Abstract
Background:
Opioid overdose deaths have disproportionately impacted Black and Hispanic populations, in part due to disparities in treatment access. Emergency departments (EDs) serve as a resource for patients with opioid use disorder (OUD), many of whom have difficulty accessing outpatient addiction programs. However, inequities in ED treatment for OUD remain poorly understood.
Methods:
This secondary analysis examined racial and ethnic differences in buprenorphine access using data from EMBED, a study of 21 EDs across five health care systems evaluating a clinical decision support system for initiating ED buprenorphine. The primary outcome was receipt of buprenorphine, ED administered or prescribed. Hospital type (academic vs. community) was evaluated as an effect modifier. Hierarchical models with cluster effects for site and clinician were used to assess buprenorphine receipt by race and ethnicity.
Results:
Black patients were less likely to receive buprenorphine (6.4% [51/801] vs. White patients 8.5% [268/3154], odds ratio [OR] 0.59, 95% confidence interval [CI] 0.45–0.78). This association persisted after adjusting for age, insurance, gender, clinician X-waiver, hospital type, and urbanicity (adjusted OR [aOR] 0.64, 95% CI 0.48–0.84) but not when discharge diagnosis was included (aOR 0.75, 95% CI 0.56–1.02). Hispanic patients were more likely to receive buprenorphine (14.8% [122/822] vs. non-Hispanic patients, 11.6% [475/4098]) in unadjusted (OR 1.57, 95% CI 1.09–1.83) and adjusted models (aOR 1.41, 95% CI 1.08–1.83) but not including discharge diagnosis (aOR 1.32, 95% CI 0.99–1.77). Odds of buprenorphine were similar in academic and community EDs by race (interaction p = 0.97) and ethnicity (interaction p = 0.64).
Conclusions:
Black patients with OUD were less likely to receive buprenorphine whereas Hispanic patients were more likely to receive buprenorphine in academic and community EDs. Differences were attenuated with discharge diagnosis, as fewer Black and non-Hispanic patients were diagnosed with opioid withdrawal. Barriers to medication treatment are heterogenous among patients with OUD; research must continue to address the multiple drivers of health inequities at the patient, clinician, and community level.
BACKGROUND
Medications for opioid use disorder (MOUD) are the most effective means to reduce opioid-related complications, including drug-related infections, overdose, and death.1–3 With the opioid epidemic worsening across the United States, health care organizations have focused efforts on increasing the availability of MOUD.4–6 However, not all patient populations with opioid use disorder (OUD) have equal access to MOUD.7–15 From 2007 to 2017, communities in the United States with higher percentages of non-White residents experienced slower growth of buprenorphine than those with more White residents.16,17 Even after age, sex, and insurance status were adjusted for, Black patients had significantly lower odds of receiving buprenorphine in ambulatory settings compared to White patients.18 The disparities in access to MOUD have likely contributed to the disproportionate increase in drug overdose deaths in Black, Hispanic, and American Indian populations.18,19
Emergency departments (EDs) have emerged as an important health care access point for patients with OUD that may address challenges accessing traditional outpatient addiction services.20–22 With an estimated 0.5–2 million annual visits for opioid-related conditions, EDs care for a disproportionate number of patients with social vulnerabilities that lead to difficulty accessing outpatient MOUD resources.23–29 In response, many EDs have implemented low barrier treatment protocols to immediately initiate buprenorphine to bridge patients until outpatient linkage is established.30–33
Despite the ED’s role as a safety net resource, recent literature has emerged demonstrating that Black and Hispanic patients are less likely to receive an outpatient prescription for buprenorphine after being treated in an ED and are less likely to initiate MOUD within 90 days following an ED visit for a nonfatal opioid-related overdose.12,34 Knowledge of whether the same inequities in treatment of OUD exist in the ED is limited. Two single-center observational studies have evaluated MOUD disparities in the ED: the first found that Black and Hispanic patients had a lower likelihood of receiving MOUD compared to White patients (35.9% and 59.9% vs. 80.3%); the second found that Black non-Hispanic (vs. non-Black) patients were less likely to receive behavioral counseling following an ED visit for an opioid overdose.35,36
To date, the literature on ED MOUD has focused on single-site practices or administrative claims. A deeper understanding of inequities in ED MOUD administration, including at the patient, provider, and hospital level, is critical to creating systems that directly address those disparities. To address this gap in knowledge, we explore differences in ED buprenorphine administration by race and ethnicity across 21 EDs using data collected from a national pragmatic group randomized clinical trial. Further, we assess hospital affiliation as a mediator of differences in ED buprenorphine by race and ethnicity, as academic EDs administered buprenorphine at higher rates compared to community hospitals.37–39
METHODS
Study design
We conducted a secondary analysis using data collected from the EMBED (EMergency department-initiated BuprenorphinE for opioid use Disorder) trial. EMBED was a multicenter cluster-randomized trial evaluating the effect of a user-centered clinical decision support system on rates of buprenorphine administration and prescriptions in 21 EDs representing five health care systems across five states from November 2019 to May 2021.39 For the EMBED trial, data were collected from the electronic health record (EHR) of each health care system, deidentified, and aggregated monthly to a central location for analysis. The study protocol was approved by a central institutional review board. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline was used to ensure the reporting of this cross-sectional study.
Study setting and population
The same inclusion and exclusion criteria for the original EMBED trial were used in this analysis. Specifically, adults 18 years or older with an OUD-related ED visit were included. OUD-related ED visits were determined using a previously validated EHR phenotype composed of two algorithms that included International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10)–related diagnoses as well as chief complaints consistent with both opioid overdose and withdrawal. Algorithm 1 utilized opioid ICD-10 diagnostic codes associated with the ED visit. Algorithm 2 identified patients who have not been captured by Algorithm 1 but had information in their ED chief complaint suggestive of OUD, flagging patients if the words heroin, opiate, opioid, or Narcan were included in the chief complaint for the ED visit. The EHR phenotype had a positive predictive value of 0.95 (95% confidence interval [CI] 0.851–0.989) and a negative predictive value of 0.92 (95% CI 0.807–0.978) for identifying OUD-related visits.40
Patients were excluded if they were on MOUD prior to the index ED visit, including buprenorphine, methadone, or naltrexone, as determined by their documented active medications in the EHR. Additionally, participants were excluded if they were admitted to the hospital or an inpatient psychiatric unit, were pregnant, or died in the ED. Future visits following the index ED visit were also excluded from data collection.
Measures
Race and ethnicity
Race and ethnicity were collected from EHR data recorded during the ED registration process or captured from a prior hospital visit. Thus, data collection strategies were not uniform and were based on each facility’s existing registration processes. Race and ethnicity were recorded in the EHR, either Epic (Epic Systems Corporation, n = 3) or Cerner (Cerner Corporation, n = 2) as separate variables. Race was categorized by study sites in the EMBED data collection forms as White, Black or African American, Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, other (including multiple races), and unknown. Ethnicity was categorized as Hispanic, non-Hispanic, and other/unknown. Race and ethnicity were treated as related but distinct dimensions of social identity; thus, race and ethnicity were both examined independently as separate social constructs for the primary outcome as well as combined into a single ethnoracial coding construct as a sensitivity analysis with the following categories: Hispanic, White non-Hispanic, Black non-Hispanic, and other.41,42
Patient-, clinician-, and site-level variables
Patient-level variables included patient age, gender, insurance, and opioid-related ED discharge diagnoses (opioid overdose, opioid withdrawal, other OUD diagnosis). ED clinician variables included gender, age (categorized as <35, 35–44, and >45 years old), professional role (attending, fellow, advanced practitioner, resident), and DEA X-waiver status of the treating clinician (not waivered, waivered before the start of the study, waivered after the start of the study). Hospital site variables include affiliation (community, academic), urbanicity (rural, suburban, urban), and annual volume of patients with OUD.
Outcomes
The primary outcome was the proportion of participants who received buprenorphine at the index ED visit, defined either as buprenorphine administration in the ED or as a discharge prescription. All concentrations and formulations of buprenorphine and buprenorphine/naloxone, including sublingual tablets, buccal/sublingual films, transdermal patches, and intravenous injections were included. Receipt of buprenorphine was further stratified into administered in the ED and written discharge prescriptions.
Secondary outcomes included the association of hospital type on receipt of ED buprenorphine by race and ethnicity. Type of hospital was chosen as a clinically relevant mediator because academic EDs had a significantly higher rate of buprenorphine administration compared to community hospitals in the original EMBED trial.39 It was unknown if academic EDs would also have a different rate of ED buprenorphine by race and ethnicity compared to community EDs.
Data analysis
Descriptive statistics were used to summarize receipt of buprenorphine among racial and ethnic groups, with frequency and percentage for categorical variables and mean and standard deviation (SD) for continuous variables. Generalized linear mixed-effect multilevel models (GLIMMIX) were used to evaluate differences in buprenorphine administration, for both unadjusted and adjusted analyses. Random effects were included to account for clustering at the level of the ED clinician and hospital sites. The adjusted analysis included the following covariates: participant age, insurance, gender, ED clinician X-waiver status, hospital type, and hospital urbanicity. The primary analyses were done for original race groups and ethnicity group separately. A sensitivity analysis was performed using a categorical variable that combined race and ethnicity. The potential heterogeneous effect between academic and community hospitals were assessed by including an interaction term in the model. To explore the potential underlying etiologies of racial disparities based on differences in demographic data by race and ethnicity, analyses were performed by adding discharge diagnosis as covariate in the model. All analyses were performed in SAS version 9.4. Statistical significance was set at p < 0.05, two-sided.
RESULTS
From November 2019 to May 2021, a total 5047 unique patients were discharged from a study ED with an OUD-related diagnosis, of whom 3613 (71.6%) were White, 858 (17.0%) were Black, 15 (0.3%) were Asian, 13 (0.3%) were American Indian or Alaska Native, seven (0.1%) were Native Hawaiian or other Pacific Islander, and 380 (7.5%) were other races, including those who may have identified as more than one race. Race was unknown or not reported for 161 (3.2%) patients. Compared to White patients, Black patients were older (mean ± SD 42.8 ± 14.3 vs. 38.6 ± 12.7), more likely to be male (70% [601/858] vs. 63.6% [2298/3613]), more likely to have Medicaid insurance (42.3% [363/858] vs. 34.3% [1226/3613]), and less likely to have an ED discharge diagnosis of opioid withdrawal (12.4% [106/858] vs. 17.8% [644/3613]; Table 1).
TABLE 1.
Patient, clinician, and hospital demographics for patients presenting to the ED for an opioid-related diagnosis stratified by race.
| Black (n = 858) | White (n = 3613) | American Indian or Alaska Native (n = 13) | Native Hawaiian or Pacific Islander (n = 7) | Asian (n = 15) | Other (n = 380) | Unknown or not reported (n = 161) | |
|---|---|---|---|---|---|---|---|
| Participant characteristics | |||||||
| Age (years) | 42.8 (±14.3) | 38.6 (±12.7) | 44.2 (±14.4) | 32.0 (±11.7) | 33.3 (±10.7) | 36.9 (±13.1) | 38.0 (±14.3) |
| Gender | |||||||
| Female | 257 (30.0) | 1315 (36.4) | 4 (30.8) | 2 (28.6) | 6 (40.0) | 98 (25.8) | 48 (29.8) |
| Male | 601 (70.0) | 2298 (63.6) | 9 (69.2) | 5 (71.4) | 9 (60.0) | 282 (74.2) | 113 (70.2) |
| Insurance | |||||||
| Medicaid | 363 (42.3) | 1226 (34.3) | 5 (38.5) | 4 (57.1) | 8 (61.5) | 236 (63.1) | 57 (35.8) |
| Medicare | 132 (15.4) | 404 (11.3) | 5 (38.5) | 1 (14.3) | 1 (7.7) | 36 (9.6) | 8 (5.0) |
| Private | 124 (14.5) | 932 (26.1) | 2 (15.4) | 1 (14.3) | 1 (7.7) | 22 (5.9) | 35 (22.0) |
| Self-pay | 209 (24.4) | 887 (24.8) | 1 (7.7) | 1 (14.3) | 3 (23.1) | 74 (19.8) | 55 (34.6) |
| Diagnosis | |||||||
| Opioid overdose | 307 (35.8) | 1255 (34.7) | 5 (38.5) | 3 (42.9) | 7 (46.7) | 116 (30.5) | 61 (37.9) |
| Opioid withdrawal | 106 (12.4) | 644 (17.8) | 2 (15.4) | 0 (0.0) | 2 (13.3) | 79 (20.8) | 25 (15.5) |
| ED clinician characteristics | |||||||
| Clinician gender | |||||||
| Female | 234 (27.3) | 1001 (27.7) | 5 (38.5) | 2 (28.6) | 3 (20.0) | 112 (29.5) | 37 (23.0) |
| Male | 592 (69.0) | 2408 (66.6) | 8 (61.5) | 5 (71.4) | 12 (80.0) | 262 (68.9) | 118 (73.3) |
| Clinician age (years) | |||||||
| <35 | 142 (17.2) | 657 (19.3) | 3 (23.1) | 0 (0.0) | 1 (6.7) | 49 (13.1) | 31 (20.0) |
| 35–44 | 367 (44.4) | 1373 (40.3) | 4 (30.8) | 0 (0.0) | 9 (60.0) | 162 (43.3) | 71 (45.8) |
| >45 | 317 (38.4) | 1379 (40.5) | 6 (46.2) | 7 (100.0) | 5 (33.3) | 163 (43.6) | 53 (34.2) |
| Clinician DEA X-waiver | |||||||
| Waivered before trial | 389 (45.3) | 1522 (42.1) | 6 (46.2) | 2 (28.6) | 7 (46.7) | 105 (27.6) | 73 (45.3) |
| Waivered during trial | 152 (17.7) | 600 (16.6) | 0 (0.0) | 0 (0.0) | 3 (20.0) | 44 (11.6) | 26 (16.1) |
| Not waivered | 285 (33.2) | 1285 (35.6) | 7 (53.8) | 5 (71.4) | 5 (33.3) | 225 (59.2) | 56 (34.8) |
| Unknown | 32 (3.7) | 206 (5.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (1.6) | 6 (3.7) |
| Hospital characteristics | |||||||
| Type of ED | |||||||
| Community | 456 (53.1) | 2027 (56.1) | 9 (69.2) | 5 (71.4) | 12 (80.0) | 328 (86.3) | 71 (44.1) |
| Academic | 402 (46.9) | 1586 (43.9) | 4 (30.8) | 2 (28.6) | 3 (20.0) | 52 (13.7) | 90 (55.9) |
| Urbanicity | |||||||
| Rural | 67 (7.8) | 318 (8.8) | 2 (15.4) | 0 (0.0) | 1 (6.7) | 19 (5.0) | 10 (6.2) |
| Suburban | 81 (9.4) | 669 (18.5) | 2 (15.4) | 1 (14.3) | 3 (20.0) | 142 (37.4) | 28 (17.4) |
| Urban | 710 (82.8) | 2626 (72.7) | 9 (69.2) | 6 (85.7) | 11 (73.3) | 219 (57.6) | 123 (76.4) |
Note: Data are reported as mean (±SD) or n (%).
With regard to ethnicity, 822 (16.3%) participants were Hispanic, 4098 (81.2%) were non-Hispanic, and 127 (2.5%) had an unknown ethnicity. Compared to non-Hispanic patients, Hispanic patients were more likely to be male (73.1% [601/822] vs. 64.1% [2627/4098]), to have Medicaid (47.5% [388/822] vs. 36.8% [1485/4098]), and to present with an opioid overdose diagnosis (41.8% [344/822] vs. 33.5% [1374/4098]; Table 2).
TABLE 2.
Patient, clinician, and hospital demographics for patients presenting to the ED for an opioid-related diagnosis stratified by ethnicity.
| Non-Hispanic (n = 4098) | Hispanic (n = 822) | Unknown or not reported (n = 127) | |
|---|---|---|---|
| Patient characteristics | |||
| Age (years) | 39.3 (±13.3) | 38.3 (±12.5) | 38.5 (±14.3) |
| Gender | |||
| Female | 1471 (35.9) | 221 (26.9) | 38 (29.9) |
| Male | 2627 (64.1) | 601 (73.1) | 89 (70.1) |
| Insurance | |||
| Medicaid | 1485 (36.8) | 388 (47.5) | 26 (21.0) |
| Medicare | 510 (12.6) | 68 (8.3) | 9 (7.3) |
| Private | 854 (21.1) | 238 (29.2) | 25 (20.2) |
| Self-pay | 1069 (26.5) | 99 (12.1) | 62 (50.0) |
| Other | 83 (2.1) | 18 (2.2) | 0 (0.0) |
| Unknown or not reported | 39 (1.0) | 5 (0.6) | 2 (1.6) |
| Diagnosis | |||
| Overdose | 1374 (33.5) | 344 (41.8) | 36 (28.3) |
| Withdrawal | 687 (16.8) | 151 (18.4) | 20 (15.7) |
| ED clinician characteristics | |||
| Clinician gender | |||
| Female | 1077 (26.3) | 292 (35.5) | 25 (19.7) |
| Male | 2798 (68.3) | 518 (63.0) | 89 (70.1) |
| Clinician age (years) | |||
| <35 | 729 (18.8) | 135 (16.7) | 19 (16.7) |
| 35–44 | 1568 (40.5) | 362 (44.7) | 56 (49.1) |
| 45+ | 1578 (40.7) | 313 (38.6) | 39 (34.2) |
| DEA X-waiver status | |||
| Waivered before trial | 1631 (39.8) | 420 (51.1) | 53 (41.7) |
| Waivered during trial | 719 (17.5) | 76 (9.2) | 30 (23.6) |
| Not waivered | 1523 (37.2) | 314 (38.2) | 31 (24.4) |
| Unknown | 225 (5.5) | 12 (1.5) | 13 (10.2) |
| Hospital characteristics | |||
| Type of ED | |||
| Community | 2432 (59.3) | 425 (51.7) | 51 (40.2) |
| Academic | 1666 (40.7) | 397 (48.3) | 76 (59.8) |
| Urbanicity | |||
| Rural | 392 (9.6) | 18 (2.2) | 7 (5.5) |
| Suburban | 739 (18.0) | 168 (20.4) | 19 (15.0) |
| Urban | 2967 (72.4) | 636 (77.4) | 101 (79.5) |
Note: Data are reported as mean (±SD) or n (%).
With respect to the ED clinician, 67.5% of participants were treated by an ED clinician who was male (3405/5407), 81.6% by an ED clinician greater than 35 years old (3916/5407), and 58% by an ED clinician who received their DEA X-waiver before (41.7%, 2104/5407) or during the trial period (16.3%, 825/5407). For hospital site variables, 42.4% of participants were seen in an academic ED (2139/5407), with 73.4% of participants were treated in an ED classified as urban (3704/5407), compared to 18.3% (926/5407) in a suburban ED and 8.3% (417/5407) in a rural ED.
Primary outcome
Overall, 12.2% (618/5047) of patients received buprenorphine (either during the ED visit or as a prescription), including 13.1% (473/3613) of White patients, 8.9% (76/858) of Black patients, 10.5% (40/380) of patients identified as other race, and 15.4% (2/13) of American Indian or Alaska Native patients. No Asian and Native Hawaiian or Pacific Islander patients (0/35) received buprenorphine. Black patients were less likely than White patients to receive buprenorphine in unadjusted (OR 0.59, 95% CI 0.45–0.78, p < 0.001) and after adjusting for age, insurance, gender, ED clinician X-waiver status, hospital type, and urbanicity (OR 0.64, 95% CI 0.48–0.84, p = 0.001). However, when discharge diagnosis was added to the model, the difference in receipt of buprenorphine between Black and White patients was no longer statistically significant (OR 0.75, 95% CI 0.55–1.02, p = 0.07). There was no significant difference between Black and other or unknown groups. Comparisons between Asian, Native Hawaiian or Pacific Islander, and American Indian or Alaska Native patients did not converge due to the small number of participants.
Hispanic patients were more likely to receive buprenorphine (administered in the ED or prescribed) than non-Hispanic patients (14.8% [122/822] vs. 11.6% [475/4098]). This finding persisted in both unadjusted models (OR 1.57, 95% CI 1.09–1.83, p = 0.01) and after adjusting for age, insurance, gender, ED clinician X-waiver status, hospital type, and urbanicity (OR 1.41, 95% CI 1.08–1.83, p = 0.01). The finding was not statistically significant when discharge diagnosis was added to the model (OR 1.32, 95% CI 0.99–1.77, p = 0.06).
Secondary outcomes
Compared to White patients, Black patients were less likely to be administered buprenorphine in the ED only in unadjusted and adjusted models excluding diagnosis (unadjusted OR 0.62, 95% CI 0.45–0.85, p = 0.003; adjusted without diagnosis OR 0.65, 95% CI 0.47–0.90, p = 0.009). When discharge diagnosis was included, the finding was not statistically significant (adjusted with diagnosis OR 0.81, 95% CI 0.56–1.17, p = 0.26).
Compared to non-Hispanic patients, Hispanic patients were more likely to be administered buprenorphine in the ED only (un-adjusted OR 1.51 95% CI 1.12–2.05, p = 0.007; adjusted without diagnosis OR 1.50, 95% CI 1.11–2.03, p = 0.009; and adjusted with diagnosis OR 1.43, 95% CI 1.01–2.01, p = 0.04). There was no difference among White and Black patients or among non-Hispanic and Hispanic patients in buprenorphine prescribed from the ED only after adjusting for clinically relevant variables (Table 3).
TABLE 3.
Odds of buprenorphine administration after an index ED visit for an opioid-related complaint by race or ethnicity.
| Unadjusted OR (95% CI)* | p-value | Adjusted OR (95% CI) | p-value | Adjusted with Dx, OR (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Buprenorphine administered in ED or prescribed at discharge | ||||||
| Black vs. White | 0.59 (0.45–0.78) | 0.0002 | 0.64 (0.48–0.84) | 0.002 | 0.75 (0.55–1.02) | 0.07 |
| Other vs. White | 1.29 (0.87–1.92) | 0.21 | 1.38 (0.92–2.06) | 0.12 | 1.25 (0.81–1.95) | 0.31 |
| Unknown vs. White | 1.01 (0.64–1.59) | 0.98 | 1.03 (0.65–1.65) | 0.89 | 1.18 (0.70–1.99) | 0.53 |
| Hispanic vs. non-Hispanic | 1.41 (1.09–1.83) | 0.01 | 1.41 (1.08–1.83) | 0.01 | 1.32 (0.99–1.77) | 0.06 |
| Unknown vs. non-Hispanic | 1.06 (0.63–1.78) | 0.82 | 1.06 (0.63–1.81) | 0.82 | 1.14 (0.64–2.02) | 0.66 |
| Buprenorphine administered in ED | ||||||
| Black vs. White | 0.62 (0.45–0.85) | 0.003 | 0.65 (0.47–0.90) | 0.009 | 0.81 (0.56–1.17) | 0.26 |
| Other vs. White | 1.40 (0.92–2.11) | 0.12 | 1.51 (0.99–2.31) | 0.06 | 1.36 (0.85–2.19) | 0.20 |
| Unknown vs. White | 0.99 (0.58–1.68) | 0.97 | 1.02 (0.59–1.75) | 0.95 | 1.27 (0.68–2.37) | 0.45 |
| Hispanic vs. non-Hispanic | 1.51 (1.12–2.05) | 0.007 | 1.50 (1.11–2.03) | 0.009 | 1.43 (1.01–2.01) | 0.04 |
| Unknown vs. non-Hispanic | 0.99 (0.54–1.83) | 0.97 | 0.97 (0.52–1.81) | 0.92 | 1.08 (0.54–2.17) | 0.83 |
| Buprenorphine prescribed at discharge | ||||||
| White vs. Black | 0.67 (0.49–0.91) | 0.01 | 0.76 (0.55–1.04) | 0.09 | 0.90 (0.64–1.25) | 0.52 |
| Other vs. White | 1.21 (0.71–2.06) | 0.49 | 1.30 (0.75–2.24) | 0.35 | 1.18 (0.66–2.09) | 0.58 |
| Unknown vs. White | 1.29 (0.79–2.13) | 0.31 | 1.37 (0.82–2.28) | 0.23 | 1.55 (0.90–2.66) | 0.11 |
| Hispanic vs. non-Hispanic | 1.38 (1.02–1.88) | 0.04 | 1.36 (1.00–1.85) | 0.05 | 1.29 (0.93–1.79) | 0.13 |
| Unknown vs. non-Hispanic | 1.24 (0.71–2.18) | 0.45 | 1.24 (0.70–2.21) | 0.46 | 1.34 (0.73–2.45) | 0.34 |
Odds ratios and P values from Glimmix hierarchical models accounting for clustering at the level of the clinician and health care system, both unadjusted, adjusted for age, insurance, gender, clinician X-waiver status, hospital type, and urbanicity with and without discharge diagnosis.
Hospital type and receipt of buprenorphine by race and ethnicity
Of participants who presented to a community ED, 5.5% (111/2027) of White participants received buprenorphine whereas 2.9% (13/456) of Black participants received buprenorphine. In comparison, of participants who presented to an academic ED, 22.8% (362/1586) of White participants received buprenorphine whereas 15.7% (63/402) of Black participants received buprenorphine. There was no significant interaction between race and hospital type. Although the overall rate of buprenorphine administration was higher in academic sites, the differences in the rate of buprenorphine administration between Black and White patients was similar in academic and community EDs (OR 0.65, 95% CI 0.48–0.89 vs. 0.58, 0.31–1.08, academic vs. nonacademic respectively, interaction p = 0.97; Figure 1).
FIGURE 1.
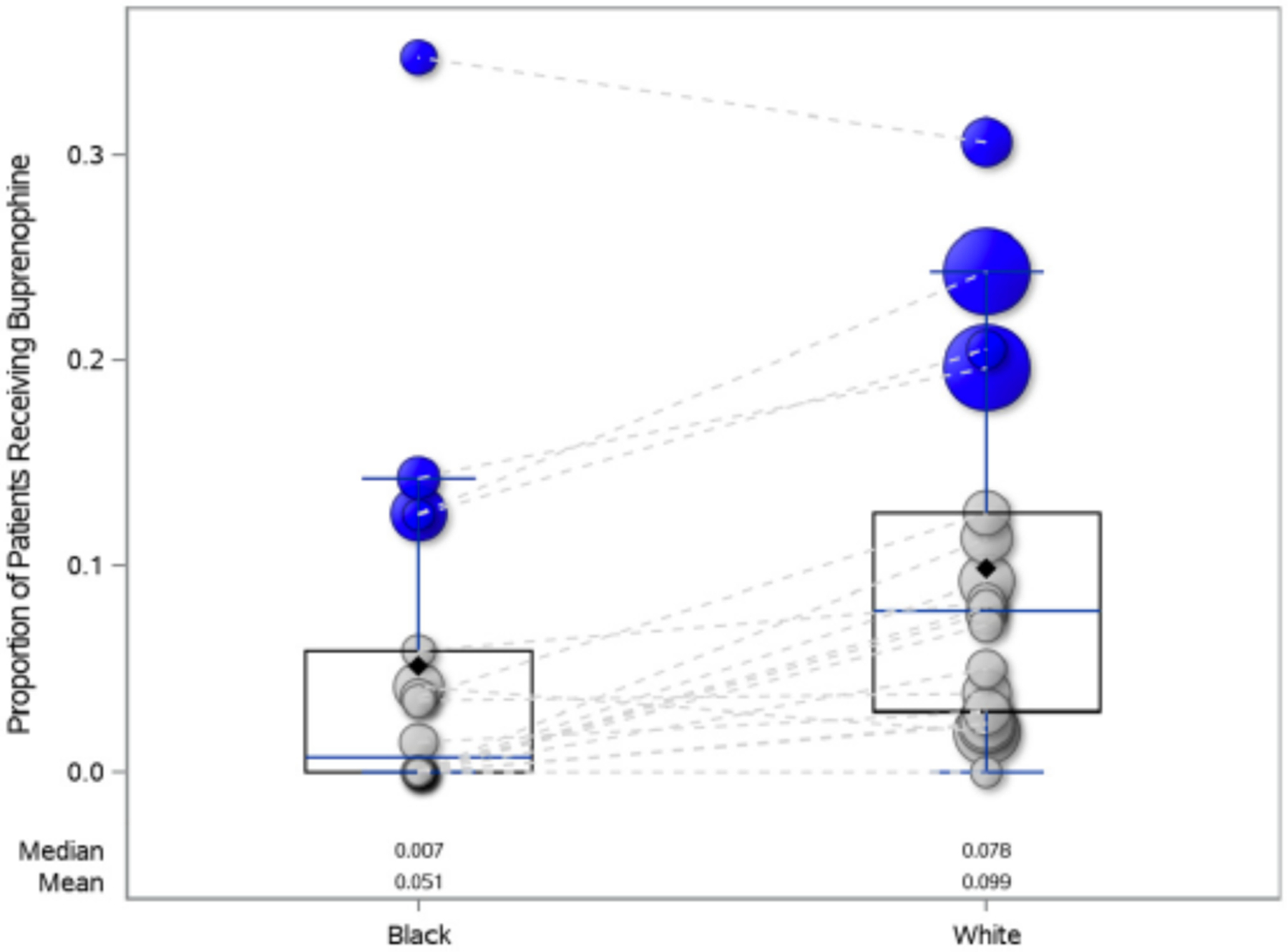
Bubble plot of the proportion of Black and White patients receiving buprenorphine (either administered or prescribed at discharge) by ED. The color of the bubble indicates the type of ED (blue is academic and gray is community) and the size of the bubble is representative of the proportion of participants from each ED in the study, with larger bubbles representing proportionally more patients. The dashed lines connect each unique ED. There was no significant interaction between type of ED and the proportion of patients receiving buprenorphine by race (interaction p = 0.97).
Of participants who presented to a community ED, 5.9% (25/425) of Hispanic participants received buprenorphine whereas 4.9% (120/2432) of non-Hispanic participants received buprenorphine. In comparison, of participants who presented to an academic ED, 24.4% (97/397) of Hispanic participants received buprenorphine whereas 21.3% (355/1666) of non-Hispanic participants received buprenorphine. There was no significant interaction between ethnicity and type of ED (OR of Hispanic vs. non-Hispanic, 1.34, 95% CI 0.98–1.83 vs. 1.60, 95% CI 0.98–2.60, academic vs. nonacademic, respectively; interaction p = 0.64); the differences in the rate of buprenorphine administration for Hispanic and non-Hispanic patients was similar in academic and community EDs (Figure 2).
FIGURE 2.
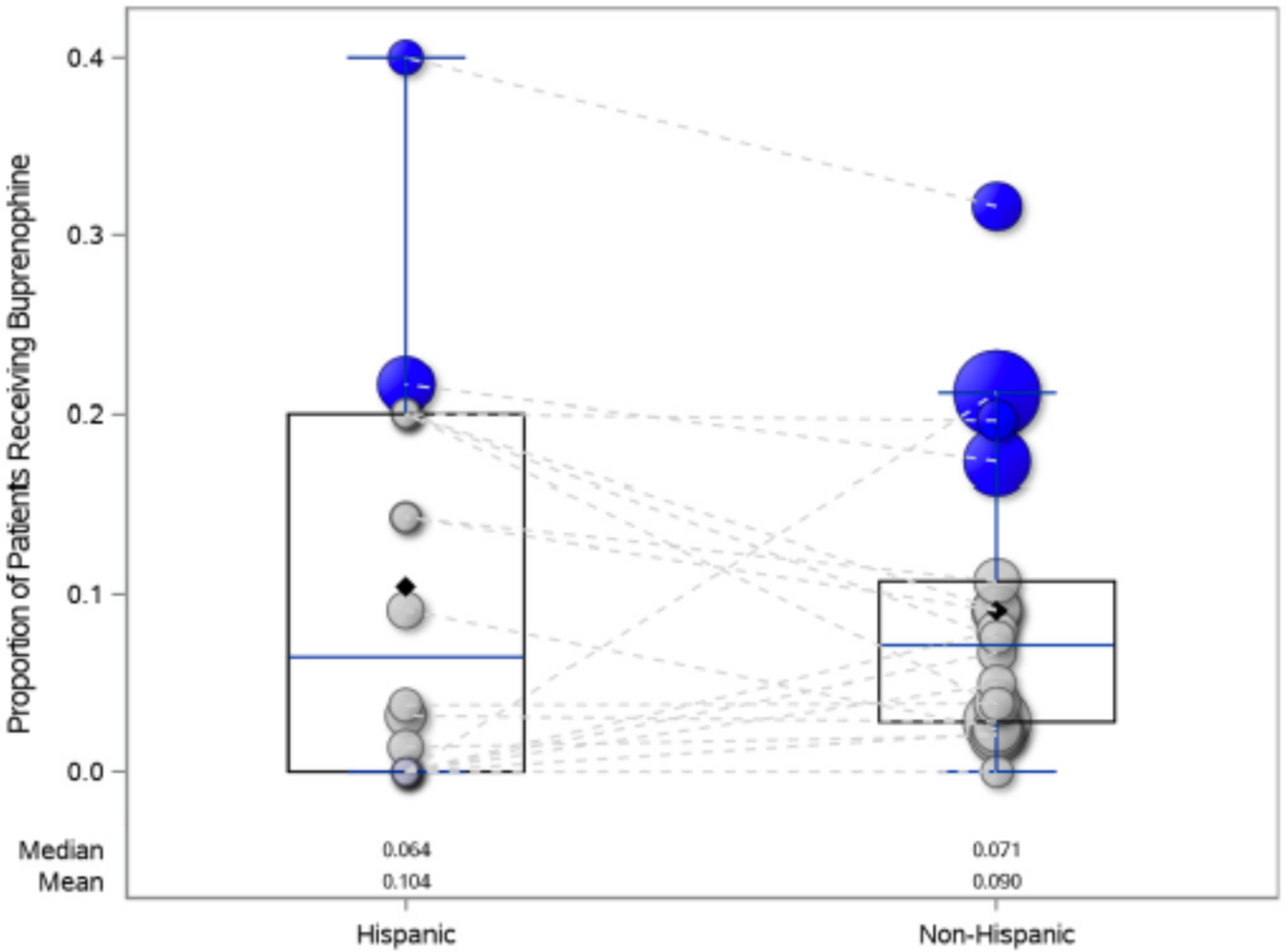
Bubble plot of the proportion of Hispanic and non-Hispanic patients receiving buprenorphine (either administered in the ED or prescribed at discharge) by ED. The color of the bubble indicates the type of ED (blue is academic and gray is community) and the size of the bubble is representative of the proportion of participants from each ED in the study, with larger bubbles representing proportionally more patients. The dashed lines connect each unique ED. There was no significant interaction between type of ED and the proportion of patients receiving buprenorphine by ethnicity (interaction p = 0.64).
Discharge diagnosis and receipt of buprenorphine by race and ethnicity
Of the 17% (858/5047) total participants with a diagnosis of opioid withdrawal, 39% (251/644) of White participants received buprenorphine whereas 35.9% (38/106) of Black participants received buprenorphine. In comparison, of the 34.8% (1754/5047) total participants with a diagnosis of opioid overdose, 4.8% (60/1255) of White participants received buprenorphine whereas 3.9% (12/307) of Black participants received buprenorphine.
With regard to ethnicity, of participants with a diagnosis of opioid withdrawal, 42.4% (64/151) of Hispanic participants received buprenorphine whereas 36.8% (253/687) of non-Hispanic participants received buprenorphine. In comparison, of participants with a diagnosis of opioid overdose, 8.1% (28/344) of Hispanic participants received buprenorphine whereas 3.7% (51/1374) of non-Hispanic participants received buprenorphine.
Sensitivity analysis
A sensitivity analysis examined race and ethnicity as a single variable with Hispanic, White non-Hispanic and Black non-Hispanic categories. Black non-Hispanic patients (n = 801) had lower odds of receiving buprenorphine (either administered or prescribed) compared to White non-Hispanic patients (n = 3154; unadjusted OR 0.63, 95% CI 0.48–0.84, p = 0.002; and adjusted OR 0.70, 95% CI 0.52–0.94, p = 0.02). Hispanic (n = 822) patients had higher odds of receiving buprenorphine compared to White non-Hispanic patients (unadjusted OR 1.31, 95% CI 1.00–1.71, p = 0.047; adjusted OR 1.33, 95% CI 1.02–1.74, p = 0.04). When discharge diagnosis was added to the model, the difference in receipt of buprenorphine between Black non-Hispanic and Hispanic patients with White non-Hispanic patients was no longer statistically significant (Table 4).
TABLE 4.
Sensitivity analysis of the Odds of buprenorphine administration after an index ED visit for an opioid-related complaint by combined race and ethnicity classifications.
| Unadjusted OR (95% CI)* | p-value | Adjusted OR (95% CI) | p-value | Adjusted with Dx, OR (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Buprenorphine administered in ED or prescribed at discharge | ||||||
| Non-Hispanic Black | 0.63 (0.48–0.84) | 0.002 | 0.70 (0.52–0.94) | 0.02 | 0.82 (0.60–1.12) | 0.21 |
| Hispanic | 1.31 (1.00–1.71) | 0.047 | 1.33 (1.02–1.74) | 0.04 | 1.29 (0.96–1.73) | 0.09 |
| Other | 0.90 (0.60–1.34) | 0.61 | 0.95 (0.63–1.43) | 0.79 | 1.11 (0.71–1.72) | 0.65 |
| Non-Hispanic White | 1 | 1 | 1 | |||
| Buprenorphine administered in ED | ||||||
| Non-Hispanic Black | 0.64 (0.46–0.89) | 0.008 | 0.70 (0.50–0.98) | 0.04 | 0.86 (0.60–1.26) | 0.44 |
| Hispanic | 1.40 (1.03–1.90) | 0.03 | 1.41 (1.04–1.92) | 0.03 | 1.40 (0.99–1.98) | 0.06 |
| Other | 1.40 (1.03–1.90) | 0.36 | 0.85 (0.52–1.37) | 0.50 | 1.03 (0.61–1.75) | 0.91 |
| Non-Hispanic White | 1 | 1 | 1 | |||
| Buprenorphine prescribed at discharge | ||||||
| Non-Hispanic Black | 0.74 (0.53–1.02) | 0.07 | 0.84 (0.60–1.17) | 0.30 | 0.98 (0.69–1.39) | 0.91 |
| Hispanic | 1.32 (0.97–1.80) | 0.08 | 1.33 (0.97–1.82) | 0.08 | 1.31 (0.94–1.83) | 0.11 |
| Other | 1.11 (0.71–1.75) | 0.64 | 1.15 (0.72–1.84) | 0.55 | 1.34 (0.83–2.19) | 0.23 |
| Non-Hispanic White | 1 | 1 | 1 | |||
Odds ratios and P values from Glimmix hierarchical models accounting for clustering at the level of the clinician and health care system, both unadjusted, adjusted for age, insurance, gender, clinician X-waiver status, hospital type, and urbanicity with and without discharge diagnosis.
DISCUSSION
Principal findings
Black patients were less likely to receive ED buprenorphine compared to White patients in this secondary analysis of 21 EDs across five diverse health care systems from the EMBED trial. This racial disparity remained after controlling for patient, clinician, and site characteristics. Despite overall higher rates of ED buprenorphine at academic hospitals, compared to White participants, Black participants still received proportionally less buprenorphine at both academic and community hospitals. The statistical association between race and receipt of ED buprenorphine was lost after adjusting for discharge diagnosis, with fewer Black patients diagnosed with opioid withdrawal.
Hispanic patients were more likely to receive buprenorphine than patients who identified as non-Hispanic in both community and academic EDs. Again, adjusting for discharge diagnosis attenuated the statistical association between ethnicity and receipt of ED buprenorphine.
Comparison of findings to other studies
Our finding that Black patients received ED buprenorphine at lower rates than White patients is consistent with existing evidence that, although EDs serve as safety net resources for marginalized patients, the effects of structural and social inequities persist, compounding disparities in care for our most vulnerable populations. Black patients experience a number of disparities in accessing care in the ED, including longer wait times, lower triage acuity ratings, and lower rates of medication administration to treat pain.43–48 The same patterns have been noted in access to OUD services, where Black, non-Hispanic patients are less likely to receive behavioral health counseling compared to White patients.36 This is despite no difference in interest in starting medication treatment for OUD in non-White patients.15 Limited research has evaluated MOUD access inequities directly in the ED. One cross-sectional analysis at a single site found that Black and Hispanic (vs. White) patients seen in the ED for OUD were less likely to receive buprenorphine or methadone.35 Our study extends this analysis by providing a more comprehensive look at racial/ethnic access disparities for obtaining ED buprenorphine from 21 EDs across five diverse health care systems.
It was thought that EDs with robust MOUD programs may better recognize and address racial and ethnic disparities. Specifically, academic centers have been highlighted for their higher rates of buprenorphine administration.13,49,50 While academic centers in our study administered ED buprenorphine at higher rates than community EDs, proportionally the disparities in receipt of buprenorphine persisted. Similar to racial inequities in undertreatment related to other conditions, this suggests that racial disparities in MOUD operate on a systemic level through shared societal biases that may impact provider prescribing patterns independent from hospital setting.
It is important to acknowledge that the association of race and ethnicity on the receipt of ED buprenorphine was consistently attenuated when discharge diagnosis was included in the model. There are multiple potential reasons for the differences in ED buprenorphine by race, ethnicity, and diagnosis. First, it is possible that the change in sample size by including discharge diagnosis resulted in insufficient power to detect a statistically significant difference. This is supported by the overall low proportion of patients with opioid withdrawal, relatively large point estimates, and the wider and lower bounds of CIs remaining very close to one.
However, if we consider discharge diagnosis as an effect modifier in the causal pathway of receipt of buprenorphine, it raises additional questions regarding the relationship of race, ethnicity, diagnosis, and medication treatment of OUD in the ED. Although any ED patient with OUD should have an equal opportunity to engage in buprenorphine, there are several reasons why patients with opioid withdrawal are more likely to receive ED buprenorphine. First, patients presenting in opioid withdrawal may appear to the ED clinician as ready to engage in medication treatment. Second, some ED clinicians may not be comfortable with home buprenorphine induction, which involves giving a prescription to allow the patient to start their buprenorphine on their own. Instead, patients not yet in opioid withdrawal may be more likely to be referred to outpatient treatment without offering buprenorphine.
The differences in ED diagnoses by race and ethnicity are more difficult to explain, specifically, that fewer Black patients were diagnosed with opioid withdrawal. It is feasible that issues regarding racial bias and inequity not only impact ED treatment, but also inform a patient’s decision to come to the ED seeking help for opioid withdrawal; Black patients in opioid withdrawal, fearing stigma, bias, and racism, may avoid the ED in seeking treatment for OUD. Additionally, intrinsic biases may result in ED clinicians spending less time with patients of minoritized racial and ethnic groups (Black patients) or being less likely to use standardized screening tools, limiting opportunities to recognize withdrawal during the patient-provider interaction.51,52 Finally, bias and stigma in the health care system may contribute to patient mistrust in the larger medical community and the medications used to treat OUD. Each of the reasons highlight potential barriers to equity in the treatment of patients with OUD that occur before the decision to give buprenorphine in the ED.
To address issues of equity in the treatment of OUD, health care systems must consider comprehensive strategies to engage historically and systematically marginalized communities. Instituting EHR-based care pathways to standardize buprenorphine administration may help to promote practice consistency. In the EMBED trial subgroup analyses, Black patients in study sites randomized to the EHR clinical decision pathway were more likely to receive buprenorphine than in control sites, a promising finding as we search for strategies to address health inequities,39 additionally, establishing quality measures that stratify outcomes by race and ethnicity and forming partnerships with trusted community-based organizations to reach Black patients who may avoid the ED due to stigma and bias and are evidence-based practices that may increase fidelity and reduce bias.53
Finally, contrary to prior literature, Hispanic patients in our study received ED buprenorphine at higher rates than both patients who did not identify as Hispanic in the primary analysis and than compared to non-Hispanic White patients in the sensitivity analysis.54 Results may be due, in part, to the differences in race and ethnicity as dimensions of social identity that are subject to different modes of discrimination, with race typically referring to the physical differences and ethnicity refers to shared values, including language, cultural practices, and beliefs.55 Further, in our study, hospital variation in buprenorphine administration for Hispanic patients may have accounted for the observed higher rates. In particular, one ED with a large number of Hispanic patients had a buprenorphine administration rate of nearly 40%. The variability of ED buprenorphine administration at the hospital level is encouraging; although issues of inequity and disparities are pervasive, individual hospital systems may be succeeding in addressing equity in ED OUD treatment in their individual communities. Each health system should work to understand their unique challenges to equity and OUD treatment disparities.
Strengths and weaknesses compared to other studies
The use of EHR data systematically collected during the EMBED trial offers benefits in evaluating the practices of ED clinicians compared to insurance claims studies. Claims data often infer an ED prescription through the linkage of a claim generated within a certain time frame after the ED visit. As such, it can be unclear if the prescription originated from the ED or if buprenorphine was prescribed by a clinician outside the ED. Further, claims data may not capture take-home buprenorphine directly distributed to patients from the ED, prescriptions that were paid out of pocket, and prescriptions that were written but not filled. In contrast, EMBED directly measured both ED administered and prescribed buprenorphine, regardless of the payer or whether the prescription was filled, as well as variables associated with the ED clinician that could impact buprenorphine administration, such as DEA X-waiver status. While claims data studies may be superior to determine if the patient ultimately received medication following their ED visit, EHR data may offer a more detailed assessment of the ED clinicians’ practice to guide efforts to improve equitable administration of MOUD in the ED.
Compared to other publications, the outcome of receipt of buprenorphine by ED patients, while important, is a limitation in that it does not capture the full breadth of ED OUD treatment and harm reduction programs, which may include admission to medication stabilization units, recovery coaching, and bridge clinic follow-up. Ensuring that patients with OUD have access to both medications and behavioral and stabilization resources should be the goal for all ED clinicians. Further, it is unclear how many patients were offered medication treatment and declined. Understanding if and how ED patients with OUD are being offered buprenorphine could help to tailor interventions to increase receipt of ED buprenorphine by addressing the patient’s readiness or stage of change.
Meaning of the study and future research
Although EDs serve as a safety net resource for many patients, disparities in the treatment of OUD by race and ethnicity persist. Beyond acknowledging the existence of disparities, our study explores the role of the hospital type as well as the influence of factors, such as discharge diagnosis, on treatment by race and ethnicity. Patients with OUD face stigma and bias, evident in the language commonly perpetuated to describe their condition.56–58 For our non-White ED patients with OUD the stigma and bias they experience may be compounded by structural racism, resulting in even higher risks of health inequities. It is important to consider how those processes may overlap and exacerbate inequities. Intersectionality is an analytic framework for investigating how different systems of power and oppression operate together to form complex social inequities such as the unique health inequities of people who are both racially and ethnically marginalized and face stigma because of their addiction.59,60 Future research in the care of patients with OUD in the ED should expand the use of an intersectionality framework to explore how systems of power in patients with OUD, including race, ethnicity, gender, class, and pregnancy impact all levels of treatment, including access to care, use of medications, referrals and admissions as we continue to address the health inequities, and the inherent stigma and bias that perpetuates distrust in the health care system.61
Limitations
The accuracy of race and ethnicity data recorded in the EHR for clinical purposes is unknown. While many EDs asked patients to identify race and ethnicity at registration, others retrieved data from prior visits. Additionally, there were not enough participants who identified as Asian, American Indian, or Pacific Islander to include in the hierarchical analyses. Finally, although the rate of missing data was low (3%), approximately 7% of the study population identified their race as other, which may have included patients who identify as multiple races. We addressed the limitations with the race and ethnicity variables by providing descriptive results for all race and ethnicity categories as well as regression analysis for participants whose race was identified as other or unknown in supplemental tables.
As a secondary analysis of an existing data set, we were limited to the inclusion criteria of the original study and were unable to include patients who were admitted or pregnant or other at-risk groups. Further, it was not possible to ensure all patients included in the study met DSM-V criteria for OUD, though the use of a validated EHR phenotype limits misclassification. Additionally, because health care systems were selected and had to agree to be a part of the original trial, the practices of ED buprenorphine administration in the study sample may be different from current real-world practice. Finally, despite the use of hierarchical modeling clustered by site, each ED had a different proportion of participants in each racial and ethnic category, causing results to be potentially more representative of hospitals with larger non-White populations.
CONCLUSIONS
In this secondary analysis of a pragmatic randomized trial across five health care systems, Black patients who presented to the ED with a diagnosis related to opioid use disorder were less likely to receive buprenorphine (administered in the ED or prescribed) than White patients. Conversely, Hispanic patients were more likely to receive ED buprenorphine than non-Hispanic patients. Differences in receipt of buprenorphine may be attributable to differences in the identified reason for presentation to the ED as well as the variability in ED buprenorphine practices at individual hospitals. Attention should be focused on identifying continued disparities in ED treatment of opioid use disorder by race and ethnicity as well as the barriers and inequities that continue to limit patients’ ability to access the ED for treatment of opioid use disorder.
Supplementary Material
FUNDING INFORMATION
This work was supported within the National Institutes of Health (NIH) Health Care Systems Research Collaboratory by the NIH Common Fund through cooperative agreement U24AT009676 from the Office of Strategic Coordination within the Office of the NIH Director and cooperative agreement (UH3DA047003) from the National Institute on Drug Abuse (NIDA) of the NIH. Dr. Soares was supported by a grant from the NIDA K08DA045933. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflict of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
Presented at the Society for Academic Emergency Medicine Annual Meeting, New Orleans, LA, May 2022.
REFERENCES
- 1.Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larochelle MR, Stopka TJ, Xuan Z, Liebschutz JM, Walley AY. Medication for opioid use disorder after nonfatal opioid overdose and mortality. Ann Intern Med. 2019;170(6):430–431. [DOI] [PubMed] [Google Scholar]
- 3.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. 10.1002/14651858.CD002207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slat S, Thomas J, Lagisetty P. Coronavirus disease 2019 and opioid use—a pandemic within an epidemic. JAMA Health Forum. 2020;1(5):e200628. doi: 10.1001/jamahealthforum.2020.0628 [DOI] [PubMed] [Google Scholar]
- 5.Soares WE III, Melnick ER, Nath B, et al. Emergency department visits for nonfatal opioid overdose during the COVID-19 pandemic across six US health care systems. Ann Emerg Med. 2022;79(2):158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkow ND, Collins FS. The role of science in addressing the opioid crisis. N Engl J Med. 2017;377(4):391–394. [DOI] [PubMed] [Google Scholar]
- 7.Abraham AJ, Andrews CM, Yingling ME, Shannon J. Geographic disparities in availability of opioid use disorder treatment for Medicaid enrollees. Health Serv Res. 2018;53(1):389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlay AK, Harris AHS, Timko C, et al. Disparities in access to medications for opioid use disorder in the veterans health administration. J Addict Med. 2021;15(2):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrilla CHA, Moore TE, Patterson DG, Larson EH. Geographic distribution of providers with a DEA waiver to prescribe buprenorphine for the treatment of opioid use disorder: a 5-year update. J Rural Health. 2019;35(1):108–112. [DOI] [PubMed] [Google Scholar]
- 10.Pashmineh Azar AR, Cruz-Mullane A, Podd JC, et al. Rise and regional disparities in buprenorphine utilization in the United States. Pharmacoepidemiol Drug Saf. 2020;29(6):708–715. [DOI] [PubMed] [Google Scholar]
- 11.Amiri S, McDonell MG, Denney JT, Buchwald D, Amram O. Disparities in access to opioid treatment programs and office-based buprenorphine treatment across the rural-urban and area deprivation continua: a US Nationwide small area analysis. Value Health. 2021;24(2):188–195. [DOI] [PubMed] [Google Scholar]
- 12.Kilaru AS, Xiong A, Lowenstein M, et al. Incidence of treatment for opioid use disorder following nonfatal overdose in commercially insured patients. JAMA Netw Open. 2020;3(5):e205852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swann WL, Kim S, Kim SY, Schreiber TL. Urban-rural disparities in opioid use disorder prevention and response activities: a cross-sectional analysis. J Rural Health. 2021;37(1):16–22. doi: 10.1111/jrh.12491 [DOI] [PubMed] [Google Scholar]
- 14.Langabeer JR, Gourishankar A, Chambers KA, Giri S, Madu R, Champagne-Langabeer T. Disparities between US opioid overdose deaths and treatment capacity: a geospatial and descriptive analysis. J Addict Med. 2019;13(6):476–482. [DOI] [PubMed] [Google Scholar]
- 15.Robbins M, Haroz R, Mazzarelli A, Clements D IV, Jones CW, Salzman M. Buprenorphine use and disparities in access among emergency department patients with opioid use disorder: a cross-sectional study. J Subst Abuse Treat. 2021;130:108405. [DOI] [PubMed] [Google Scholar]
- 16.Schuler MS, Dick AW, Stein BD. Growing racial/ethnic disparities in buprenorphine distribution in the United States, 2007–2017. Drug Alcohol Depend. 2021;223:108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein BD, Dick AW, Sorbero M, et al. A population-based examination of trends and disparities in medication treatment for opioid use disorders among Medicaid enrollees. Subst Abus. 2018;39(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagisetty PA, Ross R, Bohnert A, Clay M, Maust DT. Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiatry. 2019;76(9):979. doi: 10.1001/jamapsychiatry.2019.0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancher M, Leshner AI. Medications for Opioid Use Disorder Save Lives. 2019. [cited 2022 Aug 8]. Available from: https://pubmed.ncbi.nlm.nih.gov/30896911/ [PubMed]
- 20.Schoenfeld EM, Soares WE, Schaeffer EM, Gitlin J, Burke K, Westafer LM. “This is part of emergency medicine now”: a qualitative assessment of emergency clinicians’ facilitators of and barriers to initiating buprenorphine. Acad Emerg Med. 2022;29(1):28–40.doi: 10.1111/acem.14369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Onofrio G, McCormack RP, Hawk K. Emergency departments—a 24/7/365 option for combating the opioid crisis. N Engl J Med. 2018;379(26):2487–2490. [DOI] [PubMed] [Google Scholar]
- 22.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson G, Brown AM, DeFrances C. Opioid-involved emergency department visits in the National Hospital Care Survey and the National Hospital Ambulatory Medical Care Survey. Natl Health Stat Rep. 2020;149:1–15. [PubMed] [Google Scholar]
- 24.Wu L-T, Zhu H, Swartz MS. Treatment utilization among persons with opioid use disorder in the United States. Drug Alcohol Depend. 2016;169:117–127. doi: 10.1016/j.drugalcdep.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macrae J, Hyde P, Slavitt A. HHS launches multi-pronged effort to-combat opioid abuse. HHS Blog. 2015. [cited 2016 Aug 8]. Available from: https://www.hhs.gov/blog/2015/07/27/hhs-launches-multi-pronged-effort-combat-opioid-abuse.html [Google Scholar]
- 26.Coupet E, D’Onofrio G, Chawarski M, et al. Emergency department patients with untreated opioid use disorder: a comparison of those seeking versus not seeking referral to substance use treatment. Drug Alcohol Depend. 2021;219:108428. doi: 10.1016/j.drugalcdep.2020.108428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doran KM, Rahai N, McCormack RP, et al. Substance use and homelessness among emergency department patients. Drug Alcohol Depend. 2018;188:328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Priester MA, Browne T, Iachini A, Clone S, DeHart D, Seay KD. Treatment access barriers and disparities among individuals with Co-occurring mental health and substance use disorders: an integrative literature review. J Subst Abuse Treat. 2016;61:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goedel WC, Shapiro A, Cerdá M, Tsai JW, Hadland SE, Marshall BDL. Association of Racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw Open. 2020;3(4):e203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Onofrio G, Chawarski MC, O’Connor PG, et al. Emergency department-initiated buprenorphine for opioid dependence with continuation in primary care: outcomes during and after intervention. J Gen Intern Med. 2017;32(6):660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogan C, Jennings L, Haynes L, et al. Implementation of emergency department–initiated buprenorphine for opioid use disorder in a rural southern state. J Subst Abuse Treat. 2020;112:73–78. doi: 10.1016/j.jsat.2020.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder H, Kalmin MM, Moulin A, et al. Rapid adoption of low-threshold buprenorphine treatment at California emergency departments participating in the CA bridge program. Ann Emerg Med. 2021;78(6):759–772. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed OM, Mao JA, Holt SR, et al. A scalable, automated warm handoff from the emergency department to community sites offering continued medication for opioid use disorder: lessons learned from the EMBED trial stakeholders. J Subst Abuse Treat. 2019;102:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens MA, Tsai J, Savitz ST, et al. Trends and disparities in access to buprenorphine treatment following an opioid-related emergency department visit among an insured cohort, 2014–2020. JAMA Netw Open. 2022;5(6):e2215287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weisenthal K, Farrell N, Nentwich L, Taylor J, Manchanda EC. 264 racial inequities in emergency Department Administration of Buprenorphine and Methadone among Patients with Opioid use Disorder. Ann Emerg Med. 2021;78(4):S107. doi: 10.1016/j.annemergmed.2021.09.277 [DOI] [Google Scholar]
- 36.Reddy NG, Jacka B, Ziobrowski HN, et al. Race, ethnicity, and emergency department post-overdose care. J Subst Abuse Treat. 2021;131:108588. doi: 10.1016/j.jsat.2021.108588 [DOI] [PubMed] [Google Scholar]
- 37.Melnick ER, Jeffery MM, Dziura JD, et al. User-centred clinical decision support to implement emergency department-initiated buprenorphine for opioid use disorder: protocol for the pragmatic group randomised EMBED trial. BMJ Open. 2019;9(5):e028488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melnick ER, Nath B, Ahmed OM, et al. Progress report on EMBED: a pragmatic trial of user-centered clinical decision support to implement EMergency department-initiated BuprenorphinE for opioid use disorder. J Psychiatr Brain Sci. 2020;5. doi: 10.20900/jpbs.20200003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melnick ER, Nath B, Dziura JD, et al. User centered clinical decision support to implement initiation of buprenorphine for opioid use disorder in the emergency department: EMBED pragmatic cluster randomized controlled trial. BMJ. 2022;377:e069271. doi: 10.1136/bmj-2021-069271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chartash D, Paek H, Dziura JD, et al. Identifying opioid use disorder in the emergency department: multi-system electronic health record–based computable phenotype derivation and validation study. JMIR Med Inform. 2019;7(4):e15794. doi: 10.2196/15794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez RA, Andrabi N, Goodwin AN, Wilbur RE, Smith NR, Zivich PN. Conceptualization, operationalization, and utilization of race and ethnicity in major epidemiology journals 1995–2018: a systematic review. Am J Epidemiol. 2022. doi: 10.1093/aje/kwac146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez CE, Lopez N, Argeros G. Latinos and the Colorline. Vol 18. Sociology Faculty Publications; 2015. https://fordham.bepress.com/soc_facultypubs/18 [Google Scholar]
- 43.Qiao WP, Powell ES, Witte MP, Zelder MR. Relationship between racial disparities in ED wait times and illness severity. Am J Emerg Med. 2016;34(1):10–15. [DOI] [PubMed] [Google Scholar]
- 44.Lu FQ, Hanchate AD, Paasche-Orlow MK. Racial/ethnic disparities in emergency department wait times in the United States, 2013–2017. Am J Emerg Med. 2021;47:138–144. [DOI] [PubMed] [Google Scholar]
- 45.Schrader CD, Lewis LM. Racial disparity in emergency department triage. J Emerg Med. 2013;44(2):511–518. [DOI] [PubMed] [Google Scholar]
- 46.Shan A, Baumann G, Gholamrezanezhad A. Patient race/ethnicity and diagnostic imaging utilization in the emergency department: a systematic review. J Am Coll Radiol. 2021;18(6):795–808. doi: 10.1016/j.jacr.2020.12.016 [DOI] [PubMed] [Google Scholar]
- 47.Schrager JD, Patzer RE, Kim JJ, et al. Racial and ethnic differences in diagnostic imaging utilization during adult emergency department visits in the United States, 2005 to 2014. J Am Coll Radiol. 2019;16(8):1036–1045. [DOI] [PubMed] [Google Scholar]
- 48.Kang H, Zhang P, Lee S, Shen S, Dunham E. Racial disparities in opioid administration and prescribing in the emergency department for pain. Am J Emerg Med. 2022;55:167–173. doi: 10.1016/j.ajem.2022.02.043 [DOI] [PubMed] [Google Scholar]
- 49.Zuckerman M, Kelly T, Heard K, Zosel A, Marlin M, Hoppe J. Physician attitudes on buprenorphine induction in the emergency department: results from a multistate survey. Clin Toxicol. 2021;59(4):279–285. [DOI] [PubMed] [Google Scholar]
- 50.Hawk KF, D’Onofrio G, Chawarski MC, et al. Barriers and facilitators to clinician readiness to provide emergency department–initiated buprenorphine. JAMA Netw Open. 2020;3(5):e204561. doi: 10.1001/jamanetworkopen.2020.4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aysola J, Clapp JT, Sullivan P, et al. Understanding contributors to racial/ethnic disparities in emergency department throughput times: a sequential mixed methods analysis. J Gen Intern Med. 2022;37:341–350. doi: 10.1007/s11606-021-07028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dehon E, Weiss N, Jones J, Faulconer W, Hinton E, Sterling S. A systematic review of the impact of physician implicit racial bias on clinical decision making. Acad Emerg Med. 2017;24:895–904. https://pubmed.ncbi.nlm.nih.gov/28472533/ [DOI] [PubMed] [Google Scholar]
- 53.Khidir H, Salhi R, Sabbatini AK, et al. A quality framework to address racial and ethnic disparities in emergency department care. Ann Emerg Med. 2023;81:47–56. 10.1016/j.annemergmed.2022.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunphy CC, Zhang K, Xu L, Guy GP Jr. Racial–ethnic disparities of buprenorphine and vivitrol receipt in Medicaid. Am J Prev Med. 2022. doi: 10.1016/j.amepre.2022.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stamper K Why we confuse race and ethnicity: a lexicographer’s perspective. Conscious Style Guide. 2019;13 [cited 2022 Dec 1]. https://consciousstyleguide.com/why-we-confuse-race-ethnicity-lexicographers-perspective/ [Google Scholar]
- 56.Werder K, Curtis A, Reynolds S, Satterfield J. Addressing bias and stigma in the language we use with persons with opioid use disorder: a narrative review. J Am Psychiatr Nurses Assoc. 2022;28(1):9–22. [DOI] [PubMed] [Google Scholar]
- 57.Goodyear K, Haass-Koffler CL, Chavanne D. Opioid use and stigma: the role of gender, language and precipitating events. Drug Alcohol Depend. 2018;185:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kennedy-Hendricks A, McGinty EE, Summers A, Krenn S, Fingerhood MI, Barry CL. Effect of exposure to visual campaigns and narrative vignettes on addiction stigma among health care professionals: a randomized clinical trial. JAMA Netw Open. 2022;5(2):e2146971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crenshaw KW. Demarginalizing the intersection of race and sex: a black feminist critique of antidiscrimination doctrine. Univ Chic Leg Forum. 1989;1989:139–168. [Google Scholar]
- 60.Bauer GR, Scheim AI. Methods for analytic intercategorical intersectionality in quantitative research: discrimination as a mediator of health inequalities. Soc Sci Med. 2019;226:236–245. [DOI] [PubMed] [Google Scholar]
- 61.Gandhi P, Rouhani S, Park JN, et al. Alternative use of buprenorphine among people who use opioids in three US cities. Subst Abus. 2022;43(1):364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


