Abstract
Aims
Catheter ablation with a cryoballoon (CB) provides effective and durable pulmonary vein (PV) isolation (PVI) associated with encouraging clinical outcome data. The novel POLARx CB incorporates unique features, which may translate into improved safety, efficacy, and outcomes. The ICE-AGE-1 study aimed to assess the efficacy, safety, and 1-year clinical follow-up of the POLARx CB in comparison to the Arctic Front Advance Pro CB (AF-CB4).
Methods and results
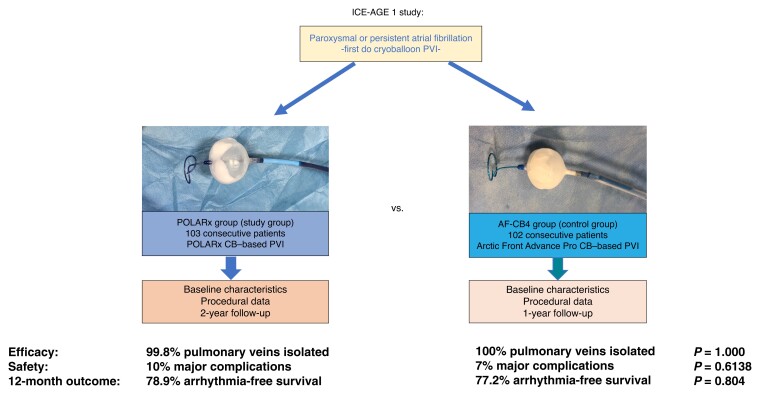
A total of 103 consecutive patients with paroxysmal or persistent atrial fibrillation (AF) who underwent POLARx-based PVI (POLARx group) were prospectively enrolled and were compared to 102 consecutive patients previously treated with the AF-CB4 (AF-CB4 group). The mean age was 68.7 ± 10.2 (POLARx) and 65.7 ± 12 (AF-CB4, P = 0.0551) years. A total of 412 (POLARx) and 404 (AF-CB4) PVs were identified. All PVs, except for one PV in the POLARx group, were successfully isolated. A significant difference regarding the mean minimal CB temperature reached using the POLARx CB (−56.1 ± 8.3°C) and AF-CB4 (−46.9 ± 10.1°C) was observed (P < 0.0001). Real-time PVI was visualized in 71% of PVs in the POLARx group and 46% of them in the AF-CB4 group (P < 0.001). The mean procedure time was comparable: 54.5 ± 17.1 min for POLARx and 59.4 ± 18.6 min for AF-CB4 (P = 0.0509). No differences were observed in terms of periprocedural complications. There were comparable rates in freedom of AF or atrial tachycardia recurrence after 12 months, beyond a 90-day long blanking period: 78.9% in the POLARx group vs. 77.2% in the AF-CB4 group (P = 0.804).
Conclusion
The novel POLARx CB showed similar safety, efficacy, and 1-year recurrence-free survival rates compared to the AF-CB4. A higher rate of real-time electrical PV recordings and significantly lower balloon temperatures were observed using the POLARx as compared to AF-CB4.
Keywords: Atrial fibrillation, Pulmonary vein isolation, Cryoballoon, Acute efficacy, Follow-up
Central Illustration
Graphical Abstract.
What’s new?
The ICE-AGE-1 study aimed to assess the efficacy, safety, and 1-year clinical follow-up of the POLARx cryoballoon (CB) (POLARx) in comparison to the Arctic Front Advance Pro CB (AF-CB4).
Real-time pulmonary vein (PV) isolation was visualized in 71% of PVs in the POLARx group and 46% in the AF-CB4 group (P < 0.001). The mean procedure time was comparable: 54.5 ± 17.1 min for POLARx and 59.4 ± 18.6 min for AF-CB4 (P = 0.0509). No differences were observed in terms of periprocedural complications.
There were comparable rates in freedom of atrial fibrillation recurrence after 12 months: 78.9% in the POLARx group vs. 77.2% in the AF-CB4 group (P = 0.804).
Introduction
Pulmonary vein (PV) isolation (PVI) is the cornerstone of interventional treatment of atrial fibrillation (AF) and is recommended as first-line therapy in patients with suspected arrhythmia-induced cardiomyopathy, as well as in patients with ineffective or non-tolerated antiarrhythmic therapy.1
The non-inferiority of cryoballoon (CB)–based PVI compared to radiofrequency (RF)–based PVI has been demonstrated in multiple clinical trials in terms of safety, efficacy, and lesion durability.2–4 The safety and efficacy profiles of the second (CB2) and fourth (CB4) generations of the Arctic Front Advance CBs (Arctic Front Advance and Arctic Front Advance Pro, Medtronic, Inc., Minneapolis, MN, USA) have been thoroughly evaluated and showed excellent acute success rates, reduced complication rates, and promising long-term follow-up (FU) results compared to earlier generations.5–9 The newly introduced POLARx cryoablation system (Boston Scientific, St Paul, MN, USA) incorporates a novel design and modern technical features aiming to increase periprocedural safety and efficacy, as well as further simplifying the balloon-based PVI.10 The POLARx system is characterized by its capability to maintain a constant balloon pressure throughout inflation and freezing cycles, which might decrease the rate of balloon dislodgement from the PV ostium (pop-out phenomenon) during energy delivery.10,11 Moreover, the POLARx system aims to increase the comfort of the operator during the procedure by offering immediate control of the inflation and deflation, energy delivery, and double-stop manoeuvre, as well as documentation of time to isolation (TTI) via a foot pedal and a remote control unit.10 In this study, we aim to assess the safety, efficacy, feasibility, and 1-year FU results of the novel POLARx cryoablation system for PVI in patients with paroxysmal AF (PAF) and persistent AF (persAF).
Methods
Inclusion and exclusion criteria
This is a prospective, non-randomized, interventional, single-centre study (Figure 1). Consecutive patients with symptomatic, drug-refractory PAF or persAF were prospectively enrolled. Between August 2020 and November 2021, 103 patients underwent PVI ablation using the POLARx cryoablation system (POLARx group; study group). A total of 102 consecutive patients previously treated with the AF-CB4 between November 2019 and July 2020 represented the control group (AF-CB4 group; control group). Exclusion criteria were prior left atrial (LA) ablation procedures, a LA diameter of >60 mm, severe valvular heart disease, or contraindications to post-interventional oral anticoagulation. All patients provided written informed consent before inclusion. All patient-related data were anonymized.
Figure 1.
ICE-AGE-1 study flow chart. Study flow chart. CB, cryoballoon; PVI, pulmonary vein isolation.
The ICE-AGE-1 study was approved by the local ethics committee (Lübeck ablation registry ethical review board number: WF-028/15) and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Pre-procedural management
Pre-procedural transoesophageal echocardiography (TEE) was performed to rule out intracardiac thrombi before the procedure. No additional pre-procedural imaging was carried out. In patients under treatment with vitamin K antagonists (VKAs), the procedure was conducted under therapeutic international normalized ratio (INR) values between 2 and 3, while in those under non-VKA oral anticoagulants (NOACs), the morning dose of NOACs was omitted on the day of the procedure.
Intraprocedural management
All procedures were performed by four physicians who were highly experienced in CB procedures. A comprehensive description of intraprocedural management has been reported in previous publications from our department.8,9,12 Briefly, the procedures were performed under deep sedation using midazolam, fentanyl, and continuous infusion of propofol. Two ultrasound-guided right femoral vein punctures were carried out, and two 8 French (F) short sheaths were inserted. One 7 F diagnostic catheter was positioned within the coronary sinus (CS) via a sheath in the right femoral vein. Single transseptal puncture (TSP) was performed under fluoroscopic guidance using a modified Brockenbrough technique and an 8.5 F transseptal sheath (either a TSX transseptal delivery system or a TSX transseptal needle; Boston Scientific or SL1; St Jude Medical, Inc.). After the TSP, LA access was confirmed by contrast medium injection via the transseptal needle. Selective angiography of all PVs was carried out utilizing a 7 F multipurpose catheter or the transseptal sheath to identify the PV ostia. Afterwards, the 15.9 F POLARSHEATH (POLARx group, Boston Scientific, St Paul, MN, USA) or the 15 F FlexCath Advance sheath (AF-CB4 group, Medtronic, Inc., Minneapolis, MN, USA) was inserted over a guidewire in the transseptal position. Both sheaths were continuously flushed with heparinized saline (20 mL/h). After the TSP, an activated clotting time (ACT) of >300 s was targeted by means of heparin boluses.9,10
Cryoballoon pulmonary vein isolation
The intraluminal oesophageal temperature was monitored during each refrigerant injection using a spiral multisite oesophageal probe (CIRCA S-CATH; Circa Scientific, Englewood, CO, USA). An intraluminal oesophageal temperature cut-off value of 15°C was used to avoid oesophageal thermal injuries. In case of a temperature drop of <15°C, the application was stopped immediately using a double-stop technique.13,14
During the isolation of the septal PVs, continuous phrenic nerve (PN) pacing using a maximum output and pulse width at a cycle length of 1000–1200 ms through a 7 F diagnostic catheter positioned in the superior vena cava was performed. Intermittent fluoroscopic evaluation, tactile feedback of the contractions of the diaphragm, and continuous motor action potential (CMAP) monitoring were used to assess the PN capture. Weakening or loss of diaphragm movement or a reduction of the CMAP amplitude of at least 30% led to an immediate termination of energy delivery using the double-stop technique.15 In case of PN palsy (PNP), no additional energy delivery was applied at the level of the right PVs.16,17 Apart from the abovementioned safety manoeuvres for PNP prevention, the novel diaphragm movement sensor (DMS) was utilized to monitor PN function in the POLARx group. The DMS cut-off was set at 60% of diaphragm movement. The freeze cycle was terminated by ‘double stop’ if the cut-off value was reached and no PN capture was detected immediately.
The ablation sequence started with the left superior PV (LSPV), followed by left inferior PV (LIPV), right inferior PV (RIPV), and right superior PV (RSPV). Before initiating the freezing cycle, the PV occlusion was verified by contrast medium injection under fluoroscopy. Additionally, a second injection was performed 5–10 s after starting the freezing cycle to assess the stability of the occlusion. If the stable occlusion was confirmed, the freezing cycle was continued; otherwise, the balloon was repositioned, and a third contrast medium injection was used to reassess the occlusion, or the freezing cycle was stopped, and another ablation attempt was made. After 70 s of freezing, a gentle pull-down manoeuvre was performed during the isolation of the inferior PVs in all cases.10
The procedure was performed using a TTI-based approach as follows: if the TTI could be assessed and achieved <60 s, a freezing cycle of 180 s without a bonus-freeze application was performed. If TTI was ≥60 s, a freezing cycle of 180 s, followed by a bonus-freeze application of 180 s was carried out. The cut-off minimal CB temperatures were set at −70°C for the POLARx group and at −60°C for the AF-CB4 group.
The procedural success was defined by the disappearance of all PV recordings on the spiral mapping catheter positioned inside the PVs after energy delivery (entrance block). Further pacing manoeuvres and adenosine testing were not performed.
In case of a pop-out phenomenon (CB dislodgement from the ostium of the PV after freezing cycle initiation), the balloon was either slightly repositioned, followed by another contrast medium injection for occlusion assessment, or the freezing cycle was stopped, and a second attempt was made.
For the patients who had AF during the procedure, electrical cardioversion was carried out after the final freezing cycle to achieve sinus rhythm (SR), followed by reconfirmation of PVI for all the PVs.
Post-procedural care
A ‘figure-of-eight’ suture and a pressure bandage were used to prevent femoral bleeding. The bandage was removed 4 h after the procedure and the suture on the next day. Pericardial effusion (PE) was ruled out in all patients, using transthoracic echocardiography (TTE) performed immediately, at 2 h and on the first post-procedural day. For patients under VKA treatment and with subtherapeutic INR values, low-molecular-weight heparin (LMWH) was administered until a therapeutic INR of 2–3 was achieved. Non-VKA oral anticoagulants were reinitiated the evening after the procedure. All patients received oral anticoagulation for at least 3 months post-procedural, which was thereafter continued based on the individual thrombo-embolic risk (CHA2DS2-VASc score). All patients received an antiarrhythmic drug for 3 months post-ablation, while a proton–pump inhibitor (PPI) was prescribed for 6 weeks.8–10
Following a 3-month blanking period, patients completed outpatient clinic visits, including electrocardiograms (ECGs) and 24 h Holter ECGs at 3, 6, and 12 months. In addition, regular telephone interviews were performed. Additional outpatient clinic visits were immediately initiated in cases of symptoms suggestive of arrhythmia recurrence.
Endpoints
Primary endpoint
The primary endpoint was defined as freedom from documented AF or atrial tachycardia (AT) recurrence 12 months after PVI, excluding a 90-day blanking period. Atrial tachycardia or AF recurrence was defined as any ECG-documented atrial tachyarrhythmia lasting for at least 30 s, including AF, AT, and atrial flutter. Patients completed outpatient clinic visits at 3, 6, and 12 months including ECGs and 24 h Holter ECGs. In addition, regular telephonic interviews were performed.
Secondary endpoints
The secondary endpoints were as follows: acute procedural success defined as confirmation of electrical isolation with a circular mapping catheter, procedural parameters (e.g. procedure time, LA dwelling time, and fluoroscopy time), number and duration of RF applications, and number of first pass isolations as well as periprocedural complications. Periprocedural complications were defined according to the latest guidelines. Adverse events were divided into the following categories: possible, probable, or definitely related to the ablation procedure, and were mentioned as safety events. An adverse event was considered serious if it resulted in a permanent injury, disability, or death, requiring interventional treatment or additional hospitalization for >24 h. All other safety events were defined as minor complications.
Statistical analysis
Categorical variables are reported as absolute and relative frequencies, n (%). They were compared using the χ2 test or Fisher’s exact test as appropriate. The continuous variables are reported as mean ± standard deviation (SD) and were compared using Student’s t-test. Recurrence-free survival was estimated with the Kaplan–Meier method. All P-values are two-sided, and a P-value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 29.0 (IBM SPSS Statistics).
Results
Patient characteristics
A total of 205 consecutive patients with PAF and persAF received CB-based PVI using either the POLARx CB (n = 103) or the AF-CB4 (n = 102). The baseline characteristics of these patients are shown in Table 1.
Table 1.
Patients’ baseline characteristics
| Variable | POLARx | AF-CB4 | P-value | |
|---|---|---|---|---|
| Patients, n | 103 | 102 | ||
| Age, years | 68.7 ± 10.2 | 65.7 ± 12 | 0.0551 | |
| Female gender, n | 47 (46) | 39 (38) | 0.3227 | |
| Paroxysmal AF, n | 53 (51) | 42 (41) | 0.1620 | |
| LA size, mL/m2 | 32.9 ± 11.4 | 31.7 ± 9.8 | 0.4201 | |
| Systolic HF, n | 11 (11) | 15 (15) | 0.4093 | |
| Arterial hypertension, n | 76 (74) | 71 (70) | 0.5378 | |
| Diabetes mellitus type II, n | 12 (12) | 11 (11) | 1 | |
| Coronary artery disease, n | 29 (28) | 27 (26) | 0.8757 | |
| 0 | 9 (9) | 10 (10) | 0.827 | |
| 1 | 21 (20) | 17 (17) | 0.439 | |
| 2 | 32 (31) | 19 (19) | 0.300 | |
| CHA2DS2-VASc score | 3 | 29 (28) | 27 (26) | 0.787 |
| 4 | 5 (5) | 16 (16) | 0.106 | |
| 5 | 6 (6) | 9 (9) | 0.410 | |
| ≥6 | 1 (1) | 4 (4) | 0.171 | |
Values are counts (n), n (%), or mean ± standard deviation.
AF, atrial fibrillation; HF, heart failure; LA, left atrium.
Acute ablation results
A total of 816 PVs were identified in 205 patients (Table 2). Four patients in the AF-CB4 group presented with a left common PV (LCPV); meanwhile, there were no patients with a LCPV in the POLARx group. The PVI rate was 99.8% in the POLARx group (one PV remained not isolated) and 100% in the AF-CB4 group. The first attempt all vein isolation (FAAVI) rate was achieved in 48% of the patients in the POLARx group and 50% in the AF-CB4 group (P = 0.7805). The minimal CB temperature was significantly lower in the POLARx patients (−56.1 ± 8.3 vs. 46.9 ± 10.1°C; P < 0.0001), however, without a significant difference in the minimal oesophageal temperature (31.6 ± 6.4 vs. 32.1 ± 9.3°C; P = 0.3704). Moreover, this distinction did not lead to a significant difference in TTI (42.4 ± 27.7 s for the study group vs. 42.2 ± 28.3 s for the control group; P = 0.9188).
Table 2.
Acute ablation results and in-hospital complications
| Variable | POLARx | AF-CB4 | P-value |
|---|---|---|---|
| Patients, n | 103 | 102 | |
| PVs, n | 412 | 404 | |
| Isolated PVs, n | 411 (99.8) | 404 (100) | 1.000 |
| Total CB cycles until PVI, n | 1.2 ± 0.4 | 1.2 ± 0.5 | 1.000 |
| Total CB cycles, n | 1.2 ± 0.4 | 1.2 ± 0.5 | 1.000 |
| FAAVI, n | 49 (48) | 51 (50) | 0.7805 |
| Minimal CB temperature, °C | −56.1 ± 8.3 | −46.9 ± 10.1 | <0.001* |
| Minimal oesophageal temperature, °C | 31.6 ± 6.4 | 32.1 ± 9.3 | 0.3704 |
| Time to PVI, s | 42.4 ± 27.7 | 42.2 ± 28.3 | 0.9188 |
| TTI recordings, n | 292 (71) | 184 (46) | <0.0001* |
| Total freezing time, s | 203 ± 68 | 209 ± 82 | 0.2552 |
| Total procedure time, min | 54.5 ± 17.1 | 59.4 ± 18.6 | 0.0509 |
| Total fluoroscopy time, min | 9.3 ± 4.3 | 12.5 ± 9.3 | 0.0018* |
| Total amount of contrast, mL | 70.5 ± 22.8 | 75.2 ± 22.6 | 0.1398 |
| Periprocedural complications | |||
| Major complications, n | 10 (10) | 7 (7) | 0.6138 |
| Cardiac tamponade, n | 0 | 1 (1) | 0.4976 |
| Severe bleeding, n | 0 | 1 (1) | 0.4976 |
| Persistent PN injury, n | 7 (7) | 3 (3) | 0.3315 |
| Stroke/TIA, n | 2 (2) | 0 | 0.4976 |
| AV block III° with intervention, n | 1 (1) | 0 | 1.000 |
| Death, n | 0 | 1 (1) | 0.4976 |
| Sinus arrest with pacemaker implantation, n | 0 | 1 (1) | 0.4976 |
| Minor complications, n | 4 (4) | 6 (6) | 0.5375 |
| Minor bleeding, n | 1 (1) | 0 | 1.000 |
| Pericardial effusion, n | 0 | 0 | 1.000 |
| Transient air embolism | 2 (2) | 0 | 0.4976 |
| Aneurysma spurium, n | 1 (1) | 2 (2) | 0.6213 |
| Transient PN injury (until discharge), n | 0 | 2 (2) | 0.2463 |
| Transient PN injury (until the end of the procedure), n | 0 | 2 (2) | 0.2463 |
Values are counts (n), n (%), or mean ± standard deviation.
AV, atrioventricular; CB, cryoballoon; FAAVI, first attempt all vein isolation; PN, phrenic nerve; PV, pulmonary vein; PVI, pulmonary vein isolation; TIA, transient ischaemic attack; TTI, time to isolation.
Statistical significance.
Interestingly, the rate of live TTI recordings was significantly higher in the POLARx group (71% vs. 46%; P < 0.0001). No difference was noted between the groups regarding the duration of total freezing time (P = 0.2552), while a significantly shorter total fluoroscopy time (9.3 ± 4.3 s vs. 12.5 ± 9.3; P = 0.0018) and a trend towards shorter procedure time (54.5 ± 17.1 vs. 59.4 ± 18.6; P = 0.0509) were observed in the POLARx group. The pop-out phenomenon was reported in four PVs in the AF-CB4 group, but no case occurred in the POLARx group (P = 0.0596).
Complications
A detailed comparison of complications is listed in Table 2. The incidence of major complications was similar between the POLARx (10%) group and the AF-CB4 group (7%; P = 0.6138). Phrenic nerve palsy was reported in seven patients from each group (P = 1); however, persistent PNP until discharge was noted in all seven (6.8%) patients in the study group (POLARx) and in only three (2.9%) patients in the control (AF-CB4) group (P = 0.3315). Two of the four patients with transient PNP in the AF-CB4 group had PNP resolution by the end of the procedure and the other two until discharge. All PNP recovered within the 12-month FU. Except for one patient from the POLARx group, in whom PNP occurred before the isolation of the RSPV, all the other patients had isolated PVs at the moment of PN injury. Two patients in the study group experienced transient air embolism, and the other two patients from the same group suffered a stroke/transient ischaemic attack (TIA). One patient experienced a pericardial tamponade, followed by pericardial puncture and aspiration with autotransfusion. Due to continuous pericardial tamponade, the decision for a surgical intervention was performed. The patient received surgical repair. The patient died after 5 weeks due to multiorgan failure.
Acute ablation results for individual pulmonary veins
The acute ablation data with regard to each PV are presented in Table 3. The difference in minimal CB temperature was noted in all PVs. The higher rate of TTI recordings in the POLARx group was significant for the LIPV (78.6% vs. 57.8%; P = 0.0056), RIPV (65% vs. 28.4%; P < 0.0001), and RSPV (63.1% vs. 39.2%; P = 0.0008), while only a trend in this direction was noted in the LSPV (77.6% vs. 62.7%; P = 0.0609). A trend towards a longer time to PVI was noted in the study group during ablation of the LIPV (37.4 ± 23.2 vs. 31.6 ± 19.9; P = 0.0591), while the difference was less significant for the other PVs. All the other parameters were similar between the groups when analysing the individual PVs. The pop-out phenomenon was reported in three patients from the AF-CB4 group when ablating at the level of the RIPV (2.9% vs. 0%; P = 0.1214) and in one patient from the same group at the level of the LSPV (1% vs. 0%; P = 0.4876).
Table 3.
Acute ablation results for individual pulmonary veins
| Variable | POLARx | AF-CB4 | P-value |
|---|---|---|---|
| LSPV, n | 103 | 98 | |
| Total cycles until PVI, n | 1.2 ± 0.5 | 1.3 ± 0.6 | 0.1999 |
| Total cycles, n | 1.2 ± 0.5 | 1.3 ± 0.6 | 0.1999 |
| FAVI, n | 78 (76) | 76 (78) | 0.8678 |
| Bonus-freeze cycles, n | 24 (23.3) | 21 (21.4) | 0.8658 |
| Minimal CB temperature, °C | −57.1 ± 6.8 | −48 ± 9.6 | <0.0001* |
| Minimal oesophageal temperature, °C | 31.6 ± 5.6 | 32.2 ± 8.7 | 0.5597 |
| Time to PVI, s | 44.6 ± 21.8 | 50 ± 32 | 0.1618 |
| TTI recordings, n | 80 (77.6) | 64 (62.7) | 0.0609* |
| Total freezing time, s | 217 ± 72 | 219 ± 102 | 0.8721 |
| Pop-out phenomenon, n | 0 | 1 (1) | 0.4876 |
| LIPV, n | 103 | 98 | |
| Total cycles until PVI, n | 1.1 ± 0.3 | 1.2 ± 0.5 | 0.0854 |
| Total cycles, n | 1.1 ± 0.3 | 1.2 ± 0.5 | 0.0854 |
| FAVI, n | 90 (87) | 85 (87) | 1.000 |
| Bonus-freeze cycles, n | 12 (11.7) | 12 (12.2) | 1.000 |
| Minimal CB temperature, °C | −55.2 ± 5.4 | −45 ± 10.4 | <0.0001* |
| Minimal oesophageal temperature, °C | 28.2 ± 8.8 | 29.8 ± 11.7 | 0.2730 |
| Time to PVI, s | 37.4 ± 23.2 | 31.6 ± 19.9 | 0.0591 |
| TTI recordings, n | 81 (78.6) | 59 (57.8) | 0.0056* |
| Total freezing time, s | 191 ± 50 | 198 ± 68 | 0.4051 |
| Pop-out phenomenon, n | 0 | 0 | 1.000 |
| LCPV, n | 0 | 4 | |
| Total cycles until PVI, n | – | 1.5 (1, 2) | |
| Total cycles, n | – | 1.5 (1, 2) | |
| FAVI, n | – | 2 (50) | |
| Bonus-freeze cycles, n | – | 2 (50) | |
| Minimal CB temperature, °C | – | −47.5 (−45, −61) | |
| Minimal oesophageal temperature, °C | – | 33.2 (11.5, 36.5) | |
| Time to PVI, s | – | — | |
| TTI recordings, n | – | 0 (0) | |
| Total freezing time, s | – | 198 (150, 360) | |
| Pop-out phenomenon, n | – | 0 | |
| RSPV, n | 103 | 102 | |
| Total cycles until PVI, n | 1.2 ± 0.4 | 1.1 ± 0.4 | 0.0750 |
| Total cycles, n | 1.2 ± 0.4 | 1.1 ± 0.4 | 0.0750 |
| FAVI, n | 85 (83) | 88 (86) | 0.5644 |
| Bonus-freeze cycles, n | 16 (15.5) | 13 (12.7) | 0.6892 |
| Minimal CB temperature, °C | −55.1 ± 12.6 | −49.5 ± 10.4 | 0.0006* |
| Minimal oesophageal temperature, °C | 33.9 ± 3.6 | 33.7 ± 8.5 | 0.8263 |
| Time to PVI, s | 42.8 ± 35 | 39.3 ± 28.3 | 0.4323 |
| TTI recordings, n | 65 (63.1) | 40 (39.2) | 0.0008* |
| Total freezing time, s | 197 ± 66 | 194 ± 60 | 0.7339 |
| Pop-out phenomenon, n | 0 | 0 | 1.000 |
| RIPV, n | 103 | 102 | |
| Total cycles until PVI, n | 1.2 ± 0.4 | 1.3 ± 0.5 | 0.1152 |
| Total cycles, n | 1.2 ± 0.4 | 1.3 ± 0.5 | 0.1152 |
| FAVI, n | 86 (83) | 78 (76) | 0.2258 |
| Bonus-freeze cycles, n | 15 (14.6) | 23 (22.5) | 0.1542 |
| Minimal CB temperature, °C | −56.7 ± 6.2 | −44.9 ± 9.6 | <0.0001* |
| Minimal oesophageal temperature, °C | 32.6 ± 5.6 | 33 ± 7.7 | 0.6708 |
| Time to PVI, s | 45.1 ± 30.6 | 49.3 ± 27.7 | 0.3043 |
| TTI recordings, n | 67 (65.0) | 29 (28.4) | <0.0001* |
| Total freezing time, s | 207 ± 78 | 225 ± 90 | 0.1274 |
| Pop-out phenomenon, n | 0 | 3 (3) | 0.1214 |
Values are counts (n), n (%), or mean ± standard deviation.
CB, cryoballoon; FAVI, first attempt vein isolation; LCPV, left common pulmonary vein; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; PVI, pulmonary vein isolation; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; TTI, time to isolation.
Statistical significance.
Follow-up and clinical success
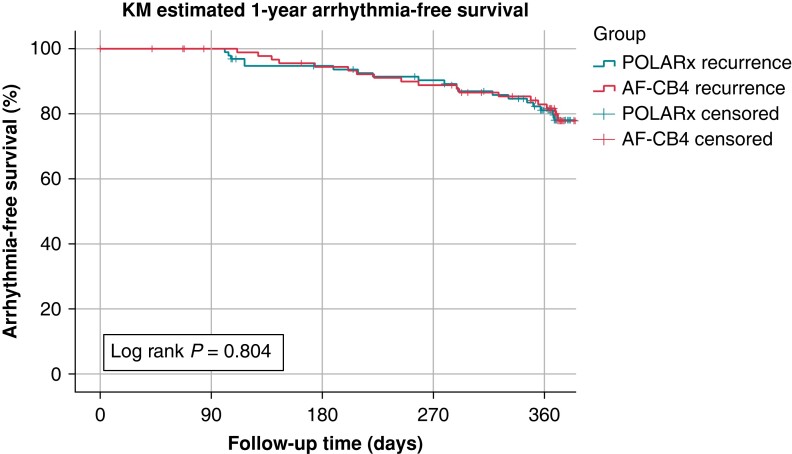
We were able to assess FU in a total of 188 of 205 patients (92%) (the rate of lost to FU was not different between the groups: POLARx: n = 7 vs. AF-CB4: n = 10, P = 0.435). The rate of 12-month AF- or AT-free survival after a 90-day blanking period was 78.9% in the POLARx group vs. 77.2% in the AF-CB4 group (P = 0.804; Figure 2). The mean time to recurrence was 253 ± 107 (POLARx) and 274 ± 100 (AF-CB4) days (P = 0.568), without a significant difference.
Figure 2.
Twelve-month follow-up. Kaplan–Meier estimates within the 12-month follow-up after the index PVI utilizing Arctic Front Advance Pro and POLARx. No statistical differences were found concerning 12-month freedom from atrial tachyarrhythmias. KM, Kaplan–Meier; PVI, pulmonary vein isolation.
For patients with PAF and persAF, the rate of 12-month AF- or AT-free survival after a 90-day blanking period was 84.0%/73.9% in the POLARx group vs. 78.9%/75% in the AF-CB4 group (P = 0.855/P = 0.902).
Discussion
The ICE-AGE-1 study sought to evaluate the acute efficacy, safety, and 1-year clinical outcome of the POLARx cryoablation system, as compared to the established Arctic Front Advance Pro system for the interventional treatment of PAF and persAF. The main findings of this study are as follows:
The minimal CB temperature was significantly lower in the POLARx group, however, without a noteworthy difference in oesophageal temperatures or time to PVI.
The ability of detecting TTI recordings was significantly increased with the POLARx system.
Significantly lower fluoroscopy time, as well as a trend towards a lower procedure time, was observed in the POLARx group.
A trend towards a lower incidence of a pop-out phenomenon was observed using the POLARx system.
No difference in terms of major and minor complications was reported between the groups.
There was no difference in AT/AF occurrence at the 12-month FU between the CB systems.
Since the FIRE AND ICE study demonstrated the non-inferiority of CB-based PVI compared to RF-based procedures, the CB procedures became the cornerstone of interventional AF treatment.1,4 The safety and efficacy profiles of the second and fourth generations of Arctic Front Advance ablation systems have been thoroughly studied.6–9,13
Recently, the POLARx cryoablation system has been introduced in clinical practice and came with several unique features, aiming to increase the safety and efficacy of the ablation procedures. Basically, the two ablation systems provide a similar configuration, consisting of a CB catheter (POLARx™/AFAP™), a steerable sheath (POLARSHEATH™/FlexCath Advance™), a circular mapping catheter (POLARMAP™/Achieve Advance™), and a console (SMARTFREEZE™/CryoConsole™).18 However, the new ablation system offers a stable inner CB pressure during inflation and refrigerant injection, aiming to reduce the rate of a pop-out phenomenon and increasing the compliance of the balloon and thus improving the balloon–tissue contact.10,18–20 Moreover, the steerable sheath of the POLARx system offers a slightly wider deflection angle (155° vs. 135°).18 The new SMARTFREEZE console provides the possibility to display the diaphragm movement monitoring and the oesophageal temperature measurements, while the incorporation of the slider switch and the pedal offers full control of the inflation–deflation process, freezing-double-stop manoeuvres, and TTI documentation.10,18,19 All these features aim to increase the comfort of the operator and to further simplify the CB procedures.
Previous studies comparing the two ablation systems showed that the minimal CB temperatures reached by the POLARx CB were significantly lower.10,11,20 Tanese et al.11 prospectively enrolled 267 consecutive patients receiving CB-based PVI using either the POLARx system or the AF-CB4 system and reported significantly lower nadir temperatures with the former, with respect to each PV. Moreover, in the recently published ANTARCTICA study, the mean minimal CB temperature for the POLARx system was −57.9 ± 7.2°C, which is considerably lower as compared to the reported equivalent of AF-CB4.7–9,19 Our results are in line with these findings, showing that the nadir CB temperature was significantly lower in the study group, as compared to the control group, for all the PVs, as well as for each individual PV. However, it is important that this difference led neither to a lower oesophageal temperature nor to a shorter time to PVI in the POLARx group. These findings are also consistent with other publications and raise questions regarding the real temperature difference at the tissue level.10,11,18,20 As suggested before, this lower CB temperature might be the result of different proprieties of the balloon’s material, as well as different inflation pressures.10
The publication from Tanese et al.11 reports similar PV signal recording rates for both catheters, except for the LIPV, in which the POLARx system showed a significantly higher rate of live recordings of potentials. In our study, this difference was significant for all the PVs (71% vs. 46%; P < 0.0001), as well as for the individual PVs, except for the LSPV, in which only a trend towards a higher rate of TTI recordings was noted. Our results regarding the rate of TTI recordings in the POLARx group (71%) are in line with the results of the ANTARCTICA study (71.9%) and prove the high efficacy of the new catheter in achieving TTI-based PVI.19
In contrast to the previously published studies, we found a shorter fluoroscopy time in the POLARx group as compared to the control group. In the recently published comparison by Menger et al.,18 the fluoroscopy time was similar between the groups (12.1 ± 6.8 min in the POLARx group vs. 13.0 ± 7.7 min in the AF-CB4 group; P = 0.55). Moreover, the initial multicentre experience published by Yap et al.20 showed a trend to a longer fluoroscopy time when using the novel ablation system (14.0 vs. 10.8 min; P = 0.141). In our study, the mean fluoroscopy time in the study group was 9.3 ± 4.3 min, which seems to be considerably lower compared to those reported in previous publications.11,18–20 This difference might be at least partially explained by the single-centre character of the study and by the fact that only highly experienced operators performed the procedures. The trend towards shorter procedure times in the POLARx group might be explained by the new features of the system (slider switch, pedal, and monitor), which might facilitate the workflow during the procedure.
As previously discussed, the main new feature of the POLARx system is the ability to maintain a constant CB pressure during inflation and freezing, as well as a constant CB diameter throughout energy delivery, thus limiting the balloon dislodgement after refrigerant injection initiation and improving the tissue contact and occlusion.10,11 Our results confirm this hypothesis, showing a trend towards a lower rate of a pop-out phenomenon in the study group (P = 0.0596), with no catheter dislodgement reported in this group. These results are in line with the ANTARCTICA study, in which no pop-out phenomenon was reported among 317 patients.19 However, in the present study, the mean number of freezing applications necessary to achieve PVI was similar between the groups, raising questions about the clinical impact of this phenomenon. These results correspond to the published data by Tanese et al.,11 who also showed a similar number of cycles needed to achieve PVI, however, without reporting any incidence of the pop-out phenomenon.
Despite the constant use of PN pacing manoeuvres, CMAP monitoring of the diaphragm as well as utilization of the novel DMS for POLARx patients, the incidence of PNP was 7% for both groups, slightly higher than that reported in the worldwide YETI registry.16 The lightly higher rate of PNP that persisted until discharge was observed in the POLARx group, which might be partially explained by the lower CB temperatures reached in this group. Even though the previously mentioned study reported that 97% of these patients show PNP recovery at 12 months post-ablation, we strongly encourage the use of pacing manoeuvres associated with the CMAP monitoring and the double-stop technique in order to limit the potential long-term consequences of this complication.16 The safety profile of the two ablation systems seems to be similar to the results confirmed also by previous publications.10,11,18,20
Besides the observed similar safety and efficacy features of the two CB systems, these observations were translated into comparable 1-year FU results, with no statistical differences in AF recurrence after a 90-day blanking period.
Limitations
We present a prospective, non-randomized, interventional study. Although the patients were not randomized, they represented consecutive patients. The current study reflects a high-volume, single-centre experience, and all the patients were treated by highly experienced operators. Thus, the results might not be applicable in everyday clinical practice of centres with less experience. The patients treated in the POLARx group represent the first cases treated with the new ablation system in our centre, and a bias regarding the learning curve, as well as a more careful manipulation of a new technology, may be invoked. No data regarding reablation procedures were available, and the PV reconnection rate has not been assessed for the two groups.
Conclusions
The POLARx ablation system proved similar safety and efficacy profiles when compared to the well-established AF-CB4 ablation system while achieving lower balloon temperatures, higher rates of live TTI recordings, and shorter fluoroscopy times. Moreover, the 1-year atrial arrhythmia-free survival was similar for both devices, certifying the long-term efficacy of the novel POLARx CB system.
Contributor Information
Christian-Hendrik Heeger, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Lübeck, Germany.
Sorin Stefan Popescu, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Tim Inderhees, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Noemi Nussbickel, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Charlotte Eitel, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Bettina Kirstein, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Huong-Lan Phan, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Sascha Hatahet, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Behnam Subin, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Anna Traub, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Niels Große, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Karl-Heinz Kuck, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Julia Vogler, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany.
Roland R Tilz, University Heart Center Lübeck, Department of Rhythmology, University Hospital Schleswig-Holstein, Ratzeburger Allee 160, D-23538 Lübeck, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Lübeck, Germany.
Funding
None declared.
Data availability
Non-digital data supporting this study are curated at the study centre of the Department of Rhythmology, University Hospital Schleswig-Holstein, Germany.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Sørensen SK, Johannessen A, Worck R, Hansen ML, Hansen J. Radiofrequency versus cryoballoon catheter ablation for paroxysmal atrial fibrillation: durability of pulmonary vein isolation and effect on atrial fibrillation burden: the RACE-AF randomized controlled trial. Circ Arrhythm Electrophysiol 2021;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle Let al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring. Circulation 2019;140:1779–88. [DOI] [PubMed] [Google Scholar]
- 4. Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KRJet al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. [DOI] [PubMed] [Google Scholar]
- 5. Ciconte G, Ottaviano L, de Asmundis C, Baltogiannis G, Conte G, Sieira Jet al. Pulmonary vein isolation as index procedure for persistent atrial fibrillation: one-year clinical outcome after ablation using the second-generation cryoballoon. Heart Rhythm 2015;12:60–6. [DOI] [PubMed] [Google Scholar]
- 6. Heeger CH, Subin B, Wissner E, Fink T, Mathew S, Maurer Tet al. Second-generation cryoballoon-based pulmonary vein isolation: lessons from a five-year follow-up. Int J Cardiol 2020;312:73–80. [DOI] [PubMed] [Google Scholar]
- 7. Straube F, Dorwarth U, Pongratz J, Brück B, Wankerl M, Hartl Set al. The fourth cryoballoon generation with a shorter tip to facilitate real-time pulmonary vein potential recording: feasibility and safety results. J Cardiovasc Electrophysiol 2019;30:918–25. [DOI] [PubMed] [Google Scholar]
- 8. Heeger C, Bohnen J, Popescu S, Meyer-Saraei R, Fink T, Sciacca Vet al. Experience and procedural efficacy of pulmonary vein isolation using the fourth and second generation cryoballoon: the shorter, the better? J Cardiovasc Electrophysiol 2021;32:1553–60. [DOI] [PubMed] [Google Scholar]
- 9. Heeger CH, Popescu SS, Saraei R, Kirstein B, Hatahet S, Samara Oet al. Individualized or fixed approach to pulmonary vein isolation utilizing the fourth-generation cryoballoon in patients with paroxysmal atrial fibrillation: the randomized INDI-FREEZE trial. Europace 2022;24:921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tilz RR, Meyer-Saraei R, Eitel C, Fink T, Sciacca V, Lopez LDet al. Novel cryoballoon ablation system for single shot pulmonary vein isolation―the prospective ICE-AGE-X study―. Circ J 2021;85:1296–304. [DOI] [PubMed] [Google Scholar]
- 11. Tanese N, Almorad A, Pannone L, Defaye P, Jacob S, Kilani MBet al. Outcomes after cryoballoon ablation of paroxysmal atrial fibrillation with the PolarX or the Arctic Front Advance Pro: a prospective multicentre experience. Europace 2023;25:873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heeger CH, Rexha E, Maack S, Rottner L, Fink T, Mathew Set al. Reconduction after second-generation cryoballoon-based pulmonary vein isolation―impact of different ablation strategies―. Circ J 2020;84:902–10. [DOI] [PubMed] [Google Scholar]
- 13. Metzner A, Burchard A, Wohlmuth P, Rausch P, Bardyszewski A, Gienapp Cet al. Increased incidence of esophageal thermal lesions using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol 2013;6:769–75. [DOI] [PubMed] [Google Scholar]
- 14. Tilz RR, Schmidt V, Pürerfellner H, Maury P, Chun KRJ, Martinek Met al. A worldwide survey on incidence, management and prognosis of oesophageal fistula formation following atrial fibrillation catheter ablation: the POTTER-AF study. Eur Heart J. Published online April2023;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghosh J, Sepahpour A, Chan KH, Singarayar S, McGuire MA. Immediate balloon deflation for prevention of persistent phrenic nerve palsy during pulmonary vein isolation by balloon cryoablation. Heart Rhythm 2013;10:646–52. [DOI] [PubMed] [Google Scholar]
- 16. Heeger CH, Sohns C, Pott A, Metzner A, Inaba O, Straube Fet al. Phrenic nerve injury during cryoballoon-based pulmonary vein isolation: results of the worldwide YETI registry. Circ Arrhythm Electrophysiol 2022;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heeger CH, Popescu SȘ, Sohns C, Pott A, Metzner A, Inaba Oet al. Impact of cryoballoon application abortion due to phrenic nerve injury on reconnection rates: a YETI subgroup analysis. Europace 2023;25:374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Menger V, Frick M, Sharif-Yakan A, Emrani M, Zink MD, Napp Aet al. Procedural performance between two cryoballoon systems for ablation of atrial fibrillation depends on pulmonary vein anatomy. J Arrhythm Published online March 17, 2023;39:341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heeger CH, Pott A, Sohns C, Riesinger L, Sommer P, Gasperetti Aet al. Novel cryoballoon ablation system for pulmonary vein isolation: multicenter assessment of efficacy and safety—ANTARCTICA study. Europace 2022;24:1917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yap S, Anic A, Breskovic T, Haas A, Bhagwandien RE, Jurisic Zet al. Comparison of procedural efficacy and biophysical parameters between two competing cryoballoon technologies for pulmonary vein isolation: insights from an initial multicenter experience. J Cardiovasc Electrophysiol 2021;32:580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Non-digital data supporting this study are curated at the study centre of the Department of Rhythmology, University Hospital Schleswig-Holstein, Germany.