Abstract
Objective This study assessed the feasibility of corticomuscular coherence measurement during a goal-directed task in children with unilateral cerebral palsy while establishing optimal experimental parameters. Methods Participants (Manual Ability Classification System levels I-III) completed a submaximal isometric goal-directed grip task during simultaneous electroencephalography and electromyography (EMG) acquisition. Results All participants (n = 11, 6 females, mean age 11.3 ±2.4 years) completed corticomuscular coherence procedures. Of the 40 trials obtained per extremity, an average of 29 (n = 9) and 27 (n = 10) trials were retained from the more- and less-affected extremities, respectively. Obtaining measurement stability required an average of 28 trials per extremity. Conclusion Findings from this work support the feasibility of corticomuscular coherence measurement in children with unilateral cerebral palsy. Acquiring 28 to 40 corticomuscular coherence trials per extremity is ideal. The experimental parameters established in this work will inform future corticomuscular coherence application in pediatric unilateral cerebral palsy.
Keywords: cerebral palsy, coherence, electroencephalography, electromyography, pediatric
Cerebral palsy is a leading cause of disability in children, with approximately 35% children experiencing motor impairment lateralized to one side of the body (unilateral cerebral palsy). 1 Despite this predominant clinical feature, there exists considerable interindividual heterogeneity in motor system function driven by the timing and etiology of neural injury and subsequent neural organization during development. 2 Such heterogeneity poses significant challenges when predicting functional outcomes and optimizing rehabilitation efforts.
By encapsulating relevant pathophysiology, brain-based measurements derived from neuroimaging, such as magnetic resonance imaging (MRI) and diffusion tensor imaging, and also transcranial magnetic stimulation (TMS) have the potential to address this heterogeneity. Research efforts have primarily focused on corticospinal tract organization and injury as a biomarker of upper extremity motor function and as a predictor of therapeutic responsiveness. 3 Many have shown significant correlations between hand motor deficits and diminished corticospinal tract integrity4,5 and also with ipsilateral corticospinal tract organization, 6 whereby corticospinal tract fibers projecting from the uninjured hemisphere control the more-affected hand. These associations are likely mediated by the timing 7 and extent 8 of injury. Relatedly, functional MRI work has demonstrated increased recruitment of sensorimotor cortical regions located on the uninjured hemisphere during voluntary movement of the more-affected hand.8,9 These structural and functional patterns in pediatric stroke and cerebral palsy were initially considered as maladaptive compensatory mechanisms resulting from diminished input from contralateral corticospinal tract projections on more-affected hand function. 10 Reinforcing this notion is additional work demonstrating greater therapeutic responsiveness in children depicting contralateral corticospinal tract organization 11 along with findings conveying different training-related neuroplasticity effects between children with ipsi- vs contralateral corticospinal tract organization. 12 However, emerging TMS research presents a contradictory role of ipsilateral corticospinal tract organization and its influence (or lack thereof) on therapeutic response. Several have concluded that the efficacy of constraint-induced movement therapy13,14 and bimanual training13,15,16 in pediatric cerebral palsy was independent of corticospinal tract organization. Complementing these findings is work showing greater hand function with larger overlap between more- and less-affected hand representations in the uninjured hemisphere. 17 Combined, this research suggests a supportive role of ipsilateral corticospinal tract organization in promoting hand function and treatment gains in cerebral palsy. The lack of consensus regarding the adaptive/maladaptive role of ipsilateral corticospinal tract projections and subsequent organization welcomes additional brain-based measures and tools to further characterize motor system function in cerebral palsy.
There exists a growing body of literature in cerebral palsy underscoring the informativeness of functional connectivity measurements between underlying neural circuits and networks. Several have shown reduced sensorimotor network connectivity in children with cerebral palsy compared to typically developing peers that related to worse upper extremity motor function.18,19 Corticomuscular coherence, a measure of connectivity between central and peripheral nervous systems (i.e., communication between corticospinal projections and motor units), 20 is another potentially informative measurement acquired from the simultaneous acquisition of brain and muscle signals typically with magnetoencephalography or electroencephalography (EEG) and electromyography (EMG). Seminal corticomuscular coherence work 21 has shown coherence between brain and muscle signals occurring most predominantly in the beta frequency range (typically 13-30 Hz), which aligns with past evidence suggesting that cortical oscillations generated from underlying pyramidal cells subserve motor behavior.22,23 By assessing the coherence between brain and muscle activity during functional movement, corticomuscular coherence may enrich our understanding of mechanisms of motor system impairment.
Of the few studies implementing corticomuscular coherence in a pediatric population, most involve infants born at term24,25 and children with typical development. 26 Collectively, this work revealed developmental increases in corticomuscular coherence between primary motor cortex (M1) and upper and lower extremity muscles in the beta frequency range during infancy that paralleled the development of normal fidgety and spontaneous movements24,25 and also during late childhood, with corticomuscular coherence values reaching adult-like levels by 10 years of age. 26 In another study examining corticomuscular coherence during a unimanual force-tracing task across 111 individuals between the ages of 8 and 30 years, investigators observed greater coherence in adults compared to children and that these differences were driven by descending (brain to muscle) vs ascending coherence, which reflects both maturation of corticomuscular networks and feedforward control during movement generation. 27 Despite these studies highlighting the capability of corticomuscular coherence in capturing motor-relevant neurophysiological information across the lifespan, only a few studies have used corticomuscular coherence in individuals with cerebral palsy, particularly in children with cerebral palsy where the generation of voluntary muscle contractions along with motor processing and planning are often compromised. 28 Initial findings of reduced corticomuscular coherence between M1 and effector muscles during the planning and execution phases of a paced hand opening and closing task have been observed in a cohort of individuals with cerebral palsy (12-54 years of age) compared with controls. 29 In a related case study involving a 4-year-old participant with a perinatal left middle cerebral artery stroke, researchers observed enhanced corticomuscular coherence between contralesional (ipsilateral) M1 and the more-affected first dorsal interossei muscle during contraction. 30 Although these findings provide initial evidence supporting the utility of corticomuscular coherence in cerebral palsy and perinatal stroke, this work motivates additional study. Building on these findings may entail implementing more goal-directed tasks during corticomuscular coherence collection and expanding corticomuscular coherence measurement beyond a specific brain region and/or frequency band. As corticomuscular coherence may reflect both muscle and cortical development, implementation of this measurement in pediatric unilateral cerebral palsy may be a beneficial step toward comprehending the role of ipsilateral corticospinal tract projections and organization.
The purpose of this study was to therefore assess the feasibility of corticomuscular coherence measurement in children and adolescents with unilateral cerebral palsy during an upper extremity goal-directed squeezing task by determining (1) the number of participants successfully completing testing procedures, (2) the proportion of trials retained from both more- and less-affected upper extremities, and (3) the proportion of trials retained from each experimental block to evaluate potential fatigue across the experiment. Limited corticomuscular coherence literature in pediatric unilateral cerebral palsy led to the hypotheses that >50% of participants would complete corticomuscular coherence procedures and that we would retain >50% of trials obtained from both extremities. Additionally, we sought to establish experimental design parameters to guide the design of future related studies by determining the minimum number of corticomuscular coherence trials necessary to achieve corticomuscular coherence measurement stability. Lastly, to extend initial corticomuscular coherence findings summarized above, we examined cortical contributions to corticomuscular coherence across the entire brain during more- and less-affected extremity performance using a 1–40 Hz frequency band. The information gained from this preliminary study has the potential to foster the development of corticomuscular coherence as an additional marker of motor behavioral status in children with unilateral cerebral palsy that may complement more established neuroimaging and neurophysiological measures such as MRI and TMS.
Methods
Participants
Individuals between 7 and 17 years of age with a diagnosis of unilateral cerebral palsy (Manual Ability Classification System levels I-III), as confirmed by medical records, were recruited. Additional inclusion criteria entailed the ability to follow 1 to 3 step directions and no surgery or botulinum toxin injections within the past 6 months. Visits occurred in either a private clinical room or laboratory space from March to July 2022.
Procedures
Electroencephalography recording.
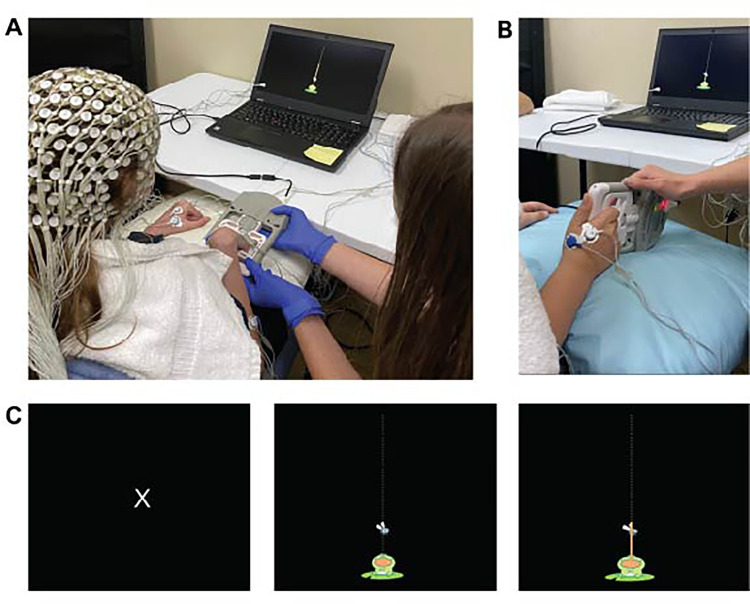
Participants wore a 256-lead Hydrocel net (Electrical Geodesics Inc, Eugene, OR) and disposable surface EMG leads applied to bilateral first dorsal interossei, flexor and extensor digitorum, and biceps brachii muscles. Synchronization of EEG and EMG signals and trial onset and offset markers occurred through a Physio16 input box (Electrical Geodesics Inc, Eugene, OR) and a Cedrus StimTracker box (Cedrus Corp, San Pedro, CA), respectively. Participants were randomized so that half performed corticomuscular coherence procedures with their more-affected extremity first followed by their less-affected extremity. We determined participants’ average maximal voluntary isometric grip force from 3 trials using a dynamometer (CAMRY) with a custom circuit board. Throughout the experiment, investigators did not require participants to maintain a specific forearm/wrist position. Rather, participants maintained a comfortable forearm/wrist position that would best accommodate task requirements and minimize compensatory movement. During the EEG recording, participants performed the same isometric grip task at 20% of their average maximal voluntary force output. A visual target (Figure 1) provided real-time force output feedback. Participants completed 5 practice trials to confirm task comprehension prior to the experiment of 2 blocks of 20 trials with a 1-minute break between blocks. Each trial lasted approximately 5 seconds, with inter-trial intervals ranging from 7 to 15 seconds to prevent habituation to the timing of onset cues. Investigators recorded the presence of mirroring and compensatory motions throughout the experiment based on EMG activity and visual assessment. Procedures were repeated on the opposite side following a brief 5-minute break where investigators checked EEG and EMG lead placement and integrity. Participants received verbal instruction to maintain a comfortable upright sitting position and to minimize talking during the recording.
Figure 1.
Participants wore a 256-lead EEG cap (A) and performed an isometric grip task using a dynamometer device (A, B) during the EEG recording. For each trial, participants observed an initial resting stimulus (C, left) followed by an activity stimulus (C, middle) where they squeezed the dynamometer to move the frog's tongue to the fly (C, right) which served as a visual target corresponding to 20% of their maximal grip force.
Clinical assessments, participant tolerance, and caregiver satisfaction
Trained physical and occupational therapists administered and scored assessments of unimanual (Box and Block Test) and bimanual (Assisting Hand Assessment) hand function in addition to the Edinburgh Handedness Inventory, Manual Ability Classification System, and the Gross Motor Function Classification System. Participants and their caregivers completed brief questionnaires regarding their tolerance and satisfaction with EEG-related procedures (Supplementary Material).
Corticomuscular Coherence Analysis
EEG signals were collected using a high-input impedance Net Amp 400 amplifier and NetStation 5.4.2 software (Electrical Geodesics Inc, Eugene, OR) with a sampling rate of 1000 Hz. Raw and unfiltered EEG data were exported to MATLAB 2017b (Natick, MA) for offline processing using EEGLAB. 31 Preprocessing steps involved re-referencing data to the mean signal across all leads following the removal of cheek and neck leads (194 leads remaining), and low- (50 Hz) and high- (0.5 Hz) pass filtering. Data were then segmented into 1-second non-overlapping epochs and visually inspected for muscle artifact before an Infomax independent component analysis 32 to remove cardiac and ocular artifacts. We completed a second data inspection to remove any remaining artifact that occurred and applied a spatial (Laplacian) filter to the preprocessed data. EMG data were also transferred to MATLAB for offline processing, which involved bandpass filtering from 0.5 to 50 Hz and passing the unrectified data through a Hilbert transform to obtain the envelope of the signal, and then rectification. EEG and EMG data were concatenated and corticomuscular coherence trial windows were defined between 1000 milliseconds (ms) before and 4000 ms after stimulus onset. We computed whole brain coherence values for each muscle across delta (1-3 Hz), theta (4-7 Hz), alpha mu (8-12 Hz), low beta (13-19 Hz), high beta (20-30 Hz), and low gamma (31-40 Hz) frequency bands. Coherence values are reported as the analogue of the squared correlation coefficient between 2 distinct signals (ie, EEG and EMG leads). Specifically, coherence is calculated with respect to the cross-spectrum density of signals S1 and S2, for each frequency of interest (f) ( ) and the auto-spectrum densities of S1 and S2 for each frequency of interest ( and ) as noted in the following equation. 33
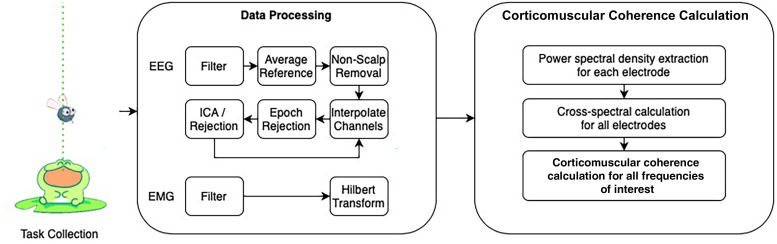
Coherence values range from 0 to 1, where the latter indicates consistent amplitude ratios and phase differences between 2 signals across time. EEG and EMG values were flipped so that the left hemisphere and right upper extremity represented the injured hemisphere and more-affected extremity for all participants. Coherence was calculated using a whole brain and hemispheric approach rather than using localized seed regions. This approach may be advantageous in this population as early neural injury alters neuroanatomical development, organization, and hand motor representation.8,10 The topographic plots, which depict 2-dimensional renderings of corticomuscular coherence magnitude across the scalp surface, were developed using functions from the open-source toolbox FieldTrip. 34 We examined preliminary differences in more- and less- affected hand performance based on visual analyses of corticomuscular coherence topoplots. An outline of our analysis pipeline is illustrated in Figure 2.
Figure 2.
The analysis of corticomuscular coherence involved a series of EEG and EMG processing steps. EEG, electroencephalography; EMG, electromyography; ICA, independent component analysis.
Statistical Analysis
We computed the percentage of enrolled participants that completed the entire corticomuscular coherence process, and we determined the proportion of corticomuscular coherence trials collected from participants’ more- and less-affected upper extremities that possessed sufficient quality (ie, low signal artifact) for subsequent analyses by recording the number of trials retained from the 40 trials acquired. We also determined if the average number of trials retained differed between the first and second blocks (a possible indicator of fatigue) for both extremities using paired t-tests. To determine the minimum number of trials required to achieve a stable estimate of corticomuscular coherence, we calculated running averages, defined for each trial as the average of all preceding trials. We used a method outlined by Goldsworthy and colleagues 35 that involved determining the minimum number of trials where the absolute percentage difference between the running average for each trial and the final average was 10%. We extended this analysis to also determine the minimum number of trials necessary to achieve percentage differences within 5% and 2%. For these analyses, we chose to assess corticomuscular coherence values involving whole brain EEG in the high beta frequency band and the first dorsal interossei muscle considering the involvement of the first dorsal interossei in precision grip tasks and the high beta band in motor control.24,25,30,36 We extended the assessment of corticomuscular coherence measurement stability to the other frequency bands within the 1–40-Hz bandwidth.
Results
Corticomuscular Coherence Feasibility & Tolerability
All 11 participants enrolled (6 females, 11.3± 2.4 years, Table 1) completed the 90-minute research visit. Data from 2 participants were partially or completely discarded because of the high presence of motion artifact throughout the EEG recording, which suggests that corticomuscular coherence measurement in these 2 participants was not feasible. On average, 29 trials (72.5%) were retained from the more-affected extremity (n = 9) and 27 trials (67.5%) from the less-affected extremity (n = 10). For the more-affected extremity, there was not a significant difference in trials retained between the first (15 trials) and second (14 trials; t = −0.97, P = .35) blocks. For the less-affected extremity, trials retained between the first (15 trials) and second blocks (12 trials) significantly differed (t = −2.90, P = .01). Participant and caregiver feedback was largely positive. All participants expressed comfort with corticomuscular coherence procedures, including EEG cap and EMG lead wear. All participants described the corticomuscular coherence computer game as fun and engaging but commented that the corticomuscular coherence experiment eventually felt repetitive and long. All caregivers expressed satisfaction with the overall research visit, including communication of procedures provided by team members and tolerance of procedures by participants.
Table 1.
Participant demographics and clinical characteristics.
| Participant | Sex | Age (y) | Side of hemiparesis | GMFCS | MACS | BBT (MA/LA) | AHA logit score | Number of trials retained (MA/LA) | Location and type of injury |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 11 | L | 2 | III | 16.5/31 | 47 | 23/24 | R MCA infarct |
| 2 | F | 15 | L | 2 | II | 19/48.5 | 46 | 34a/35 | CM-I, R MCA infarct |
| 3 | M | 7 | L | 2 | II | 22/38 | 71 | 0a/0 | R IVH |
| 4 | M | 12 | R | 1 | I | 28/43 | 81 | 33a/9a | Encephalomalacia, L MCA infarct |
| 5 | F | 13 | R | 2 | III | 6/27.5 | 19 | 0a/11a | Encephalomalacia, L MCA infarct |
| 6 | M | 8 | L | 1 | I | 38.5/41.5 | 94 | 37/35 | PVL |
| 7 | M | 10 | R | 1 | I | 40.5/56.5 | 100 | 37/36 | L MCA infarct |
| 8 | F | 10 | L | 2 | III | 17.5/25 | 80 | 17a/14 | IVH |
| 9 | F | 13 | R | 1 | I | 65.5/61.5 | 100 | 28/28 | L medial parieto-occipital lobe volume loss |
| 10 | F | 12 | R | 1 | III | 13/45 | 47 | 24a/35 | Encephalomalacia, L MCA infarct |
| 11 | M | 13 | R | 1 | III | 5.5/43.5 | 21 | 27a/38 | L MCA infarct |
Abbreviations: AHA, Assisting Hand Assessment; BBT, Box and Block Test; CM-1, Chiari Malformation Type 1; F, female; GMFCS, Gross Motor Function Classification System; IVH, interventricular hemorrhage; L, left; LA, less-affected extremity; LV, left ventricle; M, male; MA, more-affected extremity; MACS, Manual Ability Classification System; MCA, middle cerebral artery; PVL, periventricular leukomalacia; R, right.
Presence of mirroring from extremity opposite to that performing task.
Corticomuscular Coherence Stability
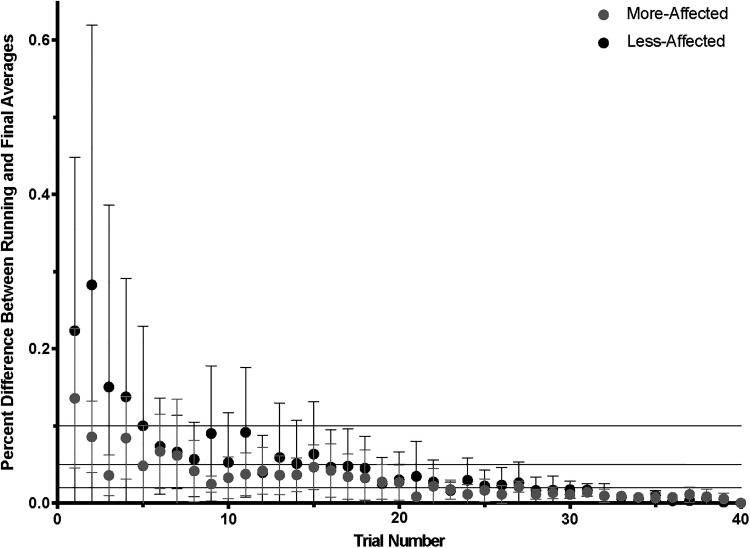
Stability analyses included data from 9 participants for the more-affected extremity and 10 participants for the less-affected extremity. For both more- and less-affected extremities, an average of 28 trials (interquartile range [IQR] = 24.5-36 for more-affected and 23-33 for the less-affected extremity) was necessary to achieve a 2% difference between the running and final corticomuscular coherence averages (Figure 3). To obtain a 5% difference, 15 trials from the more-affected extremity (IQR = 7.5-17) and 16 trials from the less-affected extremity (IQR = 10-18.5) were required. Lastly, reaching a 10% difference required 2 trials from the more-affected extremity (IQR = 1.5-10.5) and 5 trials from the less-affected extremity (IQR = 2.5-16.5). Achieving measurement stability in corticomuscular coherence measurements from other frequency bands also required an average of 28 trials per extremity (Supplementary Material).
Figure 3.
To determine stability of high beta (20-30 Hz) whole brain corticomuscular coherence with more- and less-affected first dorsal interossei across participants, the minimum number of trials (out of 40) to achieve 2% (bottom line), 5% (middle line), and 10% (top line) differences between the running and final corticomuscular coherence averages were determined. Dots and bars represent group averages and standard deviations, respectively.
Preliminary Corticomuscular Coherence Observations
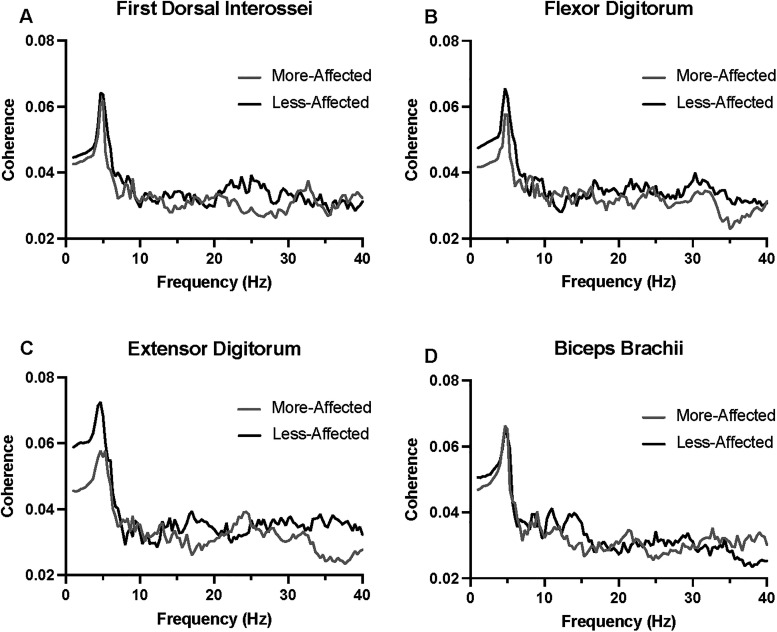
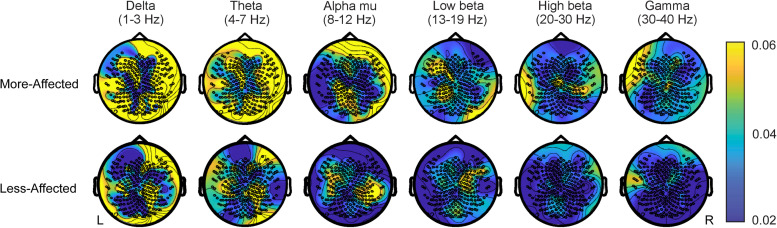
Initial inspection of corticomuscular coherence values revealed an overall reduction in corticomuscular coherence across the 1–40 Hz bandwidth for the more-affected extremity in comparison to the less-affected extremity, with prominent differences arising in the alpha mu (8-12 Hz) and low beta (13-19 Hz) frequency bands (Figure 4). Low-frequency (delta and theta) peaks were also observed across all muscles during more- and less- affected extremity performance (Figure 4). The topographic plots (Figure 5) reflect corticomuscular coherence with respect to each EEG lead and the muscle of interest to demonstrate the global cortical mapping of corticomuscular coherence during a grip task. Visual analyses of EEG topoplots depicted bilateral hemispheric EEG activity encompassing both motor and nonmotor cortical regions during more- and less-affected extremity performance (representative participant [no. 11] shown in Figure 5). This is denoted by areas of brighter colors occupying both hemispheres (dependent on the frequency band of interest). Brighter areas on the topoplots correspond to higher corticomuscular coherence values between EEG leads and the first dorsal interossei muscle during the isometric squeezing task.
Figure 4.
Corticomuscular coherence across a 1-40 Hz frequency bandwidth between all EEG leads and EMG leads from more- and less-affected first dorsal interossei (A), flexor (B) and extensor (C) digitorum, and biceps brachii (D) were acquired during an isometric grip task.
Figure 5.
Topoplots illustrating areas of cortical activity from a representative participant (no. 11) completing the isometric grip task with their more- (top row) and less-affected (bottom row) extremity. The color bar indicates the magnitude of whole brain corticomuscular coherence with the flexor digitorum muscle across various frequency bands within the 1-40 Hz spectrum. Left (L) hemisphere denotes the injured hemisphere.
Discussion
As a measure representing functional connectivity between brain and muscle, corticomuscular coherence may adjudicate heterogeneity in pediatric unilateral cerebral palsy by providing novel insight to motor prognostication and intervention response. Findings from this pilot work support the feasibility and tolerability of corticomuscular coherence collection during a goal-directed task in children with unilateral cerebral palsy. A preliminary visual analysis of EEG plots across participants also reveals the potential utility of corticomuscular coherence in the characterizing motor system organization following early neural injury.
Experimental paradigms from past related work involving corticomuscular coherence collection in pediatric populations vary considerably. The number of trials and tasks, for instance, range from 12 to 211 trials and encompass participants opening and closing their hand or contracting a specific upper extremity muscle.29,30 Our decision to implement a submaximal force value of 20% is consistent with other published work with values typically between 20% and 50% 37 and enables for the collection of multiple trials to enhance statistical power while also minimizing task-related fatigue. The high percentage of trials retained overall, 100% participant experiment completion, and no difference in trials retained between the first and second blocks for the more-affected extremity suggest that our chosen parameters (ie, number of trials/blocks and percentage of submaximal force required from the participant) are appropriate and that fatigue was not an issue.
Notably, despite only 3 trials separating the first and second blocks from the less-affected extremity, this difference was significant. Although this may indicate fatigue or diminishing attention contributing to greater instances of motion artifact, this finding may also underscore greater variability in less-affected extremity performance. Although we did not directly compare more- vs less-affected extremities when determining the number of trials required to achieve corticomuscular coherence stability, corticomuscular coherence measurement from the less-affected extremity required on average a greater number trials to achieve 2%, 5%, and 10% differences between running and final averages, which may also suggest heterogeneity in less-affected extremity performance. In a TMS study by Rich and colleagues 38 that examined hand motor function and grip strength in children with unilateral cerebral palsy, investigators observed greater mean differences between more- and less-affected upper extremities in children with ipsilateral corticospinal tract organization compared to those with contralateral corticospinal tract organization as determined by motor-evoked potentials. Significant differences also emerged when comparing the less-affected extremity from children with unilateral cerebral palsy with the dominant hand from children with typical development. 38 Combined with our findings, accounting for heterogeneity both within (more- vs less-affected extremities) and between participants is paramount when determining the total number of trials. Based on the above-mentioned observations and the likelihood of discarding 20% to 30% of trials, we assert that acquiring 28 to 40 trials per extremity is appropriate. Inclusion of a control group and combining corticomuscular coherence with TMS measures of corticospinal excitability for future corticomuscular coherence work will further elucidate the physiological underpinnings of corticomuscular coherence while also highlighting the effects of early neural injury to corticomuscular coherence.
An important difference between our work and previous work29,30 was the examination of whole brain coherence between EEG leads and each upper extremity muscle across a 1-40 Hz band. Additional insight to brain activity during motor performance in children with unilateral cerebral palsy may be captured by not restricting initial corticomuscular coherence observations to specific cortical regions like M1 or frequency bands. 39 Indeed, we observed low-frequency peaks for both extremities in all muscles (Figure 4). Others have also observed a similar low-frequency peak during corticomuscular coherence testing in primates that they attributed to residual movements generated during the hold period in the isometric task. 40 Despite seminal work depicting pronounced beta band coherence between brain and muscle during isometric contractions, 21 we did not observe a similar peak in the beta frequency range. The absence of prominent beta band contributions to corticomuscular coherence may relate to ongoing development and maturity of functional connections between brain and muscle as indicated by lower beta band coherence in children as compared to adults. 27 Observational studies entailing the examination of beta frequency corticomuscular coherence from children in narrower age ranges (ie, 7-10 years or 11-13 years) is a necessary next step to determine the relevance of beta frequency contributions to corticomuscular coherence output in unilateral cerebral palsy.
Although not a primary objective of this study, visual analyses of EEG topoplots across participants (exemplar in Figure 5) afforded a preliminary account of cortical contributions to corticomuscular coherence measures. Bilateral hemisphere activity during both more- and less-affected extremity performance involving primary and secondary motor regions and also frontoparietal regions aligns with past findings showing greater cortical activation during gait in bilateral motor, parietal, and frontal regions in children with unilateral cerebral palsy compared to children with typical development. 41 Combining these EEG topoplots with other modalities in similar fashion to Weinstein et al 42 that utilized EEG, TMS, EMG, and functional and diffusion MRI may impart valuable wisdom regarding upper extremity function in children with unilateral cerebral palsy. Notably, Weinstein et al 42 had a 64% success rate in obtaining high-quality functional MRI data and a 77% success rate with EEG, which is similar to our work. Given the lower costs and greater accessibility associated with EEG as compared to functional MRI, EEG may be the more preferred modality to assess brain activity in pediatric populations.
We acknowledge a few limitations with this pilot study. The heterogeneity within our small sample size limited sufficient statistical power necessary to determine associations between corticomuscular coherence and motor behavioral assessments, which is a critical next step to determine the utility of corticomuscular coherence in unilateral cerebral palsy. The acquisition and measurement of corticomuscular coherence may not be feasible in children with more severe motor impairment and hemiparesis that cannot complete a precision grip task. Lastly, poor spatial resolution is a universal limitation of EEG studies. The use of a high-density EEG system partially mitigated this shortcoming, and the use of whole brain coherence without the reliance of a seed region provided a more comprehensive account of regional cortical activity.
Conclusion
This study determined the feasibility of combined EEG and EMG collection in children and adolescents with unilateral cerebral palsy to measure corticomuscular coherence. Findings from this work, including our recommended experimental parameters and topographic illustrations, both guide and encourage future corticomuscular coherence studies in this pediatric population.
Supplemental Material
Supplemental material, sj-docx-1-jcn-10.1177_08830738231187010 for Corticomuscular Coherence in Children with Unilateral Cerebral Palsy: A Feasibility and Preliminary Protocol Study by Rachana R. Gangwani, Jasper I. Mark, Rachel M. Vaughn, Holly Holland, Deborah E. Thorpe, Joshua J. Alexander, Swati M. Surkar and Jessica M. Cassidy in Journal of Child Neurology
Supplemental material, sj-doc-2-jcn-10.1177_08830738231187010 for Corticomuscular Coherence in Children with Unilateral Cerebral Palsy: A Feasibility and Preliminary Protocol Study by Rachana R. Gangwani, Jasper I. Mark, Rachel M. Vaughn, Holly Holland, Deborah E. Thorpe, Joshua J. Alexander, Swati M. Surkar and Jessica M. Cassidy in Journal of Child Neurology
Acknowledgments
We acknowledge the scientific and financial support of the National Institutes of Health: Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Neurological Disorders and Stroke, National Institute of Biomedical Imaging and Bioengineering, and the American Academy of Cerebral Palsy and Developmental Medicine.
Footnotes
Author Contributions: JMC was responsible for the study conception and design. JMC, RRG, JIM, RMV, and HH were responsible for datacollection. JMC, RRG, JIM, RMV, HH, and SMS were responsible for data analysis. JIM developed code for corticomuscular coherencecomputation with guidance from JMC. JMC, RRG, and JIM wrote the manuscript and prepared figures. All authors edited andapproved the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the American Academy of Cerebral Palsy and Developmental Medicine Research Pilot Grant sponsored by the National Institutes of Health through the National Pediatric Rehabilitation Resource Center (C-PROGRESS).
Ethical Approval: Legal guardians provided written informed consent, and all participants provided written assent as approved by the Institutional Review Board (study no. 21-2301) at the University of North Carolina at Chapel Hill. Legal guardians also provided written consent for the release of photography/images for publication purposes.
ORCID iD: Jessica M. Cassidy https://orcid.org/0000-0003-3469-0399
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Auld ML, Boyd R, Moseley GL, Ware R, Johnston LM. Tactile function in children with unilateral cerebral palsy compared to typically developing children. Disabil Rehabil . 2012;34(17):1488-1494. [DOI] [PubMed] [Google Scholar]
- 2.Hankins GD, Speer M. Defining the pathogenesis and pathophysiology of neonatal encephalopathy and cerebral palsy. Obstet Gynecol . 2003;102(3):628-636. [DOI] [PubMed] [Google Scholar]
- 3.Jaspers E, Byblow WD, Feys H, Wenderoth N. The corticospinal tract: a biomarker to categorize upper limb functional potential in unilateral cerebral palsy. Front Pediatr . 2015;3:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleyenheuft Y, Grandin CB, Cosnard G, Olivier E, Thonnard JL. Corticospinal dysgenesis and upper-limb deficits in congenital hemiplegia: a diffusion tensor imaging study. Pediatrics . 2007;120(6):e1502-e1511. [DOI] [PubMed] [Google Scholar]
- 5.Glenn OA, Ludeman NA, Berman JI, et al. Diffusion tensor MR imaging tractography of the pyramidal tracts correlates with clinical motor function in children with congenital hemiparesis. Am J Neuroradiol . 2007;28(9):1796-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmström L, Vollmer B, Tedroff K, et al. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Dev Med Child Neurol . 2010;52(2):145-152. [DOI] [PubMed] [Google Scholar]
- 7.Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krägeloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann Neurol . 2004;56(6):854-863. [DOI] [PubMed] [Google Scholar]
- 8.Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krägeloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain . 2002;125(10):2222-2237. [DOI] [PubMed] [Google Scholar]
- 9.Van de Winckel A, Klingels K, Bruyninckx F, et al. How does brain activation differ in children with unilateral cerebral palsy compared to typically developing children, during active and passive movements, and tactile stimulation? An fMRI study. Res Dev Disabil . 2013;34(1):183-197. [DOI] [PubMed] [Google Scholar]
- 10.Eyre JA, Smith M, Dabydeen L, et al. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann Neurol . 2007;62(5):493-503. [DOI] [PubMed] [Google Scholar]
- 11.Kuhnke N, Juenger H, Walther M, Berweck S, Mall V, Staudt M. Do patients with congenital hemiparesis and ipsilateral corticospinal projections respond differently to constraint-induced movement therapy? Dev Med Child Neurol . 2008;50(12):898-903. [DOI] [PubMed] [Google Scholar]
- 12.Juenger H, Kuhnke N, Braun C, et al. Two types of exercise-induced neuroplasticity in congenital hemiparesis: a transcranial magnetic stimulation, functional MRI, and magnetoencephalography study. Dev Med Child Neurol . 2013;55(10):941-951. [DOI] [PubMed] [Google Scholar]
- 13.Friel KM, Ferre CL, Brandao M, et al. Improvements in upper extremity function following intensive training are independent of corticospinal tract organization in children with unilateral spastic cerebral palsy: a clinical randomized trial. Front Neurol . 2021;12:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Islam M, Nordstrand L, Holmström L, Kits A, Forssberg H, Eliasson AC. Is outcome of constraint-induced movement therapy in unilateral cerebral palsy dependent on corticomotor projection pattern and brain lesion characteristics? Dev Med Child Neurol . 2014;56(3):252-258. [DOI] [PubMed] [Google Scholar]
- 15.Smorenburg AR, Gordon AM, Kuo H-C, et al. Does corticospinal tract connectivity influence the response to intensive bimanual therapy in children with unilateral cerebral palsy? Neurorehabil Neural Repair . 2017;31(3):250-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bleyenheuft Y, Dricot L, Gilis N, et al. Capturing neuroplastic changes after bimanual intensive rehabilitation in children with unilateral spastic cerebral palsy: a combined DTI, TMS and fMRI pilot study. Res Dev Disabil . 2015;43-44:136-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marneweck M, Kuo HC, Smorenburg ARP, et al. The relationship between hand function and overlapping motor representations of the hands in the contralesional hemisphere in unilateral spastic cerebral palsy. Neurorehabil Neural Repair . 2018;32(1):62-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta D, Barachant A, Gordon AM, et al. Effect of sensory and motor connectivity on hand function in pediatric hemiplegia. Ann Neurol . 2017;82(5):766-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodward K, Carlson H, Kuczynski A, Saunders J, Hodge J, Kirton A. Sensory-motor network functional connectivity in children with unilateral cerebral palsy secondary to perinatal stroke. NeuroImage Clin . 2019;21:101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mima T, Hallett M. Corticomuscular coherence: a review. J Clin Neurophysiol . 1999;16(6):501. [DOI] [PubMed] [Google Scholar]
- 21.Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol . 1997;77(6):3401-3405. [DOI] [PubMed] [Google Scholar]
- 22.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol . 1999;110(11):1842-1857. [DOI] [PubMed] [Google Scholar]
- 23.Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci U S A . 2004;101(26):9849-9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanazawa H, Kawai M, Kinai T, Iwanaga K, Mima T, Heike T. Cortical muscle control of spontaneous movements in human neonates. Eur J Neurosci . 2014;40(3):2548-2553. [DOI] [PubMed] [Google Scholar]
- 25.Ritterband-Rosenbaum A, Herskind A, Li X, et al. A critical period of corticomuscular and EMG-EMG coherence detection in healthy infants aged 9-25 weeks. J Physiol . 2017;595(8):2699-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James LM, Halliday DM, Stephens JA, Farmer SF. On the development of human corticospinal oscillations: age-related changes in EEG-EMG coherence and cumulant. Eur J Neurosci . 2008;27(12):3369-3379. [DOI] [PubMed] [Google Scholar]
- 27.Beck MM, Spedden ME, Lundbye-Jensen J. Reorganization of functional and directed corticomuscular connectivity during precision grip from childhood to adulthood. Sci Rep . 2021;11(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surkar SM, Hoffman RM, Davies B, Harbourne R, Kurz MJ. Deficits in planning sequential goal-directed action impact motor execution in children with hemiplegic cerebral palsy: a kinematic analysis. J Motor Learn Dev . 2019;7(1):122-140. [Google Scholar]
- 29.Riquelme I, Cifre I, Muñoz MA, Montoya P. Altered corticomuscular coherence elicited by paced isotonic contractions in individuals with cerebral palsy: a case-control study. J Electromyogr Kinesiol . 2014;24(6):928-933. [DOI] [PubMed] [Google Scholar]
- 30.Basu A, Graziadio S, Smith M, Clowry GJ, Cioni G, Eyre JA. Developmental plasticity connects visual cortex to motoneurons after stroke. Ann Neurol . 2010;67(1):132-136. [DOI] [PubMed] [Google Scholar]
- 31.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods . 2004;134(1):9-21. [DOI] [PubMed] [Google Scholar]
- 32.Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage . 2007;34(4):1443-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Sheng Y, Liu H. Corticomuscular coherence and its applications: a review. Front Hum Neurosci . 2019;13:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baillet S, Friston K, Oostenveld R. Academic software applications for electromagnetic brain mapping using MEG and EEG. Comput Intell Neurosci . 2011;2011:972050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldsworthy MR, Hordacre B, Ridding MC. Minimum number of trials required for within- and between-session reliability of TMS measures of corticospinal excitability. Neuroscience . 2016;320:205-209. [DOI] [PubMed] [Google Scholar]
- 36.Reyes A, Laine CM, Kutch JJ, Valero-Cuevas FJ. Beta band corticomuscular drive reflects muscle coordination strategies. Front Comput Neurosci . 2017;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye Y, Ma L, Yan T, Liu H, Wei X, Song R. Kinetic measurements of hand motor impairments after mild to moderate stroke using grip control tasks. J Neuroeng Rehabil . 2014;11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rich TL, Menk JS, Rudser KD, Feyma T, Gillick BT. Less-affected hand function in children with hemiparetic unilateral cerebral palsy: a comparison study with typically developing peers. Neurorehabil Neural Repair . 2017;31(10-11):965-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deligianni F, Centeno M, Carmichael DW, Clayden JD. Relating resting-state fMRI and EEG whole-brain connectomes across frequency bands. Front Neurosci . 2014;8:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witham CL, Wang M, Baker SN. Corticomuscular coherence between motor cortex, somatosensory areas and forearm muscles in the monkey. Front Syst Neurosci . 2010;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Short MR, Damiano DL, Kim Y, Bulea TC. Children with unilateral cerebral palsy utilize more cortical resources for similar motor output during treadmill gait. Front Hum Neurosci . 2020;14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinstein M, Green D, Rudisch J, et al. Understanding the relationship between brain and upper limb function in children with unilateral motor impairments: a multimodal approach. Eur J Paediatr Neurol . 2018;22(1):143-154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jcn-10.1177_08830738231187010 for Corticomuscular Coherence in Children with Unilateral Cerebral Palsy: A Feasibility and Preliminary Protocol Study by Rachana R. Gangwani, Jasper I. Mark, Rachel M. Vaughn, Holly Holland, Deborah E. Thorpe, Joshua J. Alexander, Swati M. Surkar and Jessica M. Cassidy in Journal of Child Neurology
Supplemental material, sj-doc-2-jcn-10.1177_08830738231187010 for Corticomuscular Coherence in Children with Unilateral Cerebral Palsy: A Feasibility and Preliminary Protocol Study by Rachana R. Gangwani, Jasper I. Mark, Rachel M. Vaughn, Holly Holland, Deborah E. Thorpe, Joshua J. Alexander, Swati M. Surkar and Jessica M. Cassidy in Journal of Child Neurology