Abstract
Objective
Thyroid nodules are extremely common, with prevalence rate up to 68%, yet only 7–15% of these are malignant. Many nodules require surveillance and 2-dimensional ultrasound (2D US) is used. Issues include the huge workload of obtaining and labeling images and difficulty comparing sizes of nodules over time due to large inter-operator variability. Inaccuracies may result in unnecessary FNAC or missed diagnosis of malignant nodules.
Methods
We compared two techniques: freehand plain 2D US against freehand 2D US with gyroscopic guidance, both followed by 3D reconstruction using software. We measured the volume of nodules and a normal thyroid gland.
Results
We found 2D US with gyroscopic guidance to be superior to plain 2D US as 3D reconstructions of greater accuracy are produced. The volume of the thyroid lobe measured 8.42 cm3 ± 0.94 was reasonably close to the normal average volume. However, the measured volume of the ellipsoidal nodule by the software is 8.69 cm3 ± 0.97 while the measured volume of the spherical nodule is 7.09 cm3 ± 0.79. As the expected volume of the nodules were 4.24cm3 and 4.19 cm3 respectively, the measured volume of the nodule was not accurate. The time taken to characterise nodules was reduced greatly from over 30 min in usual procedure to less than 10 min.
Conclusion
We find 3D US promising for evaluating size of thyroid nodules, with potential to study other TIRAD characteristics. Freehand 2D US with gyroscopic guidance shows the most promise for producing reliable, accurate and faster 3D reconstructions of thyroid nodules.
Keywords: 3D ultrasound, Thyroid, Thyroid nodules, Thyroid volume measurement, Nodule monitoring
Introduction
Thyroid nodules are extremely common, with a prevalence rate of up to 68% in high resolution (7.5 or 10–15 MHz) ultrasound (HRUS) [1, 2], yet only 7–15% of these are malignant [1]. Conventional 2D ultrasound (2D US) has been the first step in a series of investigations when a thyroid nodule is suspected. A combination of sonographic features according to the American Thyroid Association (ATA) [1] or American College of Radiology (ACR) Thyroid Imaging Reporting and Data System (TIRADS) classification [3] helps us evaluate the likelihood of a nodule being malignant so as to make a decision based on size and features whether a fine needle aspiration cytology (FNAC) is necessary. If a nodule achieves a grade of TR3-5 but does not meet size criteria, we need to monitor these nodules regularly [3]. As other imaging modalities such as Computer Tomography (CT) or Magnetic Resonance Imaging (MRI) scans do not provide adequate soft tissue resolution, ultrasound (US) is still the first line preferred imaging modality. This represents a huge challenge for doctors, as skilled operators are necessary to obtain reliable images and much time is taken labelling every benign nodule on each follow-up, especially due to the high prevalence rate. Nevertheless, such efforts are necessary as ultrasound images are still the most useful imaging modality for the thyroid. As such, further modified ultrasound modalities such as color doppler ultrasound (CDUS) [4–9], contrast-enhanced ultrasound (CEUS) [10, 11], shear wave elastography (SWE) [12], 3D power doppler ultrasound (3D PDUS) and 3D ultrasound (3D US) have recently come into practice to improve the diagnostic accuracy of thyroid nodules. [13, 14] Modified ultrasound modalities has also been used to great extents in other fields of medicine as well such as detection of pediatric encephalic hemorrhages [15], studying the gastroesophageal region [16].
Despite the leaps made in imaging over the past few years, it is hard to characterize the size of thyroid nodules based on 2D US. The conventional method of taking the largest measurement in each dimension has a few main shortfalls. Firstly, there is high inter-operator variability as the description of the nodule can vary depending on the slice obtained by the operator [17]. Secondly, difficulty of comparison across multiple imaging time points of US assessment as it is not a static cross sectional imaging modality. This also takes a lot of time to perform. Inaccuracies arising from this may result in unnecessary FNAC or missed diagnosis of potentially malignant thyroid nodules.
This motivates us to look for more objective ways of assessing the thyroid through three-dimensional reconstruction. 3D US has been widely used in many other fields of medicine such as breast nodule characterization [18], prostate volume assessments [19] and fetal visualization [20] for better visualization of masses in the body and characterization of their exact shape and size with great efficacy. 3D reconstructed images have also been used for evaluation of other masses such as lymph nodes. [21]
Multiple attempts at utilization of 3D US for thyroidology have been attempted. These include measuring the total volume of a model balloon thyroid [22, 23] as well as characterization of nodules using CEUS [24], power doppler ultrasound [13, 14]. Most of these either make use of a freehand scan [24] or a volumetric probe [13, 14] to obtain 3D reconstructions which can be inaccurate. In order to reconstruct a 3D model, multiple images of the thyroid or the nodule have to be taken and stitched together as the probe is not able to capture the entire nodule or gland in one image. This stitching process might not be able to align all the images accurately since the images are not taken with the same frame of reference. We aim to use normal conventional B-mode 2D US with a freehand linear probe in a similar manner to Molinari et al. [24]. The images obtained will then be used for 3D reconstruction. This will be compared with a repeat scan using the same setup with an additional gyroscopic attachment to the linear probe, which allows us to perform 3D spatial based US tomography of the thyroid via software reconstruction automatically.
Materials and methods
We evaluated two 3D US modalities to determine the ideal solution. We tried doing a B-mode freehand 2D US scan of the thyroid with 3D reconstruction using ITK-SNAP [25] (IT-K SNAP v.3.8.0, PICSL and SCI, Philadelphia and Salt Lake City, Pennsylvania and Utah, United States of America), a free software available online to segment structures in 3D US images semi-automatically. We also augmented the freehand 2D US scan by adding gyroscopic guidance to provide additional data for 3D reconstruction using PIUR tUS software [26].
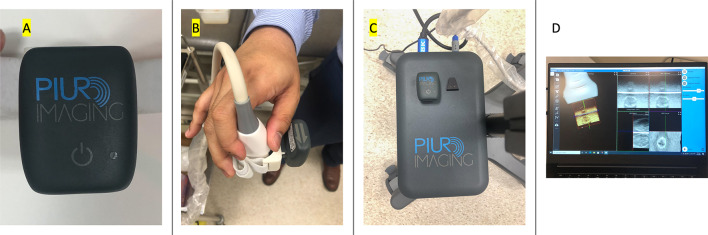
The first modality an augmented 2D US with gyroscopic guidance using the tUS Infinity platform from PIUR Imaging (PIUR tUS infinity, PIUR imaging, Vienna, Vienna, Austria). This product has three components. The first component is the gyroscopic attachment (Infinity Sensor) that is attached to the US linear probe via a clip on attachment. The next component is a video box (Infinity Video Box) that receives the US image data from the US machine and tags the US image data with gyroscopic data and sends that to the computer via Wi-Fi. The third component is the computer software (Infinity Workstation) that aggregates the data and produces 3D renderings of the thyroid by using the gyroscopic data to align the 2D US cuts taken. From these 3D renderings, nodules can be segmented semi-automatically and the thyroid volume can be calculated automatically using the Infinity Workstation software. This process is shown in Fig. 1.
Fig. 1.
Labelled picture of the tUS infinity platform with three components. A The Infinity Sensor (the gyroscopic attachment), B the Infinity Sensor attached to the probe, C infinity Video Box, D infinity workstation (the computer software)
The second modality is is a 2D US with 3D reconstruction was achieved by using freehand 2D US scans of the neck using a linear probe. We used the Mindray DC-80A US machine (DC-80A, Mindray, Nanshan, Shenzhen, China) together with a Philips L10-5 linear probe (L10-5, Philips Healthcare, Amsterdam, North Holland, Netherlands) at 10 MHz to record a 2D US video file in DICOM format. This was then analyzed using the ITK-SNAP v. 3.8.0 software and its semi-automatic segmentation approach to reconstruct a 3D image of the thyroid gland.
Apart from imaging the thyroid, we also imaged two thyroid phantoms, one with an ellipsoidal nodule and one with a spherical nodule of similar volumes. This was to test if the 3D reconstruction of nodules by this software could pick up differences in shape accurately. The phantom was created by putting a grape in a container of agar to simulate a thyroid nodule. Two phantoms were created using identical 16 cm by 16 cm containers, each with a differently shaped grape. One phantom had an ellipsoid grape while the other had a roughly spherical grape. We attempted to keep the grape volumes as close as possible to each other: the calculated volume of the ellipsoid is 4.24 cm3 and the calculated volume of the sphere is 4.19 cm3. The live thyroid model is a scan of a healthy volunteer’s thyroid. This is seen in Fig. 2. The phantoms were used only for the modality with gyroscopic guidance as the second modality did not give accurate results for the thyroid volume, thus we concluded that since the second modality was not able to reconstruct live thyroid tissue accurately, further study on phantoms would not hold much meaning. This is also because the phantom does not accurately simulate human tissue well. The density of the agar and the grape in the phantom is much more homogenous compared to human tissue. Nevertheless, the phantom was used to provide an approximation of a nodule as a proof of concept.
Fig. 2.
A–C Pictures of the grapes used in the thyroid phantom from 3 orthogonal views, D picture of the thyroid phantom. Grape on the left measured 2 cm by 2 cm by 2 cm. Grape on the right measured 1.8 cm by 2.5 cm by 1.8 cm in A–C respectively
A typical 2D US workflow follows ACR TIRADS procedure [27]. For an initial study, sonographers will take a brief overview of the entire gland to identify nodules of interest that require further studies. Thereafter, they will scan the entire gland following each individual laboratory’s protocol and should measure up to approximately four nodules that are likely to require FNAC or follow-up as well as obtain enough images to document the nodule architecture. Sonographers are recommended to label each nodule according to its location in the gland. Should the patient be here for follow-up, the sonographer should review prior images and reports before reimaging to determine nodules of interest that will require formal reassessment. If the previous sonogram was reported by using ACR TI-RADS, nodules should be numbered as before even if previously reported nodules are no longer present. Thereafter, the images are then passed on to the radiologist for interpretation and characterization of nodules. A formal report consisting of tridimensional measurements of the thyroid lobes and the isthmus, overall description of thyroid parenchyma, formal description of up to four most suspicious nodules and recommendations for management is produced by the radiologist. While ACR-TIRADS does not encompass regional lymph nodes, nodal assessment is essential in the management of patients with thyroid conditions, especially in the context of malignancy as it would alter the staging, choice of surgery and nature of adjuvant therapy for the patient. If patients have a history of thyroid cancer or there is sufficient suspicion of thyroid cancer, a comprehensive evaluation of lymph nodes is required. However, this comprehensive evaluation can be performed at a later time, either together with the US-guided biopsy or after cancer diagnosis has been made using the biopsy results as a separate pre-op US evaluation. ACR-TIRADS also borrows its definition of clinically important growth from the American Thyroid Association (ATA) [27] which is a 20% increase in at least two nodule dimensions and a minimal increase of 2 mm or a 50% or greater increase in volume. While rapid growth is suspicious, it does not reliably differentiate between benign and malignant nodules [28]. At the same time, nodules that do not grow substantially over the course of 5 years can be considered benign. This protocol takes a total time of about 30 min for a typical US with characterization in the local context.
However, if we were to adopt 3D US, these can be captured as videos in real time. Every slice would be tagged with a well precisioned positional annotation, thus allowing these slices to be re-aligned with computer software into an accurate 3D model of the thyroid. In order to test this hypothesis, we came up with a phantom model to allow objective visualization of workflow as well as a live thyroid model to test the scan. We did not inject any contrast for the ultrasound scans performed in our studies.
Results
Using the PIUR tUS infinity imaging platform, we produced 3D renderings of the phantom with volumetric assessments as shown below in Fig. 3.
Fig. 3.
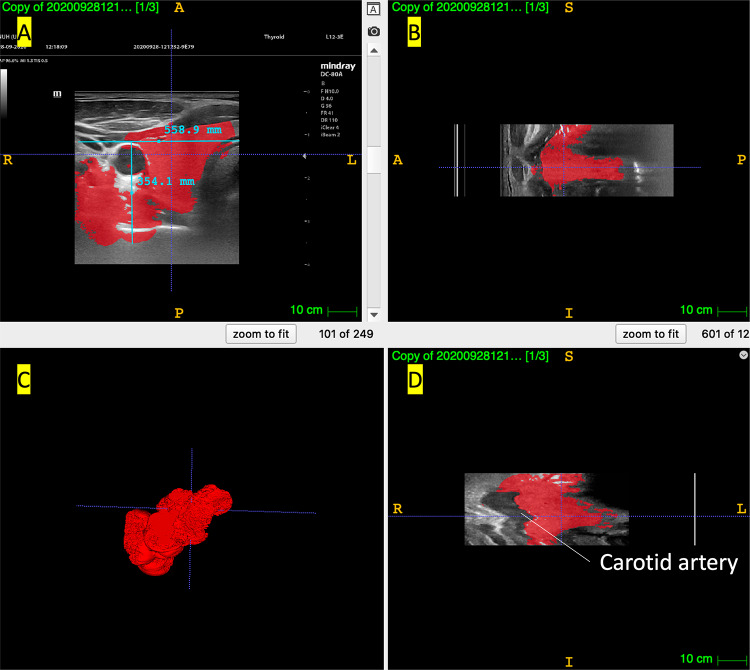
3D reconstruction of 2D thyroid US scan by ITK-SNAP. A Axial view, B Sagittal view, C 3D reconstruction, D Coronal view, E 3D reconstruction of the same scan in coronal view, with the same views (A–D)
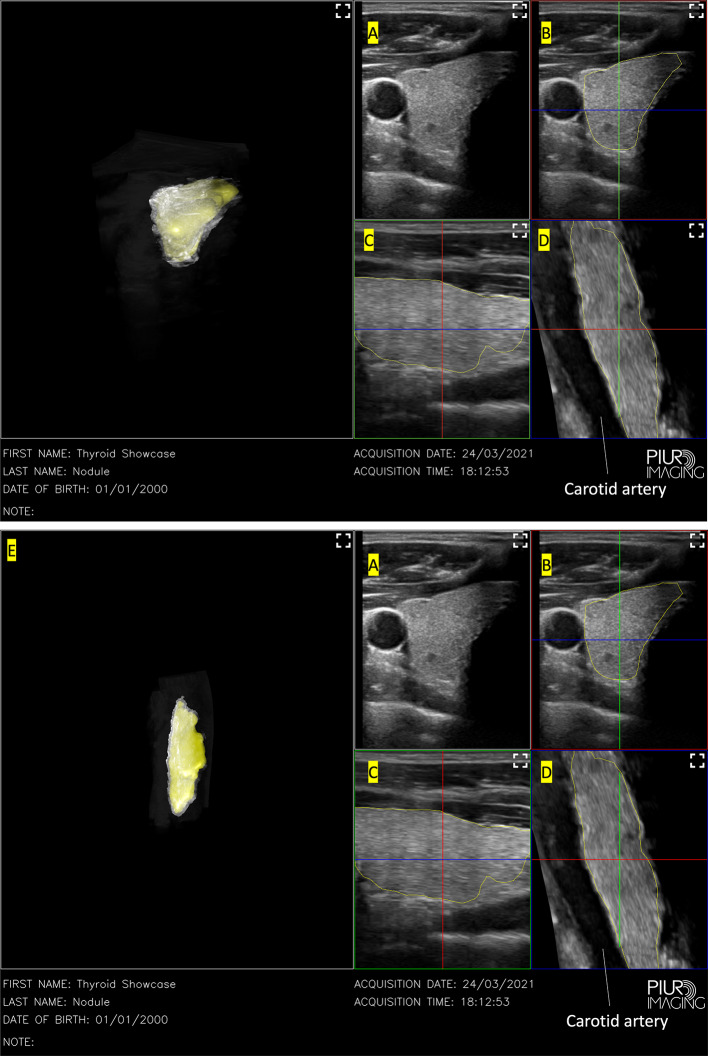
PIUR’s Infinity Workstation software allowed for multiplanar reconstruction of the axial, sagittal and coronal view while aligning the images obtained using information sent from their gyroscopic attachment to their software. The resultant semi-automatic 3D reconstruction as seen in Fig. 4 allows for a much more accurate 3D model of the thyroid to be produced in terms of volume measured as well as the regularity of the 3D model produced. The machine was able to successfully reconstruct it in under a minute of scanning time. The 3D model produced has a regular shape and is a clean reproduction of the thyroid nodule. Automatic volumetric analysis measured the thyroid volume at 8.42 cm3 ± 0.94, which is near the average volume [29, 30]. When calculated according to WHO estimation formulas [31], the calculated volume was cm3. This process did not require the operator to modify multiple variables to obtain a segmentation result and was fully automatic, generating a 3D model with the measured volume in 45 s after the scan. Thus, it was comparatively easier to operate as compared to ITK-SNAP. This could be due to the software being pre-calibrated for the thyroid gland by the manufacturer.
Fig. 4.
3D reconstruction of a 2D US scan using PIUR imaging gyroscopic guidance. Left: 3D reconstruction, A Original single frame scans from the US device, B Axial view, C Sagittal view, D Coronal view
For our two thyroid phantoms, the results are shown below in Fig. 4.
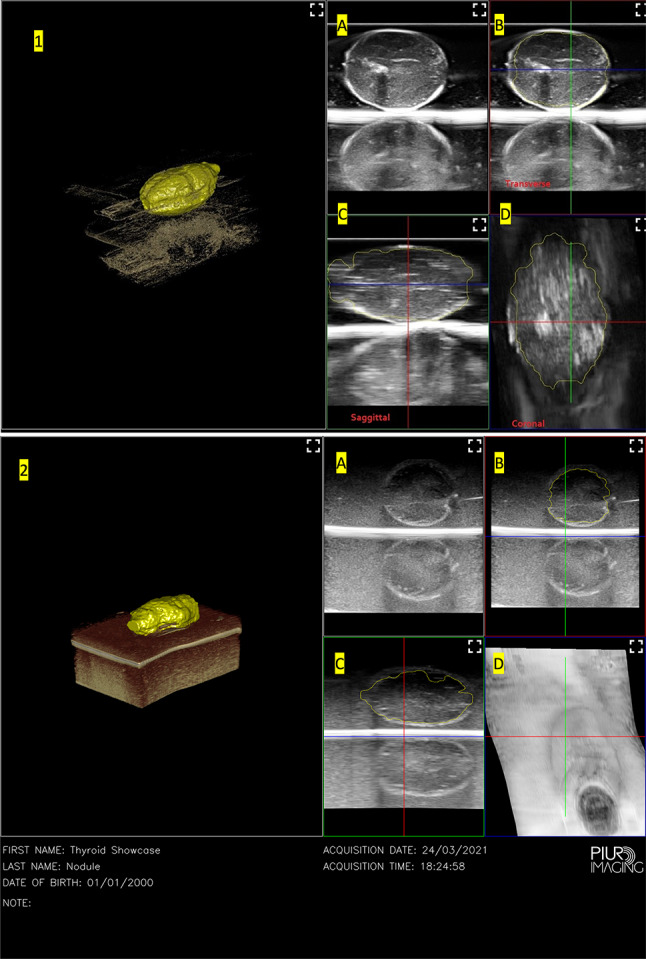
As seen in Fig. 5, the software was able to automatically identify the nodule from the surrounding tissue but was unable to consistently segment the nodule across multiple cuts. This semi-automatic process of drawing the areas of interest took much longer for the nodule around 10 min 15 s as compared to the live thyroid tissue, which had a much faster segmentation process and took under 1 min. As a result, despite both nodules being different sized as can be seen in Fig. 2, the 3D reconstruction of both nodules was similar. The volume of the ellipsoidal nodule as measured by the software is 8.69 cm3 ± 0.97 while the volume of the spherical nodule as measured by the software is 7.09 cm3 ± 0.79. Using the standard formula for a regular ellipsoid and sphere, the calculated volume of this ellipsoid is 4.24 cm3 and the calculated volume of this sphere is 4.19 cm3. These represent significantly different measured and calculated volumes which is still a current limitation of the software system.
Fig. 5.
3D reconstruction of 1: ellipsoidal nodule in thyroid phantom, 2: spherical nodule in thyroid phantom, A original single frame scans from the US device, B Axial view, C Sagittal view, D Coronal view. Viewing options for the tissue surrounding the nodule were selected in the 3D view based on which view option highlighted the nodule the most clearly
We also attempted to reconstruct a 2D scan of the thyroid using ITK-SNAP, with the results shown in Fig. 5.
ITK-SNAP arranges the DICOM images in sequential order, aligning them without using any gyroscopic correction. The software allows us to do semi-automatic segmentation by using their active contour function. This allows for the modelling of objects of interest such as the thyroid gland. However, as can be seen in Fig. 3, the carotid artery is extremely jagged as the individual slices obtained by handheld 2D US scans are not perfectly aligned. This is due to the freehand nature of the scan, which inevitably results in misalignment. The 3D model generated, while retaining an overall shape similar to the thyroid gland, is very imprecise and not ideal for clinical use and interpretation. We attempted to adjust parameters such as the threshold values for the intensity region filter and weight of the velocities for image propagation among other parameters to optimise the 3D auto-segmentation according to the tutorial provided by ITK-SNAP and have presented the most successful 3D reconstruction here. Despite this effort, the volume of the thyroid is also not calculated accurately by this software with a calculated volume of 19,700 cm3 (3sf). This could be due to the width and height of the thyroid measured by the software being 558.9 mm and 354.1 mm. These inaccuracies could be due to inadequate experience with the software among the members of the research team. Overall, while this open source program provides a good starting point for trying out 3D segmentation on medical images, freehand US + ITK-SNAP was not a suitable modality for us due to the above-mentioned limitations.
Discussion
We undertook 3D gyroscopic guided 2D US and found that this method is relatively efficient and accurate to provide 3D reconstruction of thyroid gland. In the live thyroid model, the software was able to reconstruct the thyroid despite the presence of multiple structures in the neck such as the carotid artery and trachea. On the coronal view, the carotid artery is reconstructed smoothly in Fig. 4 which shows much better alignment compared to 3D reconstruction from freehand 2D US scans without any gyroscopic guidance which can be seen in Fig. 3. Given our experience with ITK-SNAP without gyroscopic guidance, gyroscopic guidance is clearly superior in terms of image reproduction and 3D reconstruction. Hence, gyroscopic guided 2D US scans might be the ideal way to improve the workflow in capturing thyroid tomography.
This method could prove to be more objective than traditional 2D thyroid US scan by removing the inter-operator variability because the thyroid gland is being reconstructed in 3D rather than being evaluated in 2D. This method still allows for individual 2D images to be preserved for comparison with older scans as well. However, more work needed to be done to ensure its accuracy in reconstructing nodules in thyroid phantoms and eventually live patients.
Limitations
The limitation of this initial pilot study is the use of phantoms rather than actual patients to evaluate the system. This limits us from being able to conclude whether the system can accurately evaluate live thyroid nodules. With our one thyroid live sample, we did notice an accurate 3D reconstruction and volumetric assessment of the thyroid lobe. More work can be done to prove the reproducibility of our results and demonstrate the reliability and practical application of this system.
The ultrasound scans in this study were performed by a clinician doctor who specializes in Head and Neck surgery and has had more than 15 years of experience with thyroid ultrasound scans. As this is a preliminary study, we did not involve a sonographer in the image acquisition process. This is a limitation of our study as this does not properly mimic the typical 2D US workflow.
In this preliminary study, the two approaches were not compared equally as the team faced difficulty calibrating ITK-SNAP for our purposes to calculate the volume of the thyroid lobe properly. This resulted in inaccurate calculations of the thyroid volume via ITK-SNAP. Thus, we decided to move on and skip using ITK-SNAP with the thyroid phantoms since we were unable to get accurate thyroid lobe reconstructions using it. Furthermore, the image sequence files obtained via the PIUR imaging platform were not compatible with the ITK-SNAP software. Performing the same tests using both approaches would have made for a more rigorous study. More can be done in future studies to utilise ITK-SNAP, such as reaching out to experts familiar with the program for collaboration.
Operator familiarity was necessary to achieve consistent results both in scanning and segmentation. Approximately 5–10 min were required to familiarize with the system before performing a few scans of the thyroid phantom and the live thyroid model. This revealed that some training is necessary to hit the ideal 1–2 cm/s scanning speed consistently and maintain the scanning speed throughout the entire scan. However, as ultra-sonographers and doctors are likely to have a wealth of prior experience in 2D US scans, this training process is not expected to be laboriously long. Despite the training process, we did notice significant reduction in the time taken to acquire and segment images, with the total time taken to be under 10 min, while the typical US with characterization takes over 30 min in Singapore. Taking more accurate timings rather than rough timings like 10 min and 30 min is also something we could have done.
We experienced some deviation from expected values for the volume and shape when reconstructing the nodules in 3D. This can be explained by a variety of factors. The scanning speed of the probe should ideally be kept within 1–2 cm/s. Any variation in speed would affect the multiplanar rendering of the nodule, causing possible elongation or distortion of the image. The ultrasound device frame rate was 22 Hz. In contrast, the recommended frame rate is much higher at 73 Hz [PIUR tUS 26]. That could have caused the inaccuracy in reconstruction leading to an elongated image. The ultrasound device image quality may also play a role in the inaccuracies. Finally, this system is not optimized for thyroid phantoms using agar, hence segmentation in the application is not fully optimized for this use case. Further work can be done to establish the accuracy of this method, both in optimizing the software and in operator training in order to achieve our goal of highly accurate segmentation for comparative assessment of thyroid nodules. We did not measure or account for the phantom’s scattering characteristics either in this preliminary study.
We are also unable to evaluate the other TIRAD characteristics of the nodules using our current 3D reconstructions.
Conclusion
3D US Tomography with the aid of gyroscopic guidance provides a new modality in which we can assess, evaluate, and follow up thyroid nodules with greater efficacy and precision. Gyroscopic guidance for 3D reconstruction is the way forward, offering greater reproducibility of adequately reconstructed imaging. Further studies in the future are required to validate this methodology on real patients and actual thyroid nodules. Further studies into the evaluation and follow-up of thyroid gland volume using our 3D ultrasound technique in the case of goiters treated by thyroxine and/or iodine should be considered.
Acknowledgements
We would like to thank Drs. Gao Yujia and Andrew Makmur for their support and advice.
Author Contributions
Aldred Cheng: conceptualisation, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, writing—original draft. James Wai Kit Lee: conceptualisation, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, writing—review and editing. Kee Yuan Ngiam: conceptualisation, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, writing—review and editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
Aldred Cheng: no competing financial interests exist, James Wai Kit Lee: no competing financial interests exist, Kee Yuan Ngiam: no competing financial interests exist.
Ethical approval
This study has been submitted and approved by DSRB (reference number: 2021/00337). The study has been conducted in accordance with the experimental protocol submitted to and approved by DSRB. Informed consent was obtained from all human subjects for participation in the study and publishing.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aldred Cheng, Email: e0345824@u.nus.edu.
James Wai Kit Lee, Email: james_lee@nuhs.edu.sg.
Kee Yuan Ngiam, Email: kee_yuan_ngiam@nuhs.edu.sg.
References
- 1.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blum M. Ultrasonography of the Thyroid. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al., editors. Endotext. South Dartmouth (MA) 2000.
- 3.Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14:587–595. doi: 10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 4.Brillantino C, Rossi E, Minelli R, Irace D, Castelli L, Zeccolini R, et al. A rare case of renal tumor in children: clear cell sarcoma. G Chir. 2019;40:217–224. [PubMed] [Google Scholar]
- 5.Brillantino C, Rossi E, Bifano D, Minelli R, Tamasi S, Mamone R, et al. An unusual onset of pediatric acute lymphoblastic leukemia. J Ultrasound. 2021;24:555–560. doi: 10.1007/s40477-020-00461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brillantino C, Rossi E, Baldari D, Minelli R, Bignardi E, Paviglianiti G, et al. Duodenal hematoma in pediatric age: a rare case report. J Ultrasound. 2022;25:349–354. doi: 10.1007/s40477-020-00545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brillantino C, Rossi E, Pirisi P, Gaglione G, Errico ME, Minelli R, et al. Pseudopapillary solid tumour of the pancreas in paediatric age: description of a case report and review of the literature. J Ultrasound. 2022;25:251–257. doi: 10.1007/s40477-021-00587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brillantino C, Rossi E, Minelli R, Bifano D, Baldari D, Pizzicato P, et al. Mediastinal thymoma: a difficult diagnosis in the pediatric age. Radiol Case Rep. 2021;16:2579–2585. doi: 10.1016/j.radcr.2021.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rumolo M, Santarsiere M, Menna BF, Minelli R, Vergara E, Brunetti A, et al. Color doppler and microvascular flow imaging to evaluate the degree of inflammation in a case of hidradenitis suppurativa. J Vasc Ultrasound. 2022 doi: 10.1177/54431672110664. [DOI] [Google Scholar]
- 10.Tufano A, Minelli R, Rossi E, Brillantino C, Di Serafino M, Zeccolini M, et al. Inferior epigastric artery pseudoaneurysm secondary to port placement during a robot-assisted laparoscopic radical cystectomy. J Ultrasound. 2021;24:535–538. doi: 10.1007/s40477-020-00442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tufano A, Flammia RS, Antonelli L, Minelli R, Franco G, Leonardo C, et al. The value of contrast-enhanced ultrasound (CEUS) in differentiating testicular masses: a systematic review and meta-analysis. Appl Sci. 2021;11:8990. doi: 10.3390/app11198990. [DOI] [Google Scholar]
- 12.Santarsiere M, Rumolo M, Menna BF, Vergara E, Minelli R, Brillantino C, et al. A rare case of bilateral testicular metastasis from ileocecal NET: multiparametric US detection. J Ultrasound. 2022 doi: 10.1007/s40477-022-00657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slapa RZ, Jakubowski WS, Slowinska-Srzednicka J, Szopinski KT. Advantages and disadvantages of 3D ultrasound of thyroid nodules including thin slice volume rendering. Thyroid Res. 2011;4:1. doi: 10.1186/1756-6614-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cansu A, Ayan E, Kul S, Eyuboglu I, Oguz S, Mungan S. Diagnostic value of 3D power Doppler ultrasound in the characterization of thyroid nodules. Turk J Med Sci. 2019;49:723–729. doi: 10.3906/sag-1803-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitale V, Rossi E, Di Serafino M, Minelli R, Acampora C, Iacobellis F, et al. Pediatric encephalic ultrasonography: the essentials. J Ultrasound. 2020;23:127–137. doi: 10.1007/s40477-018-0349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minella R, Minelli R, Rossi E, Cremone G, Tozzi A. Gastroesophageal and gastric ultrasound in children: the state of the art. J Ultrasound. 2021;24:11–14. doi: 10.1007/s40477-020-00471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HJ, Yoon DY, Seo YL, Kim JH, Baek S, Lim KJ, et al. Intraobserver and interobserver variability in ultrasound measurements of thyroid nodules. J Ultrasound Med. 2018;37:173–178. doi: 10.1002/jum.14316. [DOI] [PubMed] [Google Scholar]
- 18.Padilla F, Roubidoux MA, Paramagul C, Sinha SP, Goodsitt MM, Le Carpentier GL, et al. Breast mass characterization using 3-dimensional automated ultrasound as an adjunct to digital breast tomosynthesis: a pilot study. J Ultrasound Med. 2013;32:93–104. doi: 10.7863/jum.2013.32.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giubilei G, Ponchietti R, Biscioni S, Fanfani A, Ciatto S, de Loro F, et al. Accuracy of prostate volume measurements using transrectal multiplanar three-dimensional sonography. Int J Urol. 2005;12:936–938. doi: 10.1111/j.1442-2042.2005.01182.x. [DOI] [PubMed] [Google Scholar]
- 20.Downey DB, Fenster A, Williams JC. Clinical utility of three-dimensional US. Radiographics. 2000;20:559–571. doi: 10.1148/radiographics.20.2.g00mc19559. [DOI] [PubMed] [Google Scholar]
- 21.Botta F, Raimondi S, Rinaldi L, Bellerba F, Corso F, Bagnardi V, et al. Association of a CT-based clinical and radiomics score of Non-Small Cell Lung Cancer (NSCLC) with lymph node status and overall survival. Cancers (Basel). 2020;12(6):1432. doi: 10.3390/cancers12061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freesmeyer M, Knichel L, Kuehnel C, Winkens T. Stitching of sensor-navigated 3D ultrasound datasets for the determination of large thyroid volumes—a phantom study. Med Ultrason. 2018;20:480–486. doi: 10.11152/mu-1687. [DOI] [PubMed] [Google Scholar]
- 23.Seifert P, Winkens T, Knichel L, Kuhnel C, Freesmeyer M. Stitching of 3D ultrasound datasets for the determination of large thyroid volumes—phantom study part II: mechanically-swept probes. Med Ultrason. 2019;21:389–398. doi: 10.11152/mu-2006. [DOI] [PubMed] [Google Scholar]
- 24.Molinari F, Mantovani A, Deandrea M, Limone P, Garberoglio R, Suri JS. Characterization of single thyroid nodules by contrast-enhanced 3-D ultrasound. Ultrasound Med Biol. 2010;36:1616–1625. doi: 10.1016/j.ultrasmedbio.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 26.tUS P. PIUR tUS—Tomographic 3D ultrasound for safe and more cost effective vascular diagnostics and treatment planning. 2019.
- 27.Tessler FN, Middleton WD, Grant EG. Thyroid Imaging Reporting and Data System (TI-RADS): a user's guide. Radiology. 2018;287:29–36. doi: 10.1148/radiol.2017171240. [DOI] [PubMed] [Google Scholar]
- 28.Ajmal S, Rapoport S, Ramirez Batlle H, Mazzaglia PJ. The natural history of the benign thyroid nodule: what is the appropriate follow-up strategy? J Am Coll Surg. 2015;220:987–992. doi: 10.1016/j.jamcollsurg.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Berghout A, Wiersinga WM, Smits NJ, Touber JL. Determinants of thyroid volume as measured by ultrasonography in healthy adults in a non-iodine deficient area. Clin Endocrinol (Oxf) 1987;26:273–280. doi: 10.1111/j.1365-2265.1987.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 30.Maravall FJ, Gomez-Arnaiz N, Guma A, Abos R, Soler J, Gomez JM. Reference values of thyroid volume in a healthy, non-iodine-deficient Spanish population. Horm Metab Res. 2004;36:645–649. doi: 10.1055/s-2004-825901. [DOI] [PubMed] [Google Scholar]
- 31.Brunn J, Block U, Ruf G, Bos I, Kunze WP. Scriba PC [Volumetric analysis of thyroid lobes by real-time ultrasound (author's transl)] Dtsch Med Wochenschr. 1981;106:1338–1340. doi: 10.1055/s-2008-1070506. [DOI] [PubMed] [Google Scholar]