Abstract
Pathological remodeling includes alterations of ion channel function and calcium homeostasis and ultimately cardiac maladaptive function during the process of disease development. Biochemical assays are important approaches for assessing protein abundance and post-translational modification of ion channels. Several housekeeping proteins are commonly used as internal controls to minimize loading variabilities in immunoblotting protein assays. Yet, emerging evidence suggests that some housekeeping proteins may be abnormally altered under certain pathological conditions. However, alterations of housekeeping proteins in aged and diseased human hearts remain unclear. In the current study, immunoblotting was applied to measure three commonly used housekeeping proteins (β-actin, calsequestrin, and GAPDH) in well-procured human right atria (RA) and left ventricles (LV) from diabetic, heart failure, and aged human organ donors. Linear regression analysis suggested that the amounts of linearly loaded total proteins and quantified intensity of total proteins from either Ponceau S (PS) blot-stained or Coomassie Blue (CB) gel-stained images were highly correlated. Thus, all immunoblotting data were normalized with quantitative CB or PS data to calibrate potential loading variabilities. In the human heart, β-actin was reduced in diabetic RA and LV, while GAPDH was altered in aged and diabetic RA but not LV. Calsequestrin, an important Ca2+ regulatory protein, was significantly changed in aged, diabetic, and ischemic failing hearts. Intriguingly, expression levels of all three proteins were unchanged in non-ischemic failing human LV. Overall, alterations of human housekeeping proteins are heart chamber specific and disease context dependent. The choice of immunoblotting loading controls should be carefully evaluated. Usage of CB or PS total protein analysis could be a viable alternative approach for some complicated pathological specimens.
Keywords: Human heart specimens, Housekeeping proteins, Aging, Diabetes, Heart failure
Introduction
Cardiovascular diseases (CVDs, such as heart failure (HF), coronary artery disease, and stroke) and cardiac arrhythmias (including atrial fibrillation (AF), the most common cardiac arrhythmia) are the leading cause of morbidity and mortality in the USA and many developing countries [17, 30, 35, 50]. Pathological molecular remodeling, including upregulated or downregulated proteins and post-transcriptional modification, has been found to be critical in the alteration of ion channel functions, calcium homeostasis, myocardial electrical and mechanical coupling, and ultimately cardiac maladaptive function during the process of disease development.
Using combined experimental approaches including cellular/whole heart functional assessments (i.e., ion currents, Ca2+ transients, myofilament contractility, cardiac output) and biochemical assays (protein expression and post-translational modification profiles of ion channel/regulatory partner complexes and signaling transduction pathways) are essential in understanding the underlying molecular and electrophysiological mechanisms of CVDs and fatal cardiac arrhythmias. Among those experimental tools, the immunoblotting assay has been used as a common technique to semi-quantitatively measure protein abundance and post-translational modification (i.e., phosphorylation) status that is detected by target-specific antibodies in cardiac tissue and cells [9, 37, 48]. An important step to minimize technique errors of uneven amounts of sample loading in immunoblotting assays is to measure a housekeeping protein as an internal loading control. This is based on a common notion that housekeeping proteins (including β-actin, calsequestrin (CSQ), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)) are stably expressed in cells under various conditions [7, 20, 51]. However, an increasing number of studies suggest that this may not be the case in some tissues or cell types in different disease states such as diabetes, cancers, spinal injury, neuronal diseases, and heart diseases [11, 12, 18, 28, 38, 44, 46, 49, 57].
To date, animal and cellular models have been widely used, and they have provided important insights into the molecular mechanisms of pathological remodeling in CVDs. However, translational studies in the human heart are crucial to transforming results from bench research work into bedside clinical applications. Whether these cardiac housekeeping proteins are altered in pathologically remodeled human hearts remains unclear, to date. Our carefully designed study here aimed to characterize housekeeping protein expression profiles in aged and diseased human hearts. We assessed three commonly used housekeeping proteins in heart specimens from human organ donors (not used for transplantation) with increasing age or with a history of non-ischemic or ischemic HF, or diabetes mellitus (DM). The findings not only provide important information for further investigation regarding altered Ca2+ binding CSQ proteins and sarcomeric structural β-actin proteins in human heart specimens but also provide important guidance for choosing a proper housekeeping protein as an internal control for immunoblotting assays.
Methods
Human heart specimens (donor hearts not used for transplantation)
Human atrial and ventricular tissues were obtained from 53 human donor hearts provided by the Illinois Gift of Hope (GOH) Organ & Tissue Donor Network. These donor hearts were not used or not suitable for heart transplantation due to technical reasons (such as impaired cardiac function, major cardiovascular diseases, and/or the age of the potential donor). The studies were approved by the Human Study Committees of Loyola University Chicago, Rush University Medical Center, and Illinois GOH. Consent was obtained by GOH from the donors’ families for the use of the donor’s hearts for research purposes and studies were performed in accordance with the Declaration of Helsinki. The diseased hearts were from organ donors who had a history of either type 2 DM or HF (ischemic or non-ischemic), whereas the control hearts were from the donors who had a normal cardiac function and without a history of any major cardiovascular disease. Tables 1–4 show de-identified demographic data (age, sex, and race) and the medical history of all the donor hearts in the current studies. Following heart explanation, right atrial (RA) and left ventricular (LV) tissue were quickly dissected and frozen in liquid nitrogen for further biochemistry assays.
Table 1.
Deidentified demographic data of human organ donors with a history of diabetes and age-matched organ donors without a history of diabetes
| Non-DM | DM | |
|---|---|---|
| N | 6 | 12 |
| Age (yrs) | 45.5 ± 15.6 | 58.4 ± 10.8 |
| EF | 53.0 ± 14.6 | 33.3 ±9.4 |
| Male (%) | 100 | 75 |
| CAU (%) | 100 | 75 |
| AA(%) | 0 | 0 |
| Hisp (%) | 0 | 25 |
| Race unknown (%) | 0 | 0 |
| BMI | 25.1 ±2.3 | 32.2 ±4.8 |
| DM (%) | 0 | 100 |
| HTN (%) | 0 | 91.6 |
| HF (%) | 0 | 0 |
AA, African American; BMI, body mass index; CAU, Caucasian; DM, diabetes mellitus; EF, ejection fraction; HF, heart failure; Hisp, Hispanic; HTN, hypertension; non-DM, non-diabetic; yrs, years of age
Table 4.
Deidentified demographic data of human organ donors who had preserved cardiac function, no history of cardiac arrhythmias, and lacked a history of valvular diseases and other major CVDs (except CAD and hypertension (HTN))
| Young (< 55 yr) | Aged (≥ 55 yr) | |
|---|---|---|
| N | 7 | 7 |
| Age (yrs) | 37.4 ± 13.8 | 65.6 ± 10.7 |
| EF | 50.8 ±3.4 | 60.0 ±8.6 |
| Male (%) | 28.6 | 14.3 |
| CAU (%) | 85.7 | 71.4 |
| AA (%) | 14.3 | 14.3 |
| Hisp (%) | 0 | 14.3 |
| Race unknown (%) | 0 | 0 |
| BMI | 25.4 ± 4.7 | 24.0 ±3.7 |
| DM (%) | 0 | 14.3 |
| HTN (%) | 0 | 57.1 |
| HF (%) | 0 | 0 |
AA, African American; BMI, body mass index; CAU, Caucasian; DM, diabetes mellitus; EF, ejection fraction; HF, heart failure; Hisp, Hispanic; HTN, hypertension; yrs, years of age
Rabbit non-ischemic heart failure model and cardiac myocytes isolation
Studies were performed on control (n = 5) and HF (n = 5) adult female New Zealand White rabbits. HF was induced by combined aortic insufficiency and aortic constriction as previously described [1–4]. The protocol was approved by the Institutional Animal Care and Use Committees of Rush University Medical Center and University of Alabama at Birmingham. Terminal studies were performed at 6 months after the procedure of aortic constriction when the left ventricular end-systolic dimension exceeded 1.4 cm. The left ventricular cardiomyocytes were isolated as previously described [1–4].
Immunoblotting
Total proteins from human RA and LV tissue and isolated rabbit myocytes were extracted using RIPA lysis buffer in the presence of a protease inhibitor cocktail (Sigma). The immunoblotting assay was performed using a well-established protocol as previously described [2, 54]. In brief, the protein concentrations of human tissue homogenates and rabbit myocyte lysates were determined using a colorimetric Bradford Protein Assay kit (Bio-Rad). Then, serial diluted total protein samples (testing the variability of linear loading) or an equal amount of 30 μg proteins per sample (testing the variability of equal loading) were loaded and separated based on molecular weight by 10% SDS-PAGE gels. Immunoblotting assays were performed using specific antibodies recognizing either β-actin, calsequestrin (CSQ), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) proteins. Immunoreactivity was detected using a chemiluminescence kit (Bio-Rad) followed by the acquisition of the immunoblotting image and then quantification using a gel imaging analysis software (Bio-Rad). Each experiment was repeated three times to ensure the reproducibility of the data.
Ponceau S and Coomassie brilliant blue staining
At the end of the electrophoretic separation, SDS-PAGE gels underwent blot transfer to a nitrocellulose membrane. Then, the blots were rinsed briefly in distilled water and immersed in the Ponceau S (PS) solution (Sigma) for 5 min, followed by a brief rinse in distilled water to visualize the protein bands. Next, PS-stained blot images were acquired using the Bio-Rad gel imaging system as previously described [24]. After the PS staining and image acquisition, blots were subjected to a thorough rinse procedure to completely remove the PS dye followed by incubation with a primary antibody for immuno-blotting as described above.
For Coomassie Brilliant Blue staining, SDS-PAGE gels were stained using a Coomassie Brilliant Blue gel staining protocol as previously described [52]. In brief, the gel was pre-fixed in 50% v/v methanol, 40% v/v H2O, 10% v/v acetic acid for approximately 30 min after electrophoresis, and then stained with 0.1% w/v Coomassie brilliant R250 in the same solution as described above for 2 h until the gels were uniformly stained with a blue color. The gel was then de-stained by soaking overnight in 5% v/v methanol, 7.5% v/v acetic acid, and 87.5% v/v H2O until the background blue was removed, and protein bands were clearly visualized. Finally, an image of the gel was acquired and intensities of the total protein bands per lane were quantified using the Bio-Rad gel imaging software as described in the “Immunoblotting” section.
Statistical analysis
Statistically significant differences were assessed by either the nonparametric test (Mann-Whitney test) or linear regression analysis using IBM SPSS Statistic 24 (SPSS, Chicago, IL, USA). All data are presented as mean ± SEM. The criterion for statistical significance was a p value < 0.05.
Results
Quantitative total proteins of Ponceau S blot-stained and Coomassie blue gel-stained images as alternative approaches to calibrate loading variabilities
We first performed PS blot staining and CB gel staining in SDS-PAGE gels loaded with a linear range of total proteins (10, 20, 30, 40, 50, 60 μg) from the same human atrial homogenate. After electrophoretic protein separation, one of the two gels from the electrophoresis set was stained with CB solution, while the other gel was used for PS staining of the protein-transferred nitrocellulose membrane blot. Three gel repeats (running on different days) were carefully performed to eliminate potential sample loading variability. Figure 1a shows summarized quantitative results of all detected bands from each lane from CB gels and PS blots, suggesting a well-correlated linear relationship between the amount of loaded total protein of each lane and the corresponding band intensities. Next, we loaded 30 μg total protein of each atrial homogenate from 7 different normal human donors in the same gel followed by CB and PS staining and image quantification. Three gel repeats (running on different days) were also carefully performed. Summarized quantitative results of either CB gel-stained or PS blot-stained images showed minimal variations between different samples (Fig. 1b). These results show that quantitative protein band intensities from CB and PS images were well correlated to the loaded amount of total proteins. In addition, we performed immunoblotting with an SDS-PAGE gel loaded with a linear range of total proteins (5, 10, 20, 30 μg) from a HF rabbit myocyte sample. The blot was stained with PS solution followed by probing with a β-actin antibody. We found that the quantitative intensity of β-actin proteins detected by the antibody was highly correlated with loaded total proteins from quantified PS-stained image (Fig. 1c). Thus, our data demonstrate that the quantitative intensity of total proteins from CB or PS images properly reflects the loaded amounts of total proteins and the relative expression levels of a target protein of interest. Thus, in the subsequent experiments in the current studies, all the immunoblotting raw data were normalized to quantitative total protein values of CB or PS images to calibrate potential loading variabilities.
Fig. 1.
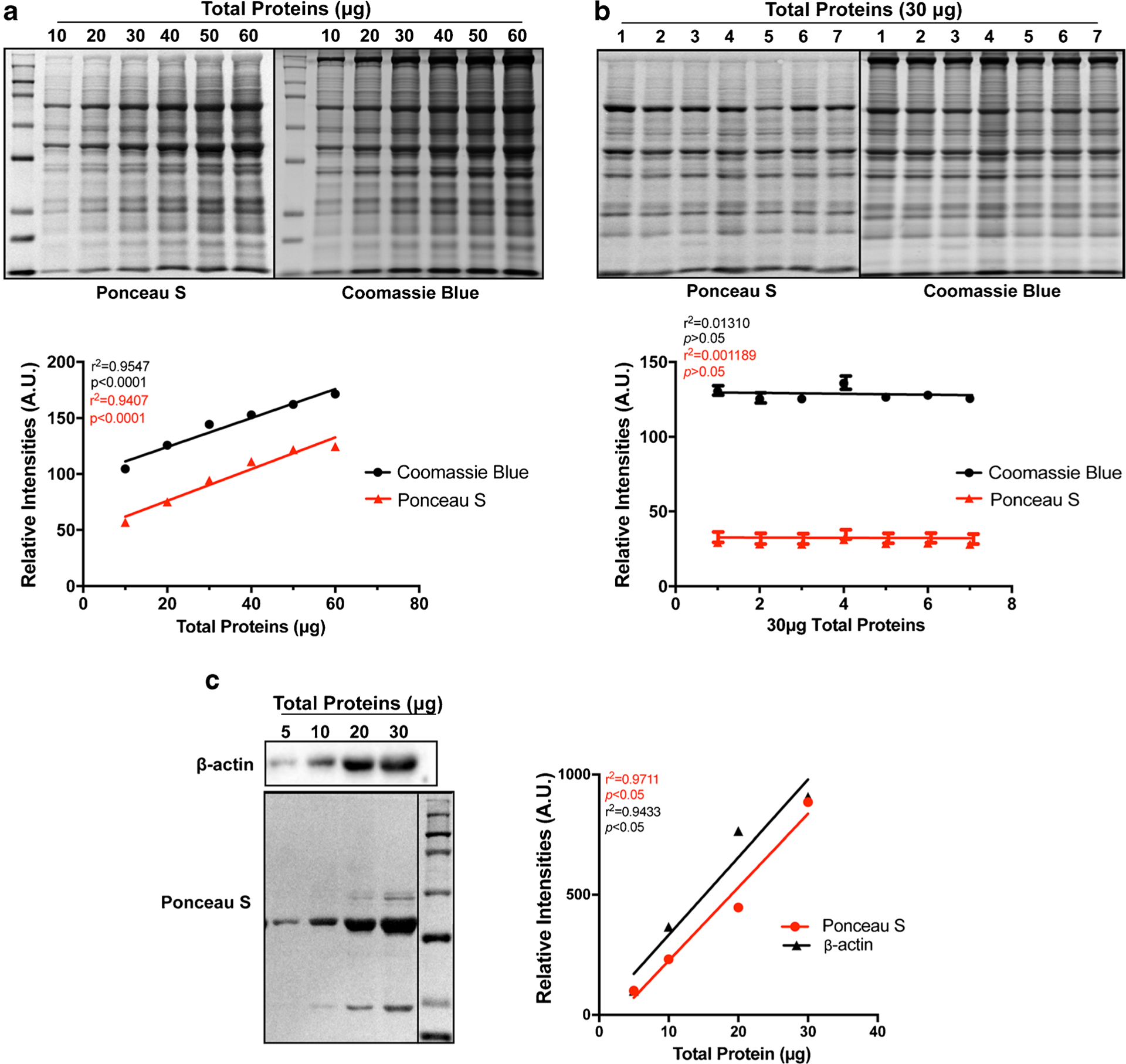
Highly correlated total protein amounts and quantified values from Ponceau S (PS) blot-stained and Coomassie Blue (CB) gel-stained images. a Representative images of PS (upper panel, left) and CB (upper panel, right) and linear regression (bottom panel) of various amounts of loaded total proteins and quantified total proteins from Ponceau S blot-stained (PS) and Coomassie blue (CB) gel-stained images. b Representative images of PS (upper panel, left) and CB (upper panel, right) and quantitative results of 30 μg total proteins of each human RA homogenate samples from PS- and CB-stained images. c Representative images of β-actin immunoblot (upper panel, left) and PS blot staining (upper panel, left) as well as linear regression (right) of various amounts of β-actin proteins and quantified total proteins from Ponceau S (PS) blot-stained image
Three altered housekeeping proteins in diabetic human hearts
Next, we assessed three housekeeping proteins including β-actin, calsequestrin (CSQ), and GAPDH in diabetic human RA and LV from organ donors with a history of type 2 diabetes and age-matched non-diabetic donors (Table 1). All of the organ donors had a normal cardiac function and no history of cardiac arrhythmias or any major cardiovascular diseases. Three sets of immunoblots probed with the three different antibodies were obtained from same-day back-to-back experiments, and each experiment was repeated three times on different days. The intensity of immunoblotting bands for each protein was quantified and all raw data were normalized to the quantified value of total proteins obtained from CB gel-stained images. We found that the protein levels of β-actin, CSQ, and GAPDH were all significantly downregulated in the diabetic human RA compared to age-matched controls (Fig. 2a, c–e and Supplemental Fig.S1a). Furthermore, we normalized the same immunoblotting raw data to the total protein intensity values from PS blot-stained images, and the results of using either CB or PS values were comparable (Fig. 2f–h and Supplemental Fig.S1a,1c). In diabetic human LV, we found significantly reduced β-actin but unchanged GAPDH and CSQ compared to age-matched controls (Fig. 2b–e and Supplemental Fig.S1b). Taken together, our results suggest that the diabetic disease state suppresses the protein expression of CSQ and GAPDH only in RA but downregulates β-actin in both RA and LV.
Fig. 2.
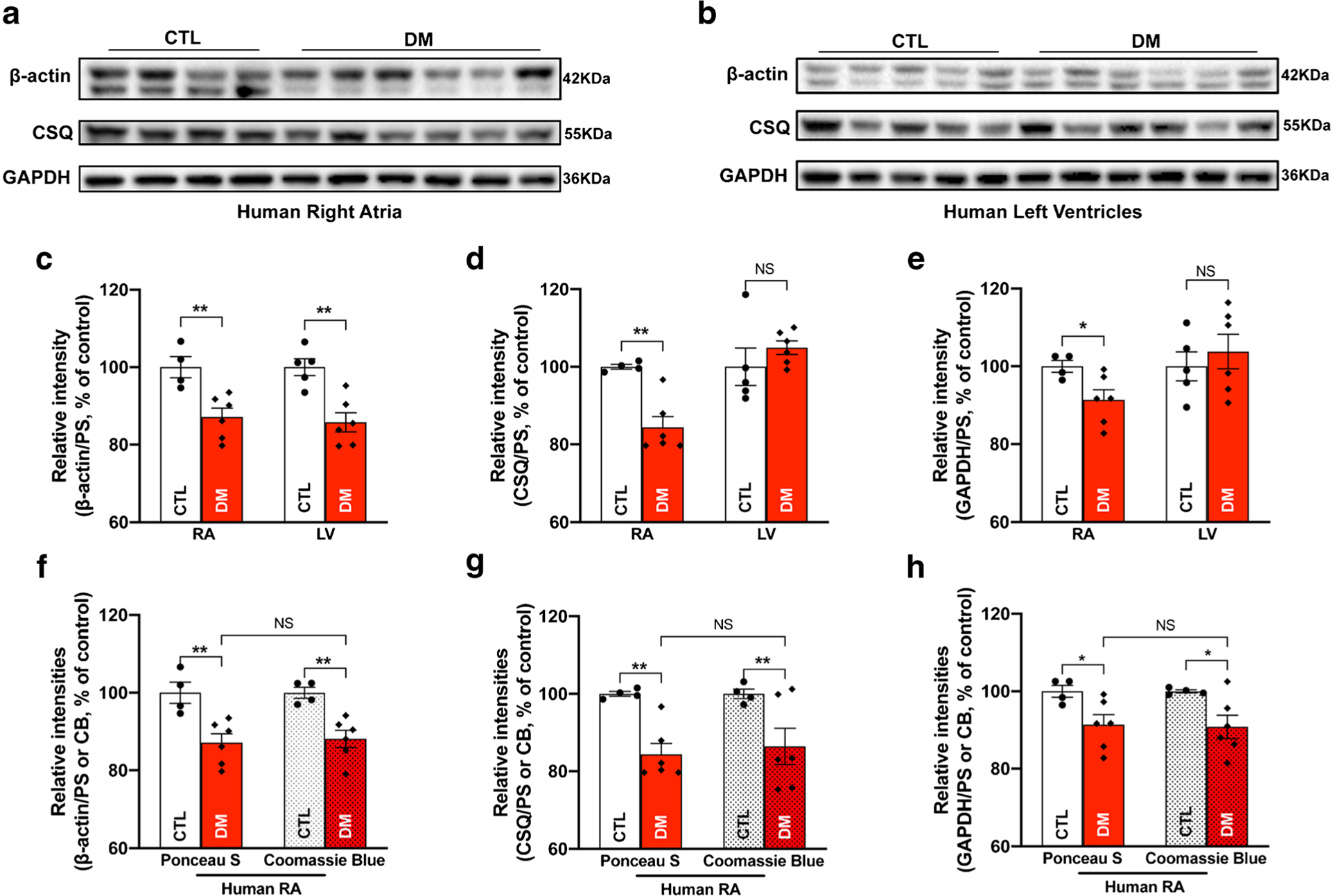
Three housekeeping proteins altered in diabetic human hearts. a Immunoblotting images of β-actin, calsequestrin (CSQ), and GAPDH in right atria (RA) of donors with diabetes mellitus (DM) compared to that of aged-matched controls (CTL); b immunoblotting images of β-actin, CSQ, and GAPDH in the left ventricle of donors with diabetes mellitus (DM) compared to that of aged-matched controls (CTL); c–e summarized data of β-actin (c), CSQ (d), and GAPDH (e) protein expression in right atria (left two bars) and left ventricle (right two bars) of diabetic hearts (DM) compared to that of aged-matched controls (CTL); f–h comparing summarized immunoblotting raw data normalized with total protein intensities from either PS-stained blot (two bars at far left) or CB-stained gel (two bars at far right) images. Presented data of the two bars at far left in f–h are the same as that of the two bars at far left in c–e. Data are represented as mean ± SEM. *p < 0.05; **p < 0.01. DM RA, n = 6; CTL RA, n = 4; DM LV, n = 6; CTL LV, n = 5
Altered abundance of CSQ proteins in ischemic failing human hearts
The protein levels of the three housekeeping proteins were measured in human RA and LV from donors with a history of ischemic HF. As shown in Table 2, the average ejection fraction (EF) of the ischemic HF donor hearts was 16.6% compared to an average of 53.1% EF in age-matched non-failing control donors, reflecting significantly impaired cardiac function in these ischemic failing hearts. Immunoblotting data showed that the abundance of CSQ proteins was markedly increased in ischemic failing RA but significantly reduced in ischemic failing LV compared to age-matched non-failing controls (Figs. 3a, b, 3d, and Supplemental Fig. S2a, 2b). In contrast, β-actin and GAPDH were unchanged in both RA and LV ischemic failing hearts compared to age-matched non-failing controls (Fig. 3a–c, e and Supplemental Fig. S2a, 2b). All quantified raw data were normalized to quantified values of CB gel-stained images. Overall, our findings showed cardiac chamber-specific changes in CSQ that suggest that CSQ apparently is not a suitable internal loading control for ischemic HF specimens, while β-actin and GAPDH could be considered as good loading controls for immunoblotting assays in this setting.
Table 2.
Deidentified demographic data of human organ donors with a history of ischemic heart failure and age-matched non-failing organ donors
| Non-failing | Ischemia | |
|---|---|---|
| N | 10 | 9 |
| Age (yrs) | 48.9 ± 12.6 | 61.1 ±7.1 |
| EF | 53.1 ± 12.2 | 16.6 ±5.8 |
| Male (%) | 60 | 100 |
| CAU (%) | 80 | 55.5 |
| AA (%) | 10 | 22.2 |
| Hisp (%) | 10 | 0 |
| Race unknown (%) | 10 | 33.3 |
| DM (%) | 0 | 33.3 |
| HF (%) | 0 | 100 |
AA, African American; CAU, Caucasian; DM, diabetes mellitus; EF, ejection fraction; HF, heart failure; Hisp, Hispanic; yrs, years of age
Fig. 3.
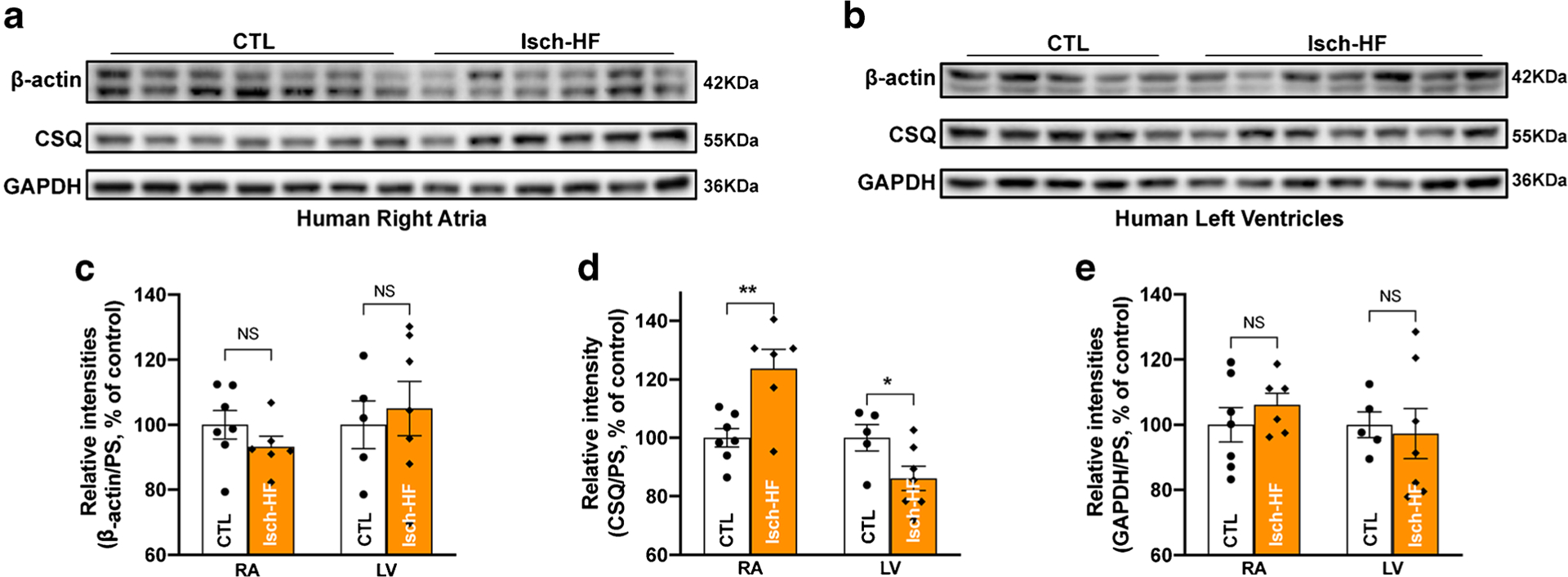
Changed abundance of CSQ proteins in ischemic failing human hearts. a Immunoblotting images of β-actin, calsequestrin (CSQ), and GAPDH in right atria of donors with of ischemic heart failure (Isch-HF) compared to that of aged-matched controls (CTL); b immunoblotting images of β-actin, CSQ, and GAPDH in the left ventricle of donors with ischemic heart failure (Isch-HF) compared to that of aged-matched controls (CTL); c–e summarized data of β-actin (c), CSQ (d), and GAPDH (e) protein expression in right atria (left panel) and left ventricle (right panel) of human organ donors with ischemic heart failure (Isch-HF) compared to that of aged-matched non-failing controls (CTL). Data are represented as mean ± SEM. *p < 0.05; **p < 0.01. Isch-HF RA, n = 6; non-HF CTL RA, n = 7; Isch-HF LV, n = 7; Non-failing CTL LV, n = 5
Unchanged housekeeping proteins in non-ischemic failing human hearts
Human donors with a history of non-ischemic HF showed an average EF of 15.5% compared to 63.0% in age-matched control donors, indicating markedly reduced cardiac function (Table 3). Due to their use in the previous studies, we assessed the three housekeeping proteins in LV specimens. We found that the protein levels of all three proteins (β-actin, CSQ, and GAPDH) were unchanged in the LV of non-ischemic HF hearts compared to age-matched non-failing controls (Fig. 4a, c–e, left panel and Supplemental Fig.S3a). All quantified immunoblotting raw data were normalized to the quantified values of PS blot-stained images. It is known that multiple cell types including myocytes, fibroblasts, and endothelial cells exist in the heart tissue, while myocytes comprise the most cellular volumes and play a predominant role in cardiac electrical and mechanical function [5]. To further confirm these findings in human heart tissue, we used isolated myocytes from our well-characterized non-ischemic HF rabbit model with a decreased mean fraction shortening (FS) by 24% compared to age-matched controls. This non-ischemic HF rabbit model exhibits similar abnormal phenotypes including molecular and electrophysiological changes seen in nonischemic failing patients as previously described [1–3]. The three housekeeping proteins were also found to be unchanged in non-ischemic HF rabbit myocytes compared to age-matched control rabbit myocytes (Fig. 4b, c–e, right panel, and Supplemental Fig.S3b). Thus, non-ischemic HF (in humans and rabbits) had no impact on the expression of these housekeeping proteins.
Table 3.
Deidentified demographic data of human organ donors with a history of non-ischemic heart failure and age-matched controls
| Non-failing | Non-Ischemia | |
|---|---|---|
| N | 6 | 5 |
| Age (yrs) | 57.7 ± 8.4 | 55.4 ± 10.1 |
| EF | 63.0 ±7.5 | 15.5 ± 3.1 |
| Male (%) | 50 | 80 |
| CAU (%) | 50 | 60 |
| AA (%) | 16.7 | 20 |
| Hisp (%) | 33.3 | 0 |
| Race unknown (%) | 0 | 20 |
| DM (%) | 0 | 40 |
| HF (%) | 0 | 100 |
AA, African American; CAU, Caucasian; DM, diabetes mellitus; EF, ejection fraction; HF, heart failure; Hisp, Hispanic; yrs, years of age
Fig. 4.
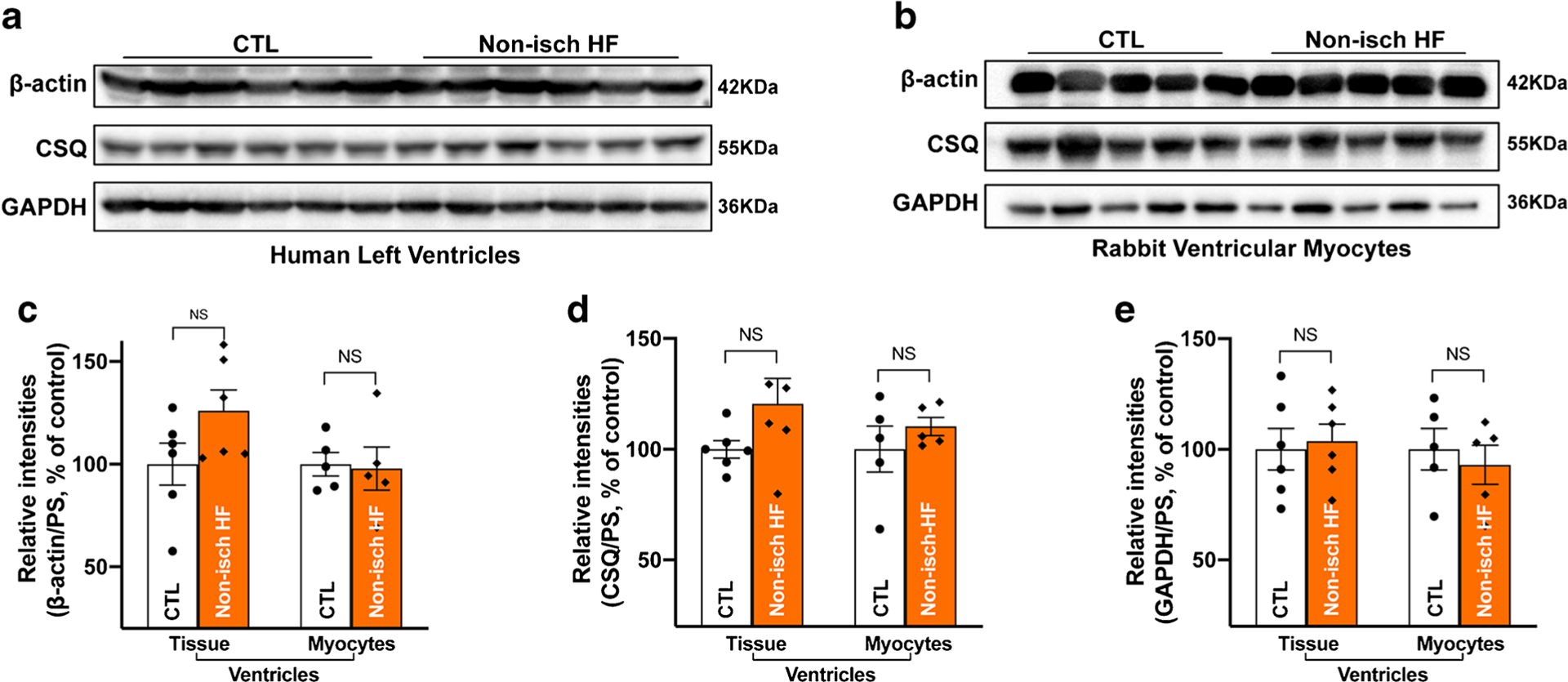
Unchanged expression of housekeeping proteins in non-ischemic failing human hearts. a Immunoblotting images of β-actin, calsequestrin (CSQ), and GAPDH in the left ventricle of donors with of non-ischemic heart failure (non-Isch-HF) compared to that of aged-matched controls (CTL); b immunoblotting images of β-actin, CSQ, and GAPDH in ventricular myocytes of heart failure rabbit (non-Isch-HF) compared to that of aged-matched controls (CTL); c–e summarized data of unchanged β-actin (c), CSQ (d), and GAPDH (e) protein expression in human HF LV (left panel) and isolated HF rabbit myocytes (right panel) compared to that of aged-matched controls. Data are represented as mean ± SEM. Non-Isch-HF LV, n = 6; non-failing CTL LV, n = 6. Non-Isch-HF rabbits, n = 5; age-matched CTL rabbits, n = 5
Evaluation of the three housekeeping proteins in the human heart with increasing age
Aging has been well recognized as an unavoidable risk factor for cardiovascular diseases because the aged heart is more susceptible to intrinsic and extrinsic stress stimuli, which could prompt molecular and electrophysiological remodeling [6, 17, 22, 23, 35, 36, 53]. To determine the age-related changes of these three housekeeping proteins in human atria, we studied RA specimens from human donors (19–82 years old) who had preserved cardiac function, no history of cardiac arrhythmias, and lacked a history of any major CVDs (except CAD and hypertension (HTN)) (Table 4). We found that the protein expression levels of β-actin in aged human atria were comparable with increasing age (Fig. 5a, b, and Supplemental Fig.S4). In contrast, the abundance of CSQ and GAPDH proteins was significantly increased with increasing age (Fig. 5a, c, d, and Supplemental Fig.S4). Our results indicate that age-related molecular remodeling affects the expression of some of the housekeeping proteins, and β-actin could be considered as a suitable internal loading control for biochemistry assays in this setting.
Fig. 5.
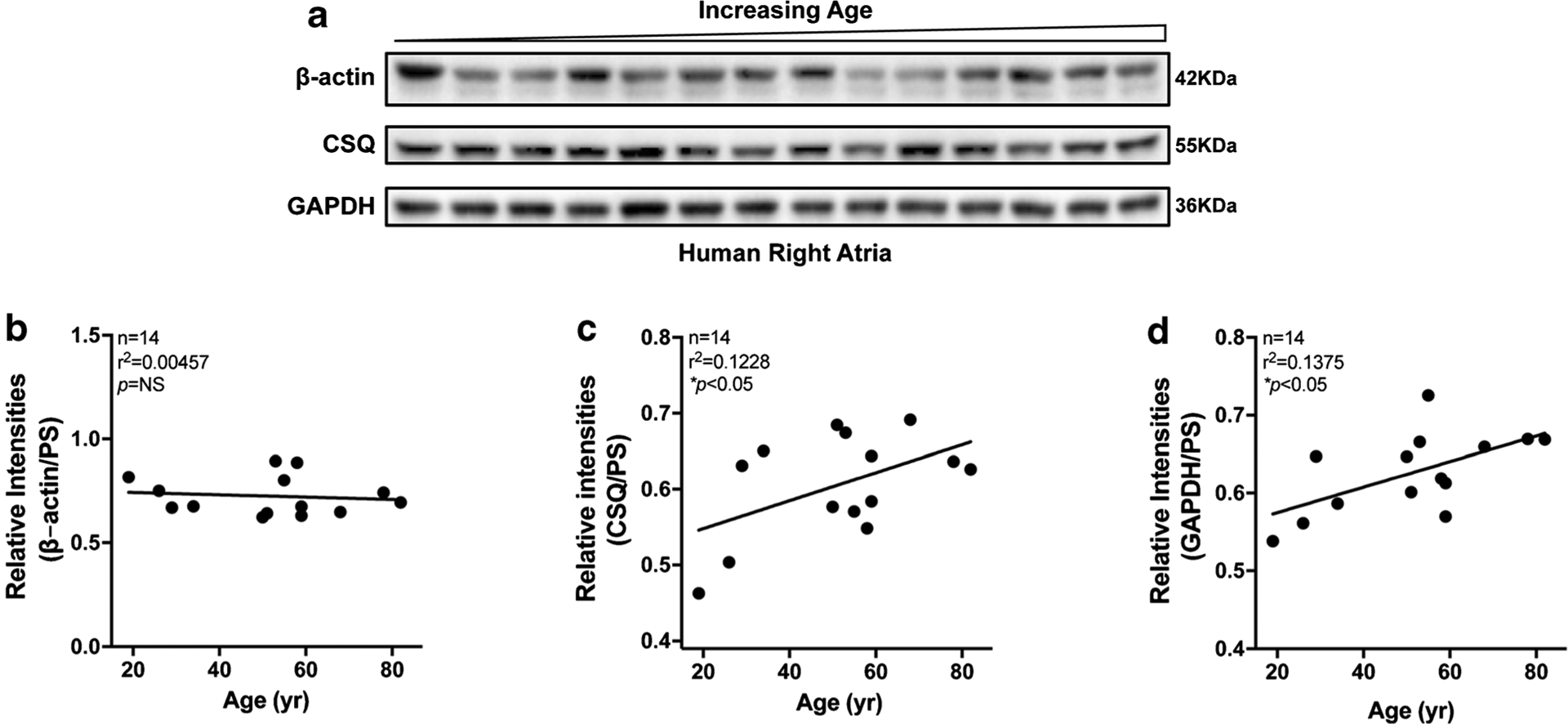
Expression of three housekeeping proteins in human atria with increasing age. a Immunoblotting images of β-actin, calsequestrin (CSQ), and GAPDH in right atria of donors with increasing age. b–d Linear regression analysis of β-actin (b), CSQ (c), and GAPDH (d) in right atria of organ donors with increasing age
Discussion
In the present study, we carefully assessed the expression profile of three commonly used housekeeping proteins (β-actin, CSQ, GAPDH) in both aged and diseased (diabetic, non-ischemic HF, and ischemic HF) human hearts. Isolated LV cardiomyocytes from a unique and well-characterized non-ischemic HF rabbit model were also used to complement the findings from non-ischemic failing human LV. We demonstrated for the first time that there are disease context-dependent and heart chamber-specific alterations in housekeeping protein expression in human heart tissue specimens. Specifically, our study provides evidence that (1) β-actin, a sarcomeric structural protein, is reduced only in diabetic RA and LV; (2) CSQ, an important sarcoplasmic reticulum (SR) Ca2+ binding protein, is altered in both diabetic, HF, and aging hearts; and (3) GAPDH is increased in RA with increasing age but reduced in diabetic RA.
β-Actin has been the most commonly used loading control for biochemistry assays. However, recent studies suggest that it may not be a valid internal control because its expression abundance may be altered under certain pathological conditions [11, 12, 14]. Along with abundantly expressed cardiac α-actin, cardiac β-actin is expressed at a lower amount in cardiomyocytes. However, it has been shown to be involved in sarcomeric actin filament dynamic structures and gene expression through control of the cellular G-actin pool [8, 34]. Under pathological conditions such as DM, altered actin-mediated cytoskeleton network and related signaling pathways were reported, and insulin may have a direct effect on the β-actin proximal promoter CCArGG motif in certain cell types [15, 31–33]. DM is a metabolic disorder with impaired insulin production or an insulin-resistant response leading to hyperglycemia, which can cause serious pathological remodeling in different organs and lead to cardiovascular diseases, stroke, and kidney dysfunction [10, 27, 42]. In the diabetic human heart, our results demonstrate for the first time significantly decreased β-actin in both diabetic human atrium and ventricle, suggesting a possible functional impact of the reduced β-actin on the actin cytoskeleton network in the diabetic heart. However, β-actin expression was unchanged in human atria with increasing age as well as in failing human hearts with either ischemic or non-ischemic HF. Therefore, there are pathologic alterations of β-actin in the heart in a disease context-specific manner, and the functional impact of this altered β-actin in the diabetic hearts is clearly worthy of further investigation.
Another important finding here is that altered CSQ protein levels were found in the human heart with increasing age as well as in pathologically remodeled human diabetic and ischemic failing hearts. In addition, there were atrial vs ventricular chamber differences shown in aging-related and disease-related changes of CSQ expression. For instance, reduced abundance of CSQ protein was only found in diabetic human atria but not ventricles, whereas increased CSQ was found in ischemic HF RA, but significantly reduced CSQ was noted in ischemic HF LV. CSQ is the most abundant Ca2+ binding protein in the SR; it regulates luminal Ca2+ concentration and cardiac Ca2+ triggered Ca2+ release channels ryanodine receptor (RyR2) as well as Ca2+ release refractoriness in each heartbeat [19, 21, 41, 45]. It is also a major determinant of the Ca2+ storage capacity of SR. Missense mutations of CSQ have been found to cause aberrant Ca2+ release, which is linked to the life-threatening, genetically inherited arrhythmogenic disease, catecholaminergic polymorphic ventricular tachycardia (CPVT) [13]. Transgenic mouse models suggest that depletion of CSQ leads to a fast rise of luminal Ca2+ concentration after each heartbeat, which causes an increased amount of systolic Ca2+ release during each heartbeat cycle and a much faster recovery of the RyR2 channels from SR release refractoriness. Therefore, reduced CSQ in ischemic HF ventricles could be a compensatory mechanism against Ca2+ triggered arrhythmic activities. While unchanged CSQ was reported in human HF [29, 40], our findings of unchanged CSQ in non-ischemic HF in humans and rabbits suggest that the etiology of HF influences the different housekeeping protein expression. In addition, aged and diabetic as well as ischemic failing atria all showed significantly increased CSQ. To our knowledge, these intriguing findings in aged and diseased atria have not been previously reported in humans; thus, future functional investigations would be worth exploring. In addition, our findings here suggest that CSQ is not a suitable internal loading control for biochemical assays in aged, diabetic, and ischemic failing hearts.
The third housekeeping protein we examined in the current study was GAPDH. This protein functions as a glycolytic enzyme involved in the cellular metabolic process [39]. In addition to its metabolic function, GAPDH is also involved in certain cellular functions including transcription activation, initiation of apoptosis, and regulation of vesicle shuttling from the ER to the Golgi apparatus [43, 58]. Extensive studies suggest that GAPDH is stably expressed in different cell types under normal and various pathological conditions [38, 59]. In our current study, we found unchanged GAPDH expression in both ischemic and non-ischemic failing hearts. However, GAPDH expression was significantly increased in human atria with increasing age and reduced in diabetic atria, although unchanged abundance of GAPDH was reported in human skeletal muscles with increasing age [26, 47].
In the current study, we also demonstrated that Coomassie Blue (CB) gel-stained and Ponceau S (PS) blot-stained total proteins could be used as alternative loading controls in immunoblotting assays. CB-stained gels and PS-stained blots are the two classical quantification methods for total loading proteins based on the advantages of the simplicity of the procedure, mass spectrometry compatibility, and low cost. PS staining has another advantage in that the staining is completely reversible and does not interfere with the procedures of the immunoblotting. Thus, using PS or CB total protein analysis could be considered under certain complicated diseased conditions. In addition, other technical aspects, including using a well-designed protocol, keeping up the rigorous protocol in every step of the immunoblotting assay, ensuring the high quality of the reagents/buffers, and validating antibodies (reactivity, specificity, and dilution rate), should also be considered to minimize other technical variances and to ensure the reliability and reproducibility of the immunoblotting data.
Taken together, our studies demonstrate that housekeeping proteins in the human heart may be altered during the process of age-related remodeling and pathological remodeling such as DM, ischemic HF, and non-ischemic HF. β-actin and GAPDH may be suitable internal loading controls for both ischemic and non-ischemic failing hearts. While β-actin can be a proper internal control for aged heart specimens, it is apparently a poor loading control for diabetic human atria and ventricles. CSQ is an important Ca2+ regulatory protein and our results suggest that CSQ may be functinally involved in pathological remodeling in aged, diabetic, and failing human hearts. Thus, our findings of altered expression profiles of these housekeeping proteins suggest that the choice of internal control should be carefully evaluated in human heart studies. It is known that the prevalence of cardiovascular diseases and arrhythmias is significantly increased in the aging population [25, 35, 53, 56]. While aging may not lead to apparent disease phenotypes, the aged heart is known to be more susceptible to stress challenges and subject to pathological remodeling [16, 47, 53, 55, 56]. Our finding of altered atrial CSQ in the current study, along with our recently reported findings of altered SR Ca2+ dynamics and enhanced atrial arrhythmogenicity in both aged human and animal models [53, 55, 56], supports that age-related cardiac remodeling may have a milder impact on the expression of housekeeping proteins than on SR Ca2 + mishandling and arrhythmogenicity in the human heart. Notably, our recently reported immunoblotting data obtained from aged human atria were all normalized to GAPDH as an internal loading control. Although our findings of enhanced stress kinase JNK activation and increased phosphorylation of JNK-regulated SR Ca2+ handling proteins were confirmed by using PS blot-stained total proteins, the data with normalization of increased GAPDH in the aged human atria likely underestimated the changes. The findings in the current studies clearly address the importance of using a suitable internal loading control in biochemistry assays. While our findings demonstrate that alterations of human housekeeping proteins are heart chamber-specific and disease context-dependent, post-translational modification and sex differences are other important physiological/pathological variables that were not included in the current studies but are clearly worthy of future investigations.
Supplementary Material
Funding
This work was supported by the National Institutes of Health [R01-HL113640 to XA].
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00424-021-02538-x.
References
- 1.Ai X, Pogwizd SM (2005) Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res 96:54–63. 10.1161/01.RES.0000152325.07495.5a [DOI] [PubMed] [Google Scholar]
- 2.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM (2005) Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res 97:1314–1322. 10.1161/01.RES.0000194329.41863.89 [DOI] [PubMed] [Google Scholar]
- 3.Ai X, Zhao W, Pogwizd SM (2010) Connexin43 knockdown or overexpression modulates cell coupling in control and failing rabbit left ventricular myocytes. Cardiovasc Res 85:751–762. 10.1093/cvr/cvp353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ai X, Jiang A, Ke Y, Solaro RJ, Pogwizd SM (2011) Enhanced activation of p21-activated kinase 1 in heart failure contributes to dephosphorylation of connexin 43. Cardiovasc Res 92:106–114. 10.1093/cvr/cvr163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee I, Yekkala K, Borg TK, Baudino TA (2006) Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Ann N Y Acad Sci 1080:76–84. 10.1196/annals.1380.007 [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA (1994) Independent risk factors for atrial fibrillation in a population-based cohort. The framingham heart study. JAMA 271: 840–844 [PubMed] [Google Scholar]
- 7.Bidasee KR, Nallani K, Besch HR Jr, Dincer UD (2003) Streptozotocin-induced diabetes increases disulfide bond formation on cardiac ryanodine receptor (RyR2). J Pharmacol Exp Ther 305: 989–998. 10.1124/jpet.102.046201 [DOI] [PubMed] [Google Scholar]
- 8.Bunnell TM, Burbach BJ, Shimizu Y, Ervasti JM (2011) Beta-actin specifically controls cell growth, migration, and the G-actin pool. Mol Biol Cell 22:4047–4058. 10.1091/mbc.E11-06-0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dennis-Sykes CA, Miller WJ, McAleer WJ (1985) A quantitative Western blot method for protein measurement. J Biol Stand 13: 309–314. 10.1016/s0092-1157(85)80044-5 [DOI] [PubMed] [Google Scholar]
- 10.Deshpande ADH-HM, Schootman M (2008) Epidemiology of diabetes and diabetes-related complications. Phys Ther 88:1254–1264. 10.2522/ptj.20080020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dittmer A, Dittmer J (2006) Beta-actin is not a reliable loading control in Western blot analysis. Electrophoresis 27:2844–2845. 10.1002/elps.200500785 [DOI] [PubMed] [Google Scholar]
- 12.Eaton SL, Roche SL, Llavero Hurtado M, Oldknow KJ, Farquharson C, Gillingwater TH, Wishart TM (2013) Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS One 8:e72457. 10.1371/journal.pone.0072457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faggioni M, Knollmann BC (2012) Calsequestrin 2 and arrhythmias. Am J Physiol Heart Circ Physiol 302:H1250–H1260. 10.1152/ajpheart.00779.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson RE, Carroll HP, Harris A, Maher ER, Selby PJ, Banks RE (2005) Housekeeping proteins: a preliminary study illustrating some limitations as useful references in protein expression studies. Proteomics 5:566–571. 10.1002/pmic.200400941 [DOI] [PubMed] [Google Scholar]
- 15.Franklin JL, Amsler MO, Messina JL (2016) Prenylation differentially inhibits insulin-dependent immediate early gene mRNA expression. Biochem Biophys Res Commun 474:594–598. 10.1016/j.bbrc.2016.04.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao X, Wu X, Yan J, Zhang J, Zhao W, DeMarco D, Zhang Y, Bakhos M, Mignery G, Sun J, Li Z, Fill M, Ai X (2018) Transcriptional regulation of stress kinase JNK2 in proarrhythmic CaMKIIdelta expression in the aged atrium. Cardiovasc Res 114:737–746. 10.1093/cvr/cvy011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE (2001) Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285:2370–2375. 10.1001/jama.285.18.2370 [DOI] [PubMed] [Google Scholar]
- 18.Greer S, Honeywell R, Geletu M, Arulanandam R, Raptis L (2010) Housekeeping genes; expression levels may change with density of cultured cells. J Immunol Methods 355:76–79. 10.1016/j.jim.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 19.Gyorke I, Hester N, Jones LR, Gyorke S (2004) The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J 86:2121–2128. 10.1016/S0006-3495(04)74271-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herraiz-Martinez A, Alvarez-Garcia J, Llach A, Molina CE, Fernandes J, Ferrero-Gregori A, Rodriguez C, Vallmitjana A, Benitez R, Padro JM, Martinez-Gonzalez J, Cinca J, Hove-Madsen L (2015) Ageing is associated with deterioration of calcium homeostasis in isolated human right atrial myocytes. Cardiovasc Res 106:76–86. 10.1093/cvr/cvv046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones LR, Suzuki YJ, Wang W, Kobayashi YM, Ramesh V, Franzini-Armstrong C, Cleemann L, Morad M (1998) Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J Clin Invest 101:1385–1393. 10.1172/JCI1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ (2005) Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res 66:233–244. 10.1016/j.cardiores. 2004.12.020 [DOI] [PubMed] [Google Scholar]
- 23.Karin M (2005) Inflammation-activated protein kinases as targets for drug development. Proc Am Thorac Soc 2:386–390; discussion 394–385. 10.1513/pats.200504-034SR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein D, Kern RM, Sokol RZ (1995) A method for quantification and correction of proteins after transfer to immobilization membranes. Biochem Mol Biol Int 36:59–66 [PubMed] [Google Scholar]
- 25.Li SY, Du M, Dolence EK, Fang CX, Mayer GE, Ceylan-Isik AF, LaCour KH, Yang X, Wilbert CJ, Sreejayan N, Ren J (2005) Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging Cell 4:57–64. 10.1111/j.1474-9728.2005.00146.x [DOI] [PubMed] [Google Scholar]
- 26.Lowe DA, Degens H, Chen KD, Alway SE (2000) Glyceraldehyde-3-phosphate dehydrogenase varies with age in glycolytic muscles of rats. J Gerontol A Biol Sci Med Sci 55: B160–B164. 10.1093/gerona/55.3.b160 [DOI] [PubMed] [Google Scholar]
- 27.Luitse MJA, Biessels GJ, Rutten GEHM, Kappelle LJ (2012) Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol 11:261–271. 10.1016/s1474-4422(12)70005-4 [DOI] [PubMed] [Google Scholar]
- 28.McLoughlin KJ, Pedrini E, MacMahon M, Guduric-Fuchs J, Medina RJ (2019) Selection of a real-time PCR housekeeping gene panel in human endothelial colony forming cells for cellular senescence studies. Front Med (Lausanne) 6:33. 10.3389/fmed.2019.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer M, Schillinger W, Pieske B, Holubarsch C, Heilmann C, Posival H, Kuwajima G, Mikoshiba K, Just H, Hasenfuss G et al. (1995) Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation 92:778–784. 10.1161/01.cir.92.4.778 [DOI] [PubMed] [Google Scholar]
- 30.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS (2006) Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 114:119–125. 10.1161/CIRCULATIONAHA.105.595140 [DOI] [PubMed] [Google Scholar]
- 31.Onyia JE, Halladay DL, Messina JL (1995) One of three CCArGG box/serum response elements of the beta-actin gene is an insulin-responsive element. Endocrinology 136:306–315. 10.1210/endo.136.1.7828546 [DOI] [PubMed] [Google Scholar]
- 32.Peravali R, Gunnels L, Alleboina S, Gerling IC, Dokun AO (2019) Type 1 diabetes alters ischemia-induced gene expression. J Clin Transl Endocrinol 15:19–24. 10.1016/j.jcte.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peravali R, Gunnels L, Dhanabalan K, Ariganjoye F, Gerling IC, Dokun AO (2019) In experimental peripheral arterial disease, type 2 diabetes alters post-ischemic gene expression. J Clin Transl Endocrinol 17:100199. 10.1016/j.jcte.2019.100199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pires RH, Shree N, Manu E, Guzniczak E, Otto O (2019) Cardiomyocyte mechanodynamics under conditions of actin remodelling. Philos Trans R Soc Lond Ser B Biol Sci 374: 20190081. 10.1098/rstb.2019.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rich MW (2009) Epidemiology of atrial fibrillation. J Interv Card Electrophysiol 25:3–8. 10.1007/s10840-008-9337-8 [DOI] [PubMed] [Google Scholar]
- 36.Rose BA, Force T, Wang Y (2010) Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heartbreaking tale. Physiol Rev 90:1507–1546. 10.1152/physrev.00054.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rybicki EP, von Wechmar MB (1982) Enzyme-assisted immune detection of plant virus proteins electroblotted onto nitrocellulose paper. J Virol Methods 5:267–278. 10.1016/0166-0934(82)90017-9 [DOI] [PubMed] [Google Scholar]
- 38.Said HM, Polat B, Hagemann C, Anacker J, Flentje M, Vordermark D (2009) Absence of GAPDH regulation in tumor-cells of different origin under hypoxic conditions in - vitro. BMC Res Notes 2:8. 10.1186/1756-0500-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarfraz A, Tunio NJO, Ala’Aldeen DAA, Wooldridge KG, Turner DPJ (2010) The role of glyceraldehyde 3-phosphate dehydrogenase (GapA-1) in Neisseria meningitidis adherence to human cells. BMC Microbiol 10:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schillinger WMM, Kuwajima G, Mikoshiba K, Just H, Hasenfuss G (1996) Unaltered ryanodine receptor protein levels in ischemic cardiomyopathy. Mol Cell Biochem 160/161:297–302 [DOI] [PubMed] [Google Scholar]
- 41.Scriven DR, Dan P, Moore ED (2000) Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. Biophys J 79:2682–2691. 10.1016/S0006-3495(00)76506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H (2015) Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol 3:105–113. 10.1016/s2213-8587(14)70219-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarze A, Deniaud A, Le Bras M, Maillier E, Molle D, Larochette N, Zamzami N, Jan G, Kroemer G, Brenner C (2007) GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization. Oncogene 26:2606–2620. 10.1038/sj.onc.1210074 [DOI] [PubMed] [Google Scholar]
- 44.Taylor SC, Posch A (2014) The design of a quantitative western blot experiment. Biomed Res Int 2014:361590–361598. 10.1155/2014/361590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terentyev D, Viatchenko-Karpinski S, Vedamoorthyrao S, Oduru S, Gyorke I, Williams SC, Gyorke S (2007) Protein protein interactions between triadin and calsequestrin are involved in modulation of sarcoplasmic reticulum calcium release in cardiac myocytes. J Physiol 583:71–80. 10.1113/jphysiol.2007.136879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Timaru-Kast R, Herbig EL, Luh C, Engelhard K, Thal SC (2015) Influence of age on cerebral housekeeping gene expression for normalization of quantitative polymerase chain reaction after acute brain injury in mice. J Neurotrauma 32:1777–1788. 10.1089/neu.2014.3784 [DOI] [PubMed] [Google Scholar]
- 47.Touchberry CD, Wacker MJ, Richmond SR, Whitman SA, Godard MP (2006) Age-related changes in relative expression of real-time PCR housekeeping genes in human skeletal muscle. J Biomol Tech 17:157–162 [PMC free article] [PubMed] [Google Scholar]
- 48.Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76:4350–4354. 10.1073/pnas.76.9.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veres-Szekely A, Pap D, Sziksz E, Javorszky E, Rokonay R, Lippai R, Tory K, Fekete A, Tulassay T, Szabo AJ, Vannay A (2017) Selective measurement of alpha smooth muscle actin: why beta-actin can not be used as a housekeeping gene when tissue fibrosis occurs. BMC Mol Biol 18:12. 10.1186/s12867-017-0089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S(2020) Heart Disease and Stroke Statistics-2020 Update: a report from the American Heart Association. Circulation 141:e139–e596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 51.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XHT, Nattel S, Dobrev D (2014) Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 129:145–156. 10.1161/CIRCULATIONAHA.113.006641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westermeier R (2006) Sensitive, quantitative, and fast modifications for Coomassie blue staining of polyacrylamide gels. Proteomics 6(Suppl 2):61–64. 10.1002/pmic.200690121 [DOI] [PubMed] [Google Scholar]
- 53.Yan J, Kong W, Zhang Q, Beyer EC, Walcott G, Fast VG, Ai X (2013) c-Jun N-terminal kinase activation contributes to reduced connexin43 and development of atrial arrhythmias. Cardiovasc Res 97:589–597. 10.1093/cvr/cvs366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan J, Thomson JK, Wu X, Zhao W, Pollard AE, Ai X (2014) Novel methods of automated quantification of gap junction distribution and interstitial collagen quantity from animal and human atrial tissue sections. PLoS One 9:e104357. 10.1371/journal.pone.0104357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan J, Thomson JK, Zhao W, Wu X, Gao X, DeMarco D, Kong W, Tong M, Sun J, Bakhos M, Fast VG, Liang Q, Prabhu SD, Ai X (2017) The stress kinase JNK regulates gap junction Cx43 gene expression and promotes atrial fibrillation in the aged heart. J Mol Cell Cardiol 114:105–115. 10.1016/j.yjmcc.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan J, Zhao W, Thomson JK, Gao X, DeMarco DM, Carrillo E, Chen B, Wu X, Ginsburg KS, Bakhos M, Bers DM, Anderson ME, Song LS, Fill M, Ai X (2018) Stress signaling JNK2 crosstalk with CaMKII underlies enhanced atrial arrhythmogenesis. Circ Res 122:821–835. 10.1161/CIRCRESAHA.117.312536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao L, Chen X, Tian Y, Lu H, Zhang P, Shi Q, Zhang J, Liu Y (2012) Selection of housekeeping genes for normalization of RT-PCR in hypoxic neural stem cells of rat in vitro. Mol Biol Rep 39:569–576. 10.1007/s11033-011-0772-8 [DOI] [PubMed] [Google Scholar]
- 58.Zala D, Hinckelmann MV, Yu H, Lyra da Cunha MM, Liot G, Cordelieres FP, Marco S, Saudou F (2013) Vesicular glycolysis provides on-board energy for fast axonal transport. Cell 152:479–491. 10.1016/j.cell.2012.12.029 [DOI] [PubMed] [Google Scholar]
- 59.Zhong H, Simons JW (1999) Direct comparison of GAPDH, beta-actin, cyclophilin, and 28S rRNA as internal standards for quantifying RNA levels under hypoxia. Biochem Biophys Res Commun 259:523–526. 10.1006/bbrc.1999.0815 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


