Abstract
Objective
The aim of this study is to evaluate the feasibility, safety, and short-term effectiveness of a high-power (150 W) microwave ablation (MWA) device for tumor ablation in the lung, liver, and kidney.
Methods
Between December 2021 and June 2022, patients underwent high-power MWA for liver, lung, and kidney tumors. A retrospective observational study was conducted in accordance with the Declaration of Helsinki. The MWA system utilized a 150-W, 2.45-GHz microwave generator (Emprint™ HP Ablation System, Medtronic). The study assessed technical success, safety, and effectiveness, considering pre- and post-treatment diameter and volume, lesion location, biopsy and/or cone beam computed tomography (CBCT) usage, MWA ablation time, MWA power, and dose-area product (DAP).
Results
From December 2021 to June 2022, 16 patients were enrolled for high-power MWA. Treated lesions included hepatocellular carcinoma (10), liver metastasis from colon cancer (1), liver metastasis from pancreatic cancer (1), squamous cell lung carcinoma (2), renal cell carcinoma (1), and renal oncocytoma (1). Technical success rate was 100%. One grade 1 complication (6.25%) was reported according to CIRSE classification. Overall effectiveness was 92.8%. Pre- and post-treatment mean diameters for liver lesions were 19.9 mm and 37.5 mm, respectively; for kidney lesions, 34 mm and 35 mm; for lung lesions, 29.5 mm and 31.5 mm. Pre- and post-treatment mean volumes for liver lesions were 3.4 ml and 24 ml, respectively; for kidney lesions, 8.2 ml and 20.5 ml; for lung lesions, 10.2 ml and 32.7 ml. The mean ablation time was 48 minutes for liver, 42.5 minutes for lung, and 42.5 minutes for renal ablation. The mean DAP for all procedures was 40.83 Gcm2.
Conclusion
This preliminary study demonstrates the feasibility, safety, and effectiveness of the new 150 W MWA device. Additionally, it shows reduced ablation times for large lesions.
Keywords: microwave ablation, high power, 150 W, lung, liver, kidney, tumor, thermal ablation
Introduction
Over the years multiple loco-regional techniques have been developed including thermal and non-thermal ablation.1–5
Thermal ablation techniques include heat-based thermal ablations, such as radiofrequency ablation (RFA) and microwave ablation (MWA) and cold-based thermal ablations, such as cryoablation (CA).6,7
Although RFA is still the most widely used ablation technique worldwide, throughout the years MWA has gained a lot of interest proving to be an effective technique, equal or even superior to RFA in some cases.5–12
Among advantages of MWA over RFA there are: shorter time of treatment, higher intra-tumoral temperatures, larger area of necrosis and less effectiveness by the heat-sink effect.8,10,13,14
The basic microwave system consists of three components: a generator, a power distribution system and antennas.15–18 The generator operates in the 915 MHz and 2.45 GHz frequency bands to directly heat tissue to lethal temperatures greater than 150 °C through dielectric hysteresis.19–21 Dielectric hysteresis is a process in which polar molecules, mainly water, are forced to continuously realign themselves with the oscillating electric field. This results in the generation of kinetic energy and the consequent generation of heat.4,18,22
In other words, MWA is a method of thermal tumor ablation where tumors are heated to damage the structure and proteins of the cell. As tumors have often a high water content, the microwaves induce rapid heating thanks to the interaction with the polar water molecules within the tumor cells. 23
With regard to percutaneous ablations, data in literature show a large collection of studies concerning systems operating at a maximum power of 100 W. 24
As far as we know there are only few studies that demonstrate the safety and efficiency in vivo of systems operating at 140 W.4,17,25–27
Recently has been developed a new system that can generate up to 150 W of power. Berber and Akbulut 10 published the first worldwide experience with this device, which they used only laparoscopically or in open surgery to ablate liver tumors.
We report our initial experience to evaluate the feasibility, safety and short-term effectiveness of this new ablation device used percutaneously to ablate tumors in lung, liver and kidney.
To our knowledge this is the first experience that reports the percutaneous employ of this ablation device.
Materials and Methods
Patients
Between December 2021 and June 2022 all patients were enrolled for high-power MWA.
MWA was applied to liver, lung and kidney.
Written informed consent was obtained from all patients.
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of IRCCS Ca’ Granda Policlinico di Milano (Project identification code: OSMAMI-23/05/2023-0021942-U).
The reporting of this study conforms to STROBE guidelines. 28
Diagnosis was made on the basis of imaging, medical history and in some cases on biopsy.
Indications to MWA were shared by clinicians, surgeons and interventional radiologists according to different selection criteria, tailored to the specific abdominal diseases and according to standard guidelines. 24
Patients undergoing anticoagulant and/or antiaggregant therapy interrupted the treatment following the current guidelines, with the introduction of fractionated heparin when necessary.29,30 Uncorrectable coagulopathy was the only absolute contraindication to the procedure.
All patients’ details were de-identified for this study and the patients were selected consecutively.
Pre-Treatment Procedure
Pre-treatment imaging consisted of multidetector computed tomography (CT) using a 64-slice CT scanner (Philips, Netherlands) and/or magnetic resonance using a 1.5-T scanner (Siemens, Germany).
Before the beginning of each procedure, local anesthesia of the antenna entrance site was achieved with 10-ml solution of 2% lidocaine.
Each patient was kept in a state of moderate sedation through intravenous administration of a combination of midazolam (0.07-0.08 mg/kg), propofol (0.5-2.0 mg/kg/h), and fentanyl (1-2 μg/kg), which was mild during the antenna placement and slightly stronger during the ablation.
Heart rate, electrocardiographic trace, oxygen saturation, respiratory frequency and blood pressure were continuously monitored throughout the procedure.
Adequate antibiotic prophylaxis was achieved with intravenous administration of 2 g of cefazolin sodium (Ancef, SmithKline Beecham Pharmaceuticals, Philadelphia, USA) shortly before the procedure.
Procedure
The ablation system device used consisted of a microwave generator (Emprint™ HP Ablation System, Medtronic) capable of producing 150 W of power at 2.45 GHz, connected by coaxial cable to a 13-gauge straight microwave antenna with a length of 15 cm or 20 cm. The antenna was continuously perfused with saline solution to prevent over-heat.
Patients were placed in the supine, prone or oblique position and ultrasound (US) with or without the additional use of cone-beam CT (CBCT) guidance or a CBCT only were performed to choose the safest insertion of antenna.
In some cases, we used CBCT-derived volumetric data fused with pre-procedural cross-sectional imaging and combined with dedicated software for needle trajectory planning and ablation volume prediction. We used as navigational software, Xperguide (Philips Allura Xper FD20; Philips Healthcare, Best, Netherlands) for needle trajectory planning, and XperCT (Philips Allura Xper FD20; Philips Healthcare, Best, Netherlands), for the ablation volume prediction.
The generator's output was moved straight up to 150 W from the beginning, avoiding a gradual increase of power, at a continuous wave of 2.45 GHz. The total ablation time was variable tailored to the dimensions of the target lesion, to obtain an optimal necrosis volume.
The cauterization of the needle tract after ablation was performed to reduce the risk of seeding of the needle tract and the risk of hemorrhages.
In some cases of high-power MWA we performed a post-procedural CBCT to exclude the presence of immediate complications.
After ablation, patients were transferred to the radiology recovery room for observation. At 1 hour from the procedure, all patients submitted to liver and kidney MWA underwent to abdominal US and all patients submitted to lung MWA underwent to chest X-ray.
Follow-Up
Each patient was scheduled to undergo follow-up at 1 month after the procedure with multidetector CT using a 64-slice CT scanner (Philips, Netherlands) before and after medium contrast injection. 1
Outcomes Measures
The established outcomes for this study were technical success, safety and effectiveness of the technique.
Technical success was defined as the correct positioning of the antennae within the lesion, evaluated by intra-procedural US/CBCT.
Safety was defined as the frequency of intraoperative, perioperative and delayed complications.
All complications were recorded and classified on a scale from 1 to 6 according to the CIRSE Classification System For Complications.30,31
Effectiveness of the technique was defined as the absence of imaging signs suggestive of residual or recurrence disease.
The parameters used to evaluate the effectiveness of the technique were defined on the basis of the lesion's site in an organ-specific fashion:
Liver lesions: the effectiveness of the technique was defined as the absence of intra-tumoral (ie, non-rim-like) arterial enhancement on contrast-enhanced CT or MRI.
Renal lesions: the effectiveness of the technique was defined adequate if the follow-up imaging did not show significant enhancement (<15 HU) inside the treated zone after contrast injection. Replacement of the ablated parenchyma by necrosis was considered as a positive predictive factor of treatment success.32–34
Lung lesions: the effectiveness of the technique was defined, after 1 month, as the absence of any significant enhancement of the ablation zone on contrast enhanced CT scan.35–37
For each case we have also evaluated multiple other parameters: pre-treatment diameter, volume and location of the lesion, usage of biopsy and/or CBCT before ablation, MWA ablation time, MWA power and total Dose area product (DAP). We also evaluated the post-operative ablation volume and diameter of treated lesions.
Statistical Analysis
Descriptive analysis was performed on the dataset and presented in simple frequencies, proportion and percentages using Microsoft Excel 2020 (Microsoft Corporation, Redmond, WA, USA). The statistical analyses and the graph were performed using Graph-Pad Prism software (Version 6; GraphPad, Inc., San Diego, CA, USA).
Results
Patients
Between December 2021 and June 2022, 16 patients (11 males and 5 females ranging in age between 64 and 83 years, mean age 72.5) were enrolled for high-power MWA.
Pre-Procedural Data
In 10 of 16 patients, pre-treatment imaging consisted of contrast enhanced CT, instead in 6 of 16 patients the pre-treatment imaging consisted of contrast enhanced MRI.
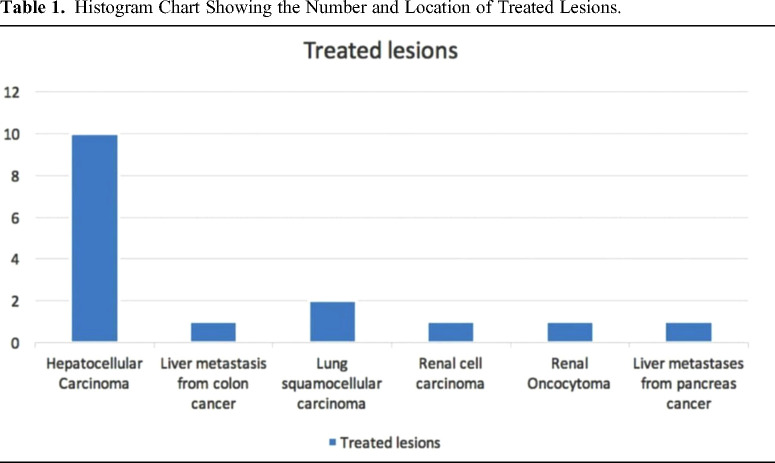
The lesions treated were the follows: hepatocellular carcinoma (HCC) (10), liver metastasis from colon cancer (1), liver metastasis from pancreas cancer (1), squamous cell lung carcinoma (2), renal cell carcinoma (1) and renal oncocytoma (1). (Table 1) (Figures 1–4)
Table 1.
Histogram Chart Showing the Number and Location of Treated Lesions.
Figure 1.
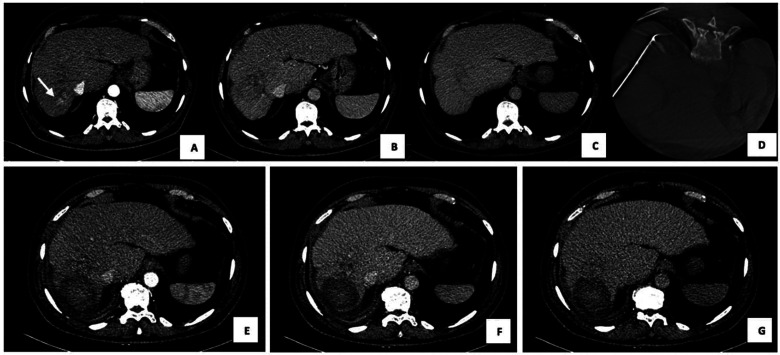
150 W microwave ablation (MWA) of HCC in SVIII in a 70-year-old man. (A) arterial (B) venous and (C) delayed phase on CECT show the presence of nodule of HCC 12 mm of maximus axial diameter (white arrow). (D) Intra-procedural CBCT shows the correct placement of antenna and MWA at 150 W for 6 minutes was performed. 1-month CECT follow-up on (E) arterial, (F) portal and (G) delayed phase show a hypodense area of thermocoagulation in all phases without residual disease. Abbreviations: CBCT, cone-beam CT; CECT, contrast-enhanced CT; HCC hepatocellular carcinoma; MWA, microwave ablation.
Figure 4.
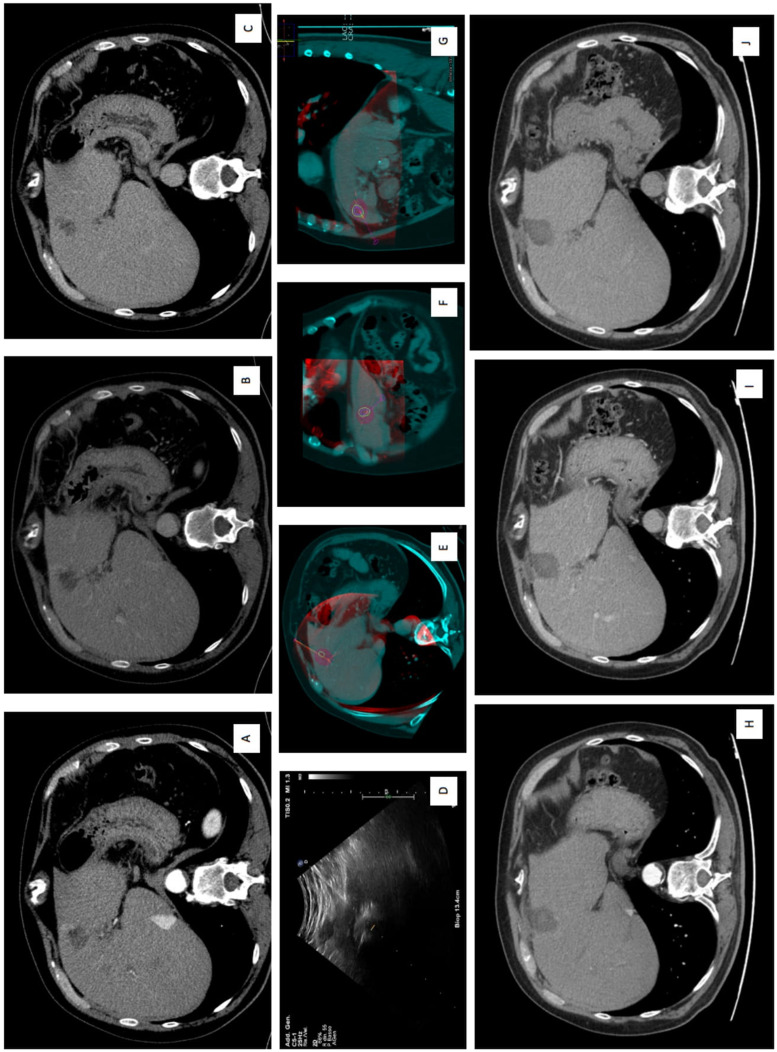
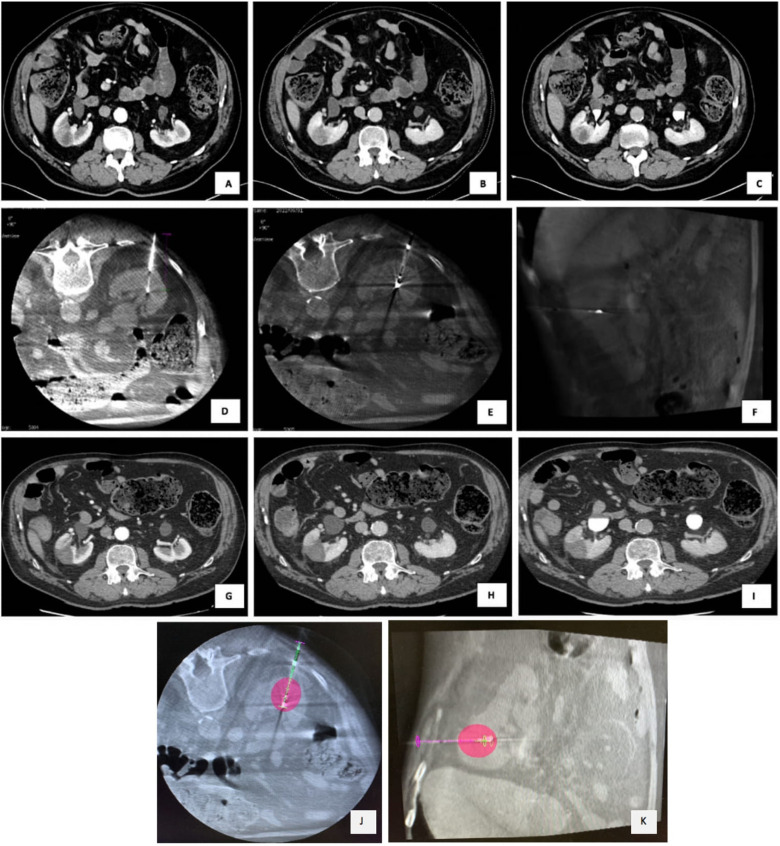
150 W microwave ablation (MWA) of residual hepatic lesion after loco-regional treatment in S4a. (A) Arterial, (B) venous, (C) delayed phase show CECT show a contrast enhanced residual liver lesion with wash-out in venous and in delayed phases. (D) Intra-procedural US guidance during the insertion of MWA antenna. (E) Axial, (F) coronal, (G) sagittal plans imaging-fusion between intra-procedural CBCT and previous CECT, using navigational softwares, Xperguide (Philips Allura Xper FD20; Philips Healthcare, Best, Netherlands) for needle trajectory planning, and XperCT (Philips Allura Xper FD20; Philips Healthcare, Best, Netherlands), for the ablation volume prediction. (H) Arterial, (I) venous, and (J) delayed phases at 1-month CT follow-up, showing a complete response with the evidence of the coagulation zone. Abbreviations: MWA, microwave ablation; CBCT, cone-beam CT; CECT, contrast-enhanced CT; HCC hepatocellular carcinoma.
Figure 3.
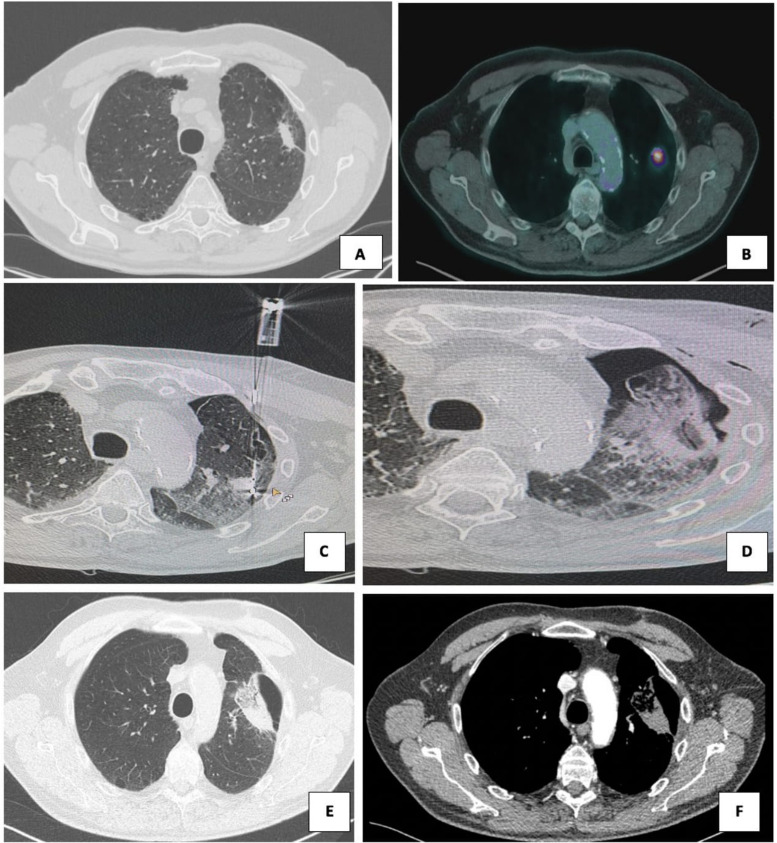
150 W microwave ablation (MWA) of pulmonary lesions in 82-year-old man. (a) Pre-procedural CT scan and (B) pre-procedural CT-PET show a left upper lobe metastatic lesion measuring 40 × 32 mm in greatest axial diameters with FDG uptake. (C) CT scan shows a single antenna, which was positioned with CT guidance into the center of the lesion; 4 minutes at 150 W MWA was performed. (D) The patient suffered a pneumothorax with an air leak that not required chest tube insertion. (E, F) Contrast-enhanced CT scans obtained at 1-month follow-up show an enlargement of consolidation area, with intralesional cavitation and no enhancement uptake. Abbreviations: CBCT, cone-beam CT; CECT, contrast-enhanced CT; computed tomography-positron emission tomography (CT-PET); HCC hepatocellular carcinoma; F-fluorodeoxyglucose (FDG);MWA, microwave ablation.
Pre-intervention biopsy was performed for all 2 renal lesions, for all 2 lung lesions and for 2 hepatic lesions. The histological results were the follows: renal cell carcinoma (1), oncocytoma (1), hepatocellular carcinoma (1) metastases from colon cancer (1), squamous cell lung carcinoma (2).
The mean diameter was considered for each organ like the average of the largest one evaluated on any axis.
The pre-treatment mean diameter of treated lesions was 19.9 mm (range 10-38 mm) for liver lesions, 34 mm for kidney lesions (range 30-38 mm) and of 29.5 mm for lung lesions (range 23-36 mm).
Hepatic lesions are located both in right and left hepatic lobe, precisely: in S8 (6 lesions, range 14-20 mm, average 17 mm), in S7 (2 lesions, range 18-30 mm, average 24 mm), in S4 (2 lesions, range 26-30 mm, average 28 mm), in S3 (1 lesion, 9 mm), and in S2 (1 lesion, 20 mm).
Two lung lesions were in the upper right lobe and in upper left lobe.
The renal lesion was located into the middle portion of right kidney.
The pre-treatment mean volume of the treated lesion was 3.4 mL (range 0.8-8.4 mL) for liver lesions, 8.2 mL for kidney lesions (range 5.8-10.7 mL), and 10.2 mL for lung lesions (range 4.9-15.5 mL).
Procedure
All procedures were conducted by a percutaneous approach.
The mean time required to perform a complete procedure was of 48 minutes for liver ablation (range 25-70 minutes); of 42.5 minutes for lung ablation (range 40-45 minutes) and of 42.5 minutes for renal ablation (range 35-50 minutes).
The generator produced 150 W of power for an average time of 3.5 minutes in the liver, 5.15 minutes for the lung and 4.65 minutes for kidney.
All procedures were performed with a power of 150 W.
We used in 9 cases (all cases of kidney and lung ablation and 7 cases of liver ablation) CBCT and navigational softwares such as Xperguide (Philips Allura Xper FD20; Philips Healthcare, Best, Netherlands) for needle trajectory planning, and XperCT (Philips Allura Xper FD20; Philips Healthcare, Best, Netherlands), for the ablation volume prediction (Figures 2 and 4).
Figure 2.
150 W microwave ablation (MWA) of renal carcinoma in a 74-year-old man. (A) Arterial phase, (B) venous phase and (C) delayed phase on CECT show a 32 × 27 mm of diameter lower polar right renal lesion. (D) Renal biopsy was performed with a histological diagnosis of clear cell renal carcinoma. (E, F) Intra-procedural CBCT shows the correct placement of antenna and MWA at 150 W for 4 minutes was performed. (G) Arterial, (H) portal and (I) delayed phase of 1-month CECT show lack of enhancement in the mass; (J) axial and (K) coronal CBCT images using navigational softwares, Xperguide (Philips Allura Xper FD20; Philips Healthcare, Best, Netherlands) for needle trajectory planning, and XperCT (Philips Allura Xper FD20; Philips Healthcare, Best, Netherlands), for the ablation volume prediction. Abbreviations: CBCT, cone-beam CT; CECT, contrast-enhanced CT; HCC hepatocellular carcinoma; MWA, microwave ablation.
The mean DAP for all procedures was 40.83 Gcm2.
Follow-Up
Follow-up imaging consisted of contrast enhanced CT at 1 month after the procedure.
Out of 16 patients, 14 had a 1-month follow-up, 2 were lost (one renal oncocytoma and one HCC) and the remaining 3 had been treated from less than a month.
The post-treatment mean diameter of the treated lesions was 37.5 mm for liver lesions (range 20-44 mm), 35 mm for kidney lesion and 31.5 mm for lung lesions (range 26-37 mm).
The post-treatment mean volume of the treated lesions was 24 mL for liver lesion (range 8-40 mL), 20.5 mL for kidney and 32.7 mL for lung lesions (range 13.1-52.3 mL).
Outcome Measures
Technical Success
Technical success was 100%, with antenna correctly placed within the lesion in all 16 cases.
Correct positioning of the antenna was confirmed with CBCT in 10/16 lesions, with the remaining of the lesions confirmed with US only.
Safety
In our study population did not showed significant complications and only two grade 1 complication (12,5%) were reported according to CIRSE classification.
The first was a case of mild endoalveolar hemorrage detected on post-procedural CBCT, consequent to lung thermoablation, which did not make it necessary to add additional therapy; the second was a small pneumothorax (PNX) in the same patient, that not needed of insertion of drainage tube (Figure 1).
Both lesions were adherent to the pleura and did not cause ablation of the ribs or great vessels; ablation of the smaller lesion caused a clinically silent saccular effusion with super-fluid density detected on follow-up CT scan at 1 month.
Effectiveness of the Technique
As previously discussed, the effectiveness of the technique was defined as the absence of early imaging signs suggestive of residual and/or recurrence disease and was evaluated in a different way for each organ targeted. These goals were achieved in all procedure except in one case of HCC where was observed a residual of disease of 20 mm at 1-month CT follow-up.
In our study:
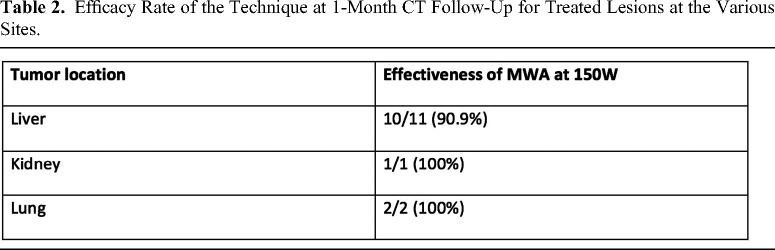
10 out of 11 (90.9%) of liver lesions did not show rim-like intra-tumoral enhancement at 1-month follow-up imaging.
1 out of 1 (100%) of renal lesions did not show significant intra-tumoral enhancement at 1-month follow-up imaging.
2 out of 2 (100%) of lung lesions did not show significant intra-tumoral contrast uptake at 1-month follow-up imaging.
The total effectiveness assessed by our study was therefore 92.8% (13 out of 14) (Table 2).
Table 2.
Efficacy Rate of the Technique at 1-Month CT Follow-Up for Treated Lesions at the Various Sites.
Discussion
Several types of ablation devices can be used in the field of interventional oncology, these include both thermal and non-thermal techniques. 38
RFA and MWA are both hyperthermic procedures that apply energy in order to heat tissues until they reach lethal temperatures. MWA has several other advantages of other ablation techniques, in particular rather than RFA, including: higher intra-tumoral temperatures, larger tumor ablation volumes, faster ablation times, ability to use simultaneously multiple applicators, optimal heating of cystic masses and tumors close to the vessels for its less susceptibility to “heat sink effect.” 39 Another considerable difference between MWA and RFA is that, since all antennas are bipolar by definition, there is no need of neutral electrodes applied to the patient, ruling out skin burns at the grounding pad site, one of the possible complications of RFA treatments.
MWA heat-generating capabilities depend upon electromagnetic radiation, causing a fast switching rotation at atomic or molecular levels of electric dipoles such as polar molecules (eg H2O), causing heating by friction of water molecules. Temperatures higher than 100 °C and tissue carbonization are not limiting the MW heating process, allowing for large coagulation zones, less susceptibility to the heat sinking effects and higher temperatures in general. 40
With the advancement of microwaves ablative techniques, several new innovations in this field of application have been introduced: the achieving of a real predictable ablation area, using the thermosphere technology, which leads to field control, thermal control and wavelength control to maintain a precise, predictable and spherical ablation zone throughout procedures; the application of increasing power systems, until 150 W, with a production of large zone of ablation in short time. 22
In the present study we report the first experience of percutaneous application of 150 W system for the MWA in different clinical applications, including liver, lung and kidney lesions.
Considering the clinical outcome of the whole population, our findings were globally satisfactory in terms of technical success, the effectiveness of the technique, and recorded complications.
Although preliminary, the data from our cases confirm the aforementioned experimental studies, demonstrating the efficacy of percutaneous ablation of lesions with high-power system (150 W), achieving a large diameter of ablation (more than 3 cm) with a single antenna and in short times without significant complications.
Thus, comparing with 100 W ablation system, the high-power ablation systems lead to a large ablation area using a single antenna whereas the necessity to use 2 antennas with consequent lower costs. Furthermore, the use of a single antenna rather than double, ensures a more predictable and spherical ablation area.
In our series, only one case of intra-alveolar hemorrhage following ablation in the lung was reported, without post-procedural sequelae and deviation from the normal post-therapeutic course (grade 1).
Hines-Peralta et al 41 evaluated the rationale of using probes with power greater than 100 W. In ex vivo and in vivo studies on animal livers, demonstrating how the use of high-powered antennas produces large zones of coagulation in shorter time, in accordance with our study. In comparison to this study, we performed all procedure in vivo humans including multiple tumor diseases.
Berber and Akbulut 10 published the first worldwide experience with 150 W ablation system, which they used only laparoscopically or in open surgery to ablate liver tumors.
In confront to this study, we performed all procedures by a percutaneous approach with its acknowledged minimally invasiveness. Furthermore, we used this new ablation system not only for liver lesions, but for different abdominal and thoracic lesions, including liver, lung, and kidney.
The generator's output was moved straight up to 150 W from the beginning, avoiding a gradual increase of power, at a continuous wave of 2.45 GHz, without significant recorded complications.
Conclusions
In conclusion, our preliminary experience demonstrates that this new 150 W MW device is feasible, safe and effective. This system also has been shown a short time of ablation time for the treatment of large lesions.
Limited number of patients and short-term follow-up represent the main limitations of our study and additional larger case series with medium and long-term follow-up will be needed to confirm these preliminary data.
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of IRCCS Ca’ Granda Policlinico di Milano (Project identification code: OSMAMI-23/05/2023-0021942-U).
Abbreviations
- CA
cryoablation
- CBCT
cone-beam CT
- DAP
dose area product
- MWA
microwave ablation
- RFA
radiofrequency ablation
- US
ultrasound
- PNX
pneumothorax
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Carolina Lanza https://orcid.org/0000-0002-8286-1562
Velio Ascenti https://orcid.org/0000-0001-8041-4075
References
- 1.Galle PR, Forner A, Llovet JM, et al. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 2.Crocetti L, de Baére T, Pereira PL, Tarantino FP. CIRSE standards of practice on thermal ablation of liver tumours. Cardiovasc Intervent Radiol. 2020;43(7):951-962. doi: 10.1007/s00270-020-02471-z [DOI] [PubMed] [Google Scholar]
- 3.Ridouani F, Srimathveeravalli G. Percutaneous image-guided ablation: From techniques to treatments. Presse Med. 2019;48(7-8 Pt 2):e219-e231. doi: 10.1016/J.LPM.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 4.Simo KA, Tsirline VB, Sindram D, et al. Microwave ablation using 915-MHz and 2.45-GHz systems: What are the differences? HPB (Oxford). Blackwell Publishing Ltd; 2013;15:991-996. doi: 10.1111/hpb.12081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan TP. Microwave ablation for cancer: physics, performance, innovation, and the future. In: Image-Guided cancer therapy. Springer; 2013:37-59. doi: 10.1007/978-1-4419-0751-6_5 [DOI] [Google Scholar]
- 6.Zhou Y, Yang Y, Zhou B, et al. Challenges facing percutaneous ablation in the treatment of hepatocellular carcinoma: Extension of ablation criteria. J Hepatocell Carcinoma. 2021;8:625. doi: 10.2147/JHC.S298709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed M, Brace CL, Lee FT, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258(2):351-369. doi: 10.1148/radiol.10081634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreland AJ, Ziemlewicz TJ, Best SL, et al. High-powered microwave ablation of T1a renal cell carcinoma: Safety and initial clinical evaluation. J Endourol. 2014;28(9):1046-1052. doi: 10.1089/end.2014.0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poggi G, Montagna B, Di Cesare P, et al. Microwave ablation of hepatocellular carcinoma using a new percutaneous device: Preliminary results. Anticancer Res. 2013;33(3):1221-1227. [PubMed] [Google Scholar]
- 10.Berber E, Akbulut S. Assessment of a new 150 W single-antenna microwave ablation system in the treatment of malignant liver tumors: The first worldwide experience. J Surg Oncol. 2022;125(2):168-174. doi: 10.1002/jso.26692 [DOI] [PubMed] [Google Scholar]
- 11.Gaia S, Ciruolo M, Giuseppe Ribaldone D, et al. Higher Efficiency of Percutaneous Microwave (MWA) Than Radiofrequency Ablation (RFA) in Achieving Complete Response in Cirrhotic Patients with Early Hepatocellular Carcinoma Higher Efficiency of Percutaneous Microwave (MWA) Than Radiofrequency Ablation (RFA) in Achieving Complete Response in Cirrhotic Patients with Early. Published online 2021. doi: 10.3390/curroncol28020101 [DOI] [PMC free article] [PubMed]
- 12.Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7(8):1054. doi: 10.4254/WJH.V7.I8.1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubner MG, Hinshaw JL, Andreano A, Sampson L, Lee FT, Brace CL. High-powered microwave ablation with a small-gauge, gas-cooled antenna: Initial ex vivo and in vivo results. J Vasc Interv Radiol. 2012;23(3):405-411. doi: 10.1016/j.jvir.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfannenstiel A, Iannuccilli J, Cornelis FH, Dupuy DE, Beard WL, Prakash P. Shaping the future of microwave tumor ablation: a new direction in precision and control of device performance. Published online 2022. doi: 10.1080/02656736.2021.1991012 [DOI] [PubMed]
- 15.Kapoor H, Nisiewicz MJ, Jayavarapu R, Gedaly R, Raissi D. Early outcomes with single-antenna high-powered percutaneous microwave ablation for primary and secondary hepatic malignancies: Safety, effectiveness, and predictors of ablative failure. J Clin Imaging Sci. 2020;10(1). doi: 10.25259/JCIS_173_2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan A, Byrne C, Pusceddu C, Buy X, Tsoumakidou G, Filippiadis D. CIRSE standards of practice on thermal ablation of bone tumours. Cardiovasc Intervent Radiol. 2022;45(5):591–605. doi: 10.1007/s00270-022-03126-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim C. Understanding the nuances of microwave ablation for more accurate post-treatment assessment. Futur Oncol. 2018;14(17):1755-1764. doi: 10.2217/fon-2017-0736 [DOI] [PubMed] [Google Scholar]
- 18.Nieuwenhuizen S, Dijkstra M, Puijk RS, et al. Microwave ablation, radiofrequency ablation, irreversible electroporation, and stereotactic ablative body radiotherapy for intermediate size (3–5 cm) unresectable colorectal liver metastases: A systematic review and meta-analysis. Curr Oncol Rep. 2022;24(6):793–808. doi: 10.1007/s11912-022-01248-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simo KA, Tsirline VB, Sindram D, et al. Microwave ablation using 915-MHz and 2.45-GHz systems: What are the differences? HPB (Oxford). 2013;15(12):991-996. doi: 10.1111/HPB.12081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peña K, Ishahak M, Arechavala S, Leveillee RJ, Salas N. Comparison of temperature change and resulting ablation size induced by a 902-928 MHz and a 2450 MHz microwave ablation system in in-vivo porcine kidneys. Int J Hyperthermia. 2019;36(1):313-321. doi: 10.1080/02656736.2019.1565788 [DOI] [PubMed] [Google Scholar]
- 21.Ierardi AM, Mangano A, Floridi C, et al. A new system of microwave ablation at 2450 MHz: preliminary experience. Updates Surg. doi: 10.1007/s13304-015-0288-1 [DOI] [PubMed] [Google Scholar]
- 22.Lubner MG, Brace CL, Hinshaw JL, Lee FT. Microwave tumor ablation: Mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21(SUPPL. 8). doi: 10.1016/J.JVIR.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gartshore A, Kidd M, Joshi LT. Applications of microwave energy in medicine. Biosensors. 2021;11:96. 10.3390/bios11040096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiter SJS, Heerink WJ, de Jong KP. Liver microwave ablation: A systematic review of various FDA-approved systems. Eur Radiol. 2019;29(8):4026-4035. doi: 10.1007/s00330-018-5842-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrafiello G, Mangini M, De Bernardi I, et al. La terapia ablativa con microonde nel trattamento delle lesioni tumorali primitive e secondarie del polmone: Nota tecnica. Radiol Medica. 2010;115(6):962-974. doi: 10.1007/s11547-010-0547-7 [DOI] [PubMed] [Google Scholar]
- 26.Filippiadis DK, Gkizas C, Chrysofos M, et al. Percutaneous microwave ablation of renal cell carcinoma using a high power microwave system: Focus upon safety and efficacy. Int J Hyperth. 2018;34(7):1077-1081. doi: 10.1080/02656736.2017.1408147 [DOI] [PubMed] [Google Scholar]
- 27.Zondervan PJ, Buijs M, Bruin D, van Delden DM, Van Lienden OM, P K. Available ablation energies to treat cT1 renal cell cancer: Emerging technologies. World J Urol. 2019;37(3):445-455. doi: 10.1007/s00345-018-2546-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med. 2007;4(10):e296; 147:573-577. [DOI] [PubMed] [Google Scholar]
- 29.Hadi M, Walker C, Desborough M, et al. CIRSE STANDARDS OF PRACTICE CIRSE Standards of Practice on Peri-operative Anticoagulation Management During Interventional Radiology Procedures. doi: 10.1007/s00270-020-02763-4 [DOI] [PubMed]
- 30.Patel IJ, Rahim S, Davidson JC, et al. Society of interventional radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part II: Recommendations: Endorsed by the Canadian association for interventional radiology and the cardiovascular and interventional radiological society of Europe. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. doi: 10.1016/J.JVIR.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 31.Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. CIRSE STANDARDS OF PRACTICE GUIDELINES Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. doi: 10.1007/s00270-017-1703-4 [DOI] [PubMed]
- 32.Vles MJD, Höppener DJ, Galjart B, et al. Local tumour control after radiofrequency or microwave ablation for colorectal liver metastases in relation to histopathological growth patterns. HPB. Published online. January 24, 2022. doi: 10.1016/J.HPB.2022.01.010 [DOI] [PubMed] [Google Scholar]
- 33.Krokidis ME, Orsi F, Katsanos K, Helmberger T, Adam A. CIRSE STANDARDS OF PRACTICE GUIDELINES CIRSE Guidelines on Percutaneous Ablation of Small Renal Cell Carcinoma. doi: 10.1007/s00270-016-1531-y [DOI] [PubMed]
- 34.Zhong J, Wah TM. Renal ablation: Current management strategies and controversies. Chinese Clin Oncol. 2019;8(6). doi: 10.21037/cco.2019.12.08 [DOI] [PubMed] [Google Scholar]
- 35.Choi SH, Kim JW, Kim JH, Kim KW. Efficacy and safety of microwave ablation for malignant renal tumors: An updated systematic review and meta-analysis of the literature since 2012. Korean J Radiol. 2018;19(5):938-949. doi: 10.3348/kjr.2018.19.5.938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maas M, Beets-Tan R, Gaubert JY, et al. Follow-up after radiological intervention in oncology: ECIO-ESOI evidence and consensus-based recommendations for clinical practice. Insights Imaging. 2020;11(1):1-15. doi: 10.1186/S13244-020-00884-5/TABLES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grieco CA, Simon CJ, Mayo-Smith WW, DiPetrillo TA, Ready NE, Dupuy DE. Percutaneous image-guided thermal ablation and radiation therapy: Outcomes of combined treatment for 41 patients with inoperable stage I/II non-small-cell lung cancer. J Vasc Interv Radiol. 2006;17(7):1117-1124. doi: 10.1097/01.RVI.0000228373.58498.6E [DOI] [PubMed] [Google Scholar]
- 38.Venturini M, Cariati M, Marra P, Masala S, Pereira PL, Carrafiello G. CIRSE Standards of practice on thermal ablation of primary and secondary lung tumours. Cardiovasc Intervent Radiol. 2020;43(5):667-683. doi: 10.1007/s00270-020-02432-6 [DOI] [PubMed] [Google Scholar]
- 39.Lee SK, Chung DJ, Cho SH. A real-world comparative study of microwave and radiofrequency ablation in treatment-naïve and recurrent hepatocellular carcinoma. J Clin Med. 2022;11(2):302. doi: 10.3390/JCM11020302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrafiello G, Laganà D, Mangini M, et al. Microwave tumors ablation: Principles, clinical applications and review of preliminary experiences. Int J Surg. 2008;6(SUPPL. 1). doi: 10.1016/j.ijsu.2008.12.028 [DOI] [PubMed] [Google Scholar]
- 41.Hines-Peralta AU, Pirani N, Clegg Pet al. et al. Microwave ablation: Results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology. 2006;239(1):94-102. [DOI] [PubMed] [Google Scholar]