Abstract
Amyotrophic lateral sclerosis (ALS) is a chronic, progressive neurodegenerative disease characterized by the loss of motor neurons. Dysregulated peripheral immunity has been identified as a hallmark of ALS. Neutrophils, as the front-line responders of innate immunity, contribute to host defense through pathogen clearance. However, they can concurrently play a detrimental role in chronic inflammation. With the unveiling of novel functions of neutrophils in neurodegenerative diseases, it becomes essential to review our current understanding of neutrophils and to recognize the gap in our knowledge about their role in ALS. Thus, a detailed comprehension of the biological processes underlying neutrophil-induced pathogenesis in ALS may assist in identifying potential cell-based therapeutic strategies to delay disease progression.
Keywords: neutrophils, innate immunity, degranulation, MPO, ALS
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by progressive degeneration of upper and lower motor neurons (1). Clinical phenotypes of ALS exhibit heterogeneity, including muscle atrophy, weakness, spasticity and pyramidal syndrome, progressive dysphasia, and dysarthria. The average incidence of ALS is estimated to be 1.5 to 2 per 100,000 personyears, with increased incidence observed worldwide over the past decade (2, 3). Several pathological events, including excitotoxicity resulting from excessive glutamate levels, oxidative stress damage, protein misfolding, disruption of the blood-brain barrier (BBB), and reduced energy metabolism, have been hypothesized to contribute to the neurodegenerative process in ALS. TDP-43 is an RNA-binding protein mainly found in the nucleus and plays an important role in RNA cleavage, transport, translation, and stability (4, 5). 95% of ALS patients exhibit abnormal localization of TDP43 in the cytoplasm, which leads to serious consequences such as miscleavage of RNA in the cytoplasm, decreased translation efficiency, and loss of stability, which may be the main cause in ALS (with the exception of SOD1 mutations) (4, 5). However, the exact pathogenesis of ALS remains unclear to date.
Compelling evidence has revealed dysregulated peripheral immunity as a pathological factor of ALS. Whereas the central nervous system (CNS) has traditionally been considered an immune-privileged tissue due to the selective permeability of the blood-brain barrier (BBB), however, a growing number of studies have revealed functional crosstalk between peripheral immune cells and the CNS (6). The peripheral immune system comprises the innate immune system, with neutrophils as its core components, and the adaptive immune system, with lymphocytes as its core components. Different subpopulations of immune cells display different functions in the progression of ALS. For instance, Tregs have shown a protective role (7), while inflammatory monocytes and CD8+ T cells may be destructive (8–10). As the key component of innate immunity, neutrophils act as front-line defenders against pathogens by being stimulated and recruited to affected sites through chemotaxis and engaging in degranulation to eliminate pathogens. However, excessive neutrophil activity can result in significant collateral tissue damage. In ALS, researchers mainly focus on monocytes or T cells previously, while potential effects and mechanisms of neutrophils in the pathogenesis of ALS remain neglected. Modulation of peripheral immune cells has been shown to delay the progression of ALS in mouse models (11, 12), offering a feasible and less invasive intervention strategy than modulation of immune cells in CNS and making peripheral immune cells an attractive therapeutic target in ALS treatment (13–15). For example, pharmacological treatment with masitinib delayed peripheral motor pathway degeneration (axonal pathology, secondary demyelination, and the loss of type 2B myofibers) by preventing mast cell infiltration (11). Garofalo et al. found antibody for the α4 integrin, Natalizumab, prolonged the survival time of ALS mice by blocking the extravasation of immune cells in the central nervous system (12). Thereby, searching for precise immunotherapies targeting specific subsets of peripheral immune cells holds great promise in ameliorating immune-mediated pathological deterioration in ALS, and has great benefits in treating ALS. This review aims to summarize the role of neutrophils in ALS in terms of their origin, activation, and functions in ALS, with a specific emphasis on the impacts of the neutrophil granules on ALS, shedding light on the overlooked contributions of neutrophils in ALS and providing a theoretical basis for potential precise immunotherapy.
2. A clinical perspective on the linkage between neutrophil counts and ALS
In recent years, the potential link between circulating neutrophil counts and ALS disease progression has been proposed ( Table 1 ). In 2017, Murdock et al. investigated the longitudinal association between changes in peripheral immune markers and ALS disease progression. They found that an early elevation of neutrophils was associated with accelerated disease progression reflected by the change in the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) (17). However, Cui et al. revealed a different conclusion. They found that neutrophil counts increased gradually over time following ALS diagnosis and were negatively correlated with ALSFRS-R score but didn’t find an association between neutrophil changes and ALS progression (9). In 2020, increased expression of CD16 on the surface of neutrophils was observed, which is considered a marker of oxidative stress and phagocytic activity of neutrophils (25) that is associated with ALS disease progression, indicating a highly-activated state of neutrophils in rapidly progressing ALS (20). In 2021, Murdock et al. conducted a prospective cohort study to explore the association between baseline neutrophil counts and ALS survival. The results showed that higher baseline neutrophil counts are associated with reduced survival in ALS. Furthermore, the effect appeared to be sex-dependent, with a more pronounced hazard ratio in females (19), suggesting a potential role of sex hormones in neutrophil activity. However, evidence from mendelian randomization analysis showed that a genetically determined increased neutrophil count was associated with a reduced risk of ALS (95% CI: 0.858-1.000, P: 0.049) (18). The controversial conclusions between mendelian randomization and observational studies may be attributed to two factors: firstly, the effect of neutrophils on ALS in mendelian randomization is weak and could be influenced by confounding biases; secondly, previous observational studies mainly focused on the link between neutrophils and ALS after diagnosis, while mendelian randomization analysis revealed the causal relationships between neutrophils and ALS incidence. These inconsistent findings also suggest that neutrophils may play both detrimental and protective roles for neurons at different stages of diseases. To date, no positive immunosuppressive drugs for ALS patients have been identified in clinical trials (26). More attention should be paid to applying precise immunotherapy in different disease stages and specific subpopulations.
Table 1.
Previous clinical studies on neutrophil counts and ALS disease progression.
| Indicator | Duration | Participant | Outcomes | Conclusions | Reference |
|---|---|---|---|---|---|
| Neutrophils | 2011.3-2014.5 | 44 control patients and 90 patients with ALS. | ALSFRS-R | ALS patients exhibited a significant increase in the percentage of neutrophils compared to controls. However, the increase was not correlated with the ALSFRS-R score. NMR was significantly increased in patients with ALS and was correlated with disease progression. |
(16) |
| 2014.6-2016.5 | 35 controls and 119 participants with ALS | change in ALSFRS-R score | Participants with ALS had increased neutrophil counts compared to healthy controls. Early changes in neutrophil counts had a significant correlation with the changes in the ALSFRS-R. |
(17) | |
| – | Mendelian randomization | – | Increased neutrophil count was suggestive association with reduced ALS risk. | (18) | |
| 2011.6-2019.10 | 269 patients with ALS | mortality rate | Participants with higher early neutrophil counts had a higher mortality rate compared to those with a lower neutrophil count. This effect was more pronounced in females. ALS participants had increased neutrophil presence in cervical and thoracic spinal cord segments compared with control participants. |
(19) | |
| 2017.3-2018.8 | 23 healthy controls and 48 patients with ALS | ALSFRS-R, bulbar subscore of the ALSFRS-R, change in ALSFRS-R, respiratory function | CD16 expression on neutrophils was associated with greater disease severity and faster rate of disease progression in patients with ALS | (20) | |
| 2015-2020 | 288 ALS patients | Primary outcome: risk of death after diagnosis of ALS Secondary outcomes: functional status and disease progression rate. |
Neutrophils increased over time since diagnosis and were negatively correlated with ALSFRS-R score but not associated with risk of death or the disease progression rate. | (9) | |
| NLR | – | 80 patients with ALS and 80 matched controls | ALSFRS-R | NLR was significantly elevated in patients with ALS compared with controls. | (21) |
| 2012.1-2017.8 | 194 patients with ALS | ALSFRS-R; survival time | A high baseline NLR was associated with a shorter survival period in patients with ALS. | (22) | |
| 2012.1-2018.12 | 1030 patients with ALS | ALSFRS-R | Higher NLR in patients with sporadic ALS was associated with faster disease progression rates and shorter survival period. | (23) | |
| 2016.3-2020.1 | 146 patient with ALS | The rate of disease progression (ΔFS score) | NLR positively correlated with ΔFS values. The ΔFS score progressively increased from the lowest to the highest NLR tertile. The mortality rate of patients with a higher NLR value was twice that of the patients with a lower value. |
(24) |
The neutrophil-derived metric, neutrophil to lymphocyte ratio (NLR), which reflects the balance of peripheral innate and adaptive immunity, has also been indicated as a promising biomarker for ALS disease progression and prognosis. As early as 2009, low-grade systemic inflammation, characterized by an elevated wide-range C-reactive protein (wrCRP), fibrinogen, erythrocyte sedimentation rate (ESR), and NLR, has been proposed as a hallmark of ALS. This correlation was consistently observed in repeated blood draws over time (21). In 2020, research conducted in South Korea divided ALS patients based on their baseline NLR into three groups and revealed that the patients with a high NLR had faster disease progression and shorter survival (22). These findings were subsequently confirmed by a single-center cohort from China and a multicenter cross-sectional cohort study from Italy (23, 24), suggesting that the balance between innate and adaptive immunity plays an important role in ALS progression.
3. The production and release of neutrophils
Neutrophils constitute 50-70% of total leukocytes, making them the most abundant leukocytes in circulation (27). They are polymorphonuclear leukocytes originating from myeloid precursors in the bone marrow (BM), characterized by lobulated or rod-shaped nuclei and large cytoplasm of neutral granules (28). These granules primarily consist of lysosomes containing rich enzymes, such as myeloperoxidase (MPO) and neutrophil elastase (NE), which play crucial roles in the phagocytic functions of neutrophils.
Under physiological conditions, neutrophils are generated and differentiated from stem cells to neutrophil precursors in the BM. Upon stimulation, neutrophil precursors can be mobilized from BM into the circulation (29), which is regulated by the interplay between the C-X-C chemokine receptors (CXCRs) family and C-X-C chemokine ligands (CXCL) family (29).
Neutrophils remain in the BM when their surface receptor CXCR4 binds to CXCL12, which is produced by hematopoietic stem cells and BM stromal cells (BMSC). Granulocyte colony-stimulating factor (G-CSF) serves as a major regulator of neutrophil homeostasis (30, 31). When external stimulation occurs, G-CSF can shift CXCR4 on neutrophils to CXCR2, thereby inducing neutrophil mobilization and release.
As the principal regulator of neutrophil biology, G-CSF, a 19.6kd hematopoietic cytokine, has garnered significant attention in the field of ALS. However, clinical and pre-clinical studies yielded inconsistent conclusions regarding the effect of neutrophils increment. Clinical observations have suggested that higher neutrophil level is associated with accelerated ALS disease progression and higher mortality rate (9, 17, 19, 22). Assuming that G-CSF-induced neutrophilia is detrimental to ALS patients, increased expression of G-CSF would be expected to accelerate the disease progression. However, the results of pre-clinical studies do not support the assumption. Animal studies have demonstrated that G-CSF has neuroprotective effects by suppressing endoplasmic reticulum stress and inhibiting pro-apoptotic proteins (32–34). In SOD1G93A transgenic mice, continuous subcutaneous delivery or CNS-targeted overexpression of G-CSF can inhibit the apoptosis of motor neurons (35). The inconsistency may arise from two aspects. First, the effects of G-CSF were complex, with dual roles in both the hematopoietic and nervous systems. Systemic delivery of G-CSF can stimulate the proliferation and differentiation of white blood cells, potentially playing a detrimental role in ALS progression. However, G-CSF receptors are highly expressed in motor neurons (35, 36), and their effect on motor neurons is primarily responsible for their neuroprotective role. As a result, researchers are exploring methods to enhance the central neuroprotective effects of G-CSF while minimizing its peripheral effects. In 2011, Henriques et al. found that intraspinal injection of G-CSF AAV significantly delayed the onset of hindlimb paralysis and prolonged 10% survival in SOD1G93A mice (37), which is improved compared with systemic injections. Second, G-CSF mobilizes neutrophil production but also modifies their activation. It is possible that the action of G-CSF is due to enhancing beneficial/pro-resolution processes in neutrophils (29). Neutrophil infiltration without degranulation may stimulate efferocytosis and repair processes (29).
Although G-CSF treatment has shown potential neurotrophic effects in both pre-clinical and clinical studies of ALS, it is important to consider its significant systemic effects, particularly its impact on neutrophilia. Researchers are currently exploring strategies to mitigate potential peripheral effects of G-CSF by administering it intrathecally in ALS, however, the harmful effects due to its invasive nature should not be ignored.
4. Potential functions of neutrophils in ALS
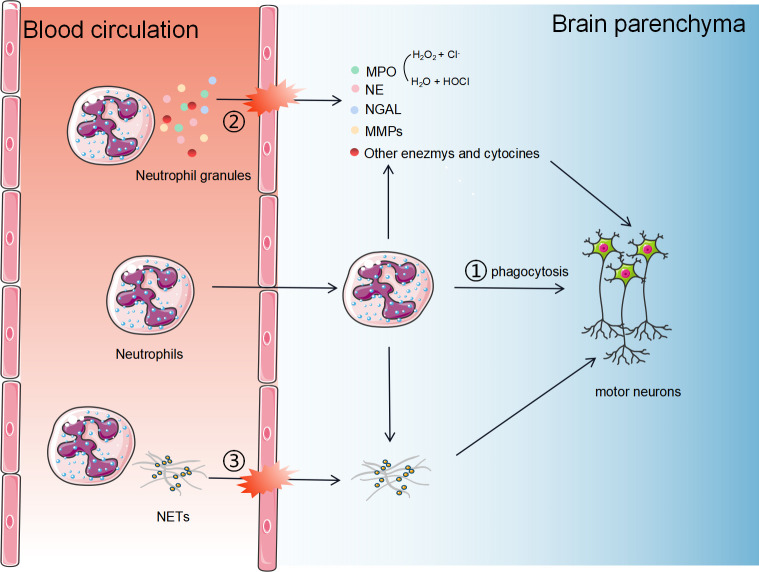
As key effector of innate immunity, neutrophils trigger host tissue damages through three major pathways ( Figure 1 ): (1) Phagocytosis: neutrophils can directly phagocytose foreign pathogens and self-components; (2) Degranulation: neutrophils secrete cytotoxic particles during maturation affecting the immune response. The main components of these particles are enzymes, such as MPO, NE, lactoferrin, and metalloproteinases (MMPs) (38); (3) Activated neutrophils can form neutrophil extracellular traps (NETs), which are composed of chromatin components, MPO and NE attached DNA fibers. (4) Neutrophils may crosstalk with other cells, such as microglia and astrocytes. The exact roles of neutrophils playing in ALS pathology and the underlying mechanisms are still unclear. In this section, we describe the three major roles of neutrophils in ALS and propose potential mechanisms by which they contribute to the disease.
Figure 1.
The potential mechanisms of neutrophils in the progression of ALS. Neutrophils contribute to host tissue damage through three major strategies: 1) Phagocytosis. Neutrophils can cross the BBB and directly phagocytose self-components; 2) Degranulation. Neutrophils secrete cytotoxic particles during maturation affecting the immune response. The main components of these particles are enzymes, such as MPO, NE, NGAL, and MMPs; (3) Activated neutrophils can form neutrophil extracellular traps, consisting of chromatin components, MPO and NE attached DNA fibers.
4.1. Phagocytosis
Whether neutrophils can directly phagocytose healthy motor neurons is not yet clear, while invading to the brain parenchyma is the prerequisite for their phagocytic function. Although it was believed that the BBB provides a privileged immune environment for the brain by blocking peripheral immune infiltration, the current understanding is that the immune privilege in neurodegenerative diseases is relative. Disruption of BBB contributes to the infiltration of peripheral immune cells (39). In disease models of multiple sclerosis (MS) and Alzheimer’s disease (AD), neutrophils have been confirmed to be infiltrated into the CNS (40, 41). Adhesion receptors on endothelial cells, known as integrins, undergo an activation process in response to various stimuli and recruit and promote the attachment of neutrophils to the inflamed endothelium (42). Concurrently, the glycocalyx, a proteoglycan structure, prevents the interaction of surface molecules (43). Once neutrophils adhere to the vasculature, the interaction of endothelial membrane protrusions, containing multiple adhesion molecules, facilitates the migration of immune cells into the brain parenchyma (44). In 2002, Scali et al. reported that peripheral blood neutrophil integrin CD11b was upregulated in AD patients (45). Increased expression of CD11b, which mediates neutrophil adhesion and migration, was positively correlated with AD severity (45). In 2004, Baik et al. showed dynamic imaging of neutrophils entering into brain parenchyma in AD mice, but no such migration was found in wild-type mice (46). In 2015, Zenaro et al. demonstrated that the binding of LFA-1, an integrin expressed on neutrophils, to adhesion molecules expressed on endothelial cells mediated the infiltration of neutrophils into the brain parenchyma (47). Depletion of neutrophils or LFA-1 improved cognitive function and reduced microglia proliferation in AD mice (47). However, whether limiting neutrophil migration and infiltration can delay neurodegeneration in ALS is still unknown.
Figueroa-Romero et al. and Trias et al. showed increasing infiltration of neutrophils with time in ALS mouse models in the spinal cord or the peripheral motor pathway (11, 48). However, previous studies have only demonstrated that neutrophils can be accumulated in the spinal cords of late-stage ALS mice (48) and ALS autopsy (19). It remains unclear whether the accumulation of neutrophils worsens neuronal degeneration or it is a byproduct of neuronal degeneration. There are no studies showing the access of neutrophils into the CNS in ALS mice or patients at the early stage of the disease. Meanwhile, neutrophils are smaller than motor neurons, with a diameter 10 ~12μm. We thus proposed that the neutrophils don’t play a direct phagocytosis role in motor neuron degeneration.
4.2. Degranulation of neutrophils
The function of neutrophils relies heavily on the release of cytoplasmic granules. Neutrophils mainly contain three types of cytoplasmic granules. Primary granules, also known as azurophilic granules, are the largest and earliest-formed granules containing proteolytic and bactericidal proteins, including MPO, NE, and arginase-1 (ARG1) (49). Secondary and tertiary granules, also known as specific and gelatinase granules, are enriched with enzymes involved in extracellular matrix (ECM) degradation and regulation, such as the lactoferrin, neutrophil gelatinase-associated lipid transport protein (NGAL) and MMPs (38).
The mobilization of neutrophils depends on the fusion of cytoplasmic granules with the plasma membrane (exocytosis) and endocytose vacuoles (endocytosis). Neutrophil degranulation plays an important role in chronic inflammation (50, 51), which is potentially linked to ALS disease progression.
4.2.1. MPO
MPO is primarily contained in the azurophilic granules of myeloid cells (mainly neutrophils and monocytes), which are among the last to exocytose (52), serving as a specific marker for myeloid cells. MPO can be rapidly released from neutrophils into the circulation in response to the inflammation occurrence. In vivo, MPO converts H2O2 and Cl- to H2O and hypochlorous acid (HOCl) (53). The MPO-HOCl system plays a dual role, serving as a defense mechanism against invading pathogens while also potentially causing tissue damage. MPO has attracted considerable attention from researchers in neurodegenerative diseases, including AD, Parkinson’s disease (PD), and multiple system atrophy (MSA) (54, 55). MPO can be detected in both peripheral blood circulation and CSF. In patients with AD and PD, serum MPO is found to be higher than in healthy controls, making it a possible diagnosis biomarker and indicator of therapeutic effects (56, 57). The elevated expression of serum MPO indicates the activation of circulating neutrophils, suggesting the participation of innate immunity in neurodegeneration. In the CNS, highly expressed MPO were observed in both CSF and postmortem brain tissues from patients with AD and PD 48,49, while the origin of central MPO is still debated: 1) Studies have suggested that microglia, astrocytes, and neurons in the CNS can release MPO (58, 59), therefore high expression of MPO in the CNS may not solely be attributed to neutrophils; 2)Smyth et al. found that, in brain tissues of AD mice, MPO is highly expressed and originated from neutrophils that infiltrated into the CNS (60). Another research demonstrated that neutrophil-specific MPO-deficient AD mice perform better in spatial learning and memory than controls (61); 3) Another possible mechanism is that the disruption of BBB allows peripheral MPO to invade the brain parenchyma.
To our knowledge, MPO has not been detected in both plasma and CSF in patients with ALS. However, HOCl was elevated in ALS patients, suggesting a potential pathogenic role of MPO in ALS. In SOD1G93A mice, activated MPO/HOCl has been found in motor neurons, and the systemic inhibition of MPO has been shown to inhibit motor neuron apoptosis and ferroptosis and improve motor functions in these mice (62). However, the specific role of neutrophil-derived MPO in ALS is still unclear.
MPO can mediate neurodegeneration through multiple mechanisms: 1) Oxidative stress. HOCl, as the downstream product of MPO, has potent oxidative activity and can induce serious oxidative damage to motor neurons; 2) Disruption of the BBB. MPO-derived oxidants could induce BBB dysfunction in vitro and in vivo ( 63). Other oxidant species produced by MPO, HOSCN, have been shown to reduce the barrier function of cerebral endothelial cells in vitro ( 64). Disruption of the BBB is an early event in ALS, while BBB hyperpermeability is involved in the late stage of ALS, which has been linked to motor neuron degeneration (65); 3) Induction of inflammatory cytokines release. In addition to its oxidative activity, HOCl can diffuse through the cell membrane and modify proteins (66) and regulate cellular apoptosis (67); 4) Disruption of energy metabolism. MPO/HOCl inhibits intracellular NAD levels, thereby reducing mitochondrial respiration as well as the production of ATP, NAD, and glutathione (68). High concentrations of HOCl can also directly interact with ATP and disrupt energy metabolism (69). 5) Axonal degeneration. HOSCN can act as a switch to trigger necroptosis (70), which also contributes to axonal degeneration in ALS (71).
Due to strong oxidative capacity and cytotoxicity, MPO has emerged as a promising strategy for the treatment of neurodegenerative diseases. Verdiperstat is an MPO inhibitor that has been granted orphan drugs and fast-track designations for the treatment of MSA by the Food and Drug Administration (FDA) and European Medicines Agency. In addition, FDA has approved Verdiperstat in the clinical trials in the HEALEY ALS platform, providing a novel therapeutic option for ALS patients.
4.2.2. NE
NE is a serine protease mainly released from distributed in the azurophilic granules of neutrophils (72), and plays roles as substrates of ECM, ezymogens, adhesion molecules, signaling receptors, cytokines, and so on (52). It removes invading pathogens and inhibits inflammatory responses caused by bacterial infections. At the same time, persistent secretion of NE may lead to tissue damage. In the extracellular environment, NE is capable of cleaving chemokines and cytokines, leading to their activation or inactivation. NE has also been demonstrated to efficiently cleave Aβ1-42 and is closely associated with the inhibition of Aβ1-42 aggregation (73). However, the correlation between NE and ALS remains unclear.
4.2.3. Neutrophil gelatinase-associated lipid transport protein
NGAL, also known as Lipocalin 2 (LCN2), is a 25kd glycoprotein identified as an acute phase protein stored and secreted by neutrophils. Given its stability and the nature of easy detection in the CSF, plasma, and urine, LCN2 is recognized as a suitable diagnostic and prognostic biomarker for neurological disorders such as AD and MS (74–76). Studies have found a high expression of LCN2 in plasma and postmortem brain tissues of ALS patients (77, 78). Additionally, the analysis of ALS patient data from the ALS Knowledge Portal (ALS KP) and Project MinE has led to the identification of 13 genetic variants of LCN2, thereby supporting the potential contribution of LCN2 variants to the pathology of ALS.
Although it’s believed that plasma LCN2 is mainly derived from neutrophils (79), it can also be expressed in other organs and cells, such as kidneys, endothelial cells, liver, smooth muscle cells, and various immune cells (80). In the CNS, LCN2 is mainly expressed in neurons and glial cells, and its expression increases in response to injury or inflammation (81, 82). Recent in vitro and in vivo studies have reported that LCN2 induces neurodegeneration via several pathways: 1) LCN2 is neurotoxic in ALS. In rodent models of ALS, TDP-43 mutation leads to the release of LCN2 from activated astrocytes. Reduction of LCN2 in astrocytes reduced neuronal death by regulating apoptosis and iron homeostasis (83, 84); 2) Promotion of inflammation. The concentration of LCN2 is correlated with a significant increase in pro-inflammatory cytokines and chemokines both in vivo or in vitro in a dose-dependent manner (83, 85, 86); 3) Promotion of pro-inflammatory microglial polarization (87, 88).
4.2.4. Matrix metalloproteinases
MMPs represent zinc-dependent proteases mainly produced by neutrophils (89), characterized by digesting components of ECM. MMPs are classified into five subgroups based on their localization and substrate specificity: collagenases, gelatinases, matrix lysins, membrane-type matrix metalloproteinases, and enamel lysins (90). MMP-9, primarily released by neutrophils and macrophages, is among the most extensively studied MMPs in ALS research (91, 92). When activated, MMP-9 hydrolyzes a wide variety of substrates, including ECM proteins (collagen, fibronectin, laminin, thrombospondin, and tendon in C), non-ECM substrates (TNFα, IL-1β, TGFβ, and CXC motif ligands), and neo-substrates (CD36 and citrate synthase).
In MMP-9 polymorphism, the C (-1562) T variant with higher promoter activity in the T allele compared to the C allele has been demonstrated to increase the risk of developing sALS nearly 2.2-fold in the Chinese population (93), suggesting the pathogenic effects of MMP-9 in ALS.
Beuche et al. reported that the levels of MMP-9 were persistently increased in the serum of ALS patients compared to healthy controls. Subsequently, Demestre et al. released that MMP-9 activation in ALS serum occurs prior to the onset of muscular atrophy or peripheral nerve degeneration, suggesting that MMP-9 activation is not merely a byproduct of nerve injury (94). However, the MMP-9 levels in the CSF of ALS patients have been subject to controversy. Two clinical cohorts from Poland showed significantly decreased MMP-9 levels in the CSF of ALS patients (95, 96), while another study reported an elevated level of MMP-9 (97)
MMP-9 appears to play dual roles in ALS. Dewil et al. found that the deletion of MMP-9 accelerated ALS progression and significantly reduced the survival of SOD1G93A mice, suggesting a protective role of MMP-9 in ALS (98). However, subsequent research by Lorenzl et al. reported that MMP inhibitor prolonged the SOD1G93A mice survival compared to control (99), suggesting a detrimental effect of MMP-9 on ALS. Mechanically, MMP-9 may play a neuroprotective role in ALS by promoting injured neuron regeneration and elongation via its interaction with the Schwann cell basal lamina, which is essential for creating a passage for sprouting axons. While several studies also proposed the possibility that MMP-9 might aggravate the progression of ALS: 1) Interruption of the BBB integrity. Neutrophil-derived MMP-9 is implicated in exacerbating BBB leakage, inflammatory cytokine infiltration, and brain injury (100); 2) Induction of axonal dieback in fast motor neurons. MMP-9 can break the ECM and disrupt the NMJ structure. Downregulation of MMP-9 in lumbar spinal neurons has been shown to delay axonal dieback and ameliorates motor neuron degeneration in ALS mice (101); 3) Amplification of the inflammatory response. MMP-9 may promote the cleavage of TNF-α and pro-inflammatory cytokines, leading to motor neuron apoptosis (102).
Interestingly, selective neuron death seems to be associated with the MMP-9 gene in both ALS mice models and patients. Kaplan et al. compared the expression profiles of distinct subpopulations of motor neurons using the microarray and found that the expression of MMP-9 in fast vulnerable motor neurons is significantly higher than that in the slow motor neurons. Moreover, MMP-9 was reported to be highly expressed in SOD1G93A mice before disease onset, while no MMP-9-positive motor neurons were detected at the end-stage of SOD1G93A mice, suggesting that MMP-9 is a major pathological factor in ALS progression. In addition, significantly delayed muscle denervation and motor function loss were recorded in genetic ablation of MMP-9 in SOD1G93A mice, and these mice demonstrated increased survival times compared to the control (103), further implying a substantial role of MMP-9 in ALS progression.
4.3. NETs
Highly-active neutrophils can release NETs, which are extracellular, web-like chromatin structures attached with cytosolic granule enzymes and histones on a scaffold of decondensed chromatin (104). The process of NET formation, known as NETosis, is functionally distinct from apoptosis and necrosis. NETs trap, neutralize and eliminate invading pathogens, including bacteria, viruses, and parasites. However, overactivated NETs contribute to the pathogenesis of neurodegenerative diseases.
Recently, NETs have been observed within the cerebral vasculature and parenchyma of AD models and AD patients, suggesting that NETs can potentially harm the BBB integrity and neural cells (47).
Circulating NETs may be a key factor in BBB collapse. During inflammation, Mac-1 and LFA-1, two forms of integrins, mediate the adhesion of neutrophils to vascular ICAM-1 (105, 106). Recent studies have shown that neutrophil adhesion, via Mac-1 or LFA-1, without transmigration, is sufficient to trigger the NET formation and subsequent BBB breakdown (107–109). Zenaro et al. proposed the formation of intravascular NETs as a mechanism of neutrophil-dependent BBB breakdown using two AD models (47). In addition to AD, NETs have also been proposed to contribute to BBB breakdown in stroke models and cerebral malaria (110, 111). Mechanically, intravascular NETs have a direct toxic effect on endothelial cells by releasing proteins such as NE and MPO. NETosis, a process of neutrophil death, further facilitates the release of enzymes mentioned above. NE increases endothelial cell permeability, while MPO and histones induce endothelial cell death (112) and endothelial barrier dysfunction (113). Several lines of evidence indicate that neutrophils can promote vascular damage and BBB breakdown (113).
NETs have also been found in brain parenchyma, which may play a deleterious role in neurons in neuronal diseases (114). NET components can enzymolyze extracellular matrix proteins and activate inflammasome pathways and mitochondrial apoptosis pathways (115, 116). Therefore, the involvement of NETs has been proposed as a novel mechanism for neutrophil-mediated neurotoxicity and neurodegeneration.
Neutrophils in the neuromuscular junction (NMJ) of ALS mice and patients have been found to form NETs, as demonstrated by extracellular web-like fibers of DNA fibers attached with MPO and NE, implying a high cytotoxic potential towards surrounding tissues (11). In the CNS, NETs have been found in the cerebral cortex in AD mice due to the disruption of BBB. However, as of now, no studies have demonstrated the formation of NETs around motor neurons in ALS.
4.4. Crosstalking with other cells
In addition to the direct phagocytosis role, neutrophils can interact with multiple cell types in the brain to exert toxic or beneficial effects.
Microglia are the innate immune cells of the brain. Neutrophil-microgial interactions affect microglia reactivity. Neutrophils release inflammatory factors to activate microglia, such as ROS, LCN2, and MMP9 (87, 117). Moxon et al. showed that a decrease in neutrophils reduces the number of microglia and decreases the activation marker CD68 on microglia after cerebral hemorrhage (118). The main component of NETs, LL37, an agonist of the P2X7 receptor, is highly expressed on the cell membrane of microglia which can promote the activation and proliferation of microglia in TBI, ischemic brain injury, and Alzheimer’s disease (119). At the same time, activated microglia produce large amounts of inflammatory cytokines and chemokines that reciprocally promote the recruitment of peripheral neutrophils to the central nervous system (120). In stroke models, reactive microglia engulfed the infiltrated neutrophils around ischemic core (121). Dedepletion of microglia by CSF1R inhibitors increased the number of neutrophils and aggravated ischemic injury (121).
During the inflammatory response, reactive astrocytes can form perivascular scars, thus limiting the spread of neutrophils from damaged to healthy tissues (122). In vitro, Xie et al. (123) found that astrocytes inhibited neutrophil apoptosis and degranulation and increased neutrophil phagocytosis and pro-inflammatory cytokine expression (123). Neutrophil-astrocyte interactions also affect astrocyte reactivity. Treatment of mice with anti-Ly6G antibody inhibited astrocyte proliferation (124). In another in vitro study, Hooshmand et al. found that neutrophils can induce astrocyte formation by producing C1q and C3a (125).
The above data suggest that neutrophils and astrocytes and microglia are the main sources of cytokines during neuroinflammation and may promote neurodegeneration by interacting with each other to promote the inflammatory cascade response. In the field of ALS, astrocytes and microglia in different stages of the disease play a very different role, in the early stages of the disease the two kinds of cells seem to play protective roles, contribute to the compensatory response of early neuron death, but with the disease progressing, glial cells shit to a neurotoxicity phenotype and cause further deterioration of the disease (126). Whether neutrophils can interact with microglia and astrocytes in ALS and the underlying mechanisms remains largely unknown. From the above studies in other disease models (87, 117), 118, 123), we speculated that neutrophils may aggravate neurodegeneration by activating microglia and astrocytes and translating them into a deleterious phenotype.
5. Concluding remarks and future directions
Research on the role of neutrophils in ALS is still in its early stages, and there are many intriguing areas yet to be explored. Firstly, in other neurodegenerative disease animal models, such as AD and PD, neutrophils have been shown to infiltrate into the brain due to the disruption of BBB (47, 127, 128). Although the presence of neutrophils in the spinal cord and NMJ of SODG93A mice is documented, their ability to cross the BBB at the early stage of ALS remains uninvestigated. Secondly, neutrophil depletion may improve the cognitive function of AD mice (47), while its role in ALS is not yet known. Thirdly, neutrophils exhibit diverse phenotypes, including a subpopulation known as low-density neutrophils (LDN), which has been reported in a variety of disease conditions (129–131). Investigating the potential role of neutrophil subpopulations in ALS is a promising direction. Lastly, ALS is a highly heterogeneous disease, displaying different levels of innate immunity. Establishing an immune stratification based on neutrophils for ALS patients could pave the way for more precise immunotherapy tailored to distinct patient groups. Further research in these areas will advance our understanding of ALS and the contribution of neutrophils to its pathogenesis and progression.
Author contributions
DF and WC contributed to conception and design of the review. WC wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version. All authors contributed to the article and approved the submitted version.
Funding Statement
This research was funded by the National Natural Science Foundation of China (81873784, 82071426).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Feldman EL, Goutman SA, Petri S, Mazzini L, Savelieff MG, Shaw PJ, et al. Amyotrophic lateral sclerosis. Lancet (2022) 400(10360):1363–80. doi: 10.1016/S0140-6736(22)01272-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marin B, Boumédiene F, Logroscino G, Couratier P, Babron MC, Leutenegger AL, et al. Variation in worldwide incidence of amyotrophic lateral sclerosis: a meta-analysis. Int J Epidemiol (2017) 46(1):57–74. doi: 10.1093/ije/dyw061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiò A, Mora G, Moglia C, Manera U, Canosa A, Cammarosano S, et al. Secular trends of amyotrophic lateral sclerosis: the piemonte and valle d'Aosta register. JAMA Neurol (2017) 74(9):1097–104. doi: 10.1001/jamaneurol.2017.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McMillan M, Gomez N, Hsieh C, Bekier M, Li X, Miguez R, et al. RNA methylation influences TDP43 binding and disease pathogenesis in models of amyotrophic lateral sclerosis and frontotemporal dementia. Mol Cell (2023) 83(2):219–36.e7. doi: 10.1016/j.molcel.2022.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suk TR, Rousseaux M. The role of TDP-43 mislocalization in amyotrophic lateral sclerosis. Mol Neurodegener (2020) 15(1):45. doi: 10.1186/s13024-020-00397-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu W, He J, Cai X, Yu Z, Zou Z, Fan D. Neuroimmune crosstalk between the peripheral and the central immune system in amyotrophic lateral sclerosis. Front Aging Neurosci (2022) 14:890958. doi: 10.3389/fnagi.2022.890958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yazdani S, Seitz C, Cui C, Lovik A, Pan L, Piehl F, et al. T cell responses at diagnosis of amyotrophic lateral sclerosis predict disease progression. Nat Commun (2022) 13(1):6733. doi: 10.1038/s41467-022-34526-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Figueroa-Romero C, Monteagudo A, Murdock BJ, Famie JP, Webber-Davis IF, Piecuch CE, et al. Tofacitinib suppresses natural killer cells in vitro and in vivo: implications for amyotrophic lateral sclerosis. Front Immunol (2022) 13:773288. doi: 10.3389/fimmu.2022.773288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui C, Ingre C, Yin L, Li X, Andersson J, Seitz C, et al. Correlation between leukocyte phenotypes and prognosis of amyotrophic lateral sclerosis. Elife (2022) 11. doi: 10.7554/eLife.74065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trolese MC, Scarpa C, Melfi V, Fabbrizio P, Sironi F, Rossi M, et al. Boosting the peripheral immune response in the skeletal muscles improved motor function in ALS transgenic mice. Mol Ther (2022) 30(8):2760–84. doi: 10.1016/j.ymthe.2022.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trias E, King PH, Si Y, Kwon Y, Varela V, Ibarburu S, et al. Mast cells and neutrophils mediate peripheral motor pathway degeneration in ALS. JCI Insight (2018) 3(19). doi: 10.1172/jci.insight.123249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garofalo S, Cocozza G, Bernardini G, Savage J, Raspa M, Aronica E, et al. Blocking immune cell infiltration of the central nervous system to tame Neuroinflammation in Amyotrophic lateral sclerosis. Brain Behav Immun (2022) 105:1–14. doi: 10.1016/j.bbi.2022.06.004 [DOI] [PubMed] [Google Scholar]
- 13. Thonhoff JR, Berry JD, Macklin EA, Beers DR, Mendoza PA, Zhao W, et al. Combined regulatory T-lymphocyte and IL-2 treatment is safe, tolerable, and biologically active for 1 year in persons with amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm (2022) 9(6). doi: 10.1212/NXI.0000000000200019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Polgár TF, Meszlényi V, Nógrádi B, Körmöczy L, Spisák K, Tripolszki K, et al. Passive transfer of blood sera from ALS patients with identified mutations results in elevated motoneuronal calcium level and loss of motor neurons in the spinal cord of mice. Int J Mol Sci (2021) 22(18). doi: 10.3390/ijms22189994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Campisi L, Chizari S, Ho J, Gromova A, Arnold FJ, Mosca L, et al. Clonally expanded CD8 T cells characterize amyotrophic lateral sclerosis-4. Nature (2022) 606(7916):945–52. doi: 10.1038/s41586-022-04844-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murdock BJ, Bender DE, Kashlan SR, Figueroa-Romero C, Backus C, Callaghan BC, et al. Increased ratio of circulating neutrophils to monocytes in amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm (2016) 3(4):e242. doi: 10.1212/NXI.0000000000000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murdock BJ, Zhou T, Kashlan SR, Little RJ, Goutman SA, Feldman EL. Correlation of peripheral immunity with rapid amyotrophic lateral sclerosis progression. JAMA Neurol (2017) 74(12):1446–54. doi: 10.1001/jamaneurol.2017.2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li C, Yang W, Wei Q, Shang H. Causal association of leukocytes count and amyotrophic lateral sclerosis: a mendelian randomization study. Mol Neurobiol (2020) 57(11):4622–7. doi: 10.1007/s12035-020-02053-7 [DOI] [PubMed] [Google Scholar]
- 19. Murdock BJ, Goutman SA, Boss J, Kim S, Feldman EL. Amyotrophic lateral sclerosis survival associates with neutrophils in a sex-specific manner. Neurol Neuroimmunol Neuroinflamm (2021) 8(2). doi: 10.1212/NXI.0000000000000953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McGill RB, Steyn FJ, Ngo ST, Thorpe KA, Heggie S, Ruitenberg MJ, et al. Monocytes and neutrophils are associated with clinical features in amyotrophic lateral sclerosis. Brain Commun (2020) 2(1):fcaa013. doi: 10.1093/braincomms/fcaa013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keizman D, Rogowski O, Berliner S, Ish-Shalom M, Maimon N, Nefussy B, et al. Low-grade systemic inflammation in patients with amyotrophic lateral sclerosis. Acta Neurol Scand (2009) 119(6):383–9. doi: 10.1111/j.1600-0404.2008.01112.x [DOI] [PubMed] [Google Scholar]
- 22. Choi SJ, Hong YH, Kim SM, Shin JY, Suh YJ, Sung JJ. High neutrophil-to-lymphocyte ratio predicts short survival duration in amyotrophic lateral sclerosis. Sci Rep (2020) 10(1):428. doi: 10.1038/s41598-019-57366-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei QQ, Hou YB, Zhang LY, Ou RW, Cao B, Chen YP, et al. Neutrophil-to-lymphocyte ratio in sporadic amyotrophic lateral sclerosis. Neural Regener Res (2022) 17(4):875–80. doi: 10.4103/1673-5374.322476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leone MA, Mandrioli J, Russo S, Cucovici A, Gianferrari G, Lisnic V, et al. Neutrophils-to-lymphocyte ratio is associated with progression and overall survival in amyotrophic lateral sclerosis. Biomedicines (2022) 10(2). doi: 10.3390/biomedicines10020354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chinda D, Nakaji S, Umeda T, Shimoyama T, Kurakake S, Okamura N, et al. A competitive marathon race decreases neutrophil functions in athletes. Luminescence (2003) 18(6):324–9. doi: 10.1002/bio.744 [DOI] [PubMed] [Google Scholar]
- 26. Meininger V, Drory VE, Leigh PN, Ludolph A, Robberecht W, Silani V. Glatiramer acetate has no impact on disease progression in ALS at 40 mg/day: a double- blind, randomized, multicentre, placebo-controlled trial. Amyotroph Lateral Scler (2009) 10(5-6):378–83. doi: 10.3109/17482960902803432 [DOI] [PubMed] [Google Scholar]
- 27. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol (2011) 11(8):519–31. doi: 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- 28. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol (2013) 13(3):159–75. doi: 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- 29. Martin KR, Wong HL, Witko-Sarsat V, Wicks IP. G-CSF - A double edge sword in neutrophil mediated immunity. Semin Immunol (2021) 54:101516. doi: 10.1016/j.smim.2021.101516 [DOI] [PubMed] [Google Scholar]
- 30. Hidalgo A, Chilvers ER, Summers C, Koenderman L. The neutrophil life cycle. Trends Immunol (2019) 40(7):584–97. doi: 10.1016/j.it.2019.04.013 [DOI] [PubMed] [Google Scholar]
- 31. Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol (2010) 31(8):318–24. doi: 10.1016/j.it.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prakash A, Medhi B, Chopra K. Granulocyte colony stimulating factor (GCSF) improves memory and neurobehavior in an amyloid-β induced experimental model of Alzheimer's disease. Pharmacol Biochem Behav (2013) 110:46–57. doi: 10.1016/j.pbb.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 33. Qiu X, Ping S, Kyle M, Chin L, Zhao LR. Long-term beneficial effects of hematopoietic growth factors on brain repair in the chronic phase of severe traumatic brain injury. Exp Neurol (2020) 330:113335. doi: 10.1016/j.expneurol.2020.113335 [DOI] [PubMed] [Google Scholar]
- 34. Dietrich J, Baryawno N, Nayyar N, Valtis YK, Yang B, Ly I, et al. Bone marrow drives central nervous system regeneration after radiation injury. J Clin Invest (2018) 128(1):281–93. doi: 10.1172/JCI90647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pitzer C, Krüger C, Plaas C, Kirsch F, Dittgen T, Müller R, et al. Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis. Brain (2008) 131(Pt 12):3335–47. doi: 10.1093/brain/awn243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest (2005) 115(8):2083–98. doi: 10.1172/JCI23559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Henriques A, Pitzer C, Dittgen T, Klugmann M, Dupuis L, Schneider A. CNS-targeted viral delivery of G-CSF in an animal model for ALS: improved efficacy and preservation of the neuromuscular unit. Mol Ther (2011) 19(2):284–92. doi: 10.1038/mt.2010.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sheshachalam A, Srivastava N, Mitchell T, Lacy P, Eitzen G. Granule protein processing and regulated secretion in neutrophils. Front Immunol (2014) 5:448. doi: 10.3389/fimmu.2014.00448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zang X, Chen S, Zhu J, Ma J, Zhai Y. The emerging role of central and peripheral immune systems in neurodegenerative diseases. Front Aging Neurosci (2022) 14:872134. doi: 10.3389/fnagi.2022.872134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu F, Cao W, Yang Y, Liu A. Extensive infiltration of neutrophils in the acute phase of experimental autoimmune encephalomyelitis in C57BL/6 mice. Histochem Cell Biol (2010) 133(3):313–22. doi: 10.1007/s00418-009-0673-2 [DOI] [PubMed] [Google Scholar]
- 41. Bajramovic JJ, Brok HP, Ouwerling B, Jagessar SA, van Straalen L, Kondova I, et al. Oligodendrocyte-specific protein is encephalitogenic in rhesus macaques and induces specific demyelination of the optic nerve. Eur J Immunol (2008) 38(5):1452–64. doi: 10.1002/eji.200737164 [DOI] [PubMed] [Google Scholar]
- 42. Smyth L, Rustenhoven J, Park TI, Schweder P, Jansson D, Heppner PA, et al. Unique and shared inflammatory profiles of human brain endothelia and pericytes. J Neuroinflamm (2018) 15(1):138. doi: 10.1186/s12974-018-1167-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jansson D, Dieriks VB, Rustenhoven J, Smyth L, Scotter E, Aalderink M, et al. Cardiac glycosides target barrier inflammation of the vasculature, meninges and choroid plexus. Commun Biol (2021) 4(1):260. doi: 10.1038/s42003-021-01787-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arts JJ, Mahlandt EK, Grönloh ML, Schimmel L, Noordstra I, Gordon E, et al. Endothelial junctional membrane protrusions serve as hotspots for neutrophil transmigration. Elife (2021) 10. doi: 10.7554/eLife.66074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scali C, Prosperi C, Bracco L, Piccini C, Baronti R, Ginestroni A, et al. Neutrophils CD11b and fibroblasts PGE(2) are elevated in Alzheimer's disease. Neurobiol Aging (2002) 23(4):523–30. doi: 10.1016/s0197-4580(01)00346-3 [DOI] [PubMed] [Google Scholar]
- 46. Baik SH, Cha MY, Hyun YM, Cho H, Hamza B, Kim DK, et al. Migration of neutrophils targeting amyloid plaques in Alzheimer's disease mouse model. Neurobiol Aging (2014) 35(6):1286–92. doi: 10.1016/j.neurobiolaging.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, et al. Neutrophils promote Alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med (2015) 21(8):880–6. doi: 10.1038/nm.3913 [DOI] [PubMed] [Google Scholar]
- 48. Figueroa-Romero C, Guo K, Murdock BJ, Paez-Colasante X, Bassis CM, Mikhail KA, et al. Temporal evolution of the microbiome, immune system and epigenome with disease progression in ALS mice. Dis Model Mech (2019) 13(2). doi: 10.1242/dmm.041947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol (2012) 30:459–89. doi: 10.1146/annurev-immunol-020711-074942 [DOI] [PubMed] [Google Scholar]
- 50. Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science (2017) 358(6359):111–6. doi: 10.1126/science.aam9690 [DOI] [PubMed] [Google Scholar]
- 51. Borregaard N, Sørensen OE, Theilgaard-Mönch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol (2007) 28(8):340–5. doi: 10.1016/j.it.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 52. Zhang N, Aiyasiding X, Li WJ, Liao HH, Tang QZ. Neutrophil degranulation and myocardial infarction. Cell Commun Signal (2022) 20(1):50. doi: 10.1186/s12964-022-00824-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ali I, Khan SN, Chatzicharalampous C, Bai D, Abu-Soud HM. Catalase prevents myeloperoxidase self-destruction in response to oxidative stress. J Inorg Biochem (2019) 197:110706. doi: 10.1016/j.jinorgbio.2019.110706 [DOI] [PubMed] [Google Scholar]
- 54. Rizo-Téllez SA, Sekheri M, Filep JG. Myeloperoxidase: regulation of neutrophil function and target for therapy. Antioxid (Basel) (2022) 11(11). doi: 10.3390/antiox11112302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ray RS, Katyal A. Myeloperoxidase: Bridging the gap in neurodegeneration. Neurosci Biobehav Rev (2016) 68:611–20. doi: 10.1016/j.neubiorev.2016.06.031 [DOI] [PubMed] [Google Scholar]
- 56. Tzikas S, Schlak D, Sopova K, Gatsiou A, Stakos D, Stamatelopoulos K, et al. Increased myeloperoxidase plasma levels in patients with Alzheimer's disease. J Alzheimers Dis (2014) 39(3):557–64. doi: 10.3233/JAD-131469 [DOI] [PubMed] [Google Scholar]
- 57. Wright JR, Deen Q, Stevenson A, Telford-Cooke LL, Parker C, Martin-Ruiz C, et al. Plasma myeloperoxidase as a potential biomarker of patient response to anti-dementia treatment in alzheimer's disease. J Alzheimers Dis (2022) 89(4):1483–92. doi: 10.3233/JAD-220642 [DOI] [PubMed] [Google Scholar]
- 58. Teismann P. [Myeloperoxidase in the neurodegenerative process of Parkinson's disease]. Dtsch Med Wochenschr (2014) 139(3):99–102. doi: 10.1055/s-0033-1359907 [DOI] [PubMed] [Google Scholar]
- 59. Gellhaar S, Sunnemark D, Eriksson H, Olson L, Galter D. Myeloperoxidase-immunoreactive cells are significantly increased in brain areas affected by neurodegeneration in Parkinson's and Alzheimer's disease. Cell Tissue Res (2017) 369(3):445–54. doi: 10.1007/s00441-017-2626-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smyth L, Murray HC, Hill M, van Leeuwen E, Highet B, Magon NJ, et al. Neutrophil-vascular interactions drive myeloperoxidase accumulation in the brain in Alzheimer's disease. Acta Neuropathol Commun (2022) 10(1):38. doi: 10.1186/s40478-022-01347-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Volkman R, Ben-Zur T, Kahana A, Garty BZ, Offen D. Myeloperoxidase deficiency inhibits cognitive decline in the 5XFAD mouse model of alzheimer's disease. Front Neurosci (2019) 13:990. doi: 10.3389/fnins.2019.00990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Peng J, Pan J, Mo J, Peng Y. MPO/HOCl facilitates apoptosis and ferroptosis in the SOD1(G93A) motor neuron of amyotrophic lateral sclerosis. Oxid Med Cell Longev (2022) 2022:8217663. doi: 10.1155/2022/8217663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Üllen A, Singewald E, Konya V, Fauler G, Reicher H, Nusshold C, et al. Myeloperoxidase-derived oxidants induce blood-brain barrier dysfunction in vitro and in vivo . PloS One (2013) 8(5):e64034. doi: 10.1371/journal.pone.0064034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. van Leeuwen E, Hampton MB, Smyth L. Hypothiocyanous acid disrupts the barrier function of brain endothelial cells. Antioxid (Basel) (2022) 11(4). doi: 10.3390/antiox11040608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maiuolo J, Gliozzi M, Musolino V, Scicchitano M, Carresi C, Scarano F, et al. The "Frail" Brain blood barrier in neurodegenerative diseases: role of early disruption of endothelial cell-to-cell connections. Int J Mol Sci (2018) 19(9). doi: 10.3390/ijms19092693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hawkins CL. Hypochlorous acid-mediated modification of proteins and its consequences. Essays Biochem (2020) 64(1):75–86. doi: 10.1042/EBC20190045 [DOI] [PubMed] [Google Scholar]
- 67. Vile GF, Rothwell LA, Kettle AJ. Hypochlorous acid activates the tumor suppressor protein p53 in cultured human skin fibroblasts. Arch Biochem Biophys (1998) 359(1):51–6. doi: 10.1006/abbi.1998.0881 [DOI] [PubMed] [Google Scholar]
- 68. Vissers MC, Winterbourn CC. Oxidation of intracellular glutathione after exposure of human red blood cells to hypochlorous acid. Biochem J (1995) 307(Pt 1):57–62. doi: 10.1042/bj3070057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Prütz WA. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch Biochem Biophys (1996) 332(1):110–20. doi: 10.1006/abbi.1996.0322 [DOI] [PubMed] [Google Scholar]
- 70. Bozonet SM, Magon NJ, Schwartfeger AJ, Konigstorfer A, Heath SG, Vissers M, et al. Oxidation of caspase-8 by hypothiocyanous acid enables TNF-mediated necroptosis. J Biol Chem (2023) 299(6):104792. doi: 10.1016/j.jbc.2023.104792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ito Y, Ofengeim D, Najafov A, Das S, Saberi S, Li Y, et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science (2016) 353(6299):603–8. doi: 10.1126/science.aaf6803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Polverino E, Rosales-Mayor E, Dale GE, Dembowsky K, Torres A. The role of neutrophil elastase inhibitors in lung diseases. Chest (2017) 152(2):249–62. doi: 10.1016/j.chest.2017.03.056 [DOI] [PubMed] [Google Scholar]
- 73. Kasus-Jacobi A, Washburn JL, Land CA, Pereira HA. Neutrophil granule proteins inhibit amyloid beta aggregation and neurotoxicity. Curr Alzheimer Res (2021) 18(5):414–27. doi: 10.2174/1567205018666210823095044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim BW, Jeong KH, Kim JH, Jin M, Kim JH, Lee MG, et al. Pathogenic upregulation of glial lipocalin-2 in the parkinsonian dopaminergic system. J Neurosci (2016) 36(20):5608–22. doi: 10.1523/JNEUROSCI.4261-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Naudé PJ, Nyakas C, Eiden LE, Ait-Ali D, van der Heide R, Engelborghs S, et al. Lipocalin 2: novel component of proinflammatory signaling in Alzheimer's disease. FASEB J (2012) 26(7):2811–23. doi: 10.1096/fj.11-202457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Al Nimer F, Elliott C, Bergman J, Khademi M, Dring AM, Aeinehband S, et al. Lipocalin-2 is increased in progressive multiple sclerosis and inhibits remyelination. Neurol Neuroimmunol Neuroinflamm (2016) 3(1):e191. doi: 10.1212/NXI.0000000000000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ngo ST, Steyn FJ, Huang L, Mantovani S, Pfluger CM, Woodruff TM, et al. Altered expression of metabolic proteins and adipokines in patients with amyotrophic lateral sclerosis. J Neurol Sci (2015) 357(1-2):22–7. doi: 10.1016/j.jns.2015.06.053 [DOI] [PubMed] [Google Scholar]
- 78. Petrozziello T, Mills AN, Farhan S, Mueller KA, Granucci EJ, Glajch KE, et al. Lipocalin-2 is increased in amyotrophic lateral sclerosis. Muscle Nerve (2020) 62(2):272–83. doi: 10.1002/mus.26911 [DOI] [PubMed] [Google Scholar]
- 79. Kjeldsen L, Bainton DF, Sengeløv H, Borregaard N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood (1994) 83(3):799–807. doi: 10.1182/blood.V83.3.799.799 [DOI] [PubMed] [Google Scholar]
- 80. Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem (1993) 268(14):10425–32. doi: 10.1016/S0021-9258(18)82217-7 [DOI] [PubMed] [Google Scholar]
- 81. Jha MK, Lee S, Park DH, Kook H, Park KG, Lee IK, et al. Diverse functional roles of lipocalin-2 in the central nervous system. Neurosci Biobehav Rev (2015) 49:135–56. doi: 10.1016/j.neubiorev.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 82. Kang SS, Ren Y, Liu CC, Kurti A, Baker KE, Bu G, et al. Lipocalin-2 protects the brain during inflammatory conditions. Mol Psychiatry (2018) 23(2):344–50. doi: 10.1038/mp.2016.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bi F, Huang C, Tong J, Qiu G, Huang B, Wu Q, et al. Reactive astrocytes secrete lcn2 to promote neuron death. Proc Natl Acad Sci U.S.A. (2013) 110(10):4069–74. doi: 10.1073/pnas.1218497110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee S, Lee WH, Lee MS, Mori K, Suk K. Regulation by lipocalin-2 of neuronal cell death, migration, and morphology. J Neurosci Res (2012) 90(3):540–50. doi: 10.1002/jnr.22779 [DOI] [PubMed] [Google Scholar]
- 85. Jin M, Jang E, Suk K. Lipocalin-2 acts as a neuroinflammatogen in lipopolysaccharide-injected mice. Exp Neurobiol (2014) 23(2):155–62. doi: 10.5607/en.2014.23.2.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee S, Jha MK, Suk K. Lipocalin-2 in the inflammatory activation of brain astrocytes. Crit Rev Immunol (2015) 35(1):77–84. doi: 10.1615/critrevimmunol.2015012127 [DOI] [PubMed] [Google Scholar]
- 87. Jang E, Lee S, Kim JH, Kim JH, Seo JW, Lee WH, et al. Secreted protein lipocalin-2 promotes microglial M1 polarization. FASEB J (2013) 27(3):1176–90. doi: 10.1096/fj.12-222257 [DOI] [PubMed] [Google Scholar]
- 88. Kim H, Lee S, Park HC, Lee WH, Lee MS, Suk K. Modulation of glial and neuronal migration by lipocalin-2 in zebrafish. Immune Netw (2011) 11(6):342–7. doi: 10.4110/in.2011.11.6.342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res (2006) 69(3):562–73. doi: 10.1016/j.cardiores.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 90. Fertin M, Lemesle G, Turkieh A, Beseme O, Chwastyniak M, Amouyel P, et al. Serum MMP-8: a novel indicator of left ventricular remodeling and cardiac outcome in patients after acute myocardial infarction. PloS One (2013) 8(8):e71280. doi: 10.1371/journal.pone.0071280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sorsa T, Tervahartiala T, Leppilahti J, Hernandez M, Gamonal J, Tuomainen AM, et al. Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclines. Pharmacol Res (2011) 63(2):108–13. doi: 10.1016/j.phrs.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 92. Chakrabarti S, Patel KD. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J Leukoc Biol (2005) 78(1):279–88. doi: 10.1189/jlb.1004612 [DOI] [PubMed] [Google Scholar]
- 93. He X, Zhang L, Yao X, Hu J, Yu L, Jia H, et al. Association studies of MMP-9 in Parkinson's disease and amyotrophic lateral sclerosis. PloS One (2013) 8(9):e73777. doi: 10.1371/journal.pone.0073777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Demestre M, Parkin-Smith G, Petzold A, Pullen AH. The pro and the active form of matrix metalloproteinase-9 is increased in serum of patients with amyotrophic lateral sclerosis. J Neuroimmunol (2005) 159(1-2):146–54. doi: 10.1016/j.jneuroim.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 95. Iłzecka J, Stelmasiak Z, Dobosz B. Matrix metalloproteinase-9 (MMP-9) activity in cerebrospinal fluid of amyotrophic lateral sclerosis patients. Neurol Neurochir Pol (2001) 35(6):1035–43. [PubMed] [Google Scholar]
- 96. Niebroj-Dobosz I, Janik P, Sokołowska B, Kwiecinski H. Matrix metalloproteinases and their tissue inhibitors in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Eur J Neurol (2010) 17(2):226–31. doi: 10.1111/j.1468-1331.2009.02775.x [DOI] [PubMed] [Google Scholar]
- 97. Fang L, Huber-Abel F, Teuchert M, Hendrich C, Dorst J, Schattauer D, et al. Linking neuron and skin: matrix metalloproteinases in amyotrophic lateral sclerosis (ALS). J Neurol Sci (2009) 285(1-2):62–6. doi: 10.1016/j.jns.2009.05.025 [DOI] [PubMed] [Google Scholar]
- 98. Dewil M, Schurmans C, Starckx S, Opdenakker G, Van Den Bosch L, Robberecht W. Role of matrix metalloproteinase-9 in a mouse model for amyotrophic lateral sclerosis. Neuroreport (2005) 16(4):321–4. doi: 10.1097/00001756-200503150-00003 [DOI] [PubMed] [Google Scholar]
- 99. Lorenzl S, Narr S, Angele B, Krell HW, Gregorio J, Kiaei M, et al. The matrix metalloproteinases inhibitor Ro 28-2653 [correction of Ro 26-2853] extends survival in transgenic ALS mice. Exp Neurol (2006) 200(1):166–71. doi: 10.1016/j.expneurol.2006.01.026 [DOI] [PubMed] [Google Scholar]
- 100. Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol (2005) 289(2):H558–68. doi: 10.1152/ajpheart.01275.2004 [DOI] [PubMed] [Google Scholar]
- 101. Spiller KJ, Khan T, Dominique MA, Restrepo CR, Cotton-Samuel D, Levitan M, et al. Reduction of matrix metalloproteinase 9 (MMP-9) protects motor neurons from TDP-43-triggered death in rNLS8 mice. Neurobiol Dis (2019) 124:133–40. doi: 10.1016/j.nbd.2018.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kiaei M, Kipiani K, Calingasan NY, Wille E, Chen J, Heissig B, et al. Matrix metalloproteinase-9 regulates TNF-alpha and FasL expression in neuronal, glial cells and its absence extends life in a transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol (2007) 205(1):74–81. doi: 10.1016/j.expneurol.2007.01.036 [DOI] [PubMed] [Google Scholar]
- 103. Kaplan A, Spiller KJ, Towne C, Kanning KC, Choe GT, Geber A, et al. Neuronal matrix metalloproteinase-9 is a determinant of selective neurodegeneration. Neuron (2014) 81(2):333–48. doi: 10.1016/j.neuron.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science (2004) 303(5663):1532–5. doi: 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 105. Neeli I, Dwivedi N, Khan S, Radic M. Regulation of extracellular chromatin release from neutrophils. J Innate Immun (2009) 1(3):194–201. doi: 10.1159/000206974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe (2012) 12(3):324–33. doi: 10.1016/j.chom.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 107. Fabene PF, Navarro Mora G, Martinello M, Rossi B, Merigo F, Ottoboni L, et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med (2008) 14(12):1377–83. doi: 10.1038/nm.1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol (2009) 30(11):547–56. doi: 10.1016/j.it.2009.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zarbock A, Ley K. Mechanisms and consequences of neutrophil interaction with the endothelium. Am J Pathol (2008) 172(1):1–7. doi: 10.2353/ajpath.2008.070502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab (2015) 35(6):888–901. doi: 10.1038/jcbfm.2015.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Boeltz S, Muñoz LE, Fuchs TA, Herrmann M. Neutrophil extracellular traps open the pandora's box in severe malaria. Front Immunol (2017) 8:874. doi: 10.3389/fimmu.2017.00874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PloS One (2012) 7(2):e32366. doi: 10.1371/journal.pone.0032366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Meegan JE, Yang X, Beard RS, Jr, Jannaway M, Chatterjee V, Taylor-Clark TE, et al. Citrullinated histone 3 causes endothelial barrier dysfunction. Biochem Biophys Res Commun (2018) 503(3):1498–502. doi: 10.1016/j.bbrc.2018.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cai W, Liu S, Hu M, Huang F, Zhu Q, Qiu W, et al. Functional dynamics of neutrophils after ischemic stroke. Transl Stroke Res (2020) 11(1):108–21. doi: 10.1007/s12975-019-00694-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pietronigro EC, Della Bianca V, Zenaro E, Constantin G. NETosis in alzheimer's disease. Front Immunol (2017) 8:211. doi: 10.3389/fimmu.2017.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Allam R, Kumar SV, Darisipudi MN, Anders HJ. Extracellular histones in tissue injury and inflammation. J Mol Med (Berl) (2014) 92(5):465–72. doi: 10.1007/s00109-014-1148-z [DOI] [PubMed] [Google Scholar]
- 117. McPherson CA, Merrick BA, Harry GJ. In vivo molecular markers for pro-inflammatory cytokine M1 stage and resident microglia in trimethyltin-induced hippocampal injury. Neurotox Res (2014) 25(1):45–56. doi: 10.1007/s12640-013-9422-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Moxon-Emre I, Schlichter LC. Neutrophil depletion reduces blood-brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J Neuropathol Exp Neurol (2011) 70(3):218–35. doi: 10.1097/NEN.0b013e31820d94a5 [DOI] [PubMed] [Google Scholar]
- 119. Monif M, Burnstock G, Williams DA. Microglia: proliferation and activation driven by the P2X7 receptor. Int J Biochem Cell Biol (2010) 42(11):1753–6. doi: 10.1016/j.biocel.2010.06.021 [DOI] [PubMed] [Google Scholar]
- 120. Loane DJ, Kumar A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp Neurol (2016) 275 Pt 3(0 3):316–27. doi: 10.1016/j.expneurol.2015.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Otxoa-de-Amezaga A, Miró-Mur F, Pedragosa J, Gallizioli M, Justicia C, Gaja-Capdevila N, et al. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol (2019) 137(2):321–41. doi: 10.1007/s00401-018-1954-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci (2009) 29(37):11511–22. doi: 10.1523/JNEUROSCI.1514-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Xie L, Poteet EC, Li W, Scott AE, Liu R, Wen Y, et al. Modulation of polymorphonuclear neutrophil functions by astrocytes. J Neuroinflamm (2010) 7:53. doi: 10.1186/1742-2094-7-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Stirling DP, Liu S, Kubes P, Yong VW. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J Neurosci (2009) 29(3):753–64. doi: 10.1523/JNEUROSCI.4918-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hooshmand MJ, Nguyen HX, Piltti KM, Benavente F, Hong S, Flanagan L, et al. Neutrophils induce astroglial differentiation and migration of human neural stem cells via C1q and C3a synthesis. J Immunol (2017) 199(3):1069–85. doi: 10.4049/jimmunol.1600064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Cipollina G, Davari Serej A, Di Nolfi G, Gazzano A, Marsala A, Spatafora MG, et al. Heterogeneity of neuroinflammatory responses in amyotrophic lateral sclerosis: A challenge or an opportunity. Int J Mol Sci (2020) 21(21). doi: 10.3390/ijms21217923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci (2017) 20(2):136–44. doi: 10.1038/nn.4475 [DOI] [PubMed] [Google Scholar]
- 128. Chen L, Wang Y, Huang J, Hu B, Huang W. Identification of immune-related hub genes in parkinson's disease. Front Genet (2022) 13:914645. doi: 10.3389/fgene.2022.914645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Blanco-Camarillo C, Alemán OR, Rosales C. Low-density neutrophils in healthy individuals display a mature primed phenotype. Front Immunol (2021) 12:672520. doi: 10.3389/fimmu.2021.672520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Obama T, Itabe H. Neutrophils as a novel target of modified low-density lipoproteins and an accelerator of cardiovascular diseases. Int J Mol Sci (2020) 21(21). doi: 10.3390/ijms21218312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Cho Y, Bukong TN, Tornai D, Babuta M, Vlachos IS, Kanata E, et al. Neutrophil extracellular traps contribute to liver damage and increase defective low-density neutrophils in alcohol-associated hepatitis. J Hepatol (2023) 78(1):28–44. doi: 10.1016/j.jhep.2022.08.029 [DOI] [PubMed] [Google Scholar]