Abstract
In recent years, the utility of serum-based diagnostic testing for Lyme disease has improved substantially; however, recovery by culture of the bacterium from skin biopsies of suspected patients is still the only definitive laboratory test. Reinfection of patients has been assumed to occur but as yet has not been documented by serial isolates from the same person. We present a case of culture-confirmed reinfection of a patient in Menominee County, Michigan. Borrelia burgdorferi was isolated from the skin punch biopsy specimens during each episode of erythema migrans (EM) and was subjected to molecular strain typing, genetic analysis of two outer surface protein genes, protein profile analysis, and serum antibody response testing. Results show that these isolates are distinct strains of the bacterium and that the two episodes of EM were caused by independent infections. This report describes the documented, culture-confirmed reinfection of a human by two different strains of B. burgdorferi.
Lyme disease is a vector-borne spirochetosis caused by Borrelia burgdorferi and transmitted by ticks of the genus Ixodes (6, 21). B. burgdorferi has been isolated and shown to be the cause of endemic disease in several areas of the United States. In Michigan, ecologic studies have demonstrated that the tick vector of Lyme disease became established in the 1980s in Menominee County in the Upper Peninsula (23). Further studies have suggested that Menominee County is the only area in the state where B. burgdorferi is currently endemic (26). In conjunction with a statewide active surveillance program for acute Lyme disease, culture-confirmed Lyme disease has been documented for eight Michigan residents, all of whom had physician-observed erythema migrans (EM) lesions following tick exposure in Menominee County (22).
Multiple distinct episodes of EM in the same patient have been well described clinically (7, 10, 17, 19). To our knowledge, however, no such cases have been documented by culture of the etiological agent in each of two consecutive episodes of EM and subsequent characterization of the two isolates, leaving open the question of whether these cases were independent infections or recrudescences of the initial infection.
The patient subject of the current report, a resident of Menominee County, experienced two distinct episodes of EM separated by more than 2 years, in 1992 and 1994, and isolates of B. burgdorferi were obtained during each infection. This has provided the opportunity to determine whether the second episode was a result of persistence and reactivation of the initial infection or a novel infection due to a new exposure to infected ticks. Analysis of these two isolates demonstrated numerous differences in protein profile, plasmid profile, and most importantly, the alleles of outer surface protein A (OspA) and outer surface protein C (OspC) genes of B. burgdorferi sensu stricto. Serum samples taken during both episodes of infection contained antibodies to B. burgdorferi.
The duration of immunity in humans to natural infection with B. burgdorferi has not been determined for patients that have been treated and that are no longer infected. Such an analysis requires separate, documented episodes of infection by isolation and characterization of the spirochete. In laboratory experiments, natural immunity in mice appears to wane at about 9 months after treatment and clearance of infection. This immunity does not correlate with anti-B. burgdorferi serum antibody titers (15). This study begins to provide data on the immune status of Lyme disease patients after treatment and resolution of infection and on their susceptibility to new episodes of infection.
MATERIALS AND METHODS
Borrelia cultures.
Modified Barbour-Stoner-Kelly (BSK) medium (2) (Sigma, St. Louis, Mo.) supplemented with 6% rabbit serum (Sigma) was used for all borrelia cultures. It was prepared as described previously, with the addition of rifampin (50 mg/liter), phosphomycin (100 mg/liter), and amphotericin B (2.5 mg/liter) (5, 13, 20). Biopsies were taken according to the procedure described by Berger et al. (5). Upon receipt at the laboratory, the punch biopsies were aseptically transferred to 3 ml of fresh modified BSK medium. Cultures were standardly incubated at 30°C for 12 weeks and were examined on alternate days under dark-field microscopy. Cultures which showed slender, highly motile, helical spirochetes measuring approximately 25 μm in length were tested by indirect immunofluorescence assay with monoclonal antibody (MAb) H5332 (3) specific for the outer surface protein A (OspA) lipoprotein of B. burgdorferi. The 1992 isolate was designated U193, and the 1994 isolate was designated U14. Aliquots were frozen in 50% glycerol in BSK-H medium and stored at −70°C.
Serologic analyses.
In 1992, all serum samples were tested for antibodies to B. burgdorferi by both immunoglobulin M (IgM)- and IgG-specific enzyme immunoassays (EIA; Zeus Scientific, Cranbury, N.J.) according to the manufacturer’s instructions. In 1994, specimens were tested for IgM and IgG antibodies according to the Centers for Disease Control and Prevention (CDC) and Association of State and Territorial Public Health Laboratory Directors protocol (1). This procedure recommends two-stage testing starting with an EIA, which in our case involved testing a whole-cell sonicate preparation of strain B31 at low passage. We also tested all serum samples with the flagellin protein preparation obtained from CDC as the antigen. All positive and equivocal samples were confirmed by Western blot testing (MarDx Diagnostics, Carlsbad, Calif.) with a collection of B. burgdorferi-specific mouse MAbs as a reference (1).
Molecular analyses.
Isolates of B. burgdorferi were cultured in BSK medium (Sigma) to a density of 108 spirochetes per ml. Lysates were prepared by pelleting 100 ml of the bacterial culture and washing twice with phosphate-buffered saline containing 5 mM MgCl2. Spirochetes were then lysed in a Dounce homogenizer and assayed for protein content. Lysates were diluted to 2 mg/ml and mixed with an equal volume of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer. Samples were separated by SDS–10% PAGE under reducing conditions. Immunoblot analysis was performed as reported previously (8), with the B31 strain of B. burgdorferi included as a reference.
Plasmid analysis was performed as previously reported (9). Samples of genomic DNA were prepared from spirochetes by pelleting 5 × 108 bacteria, washing the pellet with cold 10 mM Tris–150 mM NaCl (Tris saline), and resuspending the bacteria in 0.5 ml Tris saline. An equal volume of 2% low-melt agarose (Bio-Rad, Richmond, Calif.) in Tris saline was added, and plugs were cast in plug molds in a volume of 0.1 ml. Agarose plugs were cooled at 4°C, removed from the molds, and incubated in lysis buffer (10 mM Tris, 150 mM NaCl, 20 mM EDTA, 0.1% SDS) for 2 h with tumbling at room temperature. Plugs were then washed in 0.5× Tris-borate-EDTA (TBE) with two changes and stored in 0.5× TBE at 4°C.
Genomic DNA was separated by pulsed-field gel electrophoresis (PFGE) on a CHEF Mapper (Bio-Rad) PFGE system in 0.5× TBE as described previously (9). Gels were stained with ethidium bromide and photographed under UV. Restriction fragment length polymorphism testing was performed by the criteria of Belfaiza et al. (4) with MluI.
Further differentiation of the two isolates was carried out by PCR–single-strand confirmation polymorphism (PCR-SSCP) for the OspA gene by the method of Guttman et al. (11). The PCR amplification protocol is a combination of nested PCR and modified “touchdown” PCR (12). A volume of 0.5 μl of culture was used directly as the template for the nested PCR. Primers specific for the central region of the OspA gene were amplified with a cycling profile of 1 min at 96°C for 1 cycle followed by 30 s at 94°C, 30 s at 37°C, and 2 min at 72°C for 20 cycles in a PTC-100 thermocycler (MJ Research, Inc., Watertown, Mass.). For the touchdown PCR, the protocol was 1 min at 96°C for one cycle followed by 30 s at 94°C, 30 s at 60°C, and 1 min at 72°C for 10 cycles. The next 10 cycles used an annealing temperature of 55°C, followed by 50°C for 10 cycles. For the last five cycles, 45°C was used. External primers spanned bp 4 through 695, and internal primers were targeted for bp 220 through 565. A final 345-bp fragment internal to the OspA gene was the predicted product after this amplification procedure.
For SSCP analysis, volumes of PCR product were normalized to 5 μl and mixed with 0.4 μl of 1 M mercury hydroxide and 9.6 μl of loading buffer. After being heated at 96°C for 4 min, samples were immediately iced. Samples were run on a 20% polyacrylamide–TBE nondenaturing gel (Novex, San Diego, Calif.) with a 1.5× TBE buffer at 210 V for 16 h at a constant temperature of 4 to 8°C. Gels were stained with ethidium bromide and visualized with UV. This assay analyzes the electrophoretic mobility shift of single-stranded DNA of highly related genes and can detect a difference of as little as a single base.
RESULTS
Case history and isolation of B. burgdorferi.
The first illness in the patient was previously reported (22). Briefly, this 56-year-old male resident of Menominee County was seen by his physician in August 1992 with a 5- to 7-day history of fever, headache, myalgia, nausea, and stiff neck. Upon physical examination, a 14-cm-diameter lesion characteristic of EM was noted in the left scapular region. He denied any knowledge of recent tick bites but 2 weeks previously had spent time outdoors in an area of southern Menominee County in which B. burgdorferi-infected Ixodes scapularis ticks have been identified (23). He had not traveled outside of Menominee County during this time. A punch biopsy was obtained from the erythematous periphery of the lesion (5) and placed into modified BSK medium. The biopsy was positive for the presence of spirochetes after 11 days of culture. Serum samples were obtained at the time of the skin biopsy, 1 month later, and then 3 months after diagnosis. Therapy with doxycycline (100 mg twice a day for 3 weeks) was initiated after the biopsy specimen was obtained. The patient reported the EM resolved within 48 h of treatment, and other symptoms resolved within a week.
The patient continued to be asymptomatic for Lyme disease until 11 October 1994 when he noted the appearance of another lesion suggestive of EM. He presented to his physician the following day, and EM was confirmed. The lesion measured approximately 5 cm in diameter and was again located in the left infrascapular area. He reported mild myalgia and fatigue but was afebrile and denied knowledge of a tick bite. Generally, he reported feeling much less ill than with his previous episode of Lyme disease. Once again a biopsy specimen was obtained from the edge of the EM lesion and submitted for culture of B. burgdorferi to the Michigan Department of Community Health Laboratory. This culture of biopsy material had detectable spirochetes after 7 days of incubation. In both cases, isolation by culture of motile spirochetes occurred within the expected range of time for bacterial isolation of B. burgdorferi. Serum samples were obtained at the time of diagnosis and again 39 days later. These samples were tested for antibodies to B. burgdorferi by EIA and immunoblotting studies. After the skin punch biopsy specimen was obtained, the patient was again treated with doxycycline (100 mg twice daily), this time for 4 weeks. Clinical recovery was rapid.
Protein analysis of patient isolates.
Spirochetes reacting by indirect immunofluorescence assay to MAb H5332 specific for OspA, and therefore nominally considered to be B. burgdorferi, were isolated from skin biopsies taken from the EM lesions of the patient in 1992 and 1994. The protein profiles of these isolates were very similar (Fig. 1); the majority of bands for the two isolates were identical, with two notable exceptions. The isolate from 1994 expressed bands corresponding to molecular masses of 23 and 66 kDa that were absent from the isolate from 1992. Both of the isolates expressed a number of proteins in common with strain B31; however, both isolates expressed an OspB protein that migrated slightly faster than the OspB of B31.
FIG. 1.
Protein profiles for lysates of the two patient isolates and B. burgdorferi B31 separated by SDS-PAGE and stained with Coomassie blue. Molecular weight reference standards are indicated.
When lysates of these isolates were electrophoretically transferred to nitrocellulose filters for Western blotting, distinct patterns of reactivity with a panel of antibodies were observed. Both isolates expressed proteins reactive with the panel of MAbs (Table 1) with one exception. The isolate from 1992 lacked a protein reactive with the MAb recognizing OspD, whereas this protein was detected in the isolate from 1994. When these same nitrocellulose filters were probed with serum samples from the patient, only the convalescent-phase serum from the 1994 episode of EM was found to be significantly reactive with either of the isolates, and it reacted with both isolates. The convalescent-phase serum sample from the 1992 episode of EM had marginal reactivity with both isolates, whereas the acute-phase serum sample from the 1994 infection was not reactive with either.
TABLE 1.
Reactivities of the two patient isolates with B. burgdorferi-specific MAbs and serum samples
| Antibody or serum sample | Reactivitya with:
|
|
|---|---|---|
| 1992 isolate | 1994 isolate | |
| 1992 convalescent-phase serum | +/− | +/− |
| 1994 acute-phase serum | − | − |
| 1994 convalescent-phase serum | + | + |
| MAb anti-P22 | + | + |
| MAb anti-OspC (P23) | + | + |
| MAb anti-OspD (P28) | − | + |
| MAb anti-OspA | + | + |
| MAb anti-OspB | + | + |
| MAb anti-P39 | + | + |
| MAb anti-Fla (P41) | + | + |
| MAb anti-P60 | + | + |
| MAb anti-P66 | + | + |
| MAb anti-P72 | + | + |
| MAb anti-P93 | + | + |
+, reactive; −, not reactive; +/−, marginally reactive.
Serum antibody testing.
Results of two-stage testing by EIA followed by immunoblotting of serum samples taken in 1992 and 1994 are shown in Table 2. The acute-phase serum from 1992 was negative by EIA and immunoblotting. The second sample, taken 1 month after the initial diagnosis and after treatment had ended, was positive by EIA for IgM antibodies to B. burgdorferi but negative for IgG antibodies. Paradoxically, results of immunoblotting showed that there was reactivity with one diagnostic band when the sample was assayed for IgM and reactivity with five diagnostic bands in the IgG assay. The third serum sample, taken 3 months after the original diagnosis, contained no IgM antibodies demonstrable by EIA but was positive for IgG antibodies to B. burgdorferi. There were too few bands present in this immunoblot for it to be considered positive.
TABLE 2.
Results of EIA and immunoblotting for patient reinfected with B. burgdorferi
| Episode of EMa | Date of:
|
EIA result for:
|
Molecular masses (kDa) for diagnostic bandsb | ||
|---|---|---|---|---|---|
| Onset | Specimen | IgM | IgG | ||
| 1 | 8/17/92 | 8/24/92 | Neg | Neg | IgM, 23; IgG, 30 |
| 1 | 8/17/92 | 9/23/92 | Pos | Neg | IgM, 23; IgG, 28, 30, 39, 41, 58 |
| 1 | 8/17/92 | 11/19/92 | Neg | Pos | IgM, neg; IgG, 30 |
| 2 | 10/11/94 | 10/12/94 | Neg | Neg | IgM, 23; IgG, 18, 30, 41, 45 |
| 2 | 10/11/94 | 11/18/94 | Neg | Pos | IgM, 23, 41; IgG, 18, 23, 39, 41, 45, 58 |
1, original infection; 2, reinfection.
Immunoblotting was performed with MarDx strips according to the manufacturer’s instructions. In order to obtain a complete assessment, all samples were tested by EIA and immunoblotting for both IgG and IgM. Neg, no reactivity with diagnostic bands.
The serum sample obtained at the time of diagnosis during the episode of EM in 1994 had no antibody to B. burgdorferi detectable by EIA and was also negative for the Fla antigen by EIA. The second specimen, drawn 39 days after diagnosis, contained IgG reactive with both the whole-cell B. burgdorferi antigen and the Fla antigen. The results of immunoblotting correlated with testing results obtained by EIA in that the first specimen was neither IgM nor IgG positive but the second specimen was positive for both IgM and IgG antibodies reactive with diagnostic bands.
Genetic analysis of the two isolates.
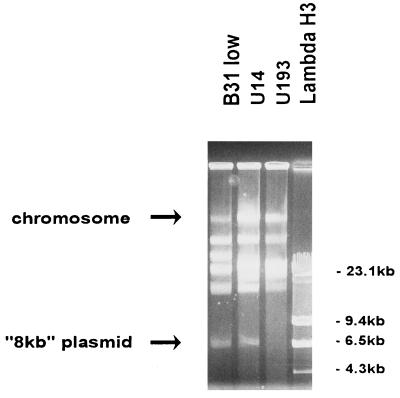
Restriction fragment length polymorphism analysis was used to confirm the identification of the two isolates and showed that both strains were B. burgdorferi sensu stricto (data not shown). It was found that the genetic profiles of the isolates differed when the chromosomal DNA and plasmids were separated by PFGE (Fig. 2). The isolate from 1992 lacked an 8-kb plasmid present in the isolate from 1994, as well as in the B31 strain. The lack of an 8-kb plasmid is not likely to be an artifact of culture, as these analyses were done on the first passage of the spirochete after isolation and no loss of plasmids has been reported for such low-passage derivatives.
FIG. 2.
Genomic DNA of the two patient isolates and B. burgdorferi B31 separated in agarose gels by PFGE. Reference size standards are provided by a HindIII digest of bacteriophage lambda (Lambda H3).
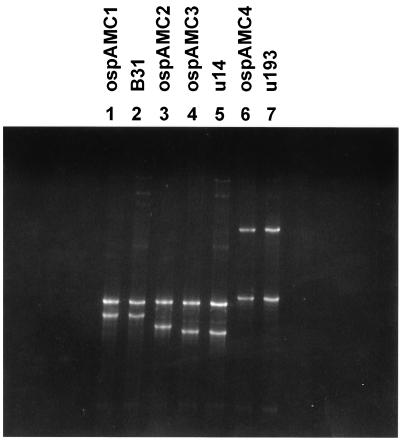
We have previously reported the use of PCR-SSCP to successfully identify OspA gene alleles of B. burgdorferi amplified from a single tick (11). A double-blind test of the two isolates from this patient, with the addition of strain B31 as a standard, was performed. Duplicate cultures of the three strains were analyzed, and the results showed that the strain in one pair of cultures was identical to the known strain B31 used as the standard. The other four samples could be sorted into two pairs, each pair member containing a strain identical to that of the other member and different from those of any other pair in the analysis. Figure 3 shows a representation of these results, with the isolates identified and run with the allelic standards previously reported. The banding pattern clearly shows the allelic differences between these isolates. A similar system has been developed for OspC, and the results confirm the OspA analysis in that all three isolates, B31 and the two patient isolates, contain distinct alleles of the OspC gene (27).
FIG. 3.
SSCP allelic analysis of the OspA genes of the two patient isolates, U14 (lane 5) and U193 (lane 7), and B. burgdorferi B31 (lane 2). Reference strains representing the four alleles of the OspA gene identified by this analysis were arranged such that each was run in the lane adjacent to that of the test strain that also has the allele.
DISCUSSION
As has been reported earlier, prompt treatment of this patient did not abrogate the immune response during the initial episode of disease (22). Upon reexposure and a new episode of infection in this patient, there was a serum antibody response to borrelia antigens detected. During both episodes, IgM and IgG antibodies were present, as detected by EIA or immunoblotting techniques, correlating with the clinical diagnosis of Lyme disease and the isolation of B. burgdorferi from this patient. In neither case, however, was any EIA positive at the time of clinical diagnosis and initiation of treatment.
Protein analysis showed that although the two isolates were similar in many respects, the 1994 isolate expressed bands corresponding to molecular masses of 23 and 66 kDa that appeared to be absent from the 1992 isolate (Fig. 1). The OspD protein was also detected in the 1994 isolate, but no reactivity with the monoclonal anti-OspD was observed with lysates from the 1992 strain (Table 1). These differences may be significant but could also be explained by variation in the stage of growth in vitro at the time of harvest or in the preparation of the lysates. These analyses did not conclusively demonstrate that the isolates were distinct and did not resolve the question of whether the second episode of EM was a new infection or reactivation of sequelae from the original infection.
Analysis of the genetic profiles of the two isolates however, yielded more definitive results. For example, the 1992 isolate is lacking the 8-kb plasmid. As we previously have reported (9), this plasmid may be lost without affecting transmission of spirochetes to ticks or mice. Given that the 1992 isolate lacks this plasmid and the 1994 isolate still maintains it, we are very confident that these are unique strains of B. burgdorferi. It has been shown that B. burgdorferi can lose plasmids during chronic infection (16) and during multiple rounds of zoonotic transmission in laboratory animals (9), yet to date there has been no description of B. burgdorferi being able to acquire genetic elements in vivo or in vitro without active manipulation in a laboratory system, such as by electroporation.
Finally, we analyzed the outer surface protein A (OspA) and outer surface protein C (OspC) genes of these isolates by SSCP. Since the OspC gene has a reported variability in nucleotide sequence (14) even in closely related strains, we predicted that these isolates would have different alleles of the OspC gene, whereas the alleles of the less-variable OspA gene would likely be similar or identical. Surprisingly, the results of these analyses show that both of the isolates from this patient have different alleles of the OspA gene and that these alleles are both distinct from those of the reference strain, B31. The more-likely result was also observed: all strains differ in the OspC gene allele. These genetic analyses conclusively demonstrate that this patient was infected by independent isolates of B. burgdorferi, and the clinical data indicate that infection resulted from two independent exposures separated by 2 years.
Previous reports have described episodes of persistence of infection after resolution of symptoms (24) and treatment failure in cases of Lyme disease (25). Further, case studies describing reinfection resulting from independent exposures to ticks, with from two years to as little as 17 weeks between episodes of infection, have been published (17, 19). Though the clinical data and risk behavior of the subjects were strong indications of independent infections, in no case were isolates made during both infections. A complicating factor illuminated by the reinfection reports is that the prompt, successful treatment of patients infected with B. burgdorferi may interfere with the generation of sufficient immunity to prevent reinfection. These reports show the necessity of careful documentation of reinfection of an individual after apparently successful antibiotic treatment of Lyme borreliosis.
The present case provides a unique opportunity to evaluate the spirochete B. burgdorferi after natural infection and treatment. To our knowledge, this is the first report of sequential, culture-confirmed infections in a single individual. Minimally, this report demonstrates that natural immunity to B. burgdorferi as a result of infection had waned in this individual 2 years after initial infection and that it is possible that new episodes of infection in Lyme disease patients can be a result of an independent exposure to a different strain of the bacterium.
These results are important to the diagnosis and treatment of this infection. First, previous exposure to the spirochete complicates serum-based diagnostics in subsequent episodes of infection since anti-B. burgdorferi antibodies are still likely to be present in the serum of these individuals. Also, it is important to differentiate between reactivation of infection in patients that were diagnosed and treated and a new infection. This further emphasizes the importance of the clinical diagnosis of Lyme disease for patients with a history of risk of exposure and clinical symptoms. As more such cases can be documented and a sufficient number of subjects can be studied, we may reach an understanding of the duration of natural immunity as well as the incidence of reinfection as opposed to the incidence of treatment failure for Lyme disease.
ADDENDUM
During the review and revision of the manuscript, Nowakowski et al. published the description of a culture-confirmed reinfection of a patient with B. burgdorferi (13a). As in the present report, the episodes of infection were apparently caused by two different strains of the bacterium.
REFERENCES
- 1.Association of State and Territorial Public Health Laboratory Directors. Proceedings from the Second National Conference on Serologic Diagnosis of Lyme Disease. Washington, D.C: Association for State and Territorial Public Health Laboratory Directors; 1995. [Google Scholar]
- 2.Barbour A B. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour A G, Tessier S L, Todd W J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigen determinant defined by a monoclonal antibody. Infect Immun. 1983;41:795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belfaiza J, Postic D, Bellenger E, Baranton G, Saint Girons I. Genomic fingerprinting of Borrelia burgdorferi sensu lato by pulsed-field gel electrophoresis. J Clin Microbiol. 1993;31:2873–2877. doi: 10.1128/jcm.31.11.2873-2877.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger B W, Johnson R C, Kodner C, Coleman L. Cultivation of Borrelia burgdorferi from erythema migrans lesions and perilesional skin. J Clin Microbiol. 1992;30:359–361. doi: 10.1128/jcm.30.2.359-361.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgdorfer W, Barbour A, Hayes S, Benach J, Grunwalt E, Davies J. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 7.Cartter M L, Hadler J L, Rhodes V J, Peterson V J. Reinfection with Borrelia burgdorferi. Conn Med. 1989;53:376–377. [PubMed] [Google Scholar]
- 8.Golde W T, Burkot T R, Sviat S, Keen M, Mayer L W, Johnson B, Piesman J. The major histocompatibility complex-restricted response of recombinant inbred strains of mice to natural tick transmission of Borrelia burgdorferi. J Exp Med. 1993;177:9–17. doi: 10.1084/jem.177.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golde W T, Dolan M C. Variation in antigenicity and infectivity of derivatives of Borrelia burgdorferi, strain B31, maintained in the natural, zoonotic cycle compared with maintenance in culture. Infect Immun. 1995;63:4795–4801. doi: 10.1128/iai.63.12.4795-4801.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafson R, Svenungsson B, Forsgren M, Gardulf A, Granström M. Two year survey of incidence of Lyme disease and tick-borne encephalitis in a high risk population in Sweden. Eur J Clin Microbiol Infect Dis. 1992;11:894–900. doi: 10.1007/BF01962369. [DOI] [PubMed] [Google Scholar]
- 11.Guttman D S, Wang P, Wang I-N, Bosler E, Luft B, Dykhuizen D. Multiple infections of Ixodes scapularis ticks by Borrelia burgdorferi as revealed by single-strand confirmation polymorphism. J Clin Microbiol. 1996;34:652–656. doi: 10.1128/jcm.34.3.652-656.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hongyo T, Buzard G S, Calvert R J, Weghorst C M. ’Cold SSCP’: a simple, rapid, and nonradioactive method for optimized single strand conformation polymorphism analysis. Nucleic Acids Res. 1993;21:3637–3642. doi: 10.1093/nar/21.16.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuiper, H., A. P. van Dam, L. Spanjaard, B. M. de Jongh, A. Widjojokusumo, T. C. P. Ramselaar, I. Cairo, K. Vos, and J. Dankert. Isolation of Borrelia burgdorferi from biopsy specimens taken from healthy-looking skin of patients with Lyme borreliosis. J. Clin. Microbiol. 32:715–720. [DOI] [PMC free article] [PubMed]
- 13a.Nowakowski J, Schwartz I, Nadelman R, Liveris D, Aguero-Rosenfeld M, Wormser G. Culture-confirmed infection and reinfection with Borrelia burgdorferi. Ann Intern Med. 1997;127:130–132. doi: 10.7326/0003-4819-127-2-199707150-00006. [DOI] [PubMed] [Google Scholar]
- 14.Padula S J, Sampieri A, Dias F, Szczepanski A, Ryan R W. Molecular characterization and expression of p23 (OspC) from a North American strain of Borrelia burgdorferi. Infect Immun. 1993;61:5097–5105. doi: 10.1128/iai.61.12.5097-5105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peisman J, Dolan M C, Happ C M, Luft B J, Rooney S E, Mather T M, Golde W T. Duration of immunity to tick-transmitted Borrelia burgdorferi in naturally infected mice. Infect Immun. 1997;65:4043–4047. doi: 10.1128/iai.65.10.4043-4047.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persing D H, Mathiesen D, Podzorski D, Barthold S W. Genetic stability of Borrelia burgdorferi recovered from chronically infected immunocompetent mice. Infect Immun. 1994;62:3521–3527. doi: 10.1128/iai.62.8.3521-3527.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfister H W, Neubert U, Wilske B, Preac-Mursic V, Einhaupl K M, Bosario G D. Reinfection with Borrelia burgdorferi. Lancet. 1986;ii:984–985. doi: 10.1016/s0140-6736(86)90640-9. [DOI] [PubMed] [Google Scholar]
- 18.Preac-Mursic V, Weber K, Pfister W, Wilske B, Gross B, Bauman A, Prokop J. Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection. 1989;17:355–359. doi: 10.1007/BF01645543. [DOI] [PubMed] [Google Scholar]
- 19.Rose C D, Fawcett P T, Klein J D, Epps S C, Caputo G M, Doughty R A. Reinfection in pediatric Lyme borreliosis. Ann Rheum Dis. 1993;52:695–696. doi: 10.1136/ard.52.9.695-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinsky R J, Piesman J. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J Clin Microbiol. 1989;27:1723–1727. doi: 10.1128/jcm.27.8.1723-1727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steere A C. Lyme disease. N Engl J Med. 1989;3219:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 22.Stobierski M G, Hall W N, Robinson-Dunn B, Stiefel H, Shiflett S, Carlson V. Isolation of Borrelia burgdorferi from two patients in Michigan. Clin Infect Dis. 1994;19:944–946. doi: 10.1093/clinids/19.5.944. [DOI] [PubMed] [Google Scholar]
- 23.Strand J, Walker E D, Merritt R. Vector control bulletin. North Central States. 1992;1:111–118. [Google Scholar]
- 24.Strle F, Cheng Y, Cimpermsn J, Maraspin V, Lotric-Furlan S, Nelson J A, Picken M M, Ruzic-Sabljic E, Picken R N. Persistence of Borrelia burgdorferi sensu lato in resolved erythema migrans lesions. Clin Infect Dis. 1995;21:380–389. doi: 10.1093/clinids/21.2.380. [DOI] [PubMed] [Google Scholar]
- 25.Strle F, Preac-Mursic V, Cimpermsn J, Ruzic E, Maraspin V, Jereb M. Azithromycin versus doxycycline for the treatment of erythema migrans: clinical and microbiological findings. Infection. 1993;21:83–88. doi: 10.1007/BF01710737. [DOI] [PubMed] [Google Scholar]
- 26.Walker E D, Smith T W, DeWitt J, Beaudo D C, McClean R G. Prevalence of Borrelia burgdorferi in host seeking ticks (Acari:Ixodidae) from a Lyme disease endemic area in northern Michigan. J Med Entomol. 1994;31:524–528. doi: 10.1093/jmedent/31.4.524. [DOI] [PubMed] [Google Scholar]
- 27.Wang, I.-N., J. Dunn, B. J. Luft, and D. Dykhuizen. Genetic diversity of OspC in a local population of Borrelia burgdorferi sensu stricto. Genetics, in press. [DOI] [PMC free article] [PubMed]