Abstract
Objective
Digital pathology (DP) is currently in the spotlight and is rapidly gaining ground, even though the history of this field spans decades. Despite great technological progress, the adoption of DP for routine clinical diagnostic use remains limited.
Methods
A systematic search was conducted in the electronic databases Pubmed-MEDLINE and Embase. Inclusion criteria were all published studies that encompassed any application of DP.
Results
Of 4888 articles retrieved, 4041 were included. Relevant articles were categorized as “diagnostic” (147/4041, 4%) where DP was utilized for routine diagnostic workflow and “non-diagnostic” (3894/4041, 96%) for all other applications. The “non-diagnostic” articles were further categorized according to DP application including “artificial intelligence” (33%), “education” (5%), “narrative” (17%) for reviews and editorials, and “technical” (45%) for pure research publications.
Conclusion
This manuscript provided temporal and geographical insight into the global adoption of DP by analyzing the published scientific literature.
Keywords: Artificial intelligence, digital pathology, image analysis, systematic review, whole slide imaging
Introduction
Digital pathology (DP) is currently in the spotlight and is quickly gaining traction, despite the fact that this field has a relatively long history spanning several decades. 1 Telepathology was one of the initial applications of DP that broke the centuries-old link between pathologist, light microscope, and glass slides. Early digital images were initially acquired using clumsy, but expensive, devices that eventually evolved into robotic microscopes, and finally whole slide imaging (WSI) scanners that we employ today. The ease of digitizing a slide, portability it offers, and ability to manipulate and analyze its pixels, has slowly lead to the widespread adoption of WSI systems. The niche telepathology applications of DP broadened to including virtual educational, research, and primary diagnosis where glass slides no longer need to be viewed using a traditional light microscope. Given the amount of published evidence that has amassed regarding the use or WSI for diagnostic purposes, the College of American Pathologists recently updated their clinical guideline for validating WSI systems for diagnostic purposes in pathology.2,3 Similarly, the European Society for Digital and Integrative Pathology published best practice recommendations for the implementation of a DP workflow. 4 However, despite great strides in recent years and although DP plays an important role in improving the progression of healthcare, the adoption of DP remains limited to only a few pathology laboratories.
The aim of the present manuscript is to provide temporal and geographical insights into the adoption of DP by analyzing the published scientific literature. Moreover, we categorized the included studies in different labels in order to provide an up to date of the literature.
Materials and methods
Literature search and article screening
A systematic review of the literature, without language restrictions, was conducted according to the guideline for Preferred Reporting Items for a Systematic Review and Meta-Analysis (PRISMA). 5 The databases PubMed and Embase were systematically searched until July 2022 to identify any article regarding DP. The search strategy comprised a combination of terms including “digital pathology,” “telepathology,” “deep learning,” “machine learning,” “telecytopathology,” “virtual microscopy,” “artificial intelligence,” “deep neural network” and their spelling variations, and was designed to be an update of the original search performed by Della Mea in 2011. 1 Two authors screened titles and abstract to decide on inclusion of the paper in this study. Inclusion criteria encompassed the application of any kind of DP technique. Data extracted included: authors, year published, country of study, and main topics indicated with a designed label (defined below).
Definition of labels
Papers dealing with DP were included and categorized in two main labels: “diagnostic,” for identifying works in which DP was actually part of routine clinical workflow, and “non-diagnostic” for all other applications, encompassing any other study design (e.g. validation, quality assurance). Additional specifications were added to the “non-diagnostic” label, including “AI” whenever artificial intelligence was involved, “education,” for works that used DP for educational/training purposes, “narrative” for reviews and editorials, and “technical” for pure research works. Some of the papers have had more than one label applied to them.
Graphical depiction
A complete list of included studies was extracted to the Rayyan web application in .csv format and choroplethic maps were generated according to the different labels using a Python-based library which can be found at https://plotly.com/python/choropleth-maps/#county-choropleth-figure-factory.
Results
Literature search findings
A total of 4888 records were found and screened with the aid of the Rayyan reference manager web application. 6 Of these, 4041 papers were finally included in our review, representing studies dealing with DP published over nearly four decades (between 1984 and 2022). The most prolific author was Liron Pantanowitz with 124 papers (3% of all papers), and the most popular journal was Journal of Pathology Informatics (n = 286, 7% of all papers).
Analysis by geographic continent
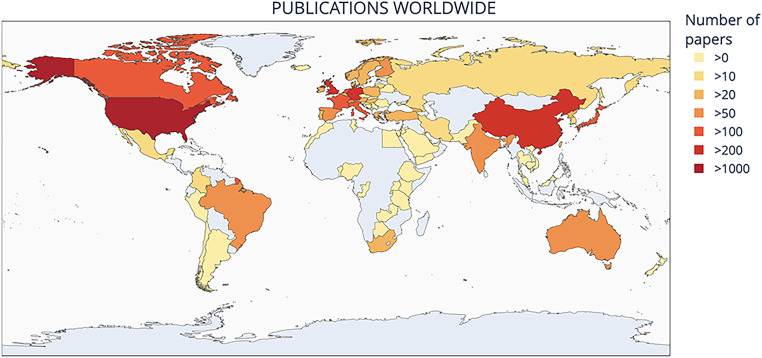
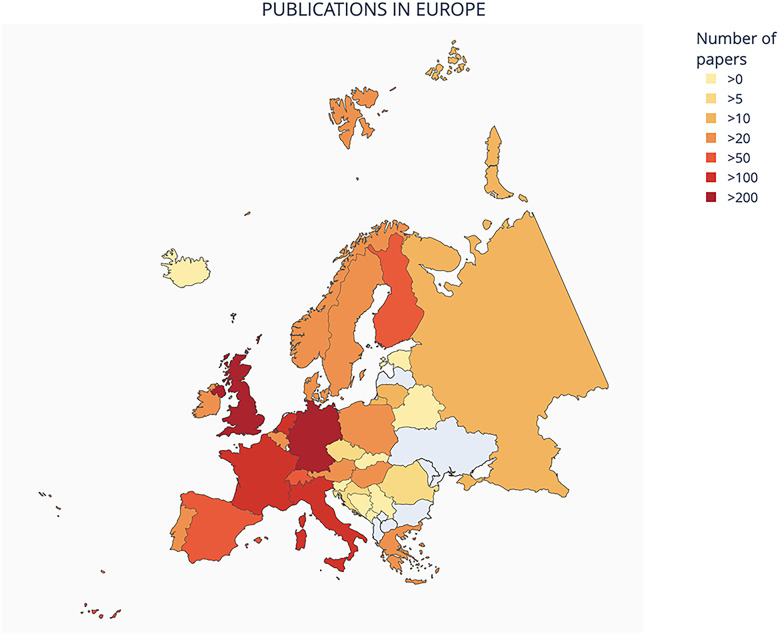
Analysis by continent (Figure 1) reveals that the majority of papers were published by groups based in Europe (n = 1552, 38%) followed by North America (n = 1536, 38%). However, in a subset of papers (n = 26, 0.6%) it was not possible to identify the geographic location of the study. Focusing on European countries, the majority of the studies were from England, followed by Germany, Switzerland, Italy, and then France (Figure 2).
Figure 1.
Number of publications worldwide.
Figure 2.
Number of publications in Europe.
Non-Diagnostic/diagnostic category
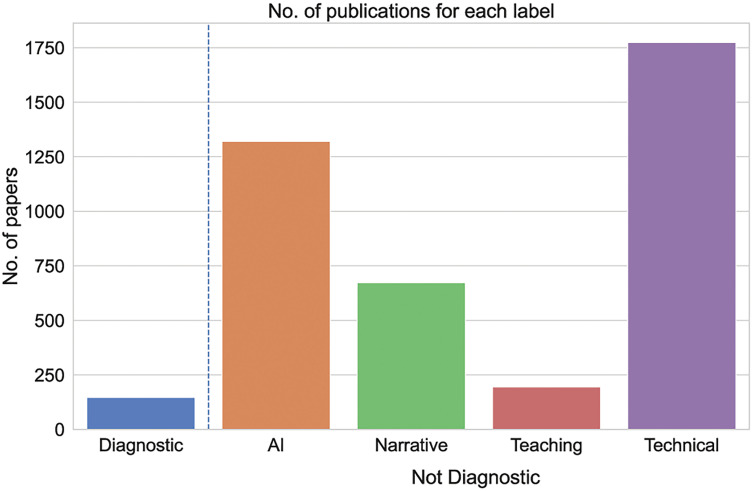
Overall analysis of papers (Figure 3) revealed that 3894 articles were applicable to the “non-diagnostic” category (96%). Among “non-diagnostic” papers, given that some studies of these had more than one label, the majority of the works (n = 1774/3894, 45%) were categorized as “technical” papers, followed by those dealing with “AI” (n = 1312/3894, 33%), “narrative” studies (n = 673/3894, 17%) and then “education” (n = 195/3894, 5%). This distribution of labels was overall similar between Europe and North America. The remaining studies (n = 147, 4%) were categorized into “diagnostic” manuscripts, including a total of eight (n = 8/147, 18%) publications focused on the use of AI.
Figure 3.
Number of publications for each label.
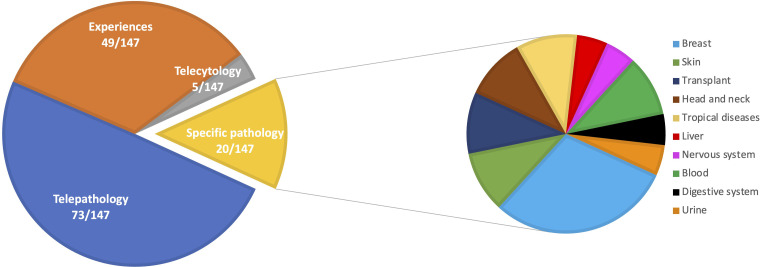
Among the 147 studies of the diagnostic category, most were categorized as telepathology (n = 73, 50%), 49 (33%) are papers that report the specific experiences of adopting DP in their laboratory, 20 (14%) articles focus on specific pathologies, and 5 (3%) articles deal with telecythology (Figure 4).
Figure 4.
Overview of papers of the diagnostic category.
Temporal analysis
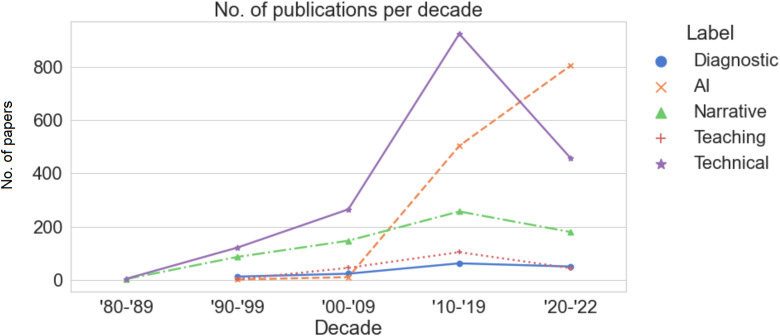
Temporal analysis (Figure 5) shows an overall increasing trend over five decades in the number of papers in each category. Specifically, “technical” papers witnessed a relatively sharp increase in the 2010s, while “AI” papers soared only in recent years.
Figure 5.
Number of publications per decade.
Cytology
Analysis of included studies found that 188 articles dealt with cytology distributed as follows: the majority were “non-diagnostic” (n = 180/188, 96%), while the remaining papers were categorized as “diagnostic” (n = 8/188, 4%).
Discussion
DP has progressed steadily over the last few decades, with content related to both “diagnostic” (clinical) and “non-diagnostic” (non-clinical) applications. The history of DP started in the late 1960s, largely with telepathology. 7 Back in the 1990s, virtual microscopy (or WSI) scanners became commercially available. 8 Since the Food and Drug Administration (FDA) in the USA approved the use of WSI for diagnostic purposes in surgical pathology using formalin-fixed paraffin embedded tissue, 9 DP has developed into a thriving field. The work summarized in this analysis offers an updated pictorial overview of the published literature related to DP and several of its applications.
In spite of all the progress made in the field of DP, deployment of this technology in human clinical environments for full diagnostic use remains limited, even in countries with relatively adequate resources (e.g. North America). Among the DP diagnostic applications studied, publications appeared to largely represent narrations about the adoption of DP in institutions of early adopters,10–15 or provided an account of DP being employed in developing countries (e.g. Tanzania, Cameroon, Pakistan) where there is a critical shortage of pathology services necessary for clinical care.16–21 Clinical applications addressed not only the benefit of digitizing slides in general surgical pathology, but also niche areas of practice such as telepathology for seeking second opinions (teleconsultation) in organ transplantation.22,23 The critical need to provide remote care and education virtually in pathology during the COVID-19 pandemic acted as a catalyst for the adoption of DP around the world.24–28
Despite advances in the application of WSI for general surgical pathology (histopathology), areas such as cytopathology that have lagged behind are slowly gaining traction.29–31 Nevertheless, further research and validation studies demonstrating the diagnostic validity of DP in routine cytology practice is still required. 32 There are several reasons why WSI usage in cytopathology has been slower than its use in histopathology.32–35 This is at least partly due to the unique nature of cytological preparations (e.g. slides with 3D cell groups, thick smears, obscuring material such as blood and/or mucin), as well as the need to screen the entire slide at high magnification in order to examine every single cell. 36 For this reason, scanning a slide with just a single focus layer is insufficient to adequately capture all of the diagnostic information across all z-planes available in a cytological slide. Techniques have been developed to overcome this issue, such as extended focusing (volumetric scanning) or multiple focus levels (z-stacking), but at the cost of longer scan times and larger WSI file sizes. Furthermore, with several of the viewers available today, the examination of z-stacked cytology WSIs remains problematic and may take longer. 34
The generation of DP data at scale creates novel challenges for the pathology community in managing, processing, and governing the use of these data. In this context, the crucial aspect concerns legal and ethical issues, such as the need to ensure the protection of patient data and the continuity of their operations with backed up or stored in secure locations, including secure cloud services. 37 Furthermore, there is a developing literature on the ethics of artificial intelligence in health, including specific legal or regulatory questions such as whether there is a right to an explanation of AI decision-making. Indeed the most dramatic trend observed is the emergence of AI, especially in recent years (publications from 2018 to 2021). In the current era of AI, several deep learning-based solutions are being deployed in practice and in several pathology laboratories justify their transition to DP. Pathologists were among the first providers, several decades ago, to seek computer-assistance for screening cervicovaginal cytology. 38 Since then, AI has been applied for multiple purposes in Pathology, as already happened in other areas of medicine such as ophthalmology and radiology.39,40 Examples range from the simple leveraging of cell detection algorithms to count a specific type of cell,41,42 up to fully analyzing a WSI and thereby rendering a diagnosis.43,44 Many of the publications on AI were very technical, and further work in this area is warranted to validate the utility and outcomes of AI for routine diagnostic use.
Many of the retrieved publications dealing with DP were labeled as “non-diagnostic.” These works are of crucial importance since these publications fuel translational research that enables diagnostic use. Further publications are necessary to support this transformation, specifically clinical validation studies. 45
Limitation
There are several potential limitations of our work. First, as with any other literature review, this review is subject to publication bias. We have evaluated the quantity and type of publications in a bibliometric manner, and publication bias can lead to an underestimation of the global adoption of DP, especially in routine clinical practice. Additionally, for the geographical analysis component we attributed each paper to the country of the primary affiliation of the corresponding author. This may not necessarily be entirely accurate, but at least served as a good approximation. Concerning the labels employed to categorize publications, they were arbitrarily chosen which might introduce some subjectivity. Nevertheless, we attempted to keep the classification of the published literature as simple as possible in order to focus on macro themes, which were useful to provide a global overview.
Conclusion
From the proposed analysis, designed to be an update of the original search performed by Della Mea in 2011, 1 it clearly depicts the evolution of DP, from the first application with telepathology to the development of AI. On 2011, 967 papers related to DP were included in Della Mea analysis; 1 today the literature reveals that DP is a mature technology that is currently deployed in many pathology laboratories and that others are in the process of, or considering, transforming to going fully digital. While there were many clinical and non-clinical publications regarding DP, there is room for more studies about diagnostic applications in the literature. The relevant difference in terms of numbers between “diagnostic” and “non-diagnostic” papers shows that a fully digital approach to the histological workflow has been implemented in a minority of pathology laboratories. One of the most important barriers has been the validation of the systems, including regulatory approval. Furthermore, we must consider that not all the laboratories have been published their own experiences with DP. These laboratories that have embarked on the journey to integrate DP into routine workflow are encouraged to publish their experience.
Footnotes
Contributorship: AE and VDM were involved in the conceptualization of the study. PCR and IG researched literature. PCR, AC, NC, and IG wrote the first draft of the manuscript. EM and VDM analyzed the data and created the figures. LP, SM, AS, APDT, MS, MB, SG, AE, VDM, EM reviewed and edited the manuscript for intellectual content. All the authors approved the final version of the manuscript.
Albino Eccher is currently affiliated with Section of Pathology, Department of Medical and Surgical Sciences for Children and Adults, University of Modena and Reggio Emilia, University Hospital of Modena, Modena, Italy.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: No ethical issues are raised by this systematic review.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: European Union - NextGenerationEU through the Italian Ministry of University and Research under PNRR - M4C2-I1.3 Project PE_00000019 “HEAL ITALIA” CUP: B33C22001030006, and PNRR project IR0000031 “Strengthening BBMRI.it” CUP: B53C22001820006; through Italian Ministry of Health, PNRR PNC-E3-2022-23683266 PNCHLS-DA “HUB Diagnostica Avanzata.” Associazione Italiana Ricerca sul Cancro (AIRC IG 26343).
Guarantor: AE.
ORCID iD: Albino Eccher https://orcid.org/0000-0002-9992-5550
References
- 1.Della Mea V. 25 Years of telepathology research: a bibliometric analysis. Diagn Pathol 2011; 6: S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pantanowitz L, Sinard JH, Henricks WH, et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med 2013; 137: 1710–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans AJ, Brown RW, Bui MM, et al. Validating whole slide imaging systems for diagnostic purposes in pathology. Arch Pathol Lab Med 2022; 146: 440–450. [DOI] [PubMed] [Google Scholar]
- 4.Fraggetta F, L’Imperio V, Ameisen D, et al. Best practice recommendations for the implementation of a digital pathology workflow in the anatomic pathology laboratory by the European Society of Digital and Integrative Pathology (ESDIP). Diagnostics 2021; 11: 2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J 2021; n71(372). doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouzzani M, Hammady H, Fedorowicz Zet al. et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstein RS. Prospects for telepathology. Hum Pathol 1986; 17: 433–434. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira R, Moon B, Humphries J, et al. The virtual microscope. Proc AMIA Annu Fall Symp 1997; n 58(449): 449–453. [PMC free article] [PubMed] [Google Scholar]
- 9.https://www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-whole-slide-imaging-system-digital-pathology.
- 10.Fraggetta F, Caputo A, Guglielmino Ret al. et al. A survival guide for the rapid transition to a fully digital workflow: the “Caltagirone example.” Diagnostics 2021; 11: 1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stathonikos N, Nguyen TQ, Spoto CPet al. et al. Being fully digital: perspective of a Dutch academic pathology laboratory. Histopathology 2019; 75: 621–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Retamero JA, Aneiros-Fernandez J, del Moral RG. Complete digital pathology for routine histopathology diagnosis in a multicenter hospital network. Arch Pathol Lab Med 2020; 144: 221–228. [DOI] [PubMed] [Google Scholar]
- 13.Eloy C, Vale J, Curado M, et al. Digital pathology workflow implementation at IPATIMUP. Diagnostics 2021; 11: 2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temprana-Salvador J, López-García P, Castellví Vives J, et al. DigiPatICS: digital pathology transformation of the Catalan Health Institute Network of 8 hospitals—planification, implementation, and preliminary results. Diagnostics 2022; 12: 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girolami I, Neri S, Eccher A, et al. Frozen section telepathology service: Efficiency and benefits of an e-health policy in South Tyrol. Digit Health 2022; 8. doi: 10.1177/20552076221116776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mremi A, Bentzer NK, Mchome B, et al. The role of telepathology in diagnosis of pre-malignant and malignant cervical lesions: implementation at a tertiary hospital in Northern Tanzania. PLoS One 2022; 17: e0266649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stauch G, Raoufi R, Sediqi A, et al. Erfahrungen mit telepathologie in Nordafghanistan. Die Pathologie 2022; 43: 303–310. [DOI] [PubMed] [Google Scholar]
- 18.Zehra T, Shaikh A, Shams M. Dawn Of artificial intelligence – enable digital pathology in Pakistan: a paradigm shift. J Pak Med Assoc 2021; 71: 2683–2684. [DOI] [PubMed] [Google Scholar]
- 19.Gruber-Mösenbacher U, Katzell L, McNeely M, et al. Digital pathology in Cameroon. JCO Glob Oncol 2021; 1380: 1380–1389. doi: 10.1200/GO.21.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voelker HU, Poetzl L, Strehl Aet al. et al. Telepathological evaluation of paediatric histological specimens in support of a hospital in Tanzania. Afr Health Sci 2020; 20: 1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery ND, Tomoka T, Krysiak R, et al. Practical successes in telepathology experiences in Africa. Clin Lab Med 2018; 38: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eccher A, Neil D, Ciangherotti A, et al. Digital reporting of whole-slide images is safe and suitable for assessing organ quality in preimplantation renal biopsies. Hum Pathol 2016; 47: 115–120. [DOI] [PubMed] [Google Scholar]
- 23.Marletta S, Pantanowitz L, Malvi D, et al. Validation of portable tablets for transplant pathology diagnosis according to the College of American Pathologists Guidelines. Acad Pathol 2022; 9: 100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ardon O, Reuter VE, Hameed M, et al. Digital pathology operations at an NYC tertiary cancer center during the first 4 months of COVID-19 pandemic response. Acad Pathol 2021; 8: 23742895211010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramaswamy V, Tejaswini BN, Uthaiah SB. Remote reporting during a pandemic using digital pathology solution: experience from a tertiary care cancer center. J Pathol Inform 2021; 12: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belfiore A, Centonze G, Maisonneuve P, et al. COVID-19 pandemic: huge stress test for health system could be a great opportunity to update the workflow in a modern surgical pathology. Cancers (Basel) 2021; 13: 3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caputo A, D’Antonio A. Digital pathology: the future is now. Indian J Pathol Microbiol 2021; 64: 6–7. [DOI] [PubMed] [Google Scholar]
- 28.Hassell LA, Peterson J, Pantanowitz L. Pushed across the digital divide: COVID-19 accelerated pathology training onto a new digital learning curve. Acad Pathol 2021; 8: 2374289521994240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al Zoughbi W, Kim D, Alperstein SA, et al. Incorporating cytologic adequacy assessment into precision oncology workflow using telepathology: an institutional experience. Cancer Cytopathol 2021; 129: 874–883. [DOI] [PubMed] [Google Scholar]
- 30.Mosquera-Zamudio A, Hanna MG, Parra-Medina Ret al. et al. Advantage of Z-stacking for teleconsultation between the USA and Colombia. Diagn Cytopathol 2019; 47: 35–40. [DOI] [PubMed] [Google Scholar]
- 31.Krishnamurthy S, Ban K. Feasibility of using digital confocal microscopy for cytopathological examination in clinical practice. Mod Pathol 2022; 35: 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antonini P, Santonicco N, Pantanowitz L, et al. Relevance of the College of American Pathologists guideline for validating whole slide imaging for diagnostic purposes to cytopathology. Cytopathology . 2022; 14(5): 5–14. doi: 10.1111/cyt.13178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanna MG, Pantanowitz L. Why is digital pathology in cytopathology lagging behind surgical pathology? Cancer Cytopathol 2017; 125: 519–520. [DOI] [PubMed] [Google Scholar]
- 34.Girolami I, Pantanowitz L, Marletta S, et al. Diagnostic concordance between whole slide imaging and conventional light microscopy in cytopathology: a systematic review. Cancer Cytopathol 2020; 128: 17–28. [DOI] [PubMed] [Google Scholar]
- 35.Eccher A, Girolami I. Current state of whole slide imaging use in cytopathology: pros and pitfalls. Cytopathology 2020; 31: 372–378. [DOI] [PubMed] [Google Scholar]
- 36.Lee RE, McClintock DS, Laver NMet al. et al. Evaluation and optimization for liquid-based preparation cytology in whole slide imaging. J Pathol Inform 2011; 2: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pantanowitz L, Dickinson K, Evans AJ, et al. American Telemedicine Association clinical guidelines for telepathology. J Pathol Inform 2014; 5: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cenci M, Giovagnoli MR, Olla SVet al. et al. [Automation of cytological analysis of cervical smears]. Minerva Ginecol 2018; 51: 291–298. [PubMed] [Google Scholar]
- 39.FDA News Release. FDA Permits Marketing of Artificial Intelligence-Based Device to detect Certain Diabetes-Related Eye Problems FDA. 2018.
- 40.FDA News Release. FDA Permits Marketing of Artificial Intelligence Algorithm for Aiding Providers in Detecting Wrist Fractures FDFA. 2018.
- 41.Caputo A, D’Antonio A, Memoli Det al. et al. Ki67 in Gleason pattern 3 as a marker of the presence of higher-grade prostate cancer. Appl Immunohistochem Mol Morphol 2021; 29: 112–117. [DOI] [PubMed] [Google Scholar]
- 42.Girolami I, Marletta S, Pantanowitz L, et al. Impact of image analysis and artificial intelligence in thyroid pathology, with particular reference to cytological aspects. Cytopathology 2020; 31: 432–444. [DOI] [PubMed] [Google Scholar]
- 43.Marini N, Marchesin S, Otálora S, et al. Unleashing the potential of digital pathology data by training computer-aided diagnosis models without human annotations. NPJ Digit Med 2022; 5: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girolami I, Pantanowitz L, Marletta S, et al. Artificial intelligence applications for pre-implantation kidney biopsy pathology practice: a systematic review. J Nephrol 2022; 35: 1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rizzo PC, Girolami I, Marletta S, et al. Technical and diagnostic issues in whole slide imaging published validation studies. Front Oncol. 2022; 12: 918580. [DOI] [PMC free article] [PubMed] [Google Scholar]