbA1c) and attainment of treatment goals.28,36,60, 61, 62,77 Significant results were achieved for reduction in diastolic blood pressure at 30-days,28 attainment of treatment goals at 180-days,62 and improvement in all measures for risk of coronary heart disease (specifically: smoking, blood sugar levels or HbA1c, blood pressure and cholesterol) at 730-days post-discharge.61
3.5. Clinical pharmacist activities and outcome effects
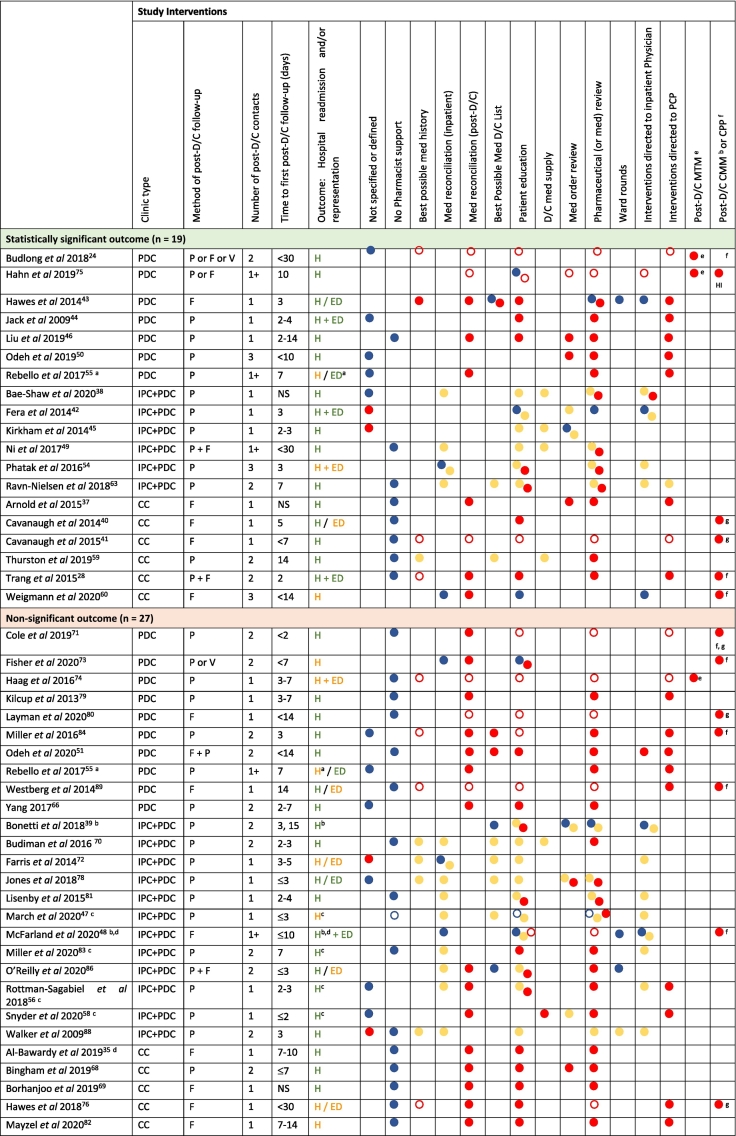
The clinical pharmacist activities are described in Table 3 for the 45 studies that reported 30-day readmissions and/or representations as an outcome. One study by Rebello et al, is represented twice as it reported a significant reduction in representations (primary outcome), but a non-significant reduction in readmissions (secondary outcome).55
Table 3.
Clinical pharmacist activities associated with 30-day hospital readmissions and representations (unless specified), categorised by clinic type.
Legend: D/C: discharge; med: medication; NS: Not stated; PCP: primary care physician; Clinic type: PDC: Post-discharge clinic; IPC+PDC: Inpatient clinical pharmacy service; CC: Collaborative post-discharge clinic. Method of follow-up: P: Phone; F: Clinic (face to face); V: Virtual. Outcome: H: 30-day hospital readmission; ED: 30-day representation; H + ED composite 30-day readmissions/representations only; Green = primary outcome, Orange = secondary outcome.
Intervention delivered:  Usual care;
Usual care;  Usual care (single intervention component only);
Usual care (single intervention component only);  Inpatient clinical pharmacy service;
Inpatient clinical pharmacy service;  Post-discharge follow-up;
Post-discharge follow-up;  Incorporated into MTM/CMM/CPP.
Incorporated into MTM/CMM/CPP.
aStatistically significant for ED/urgent care visit, not significant for 30-day readmission.
bNot statistically significant for 30-day readmission rate (all-cause), statistically significant for 30-day readmission rate (heart disease or same disease state).
cNot statistically significant for 30-day readmission rate (all-cause), statistically significant for 30-day readmission rate (sub-group analysis).
dNot statistically significant for 30-day readmission rate (all-cause), statistically significant for 90-day readmission rate.
eMTM: MTM services include the performing of a comprehensive pharmaceutical care review by ensuring all medication therapies, over the counter and herbal products are safe and effective, providing patient or carer education to optimise medication use, as well as liaising with a patients’ PCP or other health care providers to optimise medication therapy.91,92
fCMM: is an expansion on MTM and ensures that patients’ medications are appropriate, effective, safe and provides ongoing monitoring and review. CMM incorporates the development of a patient-centred care plan that assesses a patients’ clinical state and requires collaboration among members of the health care team and is continually updated as needed.93
gCPP: Clinical Pharmacist Practitioner is a licensed pharmacist advanced practice provider, who may prescribe medication therapy and order appropriate monitoring tests in accordance with an agreed protocol under the supervision of a physician.94
HIHigh Intensity.
Clinical pharmacist activities described in studies were classified into the following: no pharmacist support, best possible medication history, medication reconciliation (inpatient or post-discharge), best possible medication discharge list, patient education, discharge medication supply, medication order review, ward rounds, interventions directed to inpatient physician, interventions directed to the primary care provider (PCP), post-discharge MTM, post-discharge comprehensive medication management (CMM) or clinical pharmacist practitioner (CPP). Studies that did not define any clinical pharmacist activity were classified as ‘not specified or defined’.
Studies that did not achieve a significant reduction in hospital readmissions and/or representations provided little detail regarding clinical pharmacist activities in the usual care arm (Table 3). With a total of 21/27 studies providing no information or ‘no pharmacist support’ as the clinical pharmacist activity description,35,51,55,58,63,66,68, 69, 70, 71,74,76,78, 79, 80, 81, 82, 83, 84,88,89 compared to 13/19 of studies which achieved a significant result.24,28,37,38,40,41,44,46,49,50,55,56,59
In the 19 studies reporting a statistically significant improvement in 30-day readmissions and/or representations, 6/19 provided a full post-discharge MTM or CMM/CPP service, which is equivalent to six clinical pharmacist activities (Table 3, Table 4).24,28,40,41,60,75 For the remaining studies, 11/19 of the studies that were not MTM or CMM/CPP the number of clinical pharmacist activities ranged from 6 (n = 1) to 1 (n = 1),37,38,43,44,46,49,50,54, 55, 56,59 of which 4/11 provided four or more clinical pharmacist activities,37,43,46,56 and 2/19 of the studies did not specify the components of pharmacist post-discharge follow-up (Table 3, Table 4).42,45
Table 4.
Summary of clinical pharmacist activities provided by the post-discharge clinical pharmacist in studies reporting 30-day hospital readmission and/or representations (n = 45).
| Clinical pharmacist activity | Significant (n = 19)a |
Not significant (n = 27)a |
||
|---|---|---|---|---|
| Frequency | % | Frequency | % | |
| Best possible medication history | 7 | 37 | 8 | 30 |
| Medication reconciliation (post-discharge) | 10 | 53 | 18 | 67 |
| Best possible medication discharge listb | 1 | 5 | 2 | 7 |
| Patient educationb | 10 | 53 | 19 | 70 |
| Discharge medication supply | 0 | 0 | 1 | 4 |
| Medication order review | 8 | 42 | 9 | 33 |
| Pharmaceutical (or medication) review | 16 | 84 | 24 | 89 |
| Interventions directed to inpatient physician | 2 | 11 | 1 | 4 |
| Interventions directed to primary care provider | 12 | 63 | 13 | 48 |
Rebello et al is represented as a study with both a significant (ED/urgent care visit) and not significant (30-day readmissions) outcome.
Provided as part of the post-discharge clinic pharmacist follow-up.
In the 27 studies reporting no significant difference in 30-day readmissions and/or representations, 8/27 of the studies provided MTM or CMM/CPP services (Table 3, Table 4).48,71,73,74,76,80,83,89 The remaining 17/27 of the studies reported clinical pharmacist activities ranging from six (n = 1) to one (n = 2),35,39,47,51,55,58,63,66,68, 69, 70,78,79,81, 82, 83,86 with 4/17 providing four or more clinical pharmacist activities,51,58,68,82 and 2/27 of the studies did not specify the components of clinical pharmacist activities.72,88
The frequency of post-discharge clinical pharmacist activities reported in the intervention arm in studies achieving a significant improvement in 30-day hospital readmissions and/or representations compared to those that were not significant are summarised in Table 4. Studies that reported the intervention as MTM, CMM or CPP were assumed to have included best possible medication history, medication reconciliation (post-discharge), patient education, medication order review, pharmaceutical review and interventions directed to the PCP as clinical pharmacist activities as per the American College of Clinical Pharmacy (ACCP) guidelines.91,93,94
In the 18 inpatient clinical pharmacist service with post-discharge clinic pharmacist follow-up studies, 15 provided three or less clinical pharmacist activities in the post-discharge setting.38,39,42,45,47,49,54,63,70,72,78,81,83,86,88 Thirteen of these studies provided pharmaceutical review,38,47, 48, 49,54,56,58,63,70,78,81,83,86 and 3 provided post-discharge medication reconciliation with the pharmaceutical review.48,58,86 This compared to the 16 post-discharge clinic pharmacist review only and 11 collaborative clinic studies which provided three or less clinical pharmacist activities in the post-discharge setting in 5,44,50,55,66,79 and 3 of the studies respectively.35,59,69 However, all studies in these two groups provided pharmaceutical review as part of their study intervention,24,28,35,37,40,41,43,44,46,50,51,55,59,60,66,68,69,71,73, 74, 75, 76,79,80,82,84,89 and 14 of the post-discharge clinic studies,24,43,46,51,55,66,71,73, 74, 75,79,80,84,89 and 10 of the collaborative clinic studies,28,35,37,40,41,60,68,69,76,82 provided medication reconciliation with the pharmaceutical review.
3.6. Risk of bias
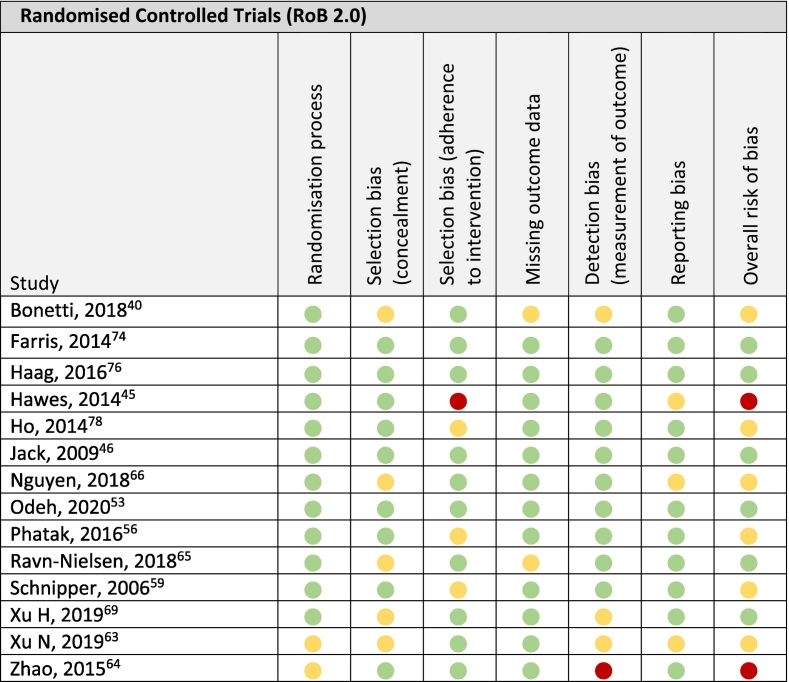
Of the 14 RCTs,39,43,44,51,54,57,61, 62, 63, 64,67,72,74,77 6 studies scored low in all six risk of bias domains.44,51,63,67,72,74 Six studies exhibited ‘some concerns’ overall due to randomisation,61,62 selection bias with regard to concealment,39,61,63,64,67 adherence to the intervention,54,57,77 missing outcome data,39,63 detection bias,39,61,67 and reporting bias.61,64 And ‘high risk of bias’ was found in 2 studies due to selection bias with regard to adherence to intervention and reporting bias,43 as well as detection bias and randomisation.62
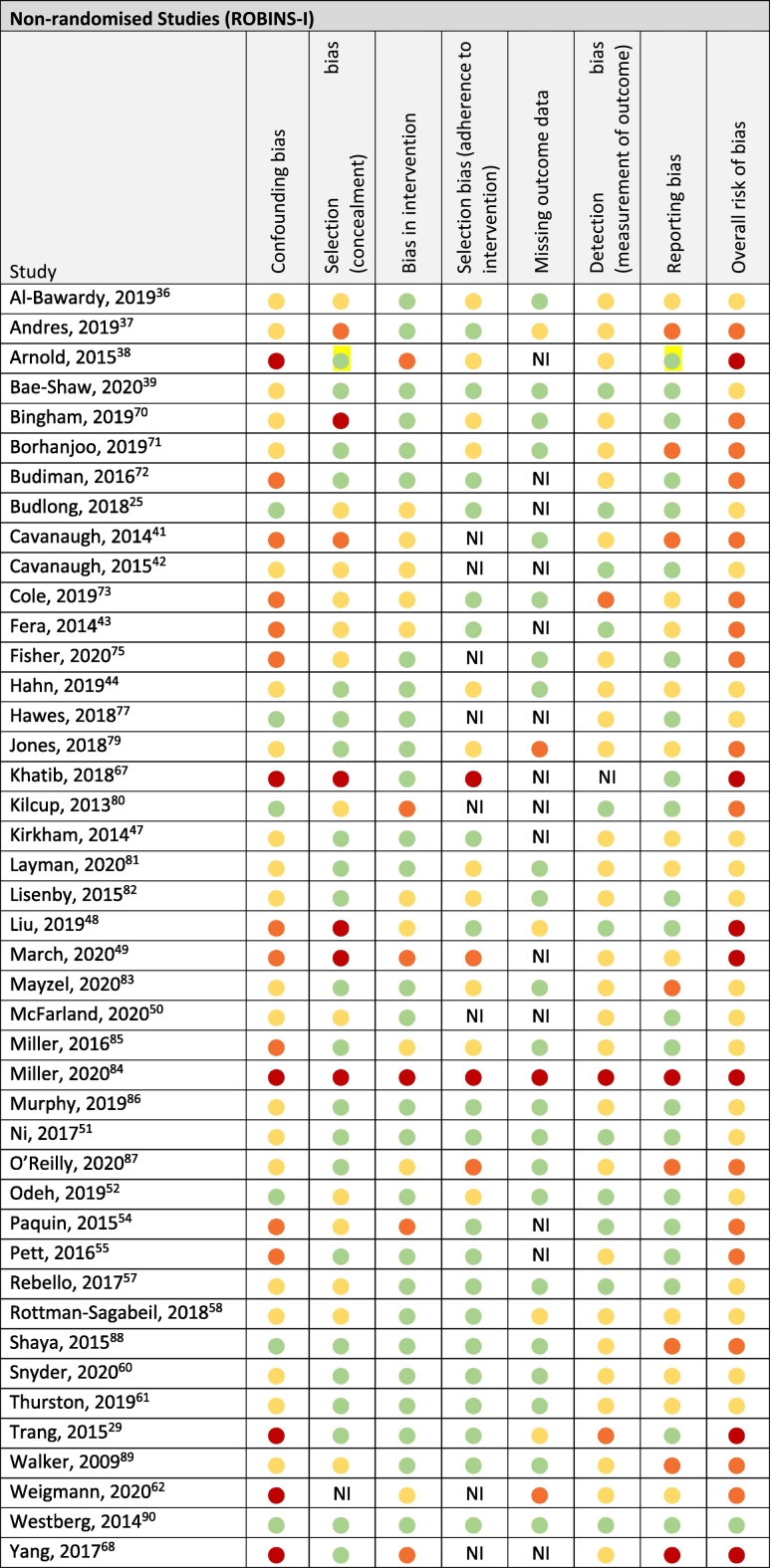
Of the 43 non-randomised studies only 1 study by Westberg et al scored low risk of bias in all seven domains.89 Nineteen studies exhibited moderate risk of bias,24,35,38,41,45,48, 49, 50,55,57, 58, 59,75,76,80, 81, 82,84,85 16 studies were scored as serious risk of bias,36,40,42,52,53,60,68, 69, 70, 71,73,78,79,86, 87, 88 and critical risk of bias was found for 7 studies.28,37,46,47,65,66,83
A complete summary of the risk of bias assessment outcomes utilising the RoB 2.0 tool for RCTs,33 and the ROBINS-I tool for non-randomised studies,34 is provided in Table 5a, Table 5b respectively.
Table 5a.
Risk of bias of randomised controlled trials (n = 14).
 Low risk of bias,
Low risk of bias,  Some concerns,
Some concerns,  High risk of bias.
High risk of bias.
Table 5b.
Risk of bias of non-randomised studies (n = 43).
 Low risk of bias,
Low risk of bias,  Moderate risk of bias,
Moderate risk of bias,  Serious risk of bias,
Serious risk of bias,  Critical risk of bias, NI = No Information.
Critical risk of bias, NI = No Information.
4. Discussion
This systematic review identified 57 studies that evaluated at least one patient clinical outcome using an intervention-control design from the addition of a hospital-based post-discharge clinical pharmacist medication review. Three key clinic types were identified and the most frequently reported outcome was 30-day hospital readmissions and/or representations. There was a mix of clinical pharmacist activities described across the studies in both the usual care and intervention groups. These activities were not always clearly defined by the study investigators and terms such as ‘medication reconciliation’ or ‘medication review’ may have also included undertaking a ‘best possible medication history’ or ‘identification and resolution of MRPs’ without explicitly stating so.
4.1. Patient clinical outcomes
This systematic review included primary and secondary outcomes in the inclusion criteria to thoroughly assess all patient clinical outcomes measured. The three main outcome metrics, hospital readmissions and/or representations, AEs and ADEs as well as disease state metrics were reported as a mixture of primary and secondary outcomes.
4.1.1. Hospital readmissions and/or representations
Hospital readmissions were the most commonly reported outcome and likely reflects that this is considered a key indicator for the quality of healthcare internationally,17,95,96 and affects how health systems are funded. Measurement of medication-related hospital readmissions is not commonly reported in studies, most likely due to the difficulty in assessing this outcome. An Australian study of patients aged 50 years and older reported 34% of patients experienced a MRP within 4 months of discharge from hospital, and 9% reported being readmitted to hospital due to a MRP.97 Only 1 study included in this systematic review assessed preventable medication-related readmissions, demonstrating a significant reduction in this outcome with pharmacist intervention.57
Health professional collaboration appears a good indicator of success with studies utilising a collaborative post-discharge clinic model of care more likely to report a significant reduction in 30-day readmissions and representations. These findings are similar to previous studies that have shown that pharmacists working with other health professionals or in multidisciplinary teams were more likely to achieve positive patient outcomes.18,98 Perhaps the pharmacist taking responsibility for the implementation of peer-agreed recommendations to optimise medicines in a collaborative environment to enhance a patient’s pharmaceutical care is a key approach to influencing patient outcomes.
4.1.2. Adverse events (AEs) and adverse drug events (ADEs)
Pharmacist interventions have been shown to reduce medication discrepancies,23,99,100 or improve the quality of medication prescribing.26,101,102 A survey of patients recently discharged from hospital revealed 9% of patients were readmitted due to a MRP.97 This is within the range of medication-related readmissions of 3-64% reported in a systematic review by El Morabet et al, of which between 5-87% were deemed potentially preventable.15 It is proposed that pharmacists are the key health professional likely to influence the prevention of MRPs,98,101 and thus any on-flow of adverse effects. However, this has not necessarily translated into a reduction in clinical outcomes such as preventing ADEs,99,102 or reducing hospital admissions or ED visits.102 These two reviews both focussed on pharmacist activities in primary or community care on preventing ADEs,99,102 which is quite different to this systematic review of hospital-based post-discharge clinical pharmacist care.
The study by Schnipper et al assessed preventable ADEs at 30-days post-discharge in an RCT design, and demonstrated a significant reduction in preventable ADEs as well as preventable medication-related readmissions and representations.57 The study design included the implementation of pharmacist-led medication reconciliation, medication review, patient education and phone follow-up after discharge compared to routine review of inpatient medication orders only.57 The observed decrease in ADEs at 30-days suggests that pharmacist input throughout transitions of care is needed to impact on medication-related patient outcomes. However, the authors reported that the measurement of these specific medication related outcomes is more labour intensive than using standard patient outcomes such as all-cause 30-day hospital readmissions, and hence, is not as commonly reported in the literature.
4.1.3. Disease state metrics
This study did not find a link between improved readmission rates and significant improvements in disease state metrics, 28,36,60 or improved adherence and improved disease state metrics.61,62,77 The two studies that reported significant improvements in reported disease state metrics by Zhao et al and Xu et al, describe a personalised monthly interaction with patients for 6 or 24 months.61,62 This suggests that ongoing education that may impact on health-related behaviours such as smoking cessation, diet and exercise, compared to improved medication taking behaviour alone may be responsible for these improved patient outcomes.
4.2. Clinical pharmacist activities
There have been several systematic reviews with or without meta-analysis, exploring the effect of pharmacist-led medication reconciliation at transitions of care both in hospital and in the community post-discharge on preventing medication errors or improving patient outcomes with predominantly favourable effects supporting pharmacist-led care.18,20,23,100 In this systematic review, the results suggest that the detail in reporting of clinical pharmacist activities varied considerably between studies. This made it difficult to draw any conclusions on the most effective clinical pharmacist activity or combination of activities required to improve patient outcomes. Other reviews highlight the high heterogeneity between studies makes it difficult to assess the impact of a single clinical pharmacist activity on patient clinical outcomes, such as medication reconciliation, or medication review.18,20,23,98,100,101,103 This is mainly due to the variability in use of different terms and lack of definition of these activities in the study methods.
Reviews in the literature have proposed several components for an optimal transition of care process, of which clinical pharmacists would ideally play a key role throughout a patient’s hospital stay as well as in the post-discharge period.19,98,104 The components include post-discharge pharmacist follow-up, and comprehensive post-discharge clinical pharmacist medication review combined with in-hospital clinical pharmacist interventions.19,98 Our results suggest that a comprehensive inpatient clinical pharmacy service, incorporating pharmacist-led medication reconciliation, medication review and patient education, with additional post-discharge clinical pharmacist follow-up, ideally in a multidisciplinary or collaborative care environment is needed to impact patient clinical outcomes.
However, recent systematic reviews to determine the most effective pharmacy intervention to influence patient outcomes,105 and evaluate hospital readmissions,18 could not identify a preferred pharmacist-led intervention that was most effective at improving patient outcomes. Like these studies, this review was unable to determine if a post-discharge medication review by a clinical pharmacist in a hospital-based clinic could be solely attributable to improving patient outcomes, as these studies often included some form of inpatient clinical pharmacist activity either as part of their usual care or the overall intervention studied. It may be that an individualised patient approach is required to influence patient outcomes.105
Improved reporting of interventions using standardised methods such as the Template for Intervention Description and Replication (TIDieR) checklist may enhance the ability to compare outcome measures of services and interventions between studies,106 and future research should ideally use this methodology. Providing post-discharge medication review by a clinical pharmacist in a hospital-based clinic to improve patient outcomes requires further exploration. And studies should provide a clear description and evaluation of the clinical pharmacist activities provided.
4.3. Strengths and limitations of the study
This study utilised broad search criteria with the aim to identify as many studies as possible that assessed patient clinical outcomes associated with a post-discharge medication review provided by a clinical pharmacist in a hospital-based clinic. This systematic review excluded studies that provided a home assessment, and studies based in community pharmacies or primary care. This enabled the focus to be on studies in which the clinical pharmacist is located within the hospital, and therefore has access to the inpatient medical records and hospital health professionals. Potentially, patients unable to attend a hospital-based clinic appointment were excluded from these studies, which may be a group at higher risk of admission or readmission to hospital due to reasons such as socioeconomic factors. This review included studies published in English only, which may have excluded significant outcomes from published research in other languages.
This systematic review excluded studies examining surrogate markers for outcomes such as identification and resolution of medication discrepancies or MRPs, or improvement in medication adherence to focus on patient clinical outcomes likely to impact on healthcare services. This may have resulted in an included study being underpowered for the patient clinical outcome reported as it may not have been a primary outcome.
A clinic pharmacist interacts with many health professionals including inpatient pharmacists, nurses or physicians. This systematic review tried to capture all studies with a post-discharge clinical pharmacist service in a hospital-based clinic incorporating some element of medication review. However, it may have contributed to the difficulty in determining the most effective activity impacting on the patient outcomes measured.
5. Conclusion
A post-discharge clinical pharmacist medication review in a hospital-based clinic appears to improve patient clinical outcomes, in particular hospital readmissions and/or representations. The most beneficial clinical pharmacist activities in a post-discharge clinic remain unclear due to the lack of clarity around the comprehensiveness of the services provided. Evidence suggests including clinical pharmacist services in the post-discharge period, particularly utilising a collaborative approach may best influence patient clinical outcomes.
Funding statement
There was no external funding support for the undertaking of this systematic review.
Declaration of Competing Interest
I declare there are no conflicts of interest to disclose for the primary author of this review.
Acknowledgements
Christine Dalais, Librarian, University of Queensland Library.
Contributor Information
Jaclyn Costello, Email: j.costello@uq.edu.au, jaclyn.costello@health.qld.gov.au.
Michael Barras, Email: m.barras@uq.edu.au, michael.barras@health.qld.gov.au.
Holly Foot, Email: h.ross1@uq.edu.au.
Neil Cottrell, Email: n.cottrell@uq.edu.au.
Appendix A. Appendices
Appendix 1.
Search strategy Pubmed.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
((((((("Medication Therapy Management"[Mesh]) OR ((medicine*[Title/Abstract] OR medication*[Title/Abstract])))) AND (((review*[Title/Abstract] OR service*[Title/Abstract] OR reconcil*[Title/Abstract] OR followup[tiab] OR "follow up"[tiab] OR clinic[tiab] OR clinics[tiab])))) AND ((((pharmacist*[Title/Abstract] OR pharmacy[Title/Abstract] OR pharmaceutical[Title/Abstract]))) OR (("Pharmacists"[Mesh]) OR "Pharmacy"[Mesh]))) AND ((((outpatient[Title/Abstract] OR outpatients[Title/Abstract] OR "Patient Discharge"[Mesh] OR discharge[Title/Abstract] OR postdischarge[Title/Abstract] OR "post discharge"[Title/Abstract] OR "hospital discharge"[Title/Abstract] OR ambulatory[Title/Abstract]))) OR ("Outpatients"[Mesh] OR "Outpatient Clinics, Hospital"[Mesh] OR "Ambulatory Care"[Mesh])))).
References
- 1.Blozik E., Signorell A., Reich O. How does hospitalization affect continuity of drug therapy: an exploratory study. Ther Clin Risk Manag. 2016;12:1277–1283. doi: 10.2147/TCRM.S109214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stowasser D.A., Collins D.M., Stowasser M. A randomised controlled trial of medication liaison services-patient outcomes. J Pharm Pract Res. 2002;32(2):133–140. [Google Scholar]
- 3.Becker C., Zumbrunn S., Beck K., et al. Interventions to improve communication at hospital discharge and rates of readmission: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(8) doi: 10.1001/jamanetworkopen.2021.19346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roughead E.E., Semple S.J., Rosenfeld E. The extent of medication errors and adverse drug reactions throughout the patient journey in acute care in Australia. Int J Evid Based Healthc. 2016;14(3):113–122. doi: 10.1097/XEB.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 5.Coleman E.A., Smith J.D., Raha D., Min S.J. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165(16):1842–1847. doi: 10.1001/archinte.165.16.1842. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Caballos M., Ramos-Diaz F., Jimenez-Moleon J.J., Bueno-Cavanillas A. Drug-related problems in older people after hospital discharge and interventions to reduce them. Age Ageing. 2010;39(4):430–438. doi: 10.1093/ageing/afq045. [DOI] [PubMed] [Google Scholar]
- 7.Chan M., Nicklason F., Vial J.H. Adverse drug events as a cause of hospital admission in the elderly. Intern Med J. 2001;31(4):199–205. doi: 10.1046/j.1445-5994.2001.00044.x. [DOI] [PubMed] [Google Scholar]
- 8.Paradissis C., Cottrell N., Coombes I., Scott I., Wang W., Barras M. Patient harm from cardiovascular medications. Ther Adv Drug Saf. 2021;12:1–22. doi: 10.1177/20420986211027451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parekh N., Ali K., Stevenson J.M., et al. Incidence and cost of medication harm in older adults following hospital discharge: a multicentre prospective study in the UK. Br J Clin Pharmacol. 2018;84(8):1789–1797. doi: 10.1111/bcp.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsilimingras D., Schnipper J., Duke A., et al. Post-discharge adverse events among urban and rural patients of an urban community hospital: a prospective Cohort study. J Gen Intern Med. 2015;30(8):1164–1171. doi: 10.1007/s11606-015-3260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mudge A.M., Kasper K., Clair A., et al. Recurrent readmissions in medical patients: a prospective study. J Hosp Med. 2011;6(2):61–67. doi: 10.1002/jhm.811. [DOI] [PubMed] [Google Scholar]
- 12.Parameswaran Nair N., Chalmers L., Bereznicki B.J., et al. Adverse drug reaction-related hospitalizations in elderly Australians: a prospective cross-sectional study in two Tasmanian Hospitals. Drug Saf. 2017;40(7):597–606. doi: 10.1007/s40264-017-0528-z. [DOI] [PubMed] [Google Scholar]
- 13.Pharmaceutical Society of Australia . Vol. 2019. PSA; Canberra: 2019. Medicine safety: take care. [Google Scholar]
- 14.Roughead E.E., Semple S.J. Medication safety in acute care in Australia: Where are we now? Part 1: a review of the extent and causes of medication problems 2002-2008. Aust New Zealand Health Policy. 2009;6(18):18. doi: 10.1186/1743-8462-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Morabet N., Uitvlugt E.B., van den Bemt B.J.F., van den Bemt P., Janssen M.J.A., Karapinar-Carkit F. Prevalence and preventability of drug-related hospital readmissions: a systematic review. J Am Geriatr Soc. 2018;66(3):602–608. doi: 10.1111/jgs.15244. [DOI] [PubMed] [Google Scholar]
- 16.Lim R., Ellett L.M.K., Semple S., Roughead E.E. The extent of medication-related hospital admissions in Australia: a review from 1988 to 2021. Drug Saf. 2022;45(3):249–257. doi: 10.1007/s40264-021-01144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Australian Commission on Safety and Quality in Health Care . ACSQHC; 2019. Indicators of Safety and Quality [Internet]https://www.safetyandquality.gov.au/our-work/indicators/ [cited 2019 June 13]. Available from: [Google Scholar]
- 18.Bach Q.N., Peasah S.K., Barber E. Review of the role of the pharmacist in reducing hospital readmissions. J Pharm Pract. 2019;32(6):617–624. doi: 10.1177/0897190018765500. [DOI] [PubMed] [Google Scholar]
- 19.Burke R.E., Kripalani S., Vasilevskis E.E., Schnipper J.L. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109. doi: 10.1002/jhm.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Oliveira G.S., Jr., Castro-Alves L.J., Kendall M.C., McCarthy R. Effectiveness of pharmacist intervention to reduce medication errors and health-care resources utilization after transitions of care: a meta-analysis of randomized controlled trials. J Patient Saf. 2017;17(5):375–380. doi: 10.1097/PTS.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 21.Freeman C.R., Scott I.A., Hemming K., et al. Reducing medical admissions and presentations into hospital through optimising medicines (REMAIN HOME): a stepped wedge, cluster randomised controlled trial. Med J Aust. 2021;214(5):212–217. doi: 10.5694/mja2.50942. [DOI] [PubMed] [Google Scholar]
- 22.Tomlinson J., Cheong V.L., Fylan B., et al. Successful care transitions for older people: a systematic review and meta-analysis of the effects of interventions that support medication continuity. Age Ageing. 2020;49(4):558–569. doi: 10.1093/ageing/afaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mekonnen A.B., McLachlan A.J., Brien J.A. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open. 2016;6(2) doi: 10.1136/bmjopen-2015-010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budlong H., Brummel A., Rhodes A., Nici H. Impact of comprehensive medication management on hospital readmission rates. Popul Health Manag. 2018;21(5):395–400. doi: 10.1089/pop.2017.0167. [DOI] [PubMed] [Google Scholar]
- 25.Ellitt G.R., Engblom E., Aslani P., Westerlund T., Chen T.F. Drug related problems after discharge from an Australian teaching hospital. Pharm World Sci. 2010;32(5):622–630. doi: 10.1007/s11096-010-9406-9. [DOI] [PubMed] [Google Scholar]
- 26.Nazar H., Nazar Z., Portlock J., Todd A., Slight S.P. A systematic review of the role of community pharmacies in improving the transition from secondary to primary care. Br J Clin Pharmacol. 2015;80(5):936–948. doi: 10.1111/bcp.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsbottom H., Rutter P., Fitzpatrick R. Post discharge medicines use review (dMUR) service for older patients: cost-savings from community pharmacist interventions. Res Social Adm Pharm. 2018;14(2):203–206. doi: 10.1016/j.sapharm.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Trang J., Martinez A., Aslam S., Duong M.T. Pharmacist advancement of transitions of care to home (PATCH) service. Hosp Pharm. 2015;50(11):994–1002. doi: 10.1310/hpj5011-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veritas Health Innovation Covidence systematic review software. 2014. app.covidence.org Available from.
- 31.Costello J., Barras M., Foot H., Cottrell N. PROSPERO: International prospective register of systematic reviews. 2018. Pharmacist medication reviews in patients recently discharged from hospital.https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018086431 31/01/2018: Available from. 31/01/2018: Available from. [Google Scholar]
- 32.Griese-Mammen N., Hersberger K.E., Messerli M., et al. PCNE definition of medication review: reaching agreement. Int J Clin Pharmacol. 2018;40(5):1199–1208. doi: 10.1007/s11096-018-0696-7. [DOI] [PubMed] [Google Scholar]
- 33.Sterne J.A.C., Savovic J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 34.Sterne J.A., Hernan M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Bawardy R., Cheng-Lai A., Prlesi L., et al. Heart failure postdischarge clinic: a pharmacist-led approach to reduce readmissions. Curr Probl Cardiol. 2019;44(10):100407. doi: 10.1016/j.cpcardiol.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Andres J., Stanton-Ameisen O., Walton S., Ruchalski C. Pharmacists’ impact on secondary stroke prevention. J Pharm Pract. 2019;32(5):503–508. doi: 10.1177/0897190018766944. [DOI] [PubMed] [Google Scholar]
- 37.Arnold M.E., Buys L., Fullas F. Impact of pharmacist intervention in conjunction with outpatient physician follow-up visits after hospital discharge on readmission rate. Am J Health Syst Pharm. 2015;72(11 Suppl 1):S36–S42. doi: 10.2146/sp150011. [DOI] [PubMed] [Google Scholar]
- 38.Bae-Shaaw Y.H., Eom H., Chun R.F., Steven Fox D. Real-world evidence on impact of a pharmacist-led transitional care program on 30- and 90-day readmissions after acute care episodes. Am J Health Syst Pharm. 2020;77(7):535–545. doi: 10.1093/ajhp/zxaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonetti A.F., Bagatim B.Q., Mendes A.M., et al. Impact of discharge medication counseling in the cardiology unit of a tertiary hospital in Brazil: a randomized controlled trial. Clinics (Sao Paulo) 2018;73 doi: 10.6061/clinics/2018/e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavanaugh J.J., Jones C.D., Embree G., et al. Implementation science workshop: primary care-based multidisciplinary readmission prevention program. J Gen Intern Med. 2014;29(5):798–804. doi: 10.1007/s11606-014-2819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavanaugh J.J., Lindsey K.N., Shilliday B.B., Ratner S.P. Pharmacist-coordinated multidisciplinary hospital follow-up visits improve patient outcomes. J Manag Care Spec Pharm. 2015;21(3):256–260. doi: 10.18553/jmcp.2015.21.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fera T., Anderson C., Kanel K.T., Ramusivich D.L. Role of a care transition pharmacist in a primary care resource center. Am J Health Syst Pharm. 2014;71(18):1585–1590. doi: 10.2146/ajhp130684. [DOI] [PubMed] [Google Scholar]
- 43.Hawes E., Maxwell W., White S., Mangun J., Lin F. Impact of an outpatient pharmacist intervention on medication discrepancies and health care resource utilization in posthospitalization care transitions. J Prim Care Community Health. 2014;5(1):14–18. doi: 10.1177/2150131913502489. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/334/CN-01014334/frame.html [Randomized Controlled Trial; Research Support, Non-U.S. Gov’t]. Available from: [Randomized Controlled Trial; Research Support, Non-U.S. Gov’t]. Available from: [DOI] [PubMed] [Google Scholar]
- 44.Jack B.W., Chetty V.K., Anthony D., et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirkham H.S., Clark B.L., Paynter J., Lewis G.H., Duncan I. The effect of a collaborative pharmacist-hospital care transition program on the likelihood of 30-day readmission. Am J Health Syst Pharm. 2014;71(9):739–745. doi: 10.2146/ajhp130457. [DOI] [PubMed] [Google Scholar]
- 46.Liu V.C., Mohammad I., Deol B.B., Balarezo A., Deng L., Garwood C.L. Post-discharge medication reconciliation: reduction in readmissions in a geriatric primary care clinic. J Aging Health. 2019;31(10):1790–1805. doi: 10.1177/0898264318795571. [DOI] [PubMed] [Google Scholar]
- 47.March K.L., Peters M.J., Finch C.K., et al. Pharmacist transition-of-care services improve patient satisfaction and decrease hospital readmissions. J Pharm Pract. 2022;35(1):86–93. doi: 10.1177/0897190020958264. [DOI] [PubMed] [Google Scholar]
- 48.McFarland M.S., Thomas A.M., Young E., et al. Implementation and effect of a pharmacist-to-pharmacist transitions of care initiative on ambulatory care sensitive conditions. J Manag Care Spec Pharm. 2020;26(4):513–519. doi: 10.18553/jmcp.2020.26.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ni W., Colayco D., Hashimoto J., et al. Impact of a pharmacy-based transitional care program on hospital readmissions. Am J Manag Care. 2017;23(3):170–176. [PubMed] [Google Scholar]
- 50.Odeh M., Scullin C., Fleming G., Scott M.G., Horne R., McElnay J.C. Ensuring continuity of patient care across the healthcare interface: Telephone follow-up post-hospitalization. Br J Clin Pharmacol. 2019;85(3):616–625. doi: 10.1111/bcp.13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Odeh M., Scullin C., Hogg A., Fleming G., Scott M.G., McElnay J.C. A novel approach to medicines optimisation post-discharge from hospital: pharmacist-led medicines optimisation clinic. Int J Clin Pharmacol. 2020;42(4):1036–1049. doi: 10.1007/s11096-020-01059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paquin A.M., Salow M., Rudolph J.L. Pharmacist calls to older adults with cognitive difficulties after discharge in a Tertiary Veterans Administration Medical Center: a quality improvement program. J Am Geriatr Soc. 2015;63(3):571–577. doi: 10.1111/jgs.13315. [DOI] [PubMed] [Google Scholar]
- 53.Pett R.G., Nye S. Evaluation of a pharmacist-managed asthma clinic in an Indian Health Service clinic. J Am Pharm Assoc (2003) 2016;56(3):237–241. doi: 10.1016/j.japh.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 54.Phatak A., Prusi R., Ward B., et al. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and postdischarge call-backs (IPITCH Study) J Hosp Med. 2016;11(1):39–44. doi: 10.1002/jhm.2493. [DOI] [PubMed] [Google Scholar]
- 55.Rebello K.E., Gosian J., Salow M., Sweeney P., Rudolph J.L., Driver J.A. The rural PILL program: a postdischarge telepharmacy intervention for rural veterans. J Rural Health. 2017;33(3):332–339. doi: 10.1111/jrh.12212. [DOI] [PubMed] [Google Scholar]
- 56.Rottman-Sagebiel R., Cupples N., Wang C.P., et al. A pharmacist-led transitional care program to reduce hospital readmissions in older adults. Fed Pract. 2018;35(12):42–50. [PMC free article] [PubMed] [Google Scholar]
- 57.Schnipper J.L., Kirwin J.L., Cotugno M.C., et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565–571. doi: 10.1001/archinte.166.5.565. [DOI] [PubMed] [Google Scholar]
- 58.Snyder M.E., Krekeler C.E., Jaynes H.A., et al. Evaluating the effects of a multidisciplinary transition care management program on hospital readmissions. Am J Health Syst Pharm. 2020;77(12):931–937. doi: 10.1093/ajhp/zxaa091. [DOI] [PubMed] [Google Scholar]
- 59.Thurston M.M., Liao Tc V., Lim T., Pounds T., Moye-Dickerson P.M. Utilization of a multidisciplinary team to reduce the rate of hospital readmissions in high-risk heart failure patients at a community teaching hospital: the pharmacist’s role in transitions of care. J Am College Clin Pharm. 2019;2(3):281–287. [Google Scholar]
- 60.Wiegmann L.E., Belisle M.S., Alvarez K.S., Kale N.J. Aiming beyond: a pharmacist impact on 90-day readmissions and clinical outcomes within a family medicine service. J Pharm Pract. 2020;33(6):738–744. doi: 10.1177/0897190019825970. [DOI] [PubMed] [Google Scholar]
- 61.Xu N.Z., Wang C.H., Wan J.W., Liu X.Q., Li Z.D., Chen M. Effectiveness of pharmacist intervention in the management of coronary artery disease after index percutaneous coronary intervention: a single center randomized controlled trial. Int J Clin Exp Med. 2019;12(6):7760–7765. [Google Scholar]
- 62.Zhao S.J., Zhao H.W., Du S., Qin Y.H. The impact of clinical pharmacist support on patients receiving multi-drug therapy for coronary heart disease in China. Indian J Pharm Sci. 2015;77(3):306–311. doi: 10.4103/0250-474x.159632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ravn-Nielsen L.V., Duckert M.L., Lund M.L., et al. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. JAMA Intern Med. 2018;178(3):375–382. doi: 10.1001/jamainternmed.2017.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen T., Nguyen T.H., Nguyen P.T., et al. Pharmacist-led intervention to enhance medication adherence in patients with acute coronary syndrome in vietnam: a randomized controlled trial. Front Pharmacol. 2018;9:656. doi: 10.3389/fphar.2018.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khatib R., Patel N., Laverty U., et al. Re-engineering the post-myocardial infarction medicines optimisation pathway: a retrospective analysis of a joint consultant pharmacist and cardiologist clinic model. Open Heart. 2018;5(2) doi: 10.1136/openhrt-2018-000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang S. Impact of pharmacist-led medication management in care transitions. BMC Health Serv Res. 2017;17(1):722. doi: 10.1186/s12913-017-2684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu H., Zou J., Ye X., et al. Impacts of clinical pharmacist intervention on the secondary prevention of coronary heart disease: a randomized controlled clinical study. Front Pharmacol. 2019;10:1112. doi: 10.3389/fphar.2019.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bingham J., Campbell P., Schussel K., et al. The discharge companion program: an interprofessional collaboration in transitional care model delivery. Pharmacy (Basel) 2019;7(2):68. doi: 10.3390/pharmacy7020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Borhanjoo P., Kouamo P., Rahman M., Norton M., Gavini M. Effect of clinical pharmacist encounters in the transitional care clinic on 30-day re-admissions: a retrospective study. AIMS Public Health. 2019;6(3):345–354. doi: 10.3934/publichealth.2019.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Budiman T., Snodgrass K., Komatsu Chang A. Evaluation of pharmacist medication education and post-discharge follow-up in reducing readmissions in patients with ST-segment elevation myocardial infarction (STEMI) Ann Pharmacother. 2016;50(2):118–124. doi: 10.1177/1060028015620425. [DOI] [PubMed] [Google Scholar]
- 71.Cole J., Wilkins N., Moss M., Fu D., Carson P., Xiong L. Impact of pharmacist involvement on telehealth transitional care management (TCM) for high medication risk patients. Pharmacy (Basel) 2019;7(4):158. doi: 10.3390/pharmacy7040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farris K.B., Carter B.L., Xu Y., et al. Effect of a care transition intervention by pharmacists: an RCT. BMC Health Serv Res. 2014;14:406. doi: 10.1186/1472-6963-14-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fisher M., Cardoza A., Gordon A., Howard M., Neighbors L., Ragan A. Enhancing clinical pharmacy specialist involvement in transitions of care focusing on ambulatory care sensitive conditions within a veterans affairs healthcare system. Pharmacy (Basel) 2020;8(1):47. doi: 10.3390/pharmacy8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haag J.D., Davis A.Z., Hoel R.W., et al. Impact of pharmacist-provided medication therapy management on healthcare quality and utilization in recently discharged elderly patients. Am Health Drug Benefits. 2016;9(5):259–268. [PMC free article] [PubMed] [Google Scholar]
- 75.Hahn L., Belisle M., Nguyen S., Snackey Alvarez K., Das S. Effect of pharmacist clinic visits on 30-day heart failure readmission rates at a county hospital. Hosp Pharm. 2019;54(6):358–364. doi: 10.1177/0018578718797263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hawes E.M., Pinelli N.R., Sanders K.A., et al. Post-hospital discharge care: a retrospective Cohort study exploring the value of pharmacist-enhanced care and describing medication-related problems. N C Med J. 2018;79(1):4–13. doi: 10.18043/ncm.79.1.4. [DOI] [PubMed] [Google Scholar]
- 77.Ho P.M., Lambert-Kerzner A., Carey E.P., et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Intern Med. 2014;174(2):186–193. doi: 10.1001/jamainternmed.2013.12944. [DOI] [PubMed] [Google Scholar]
- 78.Jones C.D., Anthony A., Klein M.D., et al. The effect of a pharmacist-led multidisciplinary transitions-of-care pilot for patients at high risk of readmission. J Am Pharm Assoc (2003) 2018;58(5):554–560. doi: 10.1016/j.japh.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 79.Kilcup M., Schultz D., Carlson J., Wilson B. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc (2003) 2013;53(1):78–84. doi: 10.1331/JAPhA.2013.11250. [DOI] [PubMed] [Google Scholar]
- 80.Layman S.N., Elliott W.V., Regen S.M., Keough L.A. Implementation of a pharmacist-led transitional care clinic. Am J Health Syst Pharm. 2020;77(12):966–971. doi: 10.1093/ajhp/zxaa080. [DOI] [PubMed] [Google Scholar]
- 81.Lisenby K.M., Carroll D.N., Pinner N.A. Evaluation of a pharmacist-specific intervention on 30-day readmission rates for high-risk patients with Pneumonia. Hosp Pharm. 2015;50(8):700–709. doi: 10.1310/hpj5008-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mayzel B., Axtell S., Richardson C., Link N. The impact of face-to-face pharmacist transitional care management visits on medication-related problems. J Pharm Technol. 2020;36(3):95–101. doi: 10.1177/8755122520905582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller D., Ramsey M., L’Hommedieu T.R., Verbosky L. Pharmacist-led transitions-of-care program reduces 30-day readmission rates for medicare patients in a large health system. Am J Health Syst Pharm. 2020;77(12):972–978. doi: 10.1093/ajhp/zxaa071. [DOI] [PubMed] [Google Scholar]
- 84.Miller D.E., Roane T.E., McLin K.D. Reduction of 30-day hospital readmissions after patient-centric telephonic medication therapy management services. Hosp Pharm. 2016;51(11):907–914. doi: 10.1310/hpj5111-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murphy J.A., Schroeder M.N., Rarus R.E., Yakubu I., McKee S.O.P., Martin S.J. Implementation of a cardiac transitions of care pilot program: a prospective study of inpatient and outpatient clinical pharmacy services for patients with heart failure exacerbation or acute myocardial infarction. J Pharm Pract. 2019;32(1):68–76. doi: 10.1177/0897190017743129. [DOI] [PubMed] [Google Scholar]
- 86.O’Reilly E.A., Kuszmaul A.K., Carter A.M., Kreft K.N., Spencer C.A. Impact of a transitions of care pilot service established by pharmacy residents within an academic medical center. J Am Pharm Assoc (2003) 2020;60(1):87–92. doi: 10.1016/j.japh.2019.09.018. e2. [DOI] [PubMed] [Google Scholar]
- 87.Shaya F.T., Chirikov V.V., Rochester C., Zaghab R.W., Kucharski K.C. Impact of a comprehensive pharmacist medication-therapy management service. J Med Econ. 2015;18(10):828–837. doi: 10.3111/13696998.2015.1052463. [DOI] [PubMed] [Google Scholar]
- 88.Walker P.C., Bernstein S.J., Jones J.N., et al. Impact of a pharmacist-facilitated hospital discharge program: a quasi-experimental study. Arch Intern Med. 2009;169(21):2003–2010. doi: 10.1001/archinternmed.2009.398. [DOI] [PubMed] [Google Scholar]
- 89.Westberg S., Swanoski M., Renier C., Gessert C. Evaluation of the impact of comprehensive medication management services delivered posthospitalization on readmissions and emergency department visits. J Manag Care Spec Pharm. 2014;20(9):886–893. doi: 10.18553/jmcp.2014.20.9.886. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/498/CN-01002498/frame.html [Controlled Clinical Trial; Research Support, Non-U.S. Gov’t]. Available from: [Controlled Clinical Trial; Research Support, Non-U.S. Gov’t]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henriksen B., Stuckey N. Effects of transitional care management services from an interprofessional team on 30-day readmission rates among medicare beneficiaries. Top Geriatr Rehabil. 2018;34(3):182–184. [Google Scholar]
- 91.American College of Clinical Pharmacy, Leadership for Medication Management . American College of Clinical Pharmacy; Bethesda, Maryland: 2019. What is medication therapy management (MTM)?https://www.accp.com/docs/govt/advocacy/Leadership%20for%20Medication%20Management%20-%20MTM%20101.pdf [19/12/2019]. Available from: [Google Scholar]
- 92.Bluml B.M. Definition of medication therapy management: development of professionwide consensus. J Am Pharm Assoc (2003) 2005;45(5):566–572. doi: 10.1331/1544345055001274. [DOI] [PubMed] [Google Scholar]
- 93.American College of Clinical Pharmacy . American College of Clinical Pharmacy; Washington DC: 2019. Comprehensive medication management in team-based care.https://www.accp.com/docs/positions/misc/CMM%20Brief.pdf [cited 19/12/2019]. Available from: [Google Scholar]
- 94.American College of Clinical Pharmacy Standards of practice for clinical pharmacists. Pharmacotherapy. 2014;34(8):794–797. doi: 10.1002/phar.1438. [DOI] [PubMed] [Google Scholar]
- 95.Centres for Medicare & Medicaid Services Hospital readmissions reduction program (HRRP) 2020. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program [updated 24/08/2020; cited 2020 19/10/2020]. Available from:
- 96.NHS Digital . NHS Digital; Leeds, United Kingdom: 2020. About the NHS outcomes framework (NHS-OF)https://digital.nhs.uk/data-and-information/publications/statistical/nhs-outcomes-framework [cited 2020 11/12/2020]. Available from: [Google Scholar]
- 97.Eassey D., Smith L., Krass I., Mc L.A., Brien J.A. Consumer perspectives of medication-related problems following discharge from hospital in Australia: a quantitative study. International J Qual Health Care. 2016;28(3):391–397. doi: 10.1093/intqhc/mzw047. [DOI] [PubMed] [Google Scholar]
- 98.Ensing H.T., Stuijt C.C., van den Bemt B.J., et al. Identifying the optimal role for pharmacists in care transitions: a systematic review. J Manag Care Spec Pharm. 2015;21(8):614–636. doi: 10.18553/jmcp.2015.21.8.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheema E., Alhomoud F.K., Kinsara A.S.A., et al. The impact of pharmacists-led medicines reconciliation on healthcare outcomes in secondary care: a systematic review and meta-analysis of randomized controlled trials. PloS One. 2018;13(3) doi: 10.1371/journal.pone.0193510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McNab D., Bowie P., Ross A., MacWalter G., Ryan M., Morrison J. Systematic review and meta-analysis of the effectiveness of pharmacist-led medication reconciliation in the community after hospital discharge. BMJ Qual Saf. 2018;27(4):308–320. doi: 10.1136/bmjqs-2017-007087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jokanovic N., Tan E.C., van den Bosch D., Kirkpatrick C.M., Dooley M.J., Bell J.S. Clinical medication review in Australia: a systematic review. Res Social Adm Pharm. 2016;12(3):384–418. doi: 10.1016/j.sapharm.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 102.Tecklenborg S., Byrne C., Cahir C., Brown L., Bennett K. Interventions to reduce adverse drug event-related outcomes in older adults: a systematic review and meta-analysis. Drugs Aging. 2020;37(2):91–98. doi: 10.1007/s40266-019-00738-w. [DOI] [PubMed] [Google Scholar]
- 103.Cebron Lipovec N., Zerovnik S., Kos M. Pharmacy-supported interventions at transitions of care: an umbrella review. Int J Clin Pharmacol. 2019;41(4):831–852. doi: 10.1007/s11096-019-00833-3. [DOI] [PubMed] [Google Scholar]
- 104.Daliri S., Boujarfi S., El Mokaddam A., et al. Medication-related interventions delivered both in hospital and following discharge: a systematic review and meta-analysis. BMJ Qual Saf. 2021;30(2):146–156. doi: 10.1136/bmjqs-2020-010927. [DOI] [PubMed] [Google Scholar]
- 105.Bethishou L., Herzik K., Fang N., Abdo C., Tomaszewski D.M. The impact of the pharmacist on continuity of care during transitions of care: a systematic review. J Am Pharm Assoc (2003) 2020;60(1):163–177. doi: 10.1016/j.japh.2019.06.020. e2. [DOI] [PubMed] [Google Scholar]
- 106.Hoffmann T.C., Glasziou P.P., Boutron I., et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348(mar07 3):g1687. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]