Abstract
Many cancers arise from sites of chronic inflammation, which creates an inflammatory microenvironment surrounding the tumor. Inflammatory substances secreted by cells in the inflammatory environment can induce the proliferation and survival of cancer cells, thereby promoting cancer metastasis and angiogenesis. Therefore, it is important to identify the role of inflammatory factors in cancer progression. This review summarizes the signaling pathways and roles of C-reactive protein (CRP) in various cancer types, including breast, liver, renal, and pancreatic cancer, and the tumor microenvironment. Mounting evidence suggests the role of CRP in breast cancer, particularly in triple-negative breast cancer (TNBC), which is typically associated with a worse prognosis. Increased CRP in the inflammatory environment contributes to enhanced invasiveness and tumor formation in TNBC cells. CRP promotes endothelial cell formation and angiogenesis and contributes to the initiation and progression of atherosclerosis. In pancreatic and kidney cancers, CRP contributes to tumor progression. In liver cancer, CRP regulates inflammatory responses and lipid metabolism. CRP modulates the activity of various signaling molecules in macrophages and monocytes present in the tumor microenvironment, contributing to tumor development, the immune response, and inflammation. In the present review, we overviewed the role of CRP signaling pathways and the association between inflammation and cancer in various types of cancer. Identifying the interactions between CRP signaling pathways and other inflammatory mediators in cancer progression is crucial for understanding the complex relationship between inflammation and cancer.
Keywords: C-reactive protein, Inflammation, Tumor progression
INTRODUCTION
Despite the various strategies for treating cancer, cancer-related mortality remains a major cause of death worldwide (Bray et al., 2018). Localized increases in immune cell infiltration and the systemic inflammatory response to tumors can be important indicators of cancer progression and prognosis (Coussens and Werb, 2002; Hanahan and Weinberg, 2011; Dolan et al., 2017). Inflammation in the tumor microenvironment has been associated with the promotion of tumor growth, invasion, and metastasis, making inflamed cancers more aggressive and prone to metastasis (Hanahan and Weinberg, 2011). Chronic inflammation creates a local microenvironment that can facilitate tumor progression through interactions between tumor cells, immune cells, and stromal cells (Gómez-Valenzuela et al., 2021). The local microenvironment formed by chronic inflammation affects cellular plasticity through complex regulatory cascades involving the tumor and stromal cells (Varga and Greten, 2017). At least 20% of all cancers are initiated by chronic inflammatory conditions, and inflammation induced by the tumor itself is highly associated with most solid malignant tumors (Grivennikov et al., 2010; Siegel et al., 2016).
Cytokines play a role in alerting immune cells to the presence of infections and tissue damage. However, persistent cytokine production at a specific site can stimulate immune cells to secrete more cytokines, leading to a chronic inflammatory state that promotes cancer growth (Greten and Grivennikov, 2019). The inflammatory cytokines associated with carcinogenesis include interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha (TNF-α), which can stimulate or inhibit tumor growth and progression (Dranoff, 2004).
C-reactive protein (CRP), an inflammatory biomarker, is an acute-phase protein primarily synthesized in the liver in response to various inflammatory stimuli (Volanakis, 2001). CRP is composed of five identical subunits forming a planar ring that gives high stability to the protein. It can bind to various endogenous and exogenous ligands exposed on damaged, necrotic, and apoptotic cell membranes. Through this binding, CRP strongly activates the classical complement pathway, which can exacerbate tissue damage and potentially lead to more severe diseases (Pepys and Hirschfield, 2003). CRP levels are increased in response to infections and tissue damage, as well as in various active disease states. Elevated CRP levels have been found to be associated with several cancers, including breast, lung, gastric, and colorectal cancer, hepatocellular carcinoma, and renal carcinoma (Roxburgh and McMillan, 2010; Wu et al., 2011). Serum CRP levels have been associated with tumor size, clinical pathological characteristics, and lymph node metastasis (Lee et al., 2009; Wang and Sun, 2009). CRP is also considered an important biomarker for tumor prognosis and treatment responses (Kishi et al., 2015; Shrotriya et al., 2015; Tang et al., 2015; Frydenberg et al., 2016; Shibutani et al., 2016).
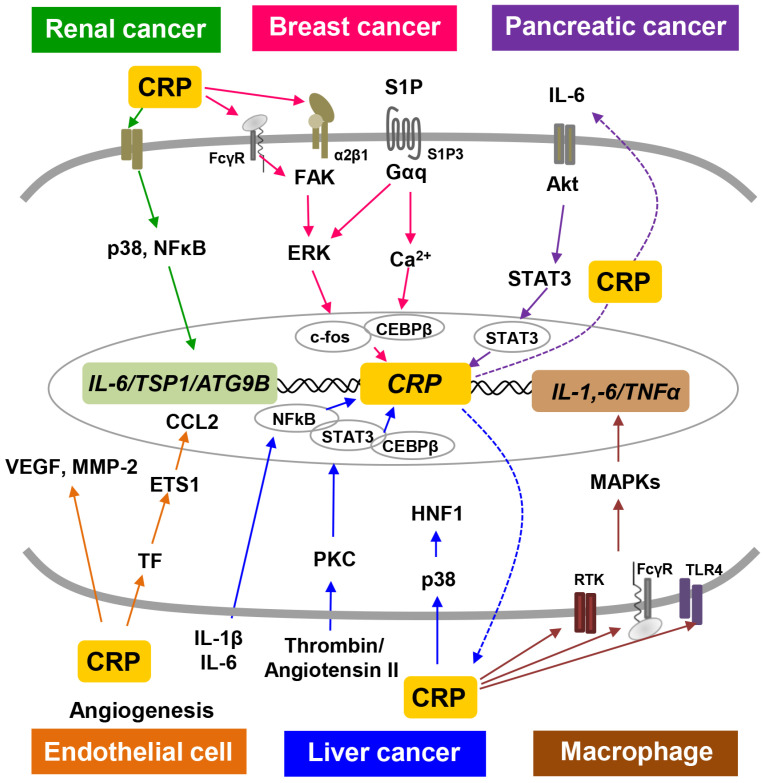
This review summarizes the role of CRP signaling in cancer progression and the interaction between inflammation and cancer in the tumor microenvironment (Fig. 1). Increased CRP levels in the inflammatory and tumor microenvironment contributes to the promotion of various cancers, including breast, liver, renal, and pancreatic cancer, through interactions with various inflammatory molecules. CRP also induces angiogenesis and increases the secretion of inflammatory factors in the tumor microenvironment. In conclusion, this review provides insights into the signaling and role of CRP as an inflammatory marker in tumorigenesis, offering potential therapeutic strategies targeting this pathway.
Fig. 1.
CRP signaling pathways in various cancers and angiogenesis. The six different colored-arrows show the representative CRP signaling pathways occurring in each cancer. Renal cancer: Green, Breast cancer: Pink, Pancreatic cancer: Purple, Endothelial cell: Orange, Liver cancer: Blue, Macrophage: Brown.
BREAST CANCER
Breast cancer is one of the most prevalent malignancies among women (Jemal et al., 2007). Triple-negative breast cancer (TNBC) has been associated with high recurrence rates and poor survival in breast cancer patients (Pierce et al., 2009; Metzger-Filho et al., 2012). Cancer metastasis is a major cause of death in breast cancer patients (Chambers et al., 2002). Many types of cancer originate from the sites of chronic inflammation, which causes an inflammatory microenvironment around the cancer (Balkwill and Mantovani, 2001). Chronic inflammatory conditions promote the development of cancer in various organs, including the breast, stomach, colon, and liver (Ames et al., 1995; Platz and De Marzo, 2004; Hojilla et al., 2008).
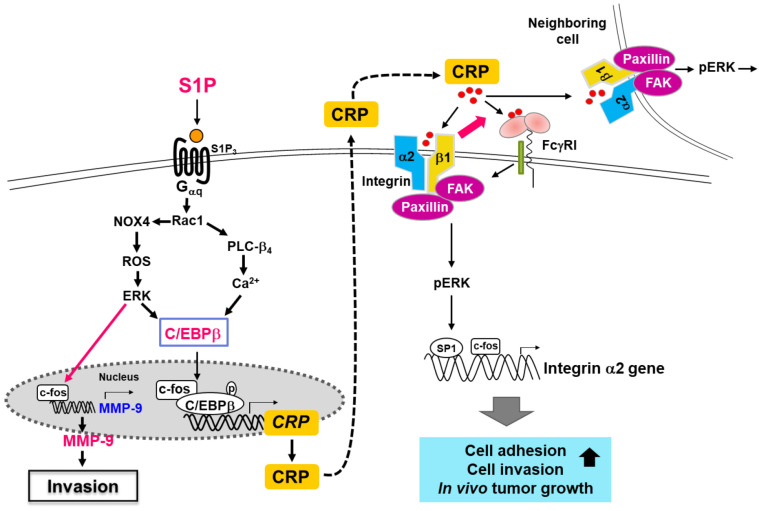
The molecular mechanisms underlying CRP increases in an inflammatory environment have been previously reported in breast epithelial cells (Kim et al., 2014). The treatment of MCF10A breast epithelial cells with the inflammatory lipid sphingosine-1-phosphate increased the expression of CRP, which was subsequently secreted into the extracellular space. The secreted CRP stimulated MCF10A cells again through receptors, increasing the expression of integrin α2. The increased expression of integrin α2 by CRP involved the transcription factors c-fos and specificity protein 1 (SP1). Additionally, the binding of integrin α2 and CRP induced the activation of focal adhesion kinase (FAK), paxillin, and extracellular signal-regulated kinase (ERK), resulting in the upregulation of matrix metalloproteinases (MMP)-9 and the induction of adhesive and invasive phenotypes (Kim et al., 2014, 2018). Thus, the molecular connection between S1P and CRP plays a significant role in increasing the invasive phenotype and promoting tumor formation in TNBC cells (Fig. 2).
Fig. 2.
The CRP signaling pathways involved in breast cancer progression (Modified from Kim et al., 2014, 2018).
CRP is highly expressed and secreted in highly invasive MDA-MB-231 TNBC cells, highlighting the significance of endogenous CRP in MDA-MB-231 TNBC cells. The knockdown of CRP reduced proliferation, invasion, and tumor formation in MDA-MB-231 TNBC cells. In vivo studies using chick chorioallantoic membrane (CAM) analysis and xenograft mouse models suggested that CRP is involved in angiogenesis and tumor growth in TNBC cells (Kim et al., 2018). Elevated serum CRP levels are closely related to breast cancer invasion, metastasis and poor prognosis (Ravishankaran and Karunanithi, 2011). A significant association between high CRP levels and TNBC and luminal B breast cancer in premenopausal women has been reported (Agresti et al., 2016). In contrast, another study found an association only with hormone receptor-positive and HER2-negative breast cancer (Hong et al., 2013). The expression levels of CRP were correlated with vascular endothelial growth factor (VEGF) expression, Ki-67 labeling index, and body mass index (BMI) values. Highly proliferative tumors, including TNBC, showed enhanced neovascularization. In breast cancer, the expression levels of VEGF were reported to be associated with poor recurrence-free survival (RFS) (Toi et al., 1995). Additionally, Ki-67 labeling index values were related to overall survival (OS) in breast cancer (Elston and Ellis, 1991). Increased breast cancer risk is associated with weight gain and obesity in women, especially in postmenopausal patients, and there is a significant correlation between CRP expression levels and BMI values (Dossus et al., 2014).
Obesity is a well-established risk factor for postmenopausal breast cancer (Calle and Kaaks, 2004). Crown-like structures (CLS) are frequently found in the breasts of obese women, and the extent of CLS is correlated with adipocyte size, reflecting the severity of peritumoral inflammation in the breast (Subbaramaiah et al., 2012). The increased secretion of CRP in obese states may be involved in the development and progression of breast cancer, although the molecular mechanisms are only partially understood. Specifically, in the inflamed breast tissue of obese women, levels of the enzyme cyclooxygenase-2 (COX-2), which is involved in inflammation and prostaglandin synthesis, are increased. This directly contributes to the increased activity of aromatase, playing a significant role in breast cancer development and stimulating the proliferation of tumorigenic breast epithelium (Subbaramaiah et al., 2012). The inhibition of COX-2 resulted in reduced CRP levels, indicating a potential connection between inflammation and estrogen production in the context of breast tumor development (Bogaty et al., 2004). This suggests that obesity-related aromatase activity is associated with inflammation in breast tumor formation. Besides its local regulation of estrogen synthesis in breast tissue, CRP can also systemically increase circulating estrogen levels in obese women.
Recently, significant CRP levels were detected in nipple aspirate fluid (NAF) collected from women with benign breast diseases. This suggests that increased levels of CRP in breast secretions may serve as an early and non-invasive biomarker for inflammation and a pre-cancerous breast microenvironment (Lithgow et al., 2006). Cancer NAF from postmenopausal women contains higher levels of CRP compared to premenopausal cancer (Mannello et al., 2010). This suggests a potential role of CRP accumulation in the breast microenvironment during the progression of breast cancer.
PANCREATIC CANCER
Pancreatic neuroendocrine neoplasms (pNENs) are known as the most aggressive neuroendocrine malignancies, and their incidence is increasing annually (Dasari et al., 2017; Daskalakis, 2021). The prognosis of pancreatic cancer has remained largely unchanged over the past 20 years, with an overall 5-year survival rate of only 10% (Siegel et al., 2019; Sun et al., 2020). Despite advancements in diagnostic technologies, the diagnosis of pancreatic cancer is often delayed due to the absence of early symptoms. Preoperative elevations in CRP levels were shown to impact the poor prognosis of pNEN patients (Wiese et al., 2016). CRP is primarily synthesized in the liver and is a protein that is rarely produced in atherosclerotic lesions, kidneys, neurons, or pulmonary mast cells (Dong and Wright, 1996; Yasojima et al., 2001). The synthesis of CRP is mainly increased by IL-6, which is secreted by macrophages and T cells (Weinhold and Ruther, 1997). All inflammatory processes have the potential to activate IL-6, leading to an increase in CRP concentrations in the systemic circulation (Pepys and Hirschfield, 2003).
The IL-6 stimulation of pancreatic neuroendocrine tumor cells (BON1 and QGP1) leads to the upregulation and secretion of CRP. IL-6 was also secreted by CRP-stimulated BON1 cells, and both CRP and IL-6 increased the invasion of BON1 and QGP1 cells (Schimmack et al., 2019). ERK, AKT, and/or signal transducer and activator of transcription 3 (STAT) signaling pathways are important effector pathways strongly associated with the intrinsic malignant characteristics of cancer cells. The phosphorylation of ERK/AKT/STAT3, along with increased CRP and IL-6 expression, was observed in tissues from pNEN patients with increased CRP serum levels (Schimmack et al, 2019). In summary, CRP was internalized through receptor binding or endocytosis in BON1 and QGP1 cells, triggering IL-6 production and activating the IL-6/AKT/STAT3-CRP axis. The cross-stimulation between CRP and IL-6 in these cells may represent a positive feedback mechanism that promotes tumor progression (Schimmack et al, 2019). STAT3 is a protein that plays a pivotal role in the response to inflammatory mediators. It is involved in regulating cell growth, survival, and differentiation and acts as a significant regulator of the expression of the IL-6 gene (Levy and Darnell, 2002; Yu et al., 2009). Both CRP and STAT3 are potential components of the connection between inflammation and cancer (Mantovani et al., 2008). Elevated CRP and tumor markers levels were both associated with poor prognostic indicators in patients with resected pancreatic ductal adenocarcinoma.
LIVER CANCER
Liver cancer is one of the most common cancers worldwide and the third cause of cancer-related mortality (Sung et al., 2021). Chronic hepatitis B and hepatitis C infections are the main causes of liver cancer. Chronic inflammation is considered a key mediator of liver cancer, leading to fibrosis, cirrhosis, and eventually, liver cancer (Cervello and Montalto, 2006; Nikolaou et al., 2013).
CRP is produced by the liver. Thus, it can potentially be influenced by chronic liver diseases. In recent studies, elevated levels of CRP were associated with the poor prognosis of patients with liver cancer (Hashimoto et al., 2005; Sieghart et al., 2013). High CRP levels were observed in patients with liver failure (Park et al., 2005) and were associated with poor outcomes in patients with liver cirrhosis (Blot et al., 1993). Studies in hepatocellular carcinoma cell lines found that CRP synthesis was mainly stimulated by cytokines, particularly IL-1β and IL-6 (Majello et al., 1990; Toniatti et al., 1990; Kleemann et al., 2003). Transcription factors involved in IL-6-mediated CRP synthesis include STAT3 and CCAAT/enhancer-binding protein (C/EBP) family, especially C/EBPα, β, and δ. NF-κB subunits p50 and p65 are also involved in cytokine-induced CRP synthesis. Thrombin, IL-8, and angiotensin II could be potential candidates to mediate CRP induction within the body via protein kinase C (PKC). Their receptors are present on hepatocytes, and they are produced during sepsis and inflammatory conditions concurrently with elevated CRP plasma levels (Maekawa and Tollefsen, 1996; Wigmore et al., 1997). The activation of PKC is known to result in the phosphorylation of Ser 105 within the activation domain of C/EBPβ, enhancing its transcriptional activity (Ghosh and Karin, 2002; Su et al., 2002; Moscat et al., 2003). Therefore, these findings demonstrate the relevance of the PKC pathway in CRP gene expression in liver cancer.
Ruxolitinib, a Janus kinase (JAK) inhibitor, was found to completely inhibit the secretion and mRNA expression of CRP induced by lipopolysaccharide (LPS) in primary human hepatocytes and human hepatoma HepaRG cells. Similarly, it suppressed the upregulation of CRP induced by various Toll-like receptor agonists, as well as pro-inflammatory cytokines IL-1β, IL-6, and TNFα, and neutralized the LPS-mediated induction of serum amyloid A, fibrinogen, haptoglobin, and serpin. Ruxolitinib also blocked the activation of the IL-6/JAK/STAT pathway induced by LPS (Febvre-James et al., 2020). These results indicate that ruxolitinib could target the IL-6/JAK/STAT signaling cascade in inflammatory human liver cells, thereby inhibiting CRP induction and highlighting the potential of ruxolitinib as a promising therapeutic agent for modulating inflammatory responses in liver cells and associated conditions.
Coronary artery disease (CAD) is a substantial global health concern and a major contributor to atherosclerosis (Hansson, 2005). Proprotein convertase subtilisin kexin 9 (PCSK9), a significant regulator of low-density lipoprotein (LDL) metabolism, plays a crucial role in lipid and cardiovascular health, primarily expressed in liver cells (Abifadel et al., 2003). Elevated plasma CRP concentrations have been positively correlated with an increased risk of cardiovascular diseases, including dyslipidemia. According to research conducted by Cui et al. (2016), CRP could activates the p38MAPK pathway, leading to increased expression of nuclear HNF1α in HepG2 cells, thereby contributing to the upregulation of PCSK9. A previous study reported that PCSK9, as a secreted factor, played a crucial role in promoting the degradation of LDL receptors (LDLRs), reducing the membrane recycling of LDLR, and consequently impeding the uptake of LDL particles. These results demonstrate the association between CRP, PCSK9, and LDL uptake in HepG2 cells.
RENAL CANCER
Renal cell carcinoma (RCC) is the most common solid lesion within the kidneys (Ozturk, 2015), comprising 2-3% of adult malignancies (Flum et al., 2016) and accounting for 85% of primary renal tumors (Bokhari and Tiscornia-Wasserman, 2017). It commonly metastasizes to the lungs, liver, bones, brain, adrenal glands, and lymph nodes, but rarely spreads to the skin, thyroid, or pancreas (Wolf et al., 2015). Clear cell renal cell carcinoma (CCRCC), a subtype of RCC, makes up approximately 75% of all RCC cases (Feng et al., 2016). Recent studies found that CRP expression was significantly associated with OS time in RCC patients (Fujita et al., 2016; Dalpiaz et al., 2017; Guo et al., 2017).
Autophagy related 9B (ATG9B) is involved in the autophagic process of delivering cellular components degraded by autophagosomes to lysosomes for digestion (Zavodszky et al., 2013), a common occurrence in the carcinogenesis process. The expression of CRP in CCRCC cells was reported to be positively associated with ATG9B expression. The inhibition of CRP expression resulted in a decrease in ATG9B expression, while overexpression led to an increase in ATG9B expression. Serum CRP protein levels were significantly higher in CCRCC patients compared to normal patients. In summary, the expression levels of CRP and ATG9B in CCRCC showed a positive correlation, indicating poor CCRCC status (Ma et al., 2017). These findings suggest that the abnormal overexpression of CRP and ATG9B may play a role in promoting CCRCC development.
CRP is significantly increased in patients with diabetic nephropathy. A study by Wang et al. (2012) suggested that CRP directly exerted pro-inflammatory effects on human renal tubular epithelial cells (HK-2 cells). CRP induced the release and mRNA expression of IL-6 and TSP-1 proteins from HK-2 cells by activating the p38MAPK and NF-κB signaling pathways. It also increased the expression of TGF-β1, indicating the significant role of CRP in propagating and prolonging inflammation in renal fibrosis (Baer et al., 2006).
ANGIOGENESIS
Tumor cells can infiltrate blood or lymphatic vessels, circulate within the vasculature, and then metastasize to other sites where they can proliferate (Folkman, 1971). Growth of the vascular network is crucial for the dissemination of cancer cells in metastatic spread within cancer tissues. CRP is known as a potent activator of angiogenesis and is associated with the formation of immature microvessels in the body (Slevin et al., 2010). CRP dissociated into monomeric CRP (mCRP) at the endothelial cell membrane, and mCRP increased tissue factor (TF) expression and induced angiogenic effects by activating the F3-TF-ETS1-CCL2 axis (Peña et al., 2016). Additionally, mCRP upregulated the endothelial expression of CD32 and CD64 (Devaraj et al., 2005), promoting migration, wound repair, and tubular formation. mCRP has also been demonstrated to promote angiogenesis by increasing vascular proliferation, migration, and tube-like structure formation in vitro, as well as stimulating blood vessel formation in vivo, according to the chorioallantoic membrane assay. mCRP induced the expression of several key molecules involved in angiogenesis, including VEGFR2/KDR, platelet-derived growth factor (PDGF-BB), inhibitor of DNA binding/differentiation-1 (ID-1), and notch family transcription factors (Notch1 and Notch3). Additionally, mCRP promoted the stabilization and maturation of cysteine-rich angiogenic inducer 61 (CYR61/CCN1), a molecule rich in cysteine that induces angiogenesis (Turu et al., 2008). These actions play a central role in the main stages of blood vessel formation and remodeling. mCRP induced the expression of Notch3 and N-cadherin while down-regulating VE-cadherin. mCRP and Notch3 acted cooperatively in endothelial cells, increasing endothelial cell proliferation, migration, and tube formation, thereby playing a role in vascular development, remodeling, and maturation. Additionally, mCRP stabilized vascular structures by regulating of VE-cadherin and N-cadherin expression (Boras et al., 2014).
CRP was demonstrated to upregulate the expression of VEGF-A in various cell types, including bovine aortic endothelial cells, human coronary artery endothelial cells, and monocytes (Bello et al., 2008; Turu et al., 2008). CRP was shown to activate the expression of hypoxia-inducible factor (HIF)-1α and MMP-2, as well as upregulate the expression of VEGF in adipose-derived stem cells. These effects lead to increased endothelial tube formation and vascular proliferation (Chen et al., 2016). CRP was found to promote angiogenesis by specifically enhancing the formation of newly developed microvessels around atherosclerotic plaques and in the ischemic penumbra following acute ischemic stroke (Krupinski et al., 2006; Slevin et al., 2010). CRP was also shown to induce inflammation and increase the permeability of abnormally developing microvessels after tissue damage (Slevin et al., 2015). These effects could contribute to an increased risk of dementia.
TUMOR MICROENVIRONMENT: MONOCYTES AND MACROPHAGES
Monocytes and macrophages, types of white blood cells, are both integral components of the immune system (Akiyama et al., 1988). Monocytes primarily function as circulating immune cells involved in immune surveillance and inflammatory responses. However, upon migration to tissues and differentiation into macrophages, their functions become more specialized. Macrophages possess a diverse range of functions critical to immune defense and tissue homeostasis. These functions include phagocytosis, which involves the engulfment and clearance of pathogens and cellular debris, as well as antigen presentation to activate immune responses. Macrophages also secrete cytokines, participate in tissue remodeling, and modulate immune responses (Schenten and Medzhitov, 2011). Importantly, the roles of monocytes and macrophages extend to cancer development and progression, with their functions within the tumor microenvironment exhibiting both pro-tumor and anti-tumor effects. Understanding the dynamic interplay between monocytes, macrophages, and the tumor microenvironment is crucial for comprehending the complexities of cancer biology and devising effective therapeutic strategies.
CRP can affect various signaling molecules in macrophages and monocytes, leading to diverse influences on their behavior and function. TLRs recognize pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs), thereby inducing innate immune responses (Tang et al., 2012). CRP was found to interact with TLR4, resulting in the activation of downstream signaling pathways (McCarthy et al., 2014). This interaction between CRP and TLR4 can lead to the promotion of pro-inflammatory cytokine production, including TNF-α and IL-6, in macrophages and monocytes. CRP can interact with cell surface receptors on macrophages and monocytes, such as Fc gamma receptors (FcγRs), complement receptors, including C1q, and TLRs. The binding of CRP to these receptors initiates intracellular signaling cascades, including mitogen-activated protein kinase (MAPK) activation. CRP can activate receptor tyrosine kinases (RTKs), such as the epidermal growth factor receptor (EGFR), or directly activate protein kinases involved in MAPK signaling. According to research conducted by Den Dunnen and colleagues, CRP has the ability to bind to phosphatidylcholine (PC) found on damaged or dying cell membranes (Newling et al., 2019). This interaction between CRP and PC, in conjunction with TLR signaling, promotes the production of cytokines, specifically TNF, IL-1β, and IL-23. Furthermore, the PC:CRP complex contributes to glycolytic reprogramming and fatty acid synthesis induced by the FcγRI and FcγRIIa signaling pathways, involving FcγRI/FcγRIIa-Syk-PI3K-AKT2 (Vergadi et al., 2017; Hansen et al., 2018; Serbulea et al., 2018).
NF-κB signaling pathway is a major pathway regulating immune and inflammatory responses. The activation of this pathway in monocytes and macrophages by CRP can lead to the production of pro-inflammatory cytokines, chemokines, and other inflammatory mediators. Pro-inflammatory cytokines, such as IL-1, IL-6 and TNF-α, can promote tumor growth, angiogenesis, tumor cell survival, proliferation, and resistance to apoptosis (Kumar et al., 2009; Takeuchi and Akira, 2010; Gong et al., 2020). As other inflammatory mediators, such as prostaglandins and MMPs, are increased due to CRP-induced NF-κB activation, leading to angiogenesis, tumor immune evasion, tissue remodeling, tumor invasion, and metastasis (Terada et al., 1990; Overall and Lopez-Otin, 2002).
The phosphatidylinositol 3-kinase (PI3K)/AKT pathway is a crucial intracellular signaling pathway involved in various cellular processes, such as cell survival, proliferation, and inflammation (Li et al., 2014; He et al., 2021). Upon activation by CRP, PI3K phosphorylates the lipid phosphatidylinositol 4,5-bisphosphate (PIP2) to generate phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3 acts as a second messenger and recruits AKT (also known as protein kinase B) to the cell membrane. The recruitment of AKT to the cell membrane enables its phosphorylation at two critical sites: threonine 308 (Thr308) by phosphoinositide-dependent kinase 1 (PDK1) and serine 473 (Ser473) by mammalian target of rapamycin complex 2 (Engelman et al., 2006; Manning and Cantley, 2007). Once activated, AKT phosphorylates and regulates numerous downstream targets involved in cell survival, proliferation, and inflammation (Manning and Cantley, 2007). The PI3K/AKT pathway’s dynamic regulation and its impact on various cellular processes make it an essential signaling pathway with potential implications in disease and therapeutic development. CRP promotes cell survival by activating the PI3K/AKT pathway and regulates inflammatory responses in macrophages and monocytes (Newling et al., 2019). This activation of the PI3K/AKT pathway plays a role in various physiological and pathological processes, including immune responses, tissue remodeling, and inflammation. It is essential to acknowledge that the effects of CRP on these signaling molecules can vary depending on the cellular context, CRP concentration, and other influencing factors. Furthermore, CRP can exert direct effects on these signaling pathways as well as indirect effects through its interactions with other molecules and cells in the microenvironment.
ATHEROSCLEROSIS
Atherosclerosis accounts for approximately 50% of deaths related to stroke and cardiovascular diseases (Tabas et al., 2015; Liu et al., 2016). Its incidence continues to rise as strong risk factors such as hypertension, obesity, type 2 diabetes, alcohol consumption, and smoking, increase (Verma et al., 2012; Martín-Timón et al., 2014). Vascular inflammation plays an important role in all stages of atherosclerosis, from initiation and the progression of atherosclerotic lesions to plaque rupture (Libby, 2002). CRP contributes to the initiation and progression of atherosclerosis by promoting endothelial cell activation and the recruitment of macrophages (Bisoendial et al., 2010; Grad and Danenberg, 2013). CRP primarily binds to cell membrane IgG FcγRs, leading to the increased expression of macrophage chemoattractant protein 1 (MCP-1) and vascular cell adhesion molecule 1 (VCAM-1), which contribute to the development of atherosclerosis (Sundgren et al., 2011; Raaz-Schrauder et al., 2014). CRP also upregulates the production of reactive oxygen species (ROS) in endothelial cells, platelets, monocytes, and vascular smooth muscle cells through specific FcγRs (Singh et al., 2005; Zhang et al., 2012). In human primary venous endothelial cells, the signaling mechanism of atherosclerosis induction by CRP involves the upregulation of the TF pathway by activating NF-κB and ERK1/2 (Chen et al., 2009). CRP activated the NF-kB pathway in Human umbilical vein cells (HUVECs) and human aortic endothelial cells (HAECs), induced VCAM-1 expression through CD32, and promoted endothelial cell-monocyte interaction, thereby contributing to the development of atherosclerosis (Liang et al., 2006). CRP induced the expression of inflammatory genes for nitric oxide (NO) formation, MCP-1, IL-6, and inducible nitric oxide synthase (iNOS) in vascular smooth muscle cells (Hattori et al., 2003). Additionally, CRP increased the activation of transcription factors NF-κB and AP-1, which are associated with intracellular redox reactions, and enhanced the activity of MAP kinases through the TLR4-dependent signaling pathway, inducing TNF-α secretion via the p38 MAPK-TLR4 pathway (Chang et al., 2012). Thus, the activation of these signals represents a significant risk factor for atherosclerosis and cardiovascular diseases.
The NLR family pyrin domain-containing (NLRP) 3 inflammasome is a multiprotein cytosolic complex that activates the IL-1 family and plays an important role in atherosclerosis. A meta-analysis of genome-wide association studies identified NLRP3 as a predictor of CRP levels (Dehghan et al., 2011). CRP increased the expression of pro-IL-1β and NLRP3 through the FcγRs/NF-κB pathway in endothelial cells. It also promoted NLRP3 inflammasome activation and IL-1β maturation by upregulating ROS levels, purinergic receptor signaling, and the activation of cysteine proteases (Bian et al., 2019; Prakash et al., 2023). These findings indicate an association between elevated levels of CRP and NLRP3 inflammasomes in atherosclerosis. Therefore, inhibiting CRP can represent a new approach to the prevention and treatment of cardiovascular diseases.
Multiple experiments and clinical studies have consistently demonstrated the significant role of TF in the pathogenesis of acute coronary syndrome. TF is known to induce the formation of intracoronary thrombus following endothelial injury (Wilcox et al., 1989; Pawashe et al., 1994; Annex et al., 1995). Interestingly, cells that are not typically exposed to flowing blood, such as smooth muscle cells, express TF constitutively on their surface (Schecter et al., 1997; van den Eijnden et al., 1997). In contrast, cells that are in direct contact with the bloodstream, like endothelial cells, express TF only on their membrane. CRP was reported to induce the expression of TF in both endothelial and smooth muscle cells, which are widely present in the arterial wall. Moreover, the effect of CRP on TF expression was dose-dependent. CRP-induced TF expression in endothelial cell appears to occur through a direct effect of CRP, while in human monocytes, it requires cell-cell interactions with leukocytes (Paffen et al., 2004). The induction of TF expression by CRP is mediated through the activation of the NF-κB transcription factor via the ERK 1/2 pathway. In fact, the selective NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) significantly reduced CRP-induced TF expression. Interestingly, low concentrations of CRP could activate NF-κB in endothelial cells, whereas, in vascular smooth muscle cells (SMCs), this phenomenon occurred only at higher CRP concentrations. These different patterns of NF-κB activation in the two cell populations may explain their differential sensitivity to CRP-induced TF expression (Cirillo et al., 2005). The finding that CRP induces TF expression by activating of NF-κB can provide a partial explanation for the observation that patients with acute coronary syndrome and elevated CRP serum levels tend to experience worse clinical outcomes compared to those with normal CRP levels.
CONCLUSIONS
A chronic inflammatory state has been suggested to create an inflammatory microenvironment that predisposes individuals to cancer (Asegaonkar et al., 2015). Many studies suggested that CRP not only plays a role as an indicator of inflammation but also possesses significant pro-inflammatory characteristics (Pasceri et al., 2000, 2001). Elevated CRP levels have been associated with poor outcomes, abnormal metabolism, hypoalbuminemia, anemia, a wide range of diseases, and increased serum IL-6 levels (Falconer et al., 1995; Nakachi et al., 2007; Pine et al., 2009; Argilés et al., 2011).
This review summarizes the role and signaling pathways of CRP in various cancer and tumor microenvironment (Fig. 1). (1) The molecular link between the inflammatory lipid S1P and CRP is crucial in the increased invasive phenotype and tumor formation of TNBC cells (Kim et al., 2018). Elevated serum CRP levels are closely associated with breast cancer invasion, metastasis, and poor prognosis. (2) CRP induces IL-6 production in pancreatic tumors and activates the IL-6/AKT/STAT3 signaling axis, thereby promoting tumor progression. (3) CRP promotes the development of renal cell carcinoma by increasing the autophagy-related molecule ATG9B. (4) CRP is produced in the liver by IL-1β and IL-6, and regulates inflammatory responses and LDL metabolism. (5) CRP induces the F3-TF-ETS1-CCL2 axis and VEGF expression, leading to increased endothelial cell tube formation and angiogenesis. Additionally, CRP interacts with various cells within the tumor microenvironment, contributing to the initiation and progression of atherosclerosis. (6) CRP contributes to immunity, inflammatory responses, and tumor development by regulating the activity of various signaling molecules in macrophages and monocytes. Comprehending the interaction between CRP signaling pathways and other inflammatory mediators is crucial for unraveling the complex relationship between inflammation and cancer. This review can offer valuable insight into the role of CRP in various pathological conditions and potential therapeutic strategies targeting this pathway.
Funding Statement
ACKNOWLEDGMENTS The present study was supported by the National Research Foundation of Korea (no. 2016R1A6A1A03007648, no. 2022R1A2C1093335, and no. 2022R1F1A1076029).
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- Abifadel M., Varret M., Rabes J. P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., Derré A., Villéger L., Farnier M., Beucler I., Bruckert E., Chambaz J., Chanu B., Lecerf J., Luc G., Moulin P., Weissenbach J., Prat A., Krempf M., Junien C., Seidah N. G., Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- Agresti R., Meneghini E., Baili P., Minicozzi P., Turco A., Cavallo I., Funaro F., Amash H., Berrino F., Tagliabue E., Sant M. Association of adiposity, dysmetabolisms, and inflammation with aggressive breast cancer subtypes: a cross-sectional study. Breast Cancer Res. Treat. 2016;157:179–189. doi: 10.1007/s10549-016-3802-3. [DOI] [PubMed] [Google Scholar]
- Akiyama Y., Griffith R., Miller P., Stevenson G. W., Lund S., Kanapa D. J., Stevenson H. C. Effects of adherence, activation and distinct serum proteins on the in vitro human monocyte maturation process. J. Leukoc. Biol. 1988;43:224–231. doi: 10.1002/jlb.43.3.224. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Gold L. S., Willett W. C. The causes and prevention of cancer. Proc. Natl. Acad. Sci. U. S. A. 1995;92:5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annex B. H., Denning S. M., Channon K. M., Sketch M. H., Jr., Stack R. S., Morrissey J. H., Peters K. G. Differential expression of tissue factor protein in directional atherectomy specimens from patients with stable and unstable coronary syndromes. Circulation. 1995;91:619–622. doi: 10.1161/01.CIR.91.3.619. [DOI] [PubMed] [Google Scholar]
- Argilés J. M., López-Soriano F. J., Toledo M., Betancourt A., Serpe R., Busquets S. The cachexia score (CASCO): a new tool for staging cachectic cancer patients. J. Cachexia Sarcopenia Muscle. 2011;2:87–93. doi: 10.1007/s13539-011-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asegaonkar S. B., Asegaonkar B. N., Takalkar U. V., Advani S., Thorat A. P. C-reactive protein and breast cancer: new insights from old molecule. Int. J. Breast Cancer. 2015;2015:145647. doi: 10.1155/2015/145647.9a21a5aeb5c5407e917494abe226ca51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer P. C., Gauer S., Wegner B., Schubert R., Geiger H. C-reactive protein induced activation of MAP-K and RANTES in human renal distal tubular epithelial cells in vitro. Clin. Nephrol. 2006;66:177–183. doi: 10.5414/CNP66177. [DOI] [PubMed] [Google Scholar]
- Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Bello G., Cailotto F., Hanriot D., Kolopp-Sarda M. N., Latger-Cannard V., Hess K., Zannad F., Longrois D., Ropars A. C-reactive protein (CRP) increases VEGF-A expression in monocytic cells via a PI3-kinase and ERK1/2 signaling dependent pathway. Atherosclerosis. 2008;200:286–293. doi: 10.1016/j.atherosclerosis.2007.12.046. [DOI] [PubMed] [Google Scholar]
- Bian F., Yang X. Y., Xu G., Zheng T., Jin S. CRP-induced NLRP3 inflammasome activation increases LDL transcytosis across endothelial cells. Front. Pharmacol. 2019;10:40. doi: 10.3389/fphar.2019.00040.4ec4f8727f88491481f18ebf385ef3c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisoendial R. J., Boekholdt S. M., Vergeer M., Stroes E. S., Kastelein J. J. C-reactive protein is a mediator of cardiovascular disease. Eur. Heart J. 2010;31:2087–2091. doi: 10.1093/eurheartj/ehq238. [DOI] [PubMed] [Google Scholar]
- Blot W. J., Li J. Y., Taylor P. R., Guo W., Dawsey S., Wang G. Q., Yang C. S., Zheng S., Gail M., Li G., Yu Y., Liu B., Tangrea J., Sun Y., Liu F., Fraumeni J. F., Zhang Y., Jr., Li B. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Natl. Cancer Inst. 1993;85:1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- Bogaty P., Brophy J. M., Noel M., Boyer L., Simard S., Bertrand F., Dagenais G. R. Impact of prolonged cyclooxygenase-2 inhibition on inflammatory markers and endothelial function in patients with ischemic heart disease and raised C-reactive protein: a randomized placebo-controlled study. Circulation. 2004;110:934–939. doi: 10.1161/01.CIR.0000139338.12464.5F. [DOI] [PubMed] [Google Scholar]
- Bokhari A., Tiscornia-Wasserman P. G. Cytology diagnosis of metastatic clear cell renal cell carcinoma, synchronous to pancreas, and metachronous to thyroid and contralateral adrenal: report of a case and literature review. Diagn. Cytopathol. 2017;45:161–167. doi: 10.1002/dc.23619. [DOI] [PubMed] [Google Scholar]
- Boras E., Slevin M., Alexander M. Y., Aljohi A., Gilmore W., Ashworth J., Krupinski J., Potempa L. A., Abdulkareem, Elobeid A., Matou-Nasri S. Monomeric C-reactive protein and Notch-3 co-operatively increase angiogenesis through PI3K signalling pathway. Cytokine. 2014;69:165–179. doi: 10.1016/j.cyto.2014.05.027. [DOI] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Calle E. E., Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- Cervello M., Montalto G. Cyclooxygenases in hepatocellular carcinoma. World J. Gastroenterol. 2006;12:5113–5121. doi: 10.3748/wjg.v12.i32.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers A. F., Groom A. C., MacDonald I. C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- Chang M. K., Hartvigsen K., Ryu J., Kim Y., Han K. H. The pro-atherogenic effects of macrophages are reduced upon formation of a complex between C-reactive protein and lysophosphatidylcholine. J. Inflamm. (Lond.) 2012;9:42. doi: 10.1186/1476-9255-9-42.c8ff0b041c7a499589ace09118779a70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Gu Z., Wu M. C-reactive protein can upregulate VEGF expression to promote ADSC-induced angiogenesis by activating HIF-1α via CD64/PI3k/Akt and MAPK/ERK signaling pathways. Stem Cell Res. Ther. 2016;7:114. doi: 10.1186/s13287-016-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wang J., Yao Y., Yuan W., Kong M., Lin Y., Geng D., Nie R. U. Crp regulates the expression and activity of tissue factor as well as tissue factor pathway inhibitor via NF-kappaB and ERK 1/2 MAPK pathway. FEBS Lett. 2009;583:2811–2818. doi: 10.1016/j.febslet.2009.07.037. [DOI] [PubMed] [Google Scholar]
- Cirillo P., Golino P., Calabro P., Cali G., Ragni M., De Rosa S., Cimmino G., Pacileo M., De Palma R., Forte L., Gargiulo A., Corigliano F. G., Angri V., Spagnuolo R., Nitsch L., Chiariello M. C-reactive protein induces tissue factor expression and promotes smooth muscle and endothelial cell proliferation. Cardiovasc. Res. 2005;68:47–55. doi: 10.1016/j.cardiores.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Coussens L. M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C. J., Li S., Zhu C. G., Sun J., Du Y., Zhang Y., Wu N., Guo Y., Xu R., Gao Y., Li J. Enhanced pro-protein convertase subtilisin/kexin type 9 expression by C-reactive protein through p38MAPK-HNF1α pathway in HepG2 cells. J. Cell. Mol. Med. 2016;20:2374–2383. doi: 10.1111/jcmm.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpiaz O., Luef T., Seles M., Stotz M., Stojakovic T., Pummer K., Zigeuner R., Hutterer G. C., Pichler M. Critical evaluation of the potential prognostic value of the pretreatment-derived neutrophil-lymphocyte ratio under consideration of C-reactive protein levels in clear cell renal cell carcinoma. Br. J. Cancer. 2017;116:85–90. doi: 10.1038/bjc.2016.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari A., Shen C., Halperin D., Zhao B., Zhou S., Xu Y., Shih T., Yao J. C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis K. Functioning and nonfunctioning pNENs. Curr. Opin. Endocr. Metab. Res. 2021;18:284–290. doi: 10.1016/j.coemr.2021.04.007. [DOI] [Google Scholar]
- Dehghan A., Dupuis J., Barbalic M., Bis J. C., Eiriksdottir G., Lu C., Pellikka N., Wallaschofski H., Kettunen J., Henneman P., Baumert J., Strachan D. P., Fuchsberger C., Vitart V., Wilson J. F., Paré G., Naitza S., Rudock M. E., Surakka I., de Geus E. J., Alizadeh B. Z., Guralnik J., Shuldiner A., Tanaka T., Zee R. Y., Schnabel R. B., Nambi V., Kavousi M., Ripatti S., Nauck M., Smith N. L., Smith A. V., Sundvall J., Scheet P., Liu Y., Ruokonen A., Rose L. M., Larson M. G., Hoogeveen R. C., Freimer N. B., Teumer A., Tracy R. P., Launer L. J., Buring J. E., Yamamoto J. F., Folsom A. R., Sijbrands E. J., Pankow J., Elliott P., Keaney J. F., Sun W., Sarin A. P., Fontes J. D., Badola S., Astor B. C., Hofman A., Pouta A., Werdan K., Greiser K. H., Kuss O., Meyer, zu Schwabedissen H. E., Thiery J., Jamshidi Y., Nolte I. M., Soranzo N., Spector T. D., Völzke H., Parker A. N., Aspelund T., Bates D., Young L., Tsui K., Siscovick D. S., Guo X., Rotter J. I., Uda, Schlessinger D., Rudan I., Hicks A. A., Penninx B. W., Thorand B., Gieger C., Coresh J., Willemsen G., Harris T. B., Uitterlinden A. G., Järvelin M. R., Rice K., Radke D., Salomaa V., Willems, van Dijk K., Boerwinkle E., Vasan R. S., Ferrucci L., Gibson Q. D., Bandinelli S., Snieder H., Boomsma D. I., Xiao X., Campbell H., Hayward C., Pramstaller P. P., van Duijn C. M., Peltonen L., Psaty B. M., Gudnason V., Ridker P. M., Homuth G., Koenig W., Ballantyne C. M., Witteman J. C. M., Benjamin E. J., Perola M., Chasman D. I. Meta-analysis of genome-wide association studies in > 80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2011;123:731–738. doi: 10.1161/CIRCULATIONAHA.110.948570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S., Du Clos T. W., Jialal I. Binding and internalization of C-reactive protein by Fcgamma receptors on human aortic endothelial cells mediates biological effects. Arterioscler. Thromb. Vasc. Biol. 2005;25:1359–1363. doi: 10.1161/01.ATV.0000168573.10844.ae. [DOI] [PubMed] [Google Scholar]
- Dolan R. D., Lim J., McSorley S. T., Horgan P. G., McMillan D. C. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta-analysis. Sci. Rep. 2017;7:16717. doi: 10.1038/s41598-017-16955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q., Wright J. R. Expression of C-reactive protein by alveolar macrophages. J. Immunol. 1996;156:4815–4820. doi: 10.4049/jimmunol.156.12.4815. [DOI] [PubMed] [Google Scholar]
- Dossus L., Jimenez-Corona A., Romieu I., Boutron-Ruault M. C., Boutten A., Dupré T., Fagherazzi G., Clavel-Chapelon F., Mesrine S. C-reactive protein and postmenopausal breast cancer risk: results from the E3N cohort study. Cancer Causes Control. 2014;25:533–539. doi: 10.1007/s10552-014-0355-9. [DOI] [PubMed] [Google Scholar]
- Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- Elston C. W., Ellis I. O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Engelman J. A., Luo J., Cantley L. C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Falconer J. S., Fearon K. C., Ross J. A., Elton R., Wigmore S. J., Garden O. J., Carter D. C. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer. 1995;75:2077–2082. doi: 10.1002/1097-0142(19950415)75:8<2077::AID-CNCR2820750808>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Febvre-James M., Lecureur V., Fardel O. Potent repression of C-reactive protein (CRP) expression by the JAK1/2 inhibitor ruxolitinib in inflammatory human hepatocytes. Inflamm. Res. 2020;69:51–62. doi: 10.1007/s00011-019-01293-1. [DOI] [PubMed] [Google Scholar]
- Feng C., Xiong Z., Jiang H., Ding Q., Fang Z., Hui W. Genetic alteration in notch pathway is associated with better prognosis in renal cell carcinoma. Biofactors. 2016;42:41–48. doi: 10.1002/biof.1250. [DOI] [PubMed] [Google Scholar]
- Flum A. S., Hamoui N., Said M. A., Yang X. J., Casalino D. D., McGuire B. B., Perry K. T., Nadler R. B. Update on the diagnosis and management of renal angiomyolipoma. J. Urol. 2016;195:834–846. doi: 10.1016/j.juro.2015.07.126. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis theraperutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Frydenberg H., Thune I., Lofterod T., Mortensen E. S., Eggen A. E., Risberg T., Wist E. A., Flote V. G., Furberg A., Wilsgaard T., Akslen L. A., McTiernan A. Pre-diagnostic high-sensitive C-reactive protein and breast cancer risk, recurrence, and survival. Breast Cancer Res. Treat. 2016;155:345–354. doi: 10.1007/s10549-015-3671-1. [DOI] [PubMed] [Google Scholar]
- Fujita T., Nishi M., Tabata K., Matsumoto K., Yoshida K., Iwamura M. Overall prognostic impact of C-reactive protein level in patients with metastatic renal cell carcinoma treated with sorafenib. Anticancer Drugs. 2016;27:1028–1032. doi: 10.1097/CAD.0000000000000417. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109:81–96. doi: 10.1016/S0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Gómez-Valenzuela F., Escobar E., Pérez-Tomás R., Montecinos V. P. The inflammatory profile of the tumor microenvironment, orchestrated by cyclooxygenase-2, promotes epithelial-mesenchymal transition. Front. Oncol. 2021;11:686792. doi: 10.3389/fonc.2021.686792.b0473a403e174260a6d3627246bf8bcd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T., Liu L., Jiang W., Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020;20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- Grad E., Danenberg H. D. C-reactive protein and atherothrombosis: cause or effect? Blood Rev. 2013;27:23–29. doi: 10.1016/j.blre.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Grivennikov S. I., Greten F. R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten F., Grivennikov S. I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., He X., Chen Q., Yang G., Yao K., Dong P., Ye Y., Chen D., Zhang Z., Qin Z., Liu Z., Xue Y., Zhang M., Liu R., Zhou F., Han H. The C-reactive protein/albumin ratio, a validated prognostic score, predicts outcome of surgical renal cell carcinoma patients. BMC Cancer. 2017;17:171. doi: 10.1186/s12885-017-3119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hansen I. S., Krabbendam L., Bernink J. H., Loayza-Puch F., Hoepel W., van Burgsteden J. A., Kuijper E. C., Buskens C. J., Bemelman W. A., Zaat S. A. J., Agami R., Vidarsson G., van den Brink G. R., de Jong E. C., Wildenberg M. E., Baeten D., Everts B., den Dunnen J. FcαRI co-stimulation converts human intestinal CD103+ dendritic cells into pro-inflammatory cells through glycolytic reprogramming. Nat. Commun. 2018;9:863. doi: 10.1038/s41467-018-03318-5.292a58835f16458d974a1336ef0a74da [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Ikeda Y., Korenaga D., Tanoue K., Hamatake M., Kawasaki K., Yamaoka T., Iwatani Y., Akazawa K., Takenaka K. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103:1856–1864. doi: 10.1002/cncr.20976. [DOI] [PubMed] [Google Scholar]
- Hansson G. K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hattori Y., Matsumura M., Kasai K. Vascular smooth muscle cell activation by C-reactive protein. Cardiovasc. Res. 2003;58:186–195. doi: 10.1016/S0008-6363(02)00855-6. [DOI] [PubMed] [Google Scholar]
- He Y., Sun M. M., Zhang G. G., Yang J., Chen K. S., Xu W. W., Li B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021;6:425. doi: 10.1038/s41392-021-00828-5.37774d4ae5334c1a9a597cb056b37cdb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojilla C. V., Wood G. A., Khokha R. Inflammation and breast cancer: metalloproteinases as common effectors of inflammation and extracellular matrix breakdown in breast cancer. Breast Cancer Res. 2008;10:205. doi: 10.1186/bcr1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T., Liu A., Cai D., Zhang Y., Hua D., Hang X., Wu X. Preoperative serum C-reactive protein levels and early breast cancer by BMI and menopausal status. Cancer Invest. 2013;31:279–285. doi: 10.3109/07357907.2013.789898. [DOI] [PubMed] [Google Scholar]
- Jemal A., Siegel R., Ward E., Murray T., Xu J., Thun M. J. Cancer statistics. CA Cancer J. Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Kim E. S., Cha Y., Ham M., Jung J., Kim S. G., Hwang S., Kleemann R., Moon A. Inflammatory lipid sphingosine-1-phosphate upregulates C-reactive protein via C/EBPβ and potentiates breast cancer progression. Oncogene. 2014;33:3583–3593. doi: 10.1038/onc.2013.319. [DOI] [PubMed] [Google Scholar]
- Kim E. S., Kim S. Y., Koh M., Lee H. M., Kim K., Jung J., Kim H. S., Moon W. K., Hwang S., Moon A. C-reactive protein binds to integrin α2 and Fcγ receptor I, leading to breast cell adhesion and breast cancer progression. Oncogene. 2018;4:28–38. doi: 10.1038/onc.2017.298. [DOI] [PubMed] [Google Scholar]
- Kishi T., Nakamura A., Itasaka S., Shibuya K., Matsumoto S., Kanai M., Kodama Y., Takaori K., Mizowaki T., Hiraoka M. Pretreatment C-reactive protein level predicts outcome and patterns of failure after chemoradiotherapy for locally advanced pancreatic cancer. Pancreatology. 2015;15:694–700. doi: 10.1016/j.pan.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Kleemann R., Gervois P. P., Verschuren L., Staels B., Princen H. M., Kooistra T. Fibrates downregulate IL-1-stimulated C-reactive protein gene expression in hepatocytes by reducing nuclear p50-NFκB-C/EBP-β complex formation. Blood. 2003;101:545–551. doi: 10.1182/blood-2002-06-1762. [DOI] [PubMed] [Google Scholar]
- Krupinski J., Turu M. M., Martinez-Gonzalez J., Carvajal A., Juan-Babot J. O., Iborra E., Slevin M., Rubio F., Badimon L. Endogenous expression of C-reactive protein is increased in active (ulcerated oncomplicated) human carotid artery plaques. Stroke. 2006;37:1200–1204. doi: 10.1161/01.STR.0000217386.37107.be. [DOI] [PubMed] [Google Scholar]
- Kumar H., Kawai T., Akira S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Lee J. G., Cho B. C., Bae M. K., Lee C. Y., Park I. K., Kim D. J., Ahn S. V., Chung K. Y. Preoperative C-reactive protein levels are associated with tumor size and lymphovascular invasion in resected non-small cell lung cancer. Lung Cancer. 2009;63:106–110. doi: 10.1016/j.lungcan.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Levy D. E., Darnell J. E., Jr. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- Li J., Luo S. H., Tang Y., Li J. J. C-reactive protein induces pulmonary artery smooth cell proliferation via modulation of ERK1/2, Akt and NF-kappaB pathways. Clin. Lab. 2014;60:1357–1363. doi: 10.7754/Clin.Lab.2013.130111. [DOI] [PubMed] [Google Scholar]
- Liang Y. J., Shyu K. G., Wang B. W., Lai L. P. C-reactive protein activates thenuclear factor-kB pathway andinduces vascular cell adhesion molecule-1expression through CD32 inhuman umbilical vein endothelial cells andaortic endothelial cells. J. Mol. Cell. Cardiol. 2006;40:412–420. doi: 10.1016/j.yjmcc.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Lithgow D., Nyamathi A., Elashoff D., Martinez-Maza O., Covington C. C-reactive protein in nipple aspirate fluid. Nurs. Res. 2006;65:418–425. doi: 10.1097/00006199-200611000-00006. [DOI] [PubMed] [Google Scholar]
- Liu X. Y., Yan F., Niu L. L., Chen Q. N., Zheng H. R., Li J. Y. Strong correlation between early stage atherosclerosis and electromechanical coupling of aorta. Nanoscale. 2016;8:6975–6980. doi: 10.1039/C5NR07398G. [DOI] [PubMed] [Google Scholar]
- Ma Z., Qi Z., Shan Z., Li J., Yang J., Xu Z. The role of CRP and ATG9B expression in clear cell renal cell carcinoma. Biosci. Rep. 2017;37:BSR20171082. doi: 10.1042/BSR20171082. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Maekawa H., Tollefsen D. M. Role of proposed serpin-enzyme complex receptor recognition sites in binding and internalization of thrombin-heparin cofactor II complexes by hepatocytes. J. Biol. Chem. 1996;271:18604–18609. doi: 10.1074/jbc.271.31.18604. [DOI] [PubMed] [Google Scholar]
- Majello B., Arcone R., Toniatti C., Ciliberto G. Constitutive and IL-6 induced nuclear factors that interact with the human C-reactive protein promoter. EMBO J. 1990;9:457–465. doi: 10.1002/j.1460-2075.1990.tb08131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannello F., Tonti G. A., Simone P., Ligi D., Medda V. Iron-binding proteins and C-reactive protein in nipple aspirate fluids: role of iron-driven inflammation in breast cancer microenvironment? Am. J. Transl. Res. 2010;3:100–113. [PMC free article] [PubMed] [Google Scholar]
- Manning B. D., Cantley L. C. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Martín-Timón I., Sevillano-Collantes C., Segura-Galindo A., Del Cañizo-Gómez F. J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes. 2014;5:444–470. doi: 10.4239/wjd.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. G., Goulopoulou S., Wenceslau C. F., Spitler K., Matsumoto T., Webb R. C. Toll-like receptors and damage-associated molecular patterns: novel links between inflammation and hypertension. Am. J. Physiol. Heart Circ. Physiol. 2014;306:H184–H196. doi: 10.1152/ajpheart.00328.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger-Filho O., Tutt A., de Azambuja E., Saini K. S., Viale G., Loi S., Bradbury I., Bliss J. M., Azim H. J., Jr., Ellis P., Di Leo A., Baselga J., Sotiriou C., Piccart-Gebhart M. Dissecting the heterogeneity of triple-negative breast cancer. J. Clin. Oncol. 2012;30:1879–1887. doi: 10.1200/JCO.2011.38.2010. [DOI] [PubMed] [Google Scholar]
- Moscat J., Diaz-Meco M. T., Rennert P. NFκB activation by protein kinase C isoforms and B-cell function. EMBO Rep. 2003;4:31–36. doi: 10.1038/sj.embor.embor704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakachi K., Furuse J., Ishii H., Suzuki E., Yoshino M. Prognostic factors in patients with gemcitabine-refractory pancreatic cancer. Jpn. J. Clin. Oncol. 2007;37:114–120. doi: 10.1093/jjco/hyl144. [DOI] [PubMed] [Google Scholar]
- Newling M., Sritharan L., van der Ham A. J., Hoepel W., Fiechter R. H., de Boer L., Zaat S. A. J., Bisoendial R. J., Baeten D. L. P., Everts B., den Dunnen J. C-reactive protein promotes inflammation through FcγR-induced glycolytic reprogramming of human macrophages. J. Immunol. 2019;203:225–235. doi: 10.4049/jimmunol.1900172. [DOI] [PubMed] [Google Scholar]
- Nikolaou K., Sarris M., Talianidis I. Molecular pathways: the complex roles of inflammation pathways in the development and treatment of liver cancer. Clin. Cancer Res. 2013;19:2810–2816. doi: 10.1158/1078-0432.CCR-12-1961. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat. Rev. Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- Ozturk H. Bilateral synchronous adrenal metastases of renal cell carcinoma: a case report and review of the literature. Oncol. Lett. 2015;9:1897–1901. doi: 10.3892/ol.2015.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffen E., Vos H. L., Bertina R. M. C-reactive protein does not directly induce tissue factor in human monocytes. Arterioscler. Thromb. Vasc. Biol. 2004;24:975–981. doi: 10.1161/01.ATV.0000126681.16619.69. [DOI] [PubMed] [Google Scholar]
- Park W. B., Lee K. D., Lee C. S., Jang H. C., Kim H. B., Lee H. S., Oh M., Choe K. W. Production of C-reactive protein in Escherichia coli-infected patients with liver dysfunction due to liver cirrhosis. Diagn. Microbiol. Infect. Dis. 2005;51:227–230. doi: 10.1016/j.diagmicrobio.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Pasceri V., Willerson J. T., Yeh E. T. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.CIR.102.18.2165. [DOI] [PubMed] [Google Scholar]
- Pasceri V., Cheng J. S., Willerson J. T., Yeh E. T. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001;103:2531–2534. doi: 10.1161/01.CIR.103.21.2531. [DOI] [PubMed] [Google Scholar]
- Pawashe A. B., Golino P., Ambrosio G., Migliaccio F., Ragni M., Pascucci I., Chiariello M., Bach R., Garen A., Konigsberg W. K. A monoclonal antibody against rabbit tissue factor inhibits thrombus formation in stenotic injured rabbit carotid arteries. Circ. Res. 1994;74:56–63. doi: 10.1161/01.RES.74.1.56. [DOI] [PubMed] [Google Scholar]
- Peña E., de la Torre R., Arderiu G., Slevin M., Badimon L. mCRP triggers angiogenesis by inducing F3 transcription and TF signalling in microvascular endothelial cells. Thromb. Haemost. 2016;117:357–370. doi: 10.1160/TH16-07-0524. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Hirschfield G. M. C-reactive protein: a critical update. J. Clin. Invest. 2003;111:1805–1812. doi: 10.1172/JCI200318921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce B. L., Ballard-Barbash R., Bernstein L., Baumgartner R. N., Neuhouser M. L., Wener M. H., Baumgartner K. B., Gilliland F. D., Sorensen B. E., McTiernan A., Ulrich C. M. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J. Clin. Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine J. K., Fusai K. G., Young R., Sharma D., Davidson B. R., Menon K. V., Rahman S. H. Serum C-reactive protein concentration and the prognosis of ductal adenocarcinoma of the head of pancreas. Eur. J. Surg. Oncol. 2009;35:605–610. doi: 10.1016/j.ejso.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Platz E. A., De Marzo A. M. Epidemiology of inflammation and prostate cancer. J. Urol. 2004;171:S36–S40. doi: 10.1097/01.ju.0000108131.43160.77. [DOI] [PubMed] [Google Scholar]
- Prakash A. V., Park I. H., Park J. W., Bae J. P., Lee G. S., Kang T. J. NLRP3 inflammasome as therapeutic targets in inflammatory diseases. Biomol. Ther. (Seoul) 2023;31:395–401. doi: 10.4062/biomolther.2023.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaz-Schrauder D., Ekici A. B., Klinghammer L., Stumpf C., Achenbach S., Herrmann M., Reis A., Garlichs C. D. The proinflammatory effect of c-reactive protein on human endothelial cells depends on the Fcγ IIa genotype. Thromb. Res. 2014;133:426–432. doi: 10.1016/j.thromres.2013.12.030. [DOI] [PubMed] [Google Scholar]
- Ravishankaran P., Karunanithi R. Clinical significance of preoperative serum interleukin-6 and C-reactive protein level in breast cancer patients. World J. Surg. Oncol. 2011;9:18. doi: 10.1186/1477-7819-9-18.d6e086e4a024468ab53d368cd8014a04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxburgh C. S. D., McMillan D. C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- Schecter A. D., Giesen P. L., Taby O., Rosenfield C. L., Rossikhina M., Fyfe B. S., Kohtz D. S., Fallon J. T., Nemerson Y., Taubman M. B. Tissue factor expression in human arterial smooth muscle cells. J. Clin. Invest. 1997;100:2276–2285. doi: 10.1172/JCI119765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenten D., Medzhitov R. The control of adaptive immune responses by the innate immune system. Adv. Immunol. 2011;109:87–124. doi: 10.1016/B978-0-12-387664-5.00003-0. [DOI] [PubMed] [Google Scholar]
- Schimmack S., Yang Y., Felix K., Herbst M., Li Y., Schenk M., Bergmann F., Hackert T., Strobel O., Yang Y. C-reactive protein (CRP) promotes malignant properties in pancreatic neuroendocrine neoplasms. Endocr. Connect. 2019;8:1007–1019. doi: 10.1530/EC-19-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbulea V., Upchurch C. M., Ahern K. W., Bories G., Voigt P., DeWeese D. E., Meher A. K., Harris T. E., Leitinger N. Macrophages sensing oxidized DAMPs reprogram their metabolism to support redox homeostasis and inflammation through a TLR2-Syk-ceramide dependent mechanism. Mol. Metab. 2018;7:23–34. doi: 10.1016/j.molmet.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani M., Maeda K., Nagahara H., Iseki Y., Ikeya T., Hirakawa K. Prognostic significance of the preoperative ratio of C-reactive protein to albumin in patients with colorectal cancer. Anticancer Res. 2016;36:995–1001. [PubMed] [Google Scholar]
- Shrotriya S., Walsh D., Bennani-Baiti N., Thomas S., Lorton C. C-reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: a systematic review. PLoS One. 2015;10:e0143080. doi: 10.1371/journal.pone.0143080.fbc208544923404d9e4949675f9444f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- Sieghart W., Pinter M., Hucke F., Graziadei I., Schöniger-Hekele M., Müller C., Vogel W., Trauner M., Peck-Radosavljevic M. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology. 2013;57:2224–2234. doi: 10.1002/hep.26057. [DOI] [PubMed] [Google Scholar]
- Singh U., Devaraj S., Jialal I. C-reactive protein decreases tissue plasminogen activator activity in human aortic endothelial cells: evidence that c-reactive protein is a procoagulant. Arterioscler. Thromb. Vasc. Biol. 2005;25:2216–2221. doi: 10.1161/01.ATV.0000183718.62409.ea. [DOI] [PubMed] [Google Scholar]
- Slevin M., Matou-Nasri S., Turu M., Luque A., Rovira N., Badimon L. Modified C-reactive protein is expressed by stroke neovessels and is a potent activator of angiogenesis in vitro. Brain Pathol. 2010;20:151–165. doi: 10.1111/j.1750-3639.2008.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slevin M., Matou S., Zeinolabediny Y., Corpas R., Weston R., Liu D., Boras E., Di Napoli M., Petcu E., Sarroca S., Popa-Wagner A., Love S., Font M. A., Potempa L. A., Al-Baradie R., Sanfeliu C., Revilla S., Badimon L., Krupinski J. Monomeric C-reactive protein-a key molecule driving development of Alzheimer's disease associated with brain ischaemia? Sci. Rep. 2015;5:13281. doi: 10.1038/srep13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T. T., Guo B., Kawakami Y., Sommer K., Chae K., Humphries L. A., Kato R. M., Kang S., Patrone L., Wall R., Teitell M., Leitges M., Kawakami T., Rawlings D. J. PKC-β controls IκB kinase lipid raft recruitment and activation in response to BCR signaling. Nat. Immunol. 2002;3:780–786. doi: 10.1038/ni823. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K., Morris P. G., Zhou X. K., Morrow M., Du B., Giri D., Kopelovich L., Hudis C. A., Dannenberg A. J. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–365. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sun J., Russell C. C., Scarlett C. J., McCluskey A. Small molecule inhibitors in pancreatic cancer. RSC Med. Chem. 2020;11:164–183. doi: 10.1039/C9MD00447E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundgren N. C., Zhu W., Yuhanna I. S., Chambliss K. L., Ahmed M., Tanigaki K., Umetani M., Mineo C., Shaul P. W. Coupling of Fcγ receptor I to Fcγ receptor IIb by src kinase mediates c-reactive protein impairment of endothelial function. Circ. Res. 2011;109:1132–1140. doi: 10.1161/CIRCRESAHA.111.254573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tabas I., García-Cardeña G., Owens G. K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tang D., Kang R., Coyne C. B., Zeh H. J., Lotze M. T. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol. Rev. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z., Sheng H., Zheng X., Ying L., Wu L., Liu D., Liu G. Upregulation of circulating cytokeratin 20, urokinase plasminogen activator and C-reactive protein is associated with poor prognosis in gastric cancer. Mol. Clin. Oncol. 2015;3:1213–1220. doi: 10.3892/mco.2015.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada K., Hayashi G., Hokama Y. C-reactive protein and 6-keto prostaglandin F 1-alpha in patients with gynecologic cancer. Gynecol. Oncol. 1990;36:212–214. doi: 10.1016/0090-8258(90)90175-K. [DOI] [PubMed] [Google Scholar]
- Toi M., Inada K., Suzuki H., Tominaga T. Tumor angiogenesis in breast cancer: its importance as a prognostic indicator and the association with vascular endothelial growth factor expression. Breast Cancer Res. Treat. 1995;36:193–204. doi: 10.1007/BF00666040. [DOI] [PubMed] [Google Scholar]
- Toniatti C., Demartis A., Monaci P., Nicosia A., Ciliberto G. Synergistic transactivation of the human C-reactive protein promoter by transcription factor HNF-1 binding at two distinct sites. EMBO J. 1990;9:4467–4475. doi: 10.1002/j.1460-2075.1990.tb07897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turu M. M., Slevin M., Matou S., West D., Rodriguez C., Luque A. C-reactive protein exerts angiogenic effects on vascular endothelial cells and modulates associated signalling pathways and gene expression. BMC Cell Biol. 2008;9:47. doi: 10.1186/1471-2121-9-47.4d66a1d30928460ca2f8370cb44d0440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Eijnden M. M., Steenhauer S. I., Reitsma P. H., Bertina R. M. Tissue factor expression during monocyte-macrophage differentiation. Thromb. Heamost. 1997;77:1129–1136. doi: 10.1055/s-0038-1656125. [DOI] [PubMed] [Google Scholar]
- Varga J., Greten F. R. Cell plasticity in epithelial homeostasis and tumorigenesis. Nat. Cell Biol. 2017;19:1133–1141. doi: 10.1038/ncb3611. [DOI] [PubMed] [Google Scholar]
- Vergadi E., Ieronymaki E., Lyroni K., Vaporidi K., Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 2017;198:1006–1014. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- Verma A. K., Kumar S., Kumar N., Verma R. K., Singh M. Study of coronary artery atherosclerosis in sudden deaths and its medicolegal relevance. J. Indian Acad. Forensic Med. 2012;34:132–134. [Google Scholar]
- Volanakis J. E. Human C-reactive protein: expression, structure, and function. Mol. Immunol. 2001;38:189–197. doi: 10.1016/S0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- Wang C. S., Sun C. F. C-reactive protein and malignancy: clinico-pathological association and therapeutic implication. Chang Gung Med. J. 2009;32:471–482. [PubMed] [Google Scholar]
- Wang H. R., Chen D. L., Zhao M., Shu S. W., Xiong S. X., Gan X. D., Chao S., Cao J. C-reactive protein induces interleukin-6 and thrombospondin-1 protein and mRNA expression through activation of nuclear factor-ĸB in HK-2 cells. Kidney Blood Press. Res. 2012;35:211–219. doi: 10.1159/000332402. [DOI] [PubMed] [Google Scholar]
- Weinhold B., Ruther U. Interleukin-6-dependent and -independent regulation of the human C-reactive protein gene. Biochem. J. 1997;327:425–429. doi: 10.1042/bj3270425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese D., Kampe K., Waldmann J., Heverhagen A. E., Bartsch D. K., Fendrich V. C-reactive protein as a new prognostic factor for survival in patients with pancreatic neuroendocrine neoplasia. J. Clin. Endocrinol. Metab. 2016;101:937–944. doi: 10.1210/jc.2015-3114. [DOI] [PubMed] [Google Scholar]
- Wigmore S. J., Fearon K. C., Maingay J. P., Lai P. B., Ross J. A. Interleukin-8 can mediate acute-phase protein production by isolated human hepatocytes. Am. J. Physiol. 1997;273:720–726. doi: 10.1152/ajpendo.1997.273.4.E720. [DOI] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Schwartz S., Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc. Natl. Acad. Sci. U. S. A. 1989;86:2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S., Obolonczyk L., Sworczak K., Czapiewski P., Sledzinski Z. Renal cell carcinoma metastases to the pancreas and the thyroid gland 19 years after the primary tumour. Prz. Gastroenterol. 2015;10:185–189. doi: 10.5114/pg.2015.49000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Fu X., Zhu X., He X., Zou C., Han Y., Xu M., Huang C., Lu X., Zhao Y. Prognostic role of systemic inflammatory response in renal cell carcinoma: a systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2011;137:887–896. doi: 10.1007/s00432-010-0951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasojima K., Schwab C., McGeer E. G., McGeer P. L. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am. J. Pathol. 2001;158:1039–1051. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9:361–367. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavodszky E., Vicinanza M., Rubinsztein D. C. Biology and trafficking of ATG9 and ATG16L1, two proteins that regulate autophagosome formation. FEBS Lett. 2013;587:1988–1996. doi: 10.1016/j.febslet.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Yang Y., Hill M. A., Wu J. Does c-reactive protein contribute to atherothrombosis via oxidant-mediated release of pro-thrombotic factors and activation of platelets? Front. Physiol. 2012;3:433. doi: 10.3389/fphys.2012.00433.820a02802d3540fba3fc577cbcb98fe0 [DOI] [PMC free article] [PubMed] [Google Scholar]