Abstract
To examine the mutual association of risk factors for both Helicobacter pylori (H. pylori) infection and chronic atrophic gastritis (CAG), a cross-sectional study on 954 residents of a rural town in Japan was conducted. Using an unconditional logistic model, we calculated the odds ratios (ORs) for H. pylori infection according to each lifestyle, as well as the ORs for CAG according to each lifestyle and H. pylori infection. A significant positive association was observed between H. pylori infection and the risk of CAG (OR = 6.29). On the other hand, a significant negative association was observed between high consumption of light-colored vegetables and the risk of CAG (OR = 0.68). We also used a path analysis to examine the direct relations of gender, age, and lifestyle variables to CAG, as well as the indirect relations of these variables to CAG through H. pylori infection. Aging had a significantly direct positive association with CAG. Although aging also had an indirect positive association with CAG through H. pylori infection, aging had no association with the consumption of light-colored vegetables. The high consumption of light-colored vegetables showed no association with H. pylori infection but had a significantly direct negative association with CAG. The results of this study suggest a possibility that high light-colored vegetables consumption contributes to the prevention of CAG.
Keywords: chronic atrophic gastritis, Helicobacter pylori, lifestyle, path analysis, risk factor
INTRODUCTION
Various epidemiological studies have demonstrated a significantly higher risk of gastric cancer in persons with chronic atrophic gastritis (CAG) than in those without CAG1). CAG has been recognized as an intermediate stage in the development of gastric cancer2). This means that the elucidation and control of risk factors for CAG may help prevent the occurrence of CAG and, consequently, lead to a prevention or reduction in the occurrence of gastric cancer.
Before the discovery of H. pylori, lifestyle factors such as smoking, alcohol consumption and dietary habits had been reported to be risk factors for CAG3). After the discovery of H. pylori in the human stomach in 19834), it was proven to be the most important risk factor for CAG5). However, the risk of CAG cannot be explained solely by H. pylori infection. Some epidemiological studies have suggested that not only H. pylori infection, but also differences in lifestyle variables appear to play an important role as risk factors for CAG6-8).
In Japan, where both the H. pylori infection rate and the prevalence of CAG are high compared to other developed countries9-11), it is important to examine the influence of lifestyle underlying these high rates. However, most epidemiological studies in Japan have so far only separately examined risk factors for either H. pylori infection or those for CAG6,8,9,12,13) and no studies have yet simultaneously examined the mutual association of risk factors for both H. pylori infection and CAG.
In most previous population-based studies, even though the study subjects were originally sampled from different areas8,9,13), the subjects were evaluated together in order to estimate the risks. These types of studies are useful in understanding the major risk factors at a national level, but they do not help identify the regional risk factors in order to reduce the occurrence of CAG for an individual area. We therefore conducted a cross-sectional study on the general residents of a rural town in Japan to obtain epidemiological evidence to be incorporated in health education programs to prevent H. pylori infection and the occurrence of CAG.
METHODS
Subjects
The study area was a rural town in a predominantly mountainous region of in Fukuoka Prefecture, Kyushu, Japan. The chief industry is agriculture and the proportion of individuals engaged in agriculture is about six times higher than that of Japan as a whole (40.2% vs.6.5% based on the 1995 census). The standardized mortality ratio (SMR) of gastric cancer in this town is not significantly different from that of Japan as a whole (males 84.8, females 112.9: 1988-1992). The total population of the town was 16,620 in 1993. In this town, annual health screening is conducted for residents aged 30 and over, and a questionnaire survey on lifestyles was administered for these residents from 1988 to 199014). We asked 2,347 residents, who participated at least once in the past in this survey, to cooperate in the present study. As a result, 1,094 residents took the health screening and gave blood samples in 1993. Out of these residents, 21 were excluded because they were over 80 years of age, and 119 were excluded because of insufficient lifestyle data. A final total of 954 residents aged 30-79 years were enrolled as subjects in this study.
Serological Method
H. pylori infection was determined by serum IgG antibodies for H. pylori using an enzyme immunoassay (HM-CAP, EPI Inc., USA). Both the sensitivity and specificity of this assay have been reported to be over 80%15,16). The level of serum pepsinogen I (PGI) and pepsinogen II (PGII) in the same samples were measured for identification of CAG using a radioimmunoassay (PG I / PG II RIABEAD, Dainabot Co. Ltd., Tokyo). CAG was determined by both serum PG I<70ng/ml and PG I/ PG II ratio<3.0. The sensitivity and specificity of the criterion have been shown to be 86% and 82%, respectively17). Recent epidemiological studies have adopted this criterion, and the reliability has been recognized6,8).
Statistical Method
Odds ratios (ORs) for H. pylori infection according to lifestyle variables were calculated, then, assuming that H. pylori infection itself was an additional risk factor, the OR for CAG of each variable was also calculated. These analyses were conducted by using an unconditional logistic model after adjusting for gender and age.
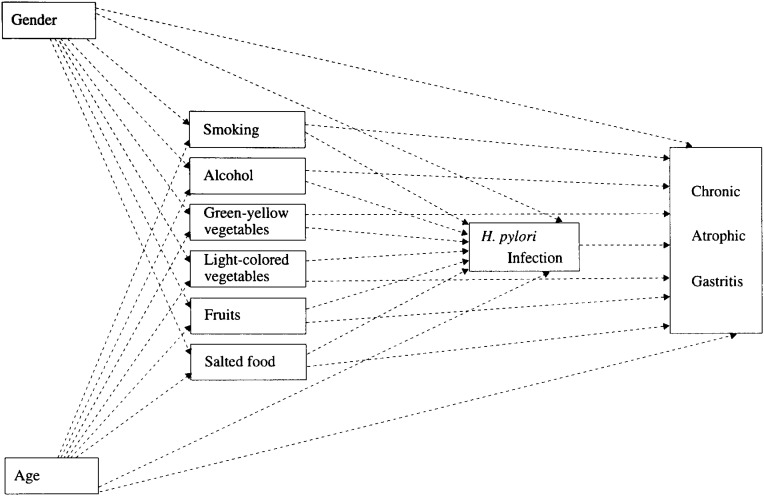
A multivariate analysis using a logistic model can evaluate the influence of each risk factor for H. pylori infection or CAG after adjustment for the influence of other factors. However, this method cannot provide a comprehensive evaluation of the mutual relationships among the variables by investigating both the direct influences and the indirect influences. The path analysis can simultaneously evaluate both the direct influence of the independent variable on the dependent variable and the indirect influence of independent variable through other variables, if many relations are set up among the variables. The relationships among the variables are illustrated according to a model prepared in advance (path diagram). This path diagram is prepared according to the epidemiological and medical findings obtained so far. Although the correlations among the variables are actually analyzed, the causal relations are inferred from those correlations according to the model. If more than one pathway is assumed, those pathways are controlled simultaneously, and the existence of causal relations are suggested for pathways between which significant correlations remained. We adopted the path analysis as well taking into consideration these advantages of the path analysis. In the first step, the following relations were defined and analyzed, such as the indirect relations of gender and age to H. pylori infection through each lifestyle variable, and the direct relations of gender and age to H. pylori infection. H. pylori infection is considered to cause CAG. As the second step, we examined the indirect relations of each lifestyle variable to CAG through H. pylori infection, as well as the direct relations of gender, age and each lifestyle variable to CAG (Figure 1). The standardized partial regression coefficient obtained by a multiple regression analysis was used as the path coefficient.
Figure 1. A hypothesized model of gender, age, lifestyle factors, H. pylori infection and CAG.
In these logistic and path analyses, the following variables, with exception of age (actual value), were assigned as dummy variables: gender (female=0, male=1), H. pylori antibody (negative=0, positive=1), presence of CAG (absence=0, presence=1), smoking (non-smoker=0, ex-smoker=1, ≦ 19/day=2, ≧ 20/day=3), alcohol consumption (non-drinker=0, ex-drinker=1, ≦ 3 days /week=2, ≧ 4 days /week=3), and consumption of each food (≦ 3 days /week=0, ≧4 days /week=1). The SAS statistical software package (version 6.11) was used in all statistical analyses.
RESULTS
Table 1 shows the distribution of the H. pylori positive rates and the prevalence of CAG, according to the gender and age group. The H. pylori positive rates in males and in females were 80.5% and 70.4%, respectively. The rate in males was significantly higher than that in females (p <0.01). These rates rose significantly as age increased (p <0.01). The prevalence of CAG in males and females was 30.1% and 24.9%, respectively, and no significant difference was found in the rates based on gender. The prevalence also rose significantly as age increased (p <0.01).
Table 1. Gender and age distribution according to the positive rates of H. pylori antibody and prevalence of chronic atrophic gastritis.
| Age group | Number of subjects | Positive rates of H. pylori antibody (%) |
Prevalence of CAG (%) |
||||||
|
|
|
|
|||||||
| Males | Females | Total | Males | Females | Total | Males | Females | Total | |
| 30-39 | 15 | 46 | 61 | 60.0 | 47.8 | 50.8 | 0.0 | 6.5 | 4.9 |
| 40-49 | 40 | 105 | 145 | 62.5 | 66.7 | 65.5 | 12.5 | 19.1 | 17.2 |
| 50-59 | 55 | 182 | 237 | 85.5 | 70.3 | 73.8 | 25.5 | 21.4 | 22.4 |
| 60-69 | 133 | 233 | 366 | 84.9 | 73.8 | 77.9 | 36.8 | 27.5 | 30.9 |
| 70-79 | 69 | 76 | 145 | 82.6 | 78.9 | 80.7 | 37.7 | 44.7 | 41.4 |
|
| |||||||||
| Total | 312 | 642 | 954 | 80.5 | 70.4 | 73.7 | 30.1 | 24.9 | 26.6 |
|
| |||||||||
| P<0.01 | P<0.01 | P<0.01 | P<0.01 | P<0.01 | P<0.01 | ||||
H. pylori : Helicobacter pylori; CAG: Chronic atrophic gastritis
The test for trend was calculated by a logistic model.
The column on the left in Table 2 shows the OR for H. pylori infection according to each lifestyle variable, while the column on the right shows the OR for CAG according to H. pylori infection and each lifestyle variable, after adjusting for gender and age. No significant relationship was seen between any of the lifestyle variables and H. pylori infection. The OR for CAG in H. pylori antibody-positive subjects compared with H. pylori antibody-negative subjects was 6.29 (95%CI: 3.73-10.61). The OR for CAG among subjects who consumed light colored-vegetables more than 4 times per week compared with subjects who consumed less than 3 times per week was 0.68 (95% CI:0.52-0.94). No significant relationship was observed between any of the other factors and CAG.
Table 2. Gender and age adjusted odds ratio(OR)and 95% confidence interval(95%CI) for H. pylori infection and CAG for each factor (n=954).
| Factor | n | No. H.pylori (+) |
OR | (95%CI) | No. CAG (+) |
OR | (95%CI) |
| H. pylori antibody | |||||||
| negative | 251 | — | 17 | 1.00 | |||
| positive | 703 | — | 237 | 6.29 | (3.73-10.61)** | ||
| Smoking | |||||||
| Non-smoker | 729 | 524 | 1.00 | 191 | 1.00 | ||
| Ex-smoker | 111 | 90 | 0.90 | (0.46-1.76) | 31 | 0.64 | (0.36-1.16) |
| ≦ 19/day | 42 | 36 | 1.32 | (0.51-3.42) | 12 | 0.64 | (0.29-1.39) |
| ≧ 20/day | 72 | 53 | 0.67 | (0.33-1.34) | 20 | 0.76 | (0.39-1.48) |
| Alcohol drinking | |||||||
| Non-drinker | 514 | 364 | 1.00 | 135 | 1.00 | ||
| Ex-drinker | 24 | 17 | 0.80 | (0.31-2.05) | 7 | 0.99 | (0.38-2.56) |
| ≦ 3days/week | 211 | 158 | 1.35 | (0.92-1.99) | 52 | 1.10 | (0.74-1.64) |
| ≧ 4days/week | 205 | 164 | 1.33 | (0.81-2.17) | 64 | 1.11 | (0.70-1.77) |
| Green-yellow vegetables | |||||||
| ≦ 3days/week | 363 | 272 | 1.00 | 95 | 1.00 | ||
| ≧ 4days/week | 591 | 431 | 0.95 | (0.70-1.29) | 159 | 1.05 | (0.78-1.44) |
| Light colored vegetables | |||||||
| ≦ 3days/week | 257 | 190 | 1.00 | 82 | 1.00 | ||
| ≧ 4days/week | 697 | 513 | 1.04 | (0.75-1.45) | 172 | 0.68 | (0.52-0.94)* |
| Fruits | |||||||
| ≦ 3days/week | 526 | 393 | 1.00 | 136 | 1.00 | ||
| ≧ 4days/week | 428 | 310 | 0.90 | (0.67-1.21) | 118 | 1.06 | (0.79-1.43) |
| Salted foods | |||||||
| ≦ 3days/week | 801 | 590 | 1.00 | 209 | 1.00 | ||
| ≧ 4days/week | 153 | 113 | 1.02 | (0.68-1.52) | 45 | 1.24 | (0.84-1.84) |
OR : Odds ratio after adjusting for gender and age
*p<0.05, **p<0.01
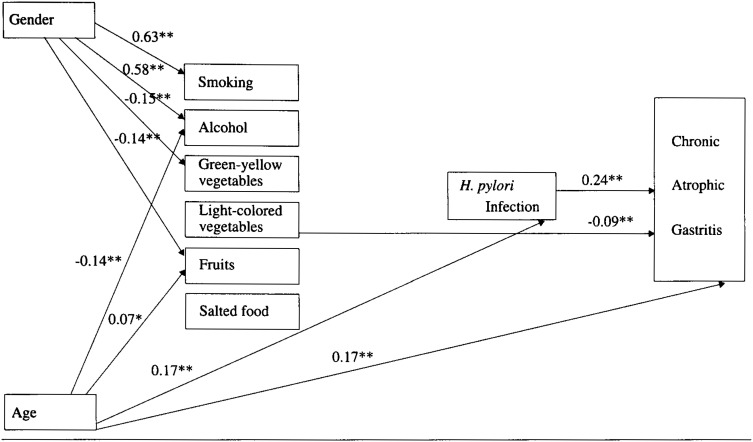
Figure 2 shows the results of the path analysis of a defined multivariate model. The overall F value from the multiple regression analysis of CAG was significant (F=12.91, p<0.001, R2=0.1096). The path coefficient between the respective variables was calculated after adjustment for the influence of the other factors.
Figure 2. Path estimates of the model with relation to gender, age, lifestyle factors, H. pylori infection and CAG(n=954).
Continuous arrows (→) represent significant relationships.
The degeree of effects are estimated by path coefficients, which are standardized partial regression coefficients.
Significant path coefficients are indicated by asterisk (*p<0.05, **p<0.01)
Being of male gender was found to have not only significantly positive relationships with the high frequencies of smoking and alcohol consumption but also significantly negative relationships with the frequency of consumption of green vegetables and fruits. Aging had a significantly negative relation with the frequency of alcohol consumption and a significantly positive relation with the frequency of fruit consumption. Significantly direct positive relations were observed between aging and H. pylori infection and between H. pylori infection and CAG. Therefore, aging had an indirect positive relation with CAG through H. pylori infection. A significantly direct positive relation between aging and CAG was also observed. Of the six investigated lifestyle variables, none were significantly related to H. pylori infection, and only high consumption of light-colored vegetables had a significantly direct negative relation with CAG. Even when the analysis was carried out by limiting the study subjects to those with H. pylori infection, a significantly direct negative relation between the consumption of light-colored vegetables and CAG was observed (path coefficient =−0.12, p<0.01).
DISCUSSION
Regarding the relation between dietary habits and H. pylori infection, a few epidemiological studies have suggested a significantly positive relationship between salt intake and the risk of H. pylori infection12,18). However, another study showed no relationship between salt intake and an increased risk7,19). As for fruit and/or vegetables consumption, although a few studies showed significant relationships between high consumption of fruit or vegetables7,12), other studies reported non-significant relationships18,20). Therefore, it is difficult to conclude that any specific food contributed to prevention of H. pylori as the incompatible findings of previous studies.
While many previous studies reported that no significant relationship was observed between smoking or alcohol consumption and H. pylori infection9,18-22), some previous studies documented significantly positive or negative relations with H. pylori infection12,13,23-25). In summary, the opinions of investigators concerning the relationships between H. pylori infection and smoking or alcohol have not come to agreement yet.
In the present study, both a logistic analysis and a path analysis showed no significant relationship between smoking, alcohol or the consumption of four foods (green-yellow vegetables, light-colored vegetables, fruits, and salted food) and H. pylori infection. These results suggest that although these lifestyle variables were not associated with H. pylori infection, aging was positively associated with H. pylori infection in the study area, which is consistent with many other studies1,12,13,26-30).
Several studies have evaluated the relation of lifestyle variables to the risk of CAG with adjustment for H. pylori infection status. The relationship between smoking and CAG is still controversial6,8,31-33). The present study does not show any positive association, but rather suggested a slight negative association. This association could be a result of CAG. That is, the manifestation of CAG could cause a person to stop smoking. We found no relation between alcohol consumption and CAG risk, which is highly consistent with other studies6,8,31,32).
Regarding green-yellow vegetables consumption, even though one study reported a significantly negative relationship between a high consumption of yellow vegetables and the risk of CAG8), other studies showed no relationship between a high consumption of green-yellow vegetables and the risk of CAG6). With regard to fruit consumption, while one study suggested a high consumption of fruit decreased the risk of CAG31), other studies reported no such relationship8,32). As a result, no definitive conclusions could be made regarding these two foods and the risk for CAG.
In our study, a logistic analysis revealed that a high consumption of light-colored vegetables negatively associated with the risk of CAG. In our path analysis, although aging did have both a direct positive association with CAG and an indirect positive association with CAG through H. pylori infection, aging had no relation with the consumption of light-colored vegetables. In addition, whereas a high consumption of this food type had no relation with H. pylori infection, a high consumption of this food type had a direct negative association with the risk of CAG. These findings support hypothesis that a high consumption of light-colored vegetables directly suppresses the risk of CAG, independent of either aging or the presence of H. pylori infection, both of which are risk factors for CAG.
Comparison of the age-adjusted prevalence of CAG determined on the basis of the same criteria set as in the previous studies in Japan30,34-36) and the age-adjusted prevalence of CAG in this study showed that CAG prevalence in this area was lower.
The results of analysis in the present study suggested involvement of the light-colored vegetables as a cause of the low CAG prevalence in the investigated area in this study. To verify this suggestion, it is necessary not only to investigate the frequency of consumption of light-colored vegetables per week but also to determine whether consumption of light-colored vegetable is really high in this area by carrying out detailed survey of the amount and kinds of those light-colored vegetables consumed and comparing the results with the findings from the high CAG prevalence areas.
Regarding intake of micro-nutrients, a few studies have shown a high intake of carotene to decrease the risk of CAG7,8). As for vitamin C, several studies have shown a high intake of vitamin C to decrease the risk of CAG7,33).
According to a national nutrition survey in Japan conducted during the same period as this study, rural residents took more vitamin C from light-colored vegetables than from green-yellow vegetables37). We therefore consider that light-colored vegetables, which are often eaten raw in this rural area, may suppress the occurrence of CAG through the effect of vitamin C, which is often destroyed during the cooking process, rather than carotene. However, in the present study no significant association was observed for the consumption of either fruit or green-yellow vegetables that also contain vitamin C. Additional research including details on the methods of cooking and/or the intake of micro-nutrients in this area are called for to clarify this discrepancy.
Recently, the hospital based eradication therapy of H. pylori has been adopted as a strategy to treat peptic ulcer patients in the Japanese clinical setting38), but such preventive eradication therapy for CAG is difficult to implement due to such problems as side-effects, the appearance of H. pylori drug resistance, the cost effectiveness and the high infection rate39,40). Therefore, health education regarding dietary habits is considered to be one of the promising methods of community based CAG prevention.
The results of this study suggest a possibility that a high consumption of light-colored vegetables contributes to the prevention of CAG. To clarify this possibility, further epidemiological studies are required in the prospective study design including detailed information of the amount and kinds of the vegetables as well as the methods of cooking the vegetables.
ACKNOWLEDGMENTS
This study was supported by the public office of a rural town in the study area. We thank the staff members of the public office and we especially thank Ms. K. Urabe (public health nurse of this town) for her valuable help.
REFERENCES
- 1.Fukao A, Hisamichi S, Ohsato N, et al. Correlation between the prevalence of gastritis and gastric cancer in Japan. Cancer Causes Control, 1993; 4: 17-20. [DOI] [PubMed] [Google Scholar]
- 2.Correa P. A human model of gastric carcinogenesis. Cancer Res, 1988; 48: 3554-3560. [PubMed] [Google Scholar]
- 3.Fontham E, Zavala D, Correa P, Rodriguez E, et al. Diet and chronic atrophic gastritis: a case-control study. J Natl Cancer Inst, 1986; 76: 621-627. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet, 1983; 1(8336): 1273-1275. [PubMed] [Google Scholar]
- 5.Blaser MJ. Hypotheses on the pathogenesis and natural history of Helicobacter pylori–induced inflammation. Gastroenterology, 1992; 102: 720-727. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe Y, Ozasa K, Higashi A, et al. Helicobacter pylori infection and atrophic gastritis. A case-control study in a rural town of Japan. J Clin Gastroenterol, 1997; 25: 391-394. [DOI] [PubMed] [Google Scholar]
- 7.Fontham ET, Ruiz B, Perez A, Hunter F, Correa P. Determinants of Helicobacter pylori infection and chronic gastritis. Am J Gastroenterol, 1995; 90: 1094-1101. [PubMed] [Google Scholar]
- 8.Tsugane S, Kabuto M, Imai H, et al. Helicobacter pylori, dietary factors, and atrophic gastritis in five Japanese populations with different gastric cancer mortality. Cancer Causes Control, 1993; 4: 297-305. [DOI] [PubMed] [Google Scholar]
- 9.Anonymous. Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. The EUROGAST Study Groop. Gut, 1993; 34: 1672-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohli Y, Kato T, Suzuki K, Tada T, Fujiki N. Incidence of atrophic gastritis with age in Japan and Canada. Jpn J Med, 1987; 26: 158-161. [DOI] [PubMed] [Google Scholar]
- 11.Imai T, Kubo T, Watanabe H. Chronic gastritis in Japanese with reference to high incidence of gastric carcinoma. J Natl Cancer Inst, 1971; 47: 179-195. [PubMed] [Google Scholar]
- 12.Hamajima N, Inoue M, Tajima K, et al. Lifestyle and anti-Helicobacter pylori immunoglobulin G antibody among outpatients. Jpn J Cancer Res, 1997; 88: 1038-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray LJ, McCrum EE, Evans AE, Bamford KB. Epidemiology of Helicobacter pylori infection among 4742 randomly selected subjects from Northern Ireland. Int J Epidemiol, 1997; 26: 880-887. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi N, Okubo T, Yamamura J, Takahashi K, Nakamura R, Funatani F. A prospective study of access to medical services following a community-based screening program. Nippon Koshu Eisei Zasshi, 1990; 37: 281-288. (in Japanese) [PubMed] [Google Scholar]
- 15.Marchildon PA, Ciota LM, Zamaniyan FZ, Peacock JS, Graham DY. Evaluation of three commercial enzyme immunoassays compared with 13C urea breath test for detection of Helicobacter pylori infection. J Clin Microbiol, 1996; 34: 1147-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tani T, Kurimoto S, Sawamura T, et al. Evaluation of five commercial kits for detection of Helicobacter pylori. Jpn Med Lab, 1997; 46: 1521-1524. [Google Scholar]
- 17.Inoue K, Miyoshi E, Aoki S, et al. The evaluation of cut-off levels of serum pepsinogens comparising with gastric endoscopy on the same time in human dry dock. Jpn J Gastroenterol, 1997; 35: 495-500. [Google Scholar]
- 18.Tsugane S, Tei Y, Takahashi T, Watanabe S, Sugano K. Salty food intake and risk of Helicobacter pylori infection. Jpn J Cancer Res, 1994; 85: 474-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinch K, Ishii H, Imanishi K, Kono S. Relationship of cigarette smoking, alcohol use, and dietary habits with Helicobacter pylori infection in Japanese men. Scand J Gastroenterol, 1997; 32: 651-655. [DOI] [PubMed] [Google Scholar]
- 20.Battaglia G, Di Mario F, Pasini M, et al. Helicobacter pylori infection, cigarette smoking and alcohol consumption. A histological and clinical study on 286 subjects. Ital J Gastroenterol, 1993; 25: 419-424. [PubMed] [Google Scholar]
- 21.Maxton DG, Srivastava ED, Whorwell PJ, Jones DM. Do non-steroidal anti-inflammatory drugs or smoking predispose to Helicobacter pylori infection? Postgrad Med J, 1990; 66: 717-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malaty HM, Kim JG, Kim SD, Graham DY. Prevalence of Helicobacter pylori infection in Korean children: inverse relation to socioeconomic status despite a uniformly high prevalence in adults. Am J Epidemiol, 1996; 143: 257-262. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi S, Kurosawa M, Sakiyama T. Helicobacter pylori risk associated with sibship size and family history of gastric diseases in Japanese adults. Jpn J Cancer Res, 1998; 89: 1109-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenner H, Rothenbacher D, Bode G, Adler G. Relation of smoking and alcohol and coffee consumption to active Helicobacter pylori infection: cross sectional study. BMJ, 1997; 315: 1489-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogihara A, Kikuchi S, Hasegawa A, et al. Relationship between Helicobacter pylori infection and smoking and drinking habits. J Gastroenterol Hepatol, 2000; 15: 271-276. [DOI] [PubMed] [Google Scholar]
- 26.Asaka M, Kimura T, Kudo M, et al. Rerationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology, 1992; 102: 760-766. [DOI] [PubMed] [Google Scholar]
- 27.Furuhashi S, Nihonyanagi H, Mishima H, et al. Evaluation of serum anti Helicobacter pylori IgG antibody titres in Nagoya Medical Examination Center Postal Life Welfare Corporation. Nippon Sogo Kenshin Igakkaishi, 1996; 23: 21-27. (in Japanese) [Google Scholar]
- 28.Horinaka M, Ohrui M, Haga T, Seta H, Hisauchi T, Watanabe N, et al. A clinical study in subjects with positive antibody to Helicobacter pylori in human dry dock: association of serum antibody to Helicobacter pylori with serum pepsinogen levels. Nippon Ningendokku Gakkaishi, 1997; 12: 249-254. (in Japanese) [Google Scholar]
- 29.Ueda M, Kasugai T, Miyake C, Tsuki S, Ohta K, et al. The results of gastric cancer check by mailing specimen: The 1st report. Shokaki Shudan Kenshin, 1997; 35: 501-507. (in Japanese) [Google Scholar]
- 30.Fukao A, Komatsu S, Tsubono Y, Hisamichi S, Ohori H, et al. Helicobacter pylori infection and chronic atrophic gastritis among Japanese blood donors: a cross-sectional study. Cancer Causes Control, 1993; 4: 307-312. [DOI] [PubMed] [Google Scholar]
- 31.Kato I, Miki K, Munoz N, et al. Determinants of plasma pepsinogen levels in a population at high risk for stomach cancer in Venezuela. Int J Cancer, 1995; 62: 512-518. [DOI] [PubMed] [Google Scholar]
- 32.Inoue M, Tajima K, Kobayashi S, et al. Protective factor against progression from atrophic gastritis to gastric cancer: data from a cohort study in Japan. Int J Cancer, 1996; 66: 309-314. [DOI] [PubMed] [Google Scholar]
- 33.Farinati F, Cardin R, Libera GD, et al. Determinants for development of chronic atrophic gastritis and intestinal metaplasia in the stomach. Eur J Cancer Prev, 1995; 4: 181-186. [DOI] [PubMed] [Google Scholar]
- 34.Miki K. At the turning point of gastric mass screening: a trial of screening of the cancer-susceptible subjects by serum pepsinogen tests. Nippon Sogo Kenshin Igakkaishi, 1993; 20: 302-307. (in Japanese) [Google Scholar]
- 35.Sasamori N, Tamura M, Iwasaki M, et al. Evaluation of serum pepsinogen tests at MHTS: a new method of gastric cancer screening. Nippon Sogo Kenshin Igakkaishi, 1993; 20: 343-354. (in Japanese) [Google Scholar]
- 36.Tomooka Y. Evaluation on a gastric mass survey: comparison with gastric mass screening using photoflurography and that using serum pepsinogen. Nippon Koshu Eisei Zasshi, 1996; 43: 118-125. (in Japanese) [PubMed] [Google Scholar]
- 37.The Ministry of Health and Welfare. The national nutrition survey in Japan.1988-1990. (in Japanese)
- 38.Satoh K. Treatment of Helicobacter pylori infection in Japan. Prog Med, 1998; 18: 1166-1169. (in Japanese) [Google Scholar]
- 39.Yamamoto I, Fukuda Y, Shintani S, et al. Diagnostic methods in eradication of H. Pylori (in general surgery and technical research). Prog Med, 1998; 18: 1183-1190. (in Japanese) [Google Scholar]
- 40.Watanabe K, Takahashi S, Saito S, Itoh T. The state of acquired drug resistance of Helicobacter pylori after eradication therapy and its counterplan. Prog Med, 1998; 18: 1178-1182. (in Japanese) [Google Scholar]