Abstract
What is already known about this topic?
Diarrhea represents a substantial public health issue, contributing globally to a high number of pediatric medical consultations, hospital admissions, and mortality rates.
What is added by this report?
An increase in diarrheal frequency serves as a critical benchmark for evaluating severity. The predominant pathogens associated with pediatric diarrhea are rotavirus and norovirus, with co-infections exerting a notable compounding effect that leads to more severe diarrhea.
What are the implications for public health practice?
Implementing sensitive diagnostic techniques and comprehensive monitoring is paramount in identifying co-infections. Such strategies can provide physicians with critical insights into disease progression, thus considerably reducing the burden of diarrhea.
Keywords: Diarrhea, Co-infection, Synergistic effect, Severity
Despite a reduction in mortality rates over the past several decades, diarrhea remains a significant factor in both morbidity and mortality among children under the age of five worldwide (1-3). Diarrhea is typified by primary clinical symptoms of diarrhea and vomiting, which can result in dehydration, sepsis, and in severe instances, death. The presence of numerous enteric viruses, bacterial pathogens, and parasites is the root cause of this condition (2-3). Among these, enteric viruses are the most prevalent source of diarrhea in children (2,4-5). The significant enteric viruses include human group A rotaviruses (RVA), noroviruses (NoVs), astroviruses (As), and adenoviruses (Ad), with transmission primarily occurring via the fecal-oral route. RVA is the leading pathogen associated with childhood diarrhea (6), and NoVs present an increased risk of diarrhea outbreaks in communal settings (5). What is particularly noteworthy is the common occurrence of co-infections, wherein multiple etiologic agents emerge in individuals with diarrhea. Prior studies have found co-infections in 10% to 40% of diarrhea cases and in 0% to 15% of non-diarrheal children, with infections from as many as 2 to 5 pathogens (2,4,7).
Current research on the impact of co-infections of enteric viral pathogens on human health and the nature of their interactions, whether synergistic or antagonistic, is lacking. This study employed hospital-based, case-control data to evaluate the prevalence and pathogenicity of common enteric viral pathogens in a population comprised of children under the age of five in northern China from January 1 through December 31, 2019. To assess pathogenicity, a clear distinction was made between single infections and co-infections. Potential interactions between co-infecting pathogens were examined by evaluating their biological synergy through the use of both additive and multiplicative models. This research received ethical approval from the Ethical Review Committee of Guangzhou Women and Children’s Medical Center (No. 2017111501) and was registered in the Chinese Clinical Trial Registry (ChiCTR-ROC-17013620). Sentinel surveillance hospitals for this research included Guangzhou Women and Children’s Medical Center, Guangzhou Children’s Hospital, and Guangzhou Maternity and Child Health Care Hospital. For the purposes of this study, acute diarrhea is characterized as children experiencing three or more bowel movements in the past 24 hours with abnormal stool consistency (4). Children with acute diarrhea were recorded as cases from the outpatient and inpatient departments of the gastroenterology department. Children without diarrhea were identified as controls from other inpatient departments and internal medicine outpatient centers. Consent was obtained from either the parents or legal guardians of the children prior to their inclusion in the study. Uniform inclusion criteria for both cases and controls included: being under the age of five, no gender bias, and parental informed consent. Exclusion criteria applicable to both groups included the presence of other gastrointestinal diseases, severe illnesses, mental disorders, and the use of antiviral drugs and antibiotics in the past two months. Additional criteria for cases included experiencing diarrhea for less than 14 days prior to a doctor’s visit. Controls were similar in age and gender to the cases experiencing diarrhea.
In this study, trained nurses performed field questionnaire surveys to gather epidemiological data. Information collected included participant’s gender, age, date of visit, any known food allergies, medical history and primary clinical manifestations. The frequency of diarrhea, for instance, was noted as a significant factor in evaluating the severity of the condition. Concurrently, stool samples were collected in sterile conditions. These were then stored in a bio-safe transport box at temperatures of 4–8 °C, and transported to the laboratory at the Guangzhou Women and Children’s Medical Center within a 12-hour timeframe. Upon receipt, the samples were frozen at −70 °C in preparation for centralized testing. The study made use of an automated system for extracting total nucleic acid from the stool samples. A real-time reverse transcription-polymerase chain reaction (real-time RT-PCR) was then administered to detect the nucleic acids of RVA, NoVs, As, and Ad (8-10).
Qualitative data were analyzed using frequencies, percentages, odds ratios (ORs), and 95% confidence intervals (CIs), and compared using the chi-square test or Fisher’s exact test. Quantitative variables were described using medians and interquartile ranges (IQRs) and compared using the Kruskal-Wallis H test or Mann-Whitney U test. The interactions among the pathogens were evaluated using logistic regression models, adjusting for relevant host factors (3-4). Statistical significance was determined at a two-tailed P-value less than 0.05. All statistical analyses were performed using R software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria).
The study was performed with a total of 930 children participants under the age of five. Of these participants, 67.7% (n=630) were identified as children with diarrhea, and 32.3% (n=300) were classified as non-diarrheic participants. The participant pool was predominantly comprised of males (n=611, 65.7%) and a significant number were under the age of 2 (n=638, 68.6%) and were originally recruited from outpatient clinics (n=760, 81.7%). The median age of the participants stood at 14 months with an IQR of 7 to 28 months. There were balanced distributions between the two groups concerning age, gender, collection season, case source, and underlying diseases (Table 1). Amongst the diarrheic cases, the median number of defecation episodes in the 24-hour period preceding the medical consultation was 5 with an IQR of 4 to 7 occurrences.
Table 1. Epidemiological characteristics and co-infections of viral enteric pathogens in children with diarrhea and healthy individuals in Guangzhou City, Guangdong Province, China, 2019.
| Variable | Subgroup |
Non-diarrhea, N=300
n (%) |
Diarrhea, N=630
n (%) |
χ 2 | P | OR (95% CIs) |
| Note: The “−” symbol indicates the data can not be calculated. Abbreviation: Cl=confidence interval; OR=odds ratio; RVA=human group A rotaviruses; NoVs=noroviruses; Ad=adenoviruses; As=astroviruses. | ||||||
| Age | < 24 months | 231 (77.0) | 472 (74.9) | 0.476 | 0.491 | 1 (reference) |
| 24–60 months | 69 (23.0) | 158 (25.1) | 1.121 (0.811, 1.549) | |||
| Gender | Female | 104 (34.7) | 215 (34.1) | 0.026 | 0.871 | 1 (reference) |
| Male | 196 (65.3) | 415 (65.9) | 1.024 (0.767, 1.368) | |||
| Season | Spring | 80 (26.7) | 255 (40.5) | 2.557 | 0.457 | 1 (reference) |
| Summer | 98 (32.7) | 144 (22.9) | 0.461 (0.322, 0.660) | |||
| Autumn | 51 (17.0) | 117 (18.6) | 0.720 (0.476, 1.089) | |||
| Winter | 71 (23.7) | 114 (18.1) | 0.504 (0.342, 0.743) | |||
| Subjects source | Outpatient | 244 (81.3) | 516 (81.9) | 0.044 | 0.833 | 1 (reference) |
| Inpatient | 56 (18.7) | 114 (18.1) | 0.963 (0.675, 1.372) | |||
| Lactose intolerance | No | 292 (97.3) | 618 (98.1) | 0.561 | 0.545 | 1 (reference) |
| Yes | 8 (2.7) | 12 (1.9) | 0.709 (0.287, 1.753) | |||
| Systemic lupus erythematosus | No | 299 (99.7) | 620 (99.8) | − | 0.541 | 1 (reference) |
| Yes | 1 (0.3) | 1 (0.2) | 0.475 (0.030, 7.626) | |||
| Allergic purpura | No | 298 (99.3) | 628 (99.7) | − | 0.598 | 1 (reference) |
| Yes | 2 (0.7) | 2 (0.3) | 0.475 (0.067, 3.385) | |||
| Milk protein allergy | No | 299 (99.7) | 623 (98.9) | 1.442 | 0.231 | 1 (reference) |
| Yes | 1 (0.3) | 7 (1.1) | 3.360 (0.411, 27.431) | |||
| Any viral pathogen | No | 249 (83.0) | 394 (62.5) | 39.873 | <0.001 | 1 (reference) |
| Yes | 51 (17.0) | 236 (37.5) | 2.924 (2.078, 4.116) | |||
| Number of viral pathogens | No | 249 (83.0) | 394 (62.5) | 41.573 | <0.001 | 1 (reference) |
| Only one pathogen | 46 (15.3) | 192 (30.5) | 2.638 (1.843, 3.776) | |||
| Only two pathogens | 5 (1.7) | 41 (6.5) | 5.182 (2.021, 13.292) | |||
| Three pathogens | 0 (0.0) | 3 (0.5) | − | |||
| RVA | No | 274 (91.3) | 460 (73.0) | 40.994 | <0.001 | 1 (reference) |
| Yes | 26 (8.7) | 170 (27.0) | 3.895 (2.511, 6.041) | |||
| NoVs GII | No | 288 (96.0) | 559 (88.7) | 13.214 | <0.001 | 1 (reference) |
| Yes | 12 (4.0) | 71 (11.3) | 3.048 (1.627, 5.712) | |||
| As | No | 287 (95.7) | 605 (96.0) | 0.069 | 0.793 | 1 (reference) |
| Yes | 13 (4.3) | 25 (4.0) | 0.912 (0.460, 1.809) | |||
| Ad | No | 295 (98.3) | 613 (97.3) | 0.937 | 0.333 | 1 (reference) |
| Yes | 5 (1.7) | 17 (2.7) | 1.636 (0.598, 4.478) | |||
| NoVs GI | No | 299 (99.7) | 629 (99.8) | 0.289 | 0.591 | 1 (reference) |
| Yes | 1 (0.3) | 1 (0.2) | 0.475 (0.030, 7.626) | |||
| Co-infection of any type | No | 294 (98.0) | 585 (92.9) | 10.371 | 0.001 | 1 (reference) |
| Yes | 6 (2.0) | 45 (7.1) | 3.769 (1.590, 8.937) | |||
| Co-infection of three pathogens | No | 300 (100.0) | 627 (99.5) | − | 0.555 | 1 (reference) |
| Yes | 0 (0.0) | 3 (0.5) | − | |||
| RVA-NoVs GII-As | No | 300 (100.0) | 629 (99.8) | − | 0.998 | 1 (reference) |
| Yes | 0 (0.0) | 1 (0.2) | − | |||
| RVA-NoVs GII-Ad | No | 300 (100.0) | 628 (99.7) | − | 0.997 | 1 (reference) |
| Yes | 0 (0.0) | 2 (0.3) | − | |||
| Co-infection of two pathogens | No | 296 (98.7) | 600 (95.2) | 6.782 | 0.009 | 1 (reference) |
| Yes | 4 (1.3) | 30 (4.8) | 3.700 (1.293, 10.601) | |||
| RVA-NoVs GII | No | 298 (99.3) | 597 (94.8) | 11.726 | 0.001 | 1 (reference) |
| Yes | 2 (0.7) | 33 (5.2) | 8.236 (1.963, 34.556) | |||
| RVA-As | No | 299 (99.7) | 623 (98.9) | 1.442 | 0.231 | 1 (reference) |
| Yes | 1 (0.3) | 7 (1.1) | 3.361 (0.412, 27.431) | |||
| RVA-Ad | No | 299 (99.7) | 623 (98.9) | 1.442 | 0.231 | 1 (reference) |
| Yes | 1 (0.3) | 7 (1.1) | 3.361 (0.412, 27.431) | |||
| NoVs GII-As | No | 299 (99.7) | 629 (99.8) | 0.289 | 0.591 | 1 (reference) |
| Yes | 1 (0.3) | 1 (0.2) | 0.475 (0.030, 7.626) | |||
| NoVs GII-Ad | No | 300 (100.0) | 628 (99.7) | − | 0.997 | 1 (reference) |
| Yes | 0 (0.0) | 2 (0.3) | − | |||
| As-Ad | No | 299 (99.7) | 629 (99.8) | − | 0.591 | 1 (reference) |
| Yes | 1 (0.3) | 1 (0.2) | 0.475 (0.030, 7.626) | |||
| RVA single | No | 278 (92.7) | 504 (80.0) | 24.367 | <0.001 | 1 (reference) |
| Yes | 22 (7.3) | 126 (20.0) | 3.159 (1.963, 5.085) | |||
| NoVs GII single | No | 291 (97.0) | 592 (94.0) | 3.893 | 0.048 | 1 (reference) |
| Yes | 9 (3.0) | 38 (6.0) | 2.089 (1.004, 4.364) | |||
| As single | No | 289 (96.3) | 612 (97.1) | 0.441 | 0.507 | 1 (reference) |
| Yes | 11 (3.7) | 18 (2.9) | 0.773 (0.360, 1.657) | |||
| Ad single | No | 296 (98.7) | 620 (98.4) | 0.088 | 0.766 | 1 (reference) |
| Yes | 4 (1.3) | 10 (1.6) | 1.194 (0.371, 3.837) | |||
| NoVs GI single | No | 299 (99.7) | 629 (99.8) | 0.289 | 0.591 | 1 (reference) |
| Yes | 1 (0.3) | 1 (0.2) | 0.475 (0.030, 7.626) | |||
In this study, the prevalence of infection with at least one viral pathogen was significantly higher among children with diarrhea compared to those without diarrhea (37.5% vs. 17.0%. χ2=39.875, P<0.001). Specifically, the detection rates of RVA and NoVs GII were substantially elevated in the group of children experiencing diarrhea compared to their counterparts [(χ2=340.994, P<0.001) & (χ2=13.214, P<0.001), respectively]. However, there was no statistically meaningful discrepancy in the detection rates of NoVs GI, As, and Ad between the two groups. Within the population of children with diarrhea, RVA presented the highest rate of infection, followed by NoVs, As, and Ad. Among all the detected viral pathogens, RVA exhibited the highest detection rate, subsequently followed by NoVs GII, As, and Ad.
The co-infection rate was decidedly higher in children with diarrhea than those without (7.1% vs. 2.0%, χ²=10.371, P=0.001). Eight distinct forms of co-infection were identified across the five viral pathogens. Remarkably, only three cases of triple co-infection were found, and all were exclusive to children with diarrhea (0.5%, 3/630). Pairwise co-infections were similarly more prevalent in children with diarrhea than those without (4.8% vs. 1.3%, χ²=6.782, P=0.009). The co-infection of RVA-NoVs GII was the most commonly observed, with a higher prevalence amongst children with diarrhea. Importantly, RVA was a key player in various co-infection combinations. Notably, after excluding co-infection cases, both RNA and NoVs GII were significantly associated with singular infection diarrhea cases[(χ2=24.367, P<0.001) & (χ2=3.893, P=0.048) (Table 1).
The frequency of diarrheal episodes among children varies substantially depending on whether they experience a co-infection or a single infection (H=44.187, P<0.001). Notably, RVA-NoVs GII-As, RVA-NoVs GII-Ad, and RVA-NoVs GII co-infections exhibit the highest frequency of diarrheal episodes, with an average of eight episodes. This is closely followed by the co-infection of RVA-Ad, which has an average of seven episodes. Single infections of RVA, NoVs GII, and the co-infection of RVA-Ad, each average around six episodes. Additional types of single infections and co-infections result in a milder form of the diarrhea illness, with the number of episodes ranging from three to six. This study suggests that the co-infection of RVA-NoVs GII is associated with a higher frequency of diarrheal episodes and a more severe diarrheal illness.
The observed association between RVA-NoVs GII co-infection and diarrhea demonstrated a strength of 8.236 (95% CI: 1.963, 34.556). In cases of RVA solo infection, diarrhea demonstrated a strength of 3.159 (95% CI: 1.963, 5.085), whereas in NoVs GII single infection cases, the strength was 2.089 (95% CI: 1.004, 4.364). Subsequently, the interaction contrast ratio (ICR) was calculated to be 3.996, and the multiplicative interaction value equated to 1.248. These findings suggest a notable additive interaction between RVA-NoVs GII in the progression of diarrhea.
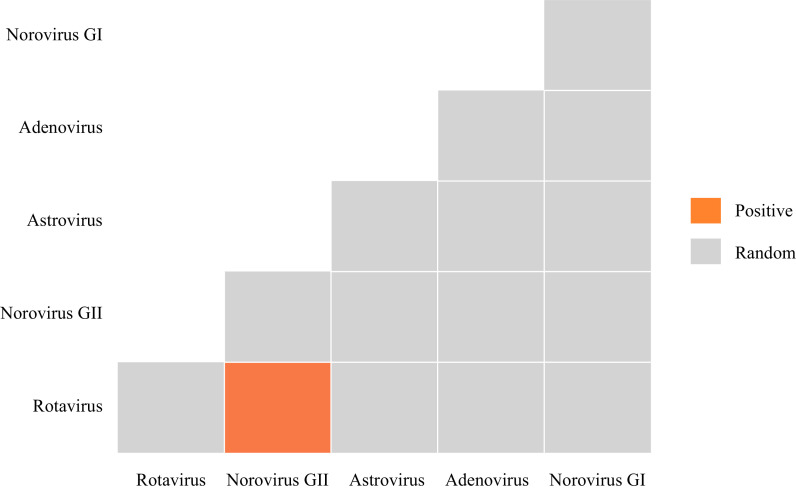
Our co-occurrence examination of two pathogens indicated that 93.3% (14/15) of classifiable species pairs exhibited random associations (Figure 1). No negative co-occurrences were identified. However, positive co-occurrences were noted in only 7.7% (1/15) of acute diarrhea cases in children, specifically with an RVA-NoVs GII co-infection.
Figure 1.
Heatmap showing associations between significant enteric viral pathogen species determined using the probabilistic co-occurrence model for viral pathogens detected in 630 children (<5 years) suffering from acute diarrhea in Guangzhou City, Guangdong Province, China, 2019.
Note: The columns and rows represent pairwise relationship between two enteric viral pathogens. Boxes in grey indicate random co-occurrences, orange boxes indicate associations that were more common than expected by chance.
DISCUSSION
Our study found that RVA is the primary cause of diarrhea in children, irrespective of their immunization status. We also identified an array of other viral etiologies and noted co-infection with various combinations of pathogens, underscoring the widespread nature of co-infection. Nevertheless, the presence of co-infecting pathogens may complicate the assertion of causal relationships and the measurement of associations between a specific pathogen and diarrheal disease. Overlooking these co-infections might result in misleading conclusions. Given the high frequency of enteric pathogen co-infections observed, a comprehensive understanding of diarrheal disease’s pathogenesis necessitates an exhaustive exploration of these pathogens’ biological interactions (6-7).
Additionally, the existence of co-infections could potentially result in an undervaluation of the protective benefits of RVA vaccines. For a comprehensive understanding of these effects, it is imperative to apply concepts rooted in core epidemiology to examine their synergistic interactions (6). It’s widely accepted that the efficacy of RVA vaccines in impoverished settings is subpar when compared to developed nations. High-quality evidence supports the fact that a decrease of 11.3% in the efficiency of RVA vaccines can be attributed to the prevalence of co-infections (6). Thus, it is vital that future efficacy studies for RVA vaccines consider these co-infections with other enteropathogens in their design.
Co-infections involving several viral pathogens are often found, even in outwardly healthy children. These co-infections can lead to unexpected health outcomes. An examination of RVA positive instances showed marked changes in their clinical characteristics when co-infections were factored in. Our study particularly led to the observation of an increased severity in diarrhea cases whenever NoVs GII co-infection was present with RVA. This correlation has been reaffirmed by other studies (4,7). It seems to assist the theory of a synergistic impact between RVA and NoVs GII on diarrheal disease development, suggesting that the pathogenic potential is further magnified in a co-infection scenario. This aligns with previous studies that have reported amplified severity of co-infection cases of diarrhea, for example, RVA-diarrheagenic Escherichia coli or RVA-Giardia lamblia (7). Within RVA-positive diarrhea cases, bacterial co-infections were related to extended periods of diarrhea, while protozoal co-infections were associated with higher hospitalization rates (7). Although it is likely that biological mechanisms underlie these synergistic events, they are not often explored or clarified. It’s possible that the inflammatory response elicited by RVA may disrupt the epithelium and modify the mucosal structure, thereby enhancing the attachment and invasion of other pathogens. It is therefore crucial for future research to focus on understanding the pathogenic mechanisms of diarrhea during co-infection with RVA in order to fully comprehend the mechanisms at play.
This investigation encompasses several limitations. Initially, it failed to account for numerous unidentified pathogens, including parasitic and bacterial variants. Second, the research did not incorporate participants suffering from chronic diarrhea nor those exceeding five years in age. Finally, due to the study’s execution in a solitary city, this restricts its generalizability.
In conclusion, the study represents a rare comprehensive exploration of pathogenicity in both singular and co-infections in children, with a specific focus on the synergistic interaction among viral pathogens. By harnessing cutting-edge technology for viral diarrhea surveillance, it is possible to establish a dynamic monitoring system with robust predictive capabilities and significant timeliness. The development and implementation of more responsive diagnostic techniques, alongside high-quality, exhaustive screening and examination of diarrhea pathogens, represent a pivotal strategy to identify co-infections. This approach enables physicians to rapidly and thoroughly comprehend co-infections and disease progression, thus allowing for timely intervention, stalling disease progression, and preventing fatalities. Ultimately, this course of action contributes to a reduction in the burden of diarrheal diseases.
Conflicts of interest
No conflicts of interest.
Funding Statement
Supported by the Three-year Action Plan for Promoting Clinical Skills and Innovation Ability of Municipal Hospitals (SHDC2022CRS039), Shanghai Natural Science Foundation (22ZR1462100, 23ZR1464000, 23ZR1463900), Medical Innovation Research Special Project of the Shanghai 2021 “Science and Technology Innovation Action Plan” (21Y11922500, 21Y11922400), the Talent Fund of Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine (LH001.007), Natural Science Foundation of Gansu Province (21JR11RA182), Research Ward Construction Project of Shanghai Hospital Development Center (SHDC2022CRW006), and Shanghai Municipal Education Commission Collaborative Innovation Center (A1-U21-205-902)
References
- 1.Wang T, Wang G, Shan CX, Sun YQ, Ren X, Yu LJ, et al Comparative study on epidemiological and etiological characteristics of patients with acute diarrhea with febrile or non-febrile symptoms in China. Infect Dis Poverty. 2023;12(1):62. doi: 10.1186/s40249-023-01108-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li LL, Liu N, Humphries EM, Yu JM, Li S, Lindsay BR, et al Aetiology of diarrhoeal disease and evaluation of viral-bacterial coinfection in children under 5 years old in China: a matched case-control study. Clin Microbiol Infect. 2016;22(4):381.e9–16. doi: 10.1016/j.cmi.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang LP, Zhou SX, Wang X, Lu QB, Shi LS, Ren X, et al Etiological, epidemiological, and clinical features of acute diarrhea in China. Nat Commun. 2021;12(1):2464. doi: 10.1038/s41467-021-22551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang SX, Zhou YM, Xu W, Tian LG, Chen JX, Chen SH, et al Impact of co-infections with enteric pathogens on children suffering from acute diarrhea in southwest China. Infect Dis Poverty. 2016;5(1):64. doi: 10.1186/s40249-016-0157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin M, Wu SY, Kong XY, Xie HP, Fu JG, He YQ, et al Norovirus outbreak surveillance, China, 2016-2018. Emerg Infect Dis. 2020;26(3):437–45. doi: 10.3201/eid2603.191183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Praharaj I, Platts-Mills JA, Taneja S, Antony K, Yuhas K, Flores J, et al Diarrheal etiology and impact of coinfections on rotavirus vaccine efficacy estimates in a clinical trial of a monovalent human-bovine (116E) oral rotavirus vaccine, rotavac, India. Clin Infect Dis. 2019;69(2):243–50. doi: 10.1093/cid/ciy896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhavnani D, Goldstick JE, Cevallos W, Trueba G, Eisenberg JNS Synergistic effects between rotavirus and coinfecting pathogens on diarrheal disease: evidence from a community-based study in northwestern Ecuador. Am J Epidemiol. 2012;176(5):387–95. doi: 10.1093/aje/kws220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannon JL, Barclay L, Collins NR, Wikswo ME, Castro CJ, Magaña LC, et al Genetic and epidemiologic trends of norovirus outbreaks in the United States from 2013 to 2016 demonstrated emergence of novel GII. 4 recombinant viruses. J Clin Microbiol. 2017;55(7):2208–21. doi: 10.1128/JCM.00455-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logan C, O'Leary JJ, O'Sullivan N Real-time reverse transcription-PCR for detection of rotavirus and adenovirus as causative agents of acute viral gastroenteritis in children. J Clin Microbiol. 2006;44(9):3189–95. doi: 10.1128/JCM.00915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett S, Gunson RN The development of a multiplex real-time RT-PCR for the detection of adenovirus, astrovirus, rotavirus and sapovirus from stool samples. J Virol Methods. 2017;242:30–4. doi: 10.1016/j.jviromet.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]