Abstract
Krüppel-like factors (KLFs) play essential roles in multiple biological functions, including maintaining vascular homeostasis. KLF11, a causative gene for maturity-onset diabetes of the young type 7, inhibits endothelial activation and protects against stroke. However, the role of KLF11 in venous thrombosis remains to be explored. Utilizing stasis-induced murine deep vein thrombosis (DVT) model and cultured endothelial cells (ECs), we identified an increase of KLF11 expression under prothrombotic conditions both in vivo and in vitro. The expression change of thrombosis-related genes was determined by utilizing gain- and loss-of-function approaches to alter KLF11 expression in ECs. Among these genes, KLF11 significantly downregulated tumor necrosis factor-α (TNF-α)-induced tissue factor (TF) gene transcription. Using reporter gene assay, chromatin immunoprecipitation assay, and co-immunoprecipitation, we revealed that KLF11 could reduce TNF-α-induced binding of early growth response 1 (EGR1) to TF gene promoter in ECs. In addition, we demonstrated that conventional Klf11 knockout mice were more susceptible to developing stasis-induced DVT. These results suggest that under prothrombotic conditions, KLF11 downregulates TF gene transcription via inhibition of EGR1 in ECs. In conclusion, KLF11 protects against venous thrombosis, constituting a potential molecular target for treating thrombosis.
Keywords: endothelial cells, early growth response protein 1, Krüppel-like factor, tissue factor, venous thrombosis
Introduction
Venous thromboembolism (VTE), ranked as the third leading vascular disease after myocardial infarction and stroke,1 is a major threat to global health with 10 million cases occurring each year.2 In the United States, the prevalence of VTE is still on the rise, and its annual economic cost is estimated to be $7 to 10 billion.3 Although VTE contributes to a high disease burden, it is considered a preventable disease, which drives the need for a deeper understanding of the mechanism underlying thrombus initiation and propagation. The endothelium plays a critical role in this process as it functions as the intersection point between inflammation and thrombus formation. Inflammation caused by either local vessel injury or systemic disease conditions (e.g., sepsis, dyslipidemia) can induce many endothelial gene expression changes.
The KLF (Krüppel-like factor) family, a group of transcription factors with Cys2/His2 zinc-finger DNA-binding domain,4 plays essential roles in the maintenance of vascular homeostasis and the pathology of various vascular diseases.5,6 Among them, KLF11 gene was initially discovered from a human genetic study which identified several KLF11 gene polymorphisms associated with diabetes.7 Maturity-onset diabetes mellitus of the young type 7, a particular type of early-onset type 2 diabetes mellitus, is defined by harboring mutations in the KLF11 gene.7 Diabetes is commonly complicated with vascular dysfunction, including thrombosis, contributing to one of its leading causes of mortality.8 Others and our laboratory proved that KLF11 is critical in maintaining vascular hemostasis. Endothelial KLF11 has been demonstrated to maintain vascular homeostasis in many diseases, including sepsis, stroke, abdominal aortic aneurysm, and type 2 diabetes mellitus.9-13 We also recently identified that selectively deficiency of KLF11 in vascular smooth muscle cells (VSMCs) aggravated arterial thrombosis formation.14 In line with the known protective effects of KLF11 in vascular diseases, in this study, we further found that KLF11 inhibits deep vein thrombosis (DVT) formation.
Methods
Mice
Conventional KLF11 knockout mice (Klf11 KO) and littermate control mice were generated and the fidelity of KLF11 deficiency was confirmed as previously described.9,14,15 Briefly, the Klf11 KO mice were backcrossed with C57BL/6J mice (The Jackson Laboratory, Stock No: 000664) for six generations, then heterozygous Klf11 KO mice were interbred to produce homozygous Klf11 KO along with wild-type (Wt) littermate control mice for experiments. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Michigan.
Reagents
Tumor necrosis factor-α (TNF-α; 210-TA) was purchased from R&D Systems. Thrombin (T1063) and lipopolysaccharide (LPS; L3012) were purchased from Sigma-Aldrich. BAY 11–7082 (10010266) and SP600125 (10010466) were obtained from Cayman.
Deep Vein Thrombosis Mouse Model
Complete stasis mouse model of inferior vena cava (IVC) was conducted as previously described.16 Briefly, Klf11 KO and Wt mice (8–10 weeks old male mice) were anesthetized with 5% isoflurane and placed in a supine position. A ventral midline incision (2 cm) was made through the skin and peritoneum to expose the abdomen. After laparotomy, the small intestine was exteriorized and covered with a wet sterile pad. Sterile saline was applied during the whole procedure to prevent drying. After gentle separation from the aorta, IVC was ligated by a 7–0 polypropylene suture immediately below the renal vein branch (toward the tail) to obtain complete blood stasis. All visible side branches were ligated or cauterized. After surgery, the peritoneum was closed by monofilament sutures, and the skin was closed by clips. Mice were sacrificed 48 hours after ligation to harvest the thrombus, which was statistically analyzed by weight and length.
Histology and Immunohistochemistry of IVC
Histology and immunohistochemistry were performed as previously described.17,18 In the stasis-induced DVT mouse model or baseline conditions, murine vessels (IVC + abdominal aorta) were harvested and the thrombus was collected for analysis. The vessels were fixed in 10% buffered formalin in phosphate buffered saline (PBS; 245685, Fisher Healthcare) overnight, embedded in paraffin, and mounted to slides at 5 μm thickness. After deparaffinization and rehydration, the slides were treated with 3% hydrogen peroxide for 15 minutes to block endogenous peroxidase activity and subjected to 10 mM citrate buffer for heat-mediated antigen retrieval. The slides were incubated with diluted rat anti-mouse tissue factor (TF) 1H1 antibody (20 μg/mL, Genentech) or control isotypic rat immunoglobulin G (IgG) at 4°C overnight, followed by goat anti-rat IgG with HRP-conjugated secondary antibody (31470, Invitrogen, 1:500 dilution) at room temperature for 1 hour. The color was developed by DAB staining system (Thermo Fisher Scientific). Slides were counterstained by hematoxylin, examined, and photographed in a blinded fashion. The expression of TF was quantified by dividing the area of TF-positive endothelial region by the total endothelial area of IVC in ImageJ software. Data were collected from 3 mice/group and presented with the Wt group at the baseline level set as 1.
Immunofluorescence Staining
The procedures were performed as previously described.13,14 In brief, after 48-hour ligation-induced DVT or sham operation, murine vessels (IVC + abdominal aorta) were collected, fixed in 4% paraformaldehyde, and embedded in Tissue-Tek O.C.T. Compound. Cryostat sections (8 μm thick) were stained with primary antibody against mouse Klf11 (X1710, Syd Labs, 1:50 dilution) and PECAM1 (DIA-310, Dianova, 1:100 dilution), or normal rabbit or rat IgG overnight. Alexa Fluor conjugated secondary antibodies (code number 711–585–152 and 112–605–143, Jackson ImmunoResearch Laboratories, 1:500 dilution) were applied for 1 hour. Slides were mounted with ProLong Gold Antifade Mountant with DAPI (P36935, Thermo Fisher Scientific), and images were taken with an Olympus IX73 microscope. Quantification of KLF11 expression in endothelial cells (ECs) was performed with ImageJ software (National Institutes of Health). Data were collected from 3 mice/group and presented with the comparison to the sham group.
Culture of Human and Bovine Endothelial Cells
Human umbilical vein ECs (HUVECs) (Lonza) were cultured in M199 medium (Gibco) supplemented with 16% fetal bovine serum (FBS) (Sigma-Aldrich), 24 mM HEPES (Gibco), 0.5 mg/mL penicillin/streptomycin (Gibco), 100 μg/mL heparin (Sagent Pharmaceuticals), and 1 ng/mL recombinant human fibroblast growth factor (Sigma-Aldrich) as previously described.19 Bovine aortic ECs (BAECs) (American Type Culture Collection) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) with 10% FBS. All cells were cultured in a 5% CO2 humidified incubator at 37°C.
Isolation and Culture of Mouse Aortic Endothelial Cells
The mouse aortic ECs (MAECs) were isolated and cultured according to a previous protocol.20 Briefly, mice were anesthetized with ketamine (50 mg/kg) and xylazine (5 mg/kg). The mouse aorta from the aortic arch to the abdominal aorta was dissected and immersed with DMEM containing 20% FBS and 100 μg/mL heparin. The surrounding fat and connective tissues were removed, and the lumen was flushed with serum-free DMEM. The aorta was then fused with DMEM-containing collagenase type 2 (2 mg/mL, LS004174, Worthington). After 45-minute incubation at 37°C, the aorta was flushed with DMEM containing 20% FBS to harvest the MAECs. Cells from the same genotype (n = 3/group) were mixed, centrifuged (1,200 rpm, 5 minutes), re-suspended (DMEM containing 20% FBS), and cultured in a collagen type 1-coated plate at 37°C for 2 hours. After PBS wash, MAECs were incubated with the culture medium (DMEM containing 20% FBS, 2 mM L-glutamine, nonessential amino acid, 1 mM sodium pyruvate, 25 mM HEPES, 100 μg/mL heparin, 0.5 mg/mL penicillin/streptomycin) for about 1 week. After reaching 80 to 100% confluence, MAECs were stimulated with TNF-α (2 ng/mL) or vehicle for 4 hours, and protein was extracted to measure TF expression.
Ex Vivo Clot Assay
The ex vivo clot assay was developed based on a previous report detecting the procoagulant activity of ECs.21 HUVECs were cultured in a 96-well plate and infected with Ad-LacZ or Ad-KLF11 for 48 hours, followed by stimulation with TNF-α (10 ng/mL) or vehicle for 4 hours. Before coagulation induction, the medium was removed, and cells were washed with warm PBS. Prewarmed human plasma (50 μL, P9523, Sigma) and calcium chloride (25 mM, 50 μL) were added to each well. Clot formation was determined by kinetic detection of optical density value at 405 nm at 37°C (Molecular Devices, Spectra Max Plus).
Messenger RNA Isolation and Quantitative Polymerase Chain Reaction
Total RNA from tissues or cells was extracted (RNeasy Kit, QIAGEN) and reverse-transcribed into cDNA with Super-Script III reverse transcriptase (Thermo Fisher Scientific). Gene expression was measured by real-time quantitative polymerase chain reaction (qPCR) with IQSYBR Green Super-mix (Bio-Rad). Primers used in qPCR are listed in ►Supplementary Table S1 (available in the online version).
Protein Extracts and Western Blot
Total protein from cells was extracted as previously reported.14 Primary antibodies used in Western blot: GAPDH (sc-32233, Santa Cruz Biotechnology, 1:1,000 dilution), TF (sc-374441, Santa Cruz Biotechnology, 1:1,000 dilution), KLF11 (H00008462-M01, Abnova, 1:1,000 dilution), and early growth response protein 1 (EGR1; 4153s, Cell Signaling Technology, 1:1,000 dilution).
Adenovirus-Mediated Gene Overexpression and Small-Interfering RNA-Mediated Gene Knockdown
These were performed as previously reported.14 For knockdown experiments, si-Control or si-KLF11 were diluted in Opti-MEM (40 nM) and incubated with Lipofectamine RNAi-MAX for 20 minutes at room temperature, and then added to cultured HUVECs. After 24 hours of incubation, the medium was changed to M199 with 0.5% FBS for 48 hours of serum starvation before thrombin stimulation. Data were calculated in triplicates from at least three independent experiments.
Co-immunoprecipitation
HUVECs were co-infected with Ad-EGR1 (5 MOI) and Ad-KLF11 (5 MOI) for 48 hours.
The cells were lysed with Pierce IP Lysis Buffer (87788, Thermo Fisher Scientific) and incubated with primary antibody against EGR1 (4153, Cell Signaling Technology, 1:50 dilution), KLF11 (H00008462-M01, Abnova, 1:50 dilution), or normal respective IgG separately at 4°C overnight with rotation. The immunocomplexes were precipitated with Protein A/G PLUS-Agarose (sc-2003, Santa Cruz Biotechnology), followed with Western blot analysis using antibodies against KLF11 and EGR1.
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation (ChIP) assays were performed using the SimpleChIP Enzymatic Chromatin IP Kit (9003s, Cell Signaling Technology) following the manufacturer’s protocol. Briefly, serum-starved HUVECs were fixed with 1% formaldehyde and quenched before enzymatic digestion. The DNA/protein complex was immunoprecipitated with control IgG or anti-Flag antibody (14793, Cell Signaling Technology) conjugated to Protein G magnetic beads. Purified DNA was used as the template for qPCR analysis with primers (listed in ►Supplementary Table S1 [available in the online version]) flanking the putative EGR1-binding region in the TF gene promoter.
Luciferase Assay
BAECs were co-transfected with plasmids using Lipofectamine 2000 (11668019, Thermo Fisher Scientific) as previously described.14 Briefly, BAECs were cultured in a 48-well plate and grown to approximately 80% confluence before transfection. The reporter plasmids (0.5 μg/well) and Lipofectamine 2000 Reagent were incubated with Opti-MEM separately and mixed to set for 20 minutes. Then, the mix was added to cultured BAECs in DMEM containing 10% FBS. Seventy-two hours after transfection, the TF gene promoter activity was measured by firefly luciferase (Promega) and normalized against Renilla activity which was co-transfected as a control.
Statistical Analysis
All quantitative data are presented as mean ± standard error of the mean. Statistical analyses were performed using the GraphPad Prism 7. The homogeneity of variances was first evaluated by the Shapiro–Wilk normality test and F-test. For data distributed normally with similar variances among groups, unpaired Student’s t-test with Welch’s correction was used for two-group comparisons. Comparisons among more than two groups were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s test. Grouped data under different conditions were analyzed with two-way ANOVA followed by Bonferroni test. All results were representative and collected from at least three independent experiments.
Results
KLF11 Expression Is Upregulated in Endothelial Cells under Prothrombotic Conditions
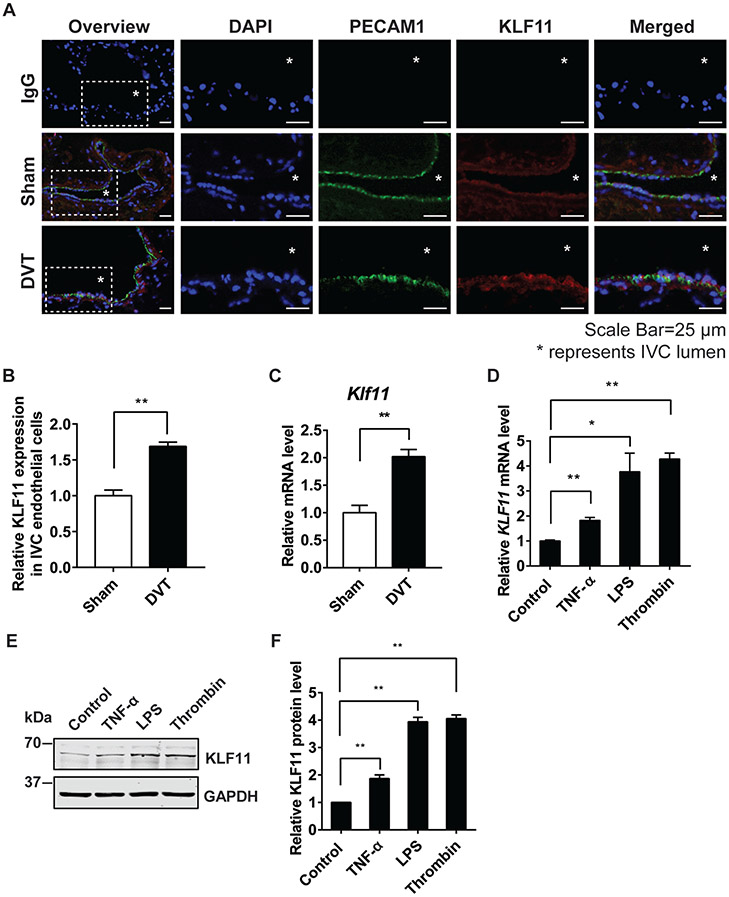
First, we performed the stasis-induced DVT model in C57BL/6 Wt male mice and compared KLF11 expression in the IVC with that in the sham-operated mice. The immunofluorescence staining shows that the induced KLF11 expression was mainly located in the endothelium (►Fig. 1A, B). The mRNA level of KLF11 was significantly doubled in the vascular wall of the DVT group (►Fig. 1C). These data suggest that vascular KLF11 may play an important role in venous thrombosis. Consistent with the in vivo results, KLF11 expression was also enhanced in the HUVECs treated with different prothrombotic stimuli, including TNF-α, LPS, and thrombin, as was measured by both qPCR and Western blot (►Fig. 1D-F). These data indicate that KLF11 is a thrombosis-responsive gene in ECs.
Fig. 1.
KLF11 expression is upregulated in endothelial cells under prothrombotic conditions. (A–C) In the stasis-induced deep vein thrombosis model (DVT), the inferior vena cava (IVC) from C57BL/6 male mice were either sham-operated or ligated for 48 hours to induce DVT (n = 3/group). The cryostat IVC sections were stained with antibodies against PECAM1 (DIA-310, Dianova, 1:100; Alexa 647, Jackson ImmunoResearch, 1:500, displayed in green) and KLF11 (X1710, Syd Labs, 1:50; Alexa 568, Jackson ImmunoResearch, 1:500, displayed in red). DAPI (4′,6-diamidino-2-phenylindole) stained for nuclear acid. Respective IgG staining was used as the negative control. Scale bars = 25 μm. The symbol “*” represents IVC lumen. The representative images are presented (A) and the expression of KLF11 in the endothelial cells of IVC was quantified by Image J (B). The vascular wall of IVC was isolated to measure Klf11 mRNA level (C). (D–F) Human umbilical vein endothelial cells (HUVECs) were treated with different stimuli for 4 hours to induce endothelial inflammation. The stimuli included: TNF-α (10 ng/mL), lipopolysaccharides (LPS, 100 ng/mL), and thrombin (5 U/mL). The mRNA level of KLF11 (D) was normalized to GAPDH and is presented relative to the control group. The representative Western blot (E) and quantification (F) of the KLF11 level are presented. N = 3/group. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01 using unpaired Student t-test. IgG, immunoglobulin G; IVC, inferior vena cava; SEM, standard error of the mean; TNF-α, tumor necrosis factor-α.
The Expression Profiles of Thrombosis-Related Genes in Response to Altered KLF11 Levels in ECs
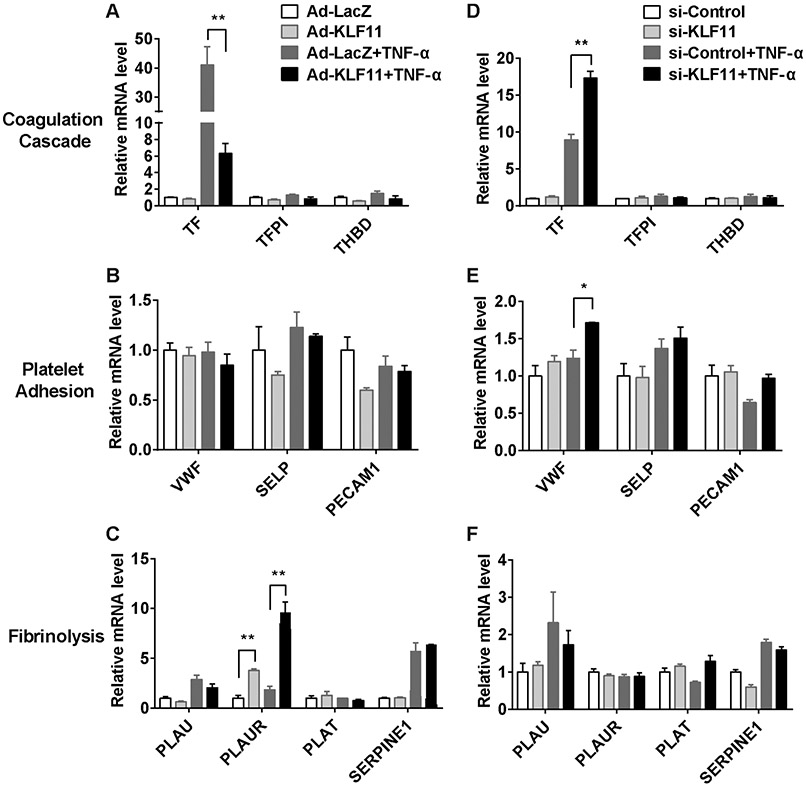
Under inflammatory conditions, endothelium is activated to express a plethora of genes regulating hemostasis and thrombosis.22,23 To gain a general view on the role of endothelial KLF11 in this process, adenovirus-mediated KLF11 overexpression and small-interfering RNA (siRNA)-mediated KLF11 knockdown were applied to cultured HUVECs, followed with TNF-α stimulation. The expression of the genes that are both highly expressed in ECs and related to thrombosis was measured by qPCR. KLF11 overexpression reduced TNF-α-induced TF gene transcription by 84.6%. Consistently, downregulation of KLF11 enhanced TF gene transcription by 1.93-fold. However, there was no significant change in anticoagulative genes, including TF pathway inhibitor (TFPI) and thrombomodulin (THBD) (►Fig. 2A, D). Next, we analyzed genes mediating the interaction between ECs and platelets. KLF11 knockdown increased TNF-α-induced transcription of von Willebrand factor (VWF), while there was no significant change after KLF11 overexpression (►Fig. 2B, E). For fibrinolysis-related genes, although the transcription of urokinase-type plasminogen activator receptor (PLAUR) was increased by KLF11 overexpression, it was not regulated by KLF11 knockdown (►Fig. 2C, F). The expression of other platelet adhesion-related genes [platelet EC adhesion molecule 1 (PECAM1), selectin P (SELP)] (►Fig. 2B, E) and fibrinolysis-related genes (►Fig. 2C, F) did not show significant change. Altogether, these data indicate that KLF11 is a negative regulator of TF gene transcription in ECs, thereby contributing to the antithrombotic phenotype.
Fig. 2.
The expression profiles of thrombosis-related genes in response to altered KLF11 levels in endothelial cells. (A–C) Human umbilical vein endothelial cells (HUVECs) were infected with Ad-LacZ or Ad-KLF11 (10 MOI) for 48 hours, followed by TNF-α (10 ng/mL) stimulation for 4 hours. (D–F) HUVECs were transfected with si-Control or si-KLF11 (20 nM). Seventy-two hours after transfection, HUVECs were stimulated with TNF-α (10 ng/mL) for 4 hours. The mRNA level of thrombosis-related genes was normalized to GAPDH and is presented relative to HUVEC infected with Ad-LacZ group. N = 3/group. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01 using two-way ANOVA followed by Bonferroni test. Abbreviations of gene names: TF, tissue factor; TFPI, tissue factor pathway inhibitor; THBD, thrombomodulin; VWF, von Willebrand factor; SELP, selectin P; PECAM1, platelet endothelial cell adhesion molecule 1; PLAU, plasminogen activator, urokinase (also known as u-PA); PLAUR, plasminogen activator, urokinase receptor; PLAT, plasminogen activator, tissue type (also known as t-PA); SERPINE1, plasminogen activator inhibitor-1 (also known as PAI-1). ANOVA, analysis of variance; SEM, standard error of the mean; TNF-α, tumor necrosis factor-α.
KLF11 Negatively Regulates TNF-α-Induced Tissue Factor Protein Expression in ECs
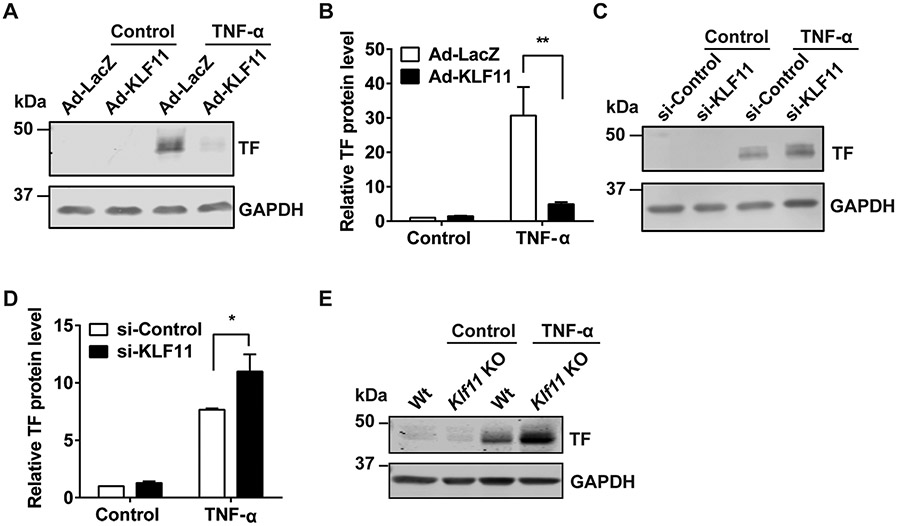
We further analyzed TF protein expression under a similar experimental setting as was performed in ►Fig. 2. Adenovirus-mediated KLF11 overexpression was confirmed by qPCR (►Supplementary Fig. S1A [available in the online version]). Consistent with the qPCR data, KLF11 overexpression reduced TNF-α-induced TF protein expression by 83.9% in ECs (►Fig. 3A, B). Similarly, siRNA-mediated KLF11 knockdown (►Supplementary Fig. S1B [available in the online version]) increased TF protein level after TNF-α stimulation by 1.44-fold (►Fig. 3C, D). Moreover, a higher TF expression was observed in the isolated MAECs from Klf11 KO mice compared with Wt mice (►Fig. 3E). Collectively, KLF11 negatively regulates TNF-α-induced TF expression in ECs.
Fig. 3.
KLF11 negatively regulates TNF-α-induced tissue factor protein expression in endothelial cells. (A, B) Human umbilical vein endothelial cells (HUVECs) were infected with Ad-LacZ or Ad-KLF11 (10 MOI) for 48 hours, followed by TNF-α (10 ng/mL) stimulation for 4 hours. The representative Western blot of tissue factor (TF) protein level (A) and quantification (B) are presented. (C, D) HUVECs were transfected with si-Control or si-KLF11 (20 nM). Seventy-two hours after transfection, HUVECs were stimulated with TNF-α (10 ng/mL) for 4 hours. The representative Western blot of TF protein level (C) and quantification (D) are presented. Mouse aortic endothelial cells (MAECs) were isolated from wild-type (Wt) or Klf11 knockout (Klf11 KO) male mice (n = 3/group) and stimulated with TNF-α (2 ng/mL) for 4 hours. The representative Western blot of TF expression is presented (E). The mRNA level was normalized to GAPDH and is presented relative to the control group. N = 3/group. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01 using two-way ANOVA followed by Bonferroni test. Antibodies: GAPDH (sc-32233, Santa Cruz Biotechnology, 1:1,000 dilution), tissue factor (sc-374441, Santa Cruz Biotechnology, 1:1,000 dilution). ANOVA, analysis of variance; SEM, standard error of the mean; TNF-α, tumor necrosis factor-α.
The Early Growth Response 1, Rather than Nuclear Factor Kappa B and Activating Protein-1, is Responsive to KLF11-Dependent Inhibition of TF gene Transcription
Under basal conditions, TF expression in the endothelium is undetectable, preventing activation of the extrinsic coagulation cascade. However, endothelial TF expression can be significantly induced upon vascular injury and thus initiates coagulation. Several proinflammatory transcription factors, including nuclear factor kappa B (NF-*κB), activating protein-1 (AP-1), and EGR1, have been well established in this process.24-26 Accordingly, we first determined whether KLF11 regulates the expression of these pathways. HUVECs infected with Ad-LacZ or Ad-KLF11 were stimulated with TNF-α for 60 minutes to evaluate the rapid gene expression changes in these three pathways. TNF-α-induced TF gene expression was increased more than 20-fold at 1 hour, which was significantly reduced in the KLF11 overexpression group (►Supplementary Fig. S2A [available in the online version]). These expression levels were either not affected (p50, c-Jun, AP-1) or slightly changed (p65 increased and EGR1 reduced) upon KLF11 overexpression compared with the LacZ control (►Supplementary Fig. S2B-D [available in the online version]). Therefore, the levels of AP-1, NF-κB, and EGR1 in ECs may not be responsible for the inhibitory effects of KLF11 on the early-phase TF gene expression (►Supplementary Fig. S2A [available in the online version]). We next determined whether KLF11 might affect their function by applying the respective inhibitors of these signal pathways. BAY11–7082 was applied to block TNF-α-induced IκB-α phosphorylation (an essential step for NF-κB activation) (►Fig. 4A). SP600125 was used to inhibit c-Jun N-terminal kinase (►Fig. 4B). NGFI-A binding protein 2 (NAB2) was known to suppress EGR1-induced transcription (►Fig. 4C).27 Consistent with the data in ►Fig. 3, overexpression of KLF11 reduced TNF-α-induced TF gene transcription in HUVECs (►Fig. 4A-C, compare the fifth and sixth columns). Of note, in the conditions of blocking a single signaling, KLF11 overexpression could still further inhibit TF gene transcription, indicating that KLF11 effects involved multiple signaling pathways (►Fig. 4A-C, compare the last two columns). In addition, with KLF11 overexpression, the NF-κB or AP-1 inhibitors but not EGR1 inhibition could further reduce TF gene transcription (►Fig. 4A-C, compare the sixth and eighth columns). As EGR1 can induce TF gene expression and contribute to thrombus formation,25,26 we determined if KLF11 inhibits the inflammation-induced EGR1 signaling. Indeed, KLF11 suppressed EGR1 overexpression-induced TF gene expression at both mRNA and protein levels (►Fig. 4D, E). Collectively, our data indicate that EGR1, at least partially, mediates the KLF11-dependent inhibition of TF gene transcription in ECs under proinflammatory conditions.
Fig. 4.
The early growth response 1 (EGR1), rather than nuclear factor kappa B (NF-κB) and activating protein-1 (AP-1), is responsive to KLF11-dependent inhibition of TF gene transcription. (A, B) Human umbilical vein endothelial cells (HUVECs) were infected with Ad-LacZ or Ad-KLF11 (10 MOI). Forty-eight hours later, cells were treated with different compounds: BAY11–7082 (0.5 μM [A]) or SP600125 (5 μM [B]) for 1 hour, followed by TNF-α (10 ng/mL) stimulation for 4 hours. DMSO serves as the vehicle control for these compounds. (C) HUVECs were co-infected with Ad-NAB2 (5 MOI) and Ad-LacZ or Ad-KLF11 (5 MOI) for 48 hours, followed by TNF-α (10 ng/mL) stimulation for 4 hours. The transcription of TF gene was determined by qPCR. (D, E) HUVECs were co-infected with Ad-EGR1 (5 MOI) and Ad-LacZ or Ad-KLF11 (5 MOI) for 48 hours, and then the transcription of TF gene was determined by qPCR (D), the protein level of TF was detected by Western blot and the representative results are presented (E). The mRNA level was normalized to GAPDH and is presented relative to the control group. N = 3/group. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01 using two-way ANOVA followed by Bonferroni test. Antibodies: GAPDH (sc-32233, Santa Cruz Biotechnology, 1:1,000 dilution), tissue factor (sc-374441, Santa Cruz Biotechnology, 1:1,000 dilution). ANOVA, analysis of variance; qPCR, quantitative polymerase chain reaction; SEM, standard error of the mean; TNF-α, tumor necrosis factor-α.
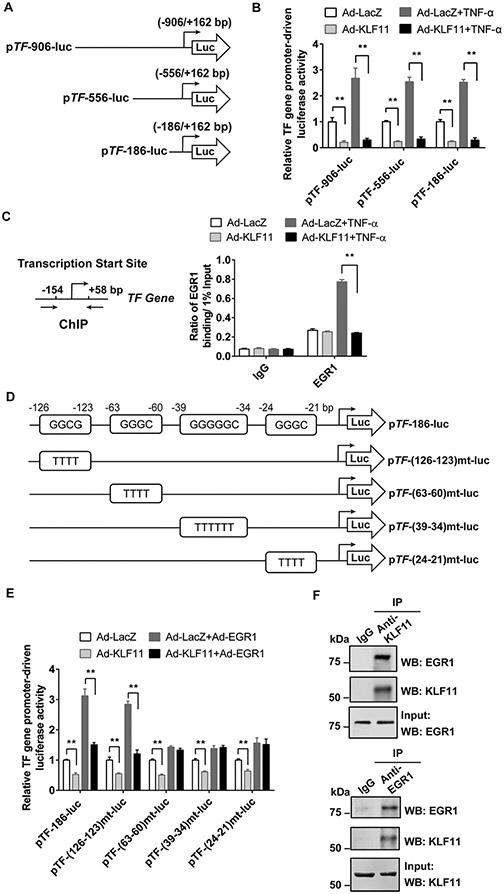
KLF11 Inhibits EGR1-Induced TF Gene Transcription
To further determine the underlying mechanism, we transfected luciferase reporter plasmids driven by different lengths of TF gene promoter into BAECs (►Fig. 5A). Under both basal and TNF-α treatment conditions, KLF11 overexpression significantly reduced all three TF gene promoter-driven luciferase activities (►Fig. 5B). These results suggest that KLF11 downregulates TF gene at the transcriptional level. Although our previous study identified that KLF11 directly binds at TF gene promoter and inhibits its transcription in VSMCs under basal conditions,14 deletions of the potential KLF11-binding region (−177/− 161 bp) failed to abolish the TNF-α-induced TF gene promoter activity in ECs (►Supplementary Fig. S3 [available in the online version]). By using ChIP assay, we found that KLF11 overexpression abolished TNF-α-induced EGR1 binding to TF gene promoter (−154/+ 58bp) (►Fig. 5C). To specifically test if KLF11 is important to counteract EGR1-induced TF gene transcription, we generated TF gene promoter-driven reporter plasmids with mutations at four putative EGR1-binding regions (predicted by online software MatInspector, ►Fig. 5D and ►Supplementary Table S2 [available in the online version]). EGR1 overexpression-induced TF gene promoter activity can be inhibited by KLF11 overexpression, while these effects were interrupted when the EGR1-binding site was mutated at −63/− 60 bp, −39/− 34 bp, or −24/− 21 bp, but not at the −126/− 123 bp region (►Fig. 5E). Furthermore, co-immuno-precipitation (Co-IP) followed by Western blot verified that KLF11 binds with EGR1 in ECs (►Fig. 5F). Therefore, KLF11 inhibits TF gene transcription via interacting with and suppressing EGR1.
Fig. 5.
KLF11 inhibits EGR1-induced TF gene transcription. (A) The simplified structure illustration of luciferase reporters driven by different lengths of human TF gene promoter (pTF). (B) Bovine aortic endothelial cells (BAECs) were transfected with pTF-luciferase reporters with varying sizes of promoter (A) for 24 hours and then infected with Ad-LacZ or Ad-KLF11 (10 MOI). Forty-eight hours after infection, BAECs were stimulated with TNF-α (10 ng/mL) for 12 hours. The luciferase activity was measured and normalized by Renilla activity. The results are presented relative to the group infected with Ad-LacZ (B). (C) Human umbilical vein endothelial cells (HUVECs) were infected with Ad-LacZ or Ad-KLF11 (10 MOI) for 48 hours, followed by TNF-α (10 ng/mL) stimulation for 4 hours. The binding of EGR1 to the TF gene promoter was determined by chromatin immunoprecipitation (ChIP) assay using an antibody against EGR1. (D) TF gene promoter-driven luciferase reporters pTF-186-luc with wild-type (Wt) or different EGR1-binding region mutants are shown in the simplified illustration. (E) BAECs were transfected with pTF-Wt-luc or several pTF-mt-luc luciferase reporters for 24 hours and then co-infected with Ad-EGR1 (5 MOI) with Ad-LacZ or Ad-KLF11 (5 MOI) for 48 hours. The luciferase activity was measured and normalized by Renilla activity. The results are presented relative to the group infected with Ad-LacZ. **p < 0.01 using two-way ANOVA followed by Bonferroni test. (F) HUVECs were co-infected with Ad-EGR1 (5 MOI) and Ad-KLF11 (5 MOI) for 48 hours. The cell lysates were subjected to immunoprecipitation (IP) with antibodies against KLF11 or IgG (upper panel), or EGR1 or IgG (lower panel). The precipitates were analyzed by Western blot (WB) using antibodies against KLF11 and EGR1. The co-immunoprecipitated EGR1 or KLF11 is presented. Data are presented as mean ± SEM. ANOVA, analysis of variance; SEM, standard error of the mean; TNF-α, tumor necrosis factor-α.
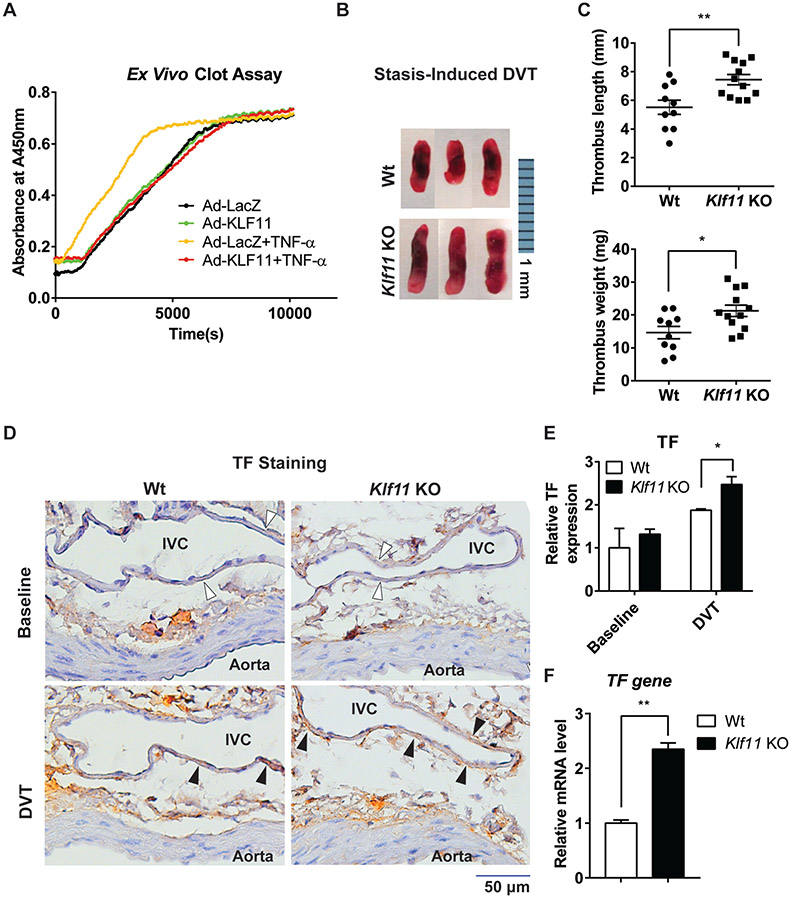
Endothelial KLF11 Protects against Thrombus Formation
To explore whether KLF11 affects venous thrombus formation, we performed ex vivo clot assay and in vivo DVT model in Klf11 KO mice. With the ex vivo clot assay, we found that KLF11 overexpression significantly reduced TNF-α-induced clot formation (►Fig. 6A). Next, we investigated the effect of KLF11 on the development of DVT in vivo. Compared with Wt mice, Klf11 KO mice were more susceptible to stasis-induced DVT formation, as was evidenced by increased thrombus length (Wt: 5.51 ± 0.50 mm vs. Klf11 KO: 7.44 ± 0.36 mm, p = 0.004) and weight (Wt: 14.6 ± 1.86 mg vs. Klf11 KO: 21.2 ± 1.73 mg, p = 0.017), after 48-hour IVC ligation (►Fig. 6B, C). Moreover, the immunohistochemistry staining showed that Klf11 KO mice had higher TF expression in the IVC endothelium under stasis conditions than Wt mice (►Fig. 6D, E). As expected, there were no significant differences for endothelial TF expression in the baseline (►Fig. 6D, E). The increased TF gene expression in the ligated IVC of Klf11 KO mice was further confirmed by qPCR (►Fig. 6F).
Fig. 6.
Endothelial KLF11 protects against thrombus formation. (A) Human umbilical vein endothelial cells (HUVECs) were infected with Ad-LacZ or Ad-KLF11 (10 MOI). Forty-eight hours after infection, HUVECs were stimulated with TNF-α (10 ng/mL) for 4 hours. Human plasma and calcium chloride were added, and the time of clot formation was measured. (B, C) The stasis-induced deep vein thrombosis (DVT) model was applied in 8–10 weeks old male wild-type (Wt, n = 10) or Klf11 KO (n = 12) mice by ligation of inferior vena cava (IVC) for 48 hours. Representative images of thrombus are shown. Bar = 1 mm (B). The length and weight of thromboses were measured (C). (D–F) The cross-sections of a combination of the abdominal aorta and IVC from Wt and Klf11 KO mice at baseline and in DVT condition (48-hour ligation) were prepared. (D) The tissue factor (TF) expression was determined by immunohistochemistry staining (filled arrowheads). The lumen of the aorta and IVC are labeled, and the endothelium of DVT is indicated with empty arrowheads. Bar = 50 μm. (E) The expression of TF was quantified by dividing the TF-positive endothelial area by the total endothelial area of IVC in ImageJ software. Data were analyzed with the Wt group at the baseline level set as 1. (F) The IVC from Wt and Klf11 KO mice were harvested after a 48-hour ligation procedure to detect the TF gene mRNA level (n = 4/group). *p < 0.05, **p < 0.01 using the unpaired Student’s t-test. TNF-α, tumor necrosis factor-α.
Discussion
The endothelium is a dynamically changing barrier that is critically involved in the maintenance of vascular hemostasis. As endothelial injury is one of the major factors leading to thrombosis development, accumulating studies focus on the role of endothelium in DVT pathology. In this study, our data showed that KLF11 is an inflammation-responsive gene in ECs and inhibits DVT in vivo. KLF11 downregulates aberrant TF gene transcription via suppressing induced-EGR1 signaling in ECs. Based on the in vitro and in vivo data, we identified that KLF11 is a protector against DVT.
As a transcription factor, KLF11 regulates insulin expression in pancreatic β cells,28,29 and increases fatty acid oxidation in hepatic cells.10 Our previous studies demonstrated that KLF11 deficiency upregulates the expression of proinflammatory adhesion molecules (e.g., ICAM1, VCAM1) by inhibiting NF-κB in ECs and thus aggravates leukocyte adhesion to vascular wall in mice.9 In this article, we discovered that KLF11 expression is induced under prothrombotic conditions, indicating that KLF11 may modulate the prothrombotic response in ECs. To extend our knowledge on the role of KLF11 in endothelial biology and DVT, we grouped the essential genes highly expressed in ECs and involved in thrombus formation into three groups according to their functions: coagulation cascade, platelet adhesion, and fibrinolysis (►Fig. 2). Among these genes, only TF gene was consistently and significantly downregulated by KLF11 under TNF-α-induced inflammation. Other genes are either not affected or only changed in one condition (i.e., KLF11 overexpression or knockdown). As a whole, we discovered TF gene as the potential downstream target of KLF11 that prevents thrombus formation under inflammatory conditions in the endothelium.
In fact, the primary mechanism by which ECs regulate the coagulation cascade lies in its upregulation of TF expression (i.e., TF gene transcription), especially upon vascular injury.22 TF is the initiator of the extrinsic coagulation cascade. Although the expression of endothelial TF is low, it is highly inducible under pathological conditions. The primary transcription factors regulating TF expression in ECs include Sp1 transcription factor (SP1), NF-κB, AP-1, and EGR1.24,25,30 In ECs, SP1 families bind at AP-1 and contribute to the constitutive TF gene transcription.24 Upon stimulation (e.g., inflammation), the NF-κB, AP-1, and EGR1 signaling can be activated and recruited to TF gene promoter, inducing TF expression.25,31
However, KLF11 does not downregulate the key factors in NF-κB, AP-1, and EGR1 signaling (►Supplementary Fig. S2 [available in the online version]), though KLF11 overexpression can significantly inhibit TF gene expression as early as 1 hour after TNF-α stimulation. This early-phase transcriptional suppression may result from the altered activity rather than the expression level of these three pathways. To test this, we applied inhibitor studies and found that KLF11 further inhibited TF gene transcription in conditions of blocking NF-κB, AP-1, or EGR1 signaling, indicating that KLF11’s effects are not mediated by a single pathway (►Fig. 4A-C, compare the last two columns). However, after KLF11 overexpression, the inhibition of NF-κB and AP-1 showed additive effects on suppressing TF gene transcription (►Fig. 4A, B, compare the sixth and eight columns), which was not observed in the EGR1 inhibition group (►Fig. 4C, compare the sixth and eight columns). These drove us to evaluate if KLF11 counteracts EGR1 signaling, which at least partially mediates the inflammation-induced TF gene transcription and contributes to thrombus formation.26
Employing multiple approaches, we demonstrated that KLF11 inhibits TF gene transcription via interrupting the binding between EGR1 and TF gene promoter. Consistent with the previous report, there are multiple EGR1-binding sites in the TF gene promoter.31 Although the mutation of three out of four EGR1-binding sites (−63/− 60 bp, −39/− 34 bp, and −24/− 21 bp) cannot entirely abolish EGR1-induced TF gene promoter activity, it is enough to abolish KLF11’s effects (no further reduction in ►Fig. 5E, compare the last two columns). Combining this data with ChIP and Co-IP results, we propose that KLF11 can at least partially interrupt the induced-EGR1 binding to the TF gene promoter.
Although our previous paper identified that KLF11 binds at TF gene promoter (−177/− 161 bp) and inhibits TF gene transcription in VSMCs,14 we did not observe similarly direct regulation in ECs (►Supplementary Fig. S3 [available in the online version ]). TF is constitutively expressed in VSMCs, but is almost undetectable in ECs at baseline. Upon inflammatory stimulation, endothelial TF is significantly induced and thus contributes to thrombus formation. Therefore, we demonstrated a novel mechanism in which KLF11, functioning as an inflammation-inducible co-repressor via binding with EGR1, inhibits EGR1-induced TF gene transcription.
DVT, characterized by clot formation in the deep veins (e.g., lower extremities), is triggered by hypercoagulative blood contents, disruption of blood flow, or endothelial damage. Inflammation, initiated by either systemic inflammatory status or local vascular injury, is an important mechanism contributing to thrombus formation.32 In the present study, we applied this model in Klf11 KO and Wt mice and found KLF11 deficiency aggravated DVT formation (►Fig. 6B, C). This is in line with the data that KLF11 deficiency enhanced TF expression in ECs (►Fig. 3) and endothelium (►Fig. 6D-F). The induced endothelial TF initiates extrinsic coagulation cascades and further triggers vascular inflammation (e.g., leukocyte adhesion), all of which can accelerate DVT formation. Apart from ECs, circulating components such as platelets, coagulation factors, monocyte-derived TFs can also contribute to DVT. Our previous study has shown that platelet activity (evaluated by ex vivo platelet activity assay) and coagulation cascade activity (assessed by prothrombin time and activated partial thromboplastin time) were not different between Klf11 KO and Wt mice.14 The overall hemostasis condition, measured by bleeding time, was also comparable between the two groups.14 These indicate that KLF11 deficiency may not affect hemostatic status. Based on our current study, the aggravated DVT formation in Klf11 KO mice can at least partially resulted from upregulated TF expression in ECs. The function of KLF11 in macrophages and monocyte-derived TF particles warrants further investigations in the future.
In summary, we demonstrated that KLF11 inhibits the EGR1-induced TF gene transcription in ECs under inflammatory conditions but not at baseline. Therefore, modulating endothelial KLF11 will be beneficial in the prevention of DVT.
Supplementary Material
What Is known about this topic?
Krüppel-like factor 11 (KLF11) is a diabetes-related transcription factor that also plays a critical role in maintaining vascular homeostasis.
Endothelial dysfunction (e.g., dysregulated gene expression) contributes to deep vein thrombosis.
What does this paper add?
KLF11 functions as a transcription co-repressor that disrupts TNF-α-induced recruitment of early growth response protein 1 (EGR1) to the tissue factor (TF) gene promoter.
KLF11 deficiency upregulates TF gene expression in endothelium and aggravates stasis-induced deep vein thrombosis in vivo.
Acknowledgments
The authors would like to thank Drs. Minerva T. Garcia-Barrio, Oren Rom, Lin Chang, Haoming Zhang, Michael Holinstat, Richard Mortensen, and Jiandie Lin at the University of Michigan for the data discussion; Dr. Daniel Kirchhofer at Genentech Inc. for providing the rat anti-mouse TF 1H1 antibody.
Funding
This work was partially supported by National Institutes of Health grants HL138139 (J.Z.), HL138094 and HL145176 (Y.F.), HL137214 and HL134569 (Y.E.C.), American Heart Association grants 18PRE34000005 (W.L) and 17PRE33400179 (H.L.).
Footnotes
Conflict of Interest
None declared.
References
- 1.Raskob GE, Angchaisuksiri P, Blanco AN, et al. ; ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol 2014;34(11):2363–2371 [DOI] [PubMed] [Google Scholar]
- 2.Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet 2016;388(10063):3060–3073 [DOI] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Benjamin EJ, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: a report from the American Heart Association. Circulation 2020;141(09):e139–e596 [DOI] [PubMed] [Google Scholar]
- 4.McConnell BB, Yang VW. Mammalian Krüppel-like factors in health and diseases. Physiol Rev 2010;90(04):1337–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alaiti MA, Orasanu G, Tugal D, Lu Y, Jain MK. Kruppel-like factors and vascular inflammation: implications for atherosclerosis. Curr Atheroscler Rep 2012;14(05):438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan Y, Lu H, Liang W, Hu W, Zhang J, Chen YE. Krüppel-like factors and vascular wall homeostasis. J Mol Cell Biol 2017;9(05):352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neve B, Fernandez-Zapico ME, Ashkenazi-Katalan V, et al. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc Natl Acad Sci U S A 2005;102(13):4807–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vazzana N, Ranalli P, Cuccurullo C, Davi G Diabetes mellitus and thrombosis. Thromb Res 2012;129(03):371–377 [DOI] [PubMed] [Google Scholar]
- 9.Fan Y, Guo Y, Zhang J, et al. Krüppel-like factor-11, a transcription factor involved in diabetes mellitus, suppresses endothelial cell activation via the nuclear factor-κB signaling pathway. Arterioscler Thromb Vasc Biol 2012;32(12):2981–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin KJ, Fan Y, Hamblin M, et al. KLF11 mediates PPARγ cerebro-vascular protection in ischaemic stroke. Brain 2013;136(Pt 4):1274–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Tang X, Ma F, et al. Endothelium-targeted overexpression of Krüppel-like factor 11 protects the blood-brain barrier function after ischemic brain injury. Brain Pathol 2020;30(04):746–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glineur C, Gross B, Neve B, et al. Fenofibrate inhibits endothelin-1 expression by peroxisome proliferator-activated receptor α-dependent and independent mechanisms in human endothelial cells. Arterioscler Thromb Vasc Biol 2013;33(03):621–628 [DOI] [PubMed] [Google Scholar]
- 13.Zhao G, Chang Z, Zhao Y, et al. KLF11 protects against abdominal aortic aneurysm through inhibition of endothelial cell dysfunction. JCI Insight 2021;6(05):141673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang W, Fan Y, Lu H, et al. KLF11 (Krüppel-like factor 11) inhibits arterial thrombosis via suppression of tissue factor in the vascular wall. Arterioscler Thromb Vasc Biol 2019;39(03):402–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song CZ, Gavriilidis G, Asano H, Stamatoyannopoulos G. Functional study of transcription factor KLF11 by targeted gene inactivation. Blood Cells Mol Dis 2005;34(01):53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wrobleski SK, Farris DM, Diaz JA, Myers DD Jr, Wakefield TW. Mouse complete stasis model of inferior vena cava thrombosis. J Vis Exp 2011;(52):2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang Z, Zhao G, Zhao Y, et al. BAF60a deficiency in vascular smooth muscle cells prevents abdominal aortic aneurysm by reducing inflammation and extracellular matrix degradation. Arterioscler Thromb Vasc Biol 2020;40(10):2494–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rom O, Xu G, Guo Y, et al. Nitro-fatty acids protect against steatosis and fibrosis during development of nonalcoholic fatty liver disease in mice. EBioMedicine 2019;41:62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu W, Lu H, Zhang J, et al. Krüppel-like factor 14, a coronary artery disease associated transcription factor, inhibits endothelial inflammation via NF-κB signaling pathway. Atherosclerosis 2018;278:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi M, Inoue K, Warabi E, Minami T, Kodama T. A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb 2005;12(03):138–142 [DOI] [PubMed] [Google Scholar]
- 21.Day SM, Reeve JL, Pedersen B, et al. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood 2005;105(01):192–198 [DOI] [PubMed] [Google Scholar]
- 22.Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord 2015;15:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M, Hao H, Leeper NJ, Zhu L Early Career Committee. Thrombotic regulation from the endothelial cell perspectives. Arterioscler Thromb Vasc Biol 2018;38(06):e90–e95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moll T, Czyz M, Holzmüller H, et al. Regulation of the tissue factor promoter in endothelial cells. Binding of NF kappa B-, AP-1-, and Sp1-like transcription factors.J Biol Chem 1995;270(08):3849–3857 [DOI] [PubMed] [Google Scholar]
- 25.Mackman N. Regulation of the tissue factor gene. Thromb Haemost 1997;78(01):747–754 [PubMed] [Google Scholar]
- 26.Shin IS, Kim JM, Kim KL, et al. Early growth response factor-1 is associated with intraluminal thrombus formation in human abdominal aortic aneurysm. J Am Coll Cardiol 2009;53(09):792–799 [DOI] [PubMed] [Google Scholar]
- 27.Houston P, Campbell CJ, Svaren J, Milbrandt J, Braddock M. The transcriptional corepressor NAB2 blocks Egr-1-mediated growth factor activation and angiogenesis. Biochem Biophys Res Commun 2001;283(02):480–486 [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Zapico ME, van Velkinburgh JC, Gutiérrez-Aguilar R, et al. MODY7 gene, KLF11, is a novel p300-dependent regulator of Pdx-1 (MODY4) transcription in pancreatic islet beta cells. J Biol Chem 2009;284(52):36482–36490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonnefond A, Lomberk G, Buttar N, et al. Disruption of a novel Kruppel-like transcription factor p300-regulated pathway for insulin biosynthesis revealed by studies of the c.−331 INS mutation found in neonatal diabetes mellitus. J Biol Chem 2011;286(32):28414–28424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mechtcheriakova D, Wlachos A, Holzmüller H, Binder BR, Hofer E. Vascular endothelial cell growth factor-induced tissue factor expression in endothelial cells is mediated by EGR-1. Blood 1999;93(11):3811–3823 [PubMed] [Google Scholar]
- 31.Bode M, Mackman N. Regulation of tissue factor gene expression in monocytes and endothelial cells: thromboxane A2 as a new player. Vascul Pharmacol 2014;62(02):57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swystun LL, Liaw PC. The role of leukocytes in thrombosis. Blood 2016;128(06):753–762 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.