Abstract
In this study, we identified 3-aminophthalic acid as a new ligand of cereblon (CRBN) E3 ubiquitin ligase and developed a phthalic acid- based O’PROTAC for degradation of ERG transcription factor. This phthalic acid-based O’PROTAC presented a comparable efficacy in degrading ERG as pomalidomide-based ERG O’PROTACs. Moreover, phthalic acid is more chemically stable and economical than classical immunomodulatory drugs (IMiDs). Therefore, phthalic acid ligand represents a new alternative for the development of PROTACs, especially O’PROTACs.
Graphical Abstract

In this study, we reported the discovery of 3-aminophthalic acid as a new ligand of cereblon (CRBN) E3 ubiquitin ligase and the development of a phthalic acid-based O’PROTAC for degradation of ERG transcription factor. This new E3 ligase ligand provides a new alternative for the development of PROTACs, especially O’PROTACs.
Proteolysis targeting chimeras (PROTACs) are heterobifunctional molecules composed of a protein of interest (POI) ligand as a warhead, an E3 ligase ligand and a linker, which induce the proximity of POI and E3 ligase with consequent ubiquitination and degradation of POI. PROTAC strategy utilizes event-driven pharmacology as the mode of action (MOA) and thus it has potential advantages over traditional inhibitor, which is occupancy-driven MOA, with respect to reducing off-target effect, drug resistance and modulating ‘undruggable’ targets,1 representing a promising approach to treat human disease.
One of the key elements of designing a potent PROTAC molecule is the E3 ligase ligand. The first PROTAC molecule was reported by Deshaies and coworkers in 2001, and it utilized a peptide ligand for E3 ligase β-TRCP.2 Peptide moieties caused poor cell permeability and biological instability, which hampered the development of PROTACs.3 In the past decade, several small-molecule ligands have been identified to recruit E3 ligases, including von Hippel–Lindau (VHL),4 MDM2,5 CRBN,6 IAPs,7 DCAF15,8 RNF4,9 RNF114,10 and DCAF16.11 However, only the CRBN and VHL ligands are frequently used in the PROTAC field.3
CRBN is a subunit of the E3 ubiquitin ligase CUL4–RBX1– DDB1–CRBN, which ubiquitinates a number of target proteins. Thalidomide derivatives, referred to as IMiDs, were shown to bind to CRBN and mediate its function in treatment of multiple myeloma and other B cell malignancies.12 Thalidomide has a legendary path as a drug. It was originally marketed in 1957 for treatment of insomnia and morning sickness. However, it was withdrawn later on from the market due to the strong teratogenicity.13 In 2010, the Hiroshi group demonstrated that the teratogenic effect is due to thalidomide binding of CRBN and inhibition of its ubiquitin ligase activity.14 Afterward, thalidomide analogs, pomalidomide and lenalidomide were reported to induce the degradation of IKZF1 and IKZF3 through the involvement of CRBN.12 The crystal structure of thalidomide with CRNB and IKZF was resolved in 2014.15 PROTAC molecules composed of CRBN ligand were first designed to degrade BET and FKBP12 by the Bradner group in 2015.6 Subsequently, the field of CRBN-recruiting PROTAC has expanded dramatically, with several PROTACs applying in clinic trials.16
Despite continuous progress in the development of potent CRBN-recruiting PROTACs, considerable challenges remain. IMiDs-based PROTACs have been described to remain the activity of IMiDs on Ikaros transcription factor, leading to the off-target effect.17 Furthermore, thalidomide showed poor stability under physiological pH 7.4 due to the hydrolysis of phthalimide and glutarimide moiety.18 Although Rankovic’s group recently reported phenyl-glutarimide analogues as alternative CRBN binders,19 more studies are needed to demonstrate the in vivo efficacy of these chemicals and broad applications in drug development. Thus, there is an urgent need to explore new ligands for CRBN.
Recently, we reported the development of O’PROTACs (OPs) by attaching DNA sequences with CRBN or VHL ligand to degrade transcriptional factors (ERG and LEF1).20 O’PROTACs were synthesized by annealing pomalidomide- and VH032- attached reverse strand with forward strand. Considering that phosphoramidite chemistry is more straightforward and efficient compared to post-synthesis conjugation of DNA oligos 20, we initially used this method to construct the pomalidomide- and VH032-attached reverse strand (ERG R-C1 to C3 and R-V1 to V3) with different linker lengths for ERG O’PROTACs. As we reported previously,20 the molecular weights of VH032- attached reverse strands (R-V1 to V3) were identical to those were expected, although none of them were effective in degradation of ERG (Fig. S1A and B). In contrast, mass spectrum analysis showed that the reverse strand (ERG R-C1) of ERG OP- C1, the most potent ERG OPs (Fig. S1A and B) was not consistent with the expected mass (Fig. S1C and D). Given that the molecular weights of pomalidomide-based ERG OPs generated through post-synthesis conjugations (e.g. NHS-ester modification and click reaction) were the same as what were expected,20 we reasoned that the pomalidomide moiety was hydrolyzed to 3-aminophthalic acid during the deprotection of all protecting groups of the ERG OP-C1 reverse strand, but not in the step of mass spectrometry (Scheme S1A). The mechanism for the conversion of pomalidomide to corresponding phthalic acid is shown in Scheme S1B. Pomalidomide was heated in fresh ammonia solution in water, hydroxide ion could attack the carbonyl group, leading to the ring opening of phthalimide or glutarimide (compound 1 and 2). Intermediates 1 or 2 was unstable with prolonged heating in the ammonia solution and was converted to the stable phthalic acid (4).21 Taken together, we hypothesized that phthalic acid can be a new E3 ligase recruiter of O’PROTACs that are effective in proteolytic degradation of a target protein.
To test this hypothesis, we synthesized a new ERG O’PROTAC (OP-C-P1) by applying a new synthetic route using phthalic acid dimethyl ester as the start material (Scheme S1C and S2). The HPLC and mass spectrometry data indicated that ERG OP-C-P1 (containing a DNA oligo composed of a phthalic acid-linked reverse strand (R-C-P1) and FITC-labeled forward strand (FITC-F)) was successfully synthesized by phosphoramidite chemistry with high purity and expected molecular mass (Fig. S2A–D). We, therefore, employed this new ERG OP-C-P1 (Fig. 1A) for further biochemical and functional studies.
Fig.1.
Phthalic acid-based ERG O’PROTAC degrades ERG oncoprotein. (A) The linear structure of ERG OP-C-P1. (B) The VCaP cells were transfected with control or four indicated ERG O’PROTACs at a final concentration of 100 nM and harvested for western blot analysis. (C and D) The VCaP cells were transfected with increasing concentrations of ERG OP-C-P1 for 36 h and treated with 20 μg/ml of cycloheximide (CHX) for another 12 h. Cells were harvested for western blot analysis of ERG protein expression (C). The remaining ERG protein (%) was calculated by normalizing the value of each group to that of the group without ERG OP-C-P1 treatment. The concentration of ERG OP-C-P1 degrading 50% of ERG protein (DC50) was calculated with Prism software in (D).
We firstly compared the efficacy of the phthalic acid-based ERG OPs (C-P1 with high purity and C1 with low purity) with two previously reported pomalidomide-based ERG OPs synthesized via post-synthesis conjugations.20 FITC-labeled ERG OPs were used to monitor the transfection efficiency of these OPs. Fluorescent microscopy analysis showed that phthalic acid- based ERG OPs were transfected as effectively as the other two previously reported OPs (C-A1 and C-N1) in both 293T and VCaP cell lines (Fig. S3A and B). Intriguingly, western blot analysis revealed that OP-C-P1 exhibited a slightly stronger effect on the downregulation of ectopically expressed full-length (FL) ERG protein than OP-C-A1 and OP-C-N1 in 293T (Fig. S3C). Similar results were obtained for the endogenous full-length (FL) ERG protein in VCaP cells (Fig. 1B). Further analysis revealed that none of these ERG OPs exerted any obvious effect on mRNA levels of both FL and truncated ERG T1/E4 derived from TMPSS2-ERG gene fusion (Fig. S3D), suggesting that ERG OP-C- P1 inhibits ERG expression at the post-transcriptional level.
We then analyzed the kinetics of OP-C-P1 potency on protein degradation. Time-course studies demonstrated that OP-C-P1 inhibited ERG protein expression starting from 24 h post-transfection (Fig. S3E). Dose-course experiments further revealed that OP-C-P1 induced a dramatic decrease in ERG protein level at a concentration as low as 50 nM (Fig. 1C). Interestingly, little or no further decrease in ERG protein level occurred with higher concentrations (100 or 500 nM), implying that the amount of ERG OP-C-P1 in cells could be saturated or its uptake by cells could be limited due to transfection efficiency. Therefore, further efforts are warranted to develop more efficient methods for O’PROTAC delivery. The degradation concentration (DC) curve demonstrated that OP-C-P1 inhibited 50% of ERG protein at 172.4 nM (Fig. 1D).
To determine whether phthalic acid-based ERG OP-C-P1- induced ERG protein downregulation is mediated through the ubiquitination and proteasome degradation pathway, VCaP cells were first transfected with OP-C-P1 and treated with the proteasome inhibitor MG132. MG132 treatment completely blocked the degradation of ERG protein (Fig. 2A), suggesting that ERG degradation is dependent on the proteasome pathway. Meanwhile, the ubiquitination assay showed that the treatment of OP-C-P1 enhanced the ubiquitination level of ERG at both endogenous (in VCaP cells) and exogenous (in 293T cells) levels (Fig. 2B and S4A).
Fig.2.
Phthalic acid-based ERG OP degrades ERG via CRBN and the proteasome pathway. (A) The VCaP cells were transfected with a final concentration of 100 nM of control OP or ERG OP-C-P1 for 36 h and treated with or without MG132 (20 μM) for another 12 h before harvested for western blot analysis. (B) The VCaP cells were transfected with the indicated plasmids and ERG OP-C-P1 at a final concentration of 100 nM for 36 h and treated with the proteasome inhibitor MG132 (20 μM) for 12 h before harvested for protein extraction. ERG protein was immunoprecipitated with ERG antibody by protein A/G beads to detect its ubiquitination level by western blot analysis. (C) The VCaP cells were transfected with a final concentration of 100 nM of control OP or ERG OP-C-P1 and siRNA control (siNS) or siCRBN for 48 h before harvested for western blot analysis. (D) The VCaP cells were transfected with control OP or ERG OP-C-P1 at a final concentration of 100 nm and incubated with 1-, 10-, or 50-fold of CRBN ligand pomalidomide for 36 h, followed by western blot analysis of ERG protein level.
To examine whether ERG OP-C-P1 can bind to ERG in vitro, we performed electrophoretic mobility shift assay (EMSA) using nuclear extract of VCaP cells. We demonstrated that biotin- labeled ERG OP-C-P1 formed a DNA-protein complex (DPC) in the nuclear extract of VCaP cells. This binding was interrupted by the addition of competitive non-biotin-labeled ERG OP-C-P1 (Fig. S4B). Moreover, the addition of ERG antibody resulted in a supershift of DPC (Fig. S4C), suggesting that the detected DPC contains ERG protein.
Next, we investigated whether OP-C-P1-mediated degradation of ERG is dependent on CRBN. We knocked down CRBN in VCaP cells and treated the cells with OP-C-P1. We found that CRBN knockdown completely abolished OP-C-P1-induced degradation of ERG (Fig. 2C). In agreement with this observation, the treatment of CRBN ligand pomalidomide also overcame the degradation of ERG protein induced by OP-C-P1, and this effect was dose-dependent (Fig. 2D). These data indicate that ERG degradation induced by OP-C-P1 is mediated through CRBN E3 ligase.
To understand the interaction between CRBN protein and 3-aminophthalic acid, we performed molecular docking using 3- N-substituted phthalic acid and CRBN (PDB: 4CI1). As shown in Fig. 3A and 3B, the interaction between CRBN and phthalic acid was observed, which is similar to the interaction between CRBN and thalidomide. The 1’-carboxylic acid group oriented toward the hydrophobic pocket and resulted in the formation of two strong hydrogen bonds. The carbonyl oxygen and the hydrogen of hydroxy groups interacted with the backbone of Tryptophan (TRP) 382 and Histidine (HIS) 380, respectively. These hydrogen bond interactions were resemblant with the glutarimide group of thalidomide, where the interaction occurred between two carbonyl and amide to residues TRP 382 and HIS 380, respectively. Additionally, the other 2’-carboxylic acid group would be more solvent-exposed. Due to the flexibility of the C- C bond between benzene and carboxylic acid, the carbonyl oxygen could position itself facing the hydrophobic pocket to retain hydrogen bond with the imidazole side chain of HIS 380. Meanwhile, the hydroxy group formed a weak water-mediated hydrogen bond with HIS 359 side-chain. Comparatively to the thalidomide, the phthalimide was completely solvent-exposed and accommodated with a water-mediated hydrogen bond with HIS 359. There were also observed pi-pi interactions between indole of TRP 388 and benzene ring of phthalic acid. Interestingly, the orientation of the 3-amino group was completely solvent-exposed similar to pomalidomide and lenalidomide, which contributed enormously to forming linkers with any potential warheads. These binding properties provide a plausible explanation for the observation that this phthalic acid-based O’PROTACs exhibited a comparable activity as pomalidomide-based O’PROTACs.
Fig.3.
Docking of CRBN binding of thalidomide (PDB:4CI1) (A) and 3-N-substituted- aminophthalic acid (B). Dotted black lines represent hydrogen bond and dotted cyan lines represent pi-pi interaction.
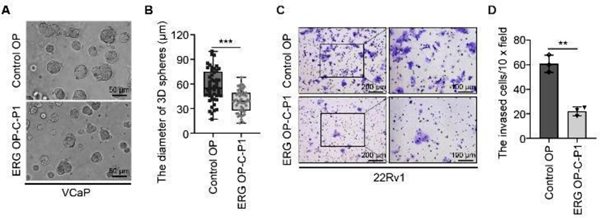
To determine whether ERG OP-C-P1 affects ERG signaling pathway, we detected the transcriptional levels of ERG target genes. We demonstrated that the downregulation of ERG by OP-C-P1 also significantly diminished mRNA expression of ERG target genes including ADAM19, MMP3, MMP9, PLAT and PLAU (Fig. S5A and B). To examine the functional effects of OP-C-P1 on cell growth, we performed three-dimensional (3D) sphere formation assay using VCaP cells. We showed that OP-C-P1 treatment largely decreased the diameters of the spheres of VCaP cells, indicating that OP-C-P1 inhibits VCaP cell growth (Fig. 4A and B). Considering the roles of ERG on metastasis,22 we also performed cell invasion assay to examine whether this ERG OP can inhibit cell invasion. Indeed, the treatment of OP-C- P1 decreased the invasion ability of VCaP cells, showing fewer cells were invaded in OP-C-P1 treated group compared with the control OP-treated group (Fig. 4C and D). Collectively, OP-C-P1- induced degradation of ERG effectively undermines the transcriptional activity of ERG and prostate cancer cell growth and invasion.
Fig.4.
Phthalic acid-based ERG OP inhibits ERG target gene expression and prostate cancer cell growth and invasion. (A and B) The VCaP cells were embedded in matrigel and cultured for 5 days, followed by the treatment of 200 nM of control OP or ERG OP-C-P1 for another five days. The representative images with three-dimension (3D) spheres are shown in (A) and the quantified diameters of 3D spheres are shown in (B). Data are demonstrated with box and whiskers; whiskers represent min to max, and each point is one value of an individual 3D sphere (n = 50). The P value was determined using the unpaired two-tailed Student’s t-test; *** P < 0.001. (C and D) The 22Rv1 cells were transfected with pCMV-HA-ERG and 100 nM of control OP or ERG OP-C-P1, followed by plating on matrigel-coated chambers and incubating for 48 h in 37 °C incubator. The invaded cells were stained with 0.5% of crystal violet. The representative fields are shown in (C) and the quantification data are shown in (D). Data represents means ± SD (n = 4). The P value was determined using the unpaired two-tailed Student’s t-test. ** P < 0.01.
In summary, we identify phthalic acid as a new ligand of CRBN ligase. We showed that phthalic acid-based ERG O’PROTAC significantly inhibited the protein level of ERG via ubiquitination-proteasome pathway and impaired ERG functions in cell growth and invasion. Further studies provide clear evidence that phthalic acid, as a new ligand of CRBN ligase, has a comparable activity with pomalidomide in O’PROTAC. Additionally, phthalic acid is more chemically stable and economical than classical IMiDs; therefore, it can be potentially employed to design O’PROTACs or canonical PROTACs to degrade other transcription factors or POIs. Moreover, considering the limited chemical scaffold of CRBN ligands for designing PROTAC, our finding sheds light on the path to develop new ligands in the field.
Supplementary Material
Acknowledgments
This work was supported in part by the Mayo Clinic Foundation (to H.H.) and the ARA grant (to H.-Y.L), and the National Institutes of Health (R01CA134514 and R01CA130908 to H.H. and R01CA197178 and R01CA249282 to H.-Y.L).
Footnotes
Electronic supplementary information (ESI) available. See DOI:
Conflicts of interest
There are no conflicts to declare.
Notes and references
- 1.Qi SM, Dong J, Xu ZY, Cheng XD, Zhang WD and Qin JJ, Front Pharmacol, 2021, 12, 692574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM and Deshaies RJ, Proceedings of the National Academy of Sciences, 2001, 98, 8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishida T and Ciulli A, SLAS discovery : advancing life sciences R & D, 2021, 26, 484–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zengerle M, Chan K-H and Ciulli A, ACS Chemical Biology, 2015, 10, 1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneekloth AR, Pucheault M, Tae HS and Crews CM, Bioorganic & Medicinal Chemistry Letters, 2008, 18, 5904–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winter GE, Buckley DL, Paulk J, Roberts JM, Souza A, Dhe-Paganon S and Bradner JE, Science (New York, N.Y.), 2015, 348, 1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itoh Y, Ishikawa M, Naito M and Hashimoto Y, Journal of the American Chemical Society, 2010, 132, 5820–5826. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Mi D, Pei H, Duan Q, Wang X, Zhou W, Jin J, Li D, Liu M and Chen Y, Signal Transduct Target Ther, 2020, 5, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward CC, Kleinman JI, Brittain SM, Lee PS, Chung CYS, Kim K, Petri Y, Thomas JR, Tallarico JA, McKenna JM, Schirle M and Nomura DK, ACS Chem Biol, 2019, 14, 2430–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo M, Spradlin JN, Boike L, Tong B, Brittain SM, McKenna JM, Tallarico JA, Schirle M, Maimone TJ and Nomura DK, Cell Chem Biol, 2021, 28, 559–566 e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Crowley VM, Wucherpfennig TG, Dix MM and Cravatt BF, Nat Chem Biol, 2019, 15, 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krönke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, Ciarlo C, Hartman E, Munshi N, Schenone M, Schreiber SL, Carr SA and Ebert BL, Science, 2014, 343, 301–305; [DOI] [PMC free article] [PubMed] [Google Scholar]; Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, Wong KK, Bradner JE and Kaelin WG Jr., Science, 2014, 343, 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito T and Handa H, Proc Jpn Acad Ser B Phys Biol Sci, 2020, 96, 189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito T, Ando H and Handa H, Cellular and molecular life sciences: CMLS, 2011, 68, 1569–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer ES, Böhm K, Lydeard JR, Yang H, Stadler MB, Cavadini S, Nagel J, Serluca F, Acker V, Lingaraju GM, Tichkule RB, Schebesta M, Forrester WC, Schirle M, Hassiepen U, Ottl J, Hild M, Beckwith REJ, Harper JW, Jenkins JL and Thomä NH, Nature, 2014, 512, 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullard A, Nat Rev Drug Discov, 2021, 20, 247–250. [DOI] [PubMed] [Google Scholar]
- 17.Schapira M, Calabrese MF, Bullock AN and Crews CM, Nat Rev Drug Discov, 2019, 18, 949–963. [DOI] [PubMed] [Google Scholar]
- 18.Goracci L, Desantis J, Valeri A, Castellani B, Eleuteri M and Cruciani G, Journal of Medicinal Chemistry, 2020, 63, 11615– 11638; [DOI] [PMC free article] [PubMed] [Google Scholar]; Steinebach C, Ng YLD, Sosič I, Lee C-S, Chen S, Lindner S, Vu LP, Bricelj A, Haschemi R, Monschke M, Steinwarz E, Wagner KG, Bendas G, Luo J, Gütschow M and Krönke J, Chemical Science, 2020, 11, 3474–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min J, Mayasundari A, Keramatnia F, Jonchere B, Yang SW, Jarusiewicz J, Actis M, Das S, Young B, Slavish J, Yang L, Li Y, Fu X, Garrett SH, Yun M-K, Li Z, Nithianantham S, Chai S, Chen T, Shelat A, Lee RE, Nishiguchi G, White SW, Roussel MF, Potts PR, Fischer M and Rankovic Z, Angewandte Chemie International Edition, 2021, 60, 26663–26670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao J, Yan Y, Ding D, Wang D, He Y, Pan Y, Yan W, Kharbanda A, Li H.-y. and Huang H, Advanced Science, 2021, 8, 2102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schumacher H, Smith RL and Williams RT, British Journal of Pharmacology and Chemotherapy, 1965, 25, 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Blee AM, Wang D, An J, Pan Y, Yan Y, Ma T, He Y, Dugdale J, Hou X, Zhang J, Weroha SJ, Zhu WG, Wang YA, DePinho RA, Xu W and Huang H, Cancer Res, 2017, 77, 6524–6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.