Abstract
Background:
People with femoroacetabular with femoroacetabular impingement syndrome (FAIS) often report pain during sports involving repeated sprinting. It remains unclear how sports participation influences running biomechanics in individuals with FAIS.
Hypothesis:
Changes in running biomechanics and/or isometric hip strength after repeated sprint exercise would be greatest in individuals with FAIS compared with asymptomatic individuals with (CAM) and without cam morphology (Control).
Study Design:
Controlled laboratory study.
Level of Evidence:
Level 3.
Methods:
Three-dimensional hip biomechanics during maximal running (10 m) and hip strength were measured in 49 recreationally active individuals (FAIS = 15; CAM = 16; Control = 18) before and after repeated sprint exercise performed on a nonmotorized treadmill (8-16 × 30 m). Effects of group and time were assessed for biomechanics and strength variables with repeated-measures analyses of variance. Relationships between hip pain (Copenhagen Hip and Groin Outcome Score) and changes in hip moments and strength after repeated sprint exercise were determined using Spearman’s correlation coefficients (ρ).
Results:
Running speed, hip flexion angles, hip flexion and extension moments, and hip strength in all muscle groups were significantly reduced from pre to post. No significant between-group differences were observed before or after repeated sprint exercise. No significant relationships (ρ = 0.04-0.30) were observed between hip pain and changes in hip moments or strength in the FAIS group.
Conclusion:
Changes in running biomechanics and strength after repeated sprint exercise did not differ between participants with FAIS and asymptomatic participants with and without cam morphology. Self-reported pain did not appear to influence biomechanics during running or strength after repeated sprint exercise in participants with FAIS.
Clinical Relevance:
A short bout of repeated sprinting may not elicit changes in running biomechanics in FAIS beyond what occurs in those without symptoms. Longer duration activities or activities requiring greater hip flexion angles may better provoke pathology-related changes in running biomechanics in people with FAIS.
Keywords: fatigue, groin, hip pain, loading, sprinting
Femoroacetabular impingement syndrome (FAIS) is a clinical condition associated with repetitive premature contact between the proximal femur and acetabulum (ie, impingement), which may lead to hip and/or groin pain and early cartilage degeneration in young to middle-aged active adults. 17 Asphericity of the femoral head-neck (cam morphology) is often reported as a cause of symptoms and soft tissue damage in people with FAIS.1,17 Nonetheless, the large incidence of cam morphology in asymptomatic people1,22 suggests pain and disability in those with FAIS may arise from a combination of abnormal morphology, altered movement patterns, and/or impaired muscle function.9,21,22 A direct comparison between people with FAIS and asymptomatic cam morphology is critical for understanding how pain and morphology independently influence hip joint mechanics.
The few studies that compared hip joint mechanics between symptomatic and asymptomatic people with cam morphology found no differences in hip angles or moments during deep squatting 6 or cross-body lunging. 16 However, the low physical demands of these tasks may explain the low pain levels reported by those with FAIS during laboratory assessments.8,16 More demanding sport-specific tasks such as running may be more likely to provoke pain and alter movement patterns in people with FAIS. However, only a single study has investigated running biomechanics in FAIS in any facet. 27
People with FAIS often report pain during sports involving repeated sprinting and cutting maneuvers.22,29 Pain provoked during these high demand and repetitive tasks likely relates to overloading and associated damage to cartilage and/or soft tissues.2,29 The insidious presentation of pain in FAIS 31 suggests neuromuscular fatigue may play a role in symptom presentation. However, this hypothesis is yet to be tested.
People with FAIS often present with lower hip strength compared with healthy controls6,9,25,26 and asymptomatic people with cam morphology. 6 Thus, it is possible that muscle weakness and task demands may prevent individuals with FAIS from avoiding pain-provoking positions of impingement (deep hip flexion combined with adduction and internal rotation).32,35 As muscles are the main shock absorbers of the musculoskeletal system,30,34 reductions in their capacity to produce force (ie, neuromuscular fatigue) 10 can lead to increases in joint loading, 4 which may further lead to hip pain. Repeated sprint exercise is a controlled approach to emulating the mechanical demands of high-intensity sports in a laboratory setting and has been shown to induce large levels of fatigue in the hip musculature. 11 Thus, investigating running biomechanics in FAIS under fatigued conditions could shed light on the link between sports participation and hip pain in FAIS.
The aims of this study were to (1) compare the effects of repeated sprint exercise on hip biomechanics during maximal sprint acceleration running (ie, accelerated sprints) and isometric hip strength between people with FAIS, asymptomatic cam morphology, and healthy controls and (2) evaluate relationships between self-reported pain and changes in hip moments and strength after repeated sprint exercise in FAIS. We hypothesized changes in running biomechanics and/or isometric hip strength after repeated sprint exercise would be greatest in the FAIS group, and within the FAIS group, people with more pain would exhibit greater reductions in hip moments and strength after repeated sprint exercise.
Methods
Full details of the methods can be found in the Online Appendix. A statistical a priori power analysis 13 determined that 8 to 12 participants were required in each group to reach a power of 0.80 and alpha level of 0.05. Forty-nine recreationally active P = 0.00 aged between 18 and 45 years were recruited from the community to participate in this exploratory study. Participants were assigned to 1 of 3 groups [FAIS = 15; asymptomatic cam morphology (CAM) = 16; healthy control (Control) = 18] depending on hip and groin symptoms, results of a clinical examination performed by 1 of 3 registered physiotherapists, and imaging findings from magnetic resonance imaging (MRI). 17 Ethical approval was obtained from the institutional Human Research Ethics Committee. Participants were informed of the procedures and provided written informed consent, consistent with the Declaration of Helsinki.
Participants attended 1 MRI session and 1 laboratory testing session. Upon arrival to the laboratory, all participants completed questionnaires about their history of sports participation and a modified Tegner Activity Scale 8 and had their anthropometrics measured. Participants in the FAIS group also completed the international Hip Outcome Tool (iHOT-33) 28 and the Copenhagen Hip and Groin Outcome Score (HAGOS). 36 Participants then performed a brief familiarization with a nonmotorized treadmill (Woodway Curve 3.0, WOODWAY). Subsequently, participants’ overground accelerated sprint running biomechanics and isometric strength (Online Appendix Table 1) were evaluated before and after a repeated sprint exercise protocol performed on the nonmotorized treadmill. 14
All data were recorded using Vicon Nexus 2.7.1 software (Vicon, Oxford Metrics Group) and processed using MATLAB R2018b (MathWorks) and OpenSim (Version 3.3; simbios, Version 3.3, https://simtk.org/projects/opensim) 7 to calculate hip joint angles and moments, maximum isometric torque, and spatiotemporal parameters. Pre to post percentage differences in spatiotemporal and torque data were calculated as a mean difference (MD = (post – pre)/pre × 100). Given the small magnitude of hip angles and moments in frontal and transverse planes, pre to post changes in hip angles and moments were calculated in raw units (post – pre). Statistical analyses were performed using MATLAB R2018b and RStudio (RStudio, PBC, Version 1.2.5) and significance was accepted for P < 0.05.
Results
Participant Characteristics
Participant descriptive data are presented in Table 1. No significant between-group differences were observed in weight, height, body mass index (BMI), lateral center edge angle, or activity level (minutes per week or Tegner scores). Both CAM and Control groups were significantly younger than the FAIS group (P = 0.00). Maximum alpha angle did not differ between FAIS and CAM groups, but both were significantly higher than the Control group (P = 0.00).
Table 1.
Participant descriptive parameters for people with femoroacetabular impingement syndrome (FAIS), asymptomatic cam morphology (CAM), and healthy controls (Control)
| FAIS (n = 15) |
CAM (n = 16) |
Control (n = 18) |
|
|---|---|---|---|
| Sex (female/male) | 3 / 11 | 1 / 15 | 9 / 9 |
| Age (years) | 30.6 (5.2) * # | 26.1(4.2) | 25.2 (5.8) |
| Weight (kg) | 80.1 (13.2) | 76.9 (9.9) | 71.2 (10.9) |
| Height (cm) | 176.7 (7.6) | 179.8 (7.2) | 173.6 (8.2) |
| BMI (kg/m2) | 25.5 (2.8) | 23.7 (2.0) | 23.5 (2.8) |
| Exercise (min per week) | 415 (231) | 368 (204) | 433 (176) |
| Alpha angle (º) | 64.6 (7.9) * | 66.7 (6.8) * | 45.9 (5.6) |
| Lateral center edge angle (º) | 20.0 (5.6) | 20.9 (5.9) | 21.4 (4.7) |
| Modified Tegner scale a | 6.4 (1.1) | 6.5 (1.0) | 6.8 (0.9) |
| FABER (n (%), positive) | 10 (67) | 0 | 0 |
| FADIR (n (%), positive) | 13 (87) | 0 | 0 |
| iHOT-33 b | 56 (23-91) | ||
| HAGOS b | |||
| Symptoms | 50 (29-93) | ||
| Pain | 73 (40-85) | ||
| Activities of daily living | 80 (45-100) | ||
| Sports and recreational activities | 61 (25-88) | ||
| Participation in physical activities | 50 (0-100) | ||
| Quality of life | 45 (20-85) | ||
Data are mean (1 SD) or median (range). FABER, flexion, abduction, external rotation test; FADIR, flexion, adduction, internal rotation test; HAGOS, Copenhagen Hip and Groin Outcome Score; iHOT-33, international Hip Outcome Tool.
0 = disability, 10 = competitive sport at the professional level.
0 = extreme hip and/or groin problems, 100 = no hip and/or groin problems.
Significant difference compared with Control (P < 0.05).
Significant difference compared with CAM (P < 0.05).
Spatiotemporal Parameters
Spatiotemporal parameters are presented in Online Appendix Table 2. There were no significant differences in the number of sprints performed by participants in the FAIS (range, 9-15), CAM (range, 8-14), and Control (range, 8-16) groups. We observed no differences in maximal speed, maximal acceleration, step time, contact time, step length, or step frequency between FAIS, CAM, and Control groups at pre or post time points.
Hip Angles and Moments
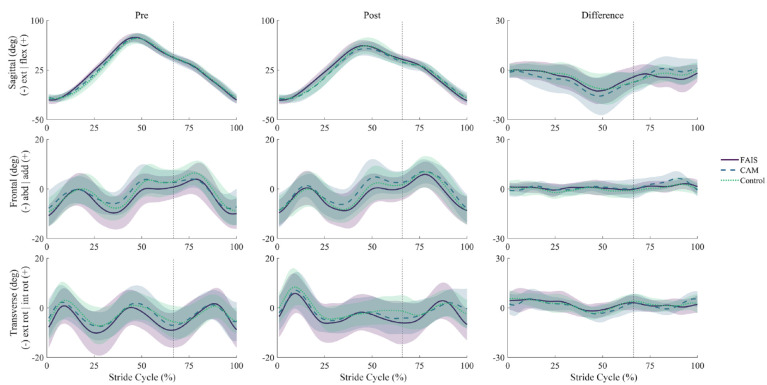
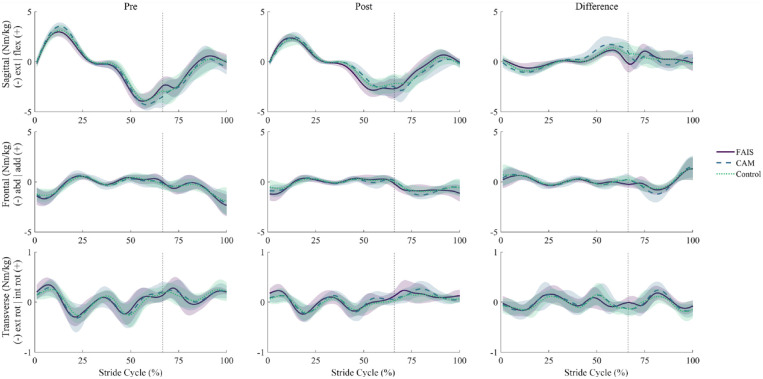
No significant group-time interactions were observed for hip angles (Figure 1) or moments (Figure 2) in any plane of movement. Main effects of time revealed a reduction, from pre to post, in hip flexion angles from mid-swing to early stance (see Online Appendix Figure 2). Similarly, hip flexion and extension moments decreased from pre to post, primarily at the beginning and end of swing (see Online Appendix Figure 3).
Figure 1.
Ensemble averages (±1 SD, shaded) for hip angles before (Pre) and after (Post) repeated sprint exercise in the sagittal (top), frontal (middle), and transverse (bottom) planes across a running stride cycle in people with femoroacetabular impingement syndrome (FAIS, n = 15), asymptomatic cam morphology (CAM, n = 16), and in healthy controls (Control, n = 18). Mean differences shown as post-pre (right). Dashed vertical lines indicate foot contact. No significant group-time interactions were observed as a result of a 2-way repeated measures analysis of variance using statistical parametric mapping. abd, abduction; add, adduction; ext, extension; ext rot, external rotation; flex, flexion; int rot, internal rotation.
Figure 2.
Ensemble averages (±1 SD, shaded) for hip internal moments before (Pre) and after (Post) repeated sprint exercise in the sagittal (top), frontal (middle), and transverse (bottom) planes across a running stride cycle in people with femoroacetabular impingement syndrome (FAIS, n = 15), asymptomatic cam morphology (CAM, n = 16), and in healthy controls (Control, n = 18). Mean differences shown as post-pre (right). Dashed vertical lines indicate foot contact. No significant group-time interactions were observed as a result of a 2-way repeated measures analysis of variance using statistical parametric mapping. abd, abduction; add, adduction; ext, extension; ext rot, external rotation; flex, flexion; int rot, internal rotation.
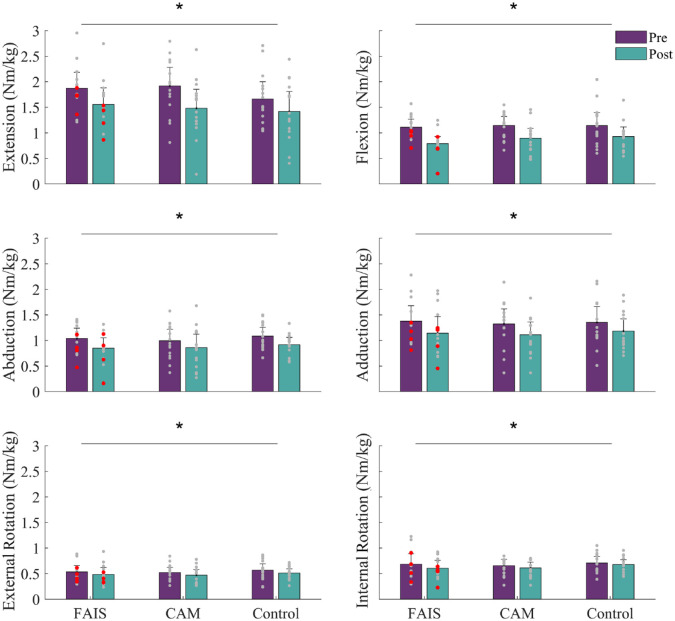
Isometric Hip Strength
No significant group-time interactions or main effects of group were observed for any strength task. However, we observed a main effect of time for all strength tasks suggesting a decrease in strength during hip extension (MD = -15% to -17%, P = 0.00), flexion (MD = -17% to -29%, P = 0.00), abduction (MD = -13% to -19%, P = 0.00), adduction (MD = -7% to -19%, P = 0.00), external rotation (MD = -5% to -11%, P = 0.00), and internal rotation (MD = -2% to -7 %, P = 0.01) after repeated sprint exercise (Figure 3).
Figure 3.
Maximal isometric hip strength before and after repeated sprint exercise compared between people with femoroacetabular impingement syndrome (FAIS), asymptomatic cam morphology (CAM), and healthy controls (Control). Data are mean (bars), 95% confidence intervals (error bars). Participants in the FAIS group shown with (red dots) and without (gray dots) hip pain (≥1/10 on numerical rating scale) after repeated sprint exercise. *Significant effect of time as a result of a 2-way analysis of variance. No between-group differences were observed.
HAGOS Subscale and NRS Pain Scores
In the FAIS group, 77% of the participants reported difficulty running, and 85% reported difficulty performing explosive movements involving accelerations or decelerations on the HAGOS subscale, and 27% reported an increase in pain (numeric rating scale [NRS] scale) after repeated sprint exercise. No significant differences in pain scores (NRS scale) were observed between pre and post at the group level [median pain (range), pre = 0 (0-3), post = 0 (0-8); P = 0.81].
Relationship Between Pain and Changes in Maximal Running Speed, Hip Moments, and Isometric Hip Strength in the FAIS Group
No significant relationship was observed between hip pain scores in the week prior testing (HAGOS scale) and changes in maximal running speed (ρ = 0.19; P = 0.49). No significant relationships were observed between HAGOS subscale scores and changes in peak hip extension (ρ = 0.23; P = 0.44), flexion (ρ = 0.24; P = 0.41), or abduction (ρ = 0.04; P = 0.89) moments during running (see Online Appendix Figure 4, top row). No significant relationships were observed between HAGOS subscale scores and changes in hip extension (ρ = 0.30;P = 0.28), flexion (ρ = 0.16; P = 0.57), or abduction (ρ = 0.30; P = 0.28) isometric strength after repeated sprint exercise (see Online Appendix Figure 4, bottom row).
Discussion
This study compared hip biomechanics during running (accelerated sprints) and isometric hip strength after repeated sprint exercise between people with FAIS, asymptomatic cam morphology, and activity matched healthy controls without cam morphology. The repeated sprint exercise resulted in significant reductions in maximal running speed, 3-dimensional hip angles and moments during running, and isometric hip strength measures for all 3 groups. In contrast to our hypotheses, we did not observe any between-group differences in running biomechanics or hip strength, either before or after repeated sprint exercise. In the FAIS group, a history of self-reported pain in the week before testing (HAGOS subscale) did not appear to influence hip biomechanics during running or hip strength after repeated sprint exercise. This novel observation highlights a discord between pain presentation and hip function during running in those with FAIS and warrants exploration in future studies.
Effect of Group and Repeated Sprint Exercise on Running Biomechanics
Consistent with the observed reductions in maximal running speed after repeated sprint exercise (14%-22%), we found decreased hip angles and moments after repeated sprint exercise compared with baseline in all 3 groups with no between-group differences. Decreases in hip flexion and extension moments were observed mainly during the swing phase, in agreement with previous findings of reduced hip moments14,15 and hip muscle activity 11 after repeated sprints during the swing phase of running. Although our results suggest hip moments during running in people with FAIS are comparable to asymptomatic people, regardless of the presence of cam morphology, our analysis does not account for muscle co-contraction or nonlinear musculotendon dynamics that influence internal hip loading. 24 Thus, future investigations of internal hip loading (eg, hip contact forces) during running in FAIS and any fatigue-related effects are warranted.
Effect of Group and Repeated Sprint Exercise on Isometric Hip Strength
Consistent with Brunner et al, 5 we observed no significant between-group differences in isometric hip strength at baseline. We also observed significant reductions in isometric hip strength (up to 34%) after repeated sprint exercise with no between-group differences, suggesting all groups likely experienced similar levels of fatigue. 12 Our results contrast previous reports of lower hip strength in FAIS compared with asymptomatic controls.6,20,33 However, previous studies recruited people with FAIS at a more advanced symptomatic stage than participants in our study and physical activity levels were often higher in the control group compared with the FAIS group.20,33 Our findings suggest people with FAIS experience similar levels of fatigue compared with those with asymptomatic cam morphology when presenting with low levels of pain and high levels of physical activity.
Effect of Repeated Sprint Exercise on Pain in FAIS
Despite observed reductions in maximal running speed and hip strength after repeated sprint exercise in all 3 groups, we found no significant changes in pain (NRS) during running in the FAIS group. Within the FAIS group, only 4 (27%) of 15 participants demonstrated increased pain (NRS) after repeated sprint exercise despite 85% of these participants having reported difficulty sprinting in the week prior testing (HAGOS subscale). The low levels of pain observed in our study are consistent with other reports during walking, bodyweight squatting, and cross-body lunging,8,16 and suggest repeated sprinting alone is not a cause for acute pain in most people with FAIS. However, we acknowledge that running in this study did not induce sufficient hip flexion to cause hip impingement 3 and the short duration of the exercise bout (<15 min) may also have contributed to low levels of hip pain reported. Thus, our findings suggest repeated sprinting may not alter running patterns sufficiently to trigger acute symptoms in people with FAIS. Future studies should aim to investigate longer duration submaximal running or more provocative tasks such as pivoting, kicking, or twisting.
Strengths and Limitations
The main limitation was our small sample size that did not allow for a subgroup analysis of participants within the FAIS group based on pain responses to repeated sprints. Second, different proportions of male/female participants within groups may explain the absence of between-group differences. However, further inspection of data separated by sex (Online Appendix Figures 5-7) suggests between-group differences in sex distribution had limited effect on reported hip biomechanics data, and a minimal effect on strength measures. Third, 60° alpha angle threshold has recently been proposed as a cut-off for cam morphology. 23 At the time of participant recruitment, a threshold was yet to be established, and thus we followed the commonly used cut-off of 55°. 17 Fourth, we did not screen participants for other hip pathologies such as cartilage or labral damage, which may have influenced our results.18,19 Last, although included participants were recreationally active, their sporting background was heterogeneous, and results may differ if sport-specific populations are compared. We included a sport-specific repeated sprint protocol, a comparison group with asymptomatic cam morphology only (no pincer morphology), and participant groups matched for physical activity levels. By assessing hip strength before and after a bout of repeated sprint exercise, we were able to verify the occurrence and extent of fatigue in each group.
Conclusion
The association between frequent participation in high-impact activities and development of cam morphology is well established in the literature22,29; however, the link between cam morphology and hip symptoms is less clear.16,22 We observed no differences in running speed, hip flexion angles, hip flexion and extension moments, and hip strength in all muscle groups between participants with FAIS and asymptomatic participants, with or without cam morphology, before or after a bout of repeated sprint exercise. We did not find any association between HAGOS scores and changes in hip biomechanics during running after repeated sprint exercise. Our results suggest a short bout of repeated sprint running (<15 min) does not elicit fatigue-related changes in running biomechanics in FAIS beyond what occurs in those without symptoms. Longer duration activities or activities requiring greater hip flexion combined with adduction and/or internal rotation may better provoke pathology-related changes in running biomechanics in those with FAIS.
Supplemental Material
Supplemental material, sj-docx-1-sph-10.1177_19417381221131570 for Running Mechanics After Repeated Sprints in Femoroacetabular Impingement Syndrome, Cam Morphology, and Controls by Basílio A.M. Gonçalves, David J. Saxby, Evy Meinders, Andrea Hams, Conor Lambert, Taryn Jones, Rod S. Barrett and Laura E. Diamond in Sports Health: A Multidisciplinary Approach
Footnotes
The following author declared potential conflicts of interest: CL is employed as a physiotherapist by Queensland Health, is a sessional lecturer at Griffith University, and is a course developer at Griffith University.
Contributor Information
Basílio A.M. Gonçalves, Griffith Centre for Biomedical and Rehabilitation Engineering (GCORE), Menzies Health Institute Queensland; and School of Health Sciences and Social Work, Griffith University, Gold Coast, Queensland, Australia.
David J. Saxby, Griffith Centre for Biomedical and Rehabilitation Engineering (GCORE), Menzies Health Institute Queensland; and School of Health Sciences and Social Work, Griffith University, Gold Coast, Queensland, Australia.
Evy Meinders, Griffith Centre for Biomedical and Rehabilitation Engineering (GCORE), Menzies Health Institute Queensland; and School of Health Sciences and Social Work, Griffith University, Gold Coast, Queensland, Australia.
Andrea Hams, Griffith Centre for Biomedical and Rehabilitation Engineering (GCORE), Menzies Health Institute Queensland; and School of Health Sciences and Social Work, Griffith University, Gold Coast, Queensland, Australia.
Conor Lambert, School of Health Sciences and Social Work, Griffith University, Gold Coast, Queensland, Australia.
Taryn Jones, School of Health Sciences and Social Work, Griffith University, Gold Coast, Queensland, Australia.
Rod S. Barrett, Griffith Centre for Biomedical and Rehabilitation Engineering (GCORE), Menzies Health Institute Queensland; and School of Health Sciences and Social Work, Griffith University, Gold Coast, Queensland, Australia.
Laura E. Diamond, Griffith Centre for Biomedical and Rehabilitation Engineering (GCORE), Menzies Health Institute Queensland; and School of Health Sciences and Social Work, Griffith University, Gold Coast, Queensland, Australia.
References
- 1. Agricola R, Waarsing JH, Arden NK, et al. Cam impingement of the hip—a risk factor for hip osteoarthritis. Nat Rev Rheumatol. 2013;9:630-634. [DOI] [PubMed] [Google Scholar]
- 2. Arokoski JPA, Jurvelin JS, Väätäinen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sport. 2000;10:186-198. [DOI] [PubMed] [Google Scholar]
- 3. Audenaert EA, Mahieu P, Pattyn C. Three-dimensional assessment of cam engagement in femoroacetabular impingement. Arthrosc - J Arthrosc Relat Surg. 2011;27:167-171. [DOI] [PubMed] [Google Scholar]
- 4. Bagwell JJ, Powers CM. The influence of squat kinematics and cam morphology on acetabular stress. Arthrosc J Arthrosc Relat Surg. 2017;33:1797-1803. [DOI] [PubMed] [Google Scholar]
- 5. Brunner R, Maffiuletti NA, Casartelli NC, et al. Prevalence and functional consequences of femoroacetabular impingement in young male ice hockey players. Am J Sports Med. 2016;44:46-53. [DOI] [PubMed] [Google Scholar]
- 6. Catelli DS, Kowalski E, Beaulé PE, Smit K, Lamontagne M. Asymptomatic participants with a femoroacetabular deformity demonstrate stronger hip extensors and greater pelvis mobility during the deep squat task. Orthop J Sport Med. 2018;6:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Delp SL, Anderson FC, Arnold AS, et al. OpenSim: Open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng. 2007;54:1940-1950. [DOI] [PubMed] [Google Scholar]
- 8. Diamond LE, Bennell KL, Wrigley TV, Hinman RS, O’Donnell J, Hodges PW. Squatting biomechanics in individuals with symptomatic femoroacetabular impingement. Med Sci Sports Exerc. 2017;49:1520-1529. [DOI] [PubMed] [Google Scholar]
- 9. Diamond LE, Dobson FL, Bennell KL, Wrigley TV, Hodges PW, Hinman RS. Physical impairments and activity limitations in people with femoroacetabular impingement: a systematic review. Br J Sports Med. 2015;49:230-242. [DOI] [PubMed] [Google Scholar]
- 10. Duchateau J, Enoka RM. Neural control of shortening and lengthening contractions: influence of task constraints. J Physiol. 2008;586:5853-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edouard P, Mendiguchia J, Lahti J, et al. Sprint acceleration mechanics in fatigue conditions: compensatory role of gluteal muscles in horizontal force production and potential protection of hamstring muscles. Front Physiol. 2018;9:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Enoka RM, Duchateau J. Translating fatigue to human performance. Med Sci Sports Exerc. 2016;48:2228-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175-191. [DOI] [PubMed] [Google Scholar]
- 14. Gonçalves BAM, Meinders E, Saxby DJ, Barrett RS, Bourne MN, Diamond LE. Repeated sprints alter mechanical work done by hip and knee, but not ankle, sagittal moments. J Sci Med Sport. 2021;24:939-944. [DOI] [PubMed] [Google Scholar]
- 15. Goodall S, Charlton K, Howatson G, Thomas K. Neuromuscular fatigability during repeated-sprint exercise in male athletes. Med Sci Sport Exerc. 2015;47:528-536. [DOI] [PubMed] [Google Scholar]
- 16. Graffos A, Mohtajeb M, Mony M, et al. Biomechanics during cross-body lunging in individuals with and without painful cam and/or pincer morphology. Clin Biomech. 2020;76. [DOI] [PubMed] [Google Scholar]
- 17. Griffin DR, Dickenson EJ, O’Donnell J, et al. The Warwick Agreement on femoroacetabular impingement syndrome (FAI syndrome): an international consensus statement. Br J Sports Med. 2016;50:1169-1176. [DOI] [PubMed] [Google Scholar]
- 18. Hall M, Allison K, Wrigley TV, et al. Frontal plane hip joint loading according to pain severity in people with hip osteoarthritis. J Orthop Res. 2018;36:1637-1644. [DOI] [PubMed] [Google Scholar]
- 19. Ismailidis P, Kaufmann M, Clauss M, et al. Kinematic changes in severe hip osteoarthritis measured at matched gait speeds. J Orthop Res. 2021;39:1253-1261. [DOI] [PubMed] [Google Scholar]
- 20. Kierkegaard S, Mechlenburg I, Lund B, Søballe K, Dalgas U. Impaired hip muscle strength in patients with femoroacetabular impingement syndrome. J Sci Med Sport. 2017;20:1062-1067. [DOI] [PubMed] [Google Scholar]
- 21. King MG, Lawrenson PR, Semciw AI, Middleton KJ, Crossley KM. Lower limb biomechanics in femoroacetabular impingement syndrome: a systematic review and meta-analysis. Br J Sports Med. 2018;52:566-580. [DOI] [PubMed] [Google Scholar]
- 22. van Klij P, Ginai AZ, Heijboer MP, Verhaar JAN, Waarsing JH, Agricola R. The relationship between cam morphology and hip and groin symptoms and signs in young male football players. Scand J Med Sci Sport. 2020;30:1221-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Klij P, Reiman MP, Waarsing JH, et al. Classifying cam morphology by the alpha angle: a systematic review on threshold values. Orthop J Sport Med. 2020;8:232596712093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lloyd DG, Besier TF. An EMG-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J Biomech. 2003;36:765-776. [DOI] [PubMed] [Google Scholar]
- 25. Malloy P, Neumann DA, Kipp K. Hip Biomechanics during a single-leg squat: 5 key differences between people with femoroacetabular impingement syndrome and those without hip pain. J Orthop Sports Phys Ther. 2019;49:908-916. [DOI] [PubMed] [Google Scholar]
- 26. Malloy P, Wichman DM, Garcia F, Espinoza-Orías A, Chahla J, Nho SJ. Impaired lower extremity biomechanics, hip external rotation muscle weakness, and proximal femoral morphology predict impaired single-leg squat performance in people with FAI syndrome. Am J Sports Med. 2021;49:2984-2993. [DOI] [PubMed] [Google Scholar]
- 27. Mentiplay BF, Kemp JL, Crossley KM, et al. Relationship between hip muscle strength and hip biomechanics during running in people with femoroacetabular impingement syndrome. Clin Biomech. 2022;92:105587. [DOI] [PubMed] [Google Scholar]
- 28. Mohtadi NGHH, Griffin DR, Pedersen ME, et al. The development and validation of a self-administered quality-of-life outcome measure for young, active patients with symptomatic hip disease: the International Hip Outcome Tool (iHOT-33). Arthrosc - J Arthrosc Relat Surg. 2012;28:595-610.e1. [DOI] [PubMed] [Google Scholar]
- 29. Nawabi DH, Bedi A, Tibor LM, Magennis E, Kelly BT. The demographic characteristics of high-level and recreational athletes undergoing hip arthroscopy for femoroacetabular impingement: a sports-specific analysis. Arthrosc - J Arthrosc Relat Surg. 2014;30:398-405. [DOI] [PubMed] [Google Scholar]
- 30. Paul ILL, Munro MB, Abernethy PJJ, Simon SRR, Radin ELL, Rose RMM. Musculo-skeletal shock absorption: relative contribution of bone and soft tissues at various frequencies. J Biomech. 1978;11:237-239. [DOI] [PubMed] [Google Scholar]
- 31. Philippon MJ, Maxwell RB, Johnston TL, Schenker M, Briggs KK. Clinical presentation of femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc. 2007;15:1041-1047. [DOI] [PubMed] [Google Scholar]
- 32. Radzak KN, Stickley CD. Fatigue-induced hip-abductor weakness and changes in biomechanical risk factors for running-related injuries. J Athl Train. 2020;55:1270-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rutherford DJ, Moreside J, Wong I. Differences in hip joint biomechanics and muscle activation in individuals with femoroacetabular impingement compared with healthy, asymptomatic individuals is level-ground gait analysis enough? Orthop J Sport Med. 2018;6:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sarvazyan A, Rudenko O, Aglyamov S, Emelianov S. Muscle as a molecular machine for protecting joints and bones by absorbing mechanical impacts. Med Hypotheses. 2014;83:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Souza RB, Powers CM. Predictors of hip internal rotation during running: An evaluation of hip strength and femoral structure in women with and without patellofemoral pain. Am J Sports Med. 2009;37:579-587. [DOI] [PubMed] [Google Scholar]
- 36. Thorborg K, Bandholm T, Schick M, Jensen J, Hölmich P. Hip strength assessment using handheld dynamometry is subject to intertester bias when testers are of different sex and strength. Scand J Med Sci Sport. 2011;23:487-493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sph-10.1177_19417381221131570 for Running Mechanics After Repeated Sprints in Femoroacetabular Impingement Syndrome, Cam Morphology, and Controls by Basílio A.M. Gonçalves, David J. Saxby, Evy Meinders, Andrea Hams, Conor Lambert, Taryn Jones, Rod S. Barrett and Laura E. Diamond in Sports Health: A Multidisciplinary Approach