Abstract
Midlife women experience changes in cardiometabolic, physical, and psychosocial health during menopause that negatively impacts their overall quality of life. Factors that contribute to these increases in cardiometabolic risk include weight gain as well as increases in fat mass (particularly abdominal adiposity), insulin resistance, and vascular dysfunction. Other deleterious changes in physical health (e. g. reduced sleep health, bone density, and balance) as well as changes in psychosocial health (e. g. mood, anxiety, and depression) often coincide and are linked to these increases in cardiometabolic risk. Physical activity and exercise are important lifestyle components that have been demonstrated to improve cardiometabolic, physical, and psychosocial health, yet physical activity and exercise is known to decline during perimenopause and into the post-menopausal years. In this narrative review, we summarize these changes in overall health during menopause as well as how declining physical activity contributes to these changes. Additionally, we discuss how incorporating physical activity and exercise during menopause can potentially ameliorate health declines. We conclude that there exists a significant, positive impact of physical activity on cardiometabolic, physical, and psychological health among midlife women, particularly if undertaken during the perimenopausal and postmenopausal years.
Keywords: menopause, physical activity, exercise, metabolism, adiposity, insulin resistance
Introduction
Menopause is a significant turning point where women experience accelerated cardiometabolic risk, as well as declines in physical and psychosocial health [1]. During the menopause transition, women experience dramatic decreases in circulating estrogens-particularly estradiol (E2)-and increases in the gonadotropin, follicle-stimulating hormone [2]. These hormonal changes are associated with increases in body weight and fat mass (mainly abdominal adiposity), as well as other cardiometabolic risk factors including dyslipidemia, hypertension, vascular dysfunction, insulin resistance, and type 2 diabetes [1, 3–5]. Additionally, declines in physical health (e. g. reduced sleep health, bone density, and balance) and psychosocial health (e. g. increased mood swings, anxiety, and depressive episodes) also become more prevalent with the menopause transition [6–8].
It is well recognized that physical activity (PA) and planned exercise can improve cardiometabolic, physical, and psychosocial health in humans. As a result, PA and exercise promotion during menopause would appear to improve cardiovascular health, accelerate metabolism, minimize weight gain, preserve bone density and balance, as well as improve mood and reduce depressive symptoms across the lifespan (Fig. 1). As a result, PA arguably becomes most important during the menopause transition and into the post-menopausal years. This narrative review details how menopause is associated with reductions in PA and the negative implications that reduced PA has on overall health. We also discuss the degree to which PA and exercise promotion can improve cardiometabolic, physical, and psychosocial health during menopause [9].
Fig. 1.
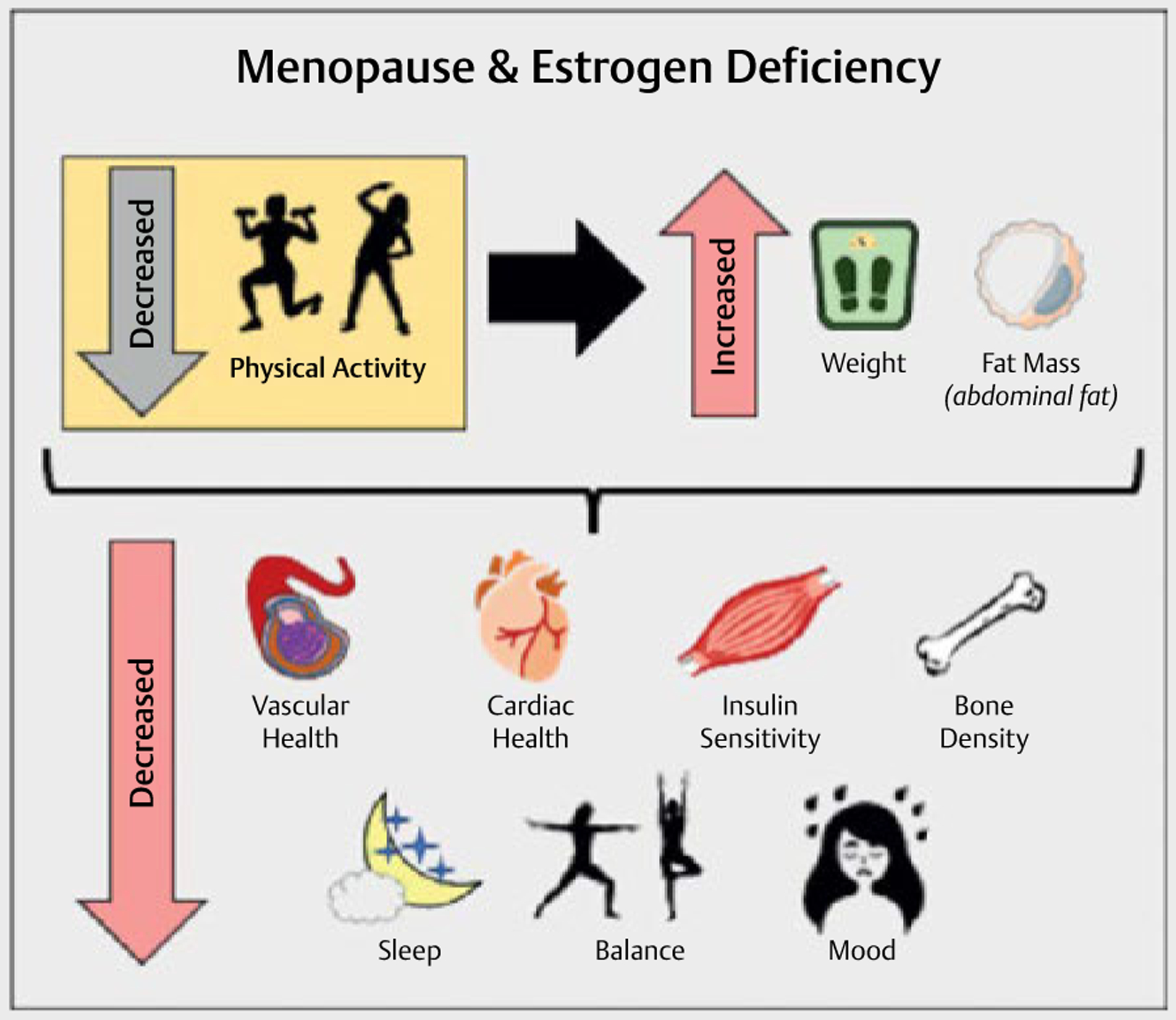
Menopause and Estrogen Deficiency Outcomes.
The Menopause Transition – A Brief Overview
The menopause transition (or perimenopause) is characterized by an increased rate of attrition of ovarian follicles, thereby causing reduced inhibin-B release from the ovaries and signals the anterior pituitary to upregulate follicle-stimulating hormone (FSH) secretion. This increased secretion of FSH works to stimulate the production of E2 from the ovaries, with FSH continuously increasing as ovarian function declines. As women transition through perimenopause (from early to late perimenopause [10]), the ovarian follicle supply becomes critically low whereby the ovary can no longer produce E2 in respond to the increased FSH. Once E2 production ceases, only a steady-state of high FSH remains – thereby becoming an important diagnostic tool when differentiating between early versus late phase perimenopause [10]. Early perimenopause is marked by increases in menstrual cycle length of ≥ 7 days with some women reporting small but noticeable increases in hallmark menopausal symptoms, such as vasomotor symptoms (e. g. hot flashes and night sweats). Late perimenopause is characterized by intervals of amenorrhea for > 60 days and the hallmark elevation of FSH > 25 mIU/mL paired with increased prevalence of menopausal symptoms. Natural menopause is defined by 12 months of amenorrhea in women traditionally aged 45 or older [11]. Furthermore, the median age of natural menopause is approximately 51 to 52 years, with an approximate 95 % confidence interval of 45 to 55 years [12, 13]. While the length of the menopause transition typically ranges from 4 to 5 years, the length of the transition is highly variable and can last from < 1 to 10 years or longer [14].
Changes in Cardiometabolic Health and the Relationship with Physical Activity
In this section, we detail how deleterious changes in cardiometabolic health – particularly weight gain, and changes in body composition, insulin sensitivity, and vascular health – are associated with the declines in PA during menopause. Furthermore, we detail how PA interventions positively impact cardiometabolic health in menopausal women.
Weight gain and body composition
Weight gain and the accrual of abdominal adiposity are two of the most common frustrations reported by women during the menopause transition [3, 15–17]. Data from the 4-year Healthy Transitions study [3], the ongoing SWAN longitudinal study [15], the 6-year Pizarra Study [16], and the 8-year Australian Longitudinal Study on Women’s Health [17] reveal that detrimental changes in body composition (i. e. increased fat mass, decreased fat-free mass, and decreased bone density) occur and exacerbate cardiometabolic risk. These menopause-related changes in body composition coincide with significant declines in PA [3, 18, 19]. Longitudinal data from the SWAN study also revealed that higher levels of vigorous PA were independently associated with lower percent body fat and smaller waist circumference, particularly among White women [20].
Experimental studies investigating the benefits of PA and exercise on weight loss and body composition tend to focus predominantly on postmenopausal women rather than also including perimenopausal women who are currently navigating the transition [9]. Data from a randomized control trial in 173 overweight, post-menopausal women revealed a significant dose-response relationship between exercise duration (i. e. treadmill walking or stationary bicycling for at least 45-min of moderate-intensity sessions, 5 days/week for 12 months) and losses in total body weight, fat mass, and intra-abdominal fat [21]. Other studies in postmenopausal women have examined whether exercise and/or caloric restriction is most effective at eliciting weight loss, particularly in the abdominal depot. The Sex Hormones and Physical Exercise (SHAPE)-2 study investigated differences in weight loss among overweight, postmenopausal women randomized to either calorie restriction plus exercise, calorie restriction alone, or control [22]. In this trial, the exercise program included both aerobic and resistance training 4 hours/week with a target of 350 kcal/day in exercise energy expenditure. Data from SHAPE-2 revealed that subcutaneous fat loss was larger in the calorie restriction plus exercise group with no differences in intra-abdominal fat loss [22]. In the Diet, Exercise, and Metabolism for Older Women Study, 60 overweight, postmenopausal women aged 45 to 80 years were randomized to either a reduced calorie diet or a reduced calorie diet plus aerobic exercise for 6 months [23]. While both groups lost similar amounts of weight (~8 %) with similar losses in subcutaneous abdominal and gluteal fat, only those women in the reduced calorie diet plus aerobic exercise maintained fat-free (muscle) mass (FFM) [23]. In a separate study, it appeared that postmenopausal women are equally capable of losing weight and reducing android fat when participating in a 3-month high-intensity exercise training program compared to premenopausal women [24]. Furthermore, data from the Women’s Healthy Lifestyle Project, a 4.5-year randomized clinical trial using long-term, dietary restriction (1,300 kcal/day) and increased PA (1,000 to 1,500 kcal/week) in menopausal women revealed that waist circumference and fat mass were reduced, yet FFM was maintained [25]. Nonetheless, while habitual PA and exercise indeed have positive effects on preventing body weight and fat mass gain during menopause, regular PA does not completely ameliorate the changes in body composition that occur [26, 27]. While not detailed extensively in this narrative review, habitual PA and exercise have a significant and positive effect on cardiorespiratory fitness and muscle strength [28], which both decline with menopause progression [29]. In summary, adding PA or exercise to any reduced calorie diet or weight loss program during menopause appears to preserve FFM or reduce the amount of FFM that is lost [30], thereby helping preserve both resting (basal) and 24-h metabolic rate [31, 32].
Insulin sensitivity
In premenopausal women, estrogens (particularly E2) exemplify a cardioprotective effect by promoting both insulin sensitivity, as well as peripheral (versus central) fat distribution [33–35]. The loss of E2 across the menopause transition places women at greater risk of developing type 2 diabetes. This is due, in part, to the E2-mediated interaction of abdominal (central) adiposity and insulin resistance. Indeed, postmenopausal women taking estrogen-based hormone therapy are less likely to develop insulin resistance and type 2 diabetes [36, 37].
Chronic exercise training and higher levels of PA are well-established lifestyle behaviors that improve insulin sensitivity and systemic glucose metabolism [38–40]. In adults with obesity and type 2 diabetes, exercise training (both aerobic and resistance) enhances the actions of insulin and non-insulin glucose transportation into the skeletal muscle [41–43]. In one cross-sectional study, postmenopausal women who regularly engaged in high levels of exercise had significantly higher insulin sensitivity compared to postmenopausal women who are sedentary, regardless of hormone therapy status (none, estrogen plus progesterone, or estrogen only) [44]. Data from this study also revealed that hormone therapy usage may be associated with lower plasma insulin concentrations, but an attenuation in improved insulin sensitivity [44]. The reason for this paradoxical attenuated improvement in insulin sensitivity is still not well understood.
Data from randomized controlled trials in postmenopausal women reveal reduced fasting insulin concentrations and HOMA-IR (a surrogate measure of insulin resistance) following 3 to 4 months of an aerobic exercise intervention [45, 46]. Longer interventions (6–12 months) do not appear to demonstrate the same degree of efficacy when there is no additional weight loss intervention (e. g. calorie restriction) [47, 48]. Combining aerobic and resistance exercise to a training program does appear to yield the most improvement in insulin sensitivity in menopausal women. Specifically, a 6-month exercise trial that introduced aerobic, resistance, and/or combined (aerobic plus resistance) exercise training to perimenopausal and postmenopausal women with overweight and obesity demonstrated improvements in insulin sensitivity with combined aerobic and resistance or aerobic exercise alone but not resistance exercise alone [49]. In both the combined and the aerobic only exercise group, but not the resistance group, improvements in body composition (reduced fat mass, increased FFM) were also observed [49]. Combined aerobic and resistance training for 9 months also reduced HbA1c in older menopausal aged women more than aerobic or resistance training alone [50].
Vascular health
The aging vascular system is associated with the expansion of the extracellular matrix (increased fibrosis), and immune cell infiltration into the endothelium [51]. These biochemical and molecular changes increase arterial stiffness and cause endothelial dysfunction. In premenopausal women, estrogen abundance increases nitric oxide (NO) production via multiple mechanisms [52, 53]. Estrogen increases gene expression of endothelial nitric oxide synthase (eNOS) and prevents smooth muscle proliferation and inhibits fibrosis while increasing elastin, resulting in vascular compliance [54, 55]. These mechanistic animal studies demonstrate the importance of estrogen in NO bioavailability and the implications of the loss of estrogen (or E2). This work also translates to human menopause as the loss of estrogen coincides with similarly lower levels of endothelial nitric oxide production (detected by reduced flow-mediated dilation, FMD) [54, 56]. Previous data has demonstrated that a decline in FMD, as well as decreased carotid artery compliance (detected by increased pulse wave velocity) accelerates during perimenopause and progressively worsens into the postmenopausal years [57]. Chronic vascular dysfunction can also lead to pathophysiological changes to the heart. For example, arterial stiffening can increase both systolic and pulse pressures, as well as create aortic impedance (via increased resistance on left ventricular ejection, thereby causing increased afterload and left ventricular hypertrophy) [58]. Evidence supporting the importance of estrogens comes from studies of OVX mice that develop left ventricular hypertrophy, which was rescued with E2 replacement [59, 60]. Furthermore, women in heart failure are more likely to have heart failure with preserved ejection fraction (HFpEF) than men, which often has better outcomes than heart failure with reduced ejection fraction (HFrEF) [61, 62]. In experimental models of postmenopausal heart failure, estrogen treatment appears to increase NO bioavailability and improve vascular health [63].
Aerobic exercise can increase NO bioavailability and FMD [64]. As expected, exercise interventions in postmenopausal women with overweight and obesity improved endothelial function [65, 66], and these improvements occur across multiple exercise intensities [67]. However, some studies among older postmenopausal women undergoing aerobic exercise training do not find these same improvements in vascular function (FMD and microvascular blood flow) [68, 69]. Whether the prevalence of women taking estrogen-based hormone therapy in the comparator (control) groups mediated this lack of significant improvement in endothelial health in the aerobic exercise groups remains unclear [68, 69]. Given menopause is associated with increased oxidative stress, which promotes eNOS uncoupling and reduced NO bioavailability, it is possible that the loss of exercise-mediated benefit in endothelial function is primarily the result of E2-deficiency in the postmenopausal state. Indeed, elegant studies from Moreau et al. highlight estrogen therapy as a positive regulator of exercise adaptions to the vascular endothelium [70]. Unfortunately, exercise intolerance and reduced cardiorespiratory fitness is a hallmark of HFpEF, thereby limiting the ability of menopausal women to engage in PA in the first place [71].
Changes in Physical Health and the Relationship with Physical Activity
Here, we summarize how changes in sleep health and bone health are related to PA level during menopause. Specifically, poor sleep health and reduced bone density are two of the most common physical changes during menopause that women experience and that can impact overall health.
Sleep health
Sleep disturbances (i. e. trouble falling asleep, early morning waking, interrupted sleep) become more prevalent in women around the menopause transition and coincide particularly with the decline of E2 and progesterone [6]. Women experiencing vasomotor symptoms (e. g. hot flashes and night sweats) – arguably the most common menopausal symptom – tend to report higher levels of sleep disturbances and are at the greatest risk for clinical insomnia [72]. Poor sleep is linked to reduced overall quality of life, as well as increased dietary intake and consumption of high-fat and high-sugar foods, reduced PA, weight gain, and obesity risk [73]. It is also well recognized that insufficient or poor sleep quality can lead to lower levels of PA the following day [74]. Given more than 50 % of perimenopausal and postmenopausal women report sleep disturbances regardless of vasomotor symptom presence [75], reduced PA following a night of poor sleep and resulting fatigue is often inevitable.
Data from the SWAN longitudinal study revealed that women aged 54 to 63 years who had greater levels of habitual and recreational PA reported having more favorable sleep characteristics, including better sleep quality and fewer nightly awakenings [76, 77]. Cross-sectional studies in physically active menopausal aged women (45 + years) also report better sleep quality and fewer sleep disturbances [78]. Indeed, the association between increased PA and better sleep health has been largely confirmed by randomized controlled trials. Data from the Dose-Response to Exercise in Post-menopausal Women (DREW) study revealed that postmenopausal women randomized to all three designated exercise groups (meeting 50 %, 100 %, or 150 % of the National Institutes of Health (NIH) physical activity recommendations) had dose-response improvements in sleep quality compared to postmenopausal control [79]. Data from a 6-month Finnish study [80], as well as the 3-month Menopause Strategies: Finding Lasting Answers for Symptoms and Health (MsFLASH) trials [81, 82] further demonstrated that moderate-intensity aerobic exercise and/or yoga also improved sleep quality. Even walking can improve sleep health in women aged 45 to 65 years, as demonstrated by significant improvements in sleep symptoms after partaking in a 24-week home-based moderate intensity walking program (compared to women of similar age who did not undertake the walking program) [83]. In summary, the link between sleep and PA appears bidirectional in nature given that poor sleep can result in reduced PA and, conversely, that increased relative PA is linked to better sleep quality.
Bone health
The SWAN longitudinal study has significantly contributed to our understanding about alterations in bone health across the menopause transition. These data demonstrate that bone mineral density declines at the greatest rate beginning 1-year before the final menstrual period and decelerates 2 years after the final menstrual period at both the lumbar spine and femoral neck sites [7]. A separate longitudinal study conducted found that bone loss began slightly earlier (~2 to 3 years before the final menstrual period) and decelerated later (ended 3 to 4 years afterwards), with spine, total body bone mineral, and femoral neck declining by 10.5, 7.7, and 5.3 %, respectively [84]. There are racial and ethnic differences in bone size, geometry, and fracture rates during the menopause transition (discussed in more detail here [85]). Indeed, preventing bone loss during menopause is inherently critical given the declines in E2 contribute to bone fractures [86] and that history of fracture is associated with higher subsequent fractures, morbidity, and mortality [87].
The evidence that weight-bearing PA (including aerobic walking and running, as well as resistance training) improves bone health comes from a large number of meta-analyses in older women demonstrating improvements in lumbar spine bone density rather than femoral neck bone density [88–91]. Another meta-analysis of walking for preservation of bone density in postmenopausal women looked at randomized and non-randomized controlled trials and concluded that regular walking had no significant effect on preservation of bone density at the spine in postmenopausal women, while significant positive effects were evident at the femoral neck [92]. Increased PA is important for bone health during menopause, but caution is needed when recommending increased PA and/or structured exercise-especially among women with already compromised bone health. Indeed, midlife women undertaking a dietary weight loss program may experience an accelerated loss of bone strength and bone density [93, 94]; therefore, adding in PA or exercise to any weight loss program is important [95]. Medical professionals should consider prescribing resistance training exercises (versus endurance only) alongside any dietary weight loss program to limit reductions in bone density and bone strength [93, 95].
One component of physical fitness that is often overlooked among older adults but that could reduce the risk of bone fractures is the promotion of balance-related activities into activities of daily living. Cross-sectional data during the menopause transition reveal clear declines in postural stability/balance, as well as declines in other functional performance tests (which inherently require balance) [96, 97] that coincide with reduced bone health. Indeed, loss of balance is also associated with concurrent loss of muscle and bone mass, increased fat mass, and other physiological changes that commonly occur during the menopausal transition [15, 98–100]. Regular engagement in balance activities (e. g. yoga, tai chi, and Pilates [101]) is a core component of meeting United States PA Guidelines for American among older adults (especially postmenopausal women) [102]. Participating in these balance activities have the potential to improve physical function and reduce the risk of bone fractures, as indicated by previous lifestyle interventions [103].
Changes in Psychosocial Health and the Relationship with Physical Activity
Data from the SWAN longitudinal study has provided important insight into changes in mood and mental health around the menopause transition. In the SWAN Mental Health ancillary study, perimenopausal and postmenopausal women tended to experience higher levels of psychological stress, depression, and anxiety compared to premenopausal women [8]. Data from SWAN also suggest that women with the greatest increases in depressive symptoms across the menopause transition had lower levels of PA, increased sleep disturbances, and a decrease in social support [104]. Furthermore, Black women appear to be less likely, whereas Hispanic women are more likely, to experience depressive symptoms over midlife [104]. Various external factors, such stressful life events, financial strain, low social support, sleep problems, and low PA, are all important contributors to depressive symptoms and anxiety in midlife women; however, these factors may also be somewhat independent of the menopause transition itself [75, 105].
A significant, positive impact of PA on psychological symptoms was consistently observed across studies in midlife women [78]. In SWAN, midlife women meeting guidelines for moderate-intensity exercise had lower odds of clinically significant depressive symptoms, and the finding persisted over ten years [106]. Other longitudinal studies, including the Harvard Study of Moods and Cycles [107], the Seattle Midlife Women’s Health Study [108], and the Penn Ovarian Aging Study [109], also examined the relationship between menopausal status and depressive symptoms and consistently found that risk for depression (symptoms or disorder) increased during the perimenopausal years. Additionally, data from the large, multi-site MsFLASH trial also found that aerobic exercise training had small improvement on depressive symptoms, insomnia, and sleep quality [81]. With regards to the mode of exercise, both aerobic and resistance exercise have been shown to elevate mood among older adults [110, 111], possibly due to its impact on the serotonergic and adrenergic systems [112].
Summary
Menopause signals the permanent cessation of ovarian production of estrogens, thereby predisposing women to increased cardiometabolic risk factors (e. g. obesity, insulin resistance, and vascular dysfunction). The menopause transition is also associated with the onset of bone loss, sleep disturbances, and mood changes, which occur alongside declines in PA. To combat these, many women undertake diets (calorie restriction), which can further accelerate bone and muscle loss if PA and/or exercise is not also prioritized in tandem. Alarmingly, the loss of bone density and muscle mass, as well as increased fat mass, is associated with loss of balance. Combining both aerobic and resistance training is better than aerobic or resistance training alone at improving body composition (i. e. increase fat mass loss, retention of fat-free (muscle) mass, maintenance of bone density). Finally, a significant, positive impact of PA on physical and psychological symptoms has been consistently observed across studies in midlife women, including better sleep and mood. Given the well-studied benefits of PA, a personalized approach should be used to encourage and maintain PA in perimenopausal and postmenopausal women. Women should be counseled on the potential benefits of PA on symptom relief (e. g. mood and sleep improvements), but it is also important to convey the broad spectrum and lifelong benefits of regular PA and exercise.
Funding Information
National Institute of General Medical Sciences — http://dx.doi.org/10.13039/100000057; P30 GM118430
Clinical Science Research and Development, VA Office of Research and Development — http://dx.doi.org/10.13039/100007496; IK2 CX002225
National Institute of Diabetes and Digestive and Kidney Diseases — http://dx.doi.org/10.13039/100000062; K01 DK128227
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- [1].El Khoudary SR, Aggarwal B, Beckie TM. et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation 2020; 142: E506–E532 [DOI] [PubMed] [Google Scholar]
- [2].Davis S, Lambrinoudaki I, Lumsden M. et al. Menopause. Nat Rev Dis Primers 2015; 1: 15004. [DOI] [PubMed] [Google Scholar]
- [3].Lovejoy JC, Champagne CM, De Jonge L et al. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes 2008; 32: 949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marlatt KL, Pitynski-Miller DR, Gavin KM et al. Body composition and cardiometabolic health across the menopause transition. Obesity (Silver Spring; ) 2022; 30: 14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol 2015; 3: 52–62 [DOI] [PubMed] [Google Scholar]
- [6].Kravitz HM, Joffe H. Sleep during the perimenopause: a SWAN story. Obstet Gynecol Clin North Am 2011; 38: 567–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Greendale GA, Sowers M, Han W et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res 2012; 27: 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bromberger JT, Kravitz HM, Chang YF et al. Major depression during and after the menopausal transition: study of women’s health across the nation (SWAN). Psychol Med 2011; 41: 1879–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cheng CC, Hsu CY, Liu JF. Effects of dietary and exercise intervention on weight loss and body composition in obese postmenopausal women: a systematic review and meta-analysis. Menopause 2018; 25: 772–782 [DOI] [PubMed] [Google Scholar]
- [10].Harlow SD, Gass M, Hall JE et al. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 2012; 97: 1159–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wallace RB, Sherman BM, Bean JA et al. Probability of menopause with increasing duration of amenorrhea in middle-aged women. Am J Obstet Gynecol 1979; 135: 1021–1024 [DOI] [PubMed] [Google Scholar]
- [12].Gold EB, Bromberger J, Crawford S et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001; 153: 865–874 [DOI] [PubMed] [Google Scholar]
- [13].Gold EB, Crawford SL, Avis NE et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol 2013; 178: 70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harlow SD. Menstrual cycle changes as women approach the final menses: what matters? Obstet Gynecol Clin North Am 2018; 45: 599–611 [DOI] [PubMed] [Google Scholar]
- [15].Greendale GA, Sternfeld B, Huang MH et al. Changes in body composition and weight during the menopause transition. JCI Insight 2019; 4: e124865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Soriguer F, Morcillo S, Hernando V et al. Type 2 diabetes mellitus and other cardiovascular risk factors are no more common during menopause: longitudinal study. Menopause 2009; 16: 817–821 [DOI] [PubMed] [Google Scholar]
- [17].Mishra GD, Carrigan G, Brown WJ et al. Short-term weight change and the incidence of diabetes in midlife: results from the Australian longitudinal study on women’s health. Diabetes Care 2007; 30: 1418–1424 [DOI] [PubMed] [Google Scholar]
- [18].Duval K, Prud’Homme D, Rabasa-Lhoret R et al. Effects of the menopausal transition on energy expenditure: a MONET group study. Eur J Clin Nutr 2013; 67: 407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pettee Gabriel K, Sternfeld B, Colvin A et al. Physical activity trajectories during midlife and subsequent risk of physical functioning decline in late mid-life: the study of women’s health across the nation (SWAN). Prev Med 2017; 105: 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sternfeld B, Bhat AK, Wang H et al. Menopause, physical activity, and body composition/fat distribution in midlife women. Med Sci Sports Exerc 2005; 37: 1195–1202 [DOI] [PubMed] [Google Scholar]
- [21].Irwin ML, Yasui Y, Ulrich CM et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA 2003; 289: 323–330 [DOI] [PubMed] [Google Scholar]
- [22].Van Gemert WA, Peeters PH, May AM et al. Effect of diet with or without exercise on abdominal fat in postmenopausal women – a randomised trial. BMC Public Health 2019; 19: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Serra MC, Blumenthal JB, Addison OR et al. Effects of weight loss with and without exercise on regional body fat distribution in postmenopausal women. Ann Nutr Metab 2017; 70: 312–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mandrup CM, Egelund J, Nyberg M et al. Effects of high-intensity training on cardiovascular risk factors in premenopausal and postmenopausal women. Am J Obstet Gynecol 2017; 216: 384. e1–384.e11 [DOI] [PubMed] [Google Scholar]
- [25].Simkin-Silverman LR, Wing RR, Boraz MA et al. Lifestyle intervention can prevent weight gain during menopause: results from a 5-year randomized clinical trial. Ann Behav Med 2003; 26: 212–220 [DOI] [PubMed] [Google Scholar]
- [26].Juppi H-K, Sipila S, Fachada V et al. Total and regional body adiposity increases during menopause – evidence from a follow-up study. Aging Cell 2022; 21: e13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hyvärinen M, Juppi HK, Taskinen S et al. Metabolic health, menopause, and physical activity – a 4-year follow-up study. Int J Obes (Lond) 2022; 46: 544–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ruiz-Rios M, Maldonado-Martin S. Physical activity on cardiorespiratory fitness and cardiovascular risk in premenopausal and postmenopausal women: a systematic review of randomized controlled trials. Menopause 2022; 29: 1222–1229 [DOI] [PubMed] [Google Scholar]
- [29].Messier V, Rabasa-Lhoret R, Barbat-Artigas S et al. Menopause and sarcopenia: a potential role for sex hormones. Maturitas 2011; 68: 331–336 [DOI] [PubMed] [Google Scholar]
- [30].Heymsfield SB, Gonzalez MCC, Shen W et al. Weight loss composition is one-fourth fat-free mass: s critical review and critique of this widely cited rule. Obes Rev 2014; 15: 310–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ravussin E, Lillioja S, Anderson TE et al. Determinants of 24-hour energy expenditure in man. methods and results using a respiratory chamber. J Clin Invest 1986; 78: 1568–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bogardus C, Lillioja S, Ravussin E et al. Familial dependence of the resting metabolic rate. N Engl J Med 1986; 315: 96–100 [DOI] [PubMed] [Google Scholar]
- [33].Godsland IF. Oestrogens and insulin secretion. Diabetologia 2005; 48: 2213–2220 [DOI] [PubMed] [Google Scholar]
- [34].Yan H, Yang W, Zhou F et al. Estrogen improves insulin sensitivity and suppresses gluconeogenesis via the transcription factor Foxo1. Diabetes 2019; 68: 291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bruns CM, Kemnitz JW. Sex hormones, insulin sensitivity, and diabetes mellitus. ILAR J 2004; 45: 160–169 [DOI] [PubMed] [Google Scholar]
- [36].Margolis KL, Bonds DE, Rodabough RJ et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the women’s health initiative hormone trial. Diabetologia 2004; 47: 1175–1187 [DOI] [PubMed] [Google Scholar]
- [37].Mauvais-Jarvis F, Manson JAE, Stevenson JC et al. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr Rev 2017; 38: 173–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Houmard JA, Tanner CJ, Slentz CA et al. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol (1985) 2004; 96: 101–106 [DOI] [PubMed] [Google Scholar]
- [39].Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol (Oxf; ) 2008; 192: 127–135 [DOI] [PubMed] [Google Scholar]
- [40].Richter EA, Garetto LP, Goodman MN et al. Muscle glucose metabolism following exercise in the rat. increased sensitivity to insulin. J Clin Invest 1982; 69: 785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ross R, Janssen I, Dawson J et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res 2004; 12: 789–798 [DOI] [PubMed] [Google Scholar]
- [42].Hansen PA, Nolte LA, Chen MM et al. Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J Appl Physiol (1985) 1998; 85: 1218–1222 [DOI] [PubMed] [Google Scholar]
- [43].Bogardus C, Lillioja S, Mott DM et al. Relationship between degree of obesity and in vivo insulin action in man. Am J Physiol 1985; 248: E286–E291 [DOI] [PubMed] [Google Scholar]
- [44].Brown MD, Korytkowski MT, Zmuda JM et al. Insulin sensitivity in postmenopausal women: independent and combined associations with hormone replacement, cardiovascular fitness, and body composition. Diabetes Care 2000; 23: 1731–1736 [DOI] [PubMed] [Google Scholar]
- [45].Frank LL, Sorensen BE, Yasui Y et al. Effects of exercise on metabolic risk variables in overweight postmenopausal women: a randomized clinical trial. Obes Res 2005; 13: 615–625 [DOI] [PubMed] [Google Scholar]
- [46].Kim JW, Kim DY. Effects of aerobic exercise training on serum sex hormone binding globulin, body fat index, and metabolic syndrome factors in obese postmenopausal women. Metab Syndr Relat Disord 2012; 10: 452–457 [DOI] [PubMed] [Google Scholar]
- [47].Choquette S, Riesco É, Cormier É et al. Effects of soya isoflavones and exercise on body composition and clinical risk factors of cardiovascular diseases in overweight postmenopausal women: a 6-month double-blind controlled trial. Br J Nutr 2011; 105: 1199–1209 [DOI] [PubMed] [Google Scholar]
- [48].Mason C, Foster-Schubert KE, Imayama I et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med 2011; 41: 366–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Davidson LE, Hudson R, Kilpatrick K et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med 2009; 169: 122–131 [DOI] [PubMed] [Google Scholar]
- [50].Church TS, Blair SN, Cocreham S et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 2010; 304: 2253–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ungvari Z, Tarantini S, Donato AJ et al. Mechanisms of vascular aging. Circ Res 2018; 123: 849–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987; 327: 524–526 [DOI] [PubMed] [Google Scholar]
- [53].Holden DP, Cartwright JE, Nussey SS et al. Estrogen stimulates dimethylarginine dimethylaminohydrolase activity and the metabolism of asymmetric dimethylarginine. Circulation 2003; 108: 1575–1580 [DOI] [PubMed] [Google Scholar]
- [54].Tan E, Gurjar MV., Sharma RV et al. Estrogen receptor-α gene transfer into bovine aortic endothelial cells induces eNOS gene expression and inhibits cell migration. Cardiovasc Res 1999; 43: 788–797 [DOI] [PubMed] [Google Scholar]
- [55].Darblade B, Pendaries C, Krust A et al. Estradiol alters nitric oxide production in the mouse aorta through the α-, but not β-, estrogen receptor. Circ Res 2002; 90: 413–419 [DOI] [PubMed] [Google Scholar]
- [56].Best PJM, Berger PB, Miller VM et al. The effect of estrogen replacement therapy on plasma nitric oxide and endothelin-1 levels in postmenopausal women. Ann Intern Med 1998; 128: 285–288 [DOI] [PubMed] [Google Scholar]
- [57].Moreau KL, Hildreth KL, Meditz AL et al. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 2012; 97: 4692–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 2003; 107: 346–354 [DOI] [PubMed] [Google Scholar]
- [59].Van Eickels M, Grohé C, Cleutjens JPM et al. 17B-estradiol attenuates the development of pressure-overload hypertrophy. Circulation 2001; 104: 1419–1423 [DOI] [PubMed] [Google Scholar]
- [60].Patten RD, Pourati I, Aronovitz MJ et al. 17 beta-estradiol differentially affects left ventricular and cardiomyocyte hypertrophy following myocardial infarction and pressure overload. J Card Fail 2008; 14: 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lam CSP, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail 2011; 13: 18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lam CSP, Carson PE, Anand IS et al. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: the irbesartan in heart failure with preserved ejection fraction (I-PRESERVE) trial. Circ Hear Fail 2012; 5: 571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fukuma N, Takimoto E, Ueda K et al. Estrogen receptor-α non-nuclear signaling confers cardioprotection and is essential to cGMP-PDE5 inhibition efficacy. JACC Basic Transl Sci 2020; 5: 282–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lewis TV, Dart AM, Chin-Dusting JPF et al. Exercise training increases basal nitric oxide production from the forearm in hypercholesterolemic patients. Arterioscler Thromb Vasc Biol 1999; 19: 2782–2787 [DOI] [PubMed] [Google Scholar]
- [65].Black MA, Cable NT, Thijssen DHJ et al. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Hear Circ Physiol 2009; 297: H1109–H1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Swift DL, Weltman JY, Patrie JT et al. Predictors of improvement in endothelial function after exercise training in a diverse sample of postmenopausal women. J Women’s Heal 2014; 23: 260–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Nyberg M, Egelund J, Mandrup CM et al. Early postmenopausal phase is associated with reduced prostacyclin-induced vasodilation that is reversed by exercise training: the Copenhagen women study. Hypertension 2016; 68: 1011–1020 [DOI] [PubMed] [Google Scholar]
- [68].Pierce GL, Eskurza I, Walker AE et al. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci 2011; 120: 13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Santos-Parker JR, Strahler TR, Vorwald VM et al. Habitual aerobic exercise does not protect against micro-or macrovascular endothelial dysfunction in healthy estrogen-deficient postmenopausal women. J Appl Physiol (1985) 2017; 122: 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Moreau KL, Stauffer BL, Kohrt WM et al. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 2013; 98: 4507–4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Salzano A, De Luca M, Israr MZ et al. Exercise intolerance in heart failure with preserved eEjection fraction. Heart Fail Clin 2021; 17: 397–413 [DOI] [PubMed] [Google Scholar]
- [72].Thurston RC. Vasomotor symptoms: natural history, physiology, and links with cardiovascular health. Climacteric 2018; 21: 96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kravitz HM, Kazlauskaite R, Joffe H. Sleep, health, and metabolism in midlife women and menopause: food for thought. Obstet Gynecol Clin North Am 2018; 45: 679–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kline CE. The bidirectional relationship between exercise and sleep: implications for exercise adherence and sleep improvement. Am J Lifestyle Med 2014; 8: 375–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].El Khoudary SR, Greendale G, Crawford SL et al. The menopause transition and women’s health at midlife: a progress report from the study of women’s health across the nation (SWAN). Menopause 2019; 26: 1213–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lambiase MJ, Thurston RC. Physical activity and sleep among midlife women with vasomotor symptoms. Menopause 2013; 20: 946–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kline CE, Irish LA, Krafty RT et al. Consistently high sports/exercise activity is associated with better sleep quality, continuity and depth in midlife women: the SWAN sleep study. Sleep 2013; 36: 1279–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pettee Gabriel K, Mason JM, Sternfeld B. Recent evidence exploring the associations between physical activity and menopausal symptoms in midlife women: perceived risks and possible health benefits. Womens Midlife Heal 2015; 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kline CE, Sui X, Hall MH et al. Dose-response effects of exercise training on the subjective sleep quality of postmenopausal women: exploratory analyses of a randomised controlled trial. BMJ Open 2012; 2: e001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mansikkamäki K, Raitanen J, Nygård CH et al. Sleep quality and aerobic training among menopausal women – A randomized controlled trial. Maturitas 2012; 72: 339–345 [DOI] [PubMed] [Google Scholar]
- [81].Sternfeld B, Guthrie KA, Ensrud KE et al. Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause 2014; 21: 330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Reed SD, Guthrie KA, Newton KM et al. Menopausal quality of life: randomized controlled trial of yoga, exercise, and omega-3 supplements. Am J Obstet Gynecol 2014; 210: 244.e1–244.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wilbur JE, Miller AM, McDevitt J et al. Menopausal status, moderate-intensity walking, and symptoms in midlife women. Res Theory Nurs Pract 2005; 19: 163–180 [PubMed] [Google Scholar]
- [84].Recker R, Lappe J, Davies K et al. Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res 2000; 15: 1965–1973 [DOI] [PubMed] [Google Scholar]
- [85].Karlamangla AS, Burnett-Bowie SAM, Crandall CJ. Bone health during the menopause transition and beyond. Obstet Gynecol Clin North Am 2018; 45: 695–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lorentzon M, Johansson H, Harvey NC et al. Osteoporosis and fractures in women: the burden of disease. Climacteric 2022; 25: 4–10 [DOI] [PubMed] [Google Scholar]
- [87].Nazrun AS, Tzar MN, Mokhtar SA et al. A systematic review of the outcomes of osteoporotic fracture patients after hospital discharge: morbidity, subsequent fractures, and mortality. Ther Clin Risk Manag 2014; 10: 937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Martyn-St. James M, Carroll S High-intensity resistance training and postmenopausal bone loss: a meta-analysis. Osteoporos Int 2006; 17: 1225–1240 [DOI] [PubMed] [Google Scholar]
- [89].Kelley GA, Kelley KS, Tran ZV. Exercise and lumbar spine bone mineral density in postmenopausal women: a meta-analysis of individual patient data. J Gerontol A Biol Sci Med Sci 2002; 57: M599–M604 [DOI] [PubMed] [Google Scholar]
- [90].Wallace BA, Cumming RG. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int 2000; 67: 10–18 [DOI] [PubMed] [Google Scholar]
- [91].Kistler-Fischbacher M, Weeks BK, Beck BR. The effect of exercise intensity on bone in postmenopausal women (part 2): a meta-analysis. Bone 2021; 143: 115697. [DOI] [PubMed] [Google Scholar]
- [92].Martyn-St James M, Carroll S. Meta-analysis of walking for preservation of bone mineral density in postmenopausal women. Bone 2008; 43: 521–531 [DOI] [PubMed] [Google Scholar]
- [93].Shea KL, Gavin KM, Melanson EL et al. Body composition and bone mineral density after ovarian hormone suppression with or without estradiol treatment. Menopause 2015; 22: 1045–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gozansky WS, Van Pelt RE, Jankowski CM et al. Protection of bone mass by estrogens and raloxifene during exercise-induced weight loss. J Clin Endocrinol Metab 2005; 90: 52–59 [DOI] [PubMed] [Google Scholar]
- [95].Villareal DT, Fontana L, Weiss EP et al. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med 2006; 166: 2502–2510 [DOI] [PubMed] [Google Scholar]
- [96].Cheng MH, Wang SJ, Yang FY et al. Menopause and physical performance - a community-based cross-sectional study. Menopause 2009; 16: 892–896 [DOI] [PubMed] [Google Scholar]
- [97].Da Câmara SMA, Zunzunegui MV, Pirkle C et al. Menopausal status and physical performance in middle aged women: a cross-sectional community-based study in Northeast Brazil. PLoS One 2015; 10: e0119480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Schultz AB, Ashton-Miller JA, Alexander NB. What leads to age and gender differences in balance maintenance and recovery? Muscle Nerve 1997; 4: S60–S64 [PubMed] [Google Scholar]
- [99].Marlatt KL, Redman LM, Beyl RA et al. Racial differences in body composition and cardiometabolic risk during the menopause transition: a prospective, observational cohort study. Am J Obstet Gynecol 2020; 222: 365.e1–365.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Polotsky HN, Polotsky AJ. Metabolic implications of menopause. Semin Reprod Med 2010; 28: 426–434 [DOI] [PubMed] [Google Scholar]
- [101].Hulteen RM, Smith JJ, Morgan PJ et al. Global participation in sport and leisure-time physical activities: a systematic review and meta-analysis. Prev Med 2017; 95: 14–25 [DOI] [PubMed] [Google Scholar]
- [102].U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. Department of Health and Human Services; Washington, DC: U.S: 2018 [Google Scholar]
- [103].Fu S, Low Choy N, Nitz J. Controlling balance decline across the menopause using a balance-strategy training program: a randomized, controlled trial. Climacteric 2009; 12: 165–176 [DOI] [PubMed] [Google Scholar]
- [104].Bromberger JT, Schott LL, Avis NE et al. Psychosocial and health-related risk factors for depressive symptom trajectories among midlife women over 15 years: study of women’s health across the nation (SWAN). Psychol Med 2019; 49: 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Bromberger JT, Schott LL, Kravitz HM et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the study of women’s health across the nation (SWAN). Arch Gen Psychiatry 2010; 67: 598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Dugan SA, Bromberger JT, Segawa E et al. Association between physical activity and depressive symptoms: midlife women in SWAN. Med Sci Sports Exerc 2015; 47: 335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Cohen LS, Soares CN, Vitonis AF et al. Risk for new onset of depression during the menopausal transition: the harvard study of moods and cycles. Arch Gen Psychiatry 2006; 63: 385–390 [DOI] [PubMed] [Google Scholar]
- [108].Woods NF, Smith-DiJulio K, Percival DB et al. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle midlife women’s health study. Menopause 2008; 15: 223–232 [DOI] [PubMed] [Google Scholar]
- [109].Freeman EW, Sammel MD, Liu L et al. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry 2004; 61: 62–70 [DOI] [PubMed] [Google Scholar]
- [110].Blumenthal JA, Babyak MA, Moore KA et al. Effects of exercise training on older patients with major depression. Arch Intern Med 1999; 159: 2349–2356 [DOI] [PubMed] [Google Scholar]
- [111].McLafferty CL, Wetzstein CJ, Hunter GR. Resistance training is associated with improved mood in healthy older adults. Percept Mot Skills 2004; 98: 947–957 [DOI] [PubMed] [Google Scholar]
- [112].Weicker H, Strüder HK. Influence of exercise on serotonergic neuromodulation in the brain. Amino Acids 2001; 20: 35–47 [DOI] [PubMed] [Google Scholar]


