Key Points
-
•
Concurrent thrombocytopenia and VTE occur frequently in cancer (∼1 in 2 hematologic malignancies and 1 in 5 solid tumors).
Visual Abstract
Abstract
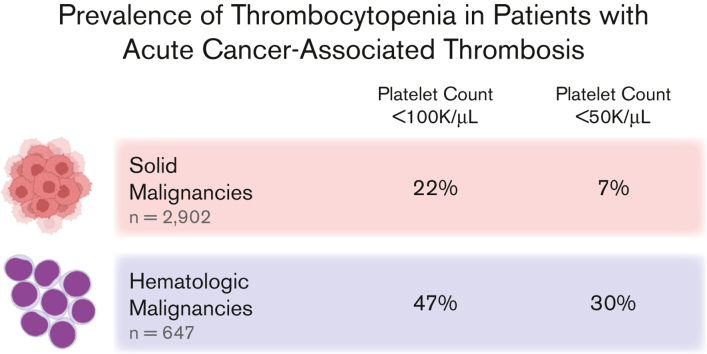
Venous thromboembolism (VTE) and thrombocytopenia are frequently encountered complications in patients with cancer. Although there are several studies evaluating the safety and efficacy of anticoagulation regimens in patients with cancer-associated thrombosis (CAT) with thrombocytopenia, there is a paucity of data assessing the scope of the concurrent diagnoses. This study evaluates the prevalence of thrombocytopenia among patients with acute CAT. A retrospective cohort analysis of adult patients with cancer was conducted at Beth Israel Deaconess Medical Center between 2010 and 2021 with CAT (acute VTE within 6 months after new diagnosis of malignancy). VTE included acute deep vein thrombosis, pulmonary embolism, abdominal or intrathoracic venous thrombosis, and cerebral sinus thrombosis. The lowest platelet count within 2 weeks of (before or after) the index VTE event was identified to assess the frequency and grade of concurrent thrombocytopenia. We identified 3635 patients with CAT (80% solid tumors, 18% hematologic malignancies, and 2% multiple concurrent cancer diagnoses). Thrombocytopenia (defined as platelet count <100 000/μL) occurred in 22% (95% CI 21%-24%) of patients with CAT with solid tumors diagnoses and 47% (95% CI 43%-51%) of patients with CAT and hematologic malignancies. Severe thrombocytopenia (platelet count <50 000/μL) occurred in 7% (95% CI 6%-8%) of patients with solid tumors and 30% (95% CI 27%-34%) of patients with hematologic malignancies. Concurrent diagnoses of CAT and thrombocytopenia are very common, especially among patients with hematologic malignancies.
Introduction
Venous thromboembolism (VTE) is a common complication in patients with cancer, with thrombosis being the second leading cause of death among outpatients undergoing chemotherapy.1,2 Because of the unique risk profile of patients with cancer, management of cancer-associated thrombosis (CAT) requires special considerations as compared with VTE in patients without cancer. Thrombocytopenia is also a common complication in patients with cancer, either due to the underlying malignancy or the toxicity of cancer-directed therapy. Clinical decision-making in patients with cancer who develop both thrombosis and thrombocytopenia is challenging, as thrombocytopenia increases the risk of bleeding without conferring protection against thrombosis. Randomized phase 3 anticoagulation trials in CAT often excluded patients with thrombocytopenia.3, 4, 5, 6, 7, 8, 9 Recently released European Hematology Association clinical guidelines on the management of these complex scenarios further emphasize the everyday challenges faced by clinicians in this population.10
Data on the true prevalence of thrombocytopenia in patients with cancer and concomitant thrombosis are extremely limited.11 Thrombocytopenia is common in hematologic malignancies and is often observed following certain cytotoxic chemotherapies in patients with solid tumors.12 Accordingly, the Flatiron Health Electronic Health Record database of patients with cancer reported a 3-month cumulative incidence of thrombocytopenia of 13% (any grade, platelet count <100 × 103/μL) in patients with solid tumors. Severe thrombocytopenia (platelet count <50 × 103/μL) occurred in 6% of patients with solid tumors receiving chemotherapy and in 28% of patients with hematologic malignancies receiving chemotherapy.13 However, how frequently thrombocytopenia occurs in the setting of acute CAT is unknown. Although practicing clinicians frequently deal with the challenge of managing anticoagulation in patients with cancer and thrombocytopenia, the true scope of the problem is undefined.
Methods
Study design and patient population
This retrospective cohort study included adult patients (age ≥ 18 years) with cancer at Beth Israel Deaconess Medical Center (BIDMC) from the years 2010 through 2021 who had an index CAT event in any clinical setting (inpatient, outpatient, or emergency department). All study procedures were approved by the Institutional Review Board at BIDMC. The study was conducted according to the Declaration of Helsinki. ICD-9 and ICD-10 codes associated with clinical encounters were used to identify diagnoses of VTE (ICD-9 415, 451-453, and ICD-10 I26, I67, I80, and 182) and cancer (ICD-9 140-239 and ICD-10 C00-D49). The positive predictive value of ICD coding for VTE has previously been validated.14, 15, 16, 17, 18, 19, 20, 21, 22 Eligibility criteria required a new VTE diagnosis (first occurrence of VTE diagnosis during the study period) associated with a first or new occurrence of a cancer diagnosis (within prior 6-month period). The 6-month temporal association of VTE and cancer diagnosis was implemented to exclude patients with a remote history of cancer. Qualifying VTE diagnoses included acute pulmonary embolism (PE), deep vein thrombosis (DVT; divided into proximal, distal, unspecified, and upper extremity DVT), cerebral venous thrombosis (CVT), abdominal thrombosis (portal vein thrombosis, Budd-Chiari Syndrome, inferior vena cava thrombosis, and renal vein thrombosis), and intrathoracic thrombosis (superior vena cava, brachiocephalic, and other unspecified thoracic veins). In instances of multiple entries, the initial occurrence of VTE was used. Chronic, superficial, or unspecified clots were excluded. Cancer type was classified and divided by system. Solid tumors included gastrointestinal (GI), lung/intrathoracic, genitourinary (GU), gynecologic, breast, head and neck, melanoma, nervous system, neuroendocrine (NET), and sarcoma. Hematologic malignancies included lymphoma, multiple myeloma, and myeloid malignancies. Remaining “neoplasm” diagnoses that were classified as benign, nonmelanoma skin cancer, secondary malignancy (metastasis), unspecified behavior, or not otherwise specified were excluded from the study. Those patients without available platelet count data in the 2 weeks before or after the index CAT event were excluded.
The lowest platelet count in the 2 weeks before or after the index CAT event was collected and classified according to Common Terminology Criteria for Adverse Events (CTCAE v.5.0) grading in relation to the lower limit of normal (LLN): not thrombocytopenic (>LLN), grade 1 (75 × 103/μL – LLN), grade 2 (50-75 × 103/μL), grade 3 (25-50 × 103/μL), and grade 4 (<25 × 103/μL).23 The LLN was chosen to be 100 × 103/μL in this study to exclude clinically insignificant or spurious decreases in platelet count in the 100 × 103 to 150 × 103/μL range and to preserve equal intervals of 25 × 103/μL between grades of thrombocytopenia. Given the retrospective nature of the study, the specific timeframe of 2 weeks before or after the index CAT event was chosen to ensure availability of platelet count data around the time of VTE diagnosis, as the coding date may differ slightly from the clinical event date. The proportion of index CAT events associated with different grades of thrombocytopenia was analyzed according to cancer type (solid vs hematologic). The temporal trend in proportions of thrombocytopenia and cancer types were also analyzed by year (2010-2020) to assess whether there were changes in relative proportion or the number of VTE over time. No formal sample size estimates were performed. Exact binomial confidence intervals were calculated using a 95% confidence level.
Results
Cohort construction
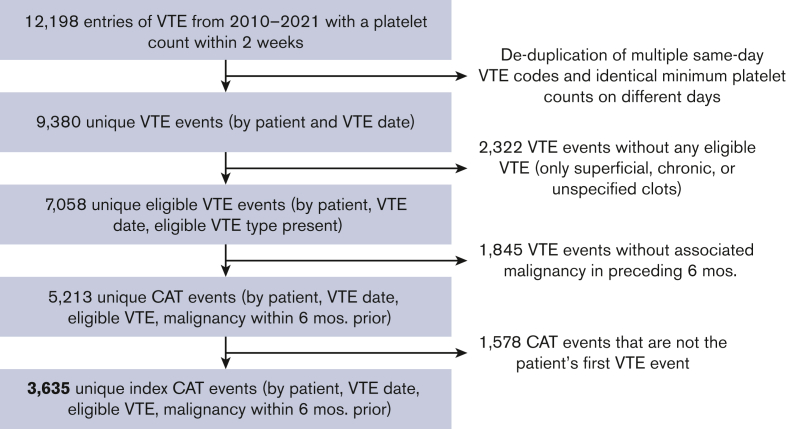
A total of 12 198 VTE were identified between October 2010 and June 2021. Excluding duplicate records, ineligible VTE, and absence of qualifying malignancy, the final number of unique patients identified meeting eligibility criteria was 3635 index CAT (Figure 1).
Figure 1.
Cohort construction. Selection process of appropriate index CAT events.
The overall cohort comprised 47% females, with a mean age 65.7 years (standard deviation of 12.8 years). Of the 3635 unique patients with CAT identified, 80% were associated with solid tumors, 18% with hematologic malignancies, and 2% with multiple concurrent cancer diagnoses (Table 1).
Table 1.
Patient characteristics at baseline, underlying malignancy, and type of incident venous thromboembolism
| Degree of thrombocytopenia | Total | Solid tumor | Hematologic malignancy | Multiple cancers |
|---|---|---|---|---|
| Number of patients, n, (%) | 3635 (100) | 2902 (80) | 647 (18) | 86 (2) |
| Age, mean ± standard deviation | 65.7 ± 12.8 | 66 ± 12.1 | 63.8 ± 15.5 | 70.7 ± 11 |
| Female sex, % | 47 | 47 | 45 | 56 |
| Encounter type | ||||
| Inpatient, % | 56 | 54 | 63 | 60 |
| Outpatient, % | 27 | 26 | 28 | 24 |
| Emergency department, % | 18 | 20 | 9 | 15 |
| VTE type, n (% of group) | ||||
| PE | 1490 (41) | 1249 (43) | 205 (32) | 36 (42) |
| PE with DVT | 293 (8) | 232 (8) | 51 (8) | 10 (12) |
| DVT | 1691 (47) | 1199 (41) | 444 (69) | 48 (56) |
| Upper extremity DVT | 582 (16) | 346 (12) | 220 (34) | 16 (19) |
| Abdominal thrombosis | 752 (21) | 697 (24) | 43 (7) | 12 (14) |
| Intrathoracic thrombosis | 54 (1) | 39 (1) | 14 (2) | 1 (1) |
| Cerebral venous thrombosis | 8 (<1) | 4 (<1) | 3 (<1) | 1 (1) |
Patients with an incident CAT event may have concurrent diagnoses of multiple VTE types (eg, concurrent PE and DVT), so percentages of VTE types are not mutually exclusive and sum in excess of 100%. “Intrathoracic thrombosis” includes thrombosis of superior vena cava, brachiocephalic, and other unspecified thoracic veins.
Of all unique CAT events identified, 41% were PE (8% with concurrent DVT), 47% DVT, 21% abdominal thrombosis (predominantly portal vein thrombosis), and 2% intrathoracic thrombosis or cerebral sinus thrombosis (Table 1). Notably, PE occurred more often in solid tumors (43%, 95% CI 41%-45%) than in hematologic malignancies (32%, 95% CI 28%-35%). CAT events in hematologic malignancies predominantly comprised DVT (69%, 95% CI 65%-72%) compared with solid tumors (41%, 95% CI 40%-43%). This was in large part due to an increased proportion of upper extremity DVT (34%, 95% CI 30%-38%) in hematologic malignancies vs solid tumors (12%, 95% CI 11%-13%). A high proportion of solid tumor CAT events were abdominal thrombosis (24%, 95% CI 22%-26%), predominantly in gastrointestinal cancers (44%, 95% CI 41%-46%), whereas the proportion was far fewer in hematologic malignancies (7%, 95% CI 5%-9%) (supplemental Table 1).
Prevalence of thrombocytopenia in CAT by cancer type
In the overall cohort, CAT with concurrent thrombocytopenia of any grade (<100 × 103/μL) was identified in 27% (95% CI 25%-28%) of patients, and grade 3 or 4 thrombocytopenia (<50 × 103/μL) was observed in 11% (95% CI 10%-13%) of patients. In the cohort with CAT and solid tumors, thrombocytopenia of any grade occurred in 22% (95% CI 21%-24%), and grade 3 or 4 thrombocytopenia occurred in 7% (95% CI 6%-8%) of cases. Gastrointestinal malignancies had the highest prevalence of thrombocytopenia among solid tumor diagnoses (32% any grade and 10% grade 3-4) (Table 2).
Table 2.
Prevalence of thrombocytopenia in patients with cancer-associated thrombosis by cancer type
| Degree of thrombocytopenia by cancer type, n (% of row) | Total CAT population | Normal >100 × 103/μL |
Grade 1 75-100 × 103/μL |
Grade 2 50-75 × 103/μL |
Grade 3 25-50 × 103/μL |
Grade 4 <25 × 103/μL |
|---|---|---|---|---|---|---|
| All cancers | 3635 (100) | 2657 (73) | 293 (8) | 269 (7) | 229 (6) | 187 (5) |
| Solid tumor | 2902 (100) | 2256 (78) | 235 (8) | 203 (7) | 156 (5) | 52 (2) |
| Gastrointestinal | 1326 (100) | 905 (68) | 144 (11) | 135 (10) | 112 (8) | 30 (2) |
| Lung / Intrathoracic | 472 (100) | 394 (83) | 30 (6) | 19 (4) | 19 (4) | 10 (2) |
| Genitourinary | 361 (100) | 313 (87) | 21 (6) | 16 (4) | 9 (2) | 2 (1) |
| Gynecologic | 222 (100) | 199 (90) | 10 (5) | 8 (4) | 2 (1) | 3 (1) |
| Breast | 204 (100) | 182 (89) | 10 (5) | 6 (3) | 6 (3) | 0 (0) |
| Other solid | 317 (100) | 263 (83) | 20 (6) | 19 (6) | 8 (3) | 7 (2) |
| Hematologic malignancy | 647 (100) | 343 (53) | 48 (7) | 61 (9) | 68 (11) | 127 (20) |
| Lymphoma | 400 (100) | 232 (58) | 32 (8) | 40 (10) | 46 (12) | 50 (13) |
| Multiple myeloma | 100 (100) | 59 (59) | 7 (7) | 8 (8) | 8 (8) | 18 (18) |
| Myeloid | 147 (100) | 52 (35) | 9 (6) | 13 (9) | 14 (10) | 59 (40) |
| Multiple cancers | 86 (100) | 58 (67) | 10 (12) | 5 (6) | 5 (6) | 8 (9) |
“Other solid” includes cancers of the nervous system, sarcoma, head and neck tumors, melanoma, and neuroendocrine tumors.
In CAT of hematologic malignancies, the corresponding proportions were 47% (95% CI 43%-51%) for any grade thrombocytopenia and 30% (95% CI 27%-34%) for grade 3 or 4 thrombocytopenia. Myeloid malignancies had the highest prevalence of concurrent thrombocytopenia (65% any grade and 50% grade 3-4), with a significant portion (40%) qualifying as grade 4 (Table 2).
For CAT events with PE, thrombocytopenia of any grade occurred in 19% of cases, and grade 3 to 4 thrombocytopenia occurred in 8% of cases. CAT events with DVT had higher rates of thrombocytopenia, with 28% of any grade and 14% of grade 3 to 4. Abdominal thrombosis was also commonly associated with thrombocytopenia (41% any grade and 14% grade 3-4).
Temporal trends in tumor type, VTE type, and thrombocytopenia
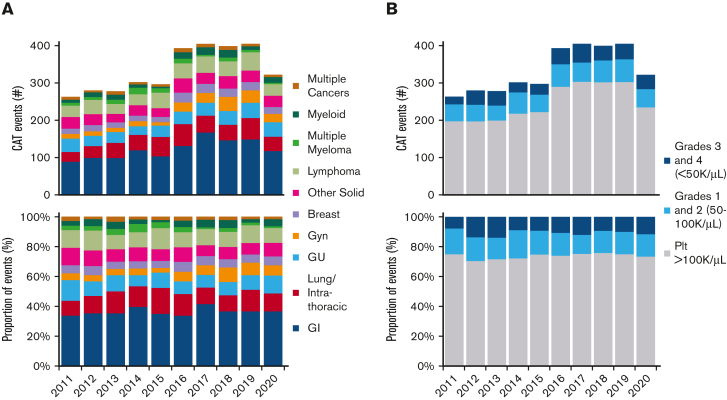
Temporal trends were also analyzed within the study period. The mix of cancer types during this period also remained similar, with 77% to 82% in solid tumors, 16% to 21% in hematologic malignancies, and 2% to 4% with multiple malignancies (Figure 2A). The proportion of PE associated with CAT cases over the study period remained stable, averaging 41% with a range of 37% to 49%. The proportion of DVT averaged 47% and ranged from 40% to 54%.
Figure 2.
Trends in cancer-associated thrombosis and thrombocytopenia. (A) Trend in CAT by cancer type over time in absolute number of events, top, and as proportion of total events, bottom. (B) Trend in CAT-associated thrombocytopenia by severity over time in absolute number of events, top, and as proportion of total events, bottom. Gyn, gynecologic; Plt, platelet count.
The proportion of CAT with thrombocytopenia remained fairly stable across the 10-year study period, with normal platelet count in 70% to 75% of CAT events, any grade thrombocytopenia in 25% to 30% of events, and grade 3 to 4 thrombocytopenia in 8% to 14% of events (Figure 2B).
Discussion
Thrombocytopenia is known to frequently occur in the setting of CAT, but to the best of our knowledge, this is the first study providing point estimates of the prevalence of concurrent thrombocytopenia and CAT. Nearly 1 in 4 patients with solid tumor diagnosis and 1 in 2 with hematologic malignancies were diagnosed with CAT and thrombocytopenia. Notably, severe thrombocytopenia (<50 × 103/μL) was present in 30% of all patients with hematologic malignancy and a diagnosis of new VTE.
The distribution of VTE in the cohort differed between the solid tumor and hematologic malignancy groups. Among the solid tumor cohort, the distribution between DVT and PE was fairly even, except in the gastrointestinal group, who frequently were diagnosed with intra-abdominal DVT. Concurrent thrombocytopenia and intra-abdominal thrombosis were particularly common (every 2.5 cases). This high rate of concurrent thrombocytopenia and abdominal thrombosis is likely a reflection of the myelosuppressive regimens used to manage advanced gastrointestinal malignancies such as pancreatic cancer.24, 25, 26, 27 Cirrhosis is also associated with an increased risk for portal vein thrombosis and often leads to thrombocytopenia. There was a higher prevalence of DVT in the hematologic malignancy group compared with the solid tumor group (69% vs 41%), driven largely by higher rates of upper extremity DVT (34% vs 12%), which is attributed to the frequent use of catheter thrombosis in hematologic malignancy.28,29
Severe thrombocytopenia is commonly observed in patients with hematologic malignancies. Chemotherapy-induced thrombocytopenia in diffuse large B-cell lymphoma patients receiving multiagent chemotherapy is associated with grade 3 or 4 thrombocytopenia in ∼7% of patients on clinical trials.30, 31, 32 In our cohort with lymphoma, grade 3 or 4 thrombocytopenia was present in 25% of patients with acute VTE. Similarly, in clinical trials for myeloma, the incidence of thrombocytopenia (any grade) is between 6% and 15%, whereas in our cohort, 41% of patients had thrombocytopenia.33, 34, 35 These data suggest that thrombocytopenia may be more common among patients with VTE compared with those without VTE. Thrombocytopenia has been linked to VTE in cancer via direct activation of platelets.36
As the management of malignancies is transitioning to more targeted therapies rather than conventional cytotoxic regimens (that are often more myelosuppressive), we analyzed the trends in VTE and thrombocytopenia over time.37 Interestingly, the prevalence of thrombocytopenia with CAT has remained largely unchanged over the last decade. Similarly, the distribution of cancer diagnosis and the prevalence of severe thrombocytopenia was fairly consistent over the timeframe assessed. There was a transition from ICD-9 to ICD-10 during the study period (starting late 2015), and there were numerically more VTE diagnosed following the transition. However, we did not observe an impact on tumor type, VTE type, or the prevalence of concurrent thrombocytopenia. The increase in VTE diagnosis in cancer cohorts is consistent with the findings of a recent Danish population-based study demonstrating a continual rise in VTE diagnosis over the decade.38 We note that in 2020 there was a sudden decrease in CAT diagnosis, which is likely due to the COVID-19 pandemic and changes in health care use and diagnosis of nonrespiratory illnesses.32,39
Inherent to retrospective analyses, we recognize there are limitations to this study. Inclusion into the VTE cohort required a VTE diagnosis within 6 months of a new cancer diagnosis. The purpose of this requirement was to better ensure attribution of VTE relative to the underlying cancer diagnosis or cancer-directed therapy rather than include VTE many years after early cancer diagnosis. However, we acknowledge that the true incidence of CAT with thrombocytopenia may be underestimated, as a population of patients with more advanced disease many years after their initial diagnosis were excluded. This study was retrospective and cross-sectional in nature, focusing on thrombocytopenia at the time of the index CAT event; however, thrombocytopenia further removed in time and duration of thrombocytopenia are also of clinical relevance. We speculate that it is likely that the incidence of thrombocytopenia in the 3- to 6-month period following VTE is higher than the already high baseline coincidence of thrombocytopenia and VTE in this cohort. Future analyses of these aspects may provide further insight into the evolving thrombotic vs bleeding risk profile of CAT. The database is limited to a single institution such that there may be favored chemotherapeutic regimens that could affect the co-occurrence of thrombocytopenia and CAT. However, we believe that the relative proportion of VTE diagnoses in our thrombocytopenia cohort is generally applicable. In prior studies defining the risk and overall incidence of VTE according to solid tumor diagnosis, the greatest number of events were GI, followed by lung, breast, GU, and gynecologic malignancies.40, 41, 42 Similarly, in our cohort, the highest proportion were GI, followed by lung, GU, and gynecologic malignancies. Although chemotherapeutic regimens have evolved over the last decade, the relative prevalence of thrombocytopenia and CAT was consistent over an extended period across tumor diagnosis. The use of ICD coding as the basis for establishing diagnoses raises the possibility of mislabeling. The accuracy of ICD coding has been studied and validated in several other studies for both VTE and various cancer subtypes, with positive predictive values between 75% to 90%.14, 15, 16, 17, 18, 19, 20 What is less well established is the sensitivity of ICD coding for VTE, as we cannot exclude the possibility of an underestimation due to the absence of coding, as is suggested by the increase in VTE diagnoses after changing to ICD-10 coding. Nonetheless, even with the increased diagnosis of CAT with ICD-10 implementation, the relative proportion of patients with thrombocytopenia remained stable. We also acknowledge that some VTE events or platelet counts may have been diagnosed or measured outside our hospital medical record system.
This study highlights the high coprevalence of thrombocytopenia and CAT. Approximately 1 in 5 patients with solid tumors and 1 in 2 patients with hematologic malignancies with a diagnosis of VTE have concurrent thrombocytopenia. These point estimates serve to better define the scope of the problem and serve as justification for additional clinical trials addressing appropriate anticoagulation for acute CAT with thrombocytopenia.
Conflict-of-interest disclosure: C.H. has received consulting fees from Anthos Therapeutics and was previously employed at Baxter/Baxalta. J.I.Z. received research funding from Incyte and Quercegen; provided consultancy services to Sanofi, CSL, and Parexel; and reports honoraria from/advisory board participation with Pfizer/Bristol Myers Squibb, Portola, and Daiichi. R.P. declares no competing financial interests.
Acknowledgments
The authors thank George Silva of the BIDMC InSIGHT Core for his collaboration and support in database query design and data extraction. The visual abstract was created on BioRender.com.
Authorship
Contribution: C.H., R.P., and J.I.Z. were involved in the conception and design of the study, and revised the manuscript; C.H. performed the primary data collection, analysis, and drafted the manuscript.
Footnotes
Data requests can be made by email to the corresponding author, Jeffrey I. Zwicker: zwickerj@mskcc.org.
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Falanga A. The incidence and risk of venous thromboembolism associated with cancer and nonsurgical cancer treatment. Cancer Invest. 2009;27(1):105–115. doi: 10.1080/07357900802563028. [DOI] [PubMed] [Google Scholar]
- 2.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 3.Agnelli G, Becattini C, Meyer G, et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N Engl J Med. 2020;382(17):1599–1607. doi: 10.1056/NEJMoa1915103. [DOI] [PubMed] [Google Scholar]
- 4.Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187–2191. doi: 10.1111/jth.13153. [DOI] [PubMed] [Google Scholar]
- 5.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 6.McBane RD, 2nd, Wysokinski WE, Le-Rademacher JG, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J Thromb Haemost. 2020;18(2):411–421. doi: 10.1111/jth.14662. [DOI] [PubMed] [Google Scholar]
- 7.Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 2018;378(7):615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- 8.Young AM, Marshall A, Thirlwall J, et al. Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D) J Clin Oncol. 2018;36(20):2017–2023. doi: 10.1200/JCO.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- 9.Carney BJ, Wang TF, Ren S, et al. Anticoagulation in cancer-associated thromboembolism with thrombocytopenia: a prospective, multicenter cohort study. Blood Adv. 2021;5(24):5546–5553. doi: 10.1182/bloodadvances.2021005966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falanga A, Leader A, Ambaglio C, et al. EHA Guidelines on Management of Antithrombotic Treatments in Thrombocytopenic Patients With Cancer. Hemasphere. 2022;6(8):e750. doi: 10.1097/HS9.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopolovic I, Lee AY, Wu C. Management and outcomes of cancer-associated venous thromboembolism in patients with concomitant thrombocytopenia: a retrospective cohort study. Ann Hematol. 2015;94(2):329–336. doi: 10.1007/s00277-014-2198-6. [DOI] [PubMed] [Google Scholar]
- 12.Kilpatrick K, Shaw JL, Jaramillo R, et al. Occurrence and Management of Thrombocytopenia in Metastatic Colorectal Cancer Patients Receiving Chemotherapy: Secondary Analysis of Data From Prospective Clinical Trials. Clin Colorectal Cancer. 2021;20(2):170–176. doi: 10.1016/j.clcc.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Shaw JL, Nielson CM, Park JK, et al. The incidence of thrombocytopenia in adult patients receiving chemotherapy for solid tumors or hematologic malignancies. Eur J Haematol. 2021;106(5):662–672. doi: 10.1111/ejh.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):154–162. doi: 10.1002/pds.2341. [DOI] [PubMed] [Google Scholar]
- 15.Ammann EM, Cuker A, Carnahan RM, et al. Chart validation of inpatient International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) administrative diagnosis codes for venous thromboembolism (VTE) among intravenous immune globulin (IGIV) users in the Sentinel Distributed Database. Medicine (Baltimore) 2018;97(8):e9960. doi: 10.1097/MD.0000000000009960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White RH, Garcia M, Sadeghi B, et al. Evaluation of the predictive value of ICD-9-CM coded administrative data for venous thromboembolism in the United States. Thromb Res. 2010;126(1):61–67. doi: 10.1016/j.thromres.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Baldi I, Vicari P, Di Cuonzo D, et al. A high positive predictive value algorithm using hospital administrative data identified incident cancer cases. J Clin Epidemiol. 2008;61(4):373–379. doi: 10.1016/j.jclinepi.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Setoguchi S, Solomon DH, Glynn RJ, et al. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between medicare claims and cancer registry data. Cancer Causes Control. 2007;18(5):561–569. doi: 10.1007/s10552-007-0131-1. [DOI] [PubMed] [Google Scholar]
- 19.Parlett LE, Beachler DC, Lanes S, et al. Validation of an Algorithm for Claims-based Incidence of Prostate Cancer. Epidemiology. 2019;30(3):466–471. doi: 10.1097/EDE.0000000000001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraha I, Serraino D, Montedori A, et al. Sensitivity and specificity of breast cancer ICD-9-CM codes in three Italian administrative healthcare databases: a diagnostic accuracy study. BMJ Open. 2018;8(7):e020627. doi: 10.1136/bmjopen-2017-020627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein MM, Dutcher SK, Maro JC, et al. Validation of an electronic algorithm for Hodgkin and non-Hodgkin lymphoma in ICD-10-CM. Pharmacoepidemiol Drug Saf. 2021;30(7):910–917. doi: 10.1002/pds.5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verma AA, Masoom H, Pou-Prom C, et al. Developing and validating natural language processing algorithms for radiology reports compared to ICD-10 codes for identifying venous thromboembolism in hospitalized medical patients. Thromb Res. 2022;209:51–58. doi: 10.1016/j.thromres.2021.11.020. [DOI] [PubMed] [Google Scholar]
- 23.US Department of Health and Human Services . 2017. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf [Google Scholar]
- 24.GEMZAR (gemcitabine). Package insert . Revised May 2019. Eli Lilly and Company.https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020509s077lbl.pdf [Google Scholar]
- 25.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 26.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 27.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 28.Hansen RS, Nybo M, Hvas AM. Venous Thromboembolism in Pediatric Cancer Patients with Central Venous Catheter-A Systematic Review and Meta-analysis. Semin Thromb Hemost. 2021;47(8):920–930. doi: 10.1055/s-0041-1729886. [DOI] [PubMed] [Google Scholar]
- 29.Tran H, Arellano M, Chamsuddin A, et al. Deep venous thromboses in patients with hematological malignancies after peripherally inserted central venous catheters. Leuk Lymphoma. 2010;51(8):1473–1477. doi: 10.3109/10428194.2010.481065. [DOI] [PubMed] [Google Scholar]
- 30.Lu R, Lin Q, Chen S, et al. Chemotherapy-induced thrombocytopenia and platelet transfusion in patients with diffuse large B-cell lymphoma. Transl Cancer Res. 2020;9(3):1640–1651. doi: 10.21037/tcr.2020.01.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poeschel V, Held G, Ziepert M, et al. Four versus six cycles of CHOP chemotherapy in combination with six applications of rituximab in patients with aggressive B-cell lymphoma with favourable prognosis (FLYER): a randomised, phase 3, non-inferiority trial. Lancet. 2019;394(10216):2271–2281. doi: 10.1016/S0140-6736(19)33008-9. [DOI] [PubMed] [Google Scholar]
- 32.Purroy N, Bergua J, Gallur L, et al. Long-term follow-up of dose-adjusted EPOCH plus rituximab (DA-EPOCH-R) in untreated patients with poor prognosis large B-cell lymphoma. A phase II study conducted by the Spanish PETHEMA Group. Br J Haematol. 2015;169(2):188–198. doi: 10.1111/bjh.13273. [DOI] [PubMed] [Google Scholar]
- 33.Mellors PW, Binder M, Buadi FK, et al. Development of thrombocytopenia during first-line treatment and survival outcomes in newly diagnosed multiple myeloma. Leuk Lymphoma. 2019;60(12):2960–2967. doi: 10.1080/10428194.2019.1613536. [DOI] [PubMed] [Google Scholar]
- 34.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116(5):679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119(19):4375–4382. doi: 10.1182/blood-2011-11-395749. [DOI] [PubMed] [Google Scholar]
- 36.Riedl J, Preusser M, Nazari PM, et al. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood. 2017;129(13):1831–1839. doi: 10.1182/blood-2016-06-720714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shahid K, Khalife M, Dabney R, et al. Immunotherapy and targeted therapy-the new roadmap in cancer treatment. Ann Transl Med. 2019;7(20):595. doi: 10.21037/atm.2019.05.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulder FI, Horvath-Puho E, van Es N, et al. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. 2021;137(14):1959–1969. doi: 10.1182/blood.2020007338. [DOI] [PubMed] [Google Scholar]
- 39.Nopp S, Janata-Schwatczek K, Prosch H, et al. Pulmonary embolism during the COVID-19 pandemic: Decline in diagnostic procedures and incidence at a university hospital. Res Pract Thromb Haemost. 2020;4(5):835–841. doi: 10.1002/rth2.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blom JW, Vanderschoot JP, Oostindier MJ, et al. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4(3):529–535. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 41.Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275. doi: 10.1371/journal.pmed.1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khorana AA, Francis CW, Culakova E, et al. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110(10):2339–2346. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.