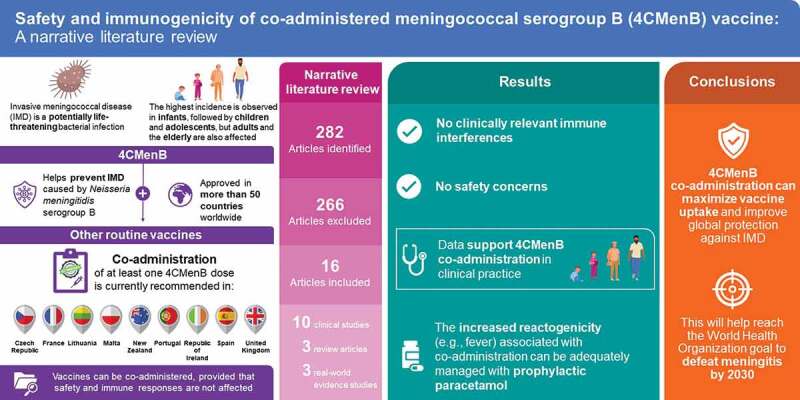
ABSTRACT
The four-component meningococcal serogroup B vaccine (4CMenB) is indicated for the prevention of invasive meningococcal disease (IMD) caused by Neisseria meningitidis serogroup B. Co-administering 4CMenB with other vaccines may improve vaccine uptake provided that the safety and immunogenicity of either are not affected. Published literature on the immunogenicity and reactogenicity of 4CMenB co-administered with other routine childhood and adulthood vaccines was reviewed. From 282 publications identified, data were collated from 10 clinical studies, 3 real-world studies, and 3 reviews. The evidence showed that 4CMenB co-administration is not associated with significant safety concerns or clinically relevant immunological interferences. The increased reactogenicity (e.g., fever) associated with 4CMenB co-administration can be adequately managed with prophylactic paracetamol in children. Thus, 4CMenB co-administration has the potential to maximize vaccine coverage and improve protection against IMD globally.
KEYWORDS: 4CMenB, meningitis B, invasive meningococcal disease, co-administration, concomitant administration
Graphical abstract
Introduction
Owing to the complex vaccination schedules offered by many countries, especially in the first years of life, co-administration of multiple vaccines during the same clinic visit may be a desirable public health strategy.1 The four-component meningococcal serogroup B vaccine (4CMenB) is approved for the prevention of invasive meningococcal disease (IMD) caused by Neisseria meningitidis serogroup B (MenB) in more than 50 countries worldwide. 4CMenB vaccination in infants is currently recommended and publicly funded in several European countries, either nationally or regionally. In most European countries, 4CMenB is licensed for infants from 2 months of age as a 2- or 3-dose series plus booster, and as a 2-dose series for children aged ≥2 y. In the Czech Republic, New Zealand, and South Australia, 4CMenB vaccination is recommended not only for infants but also for adolescents, specifically those aged 14–15 y in the Czech Republic,2,3 aged 13–25 y in specified close-living situations in New Zealand,4,5 and aged 15–16 y (Year 10 students) in South Australia.6 In the US, 4CMenB is licensed for individuals aged 10–25 y as a 2-dose series.7,8 The Advisory Committee on Immunization Practices (ACIP) recommends vaccination against meningococcal disease, including with 4CMenB, in adolescents and young adults aged 16–23 y (preferably aged 16–18 y) on the basis of shared clinical decision-making. ACIP also recommends routine 4CMenB vaccination in individuals aged ≥10 y who are at increased risk of MenB disease.9,10 As highlighted by a recent review, 4CMenB has been consistently well tolerated across clinical trials and post-licensure surveillance studies, with no significant safety issues and an acceptable safety profile.11
In addition to MenB vaccination, national immunization programs (NIPs) worldwide include vaccinations against many different infectious diseases in infants, toddlers, and adolescents; these include (but are not limited to) diphtheria, tetanus, pertussis, hepatitis B, poliovirus, influenza, rotavirus, measles, mumps, rubella, varicella, tuberculosis, Haemophilus influenzae type b infections, and meningococcal infections with different serogroups (e.g., A, C, W, and Y). Additionally, immunization programs in certain countries or regions include vaccination against hepatitis A and yellow fever. The co-administration of multiple vaccines offers various benefits, including fewer visits required to receive the full vaccination schedule, improved timeliness of vaccination, and greater vaccination compliance, which may contribute to improved vaccine uptake.12 4CMenB is co-administered with other routine childhood vaccines in certain NIPs, based on clinical evidence of tolerability and an absence of significant interferences with the immune responses elicited by either of the co-administered vaccines.2 The aim of this narrative literature review was to collate published immunogenicity and reactogenicity data from clinical and real-world studies evaluating 4CMenB co-administration with other vaccines.
Materials and methods
A narrative literature review was performed in April 2022 (no specified timeframe) using PubMed. The following search terms were used: (Bexsero OR meningococcal vaccines OR meningococcal vaccine OR meningococcal group B vaccine OR meningitis B vaccine OR MenB OR 4CMenB) AND (Coadministration OR coadminister OR coadministered OR co-administration OR co-administer OR co-administered OR concomitant OR concomitantly).
Overall, 282 publications were manually reviewed, 266 of which were deemed irrelevant to the scope of this article and were removed. In total, 16 publications were included in this review, specifically 10 clinical studies,13–22 3 real-world evidence studies,23–25 and 3 review articles.12,26,27 Relevant immunogenicity and reactogenicity data are presented herein, organized according to co-administered vaccine type where possible. Owing to methodological differences in the definition and collection of immunogenicity data across studies, the immunogenicity parameters are reported for each study.
Results
Immunogenicity
Multiple clinical studies have investigated the effects of the co-administration of 4CMenB with other childhood and adulthood vaccines, specifically focusing on the immunogenicity of each co-administered vaccine. In summary, the available clinical evidence suggests that the co-administration of 4CMenB with other vaccines, including those given as part of routine childhood vaccination schedules, is not associated with clinically relevant immunological interferences with 4CMenB or the co-administered vaccines.
Clinical studies in infants and toddlers
Seven clinical studies of infants 2–12 months of age conducted in Europe, Asia, and Central and South America between 2012 and 2021 did not report clinically relevant interferences of 4CMenB co-administration with combinations of the diphtheria-tetanus-acellular pertussis, inactivated poliovirus, H. influenzae type b plus hepatitis B vaccine (DTaP/IPV/Hib/HepB), 7-valent and 13-valent pneumococcal vaccines (PCV7 and PCV13), 10-valent pneumococcal nontypeable H. influenzae protein D conjugate vaccine (PHiD-CV), meningococcal serogroup C-CRM conjugated vaccine (MenC-CRM), rotavirus vaccines (monovalent human rotavirus vaccine and pentavalent bovine-human reassortant vaccine), measles-mumps-rubella (MMR) and MMR-varicella (MMRV) vaccines, and the meningococcal serogroups A, C, W, and Y-CRM conjugated vaccine (MenACWY-CRM).
DTaP/IPV/Hib/HepB plus PCV7 or PCV13 and rotavirus vaccines
In a phase 3 study conducted in Europe (N = 3630), 4CMenB vaccination elicited immune responses against the indicator strains selective for the four vaccine antigens at 1 month post-third dose (immunogenicity subset, 4CMenB primary schedule, N = 2627), with human complement serum bactericidal assay (hSBA) titers ≥5 reported in 100% of infants for strains 44/76-SL (factor H binding protein) and 5/99 (Neisseria adhesin A), 84% for strain NZ98/254 (PorA1.4, i.e., the major component of New Zealand strain outer-membrane vesicle), and 84% for strain M10713 (Neisserial Heparin Binding Antigen; post hoc calculation).19 Based on predefined noninferiority criteria (i.e., a lower limit of the 95% confidence interval [CI] of ≤10% for the difference in the proportion of infants with hSBA titers ≥5), there was no evidence of significant interference of the 4CMenB vaccine when co-administered with the DTaP/IPV/Hib/HepB and PCV7 vaccines, except for a slightly lower proportion of infants with poliovirus type 2 titers ≥8 (−5%; 95% CI −11, −1).19
A phase 2b study of 1885 infants in Europe also reported that 4CMenB was immunogenic when co-administered with the DTaP/IPV/Hib/HepB and PCV7 vaccines at either 2, 4, and 6 months or 2, 3, and 4 months of age; specifically, hSBA titers ≥5 at 1 month post-third dose were observed in ≥99% of infants with all schedules for strains 44/76-SL and 5/99, and 79–82% for strain NZ98/254 depending on the schedule (compared with 86% with 4CMenB alone).15,26 Based on a predefined noninferiority analysis (i.e., a lower limit of the 95% CI greater than −10% for the difference in the proportion of infants with hSBA titers ≥5), there was no evidence of significant interference of the 4CMenB vaccine when co-administered with the DTaP/IPV/Hib/HepB and PCV7 vaccines.15
Based on data from more than 300 participants enrolled across these studies and reviewed by O’Ryan and colleagues, immune responses to 4CMenB (measured as the proportion of individuals with hSBA titers ≥5) were not significantly impacted by co-administration with routine vaccine regimens including the monovalent human rotavirus vaccine or pentavalent bovine-human reassortant rotavirus vaccine.15,19,27 Specifically, after three doses of 4CMenB in 200 of these participants, 99–100% of infants achieved hSBA titers ≥5 against strains 44/76-SL and 5/99, regardless of receipt or nonreceipt of the rotavirus vaccine. Overall, 76–84% of infants who received a rotavirus vaccine achieved hSBA titers ≥5 against strain NZ98/254 compared with 83–84% of those who had no history of rotavirus vaccination.27
Finally, in a phase 3 study of 225 infants and toddlers in Taiwan, the immunogenicity of 4CMenB was not impacted by co-administration with routine vaccines (i.e., DTaP/IPV/Hib and PCV13 at 2, 4, and 6 months of age and HepB vaccine at 6 months of age), with hSBA titers ≥5 at 1 month post-third dose observed in 100% of infants for strains 44/76-SL and 5/99, 79% for strain NZ98/254, and 59% for strain M10713.13
PCV13 or PHiD-CV plus MenC-CRM vaccines
In a phase 2 study of 213 infants in the UK, co-administration of 4CMenB with PCV13 in either a 2 + 1 (2, 4, and 12 months of age) or a 1 + 1 schedule (3 and 12 months of age) did not affect the immunogenicity of 4CMenB, with hSBA titers ≥4 at 1 month post-dose 2 observed in 100% of infants for strain 5/99, 95% for strain 44/76-SL, and 89% for strain NZ98/254.14
A phase 3 study in 251 infants and toddlers in Brazil evaluated co-administration of 4CMenB with MenC-CRM plus PHiD-CV.17 The study reported sufficient responses to 4CMenB after the first two of the three primary doses at 3 and 5 months and the booster vaccination at 12 months (defined as a lower limit of the 95% CI of ≥70% at month 6 or ≥75% at month 13 for the proportion of participants with hSBA titers ≥4). Additionally, co-administration of MenC-CRM with 4CMenB was noninferior to MenC-CRM alone (defined as a lower limit of the 95% CI greater than −10% for the difference in the proportion of infants with hSBA titers ≥8).
A post hoc analysis of this study evaluated immune responses to PHiD-CV in 213 infants and toddlers in Brazil when the first two of the three primary doses and the booster dose were co-administered with 4CMenB and/or MenC-CRM vaccine at 3, 5, and 12 months of age.18 Immune responses to PHiD-CV 1 month after the second primary dose were similar in those who were also co-administered 4CMenB and/or MenC-CRM, with similar geometric mean concentrations of antibodies for each pneumococcal serotype, and similar proportions of infants with antibody concentrations ≥0.35 µg/mL for the PHiD-CV serotypes and PHiD-CV-related serotypes 6A and 19A.
MMR and MMRV vaccines
In an extension to the previously described phase 3 study in 1555 toddlers aged 12 months in Europe, administration of a 4CMenB booster dose was immunogenic when co-administered with MMRV vaccine at 1 month post-booster dose, with no evidence of significant interference due to co-administration (95−100% of children had hSBA titers ≥5 for all antigens, with or without MMRV vaccine).19 Similarly, in the previously described Taiwanese phase 3 study, a booster dose of 4CMenB at 12 months of age was immunogenic at 1 month post-booster dose (92–99% of infants with hSBA titers ≥5 against strains 44/76-SL, 5/99, and M10713) and was not impacted by co-administration with MMR and MMRV vaccines.13
MenACWY vaccine
In a multicenter phase 3b study, 750 infants and toddlers in Mexico and Argentina were randomized to receive 4CMenB alone, MenACWY-CRM alone, or 4CMenB and MenACWY-CRM co-administered at 3, 5, 7, and 13 months of age.16 Immunogenicity associated with co-administration was non-inferior compared with either vaccine alone (defined as a lower limit of the 95% CI >0.5 for the between-group ratio of hSBA geometric mean titers) at 1 month post-booster (primary objective).16 Across all groups and serogroup B strains, 68–100% and 87–100% of infants and toddlers had hSBA titers ≥5 at 1 month post-third primary dose and 1 month post-booster, respectively.16
Clinical studies in adults
MenACWY vaccine
While most studies published to date evaluate co-administration in pediatrics, evidence related to adults is also of interest. A phase 2 trial published in 2015 investigated a three-dose schedule of 4CMenB in 38 adult laboratory workers aged 18–65 y in England.22 In this study, co-administration of the first dose with MenACWY-CRM did not adversely affect immunogenicity of either vaccine. Specifically, 95–100% and 90–100% of subjects developed protective SBA titers against A, C, W, and Y strains (exogenous complement source [rSBA] titers ≥8) and a panel of MenB strains (hSBA titers ≥4), respectively.
Reactogenicity
Multiple clinical and real-world studies have investigated the effects of the co-administration of 4CMenB with other childhood and adulthood vaccines, specifically focusing on the reactogenicity of each co-administered vaccine. In summary, the published clinical and real-world evidence suggests that there are no significant safety concerns associated with the co-administration of 4CMenB with other vaccines. Moreover, the increased reactogenicity (e.g., fever) associated with co-administration can be effectively managed with prophylactic paracetamol. Crucially, emerging real-world evidence supports clinical evidence, reflecting the higher rate of adverse events (AEs) but no safety concerns associated with 4CMenB co-administration.
Clinical studies in infants and toddlers
Six clinical studies conducted in Europe, Asia, and Central and South America between 2012 and 2018 evaluated safety and reactogenicity of 4CMenB co-administration with routine childhood vaccines. Common solicited AEs associated with 4CMenB in infants and toddlers include both local (e.g., local pain and erythema) and systemic (e.g., fever and irritability) reactions.7,8 Clinical studies have shown that co-administration of 4CMenB with routine childhood vaccines is associated with greater reactogenicity compared with separate administration, including higher rates of fever ≥38°C (4CMenB co-administration, 17–75%; 4CMenB alone, 18–43%),13,15–17,20 moderate fever ≥39°C (4CMenB co-administration, 10–18%; 4CMenB alone, 3–8%),15,20 and injection-site pain/tenderness (4CMenB co-administration, 48–87%; 4CMenB alone, 55–70%)13,15–17,19,20 in all vaccination groups across studies, <1% of infants had severe fever ≥40°C.13,15–17,20; Overall, no safety concerns were reported in association with co-administration (Table 1).
Table 1.
Studies evaluating the safety and reactogenicity of 4CMenB co-administration with other routine childhood and adulthood vaccines.
| Study (N enrolled) | Vaccines evaluated | Most common solicited local AE ≤7 d post vaccination | Severe solicited local AEs ≤7 d post vaccination | Most common solicited systemic AE and fever ≤7 d post vaccination | Severe solicited systemic AEs ≤7 d post vaccination | Unsolicited AEs and SAEs in the study period |
|---|---|---|---|---|---|---|
| Clinical evidence | ||||||
| Phase 2b, open-label, multicenter study of infants aged ≤7 months in Europe (N = 1885)14 | (1) 4CMenB + routine vaccines (DTaP/IPV/Hib/HepB, PCV7) at 2, 4, and 6 months (co-administration) (2) 4CMenB at 2, 4, and 6 months and routine vaccines at 3, 5, and 7 months (intercalated administration) (3) 4CMenB + routine vaccines at 2, 3, and 4 months (accelerated co-administration) (4) Routine vaccines only at 2, 3, and 4 months |
Erythema: Co-administration: 62–69% after each dose of co-administered 4CMenB Routine vaccines only: 47–58% |
Severe local pain: Co-administration: 13–16% after each dose of co-administered 4CMenB Routine vaccines only: 1–3% |
Irritability: Co-administration: 71–74% after each dose of co-administered 4CMenB Routine vaccines only: 44–57% Fever ≥38°C: Co-administration: 51–62% after each dose of co-administered 4CMenB Routine vaccines only: 23–36% |
Fever ≥39°C: Co-administration: 10–15% after each dose of co-administered 4CMenB Routine vaccines only: 3–4% |
At least possibly vaccine-related: one hypotonic hyporesponsive episode within 12 h of co-administration, aseptic meningitis, retinal dystrophy (believed to be congenital), transient synovitis of the right hip, transient hearing loss noted by a parent, and transient apnea SAEs: Co-administration: 10% Routine vaccines only: 6% |
| Phase 3, multicenter, open-label (N = 2627) and observer-blind (N = 1003) study of infants and toddlers aged ≤12 months in Europe18 | Stage 1 (primary series; N = 3630): (1) 4CMenB + routine vaccines (DTaP/IPV/Hib/HepB + PCV7) (2) Routine vaccines only (3) Routine vaccines + MenC* Stage 2 (booster; N = 1555): (1) 4CMenB + routine vaccines (MMRV) (2) 4CMenB only |
Local pain: Co-administration (primary series): 87% after any dose of 4CMenB and 79–80% after any dose of routine vaccines Routine vaccines only: 53–59% Co-administration (booster): 71% 4CMenB only: 71% |
Severe local pain: Co-administration (primary series): 29% after any dose of 4CMenB and 24% after any dose of routine vaccines Routine vaccines only: 6–8% Co-administration (booster): 14% 4CMenB only: 15% |
Fever ≥38.5°C within 6 h of vaccination: Co-administration (primary series): 65% Routine vaccines only: 32% Fever ≥38°C within 6 h of vaccination: Co-administration (booster): 31% 4CMenB only: 32% |
Fever ≥40°C within 6 h of vaccination: Co-administration (primary series): 1% Routine vaccines only: 0% Co-administration (booster): <1% 4CMenB only: 0% |
At least possibly vaccine-related: two cases of seizures and two cases of febrile seizures within 24 h of co-administration, and three confirmed cases of Kawasaki disease in the following weeks after the primary series SAEs: Co-administration (primary series): 8% (16 events possibly vaccine-related) Routine vaccines only: 8% (one event possibly vaccine-related) Co-administration (booster): <1% (two events possibly vaccine-related) |
| Pooled analysis of three multicenter, open-label trials of infants and toddlers aged 2–15 months in Europe (N = 5026)14,18,19 | Stage 1 (primary series; N = 5026): (1) 4CMenB + routine vaccines (DTaP/IPV/Hib/HepB + PCV7, multiple schedules)14,18 (2) Routine vaccines only (multiple schedules)14,18 (3) 4CMenB only Stage 2 (booster; N = 1957): (1) 4CMenB + routine vaccines (MMRV, multiple schedules)18 (2) 4CMenB only (multiple schedules)18 |
Local pain: Co-administration (primary series): 66% 4CMenB only: 55% Routine vaccines only: not measured |
Severe local pain: Co-administration (primary series): 16% 4CMenB only: 9% Routine vaccines only: not measured |
Fever ≥38.5°C: Co-administration (primary series): 75% 4CMenB only: 43% Routine vaccines only: 43% Risk interval analysis of interaction between vaccines: Fever ≥38°C: Co-administration (primary series): 75% Separate administration: 86% Fever ≥39°C: Co-administration (primary series): 18% Separate administration: 14% Fever ≥38°C lasting > 1 d: Co-administration (primary series): 33% Separate administration: 23% |
Fever ≥40°C: Co-administration (primary series): <1% 4CMenB only: <1% Routine vaccines only: <1% |
See primary publications14,18 |
| Phase 3b, open-label, multicenter study of infants aged 3–12 months in Brazil (N = 251)16 | (1) 4CMenB + MenC-CRM (+ PHiD-CV) (2) MenC-CRM only (+ PHiD-CV) |
Local pain: Co-administration: 53–63% after each dose of co-administered 4CMenB (50–59% after routine vaccines) MenC-CRM only: 34–46% |
Severe local pain: Co-administration: 5–11% after each dose of co-administered 4CMenB (3–8% after routine vaccines) MenC-CRM only: 1–3% |
Unusual crying: Co-administration: ~50–75% after each 4CMenB + MenC-CRM vaccination MenC-CRM only: ~30–60% Fever ≥38°C: Co-administration: 39–48% after each 4CMenB + MenC-CRM vaccination MenC-CRM only: 10–20% |
Fever ≥40°C: Co-administration: <1% after the second dose of co-administered 4CMenB MenC-CRM only: 0% |
Any unsolicited AEs: Co-administration: 82% MenC-CRM only: 73% SAEs (% possibly vaccine-related): Co-administration: 4% (0%) MenC-CRM only: 6% (0%) |
| Phase 3, open-label study of infants aged 2–12 months in Taiwan (N = 225)12 |
(1) 4CMenB at 2, 4, and 6 months (primary series) and 12 months (booster) + routine vaccines (DTaP/IPV/Hib/HepB + PCV13 at 2, 4, and 6 months, HBV at 6 months, and MMRV at 12 months) (2) Routine vaccines only |
Local pain: Co-administration: 48–51% after each dose of co-administered 4CMenB (27–34% after routine vaccines) Routine vaccines only: 7–16% |
Severe local pain: Co-administration: ≤5% after each dose of co-administered 4CMenB (≤2% after routine vaccines) Routine vaccines only: not reported |
Irritability: Co-administration: 52–75% after each co-administered dose of 4CMenB or routine vaccine Routine vaccines only: 22–44% Fever ≥38°C: Co-administration: 44–51% after each co-administered dose of 4CMenB or routine vaccine Routine vaccines only: 8–17% |
Fever ≥40°C: Co-administration: 0–1% after each co-administered dose of 4CMenB or routine vaccine Routine vaccines only: 0–1% |
Any unsolicited AEs: Co-administration: 72% Routine vaccines only: 42% SAEs (% possibly vaccine-related): Co-administration: 9% (0%) Routine vaccines only: 11% (0%) |
| Phase 3b, open-label, multicenter study of infants and toddlers aged 3–13 months in Mexico and Argentina (N = 750)15 | (1) 4CMenB + MenACWY-CRM (2) 4CMenB only (3) MenACWY-CRM only |
Local pain: Co-administration: 62–68% after each dose of 4CMenB + MenACWY-CRM (59–65% after 4CMenB and 36–48% after MenACWY-CRM) 4CMenB only: 60–70% MenACWY-CRM only: 27–31% |
Severe local pain: Co-administration: 6–15% after each 4CMenB + MenACWY-CRM vaccination 4CMenB only: 7–15% MenACWY-CRM only: ≤1% |
Persistent crying: Co-administration: 41–52% after each dose of 4CMenB + MenACWY-CRM 4CMenB only: 43–58% MenACWY-CRM only: 27–36% Fever ≥38°C: Co-administration: 17–26% after each 4CMenB + MenACWY-CRM vaccination 4CMenB only: 18–25% MenACWY-CRM only: 4–11% |
Fever >40°C: Co-administration: <1% after each dose of 4CMenB + MenACWY-CRM 4CMenB only: ≤1% MenACWY-CRM only: 0% |
Any unsolicited AEs (% possibly vaccine-related): Co-administration: 31–62% (37%) 4CMenB only: 40–66% (41%) MenACWY-CRM only: 20–62% (11%) SAEs (% possibly vaccine-related): Co-administration: 2% (0%) 4CMenB only: 5% (1%) MenACWY-CRM only: 4% (0%) |
| Phase 2, open-label, single-center study of adult laboratory workers aged 18–65 y in England (N = 38)21 | (1) 4CMenB + MenACWY-CRM at 0 months (2) 4CMenB only at 2 and 6 months |
Local pain: Co-administration: 97% after 4CMenB and 21% after MenACWY-CRM 4CMenB only: 97–100% |
Severe local pain: Co-administration: 16% after 4CMenB and 0% after MenACWY-CRM 4CMenB only: ~20% |
Headache: Co-administration: ~37% after 4CMenB + MenACWY-CRM 4CMenB only: ~18% Nausea: Co-administration: ~25% after 4CMenB + MenACWY-CRM 4CMenB only: ~18% Fever: Co-administration: <5% after 4CMenB + MenACWY-CRM 4CMenB only: <10% |
Not reported | Not reported |
| Real-world evidence | ||||||
| UK immunization program, infants and toddlers aged 1–18 months (N = 107,231)22 | 93% 4CMenB exposure co-administered with routine vaccines (primary series [DTaP/IPV/Hib (with HepB from 2017), PCV13, rotavirus] or booster [Hib, MenC, PCV13, MMR]) | Not reported | Not reported | Not reported | Not reported | Not possible to differentiate whether the ~ 1.5 times higher risk of seizures (IRR 1.43; 95% CI 1.02, 2.02) and febrile seizures (IRR 1.72; 95% CI 1.08, 2.75), was due to one or other (or the combination) of vaccines |
| Post-marketing active SMS/e-mail-based safety surveillance of influenza vaccines in infants, toddlers, and children aged 6 months to 4 y in Australia (N = 7402)23 | 66 children (8%) received the non-NIP funded 4CMenB vaccine at the same time as the NIP-funded influenza vaccine | 10% of all children in the study had systemic and/or local AEs ≤3 d of vaccination, including fever (4%) and local pain/swelling/redness (3%) 30% of parents/caregivers of children co-administered 4CMenB with an influenza vaccine reported AEs vs 7% of parents/caregivers of children receiving an influenza vaccine only |
Not reported | |||
| Short-term safety surveillance during a mass 4CMenB vaccination campaign in individuals aged ≤20 y in the Saguenay-Lac-Saint-Jean region, Quebec, Canada (N = 59,098)24 | Among infants and toddlers aged <2 y, 92% of targeted individuals received a first dose of 4CMenB, and 88% received a second dose (37% and 49% co-administered with other vaccines, respectively) | Injection-site reactions reported in 58% of all reviewed open comments in the questionnaires (95% reporting pain) | Severe or long-lasting (≥4 d) local pain reported in 21% of all reviewed open comments in the questionnaires | OR for fever (onset on days 1–2) with 4CMenB co-administration vs separate administration: 2–6 months: OR 2.8 (95% CI 1.7, 4.4) 12–14 months: OR 2.0 (95% CI 1.1, 3.6) 18–23 months: OR 1.8 (95% CI 1.1, 3.1) OR for fever (onset on days 1–2) with ≥2 doses of antipyretic prophylaxis vs no antipyretics: 2–6 months: OR 0.4 (95% CI 0.2, 0.6) 12–14 months: OR 0.3 (95% CI 0.1, 0.7) 18–23 months: OR 0.4 (95% CI 0.2, 0.8) |
Not reported | |
*See original publication for data on the routine vaccines + MenC arm.18
4CMenB, four-component meningococcal serogroup B; AE, adverse event; CI, confidence interval; DTaP, diphtheria-tetanus-acellular pertussis; HBV, hepatitis B virus; HepB, hepatitis B; Hib, Haemophilus influenzae type b; IPV, inactivated poliovirus; IRR, incidence rate ratio; MenACWY, meningococcal serogroups A, C, W, and Y; MenC, meningococcal serogroup C; MMR, measles-mumps-rubella; MMRV, measles-mumps-rubella-varicella; NIP, national immunization program; OR, odds ratio; PCV7, seven-valent pneumococcal vaccine; PCV13, 13-valent pneumococcal vaccine; PHiD-CV, 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine; SAE, serious adverse event; SMS, short message service.
DTaP/IPV/Hib/HepB plus PCV7 or PCV13 and rotavirus vaccines
In a pooled analysis of 5026 infants and toddlers aged 2–15 months20 who were enrolled in three open-label, randomized clinical studies in Europe,15,19 co-administration of 4CMenB with other routine vaccines (i.e., DTaP/IPV/Hib/HepB and PCV7) was associated with an increased incidence of fever ≥39°C (18% vs 14%), long-lasting fever (>1 d; 33% vs 23%), and injection-site pain (66% vs 55%), but an overall reduction in incidence of fever ≥38°C (75% vs 86%) and other systemic AEs compared with separate administration.20 Based on pooled data, vaccine co-administration decreased the risk of AEs following immunization (AEFIs; i.e., fever ≥38°C, crying, diarrhea, and change in eating habits) by 4–49%, with the greatest reduction in risk demonstrated in those with versus without a history of the same prior AEFI(s).20 Although the pooled analysis did not report on febrile and non-febrile seizures, Kawasaki disease, and hypotonic hyporesponsive episodes,20 these AEs were temporally associated with 4CMenB vaccination in the original trials15,19; according to expert opinion, the rarity of these AEs did not allow for a definitive assessment of causality.1 Importantly, real-world data following widespread administration of 4CMenB in infants in the UK did not identify an increased risk of these AEs associated specifically with 4CMenB vaccination.28
Based on pooled data from 303 participants enrolled in these studies,15,19 similar reactogenicity profiles were observed in infants who did or did not receive a rotavirus vaccine co-administered with 4CMenB and other recommended vaccines. Overall, 80.5% of infants who received a rotavirus vaccine with 4CMenB and other routine vaccines and 75.3% of infants who did not receive a rotavirus vaccine experienced a systemic AE, whereas severe systemic reactions were recorded for 19.5% and 24.7% of participants, respectively. The rate of fever was also similar between the two groups of infants, with similar rates of high fever (≥39.5°C) in those receiving (2.2–4.2%) and not receiving (2.6–4.5%) the rotavirus vaccine in the two studies.27
In the previously described study in infants and toddlers in Taiwan, the proportion of participants who experienced ≥1 solicited AE, regardless of vaccination, was 100% with 4CMenB co-administered with routine vaccinations (i.e., DTaP/IPV/Hib/HepB, PCV13, and MMRV) compared with 93% with routine vaccines alone.13 The proportion of infants experiencing ≥1 event decreased from the first to subsequent vaccinations in both groups. Unsolicited AEs were reported in 72% of infants in the 4CMenB plus routine vaccination co-administration group compared with 42% of infants who did not receive 4CMenB. The most frequently reported unsolicited AEs (by preferred term) occurring with 4CMenB co-administration compared with routine vaccines alone were injection-site induration (52% and 17%, respectively), injection-site swelling (28% and 4%, respectively), injection-site erythema (16% and 1%, respectively), and eating disorders (12% and 8%, respectively). Overall, no safety concerns were identified with 4CMenB co-administration.13
PCV13 or PHiD-CV plus MenC-CRM vaccines
In the phase 3b study of infants and toddlers in Brazil who received MenC-CRM plus PHiD-CV with or without 4CMenB, rates of solicited AEs were 100% and 95%, respectively, after any vaccination, with no safety concerns identified.17 As expected, rates of unsolicited AEs were higher in those who received co-administered 4CMenB and MenC-CRM plus PHiD-CV compared with those who received MenC-CRM plus PHiD-CV alone (82% vs 73%, respectively). The most commonly reported AE by the Medical Dictionary for Regulatory Activities (MedDRA) preferred term was upper respiratory tract infection (47% vs 40%, respectively).
MenACWY vaccine
In the clinical study of infants and toddlers in Mexico and Argentina, no safety concerns were raised regarding the co-administration of 4CMenB and MenACWY-CRM compared with either vaccine alone.16 Across all time points, unsolicited AEs were reported by 31–62% of participants who received 4CMenB co-administered with MenACWY, 40–66% of participants who received 4CMenB alone, and 20–62% of participants who received MenACWY alone.16
Real-world studies in infants, toddlers, adolescents, and adults
In a real-world study of 107,231 infants and toddlers within the UK routine immunization program observed between 1 and 18 months of age,23 the approximately 1.5 times higher risk of seizures (incidence rate ratio [IRR] 1.43; 95% CI 1.02, 2.02) and febrile seizures (IRR 1.72; 95% CI 1.08, 2.75) after vaccination was not specifically attributable to 4CMenB, as 93% of 4CMenB vaccinations were co-administered with other routine childhood vaccines (i.e., DTaP/IPV/Hib [with HepB from 2017], PCV13 and rotavirus at 2 months; DTaP/IPV/Hib [with HepB from 2017] and PCV13 at 4 months; and Hib, MenC, PCV13, and MMR at 12–13 months).
In a real-world vaccination campaign of 59,098 individuals aged ≤20 y in Saguenay-Lac-Saint-Jean, Quebec, Canada, co-administration of 4CMenB with other routine childhood vaccines resulted in a significantly higher risk of fever within 2 d of vaccination compared with 4CMenB alone in infants aged 2–6 months (odds ratio [OR] 2.77; 95% CI 1.74, 4.41; p < .001), 12–14 months (OR 1.95; 95% CI 1.06, 3.57; p = .03), and 18–23 months (OR 1.83; 95% CI 1.09, 3.07; p = .02).25
In a small subset of a real-world study including 66 infants and toddlers aged between 6 months and 4 y who received the seasonal influenza vaccine in sentinel immunization clinics in Australia, co-administration with 4CMenB resulted in a significantly higher rate of AEs (30.3%) compared with children who received seasonal influenza vaccine alone (7.3%; p < .001).24
Importantly, a large-scale pharmacovigilance study conducted in the UK showed that the anticipated reactogenicity did not adversely affect compliance with subsequent vaccine doses.28
Use of prophylactic paracetamol in infants, toddlers, and children
A multicenter, randomized phase 2 study of 1507 infants showed that most participants who received the licensed formulation of 4CMenB co-administered with routine vaccinations (DTaP/IPV/Hib/HepB and PCV7) at 2, 3, 4, and 12 months of age experienced ≥1 systemic and/or local reaction, with slightly reduced reactogenicity after the third dose versus the other doses.29 Notably, fever ≥38.5°C was reported in 31–53% of infants receiving 4CMenB co-administration.29 Importantly, the rate of fever was reduced after the administration of prophylactic paracetamol (at the time of vaccination and at 4- to 6-h intervals post vaccination), without interfering with the immunogenicity of either 4CMenB or the co-administered vaccines.21 In the UK, these findings led the Joint Committee on Vaccination and Immunisation to recommend prophylactic paracetamol for management of the reactogenicity associated with co-administration, with paracetamol administered just before or at the time of vaccination, followed by two additional doses at 4- to 6-h intervals.30,31
The previously described Canadian real-world study provided supporting evidence of the effectiveness of prophylactic paracetamol in clinical practice, with ≥2 doses resulting in significantly lower rates of fever within 2 d of vaccination versus no antipyretic prophylaxis in infants aged 2–6 months (OR 0.35; 95% CI 0.21, 0.60; p < .001), 12–14 months (OR 0.28; 95% CI 0.11, 0.72; p = .008), and 18–23 months (OR 0.40; 95% CI 0.22, 0.76; p = .005).25 The real-world study in Australia did not routinely record the use of prophylactic paracetamol in children who received the seasonal influenza vaccine alone or co-administered with 4CMenB; however, the authors noted that the known association between 4CMenB and fever reinforces the need to recommend prophylactic paracetamol when 4CMenB is co-administered.24
Clinical studies in adults
In a clinical study of adult laboratory workers in England, local injection-site reactions (i.e., erythema, induration, and/or pain) were reported by all participants who received 4CMenB; this was compared with 23% of participants who reported a reaction with MenACWY-CRM. Fewer than 10% of participants who received 4CMenB, either alone or co-administered with MenACWY-CRM, experienced fever, which the authors considered likely related to 4CMenB based on the published literature. Although prophylactic paracetamol was not administered as part of the trial, the authors suggested that it could be used to reduce reactogenicity in adults based on the recommendation in infants.22
Discussion
The aim of this narrative literature review was to collate available immunogenicity and reactogenicity data related to 4CMenB co-administration with other routine childhood and adulthood vaccines. The available clinical and real-world evidence shows that 4CMenB co-administration in infants/toddlers and adults is not associated with clinically relevant immunological interferences with either of the co-administered vaccines. Although an increase in reactogenicity is observed when 4CMenB is co-administered with other routine vaccines, AEs tend to be mild and transient and no significant safety concerns have been identified. A study pooling data from multiple clinical trials suggested that, while vaccine-related AEs including fever were higher with 4CMenB co-administration, the overall cumulative risk of AEs was significantly lower than if the vaccines were given at separate immunization visits.20,32 Finally, the reviewed evidence shows that the increased reactogenicity (e.g., fever) associated with co-administration can be adequately managed with prophylactic paracetamol.
Collectively, these data support the continuation of 4CMenB co-administration in clinical practice. Maximizing 4CMenB uptake, including through co-administration with other routine childhood vaccines, will be important in the fight against IMD caused by MenB and in reaching the goal set out by the World Health Organization to defeat meningitis by 2030.33 Following an updated search of the literature in April 2023, 12 countries currently include 4CMenB in their NIP in infants, toddlers, and/or adolescents (Andorra, the Czech Republic, France, Italy, Lithuania, Malta, New Zealand, Portugal, the Republic of Ireland, San Marino, Spain, and the UK), including nine countries recommending co-administration of ≥1 dose of 4CMenB with other routine vaccines (the Czech Republic, France, Lithuania, Malta, New Zealand, Portugal, the Republic of Ireland, Spain, and UK) (Table 2).2–6,34–48
Table 2.
National immunization program recommendations for 4CMenB.
| Country | Schedule (age) | Co-administration regimen(s) | Use of prophylactic paracetamol |
|---|---|---|---|
| Andorra33,34 | 2, 4, and 13 months | Given alone (ideally 2 weeks apart from other vaccines) | Recommended |
| Czech Republic2,3,35 | 2–3 months, 4–6 months, 12–15 months; 14–15 y | Plus DTaP/IPV/Hib/HepB at 3 and 5 months Plus PCV at 2–3 months |
Recommended |
| France36 | 3, 5, and 12 months | Given alone at 3 months Plus MenC at 5 months Plus MenC and/or MMR at 12 months |
No recommendations |
| Italy2,37 | 3, 4, 6, and 13 months* | Given alone | No recommendations |
| Lithuania2,38 | 3, 5, and 12–15 months | Given alone at 3 and 5 months Plus PCV and/or MMR vaccine at 12–15 months |
Recommended only if co-administered |
| Malta39 | 2, 4, and 12 months | Plus DTaP/IPV/Hib/HepB vaccine and PCV at 2 and 4 months Plus PCV at 12 months |
No recommendations |
| New Zealand4,5 | 3, 5, and 12 months; 13–25 y in specified close-living situations† |
Can be administered with other routine vaccines | Recommended in children aged ≤2 y |
| Portugal40,41 | 2, 4, and 12 months | Plus DTaP/IPV/Hib/HepB vaccine and PCV at 2 months Plus DTaP/IPV/Hib vaccine and PCV at 4 months Plus MenC vaccine, PCV, and MMR vaccine at 12 months |
Recommended |
| Republic of Ireland42 | 2, 4, and 12 months | Plus DTaP/IPV/Hib/HepB vaccine, PCV, and oral rotavirus vaccine at 2 months Plus DTaP/IPV/Hib/HepB vaccine and oral rotavirus vaccine at 4 months Plus MMR vaccine at 12 months |
Recommended at 2 and 4 months |
| San Marino43 | 4, 7, and 12 months‡ | Given alone | No recommendations |
| Spain44–46 | 2, 4, and 12 months | Plus DTaP/IPV/Hib/HepB vaccine and PCV at 2 months Plus MenC, DTaP/IPV/Hib/HepB vaccine, and PCV at 4 months Plus MenC vaccine, and MMR vaccine at 12 months |
No recommendations |
| South Australia6 | 2, 4, and 12 months; Year 10 students (aged 15–16 y) | Plus DTaP/IPV/Hib/HepB vaccine, PCV, and oral rotavirus vaccine at 2 and 4 months Plus MenACWY, PCV, and MMR at 12 months Plus MenACWY in Year 10 students |
Recommended at 2, 4, and 12 months |
| UK47 | 2, 4, and 12 months | Plus DTaP/IPV/Hib/HepB vaccine and oral rotavirus vaccine at 2 months Plus DTaP/IPV/Hib/HepB vaccine at 4 months Plus Hib/MenC vaccine, PCV, and MMR vaccine at 12 months |
Recommended at 2 and 4 months |
*Differs by region. †Approved alternative schedule for the primary course (no prescription required): 8 weeks, 4 months, and a booster at 12 months; additionally, 4CMenB is recommended and funded for people aged 13–25 y in specified close-living situations (i.e., boarding school hostels, tertiary education halls of residence, military barracks, or prisons). ‡Booster dose by the second year of life.
4CMenB, four-component meningococcal serogroup B vaccine; DTaP, diphtheria-tetanus-acellular pertussis; HepB, hepatitis B; Hib, Haemophilus influenzae type b vaccine; IPV, inactivated poliovirus vaccine; MenC, meningococcal serogroup C; MMR, measles-mumps-rubella; PCV, pneumococcal vaccine.
Although 4CMenB is not part of the NIP in Australia, it is funded in infants, toddlers, and adolescents as part of a regional program in South Australia, where the rate of MenB cases is high compared with the national rate.49,50 Co-administration of 4CMenB with several vaccines, including MenACWY, is approved in the above-mentioned European countries and Australia.8,50 Uniquely in the US, MenB vaccination is recommended in adolescents and young adults aged 16–23 y (preferred age 16–18 y) on the basis of shared clinical decision-making and in individuals aged ≥10 y who are at increased risk of MenB disease; these can be co-administered with other vaccines indicated for this age group using a different anatomic site, if feasible.9
Studies evaluating the co-administration of vaccines other than 4CMenB further support the notion that vaccine co-administration is an effective public health strategy. In a Kenyan study of 10,385 children aged 1–4 y, co-administration of recommended vaccines (e.g., DTP/Hib/HepB, PCV, and rotavirus vaccines) was associated with increased odds of timely vaccination and series completion.51 In a secondary analysis of 246 children in Quebec, failure to co-administer the recommended 18-month vaccines (i.e., DTaP/IPV/Hib and MMR) was negatively associated with being up to date with all vaccinations at 24 months.52
Real-world evidence showing no significant safety concerns associated with 4CMenB has been produced following vaccination campaigns for outbreak control in the US and France,53–55 as well as post-licensure surveillance in Australia and Italy.56–58 Importantly, the real-world effectiveness of 4CMenB has been shown not only for NIPs currently recommending 4CMenB alone (Italy) but also for NIPs recommending co-administration of ≥1 dose of 4CMenB with other vaccines (England, Portugal, and Spain).59–62 During the first 5 y of vaccine availability in Portugal, children who developed IMD were less likely to have received 4CMenB vaccination compared with matched controls who did not develop IMD, with an estimated vaccine effectiveness of 79% among children receiving ≥2 doses.60 In England, the 4CMenB program was associated with continued positive effect against MenB disease in children during the first 3 y, and the adjusted vaccine effectiveness among children who received three doses was 59%.59 In contrast, vaccination had no discernible effect on the oropharyngeal carriage of disease-causing meningococci, including serogroup B, among adolescents in South Australia.63
Importantly, there is evidence that co-administration may not always be implemented in real-world clinical practice, despite existing recommendations. This was demonstrated by a recent study reviewing medical records of over 6 million vaccinations in children in England between 2008 and 2018.64 In this study, 4CMenB was administered separately in 3% of first doses at 8 weeks of age (co-administration recommended with DTaP/IPV/Hib/HepB, PCV13, and rotavirus vaccine), 3% of second doses at 16 weeks of age (co-administration recommended with DTaP/IPV/Hib/HepB and PCV13), and 2% of booster doses at 1 year of age (co-administration recommended with Hib/MenC, PCV13, and MMR). In the US, vaccine coverage among those aged 17 y was 60.0% for ≥2 doses of MenACWY and 31.4% for ≥1 dose of 4CMenB in 2021.65 In a real-world study of health claims data from 2017 to 2020 reported overall low MenB vaccine series initiation rates among commercially insured and Medicaid-covered adolescents and young adults, despite the existing ACIP recommendations; notably, one of the factors associated with increased likelihood of MenB series initiation was co-administration of MenACWY.66 These findings might be relevant to other countries with similar immunization programs and highlight that there is room for improvement in clinical practice to maximize the known benefits of co‑administration.64
Attitudes toward and acceptance of vaccine co-administration are important aspects to consider. In a global survey that examined attitudes toward co-administration of infant vaccines, 42% of parent respondents noted that two vaccine injections were the maximum number they were comfortable with during a single medical visit, with less than one-third (28%) stating they were comfortable with whatever their doctor recommended.67 In the same survey, 83% of healthcare providers stated that they administered multiple vaccines per visit because they wanted to follow the official immunization schedule in their country; other reasons included a preference to vaccinate while the child was in the clinic (48%) and a fear that the child may not return to the clinic (36%).67 Collectively, these data highlight the importance of alleviating parent/caregiver concerns regarding the co-administration of multiple vaccines, with healthcare providers playing a key role in vaccine education and recommendations.
4CMenB appeared to be well accepted by parents in a large-scale pharmacovigilance study conducted in the UK recording suspected AEs using data from the Yellow Card Scheme and the Clinical Practice Research Datalink over a 20-month surveillance period, with compliance with subsequent doses seemingly not adversely affected by any anticipated reactogenicity.28 Effective communication regarding management of the increased mild and transient reactogenicity associated with co-administration will be important in improving acceptance. Indeed, the reviewed evidence indicates that the rate of mild and transient fever is generally increased with 4CMenB co-administration in infants compared with 4CMenB alone, which is consistent with the European label for 4CMenB.8 However, evidence from clinical and real-world studies has shown that fever can be effectively managed with prophylactic paracetamol.21,25 From a public health perspective, it is important that any mild and transient reactogenicity associated with co-administration is weighed against the known benefits, such as improved timeliness of vaccination and increased vaccination coverage.12 Indeed, in the UK, it is now recommended that infants receive three doses of prophylactic paracetamol after co-administration of 4CMenB with routine vaccinations at age 2 and 4 months.2,31,68 The use of prophylactic antipyretics for 4CMenB is also recommended in Andorra, the Czech Republic, Lithuania (only if co-administered), New Zealand, Portugal, the Republic of Ireland, and South Australia (Table 2).2,4–6,35,39,41,43 Evidence suggests that prophylactic paracetamol does not reduce immunogenicity of 4CMenB or the co-administered routine infant vaccines.21,68 Although prophylactic paracetamol may decrease the immunogenicity of other vaccines (e.g., PCV7, 10-valent pneumococcal conjugate vaccine, PCV13, and PHiD-CV), it is not known whether this has clinical relevance in real-world clinical practice.69,70
Although the incidence of IMD is highest in infants and young children, a secondary peak is observed during adolescence, a phenomenon that is attributed to age-related changes in social activities, which increase exposure to new strains.71 Most cases of IMD are caused by N. meningitidis serogroups A, B, C, W, Y, and X.72 Since an effective conjugate vaccine against all relevant strains is not yet available, administration of multiple vaccines that target different meningococcal serogroups is currently needed. Co-administration may be particularly important in adolescents owing to the low vaccine adherence observed in this age group.73 Although no studies evaluating the co-administration of 4CMenB with MenACWY vaccines in adolescents have been identified, a study that included adults and young adults aged 18–65 y showed that co-administration of MenACWY-CRM and 4CMenB was immunogenic with no safety concerns.22 Co-administration provides an opportunity to maximize vaccination uptake in adolescents and young adults, a group that has been typically difficult to target with vaccination campaigns,22 providing broad-spectrum protection against the most prevalent meningococcal serogroups. Additionally, the pentavalent MenABCWY vaccine currently in late-stage development has the potential to provide further public health benefits through broad IMD protection programs encompassing serogroups A, B, C, W, and Y.74,75
In conclusion, the published evidence collectively shows that co-administration of 4CMenB with other routine vaccines is not associated with safety concerns and does not interfere with the anticipated immune responses elicited by each vaccine. These data support the inclusion of 4CMenB co-administration in NIPs to help increase coverage of each co-administered vaccine. Additionally, these data encourage further research to extend national and international recommendations for 4CMenB co-administration to different age groups and vaccines, with the overarching goal of maximizing vaccination coverage and improving protection against IMD globally.
Acknowledgments
The authors would like to thank Daniela Toneatto (GSK) for the insights and review of this manuscript. Medical writing support was provided by Silvia Pregnolato of Apollo, OPEN Health Communications, and funded by GSK, in accordance with Good Publication Practice (GPP) guidelines (www.ismpp.org/gpp-2022).
Funding Statement
This article was funded by GSK.
Author contributions
All authors were involved in the planning, discussion, and interpretation of the data. All reviewed and revised the manuscript and approved the final manuscript as submitted.
Disclosure statement
VA and WS are employees of GSK and may hold stock or stock options. MH has received honoraria from AstraZeneca, Bavaria Nordic, GSK, MSD, Novartis Vaccines, Pfizer, and Sanofi as an investigator in vaccine clinical trials and a member of advisory boards and has participated in speaker forums for these companies. MS has received research grants and personal fees for advisory boards from GSK, Pfizer, and Sanofi.
References
- 1.Deghmane AE, Taha MK.. Product review on the IMD serogroup B vaccine Bexsero®. Hum Vaccin Immunother. 2022;18(1):2020043. doi: 10.1080/21645515.2021.2020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohn W-Y, Tahrat H, Novy P, Bekkat-Berkani R. Real-world implementation of 4-component meningococcal serogroup B vaccine (4CMenB): implications for clinical practices. Expert Rev Vaccines. 2022;21(3):325–13. doi: 10.1080/14760584.2022.2021881. [DOI] [PubMed] [Google Scholar]
- 3.Czech Vaccinology Society . Recommendations of the Czech Vaccinology Society of the J. E. Purkyně Czech Medical Association for Vaccination against Invasive Meningococcal Disease; 2020[accessed 2023 Feb] http://www.szu.cz/uploads/IMO/2020_Recommendation_vaccination_IMD.pdf.
- 4.New Zealand Immunisation Advisory Centre . MenB Bexsero quick facts . [accessed 2023. Apr] https://www.immune.org.nz/factsheets/menb-bexsero.
- 5.Ministry of Health New Zealand . Meningococcal disease . [accessed 2023. Apr] https://www.health.govt.nz/our-work/immunisation-handbook-2020/13-meningococcal-disease.
- 6.Government of South Australia . National immunisation program March 2023 South Australia schedule. [accessed 2023. Apr] https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/resources/national+immunisation+program+south+australia+schedule.
- 7.FDA , Bexsero prescribing information. [accessed 2021 Nov] https://www.fda.gov/media/90996/download.
- 8.EMA . Bexsero summary of product characteristics. [accessed 2021 Nov]. https://www.ema.europa.eu/en/documents/product-information/bexsero-epar-product-information_en.pdf.
- 9.Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, MacNeil JR. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69(9):1–41. doi: 10.15585/mmwr.rr6909a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulis G, Horn M, Borrow R, Basta NE. A comparison of national vaccination policies to prevent serogroup B meningococcal disease. Vaccine. 2022;40(26):3647–54. doi: 10.1016/j.vaccine.2022.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall GS, Abbing-Karahagopian V, Marshall HS, Cenci S, Conway JH, Occhipinti E, Bekkat-Berkani R, Banzhoff A, Sohn W-Y. A comprehensive review of clinical and real-world safety data for the four-component serogroup B meningococcal vaccine (4CMenB). Expert Rev Vaccines. 2023;22(1):530–44. doi: 10.1080/14760584.2023.2222015. [DOI] [PubMed] [Google Scholar]
- 12.Dolhain J, Janssens W, Dindore V, Mihalyi A. Infant vaccine co-administration: review of 18 years of experience with GSK’s hexavalent vaccine co-administered with routine childhood vaccines. Expert Rev Vaccines. 2020;19(5):419–43. doi: 10.1080/14760584.2020.1758560. [DOI] [PubMed] [Google Scholar]
- 13.Chiu NC, Huang LM, Willemsen A, Bhusal C, Arora AK, Reynoso Mojares Z, Toneatto D. Safety and immunogenicity of a meningococcal B recombinant vaccine when administered with routine vaccines to healthy infants in Taiwan: a phase 3, open-label, randomized study. Hum Vaccin Immunother. 2018;14(5):1075–83. doi: 10.1080/21645515.2018.1425659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis K, Valente Pinto M, Andrews NJ, Goldblatt D, Borrow R, Findlow H, Southern J, Partington J, Plested E, Patel S, et al. Immunogenicity of the UK group B meningococcal vaccine (4CMenB) schedule against groups B and C meningococcal strains (Sched3): outcomes of a multicentre, open-label, randomised controlled trial. Lancet Infect Dis. 2021;21(5):688–96. doi: 10.1016/S1473-3099(20)30600-9. [DOI] [PubMed] [Google Scholar]
- 15.Gossger N, Snape MD, Yu LM, Finn A, Bona G, Esposito S, Principi N, Diez-Domingo J, Sokal E, Becker B, et al. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA. 2012;307(6):573–82. doi: 10.1001/jama.2012.85. [DOI] [PubMed] [Google Scholar]
- 16.Macias Parra M, Gentile A, Vazquez Narvaez JA, Capdevila A, Minguez A, Carrascal M, Willemsen A, Bhusal C, Toneatto D. Immunogenicity and safety of the 4CMenB and MenACWY-CRM meningococcal vaccines administered concomitantly in infants: a phase 3b, randomized controlled trial. Vaccine. 2018;36(50):7609–17. doi: 10.1016/j.vaccine.2018.10.096. [DOI] [PubMed] [Google Scholar]
- 17.Safadi M, Martinon-Torres F, Weckx LY, Moreira EDJ, da Fonseca Lima EJ, Mensi I, Calabresi M, Toneatto D. Immunogenicity and safety of concomitant administration of meningococcal serogroup B (4CMenB) and serogroup C (MenC-CRM) vaccines in infants: a phase 3b, randomized controlled trial. Vaccine. 2017;35(16):2052–9. doi: 10.1016/j.vaccine.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Safadi M, Martinon-Torres F, Weckx LY, Moreira EDJ, da Fonseca Lima EJ, Willemsen A, Toneatto D, Habib MA, Borys D. Immunogenicity of the pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) administered concomitantly with the meningococcal serogroup B (4CMenB) vaccine in infants: a post-hoc analysis in a phase 3b, randomised, controlled trial. Vaccine. 2019;37(35):4858–63. doi: 10.1016/j.vaccine.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Vesikari T, Esposito S, Prymula R, Ypma E, Kohl I, Toneatto D, Dull P, Kimura A. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet. 2013;381(9869):825–35. doi: 10.1016/S0140-6736(12)61961-8. [DOI] [PubMed] [Google Scholar]
- 20.Zafack JG, Bureau A, Skowronski DM, De Serres G. Adverse events following immunisation with four-component meningococcal serogroup B vaccine (4CMenB): interaction with co-administration of routine infant vaccines and risk of recurrence in European randomised controlled trials. BMJ Open. 2019;9(5):e026953. doi: 10.1136/bmjopen-2018-026953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prymula R, Esposito S, Zuccotti GV, Xie F, Toneatto D, Kohl I, Dull PM. A phase 2 randomized controlled trial of a multicomponent meningococcal serogroup B vaccine (I). Hum Vaccin Immunother. 2014;10(7):1993–2004. doi: 10.4161/hv.28666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Findlow J, Bai X, Findlow H, Newton E, Kaczmarski E, Miller E, Borrow R. Safety and immunogenicity of a four-component meningococcal group B vaccine (4CMenB) and a quadrivalent meningococcal group ACWY conjugate vaccine administered concomitantly in healthy laboratory workers. Vaccine. 2015;33(29):3322–30. doi: 10.1016/j.vaccine.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Hall GC, Douglas I, Heath PT, Prabhakar P, Rosillon D, Khan J, Abbing-Karahagopian V. Post-licensure observational safety study after meningococcal B vaccine 4CMenB (Bexsero) vaccination within the routine UK immunisation program. Vaccine. 2021;39(24):3296–303. doi: 10.1016/j.vaccine.2021.02.065. [DOI] [PubMed] [Google Scholar]
- 24.Pillsbury A, Quinn H, Cashman P, Leeb A, Macartney K. Active SMS-based influenza vaccine safety surveillance in Australian children. Vaccine. 2017;35(51):7101–6. doi: 10.1016/j.vaccine.2017.10.091. [DOI] [PubMed] [Google Scholar]
- 25.De Serres G, Billard MN, Gariépy MC, Rouleau I, Toth E, Landry M, Boulianne N, Gagné H, Gilca V, Deceuninck G, et al. Short-term safety of 4CMenB vaccine during a mass meningococcal B vaccination campaign in Quebec, Canada. Vaccine. 2018;36(52):8039–46. doi: 10.1016/j.vaccine.2018.10.095. [DOI] [PubMed] [Google Scholar]
- 26.Alderfer J, Srivastava A, Isturiz R, Burman C, Absalon J, Beeslaar J, Perez J. Concomitant administration of meningococcal vaccines with other vaccines in adolescents and adults: a review of available evidence. Hum Vaccin Immunother. 2019;15(9):2205–16. doi: 10.1080/21645515.2019.1581542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Ryan M, Stoddard J, Toneatto D, Wassil J, Dull PM. A multi-component meningococcal serogroup B vaccine (4CMenB): the clinical development program. Drugs. 2014;74(1):15–30. doi: 10.1007/s40265-013-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryan P, Seabroke S, Wong J, Donegan K, Webb E, Goldsmith C, Vipond C, Feavers I. Safety of multicomponent meningococcal group B vaccine (4CMenB) in routine infant immunisation in the UK: a prospective surveillance study. Lancet Child Adolesc Health. 2018;6:395–403. doi: 10.1016/S2352-4642(18)30103-2. [DOI] [PubMed] [Google Scholar]
- 29.Esposito S, Prymula R, Zuccotti GV, Xie F, Barone M, Dull PM, Toneatto D. A phase 2 randomized controlled trial of a multicomponent meningococcal serogroup B vaccine, 4CMenB, in infants (II). Hum Vaccin Immunother. 2014;10(7):2005–14. doi: 10.4161/hv.29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubus M, Ladhani S, Vasu V. Prophylactic paracetamol after meningococcal B vaccination reduces postvaccination fever and septic screens in hospitalized preterm infants. Pediatr Infect Dis J. 2020;39(1):78–80. doi: 10.1097/INF.0000000000002507. [DOI] [PubMed] [Google Scholar]
- 31.Joint Committee on Vaccination and Immunisation (JCVI) . JCVI position statement on use of BEXSERO® meningococcal B vaccine in the UK ; 2014. [accessed 2022 Feb] https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/294245/JCVI_Statement_on_MenB.pdf.
- 32.Isitt C, Cosgrove CA, Ramsay ME, Ladhani SN. Success of 4CMenB in preventing meningococcal disease: evidence from real-world experience. Arch Dis Child. 2020;105(8):784–90. doi: 10.1136/archdischild-2019-318047. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization . Defeating meningitis by 2030; 2020. [accessed 2023 Apr] https://www.who.int/initiatives/defeating-meningitis-by-2030.
- 34.Government of Andorra . Calendari de vacunacions; 2019. [accessed 2023 Apr] https://www.salut.ad/images/stories/Salut/pdfs/temes_salut/Calendari_vacunacions.pdf.
- 35.Ministry of Health Andorra . Recomanacions sobre l’administració de la vacunació enfront sel meningococ B (Bexsero) ; 2016. [accessed 2023 Apr] http://www.salut.ad/images/stories/Salut/pdfs/temes_salut/Recomanacions_Vacuna_Bexero.pdf.
- 36.Vomáčka J. Vaccination against infectious diseases in the Czech Republic. [accessed 2023 Apr] http://www.mudrvomacka.cz/index.php?option=com_content&view=article&id=8&Itemid=152&lang=en.
- 37.Ministry of Health France . Calendrier des vaccinations et recommandations vaccinales 2022 ; 2022. [accessed 2023 Mar]https://sante.gouv.fr/IMG/pdf/calendrier_vaccinal_2022_mis_a_jour_juin_2022_v2.pdf.
- 38.Ministry of Health Italy . Piano nazionale prevenzione vaccinale 2017–2019 ; 2017. [accessed 2023 Apr] http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf.
- 39.Ministry of Health Republic of Lithuania . Dėl lietuvos respublikos sveikatos apsaugos ministro. 2015 m. BIRŽELIO 12 d. įsakymo nr. V-757 „DĖL Lietuvos respublikos vaikų profilaktinių skiepijimų kalendoriaus patvirtinimo“pakeitimo ; 2015. [accessed 2023 Apr] https://eseimas.lrs.lt/portal/legalAct/lt/TAD/f4a925d0f50f11e79a1bc86190c2f01a?positionInSearchResults=0&searchModelUUID=1561434a-b283-4be2-87f5-4f556ad37c32.
- 40.Government of Malta . National immunisation schedule. [accessed 2023 Apr] https://deputyprimeminister.gov.mt/en/phc/pchyhi/Pages/National-Immunisation-Schedule.aspx#.
- 41.Comissão de Vacinas da Sociedade de Infeciologia Pediátrica da Sociedade Portuguesa de Pediatria . Recomendações sobre vacinas extra programa nacional de vacinação 2020 ; 2020. [accessed 2023 Apr] https://www.spp.pt/UserFiles/file/Seccao_Infecciologia/recomendacoes%20vacinas_sip_final_28set_2.pdf.
- 42.Ministry of Health Portugal . Aprova o novo esquema vacinal do Programa Nacional de Vacinação (PNV), revogando, com exceção do seu n. 6, o Despacho n.° 10441/2016, de 9 de agosto ; 2019. [accessed 2023 Apr] https://dre.pt/application/conteudo/127608823.
- 43.Republic of Ireland Health Service Executive . Immunisation schedule ; 2020. [accessed 2023 Apr] https://www.hse.ie/eng/health/immunisation/pubinfo/pcischedule/immschedule/.
- 44.Institute for Social Security Republic of San Marino . Vaccinazioni pediatriche raccomandate: calendario vaccinale; 2017. [accessed 2023 Apr] https://www.iss.sm/on-line/home/vaccini-e-vaccinazioni/vaccinazioni-raccomandate.html.
- 45.Sistema Nacional de Salud . Consejo Interritorial. Calendario común de vacunación a lo largo de toda la vida. Calendario recomendado año 2023. [accessed 2023 Mar] https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/calendario-y-coberturas/docs/CalendarioVacunacion_Todalavida.pdf .
- 46.Ministry of Health Spain . Recomendaciones de vacunación frente a la enfermedad meningocócica invasiva por serogrupo B ; 2022. [accessed 2023 Apr] https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/comoTrabajamos/docs/MenB_2022.pdf.
- 47.Asociación Española de Pediatría (AEP) . Calendario de immunizaciones AEP 2023. [accessed 2023 Apr] https://vacunasaep.org/profesionales/calendario-de-inmunizaciones-de-la-aep-2023.
- 48.UK Health Security Agency . The complete routine immunisation schedule from February 2022 ; 2022. [accessed 2023 Apr] https://www.gov.uk/government/publications/the-complete-routine-immunisation-schedule/the-complete-routine-immunisation-schedule-from-february-2022.
- 49.Marshall HS, Lally N, Flood L, Phillips P. First statewide meningococcal B vaccine program in infants, children and adolescents: evidence for implementation in South Australia. Med J Aust. 2020;212(2):89–93. doi: 10.5694/mja2.50481. [DOI] [PubMed] [Google Scholar]
- 50.Australian Technical Advisory Group on Immunisation (ATAGI) . Australian immunisation handbook. Canberra: Care AGDoHaA. [accessed 2022 Feb] https://immunisationhandbook.health.gov.au/. [Google Scholar]
- 51.Masters NB, Wagner AL, Carlson BF, Boulton ML. Vaccination timeliness and co-administration among Kenyan children. Vaccine. 2018;36(11):1353–60. doi: 10.1016/j.vaccine.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 52.O’Donnell S, Dubé E, Tapiero B, Gagneur A, Doll MK, Quach C. Determinants of under-immunization and cumulative time spent under-immunized in a Quebec cohort. Vaccine. 2017;35(43):5924–31. doi: 10.1016/j.vaccine.2017.08.072. [DOI] [PubMed] [Google Scholar]
- 53.McNamara LA, Shumate AM, Johnsen P, MacNeil JR, Patel M, Bhavsar T, Cohn AC, Dinitz-Sklar J, Duffy J, Finnie J, et al. First use of a serogroup B meningococcal vaccine in the US in response to a university outbreak. Pediatrics. 2015;135(5):798–804. doi: 10.1542/peds.2014-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watson PS, Turner DP. Clinical experience with the meningococcal B vaccine, Bexsero®: prospects for reducing the burden of meningococcal serogroup B disease. Vaccine. 2016;34(7):875–80. doi: 10.1016/j.vaccine.2015.11.057. [DOI] [PubMed] [Google Scholar]
- 55.Thabuis A, Tararbit K, Taha MK, Dejour-Salamanca D, Ronin V, Parent du Chatelet I, Spaccaferri G. Community outbreak of serogroup B invasive meningococcal disease in Beaujolais, France, February to June 2016: from alert to targeted vaccination. Euro Surveill. 2018;23(28):1700590. doi: 10.2807/1560-7917.ES.2018.23.28.1700590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Australia DoHaAC . Bexsero meningococcal B vaccine. Final update - monitoring finds no new or unexpected safety issues ; 2016. [accessed 2023 Apr] https://www.tga.gov.au/news/safety-alerts/bexsero-meningococcal-b-vaccine.
- 57.Italian Medicines Agency . Rapporto vaccini 2017. La sorveglianza post-marketing in Italia ; 2017. [accessed 2023 Apr] www.aifa.gov.it/sites/default/files/Rapp_Vaccini_2017_0.pdf.
- 58.Marshall HS, Koehler AP, Wang B, A’Houre M, Gold M, Quinn H, Crawford N, Pratt N, Sullivan TR, Macartney K, et al. Safety of meningococcal B vaccine (4CMenB) in adolescents in Australia. Vaccine. 2020;38(37):5914–22. doi: 10.1016/j.vaccine.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 59.Ladhani SN, Andrews N, Parikh SR, Campbell H, White J, Edelstein M, Bai X, Lucidarme J, Borrow R, Ramsay ME, et al. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N Engl J Med. 2020;382(4):309–17. doi: 10.1056/NEJMoa1901229. [DOI] [PubMed] [Google Scholar]
- 60.Rodrigues FMP, Marlow R, Simões MJ, Danon L, Ladhani S, Finn A. Association of use of a meningococcus group B vaccine with group B invasive meningococcal disease among children in Portugal. JAMA. 2020;324(21):2187–94. doi: 10.1001/jama.2020.20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Azzari C, Moriondo M, Nieddu F, Guarnieri V, Lodi L, Canessa C, Indolfi G, Giovannini M, Napoletano G, Russo F, et al. Effectiveness and impact of the 4CMenB vaccine against group B meningococcal disease in two Italian regions using different vaccination schedules: a five-year retrospective observational study (2014–2018). Vaccines (Basel). 2020;8(3):469. doi: 10.3390/vaccines8030469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castilla J, García Cenoz M, Abad R, Sánchez-Cambronero L, Lorusso N, Izquierdo C, Cañellas Llabrés S, Roig J, Malvar A, González Carril F, et al. Effectiveness of a meningococcal group B vaccine (4CMenB) in children. N Engl J Med. 2023;388(5):427–38. doi: 10.1056/NEJMoa2206433. [DOI] [PubMed] [Google Scholar]
- 63.Marshall HS, McMillan M, Koehler AP, Lawrence A, Sullivan TR, MacLennan JM, Maiden MCJ, Ladhani SN, Ramsay ME, Trotter C, et al. Meningococcal B vaccine and meningococcal carriage in adolescents in Australia. N Engl J Med. 2020;382(4):318–27. doi: 10.1056/NEJMoa1900236. [DOI] [PubMed] [Google Scholar]
- 64.Bauwens J, de Lusignan S, Sherlock J, Ferreira F, Künzli N, Bonhoeffer J. Co-administration of routine paediatric vaccines in England often deviates from the immunisation schedule. VaccineX. 2021;9:100115. doi: 10.1016/j.jvacx.2021.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pingali C, Yankey D, Elam-Evans LD, Markowitz LE, Valier MR, Fredua B, Crowe SJ, Stokley S, Singleton JA. National vaccination coverage among adolescents aged 13–17 years — National Immunization Survey-Teen, United States, 2021. Morb Mortal Wkly Rep. 2022;71(35):1101–8. doi: 10.15585/mmwr.mm7135a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Packnett ER, Zimmerman NM, Kim G, Novy P, Morgan LC, Chime N, Ghaswalla P. A real-world claims data analysis of meningococcal serogroup B vaccine series completion and potential missed opportunities in the United States. Pediatr Infect Dis J. 2022;41(4):e158–e65. doi: 10.1097/INF.0000000000003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bakhache P, Rodrigo C, Davie S, Ahuja A, Sudovar B, Crudup T, Rose M. Health care providers’ and parents’ attitudes toward administration of new infant vaccines—a multinational survey. Eur J Pediatr. 2013;172(4):485–92. doi: 10.1007/s00431-012-1904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ladhani SN, Riordan A. The yin and yang of fever after meningococcal B vaccination. Arch Dis Child. 2017;102(10):881–2. doi: 10.1136/archdischild-2017-313419. [DOI] [PubMed] [Google Scholar]
- 69.Koufoglou E, Kourlaba G, Michos A. Effect of prophylactic administration of antipyretics on the immune response to pneumococcal conjugate vaccines in children: a systematic review. Pneumonia (Nathan). 2021;13(1):7. doi: 10.1186/s41479-021-00085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Falup-Pecurariu O, Man SC, Neamtu ML, Chicin G, Baciu G, Pitic C, Cara AC, Neculau AE, Burlea M, Brinza IL, et al. Effects of prophylactic ibuprofen and paracetamol administration on the immunogenicity and reactogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugated vaccine (PHiD-CV) co-administered with DTPa-combined vaccines in children: an open-label, randomized, controlled, non-inferiority trial. Hum Vaccin Immunother. 2017;13(3):649–60. doi: 10.1080/21645515.2016.1223001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muse D, Christensen S, Bhuyan P, Absalon J, Eiden JJ, Jones TR, York LJ, Jansen KU, O’Neill RE, Harris SL, et al. A phase 2, randomized, active-controlled, observer-blinded study to assess the immunogenicity, tolerability and safety of bivalent rLP2086, a meningococcal serogroup B vaccine, coadministered with tetanus, diphtheria and acellular pertussis vaccine and serogroup A, C, Y and W-135 meningococcal conjugate vaccine in healthy US adolescents. Pediatr Infect Dis J. 2016;35(6):673–82. doi: 10.1097/INF.0000000000001124. [DOI] [PubMed] [Google Scholar]
- 72.Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Eskola J, Fermon F, Klugman KP, Ramsay M, Sow S, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11(1):17. doi: 10.1186/1478-7954-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Niccolai LM, Hansen CE. Suboptimal uptake of meningococcal vaccines among older adolescents: barriers, solutions, and future research directions. Hum Vaccin Immunother. 2020;16(12):3208–12. doi: 10.1080/21645515.2020.1754052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.ClinicalTrials.gov. NCT04502693 . Effectiveness of GlaxoSmithKline Biologicals S.A's (GSK's) meningococcal group B and combined ABCWY vaccines in healthy adolescents and young adults. [accessed 2023 Apr] https://classic.clinicaltrials.gov/ct2/show/NCT04502693 .
- 75.Bekkat-Berkani R, Fragapane E, Preiss S, Rappuoli R, Sohn WY, Soumahoro L, Vadivelu K. Public health perspective of a pentavalent meningococcal vaccine combining antigens of MenACWY-CRM and 4CMenB. J Infect. 2022;85(5):481–91. doi: 10.1016/j.jinf.2022.09.001. [DOI] [PubMed] [Google Scholar]


