Abstract
Organ transplantation is a highly utilized treatment for many medical conditions, yet the number of patients waiting for organs far exceeds the number available. The challenges and limitations currently associated with organ transplantation and technological advances in gene editing techniques have led scientists to pursue alternate solutions to the donor organ shortage. Growing human organs in animals and harvesting those organs for transplantation into humans is one such solution. These chimeric animals usually have certain genes necessary for a specific organ’s development inhibited at an early developmental stage, followed by the addition of cultured pluripotent human cells to fill that developmental niche. The result is a chimeric animal that contains human organs which are available for transplant into a patient, circumventing some of the limitations currently involved in donor organ transplantation. In this review, we will discuss both the current scientific and legal landscape of human–animal chimera (HAC) research. We present an overview of the technological advances that allow for the creation of HACs, the patents that currently exist on these methods, as well as current public attitude and understanding that can influence HAC research policy. We complement our scientific and public attitude discussion with a regulatory overview of chimera research at both the national and state level, while also contrasting current U.S. legislation with regulations in other countries. Overall, we provide a comprehensive analysis of the legal and scientific barriers to conducting research on HACs for the generation of transplantable human organs, as well as provide recommendations for the future.
Keywords: chimeras, human–animal hybrids, organ transplantation, exogenic organs and cells, chimera law, regulation, public opinion, patents
Introduction
Human–Animal Chimeras for Regenerative Medicine
Since the first successful kidney transplantation in 1954 1 , and the first human heart transplant in 1967 2 , the idea of transferring organs to save lives has been a scientific and humanitarian breakthrough 1 . Physicians now routinely transplant kidneys, livers, hearts, lungs, pancreas, and other organs from living or recently deceased donors. But despite these medical breakthroughs on transplantation, the supply of viable organs is still far below demand. In 2021, the waiting list for kidneys was three times greater than the number of kidney transplants actually performed 3 . The organs most sought for transplant are the kidney, liver, heart, lungs, pancreas, and intestines, and of the approximately 75,000 people on the active waiting list for an organ 4 , 17 will die each day without receiving a transplant 5 . Although doctors try to ensure the health of the organs donated, by necessity many are donated after a trauma occurs, or by older individuals. Organs from deceased donors make up over half of all organs transplanted4,6, and over half of those donors were over the age of 50 years, implying their organs are aged as well 7 . As the U.S. population increases and lives longer, organ demand will likely increase, while the organ supply will likely decrease as advances in vehicle safety and improved medical interventions result in fewer deceased donors 8 .
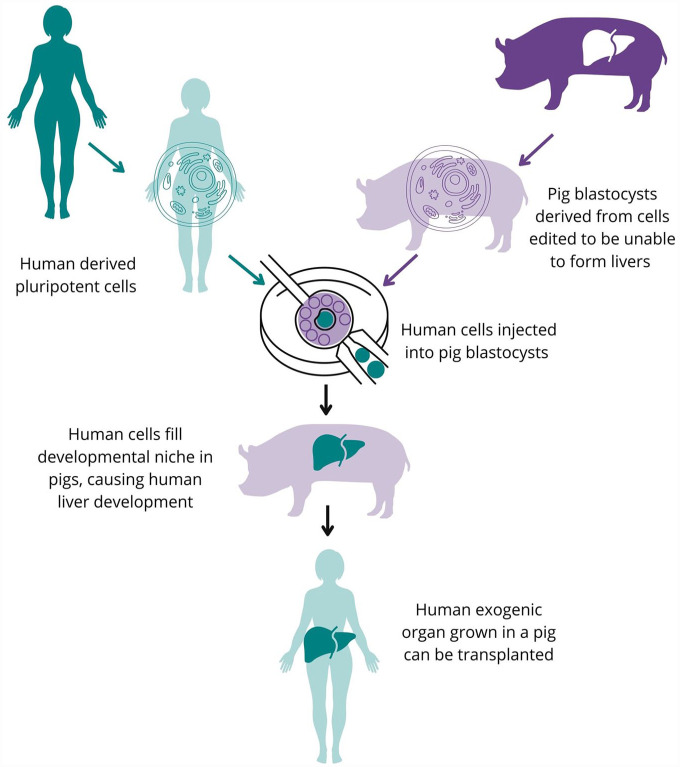
If an organ becomes available for transplant, recipient accessibility is currently limited by geographical proximity and travel efficiency since some organs only remain viable outside of the body for a few hours. In addition, the issue of organ rejection and the dangers of immunosuppression for patients remain 9 . The number of available organs can also be impacted by current events, as demonstrated when the COVID-19 pandemic initially caused donations to plunge, costing people on the waitlist their lives 5 . Technological improvements in the fields of genetics and immunology are advancing solutions to the organ shortage problem that address some of the current system’s major disadvantages. The creation of exogenic human organs in human–animal chimeras (HACs) is one such approach (Figure 1).
Figure 1.
Generating exogenic human organs in interspecies chimeras using blastocyst complementation. Human cells (teal) are added to gene-edited porcine (purple) blastocysts. The porcine cells are genetically modified to be incapable of specific organogenesis (eg, liver) by single or multiple gene manipulation. The human pluripotent cells can integrate into the modified porcine blastocyst to form the missing organ. The blastocyst is then implanted into a surrogate and allowed to grow normally. The organ produced in the chimeric animal can then be used for transplantation into the human cell donor.
Chimeras are organisms composed of at least two genetically distinct populations of cells, such as a dog and a wolf, or a cat and a human. A type of chimerism called mosaicism or micro-chimerism happens when genetically distinct cells are present in a single organism, as sometimes happens when cells from a human fetus transmigrate from the placenta and incorporate into the mother 10 . The distinct cell population can be small or large, scattered throughout the organism, or concentrated in a particular location or niche 11 . Chimerism is a natural phenomenon since it happens in human pregnancies between mothers and their fetus, as well as occasionally between developing fraternal twins.
Chimeras developed for human organ production can be created in various ways, including blastocyst complementation and xenotransplantation, but they are not the result of sexual reproduction 12 (such as a mule). A neural chimera refers to a chimera in which some cells from an independent source become part of the central nervous system, meaning the brain or the spinal cord. The concerns about creating neural chimeras echo the concerns about chimera creation in general—crossing species boundaries through reproduction, playing God, human uniqueness, the violation of natural laws—but also give rise to more nuanced and complex issues. Since the brain contributes to intelligence, emotion, memory, and learning, all traits that are frequently associated with humans as a category, the integration of human and nonhuman cells in the central nervous system leads us to ask questions about the nature of human consciousness and intelligence and the reasons that humans receive special considerations over other species.
Chimeras have the potential to address several problems in the field of clinical research. First, they are important for modeling human development and disease. Neural chimeras, in particular, would enable researchers to model traditionally high-risk or protected populations, including young developing brains or brains with neurological disorders, without risk to those populations. Creation of chimeric models would also avoid most consent issues involved with vulnerable populations, such as children and people with cognitive impairments 13 .
Second, animal models are imperfect predictors of what therapies are efficacious in humans, especially when studying diseases unique to humans 14 . Even when using nonhuman primates, long considered the gold standard of animal models, human vaccines tested in our closest genetic relative, the chimpanzee, failed 90% of the time 15 . Therefore, being able to create a human organ in an animal would be invaluable for drug testing and disease modeling. Currently, we are unable to keep large lab-grown organs and organoids alive outside of a body for more than a few days 16 . Creating humanized tissues and organs in animals would enable scientists to test possible therapies in a system much closer to that of a human for much longer durations than currently available in vitro, therefore potentially increasing predicative validity. Chimeras would also be useful for device testing because many medical devices cannot be made small enough to test on a mouse without major redesigning. However, pig organs are similar in size to human organs, and producing a human organ in a pig could be a way of testing new devices on human-sized organs 17 .
Third, chimeras could help with the organ scarcity problem as well as provide a source of cells for cellular transplantation. Animals like pigs develop faster than humans, so it would be possible to grow organs in a single year, which is a shorter time than most people spend on the transplant list 18 . Autologous transplants are better clinically because less immunosuppression is needed to prevent rejection of the transplanted tissue. The transplantable organ can be produced from cells from the recipient themself through induced pluripotent stem cell (iPSC) production 19 . Immunological chimeras could enable the patient’s own cells to be grown in an animal to create immune cells. Further, under the National Organ Transplant Act (NOTA; Pub.L. 98–507 20 ), “[o]rgan donor means a human being who is the source of an organ for transplantation into another human being.” If the organ donor was a chimeric animal, it would not fall under the auspices of NOTA and the normal consent requirements would not be a barrier. Alternatively, the patient who donated the cells to generate their own tissue grown in the animal could be considered the donor; either way avoids issues of consent. HACs, once thought a myth and an impossibility, have huge potential to address problems in medicine and are now squarely within the capabilities of modern science as a result of technological advances.
Technological Developments
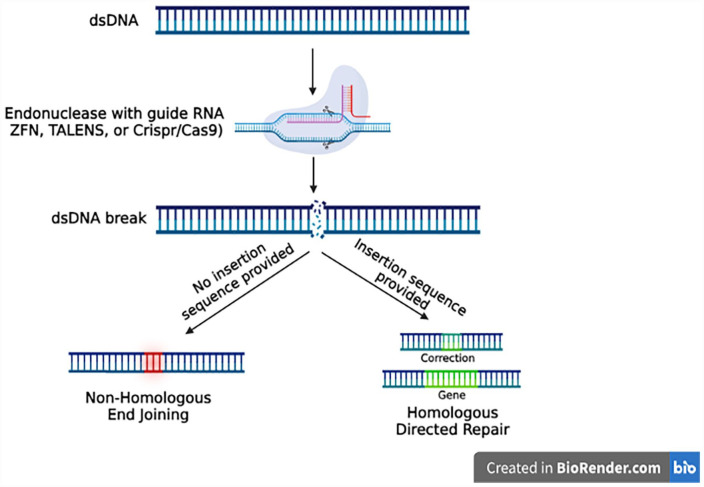
The generation of exogenic organs using blastocyst complementation requires targeted knockout of gene(s) that are critical for the development of specific organs/cells. Several technological advances for the deletion of genes have recently been developed and have been shown to facilitate the generation of exogenic organs in chimeras. These genome engineering technologies include transcription activator-like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeats (CRISPR) for gene editing. TALENs uses TALE proteins that bind to specifically targeted regions of DNA, and a nuclease that cuts the DNA strand at the targeted location. In addition, nuclear localization signals can enable TALE proteins to access and bind specific DNA sequences to specifically edit portions of the genome 21 . While this technique has been essential to the process of gene editing for successful DNA sequence recognition and allowing target specificity, TALENs have drawbacks that make CRISPR a more useful gene editing tool. TALENs can easily target specific sequences; however, they are known to be much greater in size, which makes it more difficult for TALENs to reach and enter the cells that are being targeted 22 .
CRISPR also has the advantage of being highly accurate, which has resulted in its increased utilization in the chimera creation process. CRISPR involves the use of customized single-guide RNA (sgRNA); the endonuclease Cas-9; and insertion, deletion, or modification of DNA using homologous DNA Repair (HDR) 23 (Figure 2). Off-target binding is very uncommon with CRISPR even when compared with TALENs, minimizing extraneous genome alterations beyond the intended sequence targeted. CRISPR/Cas9 is commonly used in research settings to knock out genes within developing embryos, creating a vacated niche which IPS cells can integrate and occupy. This is the basic setup for most blastocyst complementation experiments.
Figure 2.
Gene editing to generate niches for exogenic organ and cell development. TALENs: transcription activator-like effector nucleases; CRISPR: clustered regularly interspaced short palindromic repeats.
Intraspecies and Interspecies Chimeras
The chimera of Greek mythology features a creature with the head of a lion, the body and additional head of a goat, and the tail of a snake, but while interspecies chimeras where there is a fusion of zygotes of different lineages within a single embryo (Figure 3) are possible, that exact combination remains unlikely 24 . There are limits to what species can sexually reproduce to create chimeras. The science on this topic is well established: Human germ cells would not be able to integrate and produce a viable embryo in any organism other than a human germ cell. Discussion of the chances of a human sperm or egg combining with a mouse sperm or egg cell is moot because they will not combine and form a viable embryo. There are specific instances where the risk of germline contribution would be of crucial importance, specifically, any chimera studies that would attempt to combine human and nonhuman primate cells. The more similar the species are in genetic lineage, size, and developmental progression, the more likely it is that a chimera can be created 25 , and the more careful scientists need to be of off-target effects. Several mammalian intraspecies and interspecies chimeras have already been generated in laboratory settings, as shown in Tables 1 and 2.
Figure 3.
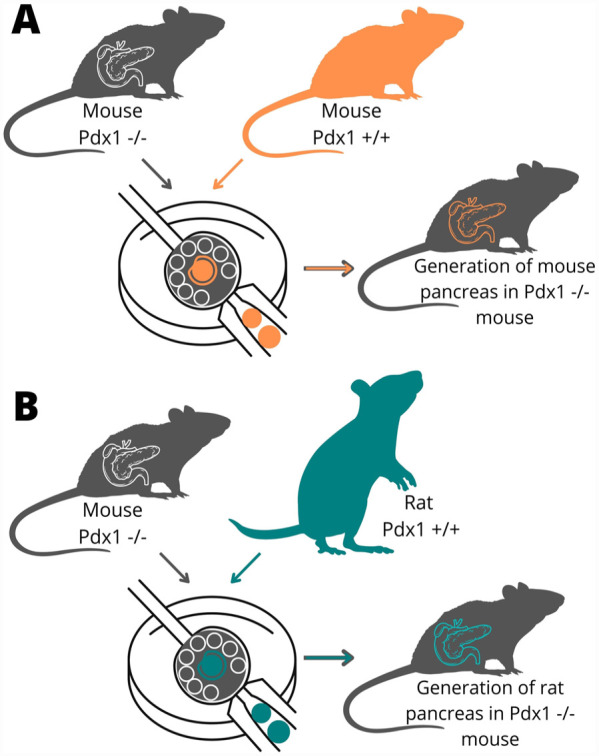
Generating exogenic organs in intraspecies and interspecies chimeras. (A) Exogenic pancreas generated in intraspecies mouse–mouse chimeras by targeted knockout of the PDX1 gene. (B) Exogenic pancreas generated in intraspecies rat–mouse chimera.
Table 1.
Generation of Intraspecies Exogenic Organs and Cells.
| Chimera type | Organs and cells generated | Gene knocked out | References |
|---|---|---|---|
| Mouse > Mouse | Immune system cells | RAG2 | Chen et al. 26 |
| RAG | Young et al. 27 | ||
| c-KIT | Jansson and Larsson 28 | ||
| RUNX2 | Chubb et al. 29 | ||
| FOXN1 | Yamazaki et al. 30 | ||
| Lens | PITX3 | Liégeois et al. 31 | |
| Pancreas and islet cells | PDX1 | Kobayashi et al. 32 | |
| Kidney | SALL1 | Usui et al. 33 | |
| Liver | HHEX | Ruiz-Estevez et al. 34 | |
| Lung | CTNNB1 | Mori et al. 35 | |
| FGFR2 | Mori et al. 35 | ||
| FGF10 | Kitahara et al. 36 | ||
| NKX2.1 | Wen et al.
37
Li et al. 38 |
||
| Thyroid | FGF10 | Ran et al. 39 | |
| CNS and PNS | EMX1 | Chang et al. 40 | |
| DCX | Chang et al. 40 | ||
| NEUROG1 | Steevens et al. 41 | ||
| Endothelial cells | VEGFR2 | Hamanaka et al. 42 | |
| Bone and skull | RUNX2 | Chubb et al. 29 | |
| Pig > Pig | Pancreas and islet cells | PDX1 | Matsunari et al. 43 |
| Liver | HHEX | Matsunari et al.
44
Ruiz-Estevez et al. 34 |
|
| Kidney | SALL1 | Matsunari et al. 44 | |
| Endothelial vasculature | FLK1 | Matsunari et al. 44 | |
| KDR | Matsunari et al. 44 | ||
| Retinal pigment epithelium | MITF | Zhang et al. 45 | |
| Skin, heart, and kidney | WILD TYPE | Ji et al. 46 | |
| Cow > Cow | Germline | NANOS3 | Ideta et al. 47 |
PNS: peripheral nervous system; CNS: central nervous system.
Table 2.
Generation of Interspecies Exogenic Organs and Cells.
| Chimera type | Organs and cells generated | Gene knocked out | References |
|---|---|---|---|
| Rat > Mouse | Pancreas and islet cells | PDX1 | Kobayashi et al. 32 |
| Endothelial vasculature | FLK1 | Wang et al. 48 | |
| Immune cells | FLK1 | Wang et al. 48 | |
| Enhanced general chimera | IGFR1 | Nishimura et al. 49 | |
| Mouse > Rat | Pancreas and islet cells | PDX1 | Yamaguchi et al. 50 |
| Kidney | SALL1 | Goto et al. 51 | |
| Germline | PRDM14 | Kobayashi et al. 52 | |
| Monkey > Pig | General chimera | Wild-type | Fu et al. 53 |
| Human > Mouse | Skin | Wild-type | Cohen et al. 54 |
| PNS cells | Wild-type | Cohen et al. 54 | |
| Human > Pig | Endoderm, mesoderm, ectoderm | Wild-type | Wu et al. 55 |
| Endothelial vasculature | ETV2 | Das et al. 56 | |
| Skeletal muscle | MYF5 MYF6 MYOD |
Maeng et al. 57 | |
| Human > Cow | Endoderm, mesoderm, ectoderm | Wild-type | Wu et al. 55 |
| Human > Monkey | Epiblast, hypoblast, extraembryonic mesenchymal cells | Wild-type | Tan et al. 58 |
PNS: peripheral nervous system.
The public reaction to HACs has been varied. Some see them as a mark of scientific advancement and an opportunity to cure human disease 59 . Others see chimeras as a stain on nature and a violation of the dignity of humans and animals alike 60 . The regulation of chimeras reflects this lack of consensus and overall misunderstanding of the technologic capability. Society now must react to and regulate something most people were skeptical could ever exist, with limited understanding of the technical details. Throughout this article, we focus on germline contributions and the creation of neural chimeras, as surveys of public opinion61,62 have indicated those are the primary areas of public concern. Neural chimeras further pose more complex questions than other types of chimeras about the nature of consciousness and humanity, as well as pose unique regulatory challenges, and so will be given special attention.
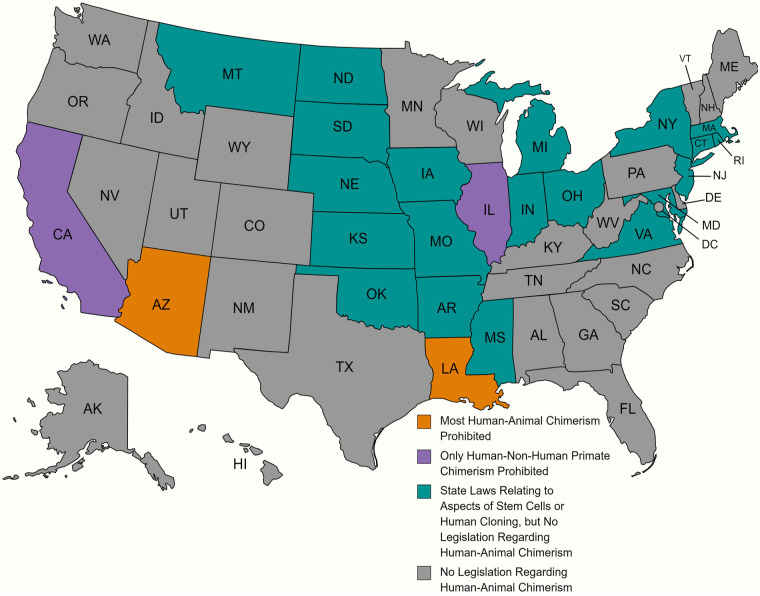
In regard to germline contribution and the generation of chimeric animals through unintended sexual reproduction, the fear is greater than the biological reality. Integration of germ cells from two disparate species would not result in viable offspring. The greatest risk of such a cross occurring would be with nonhuman primates due to their genetic similarity to humans. However, nonhuman primate chimera research is already banned in two states (see Figure 4), and nonhuman primate research in general is already heavily regulated. Thus, if germline contribution in chimera research needs regulation, specifically preventing human and nonhuman primate chimerism might be the best option to mitigate the greatest risks while allowing other research to continue. In addition, off-target analyses of reproductive tissues could be required by any group producing chimeras, to ensure no unintended germline integration occurs. A further failsafe is not allowing any neural chimera to proceed to full term, thus preventing the risk of germline contribution from animals manifesting in a mostly-human seeming being. The biological realities of chimera creation are only one part of the discussion when it comes to chimera research, however, as the existence of HACs implicates deeper philosophical and legal issues.
Figure 4.
Overview of U.S. states with human cloning, stem cells, or chimera research regulations. Orange states are the most restrictive, with direct legislation restricting chimera research. Purple states have existing legislation restricting aspects of human–nonhuman primate chimerism but not other types of chimerism. Teal states primarily have legislation banning human cloning, but no mention or discussion of chimeras at all 63 . States in gray do not have cloning laws or laws related to human–animal chimeras. Figure made using MapChart.
The ethical discussion regarding chimera research has been active even before chimera research began, and incorporates ideas discussed in early debates about human cloning and in vitro fertilization (IVF). Hence, there already exists a robust and comprehensive literature regarding the ethical issues involving chimeric research. Because this article is focused on public attitudes and current legal frameworks, we will not discuss these arguments in depth, but we reference several64–71 because of their impact on current public attitudes and regulatory schemes.
Public Attitudes Toward Creating HACs for Generating Exogenic Organs and Cells
U.S. Public Attitudes
In 2020, Kwisda et al. conducted a review of the ethical reasons cited in academic publications, both for and against HAC research 72 . Four hundred thirty-one articles published through 2017 were included in their analyses, with arguments falling into several general categories. The main arguments against HAC research, which included concerns regarding creation, treatment, future effects, and social issues, have all been discussed in this article 72 . Interestingly, of the arguments against HAC research, metaphysical and social concerns made up almost half of the entire category 72 . This indicates that there is an inherent distaste for the idea of an animal–human hybrid that stems from cultural norms and ethical worries. But once examined and dissected more closely, many of these concerns can be addressed through education on technical methodology and the potential positive impact of chimera research. As the public better understands what this work entails and the restrictions upon it, cultural norms may shift and some of the emotional revulsion may recede. Another meta-analysis indicates that research into public perceptions on this issue is scarce 73 .
A previous study by our group attempted to fill this gap by asking about public perceptions of HAC research in the United States 61 . Our study population, which was recruited online, was skewed toward younger individuals and to higher education levels 74 ; however, our findings were as follows. Following a brief explanation of the concept of blastocyst complementation and how functional organs could be developed, we asked 430 individuals in the United States various questions to assess their support for or against chimera research, as well as demographic information to identify subpopulations that may be more accepting of, or resistant to, chimera research. We found that 59% of respondents were in support of all components of chimera research regarding generation of a functional human pancreas in pigs, including creation of human–pig chimeras and subsequent transplantation of the resulting human pancreas into a human recipient. A total of 12% supported chimera production but not transplantation of produced organs, 11% only supported injection of human iPSCs into porcine embryos, and 17% supported no aspects of chimera research. Further, total support for neural chimerism (51%) and germline-related chimerism (44%) was lower than other chimera types, likely for reasons involving cognition and reproduction. Together, these findings suggest the U.S. populace is generally supportive of chimera production and the transplantation of generated organs, with the greatest areas of concern being neural and germline chimerism, which show reduced support compared with other organs such as the liver or kidney.
Self-identified conservative individuals showed 55% support for all aspects of chimera research, from development of organs to transplant into humans. According to the Pew Religious Landscape Study, 85% of conservative Americans are Christian 75 , and we expected this population to show less support for chimera research than other populations due to historic beliefs regarding similar topics such as stem cells and metaphysical concerns. However, our study showed similar support between conservative participants and the total population surveyed. A possible reason for this finding is the impact of the communication strategy used in our survey, or the population skewing toward younger individuals with more years of schooling. Without a clear description of the process and purpose, people are left to imagine their worst-case scenarios. When the benefits to patients and the risk mitigation strategies are presented in an easily digestible framework, HAC research might receive more support than previously thought possible from the general public, highlighting the need for outreach and public engagement on this topic.
Legal Frameworks for HAC Research
U.S. Federal Laws and HAC Research
Chimera regulation in the United States is currently a patchwork system, coming from the National Institutes of Health (NIH), individual state laws, as well as reports from the National Academy of Sciences (NAS), President’s Commissions professional agencies, and other advisory groups 10 . While few laws directly regulate chimeras, several do impose guidelines on related areas. For instance, 1 U.S.C. § 8 states that
[i]n determining the meaning of any Act of Congress, or of any ruling, regulation, or interpretation of the various administrative bureaus and agencies of the U.S., the words “person,” “human being,” “child,” and “individual,” shall include every infant member of the species Homo sapiens who is born alive at any stage of development. 76
1 U.S.C. § 8 is the only guidance provided on what “human” means in terms of the statutory law. As HACs would not be members of the species Homo sapiens, human-level protections would likely not apply to them. But granting HACs human-level protections is a solution that might not be necessary, as other regulations apply to nonhuman animals and could cover HACs as well.
Society has already weighed the risks and consequences inherent in using nonconsenting animals for research, food, and labor. Although debate continues on the legitimacy of such use, it is generally seen as a necessary sacrifice, and one that already has clearly defined limits. Research animals are currently protected from unnecessary suffering in research by the Animal Welfare Act, and chimeras would likely fall under its protections 77 . The Animal Welfare Act has three main components that must be evaluated in the experiment review process. The necessary factors are (1) research should not exceed a minimal amount of pain and steps should be taken to minimize pain, (2) no alternative to using animals is available, and (3) the research must be monitored by a veterinarian. The committees that review proposals to use animals in research, commonly called Institutional Animal Care and Use Committees (IACUCs), serve a similar function as an institutional review board (IRB) for human research. The factors IACUCs are mandated to consider could apply to research using HACs as well. There is even special statutory protection for nonhuman primates—animals that provoke the highest degree of concern due to their intelligence and genetic and evolutionary similarity to humans. The 1985 amendment to the Animal Welfare Act in the United States, §43(a)(2)(A) 78 requires that special steps be taken to ensure the psychological well-being of nonhuman primates. If, for example, pig–monkey chimeras were created, and had cognitive and emotional abilities similar to those normally possessed by monkeys, they could be protected by these same laws 79 .
In terms of new technology and procedures, the Coordinated Framework for Regulation of Biotechnology, which describes the federal system for evaluating products developed using modern biotechnology, states the following: “[n]o single agency, however, provides for the comprehensive regulation of genetically modified organisms, and none of the agencies address modern chimera technology directly” 80 . Despite chimeras clearly falling into the category of “genetically modified organisms,” it is not clear which agency has jurisdiction over them. Human IVF techniques also sparked debate and stretched the limits of the regulatory system 81 . The Food and Drug Administration (FDA) regulates drugs, devices, and donor tissue related to IVF in conjunction with genetic modification, but otherwise State regulations, which have jurisdiction over the practice of medicine 82 , dominate.
Currently, the authors could identify no federal law prohibiting human cloning outright. However, it is illegal to use federal funds to create or use human embryos for research purposes 83 . The Dickey–Wicker Amendment is a federal budget rider that has been renewed every year since 1996 and limits funding of research involving the creation or destruction of embryos 84 . The most recent iteration can be found in the 2022 Consolidated Appropriations Act 85 . Thus, federal funds cannot be utilized to create or use human embryos for research purposes 83 .
The only federal legislation touching directly on neural chimeras was a failed bill called the Human Chimera Prohibition Act of 2005 86 . The Bill was introduced by Samuel Brownback, a republican senator from Kansas. Senator Brownback tried several times to push his bill through the senate, all of which failed. Provision I of the bill would have prohibited “a non-human life form engineered such that it contains a human brain, or a brain derived wholly or predominantly from human neural tissues.” Although the Brownback bill did not make it out of committee, two states, Arizona 87 and Louisiana 88 , passed laws nearly identical to the Brownback Bill. Senator Brownback put forth three reasons chimeras should be prohibited: (1) to prohibit the most ethically challenging chimeras, such as neural chimeras, (2) to prohibit science that compromises human dignity by blurring the line between animal and human, and (3) to prevent the transfer of infectious disease 89 . This was the prevailing viewpoint of many advisory panels and elected officials for years90–92.
In 2009, President Obama removed some of the barriers his predecessors had placed on using stem cells for research purposes 93 . The NIH, freed from previous prohibitions, then issued guidelines for using stem cells in research that encompassed the creation of chimeras 94 . In 2016, scientific advances such as the use of CRISPR-CAS9 for targeted genome editing and a greater understanding of developmental processes pushed the NIH to halt funding for chimera research95,96. This moratorium on publicly funded chimera research was to give the NIH time to restructure their review process for chimeras and specifically, chimeras where there was a “substantial contribution or a substantial functional modification to the animal brain by the human cells.” Despite constant rumors that the moratorium will soon be repealed, it is currently still in place over seven years later. The NAS guidelines from 2010 state that Embryonic Stem Cell Research Oversight (ESCRO) approval should be required for experiments where cells introduced into animals could possibly develop into neural tissue and suggested prohibiting the introduction of human stem cells to nonhuman primate blastocysts 97 . The ESCRO approval would be on a case-by-case basis for chimera work. The NAS, while taken very seriously by states and researchers, is not a governmental agency and has no enforcement power. Thus, despite existing review structures and protections for both humans and animals, the moratorium on federally funding HAC research continues.
Overall, federal funding for research using human embryos and neural chimeras is severely restricted in the United States, despite the existence of regulations and committees that could be adapted to cover many of the associated risks. The outright banning of an entire field of technology research and development, such as the one currently in place for chimera research, not only prevents potential lifesaving alternative methods of procuring viable transplantable organs but also directly places the United States behind other countries that are still actively pursuing chimera research. The longer the moratorium remains, the greater the deficit will be, and the greater the harm to living patients.
State and Institutional Guidelines for HAC Research
While most of the states with regulations relating to HACs also ban attempts at human cloning—where the result is a human being genetically identical to an existing or previously existing human—state rules regarding human–animal mixes are much more disparate (Figure 4). Many states do not explicitly mention human–animal hybrids or chimeras at all, while at the opposite end of the spectrum, some states provide guidance on the percentage of cells that are acceptable in specific systems, as well as an approval process for conducting such research. A brief overview of such regulations by state is provided in Table 3. One potentially confusing variable is that many states utilize terms such as “embryo” or “blastocyst” or “human-animal hybrid” as terms of art with explicitly stated definitions, so anyone using these regulations should also consult the definition sections of individual state statutes.
Table 3.
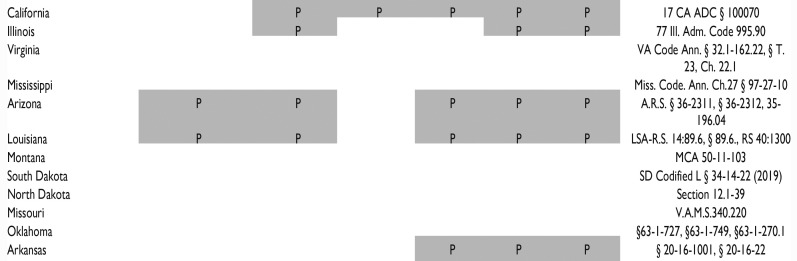
Aspects of Chimera-related Science Regulated or Prohibited by Existing Legislation in Each State.
| Animal cells transferred into another animal | Human stem cells transferred into mammalian (nonprimate) blastocyst, embryo, or fetus | Human stem cells into nonhuman primate blastocyst, embryo, or fetus | Human progenitor cells into brain/CNS of nonprimate animals | Transfer of an animal somatic cell nucleus into a human oocyte | Animal cells into human blastocyst/embryo | Live born human with animal haploid cells | Relevant state statutes | |
|---|---|---|---|---|---|---|---|---|
| ||||||||
“P” and a gray box indicate that at least part of the specific concept is regulated or prohibited in this state. Statutes were identified through WestLaw Edge database search for the term “chimera OR human animal chimera OR human animal hybrid OR human animal blastocyst complementation”. Each state included in this table was identified via the database search, even if statutes did not prohibit any of the categories identified.
CNS: central nervous system.
For example, one of the Arizona definitions of human–animal hybrid is “A human embryo into which a nonhuman cell or cells, or any component part of a nonhuman cell or cells, have been introduced.” 87 The Arizona statute is almost identical to the Louisiana statute; however, Louisiana 88 added some language that expands the definition of human–animal hybrid to include “nonhuman embryo[s] into which a human cell or cells or the component parts thereof have been introduced,” altering the application of these nearly identical laws. Many states are also careful to mention that their regulations are not intended to restrict biomedical research unless expressly prohibited, and restrict the use of funds for these activities, rather than banning them outright. States that do not specifically address human–animal hybrids do tend to have laws prohibiting human cloning, indicating that the primary concern of these laws is the protection of human genetic identity rather than general animal welfare.
Louisiana and Arizona each allow research in which human-derived cells contribute to brain tissue in animals, if the percentage of cells is less than 51%. Illinois could allow similar work, but instead of specifying that the human cells must not predominate the brain, the Illinois ESCROs instead consider what functional contributions the human cells will have to the brain of the developing organism when considering research approval 98 . California seems to have taken the most inclusive approach by limiting state funding but allowing many of these activities with Stem Cell Research Oversight (SCRO) approval and including restrictions on how long such crosses can be cultured 99 . California includes more explicit regulations that differentiate between nonhuman primates and other animals, consider the addition of animal stem cells into human embryos, and call for special consideration of any contribution of human cells to nonhuman mammalian central nervous systems and their potential functional impact. Arizona similarly has considered the contribution of human cells to animal brains, but instead of using a functional analysis, it allows contributions to nonhuman brains if the system is not “predominantly” created from human neural tissues while not clearly defining what “predominantly” means 87 .
The states with such regulations are fundamentally concerned with the same issue: limiting the contribution of human cells to animal brains to protect the Homo sapiens identity, but their approaches differ. One approach focuses on the exact percentage of cells or organ composition, while the other is concerned with the functional impact of those cells, but neither is foolproof. There may be brain regions where less than 51% of human-derived cells are enough to induce significant changes in an animal. Conversely, it is difficult to try and predict the extent of functional contributions of human cells in an animal before any testing has been done. While creation of a neural chimera capable of higher order cognition or of feeling existential angst is to be avoided, the current state laws, even those focused on functional impact, are not adequate to regulate this area. The phrasing of these state laws directly relates to the commonly cited ethical concern about blurring the line between animals and humans, but neither explains why this problem should be solved through legislation about the percent contribution of cells to an organism or the functional impact of those cells. We are left to indirectly infer that either a majority of human cells in the central nervous system or a set of “human-like” characteristics could be enough to potentially trigger human-level protections. If this is the real concern, there are likely better ways to handle it than state-level legislation, but at minimum, clearly defining what aspects of cognition should be assessed, how much change is considered “too much,” and other variables is necessary.
There are clear gaps in the state-level frameworks already. Most states are focused on animal contributions to human cells, primarily germline cells and to a lesser extent, the brain, but not on human contributions to animal cells. No state currently regulates animal–animal chimeras, few regulate human cells transferred into animals, and only two states specifically prohibit human cells transferred into early developing nonprimate mammals (Table 3). Therefore, significant regulatory gaps exist on the topic of HACs, and the extent of those gaps and what science is currently permitted with state funding differ wildly. Without clear guidance on what we are aiming to avoid with chimeras and especially neural chimeras, it is difficult to write laws that prevent the worst-case scenarios without unduly stifling other areas of research. While state lawmakers might not be familiar with some of these topics and pitfalls, many of the issues have been well thought out in the animal research space, and those regulatory schemes (IACUCs, IRBs, etc.) might be better equipped to deal with HACs than state legislatures.
International Laws on HACs
The international law on HAC production is largely undeveloped to date, with most legislation in existence being directed toward stem cell biology in general or human embryos containing animal cells specifically, not the inverse. For instance, Australia and Canada both have regulations prohibiting the production of human embryos containing animal material. Australia has the “Prohibition of Human Cloning for Reproduction Act” 100 , while Canada has the “Assisted Human Reproduction Act 2004” 101 . Both lack any specific guidance regarding the status of animal embryos with introduced human cells while clearly prohibiting human embryos with animal cells introduced. This is similar to the primary concern of most of the U.S. laws touching on HACs, which is human species protection. Similar to the U.S. NIH, Canada’s main funding agencies do not allow funding for chimera research in either human–animal or animal–human capacity, while Australia has no further regulation. This is a key point in existing chimera legislation, namely that chimera research is currently residing in a loophole in most countries, in a similar fashion to individual U.S. states as discussed above (for review, see Koplin and Savulescu 102 ).
Scientists and regulators in the United Kingdom have been discussing chimera research for decades, with two major publications of note. The first is the seminal “Animals Containing Human Material” (ACHM) publication from July 2011 103 , which discusses all aspects of stem cell and chimera research as pertains to animal systems that include human material introduction at any point. The ACHM was produced by the U.K. Academy of Medical Sciences, and although providing all necessary material to develop sound regulatory practices, has not materialized into codified U.K. legislation to date. The ACHM also discusses the primary ethical concerns regarding chimera generation in all various forms, synthesizing the robust ethics literature on chimera research and the public opinion of the U.K. populace. The second U.K. work was a joint statement by the U.K. Academy of Medical Sciences and the U.S. Committee on Guidelines for Human Embryonic Stem Cell Research, which recommended against the creation of HACs capable of human gamete production and against allowing HACs to breed 79 . Again, these are recommendations that have not manifested in codified law. This contrasts with Japan, where debate and public survey has led to government policy to regulate chimera research.
In 2019, the Japanese Ministry of Education, Culture, Sports, Science and Technology updated their guidelines regarding HACs in two significant ways104,105. First, they now will allow human brain cell–containing chimeras, in addition to in utero development beyond the previously established 14-day point. This update is likely a response to the recent national survey conducted in Japan, which found 81% of public and 92.4% of researchers supported HACs in some capacity 106 . The primary concerns regarding HACs in Japan were human–animal neurological chimerism and germline integration, similar to our previous survey in the United States 61 and Kantor 62 . Prior to 2019, Japan’s legislature established a 14-day limit on any chimera development in place under the 2001 Guidelines for Handling of a Specified Embryo 105 . This update specifically regarding chimera production was influenced by two major developments, the first being successful chimera research being conducted internationally (by Hiromitsu Nakauchi, currently at Stanford University but also faculty at the University of Tokyo), necessitating regulations; and the second being a survey conducted by the Japanese Ministry of Education, Culture, Sports, Science and Technology assessing the likelihood of any animal model receiving human neural cells to develop human-like brain functions, which was found to be very unlikely 105 . Thus, Japan made logical legislative updates to specifically address the unique concerns regarding chimeras compared with other stem cell and embryo research, allowing development of the field.
In summary, there is a lack of consensus on chimera regulation internationally that mirrors the state-by-state variability present in the United States, with most countries lacking any regulation at all. This ambiguity and lack of legislation has led the International Society for Stem Cell Research (ISSCR) to develop and include specific recommendations for human cells in animal chimeras in their semi-regularly updated “Guidelines for Stem Cell Research and Clinical Translation” 107 . This publication and the accompanying white paper published in Stem Cell Reports by the same authors 108 provide the most cohesive and moderate set of regulations and processes for organizations and governments to implement legislation by incorporating the ethical concerns, public opinion, and scientific literature into their recommendations. Their general conclusion is to regulate chimera research in a stepwise or incremental fashion by primarily extending current existing guidelines for animal and stem cell research to chimera research107,108.
As discussed above, animal research in the United States already necessitates several requirements that are equally important in chimera work: sound scientific rationale and experimental design, rationale for necessity of animal model versus other models, minimizing suffering and number of animals needed, and so on. The ISSCR largely concludes that non-neurological or germline chimera research should not require much beyond standard institutional review paradigms already in use. However, ISSCR also addressed the unique concerns and challenges neurological human chimera research entails. They recommended that researchers should identify why the proposed host species was chosen, confirm that researchers have established concrete baseline measures of cognition and intelligence, and can test whether human cells augment these measures, the use of methodologies to target introduced stem cells to a particular niche (blastocyst complementation), and potentially establishing a unique review board of experts in stem cell biology, neurodevelopment, and ethics to provide additional scrutiny to any neurological chimera work before approval.
Finally, ISSCR adamantly claims that neurological chimeras should be allowed, but the key is that we progress in a controlled and gradual process with low sample sizes and early developmental endpoints until we develop a more concrete understanding of how HACs develop and whether there is cause for concern. This stepwise process includes beginning with low numbers of produced chimeras, minimizing gestational times to the shortest period necessary to determine end results of study (and never to full term), and consistent and bidirectional status updates to regulatory boards to ensure transparency. They rightly identify that nearly all concern at present is theoretical regarding human traits being developed in animals, and thus, entirely prohibiting this science is not in line with the scientific endeavor, where data guide the science rather than emotion. Further, our review 109 of most of the literature concerning human cells introduced into animal nervous systems found at most small benefits in cognitive function, thus suggesting that the animal host likely constrains any introduced foreign cells to function within the existing brain connectome system. However, until more neurological chimeras are made, especially ones that include human stem cells introduced in animal embryos that incorporate and develop in utero, guidelines like the ones provided by ISSCR are reasonable and sufficient to allow HAC research to progress.
Patent Law and HACs
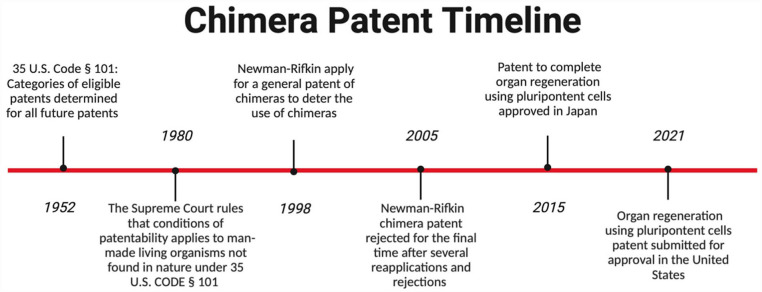
Patents are an integral part of the legal framework used to provide protection of intellectual property for the purpose of commercialization. When thinking about patents as applied to chimeras, the first basic question is whether man-made living organisms can be patented, and thus become eligible for patent protection. For many years, federal courts held firm against patenting living organisms. However, in 1980, the courts held that man-made living organisms not found in nature could be patented 110 (Figure 5). This change in patentable subject matter resulted from the ruling in Diamond v. Chakrabarty, in which Chakrabarty attempted to patent a new type of bacterium. It was determined that it fell under the category of manufactured material, allowing certain man-made living organisms to be patented, and was a significant event in the patent legal system 111 .
Figure 5.
Timeline of landmark patent approvals and rejections. The initial guidelines regarding patentable material were published in 1952. The guidelines were altered in 1980 to include non-naturally occurring man-made living organisms. In 1998, Newman–Rifkin applied for a general chimera patent and continued to apply until all reapplications were exhausted. A patent for organ regeneration using pluripotent cells was approved in Japan in 2015 and submitted for U.S. approval in 2021.
But the ability to patent living organisms that are man-made did not fully translate to the issue of chimeras. The first patent filed on chimeras was the Newman–Rifkin patent of 1998, which sought to cover a general description of chimera work. The patent discussed the use of embryos from monkeys, apes, or other animals in combination with humans without explicitly stating the combination percentages that the patent attempted to cover 112 . This patent was the start of an HAC dialogue in the patent world. Newman and Rifkin attempted to deter the use of chimeras either through the execution of a strict patent or through a general strike down of the patentability of chimeras. Their patent employed such general terminology that it would have prevented any others from using almost any chimeras in their work.
Patent approval requires meeting five criteria: (1) be a patentable subject, (2) be a useful invention, (3) not be used, created, or published in the United States or other countries, (4) be a process that is not obvious, and (5) the invention, methods, and claims of the subject matter must be disclosed 113 . The Rifkin–Newman patent was finally denied in 2005 because it was unable to show that it was not being used or created by others. The main motive for patent rejection other than standard patent criteria released by the U.S. Patent and Trademark Office (USPTO) included the inability to own a human being (U.S. Const. amend. XIII) 114 . Although other rulings allowed patents of living organisms, the USPTO believed that these rulings did not intend to include human beings and that humans were not considered to be patentable subject matter. This is very interesting given the statutory definition of “human”; the USPTO seems to have taken a much wider view because the patent was focused on primate embryos with human cells, not human embryos with animal cells. Slow progress has been made on patents involving human cells and transfected cell lines, but no ruling has been made that would allow the patent of HACs in the United States. We do not propose here that they should be patentable, merely draw attention to the differences between the USPTO’s wider view and the current narrower state legislation. The lack of legislative action providing general guidelines for patentability of partially human subjects and the continuous refiling and ultimate rejection of the Newman–Rifkin patent in 2005 helped to define the limits of what might be acceptable in a future chimera patent.
Following the strike down of the general chimera patent, several chimera-related patents such as organ regeneration using blastocyst complementation 115 , chimeric embryonic auxiliary organs, 116 and compositions and methods for chimeric embryo-assisted organ production 117 have been submitted worldwide. These utility patents focus on generating various mouse organs using chimeras. While only a few patents have been approved in other countries like Japan, the continual submission of patents involving specific complementation techniques and gene knockouts shows a movement toward the acceptance of chimera patents in specific contexts.
In the approved Japanese utility patent on organ regeneration using blastocyst complementation, the application claims to produce target organs or body parts created from nonhuman mammals via organ deficits at the developmental stage. It also claims that the method uses pluripotent stem cells derived from humans, rats, or mice to target the pancreas, kidney, thymus, or hair. The patent uses SAL1 knockout, PDX1 knockout, PDX1-Hes1 transgenic, or nude mice to induce a deficit in the target organs that are then targeted by the pluripotent stem cells (Figure 3). The subsequent claim details the process of producing the target organ by introducing a defective causative gene preventing organ development for function, which is then supplemented by blastocyst complementation. An egg from the donor animal is collected, grown to the blastocyst stage, and exposed to pluripotent stem cells that can target the deficit, creating a chimeric blastocyst.
This procedural patent has been submitted for approval in the United States, which will necessitate a reevaluation of chimera patents by the USPTO. Although human chimeras have yet to be patented in any country, the fundamental methods for HAC creation have been granted patent protection. The next step would be to go beyond animal–animal chimera patents and use the methods from those patents to begin the conversation of possibly integrating HACs themselves into patent law. This will require more discussion and development, as being able to patent HAC technology would allow research institutes (both commercial and academic) to protect their intellectual property, thus incentivizing further development and utilization of this technology. However, such patenting could also restrict access to the technology and advances based on the fee structures.
Conclusion
With the rapid development and advancement of transplantation and stem cell science, the technologies to develop human organs in other organisms are nearly a reality. In 2015, the NIH made the decision to prohibit any federal funding of HAC research, claiming the field was advancing into unknown territory and the moratorium would allow for discussion and necessary regulatory frameworks to be established and implemented95,96. Now, the moratorium has existed for many years, with minimal progress on regulation. Although the guidance regarding HACs has not progressed, the science of chimera research has continued, primarily through private funding sources and international efforts that circumvent the NIH moratorium. This observation highlights two key points: first, that chimera research continued and will likely continue regardless of whether federal funding and oversight is provided, and second, that a federal moratorium did not result in the intended outcome of halting chimera work until agreed-upon limitations were in place. In contrast, the federal moratorium likely had the opposite effect, stymying chimera research in the United States by restricting the largest public research funding agency (NIH) from funding this research, and therefore simultaneously limiting their ability to regulate the developing field and allowing other countries to continue to advance their chimera research programs. A moratorium to ensure that proper ethical consideration is given to the impact of new technologies is sensible, but the moratorium on chimera funding has been in place too long when some common sense and scientifically backed limits and guidelines already have been developed and could be quickly applied to chimera work.
Most of the concerns regarding human and nonhuman chimera production are already properly accounted for in existing animal welfare legislation or research advisory boards. These frameworks simply need to explicitly apply to chimera research as well, which has already been proposed by Insoo Hyun in 2019 118 . Most scientific evidence analyzing chimeras reported only incremental and restricted changes to behaviors in the host animal, as our review of neurological chimerism shows.
Three areas of chimera research deserve special attention, namely, human–nonhuman primate chimerism, neurological chimerism, and germline contribution, as these are likely the areas where human cell integration into host animals could have the largest unintended effects and/or are the most concerning to the general populace. Although nonhuman primates likely would be the best host organism for human organs considering evolutionary relation, the risks of off-target incorporation are greater as well. Considering that pig tissues have been used for transplantation in humans as well as in chimera research, we suggest using pigs for chimera studies on human organ generation instead of nonhuman primates to prevent some of these high-risk consequences altogether.
No “human” characteristics have been found in nonhuman organisms that have received human cells in their brains, as of the writing of this article. This does not preclude the possibility that future procedures may produce more substantial changes, but current scientific evidence suggests the host organism significantly constrains the human cells to function along host species lines 109 . Thus, we suggest expanding current regulation via IRBs, IACUCs, and other boards that regulate animal research and research involving human genetic material to also review chimera research, with additional requirements of surveillance for cognitive changes, scale of incorporation of human cells, and off-target incorporation. By requiring scientists to interrogate these aspects in each chimera study, regardless of whether the goal is making neural tissue or not, the governing bodies can promptly identify any issues as they arise and prevent the science from surpassing the regulatory framework. Science primarily progresses in incremental steps, and this method of experimental design with added surveillance should prevent the creation of anything remotely close to human-like consciousness in animals, which is one of the ethically least desirable outcomes.
In conclusion, we recommend the following changes to develop and refine policy toward chimera research. First, using the existing stem cell advisory boards like ESCRO and the existing animal welfare committees like IACUC to review and critique proposed experiments utilizing HACs. This is in line with ISSCR’s recommendations on chimera research, and as previously discussed, many of the issues raised are already being addressed by these committees. In addition, updating existing stem cell and cloning laws to include chimera research or creating new legislation addressing chimeras should be done as soon as possible. As we demonstrated, there are few states that directly regulate HAC research, and usually only in a narrow scope. Because chimera research is a rapidly advancing field where unintended consequences are possible (albeit very unlikely), we recommend the implementation of additional surveillance measures such as assessment of cognitive capacity; human cell integration into neural, germline, and other off-target tissues; and any other scientifically backed concerns that are determined by ESCRO, IACUC, NIH, or other governing bodies. These steps will ensure unintended consequences can be rapidly identified and addressed in the unlikely event they occur. Proactive regulation and oversight are far more effective and desirable in this type of research than reactionary responses. Lifting the NIH moratorium on funding HAC research will allow the scientific community to make more informed and calculated decisions regarding research directions.
Finally, investments in public education and outreach regarding chimera research will likely serve multiple beneficial functions. In addition to increasing public awareness and understanding, our previous survey hinted at how a clear understanding of actual chimera research methods and their possible beneficial impact on the donor organ shortage problem seems to alleviate some of the concerns regarding HAC research. Thus, we expect education and outreach will allow the public to engage with this new avenue of research, to understand the scientifically backed concerns and what the governing bodies are doing to ensure those concerns are addressed. Only by closely examining the possible pitfalls and ethical quandaries presented by chimera research can we develop a system that utilizes the technology to its fullest potential while respecting humans and animals alike.
Acknowledgments
The authors would like to thank Francis X. Shen for his contributions on initial discussions regarding this article.
Footnotes
Authors’ Contributions: W.C.L, J.L.B., and J.P.V conceptualized the article. W.C.L provided review and guidance. J.L.B., J.P.V., and K.P. wrote the article. J.L.B. and J.P.V. contributed equally and generated the figures and provided edits.
Ethical Approval: This review did not require institutional review board approval.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institute of Neurological Disorders and Stroke Grant F31NS125963 (J.L.B.) and National Institute on Aging Grant 2T32AG052354-06A1 (J.P.V.)
ORCID iDs: Jennifer L. Brown  https://orcid.org/0000-0002-7525-5986
https://orcid.org/0000-0002-7525-5986
Walter C. Low  https://orcid.org/0000-0001-8593-0175
https://orcid.org/0000-0001-8593-0175
References
- 1. Ghods AJ. The history of organ donation and transplantation in Iran. Exp Clin Transplant. 2014;12(Suppl 1):38–41. [DOI] [PubMed] [Google Scholar]
- 2. Cooper DKC. Christiaan barnard—the surgeon who dared: the story of the first human-to-human heart transplant. Glob Cardiol Sci Pract. 2018;2018(2):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Health Resources & Services Administration. Organ donation statistics; 2020. https://www.organdonor.gov/learn/organ-donation-statistics [accessed 2022 September 6].
- 4. Centers for Disease Control and Prevention. Key facts. Transplant safety overview. https://www.cdc.gov/transplantsafety/overview/key-facts.html [accessed 2022 September 6].
- 5. Organ Procurement and Transplantation Network. Annual record trend continues for deceased organ donation, deceased donor transplants. https://optn.transplant.hrsa.gov/news/annual-record-trend-continues-for-deceased-organ-donation-deceased-donor-transplants/ [accessed 2022 September 6].
- 6. Black CK, Termanini KM, Aguirre O, Hawksworth JS, Sosin M. Solid organ transplantation in the 21st century. Ann Transl Med. 2018;6(20):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donate Life America. Organ, eye and tissue donation statistics. Donate Life America; 2022. https://donatelife.net/donation/statistics/ [accessed 2022 September 6].
- 8. Brewer B. Click it or give it: increased seat belt law enforcement and organ donation. Health Econ. 2020;29(11):1400–21. [DOI] [PubMed] [Google Scholar]
- 9. Costello JP, Mohanakumar T, Nath DS. Mechanisms of chronic cardiac allograft rejection. Tex Heart Inst J. 2013;40(4):395–99. [PMC free article] [PubMed] [Google Scholar]
- 10. Bonnicksen AL. Chimeras, hybrids and interspecies research: politics and policymaking. Washington (DC): Georgetown University Press; 2009. [Google Scholar]
- 11. Chen HI, Wolf JA, Blue R, Song MM, Moreno JD, Ming G-l, Song H. Transplantation of human brain organoids: revisiting the science and ethics of brain chimeras. Cell Stem Cell. 2019;25(4):462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sherringham T. Mice, men, and monsters: opposition to chimera research and the scope of federal regulation. Calif Law Rev. 2008;96(3):765–800. [Google Scholar]
- 13. Garry MG, Garry DJ. Humanized organs in gene-edited animals. Regen Med. 2016;11(7):617–19. [DOI] [PubMed] [Google Scholar]
- 14. Linzey A, Linzey C. editors. The ethical case against animal experiments. Champaign (IL): University of Illinois Press; 2017. [Google Scholar]
- 15. Archibald K, Coleman R. How human biology can prevent drug deaths. New Sci. 2012. Dec 12:12–14. https://www.newscientist.com/article/mg21628950-200-how-human-biology-can-prevent-drug-deaths/ [accessed 2022 September 6].
- 16. Spence JR. Clocking the pace of organoid research. Cell Mol Gastroenterol Hepatol. 2017;4(1):203–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Groth CG. The potential advantages of transplanting organs from pig to man: A transplant Surgeon’s view. Indian J Urol. 2007;23(3):305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang H, Wu Z. Genome editing of pigs for agriculture and biomedicine. Front Genet. 2018;9:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jeevani T. Stemcell transplantation- types, risks and benefits. J Stem Cell Res Ther. 2011;1(3):114. [Google Scholar]
- 20. Title I-Task force on organ procurement and transplantation. 1984. S.2048 - 98th Congress (1983-1984): National Organ Transplant Act. (1984, October 19). https://www.congress.gov/bill/98th-congress/senate-bill/2048 [accessed 2022 September 6].
- 21. Nemudryi AA, Valetdinova KR, Medvedev SP, Zakian SM. TALEN and CRISPR/Cas genome editing systems: tools of discovery. Acta Naturae. 2014;6(3):19–40. [PMC free article] [PubMed] [Google Scholar]
- 22. Gaj T, Sirk SJ, Shui SL, Liu J. Genome-editing technologies: principles and applications. Cold Spring Harb Perspect Biol. 2016;8(12):a023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asmamaw M, Zawdie B. Mechanism and applications of CRISPR/cas-9-mediated genome editing. Biologics. 2021;15:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dunlap G, Mcardel S. The end of the waitlist: how chimeras could solve the organ transplant problem. Chimeras: mythological beasts or useful research tools? 2017. https://sitn.hms.Harvard.edu/flash/2017/end-waitlist-chimeras-solve-organ-transplant-problem/ [accessed 2023 June 15].
- 25. Rossant J. Genetic control of early cell lineages in the mammalian embryo. Annu Rev Genet. 2018;52:185–201. [DOI] [PubMed] [Google Scholar]
- 26. Chen J, Lansford R, Stewart V, Young F, Alt FW. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci U S A. 1993;90:4528–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Young FM, Pinkert CA, Bottaro A. Analysis of lymphocyte development and function using the RAG-deficient blastocyst complementation system. Methods Mol Biol. 2004;271:77–90. [DOI] [PubMed] [Google Scholar]
- 28. Jansson L, Larsson J. W41/W41 blastocyst complementation: a system for genetic modeling of hematopoiesis. Blood. 2010;115(1):47–50. [DOI] [PubMed] [Google Scholar]
- 29. Chubb R, Oh J, Riley AK, Kimura T, Wu SM, Wu JY. In vivo rescue of the hematopoietic niche by pluripotent stem cell complementation of defective osteoblast compartments. Stem Cells. 2017;35(10):2150–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamazaki K, Kubara K, Ishii S, Li P, Dairiki R, Hihara T, Ishizuka Y, Izumi Y, Kumai M, Kamisako T, Ishizaki H, et al. In vitro and in vivo functions of T cells produced in complemented thymi of chimeric mice generated by blastocyst complementation. Sci Rep. 2022;12(1):3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liégeois NJ, Horner JW, DePinho RA. Lens complementation system for the genetic analysis of growth, differentiation, and apoptosis in vivo. Proc Natl Acad Sci U S A. 1996;93(3):1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kobayashi T, Yamaguchi T, Hamanaka S, Kato-itoh M, Yamazaki Y, Ibata M, Sato H, Lee Y, Usui J, Knisely AS, Hirabayashi M. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142(5):787–99. [DOI] [PubMed] [Google Scholar]
- 33. Usui J, Kobayashi T, Yamaguchi T, Knisely AS, Nishinakamura R, Nakauchi H. Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol. 2012;180(6):2417–26. [DOI] [PubMed] [Google Scholar]
- 34. Ruiz-Estevez M, Crane AT, Rodriguez-Villamil P, Ongaratto FL, You Y, Steevens AR, Hill C, Goldsmith T, Webster DA, Sherry L, Lim S, et al. Liver development is restored by blastocyst complementation of HHEX knockout in mice and pigs. Stem Cell Res Ther. 2021;12(1):292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mori M, Furuhashi K, Danielsson JA, Hirata Y, Kakiuchi M, Lin CS, Ohta M, Riccio P, Takahashi Y, Xu X, Emala CW, et al. Generation of functional lungs via conditional blastocyst complementation using pluripotent stem cells. Nat Med. 2019;25(11):1691–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kitahara A, Ran Q, Oda K, Yasue A, Abe M, Ye X, Sasaoka T, Tsuchida M, Sakimura K, Ajioka Y, Saijo Y, et al. Generation of lungs by blastocyst complementation in apneumic Fgf10-deficient mice. Cell Rep. 2020;31(6):107626. [DOI] [PubMed] [Google Scholar]
- 37. Wen B, Li E, Ustiyan V, Wang G, Guo M, Na C-L, Kalin GT, Galvan V, Xu Y, Weaver TE, Kalin T, et al. In vivo generation of lung and thyroid tissues from embryonic stem cells using blastocyst complementation. Am J Respir Crit Care Med. 2021;203(4):471–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li E, Ustiyan V, Wen B, Kalin GT, Whitsett JA, Kalin TV, Kalinichenko VV. Blastocyst complementation reveals that NKX2-1 establishes the proximal-peripheral boundary of the airway epithelium. Dev Dyn. 2021;250(7):1001–20. [DOI] [PubMed] [Google Scholar]
- 39. Ran Q, Zhou Q, Oda K, Yasue A, Abe M, Ye X, Li Y, Sasaoka T, Sakimura K, Ajioka Y, Saijo Y. Generation of thyroid tissues from embryonic stem cells via blastocyst complementation in vivo. Front Endocrinol. 2020;11:609697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang AN, Liang Z, Dai HQ, Chapdelaine-Williams AM, Andrews N, Bronson RT, Schwer B, Alt FW. Neural blastocyst complementation enables mouse forebrain organogenesis. Nature. 2018;563(7729):126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Steevens AR, Griesbach MW, You Y, Dutton JR, Low WC, Santi PA. Generation of inner ear sensory neurons using blastocyst complementation in a Neurog1+/−−deficient mouse. Stem Cell Res Ther. 2021;12(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hamanaka S, Umino A, Sato H, Hayama T, Yanagida A, Mizuno N, Kobayashi T, Kasai M, Suchy FP, Yamazaki S, Masaki H, et al. Generation of vascular endothelial cells and hematopoietic cells by blastocyst complementation. Stem Cell Reports. 2018;11(4):988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matsunari H, Nagashima H, Watanabe M, Umeyama K, Nakano K, Nagaya M, Kobayashi T, Yamaguchi T, Sumazaki R, Herzenberg LA, Nakauchi H. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci U S A. 2013;110(12):4557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matsunari H, Watanabe M, Hasegawa K, Uchikura A, Nakano K, Umeyama K, Masaki H, Hamanaka S, Yamaguchi T, Nagaya M, Nishinakamura R, et al. Compensation of disabled organogeneses in genetically modified pig fetuses by blastocyst complementation. Stem Cell Reports. 2020;14(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang H, Huang J, Li Z, Qin G, Zhang N, Hai T, Hong Q, Zheng Q, Zhang Y, Song R, Yao J, et al. Rescuing ocular development in an anophthalmic pig by blastocyst complementation. EMBO Mol Med. Published online Nov 16, 2018. doi: 10.15252/emmm.201808861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ji H, Long C, Feng C, Shi N, Jiang Y, Zeng G, Li X, Wu J, Lu L, Lu S, Pan D. Generation of chimeric minipigs by aggregating 4- to 8-cell-stage blastomeres from somatic cell nuclear transfer with the tracing of enhanced green fluorescent protein. Xenotransplantation. Published online April 11, 2017. doi: 10.1111/xen.12300 [DOI] [PubMed] [Google Scholar]
- 47. Ideta A, Yamashita S, Seki-Soma M, Yamaguchi R, Chiba S, Komaki H, Ito T, Konishi M, Aoyagi Y, Sendai Y. Generation of exogenous germ cells in the ovaries of sterile NANOS3-null beef cattle. Sci Rep. 2016;6:24983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang X, Shi H, Zhou J, Zou Q, Zhang Q, Gou S, Chen P, Mou L, Fan N, Suo Y, Ouyang Z, et al. Generation of rat blood vasculature and hematopoietic cells in rat-mouse chimeras by blastocyst complementation. J Genet Genomics. 2020;47(5):249–61. [DOI] [PubMed] [Google Scholar]
- 49. Nishimura T, Suchy FP, Bhadury J, Igarashi KJ, Charlesworth CT, Nakauchi H. Generation of functional organs using a cell-competitive niche in intra- and inter-species rodent chimeras. Cell Stem Cell. 2021;28(1):141–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamaguchi T, Sato H, Kato-Itoh M, Goto T, Hara H, Sanbo M, Mizuno N, Kobayashi T, Yanagida A, Umino A, Ota Y, et al. Interspecies organogenesis generates autologous functional islets. Nature. 2017;542(7640):191–96. [DOI] [PubMed] [Google Scholar]
- 51. Goto T, Hara H, Sanbo M, Masaki H, Sato H, Yamaguchi T, Hochi S, Kobayashi T, Nakauchi H, Hirabayashi M. Generation of pluripotent stem cell-derived mouse kidneys in Sall1-targeted anephric rats. Nat Commun. 2019;10(1):451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kobayashi T, Goto T, Oikawa M, Sanbo M, Yoshida F, Terada R, Niizeki N, Kajitani N, Kazuki K, Kazuki Y, Hochi S, et al. Blastocyst complementation using Prdm14-deficient rats enables efficient germline transmission and generation of functional mouse spermatids in rats. Nat Commun. 2021;12(1):1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fu R, Yu D, Ren J, Li C, Wang J, Feng G, Wang X, Wan H, Li T, Wang L, Zhang Y, et al. Domesticated cynomolgus monkey embryonic stem cells allow the generation of neonatal interspecies chimeric pigs. Protein Cell. 2020;11(2):97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cohen MA, Markoulaki S, Jaenisch R. Matched developmental timing of donor cells with the host is crucial for chimera formation. Stem Cell Reports. 2018;10(5):1445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu J, Platero-Luengo A, Sakurai M, Sugawara A, Gil MA, Yamauchi T, Suzuki K, Bogliotti YS, Cuello C, Morales Valencia M, et al. Interspecies chimerism with mammalian pluripotent stem cells. Cell. 2017;168(3):473–486.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Das S, Koyano-Nakagawa N, Gafni O, Maeng G, Singh BN, Rasmussen T, Pan X, Choi KD, Mickelson D, Gong W, Pota P, et al. Generation of human endothelium in pig embryos deficient in ETV2. Nat Biotechnol. 2020;38(3):297–302. [DOI] [PubMed] [Google Scholar]
- 57. Maeng G, Das S, Greising SM, Gong W, Singh BN, Kren S, Mickelson D, Skie E, Gafni O, Sorensen JR, Weaver CV, et al. Humanized skeletal muscle in MYF5/MYOD/MYF6-null pig embryos. Nat Biomed Eng. 2021;5(8):805–14. [DOI] [PubMed] [Google Scholar]
- 58. Tan T, Wu J, Si C, Dai S, Zhang Y, Sun N, Zhang E, Shao H, Si W, Yang P, Wang H, et al. Chimeric contribution of human extended pluripotent stem cells to monkey embryos ex vivo. Cell. 2021;184(8):2020–203214. [DOI] [PubMed] [Google Scholar]
- 59. Moreno JD. The body politic: the battle over science in America. New York: Bellevue Literary Press; 2011. [Google Scholar]
- 60. Robert J, Baylis F. Crossing species boundaries. American Journal of Bioethics. 2003;3(3):1–13. [DOI] [PubMed] [Google Scholar]
- 61. Crane AT, Shen FX, Brown JL, Cormack W, Ruiz-Estevez M, Voth JP, Sawai T, Hatta T, Fujita M, Low WC. The American public is ready to accept human-animal chimera research. Stem Cell Reports. 2020;15(4):804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kantor J. Public support in the U.S. for human-animal chimera research: results of a representative cross-sectional survey of 1,058 adults. Stem Cells Transl Med. 2017;6(5):1442–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Matthews KRW, Morali D. Can we do that here? an analysis of US federal and state policies guiding human embryo and embryoid research. J Law Biosci. 2022;9(1):lsac014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Johnston J, Hyun I, Neuhaus CP, Maschke KJ, Marshall P, Craig KP, Matthews MM, Drolet K, Greely HT, Hill LR, Hinterberger A, et al. Clarifying the ethics and oversight of chimeric research. Hastings Cent Rep. 2022;52(Suppl 2):S2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Greely HT. Defining chimeras. . .and chimeric concerns. Am J Bioeth. 2003;3(3):17–20. [DOI] [PubMed] [Google Scholar]
- 66. Korsgaard CM. Fellow creatures: Kantian ethics and our duties to animals. Tanner Lect Human Value. 2004;4:77–110. [Google Scholar]
- 67. Palacios-González C. Human dignity and the creation of human–nonhuman chimeras. Med Health Care Philos. 2015;18(4):487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Johnston J, Eliot C. Chimeras and “human dignity.” The American Journal of Bioethics. 2003;3(3):6–8. [DOI] [PubMed] [Google Scholar]
- 69. Koplin JJ. Human-animal chimeras: the moral insignificance of uniquely human capacities. Hastings Cent Rep. 2019;49(5):23–32. [DOI] [PubMed] [Google Scholar]
- 70. Streiffer R. Chimeras, moral status, and public policy: implications of the abortion debate for public policy on human/nonhuman chimera research. J Law Med Ethics. 2010;38(2):238–50. [DOI] [PubMed] [Google Scholar]
- 71. Festing S, Wilkinson R. The ethics of animal research. EMBO Rep. 2007;8(6):526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kwisda K, White L, Hübner D. Ethical arguments concerning human-animal chimera research: a systematic review. BMC Med Ethics. 2020;21(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mitchell C, Lipps A, Padilla L, Werkheiser Z, Cooper DKC, Paris W. Meta-analysis of public perception toward xenotransplantation. Xenotransplantation. 2020;27(4):e12583. [DOI] [PubMed] [Google Scholar]
- 74. Bolo I, Wills BC, Maschke KJ. Public attitudes toward human-animal chimera research may be more complicated than they appear. Stem Cell Reports. 2021;16(2):225–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cooperman A, Smith GA, Cornibert SS. U.S. Public becoming less religious; 2014. https://www.pewresearch.Org/religion/2015/11/03/u-s-public-becoming-less-religious/ [accessed 2022 August 15].
- 76. 1 U.S.C. 8—Person,” “human being,” “child,” and “individual” as including born-alive infant. Content details. USCODE-2011-title1-chap1-sec8. https://www.govinfo.gov/app/details/USCODE-2011-title1/USCODE-2011-title1-chap1-sec8 [accessed 2022 August 15].
- 77. 7 U.S.C. 2143—Standards and certification process for humane handling, care, treatment, and transportation of animals. Content details. USCODE-2015-title7-chap54-sec2143. https://www.govinfo.gov/app/details/USCODE-2015-title7/USCODE-2015-title7-chap54-sec2143 [accessed 2022 August 15].
- 78. S.1233—99th Congress (1985-1986): Improved Standards for Laboratory Animals Act—Congress.gov—Library of Congress. https://www.congress.gov/bill/99th-congress/senate-bill/1233 [accessed 2022 August 15].
- 79. Devolder K, Yip LJ, Douglas T. The ethics of creating and using human-animal chimeras. ILAR J. 2019;60(3):434–38. [DOI] [PubMed] [Google Scholar]
- 80. The coordinated framework for regulation of biotechnology. 51 C.F.R. 23302. 1986. https://www.aphis.usda.gov/brs/fedregister/coordinated_framework.pdf [accessed 2023 June 15]. [PubMed]
- 81. Lewis MS. Is germline gene editing exceptional? Seton Hall Law Review. 2021;51:735–813. [Google Scholar]
- 82. Carlson D, Thompson JN. The role of state medical boards. Virtual Mentor. 2005;7(4):311–14. [DOI] [PubMed] [Google Scholar]
- 83. 48 CFR §352.270-13 continued ban on funding abortion and continued ban on funding of human embryo research. https://www.acquisition.gov/hhsar/352.270-13-continued-ban-funding-abortion-and-continued-ban-funding-human-embryo-research [accessed 2023 June 15].
- 84. The Embryo Project Encyclopedia. Dickey-Wicker Amendment, 1996. https://embryo.asu.edu/pages/dickey-wicker-amendment-1996 [accessed 2022 August 15].
- 85. Consolidated Appropriations Act, 2022, Sec. 508. 2022. [Google Scholar]
- 86. S.1373—109th Congress (2005-2006): Human Chimera Prohibition Act of 2005—Congress.gov—Library of Congress. https://www.congress.gov/bill/109th-congress/senate-bill/1373?s=1&r=40 [accessed 2022 August 15].
- 87. Ariz. Rev. Stat. Ann. § 36-2312. [Google Scholar]
- 88. La. Stat. Ann. § 14:89.6. [Google Scholar]
- 89. U.S. Sen. Sam Brownback. Keynote address at the Harvard Law School Society for Law, Life & Religion 2005. symposium: law & ethics in a brave new world: what should government do about cloning and stem cell research? 2005. April 15. https://www.priestsforlife.org/government/brownback05-04-15cloningstemcell.pdf [accessed 2023 June 15].
- 90. Report of the human embryo research panel volume 1. https://repository.library.georgetown.edu/handle/10822/559352 [accessed 2023 June 15]. [PubMed]
- 91. Dolgin E. Chimeras keep courting controversy. Proc Natl Acad Sci U S A. 2016;113(43):11984–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. President George W. Bush, State of the Union Address; 2006.https://georgewbush-whitehouse.archives.gov/stateoftheunion/2006/ [accessed 2022 August 15].
- 93. 3 CFR 13505—Executive Order 13505 of March 9, 2009. Removing barriers to responsible scientific research involving human stem cells—content details—CFR-2010-title3-vol1-eo13505. https://www.govinfo.gov/app/details/CFR-2010-title3-vol1/CFR-2010-title3-vol1-eo13505 [accessed 2022 August 15].
- 94. Federal Register, Volume 74 Issue 128 (Tuesday, July 7, 2009). https://www.govinfo.gov/content/pkg/FR-2009-07-07/html/E9-15954.htm [accessed 2022 August 15].
- 95. NOT-OD-15-158: NIH research involving introduction of human pluripotent cells into non-human vertebrate animal pre-gastrulation embryos. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-158.html [accessed 2022 August 15].
- 96. 81 C.F.R. 51921. Request for public comment on the proposed changes to the NIH guidelines for human stem cell research and the proposed scope of an NIH Steering Committee’s consideration of certain human-animal chimera research. https://www.federalregister.gov/documents/2016/08/05/2016-18601/request-for-public-comment-on-the-proposed-changes-to-the-nih-guidelines-for-human-stem-cell [accessed 2022 August 15].
- 97. Final report of the National Academies’ Human Embryonic Stem Cell Research Advisory Committee and 2010 Amendments to the National Academies’ Guidelines for Human Embryonic Stem Cell Research. Washington (DC): National Academies Press; 2010. [PubMed] [Google Scholar]
- 98. 77 Ill. Adm. Code 995.90. [Google Scholar]
- 99. 17 CA ADC § 100070. SCRO committee review and notification. https://www.cirm.ca.gov/sites/default/files/files/funding_page/100070_SCROCommitteeReviewandNotification_MES_18.03.02.pdf [accessed 2022 August 15].
- 100. Federal Register of Legislation, Australian Government. Prohibition of Human Cloning for Reproduction Act 2002; 2002. https://www.legislation.gov.Au/Details/C2017C00306#:~:text=(1)%20A%20person%20commits%20an,pregnancy%20in%20a%20particular%20woman [accessed 2023 June 15]. [Google Scholar]
- 101. Minister of Justice, Government of Canada. Assisted Human Reproduction Act; 2004. https://laws-lois.justice.gc.Ca/eng/annualstatutes/2004_2/FullText.html [accessed 2023 June 15].
- 102. Koplin JJ, Savulescu J. Time to rethink the law on part-human chimeras. J Law Biosci. 2019;6(1):37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. The Academy of Medical Sciences. Animals containing human material; 2011. https://acmedsci.ac.Uk/policy/policy-projects/animals-containing-human-material [accessed 2023 June 15].
- 104. Raposo VL. The new Japanese regulation on human/non-human chimeras: should we worry? J Bras Reprod Assist. 2021;25(1):155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sawai T, Hatta T, Fujita M. Japan significantly relaxes its human-animal chimeric embryo research regulations. Cell Stem Cell. 2019;24(4):513–14. [DOI] [PubMed] [Google Scholar]
- 106. Sawai T, Hatta T, Fujita M. The Japanese generally accept human-animal chimeric embryo research but are concerned about human cells contributing to brain and gametes. Stem Cells Transl Med. 2017;6(8):1749–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. International Society for Stem Cell Research. Guidelines for stem cell research and clinical translation; 2016. https://www.isscr.Org/guidelines [accessed 2023 June 15].
- 108. Hyun I, Clayton EW, Cong Y, Fujita M, Goldman SA, Hill LR, Monserrat N, Nakauchi H, Pedersen RA, Rooke HM, Takahashi J, et al. ISSCR guidelines for the transfer of human pluripotent stem cells and their direct derivatives into animal hosts. Stem Cell Reports. 2021;16(6):1409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Crane AT, Voth JP, Shen FX, Low WC. Concise review: human-animal neurological chimeras: humanized animals or human cells in an animal? Stem Cells. 2019;37(4):444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hagglund R. Patentability of human-animal chimeras. Santa Clara High Tech L J. 2012;25:51–104. [Google Scholar]
- 111. U.S. Patent and Trademark Office. 2105. Patent eligible subject matter—living subject matter [R-10.2019]. https://www.uspto.gov/web/offices/pac/mpep/s2105.html [accessed 2022 August 15].
- 112. Rick Weiss Washington Post Staff Writer. Rifkin files human-chimp chimaera patent. https://www.foet.org/wp-content/uploads/2021/07/Rifkin-Files-Human-Animal-Patent-WPost-April-2-1998.pdf [accessed 2023 June 15].
- 113. Cornell law School. Patent. Legal Information Institute. https://www.law.cornell.edu/wex/patent [accessed 2023 June 15].
- 114. Rabin S. The human use of humanoid beings: chimeras and patent law. Nat Biotechnol. 2006;24(5):517–19. [DOI] [PubMed] [Google Scholar]
- 115. Nakauchi H, Yamaguchi T, Hamanaka S. ORGAN REGENERATION METHOD UTILIZING iPS CELL AND BLASTOCYST COMPLEMENTATION; 2015. https://patentimages.storage.googleapis.Com/9e/9e/28/e5ca8f1e1c8113/US20140338008A1.pdf [accessed 2023 June 15].
- 116. Asakura A, Aravali R, Qi Lan MC, Dutton J, Gary DJ, Gary MG, Graham M, Lee L, Law W, Panoscartis-Motari A, O’Brien T, et al. Compositions and methods for preparing chimeric embryonic auxiliary organs. 2016. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017075276
- 117. Australia I. (12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International publication number (43) International publication date. WIPOI PCT. 2017. https://patents.google.com/patent/AU2016344152A1/en
- 118. Hyun I. Ethical standards for chimera research oversight. Methods Mol Biol. 2019;2005:165–71. [DOI] [PubMed] [Google Scholar]