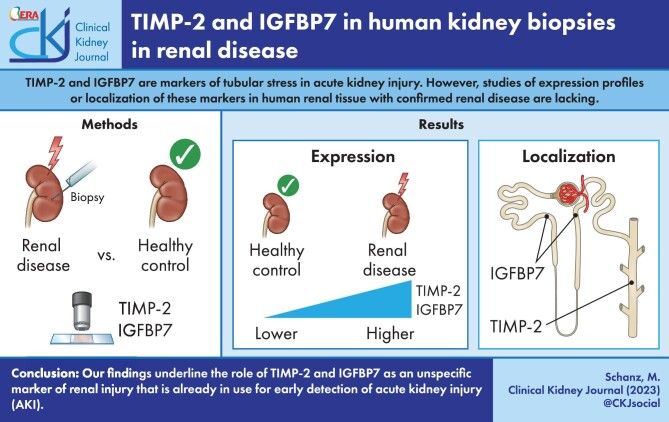
ABSTRACT
Background
Tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7) are markers of tubular stress and urinary [TIMP-2]*[IGFBP7] is an established biomarker for risk assessment of acute kidney injury. There are no studies of expression profiles or localization of these markers in human renal tissue with confirmed renal disease.
Methods
We analysed 37 kidney biopsies of patients with renal disease and 10 non-diseased control biopsies for TIMP-2 and IGFBP7 expression using immunohistochemistry. Changes in glomerular morphology were evaluated by a semi-quantitative glomerulosclerosis score (GSI) and tubular interstitial changes were graded by the tubular injury score (TSI) using periodic acid–Schiff-stained paraffin sections. Interstitial fibrosis and tubular atrophy (IF/TA) were graded according to the Banff classification. Urinary [TIMP-2]*[IGFBP7] was collected at the time of biopsy.
Results
TIMP-2 and IGFBP7 had significantly greater expression in kidney biopsies from patients with renal disease compared with control tissue, especially in the tubular compartment. Here, IGFBP7 was detected in proximal and distal tubules while TIMP-2 was predominantly localized in the collecting ducts. Renal injury significantly correlated with staining intensity for TIMP-2 and IGFBP7: GSI weakly correlated with glomerular TIMP-2 (r = 0.36) and IGFBP7 (r = 0.35) and TSI correlated with tubular TIMP-2 (r = 0.41) and IGFBP7 (r = 0.43). Urinary [TIMP-2]*[IGFBP7] correlated weakly with the histopathological damage score but not with glomerular and tubular expression.
Conclusion
Our findings underline the role of TIMP-2/IGFBP7 as an unspecific marker of renal injury that is already in use for early detection of acute kidney injury.
Keywords: acute kidney injury, AKI, IGFBP7, kidney biopsy, renal disease, TIMP-2
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Evidence of renal damage, consequently leading to acute kidney injury (AKI), is a very common finding, especially in patients with pre-existing kidney disease. Incidences of in-hospital AKI have been reported to range in high-risk collectives even up to 67% [1, 2]. The development of AKI is associated with a significantly worse outcome, with longer hospitalization and higher mortality [3]. Serum creatinine is still one of the pillars of AKI diagnosis, but unfortunately it is not an optimal biomarker for the timely evaluation of renal damage, due to its filtration marker nature, with a certain latency of increase after the occurrence of kidney damage. Consequently, preventive/therapeutic measures may not have a sufficient effect because the damage that has occurred is already irreversible. It can be speculated that the recent stagnation in therapeutic efforts to improve AKI incidence and prognosis may be due to a delayed start of therapy [4].
Two urinary biomarkers are intended to counteract this problem and indicate kidney damage even before an increasing serum creatinine is evident: the product of urinary tissue inhibitor metalloproteinase-2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7) have been established as biomarkers for early detection of AKI in various settings [5–7]. This combination was found to be superior to other known biomarkers in different patient populations, especially critically ill patients [8, 9]. The proposed mechanism for TIMP-2/IGFBP7 is based partially on theoretical pathophysiologic considerations and involves induction of G1 cell cycle arrest. It is hypothesized that in renal stress, the release of TIMP-2/IGFBP7 occurs in a paracrine manner and causes G1 cell cycle arrest to prevent further cell damage, as DNA damage that has occurred can be repaired [5]. Tubular cell release and failed tubular reabsorption of TIMP-2/IGFBP7 in response to tubular injury in the absence of gene activation were recently proposed as major mechanisms for increased urinary TIMP-2/IGFBP7 [10].
Without a specific relation to kidney tissue, for both proteins involvement in G1 cell cycle arrest is described [11–16]. IGFBP7 plays a role in growth suppression and is involved in angiogenesis and tumour diseases, but specific data on expression in kidney tissue are sparse and inconsistent [13–16]. Involvement and expression of the TIMP family and its subgroups is described in renal tissues for podocytes, proximal renal tubular epithelial cells (for the latter, mainly in response to damage, e.g. cisplatin) and renal cell carcinoma [17–20]. TIMP-2 appears to play a role not only in response to acute kidney damage, but also in non-acute renal disease such as glomerular diseases (e.g. focal segmental glomerulosclerosis) and renal fibrosis [21, 22].
Data on immunohistochemical TIMP-2/IGFBP7 expression in human kidney tissue are scarce. Previous findings are available only in cell cultures of human kidney cells, in human tissue from deceased donors without evidence of specific kidney disease and in experimental AKI models in mice [10, 23]. In particular, there are no findings on TIMP-2/IGFBP7 expression in human kidney biopsies of renal disease to date. There are also no conclusive results so far on the exact localization and expression of TIMP-2/IGFBP7 in human tissue.
In this study we examined the renal expression of these two proteins in kidney disease with and without evidence of clinically diagnosed AKI.
MATERIALS AND METHODS
Patient selection and clinical data
In our analysis, 37 patients with renal disease were consecutively included. The patients were undergoing routine kidney biopsy between December 2012 and May 2015 in the Robert-Bosch Hospital, Stuttgart, Germany. Inclusion criteria were evidence of acute tubular injury in routine histology, available tissue for preparation of TIMP-2/IGFBP7 staining and urinary biomarker measurements (NephroCheck, Marcy-l’Étoile, France) as well as clinical data to analyse.
Renal tissue specimens
We analysed TIMP-2 and IGFBP7 in 37 patients with renal disease. In addition, control tissues without evidence of renal disease (n = 10) were obtained from distant portions of kidneys surgically excised because of the presence of a localized neoplasm. All diagnoses were made by expert nephropathologists. An overview of the composition and clinical data of the patients is provided in Table 1.
Table 1:
Baseline characteristics of renal biopsy cohort: biopsy indication, baseline parameters, comorbidities and laboratory results.
| Characteristics | Total (N = 37) | AKI positive (n = 19) | AKI negative (n = 18) | P-value |
|---|---|---|---|---|
| Biopsy (major reason), n | ||||
| AKI | 10 | 10 | 0 | |
| Nephrotic syndrome | 6 | 2 | 4 | |
| Nephritic sediment | 7 | 2 | 5 | |
| Acute on chronic kidney injury | 5 | 5 | 0 | |
| Persistent haematuria | 3 | 0 | 3 | |
| Progressive proteinuria | 3 | 0 | 3 | |
| Progressive serum creatinine | 3 | 0 | 3 | |
| Baseline parameters, median (IQR) | ||||
| Age (years) | 62 (47–75) | 69 (59–76) | 52 (43–70) | .06 |
| Height (cm) | 172 (166–178) | 172 (168–181) | 171 (164–176) | .52 |
| Weight (kg) | 75 (65–86) | 75 (65–92) | 75 (64–85) | .71 |
| Body surface area (kg/m2) | 25 (24–28) | 25 (24–28) | 25 (23–28) | .99 |
| Systolic blood pressure (mmHg) | 140 (120–154) | 140 (120–155) | 130 (115–153) | .29 |
| Diastolic blood pressure (mmHg) | 80 (70–80) | 80 (62–80) | 80 (73–80) | .23 |
| Comorbidities, n | ||||
| Arterial hypertension | 23 | 14 | 9 | .18 |
| Diabetes | 7 | 2 | 5 | .23 |
| Coronary heart disease | 6 | 2 | 4 | .41 |
| Active malignancy | 3 | 2 | 1 | .99 |
| Peripheral arterial disease | 1 | 0 | 1 | .49 |
| Laboratory results, median (IQR) | ||||
| Serum creatinine at biopsy (mg/dl) | 2.50 (1.35–4.05) | 3.80 (2.70–5.10) | 1.35 (0.78–1.53) | <.0001* |
| Baseline serum creatinine (mg/dl) | 1.80 (1.20–2.95) | 2.50 (2.20–4.10) | 1.20 (0.70–1.43) | <.0001* |
| Baseline eGFR (CKD-EPI; ml/min/1.73 m2) | 38 (22–71) | 23 (13–29) | 71 (49–106) | <.0001* |
| Follow-up serum creatinine (mg/dl) | 2.00 (1.20–3.80) | 2.20 (1.60–3.90) | 1.35 (0.88–3.00) | .04* |
| Follow-up eGFR (CKD-EPI; ml/min/1.73 m2) | 35 (15–74) | 32 (15–44) | 61 (22–103) | .04* |
| Follow-up (days), median (IQR) | 204 (48–2648) | 109 (38–1836) | 1782 (44–2874) | .12 |
| White blood cell count (×109/l) | 7.7 (6.6–11.4) | 9.6 (6.4–13.4) | 7.6 (6.7–9.1) | .19 |
| Haemoglobin (g/l) | 106 (89–128) | 93 (85–106) | 121 (106–136) | .0005 |
| Platelets (×109/l) | 259 (209–336) | 253 (192–349) | 260 (210–333) | .78 |
| pH | 7.365 (7.336–7.397) | 7.344 (7.309–7.391) | 7.378 (7.349–7.404) | .07 |
| Bicarbonate (mmol/l) | 22.7 (20.6–25.1) | 20.8 (18.7–23.3) | 24.1 (22.4–25.6) | .01* |
| Sodium (mmol/l) | 139 (138–141) | 139 (138–141) | 139 (138–141) | .70 |
| Potassium (mmol/l) | 4.1 (3.7–4.4) | 3.8 (3.4–4.3) | 4.2 (3.9–4.4) | .15 |
| Calcium (mmol/l) | 2.24 (2.02–2.32) | 2.15 (1.9–2.3) | 2.27 (2.23–2.38) | .04* |
| Phosphate (mmol/l) | 0.93 (0.74–1.47) | 0.88 (0.73–1.58) | 0.98 (0.74–1.06) | .99 |
| GOT (U/l) | 21 (17–31) | 17 (12–22) | 29 (20–34) | .005* |
| GPT (U/l) | 18 (12–29) | 11 (8–18) | 28 (17–33) | .006* |
| GGT (U/l) | 38 (22–59) | 36 (23–79) | 38 (19–58) | .59 |
| Urinary results, median (IQR) | ||||
| Urinary [TIMP-2]*[IGFBP7] at biopsy | 0.56 (0.23–1.28) | 0.27 (0.15–4.16) | 0.57 (0.30–1.24) | .44 |
| UPCR (g/g) | 1.83 (0.69–4.30) | 1.83 (0.76–3.76) | 1.75 (0.38–4.97) | .69 |
| Haematuria (erythrocytes/µl) | 150 (25–250) | 150 (25–250) | 150 (25–250) | .67 |
IQR: interquartile range; GGT: gamma-glutamyltransferase; GOT: glutamate oxaloacetic transaminase; GPT: glutamate pyruvate transaminase; UPCR: urine protein:creatinine ratio.
*P < .05 (Mann-Whitney or Fisher's exact test).
The use of formalin-fixed paraffin-embedded tissue from the archive of the Department of Nephropathology was approved by the Ethics Committee of the Friedrich-Alexander-University of Erlangen-Nürnberg (no. 4415). The Ethics Committee of the University of Tuebingen (857/2019BO2) also approved the study. Because of its retrospective nature, consent for the use of archived material and the use of clinical data was not necessary, as approved by both ethics committees.
Immunohistochemistry and immunofluorescence double staining
For all single staining and co-localization studies, kidneys were fixed in 4% formalin, embedded in paraffin and cut into sections of 2 µm. Antigen retrieval was done using citrate buffer pH 6 and cooking in a pressure cooker for 2.5 minutes. After blocking with normal goat serum and 1% blotto, sections were incubated overnight at 4°C using the following antibodies diluted in 1% bovine serum albumin in 50 mM Tris pH 7.4:
TIMP-2, a mouse monoclonal antibody against human TIMP-2 (MS-1485, Neomarkers, Fremont, CA, USA); IGFBP7, a rabbit polyclonal antibody against human IGFBP7 (ab74169, Abcam, Cambridge, UK and sc-13095, Santa Cruz Biotechnology, Heidelberg, Germany); CD31, a mouse monoclonal antibody against human CD31 (M0823, Dako Deutschland, Hamburg, Germany); megalin, a mouse monoclonal antibody to human megalin (DM3613P, Acris Antibodies, Herford, Germany); aquaporin 2, a polyclonal rabbit antibody against human aquaporin 2 (HPA046834, Sigma-Aldrich, St. Louis, MO, USA); Ki67, a rabbit monoclonal antibody against human Ki67 (RM-9106, Neomarkers, Fremont, CA, USA); synaptopodin, a mouse monoclonal antibody against human synaptopodin (#65294, ProGen Biotechnik, Heidelberg, Germany); podocalyxin, a goat polyclonal antibody against human podocalyxin (AF1556, R&D Systems, Wiesbaden, Germany); parvalbumin, a goat polyclonal antibody against human parvalbumin (PVG-213, Swant, Marly, Switzerland); sodium/calcium exchanger, a mouse monoclonal antibody against sodium/calcium exchanger (R3F1, Swant); and uromodulin, a mouse monoclonal antibody against human uromodulin (gift from Prof. Jürgen Scherberich, München, Germany).
Negative controls for immunostaining included either deletion or substitution of the primary antibody with equivalent concentrations of an irrelevant murine monoclonal antibody or pre-immune rabbit immunoglobulin G (IgG). For single staining of TIMP-2 and IGFBP7, primary antibodies were detected using biotinylated horse anti-mouse IgG (BA-2001) and goat anti-rabbit IgG (BA-1000), ABC-kit (PK-6100) and ImmPACT-DAB (SK-4105) as substrate (all from Vector Laboratories, Burlingame, CA, USA). Double staining of TIMP-2 and IGFBP7 with renal cell markers was performed using fluorescence-labelled secondary antibodies: donkey anti-mouse IgG Alexa647 (A31571), donkey anti-rabbit Alexa647 (A31573), donkey anti-goat IgG Alexa488 (A11055), donkey anti-mouse IgG Alexa568 (A21202), donkey anti-rabbit Alexa568 (A10042) and donkey anti-rabbit Alexa488 (A21206) (all from Thermo Fisher Scientific, Waltham, MA, USA).
Semi-quantitative evaluation of TIMP-2 and IGFBP7 and the proliferation marker Ki67 in renal biopsies
TIMP-2 and IGFBP7 staining was evaluated in the cortex of renal biopsies by light microscopy at 400× magnification using a semi-quantitative score for glomerular and tubulointerstitial localization separately: 0, absent staining; 1, only weak staining; 2, moderate staining; 3, intense staining; and 4, strong staining. Proliferative activity was evaluated by counting Ki67-positive cells per glomerular cross section and tubular and interstitial cells per millimetre squared. Quantification of Ki67 was performed separately for the glomerulus, the cortical tubulointerstitium and tubular cells.
Histopathological evaluation
Changes in glomerular morphology including mesangial matrix accumulation and sclerosis were evaluated by a semi-quantitative glomerulosclerosis score (GSI) and tubular interstitial changes were graded by the tubular injury score (TSI) using periodic acid–Schiff-stained paraffin sections as described previously [24]. Interstitial fibrosis and tubular atrophy (IF/TA) were graded according to the Banff classification for renal transplant biopsies [25].
Evaluation of retrospective clinical data
Clinical routine parameters from patients were retrospectively acquired from the time point of biopsy collection. The following parameters were included for analysis: major reason for biopsy, histological diagnosis, baseline parameters, comorbidities and laboratory results for AKI diagnosis and urinary [TIMP-2]*[IGFBP7]. Groups of renal disease were formed according to sample size and relevance: ≥2 were considered as a separate group whereas ≤2 were grouped in ‘others’. AKI was defined according to serum creatinine–based on the Kidney Disease: Improving Global Outcomes classification [26].
Statistical analyses
After testing for normal distribution of values using the Kolmogorov–Smirnov test and finding that many datasets are not normally distributed, data were analysed using the Mann–Whitney U test. For comparison of comorbidities, Fisher's exact test was used. In all tests, P < .05 was accepted as statistically significant. Spearman's test was used to test correlation of renal TIMP-2/IGFBP7 with renal injury scores, the proliferation marker Ki67 and clinical data. Differences between groups of TIMP-2/IGFBP7 histological and clinical parameters was tested using the Mann–Whitney U test. Statistical analyses were performed using SPSS for Windows (version 19.0; IBM, Armonk, NY, USA) or GraphPad Prism 9 for Mac (version 9.02; GraphPad Software, San Diego, CA, USA).
RESULTS
Baseline characteristics
In our cohort, approximately half of the patients [19 (51%)] had AKI as the leading clinical finding (AKI group). In the non-AKI group, the main reason for kidney biopsy was either nephritic or nephrotic urinary sediment, progressive proteinuria or persistent haematuria (Table 1). No significant differences were found regarding the patients’ baseline parameters. Regarding laboratory results, the group with AKI had significantly higher serum creatinine and lower bicarbonate, as well as significantly lower haemoglobin, total calcium and some liver enzymes. Baseline kidney function in the AKI group was significantly lower compared with the non-AKI group, indicating that many subjects had acute or chronic kidney injury. Urinary results showed no significant differences in urinary [TIMP-2]*[IGFBP7], proteinuria or haematuria (Table 1).
Histological characteristics of controls and renal disease with or without AKI
Basic histological damage/injury characteristics were significantly different in controls, renal disease or AKI-positive and AKI-negative subjects: IF/TA, suggesting of chronic kidney damage, was significantly higher in biopsies with renal disease (P = .0006) and AKI (P = .0004), as was TSI in biopsies with renal disease (P = .0006) and AKI (P = .001). GSI was also higher in kidney biopsies from patients with renal disease and AKI, but this difference was only significant in the renal disease group (P < .0001) (Table 2).
Table 2:
Comparison of histological and immunohistochemical characteristics of control and renal disease specimens and the AKI-positive and AKI-negative subgroups.
| IHC staining score, median (IQR) | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Controls (n = 10) | Renal disease (n = 37) | P-value | AKI positive (n = 19) | AKI negative (n = 18) | P-value |
| Histological | ||||||
| IF/TA | 0.68 (0.50–0.94) | 2.00 (0.96–2.67) | .0006* | 2.50 (2.25–2.75) | 1.00 (0.70–1.89) | .0004* |
| TSI | 0.77 (0.71–0.95) | 2.17 (0.95–2.92) | .0006* | 2.67 (2.13–3.00) | 1.11 (0.79–2.20) | .001* |
| GSI | 0.27 (0.21–0.36) | 1.35 (0.51–2.45) | <.0001* | 1.78 (1.09–2.48) | 0.79 (0.39–2.40) | .22 |
| Proliferation (Ki67-positive cells) | ||||||
| Ki67 glomerulara | 1.45 (0.94–1.93) | 1.09 (0.54–3.28) | .58 | 1.18 (0.58–1.89) | 0.93 (0.50–6.88) | .56 |
| Ki67 tubularb | 23.01 (11.35–76.57) | 9.34 (3.25–18.54) | .01* | 9.34 (2.56–36.15) | 9.48 (3.71–15.38) | .66 |
| Ki67 tubular and interstitialb | 91.35 (59.42–161.8) | 46.15 (10.66–79.57) | .008* | 46.15 (10.14–80.77) | 46.05 (11.63–81.03) | .71 |
| IHC staining score | ||||||
| IGFBP7 glomerular | 0.56 (0.44–0.66) | 1.40 (0.92–1.72) | .0001* | 1.36 (0.90–1.67) | 1.50 (0.88–1.88) | .53 |
| IGFBP7 tubular | 0.72 (0.52–0.94) | 1.38 (0.95–1.88) | .003* | 1.43 (1.14–1.79) | 1.33 (0.72–1.91) | .57 |
| TIMP-2 glomerular | 0.08 (0.04–0.12) | 0.27 (0.00–0.71) | .09 | 0.30 (0.00–0.75) | 0.23 (0.00–0.71) | .85 |
| TIMP-2 tubular | 0.67 (0.48–0.80) | 1.14 (0.85–1.55) | <.0001* | 1.23 (0.93–1.60) | 1.10 (0.84–1.39) | .29 |
Ki67-positive cells aper glomerular cross section or bper mm2.
*P < .05 (Mann–Whitney test).
IHC: immunohistochemistry; IQR: interquartile range.
Proliferation of glomerular, tubular and interstitial cells in controls and renal disease with or without AKI
Overall, the median proliferative activity, as assessed by Ki67 staining, was significantly lower in renal disease, whereas no significant differences were seen between the AKI- and non-AKI group. In particular, tubular Ki67 and tubular/interstitial Ki67 proliferative activity was significantly lower (P = .01 and P = .008, respectively) in biopsies with renal disease compared with controls (Fig. 1A and Table 2), while the median glomerular KI67 proliferative activity did not differ significantly between groups. Surprisingly, renal cell proliferation was similar in AKI and non-AKI groups (Table 2).
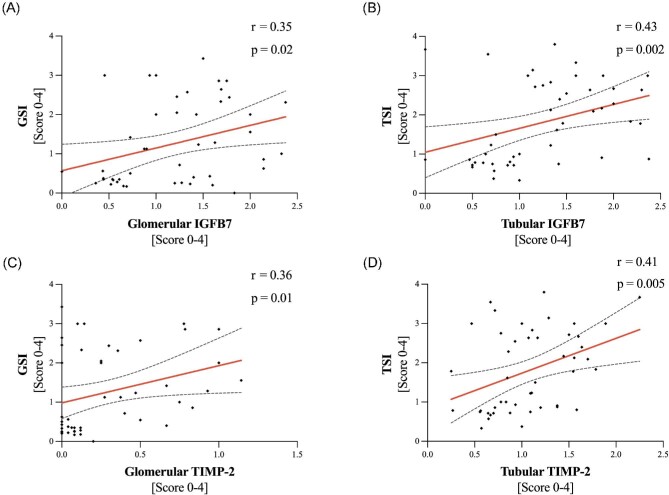
Figure 1:
Association of IGFBP7/TIMP-2 and histological injury scores. Association of GSI and glomerular (A) IGFBP7 and (C) TIMP-2 and TSI and tubular (B) IGFBP7 and (D) TIMP-2. Spearman's r and P-values for correlations.
Association of TIMP-2/IGFBP7 and histological characteristics
Correlation analysis showed a significant association of renal injury scores and staining intensity for TIMP-2/IGFBP7, as assessed by immunohistochemistry, especially tubular damage, as assessed by TSI, which correlated significantly with tubular TIMP-2 (r = 0.41, P = .005) and for TSI and tubular IGFBP7 (r = 0.43, P = .002). Also, in the glomerular compartment, glomerulosclerosis correlated significantly with glomerular TIMP-2 (r = 0.36, P = .01) and glomerular IGFBP7 (r = 0.35, P = .02) (Fig. 1).
Compartment-specific analysis of TIMP-2 and IGFBP7 in various renal diseases
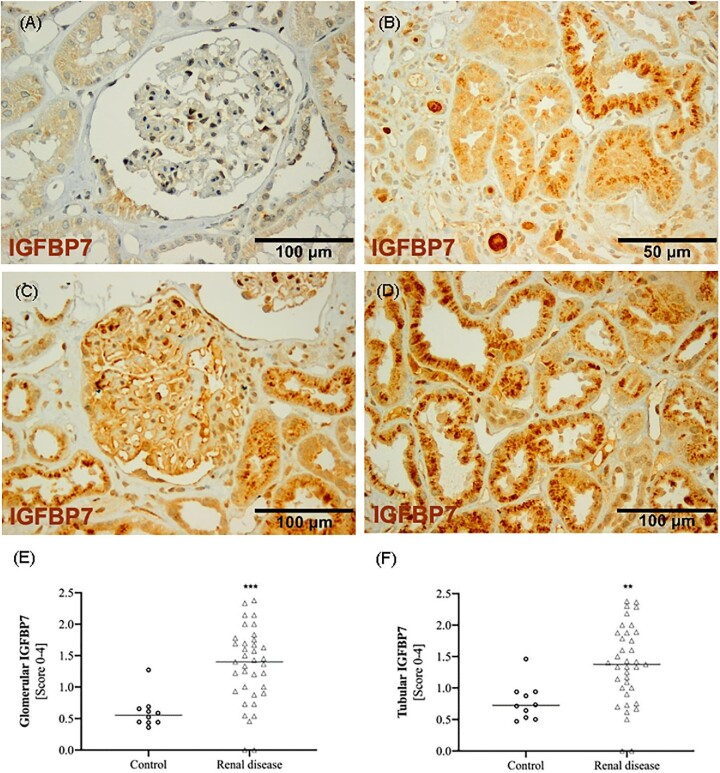
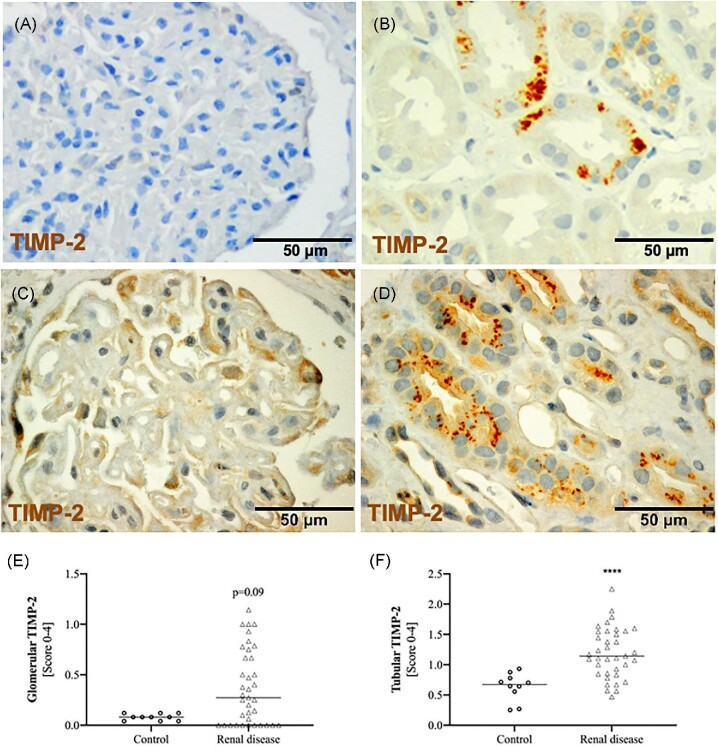
In kidney biopsies from controls, glomeruli were barely IGFBP7 positive and tubules showed weak, diffuse staining by immunohistochemistry (Fig. 2A). In contrast, both glomeruli and tubules from biopsies with kidney disease showed strong staining for IGFBP7 (Fig. 2B–D). In the tubules, in addition to the diffuse cytosolic staining, we also frequently detected a vesicular distribution (Fig. 2B and D). TIMP-2 was absent in the glomeruli of healthy controls and could only rarely be detected in tubules (Fig. 3A). In biopsies from patients with kidney disease, TIMP-2 was detected with weak staining in the glomeruli and showed a vesicular staining that was restricted to a few tubules (Fig. 3B–D). Consequently, semi-quantitative evaluation of TIMP-2 and IGFBP7 staining showed significantly higher median tubular scores in biopsies with renal disease (TIMP-2: P < .0001; IGFBP7: P = .003). In contrast, mean glomerular staining was higher for both markers but only reached significant levels for IGFBP7 (P = .0001; Fig. 2 and Fig. 4E and F). Surprisingly, tubular and glomerular TIMP-2/IGFBP7 expression did not differ significantly between the AKI and non-AKI group, although slightly higher tubular TIMP-2/IGFBP7 and glomerular IGFBP7 scores were evident in AKI (Table 2).
Figure 2:
Expression of IGFBP7 as assessed by immunohistochemistry in (A) a control and in biopsy examples with renal disease showing (B, D) vesicular tubular staining as well as (C) weak glomerular staining. Median expression of (E) glomerular and (F) tubular TIMP-2 in controls and renal diseases are shown. *P < .05, **P < .01 and ***P < .005 (Mann–Whitney U test).
Figure 3:
Expression of TIMP-2 as assessed by immunohistochemistry in (A) a control and in biopsy examples with renal disease showing (B) moderate to (D) strong tubular as well as (C) glomerular staining. Median expression of (E) glomerular and (F) tubular TIMP-2 in controls and renal diseases are shown. ****P < .001 (Mann–Whitney U test).
Figure 4:
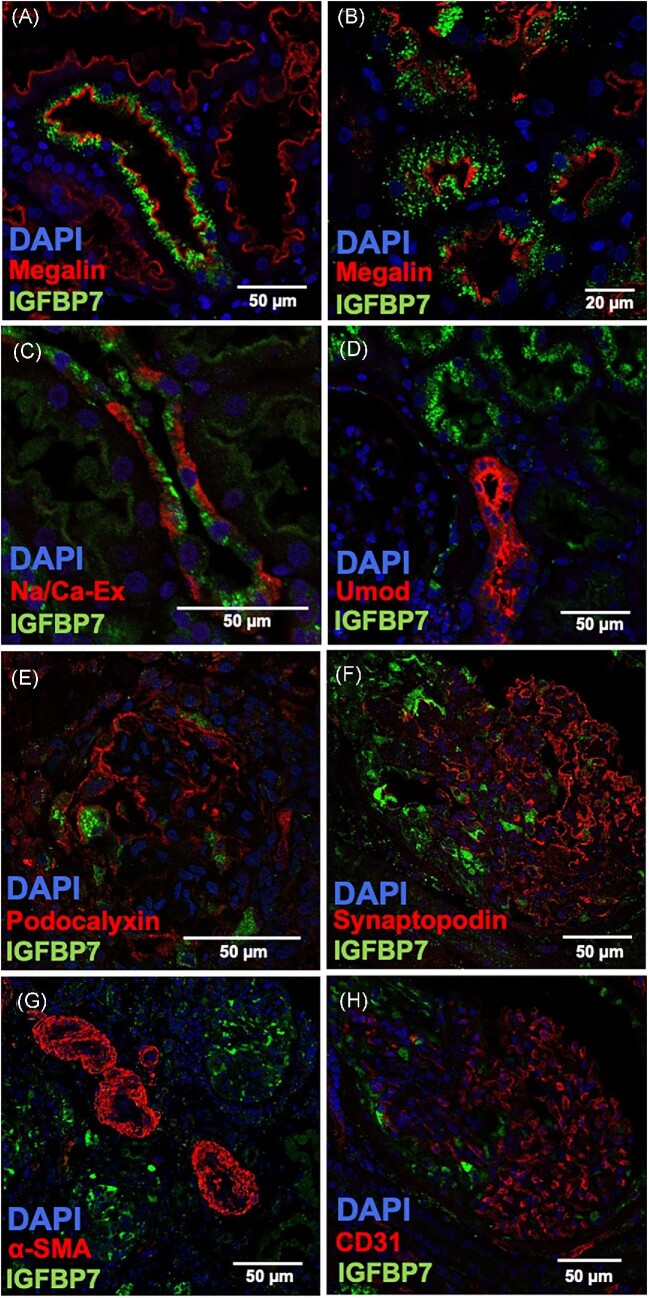
Merged images of IGFBP7 and cell-specific markers are shown using immunofluorescence microscopy. IGFBP7 staining was combined with the marker megalin for (A, B) proximal tubules, (C) sodium/calcium exchanger (Na/Ca-Ex) for distal tubules, (D) uromodulin for the thick ascending limb, (E) podocalyxin and (F) synaptopodin for podocytes, (G) alpha smooth muscle actin (SMA) for vascular muscle cells and (H) CD31 for endothelial cells.
Regarding each individual renal disease, mainly glomerular IGFBP7 and tubular TIMP-2 expression is significantly elevated in several renal diseases. In particular, membranous nephropathy exhibits significantly higher expression of glomerular/tubular IGFBP7 (P < .05) and tubular TIMP-2 (P = .003) (Table 3).
Table 3:
Immunohistochemical expression of glomerular and tubular IGFBP7 and TIMP-2 in different renal diseases.
| IHC staining score, median (IQR) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Disease | IGFBP7 glomerular | P-value | IGFBP7 tubular | P-value | TIMP-2 glomerular | P-value | TIMP-2 tubular | P-value |
| Control | 0.56 (0.44–0.66) | 0.72 (0.52–0.94) | 0.08 (0.04–0.12) | 0.67 (0.48–0.80) | ||||
| Renal disease | ||||||||
| Interstitial nephritis (n = 2) | 0.77 (0.55–1.00) | –a | 1.35 (1.09–1.60) | –a | 0.00 (0.00–0.00) | –a | 0.59 (0.47–0.71) | –a |
| Hereditary nephropathy (n = 3) | 1.40 (1.25–1.60) | –a | 1.00 (0.90–1.42) | –a | 0.40 (0.00–0.67) | –a | 1.09 (0.57–1.58) | –a |
| IgA nephropathy (n = 10) | 0.80 (0.34–1.45) | 0.12 | 1.29 (0.38–1.76) | 0.20 | 0.31 (0.00–0.70) | 0.21 | 1.14 (0.83–1.39) | 0.001* |
| Membranous glomerulopathy (n = 5) | 1.69 (1.31–2.07) | 0.001* | 2.00 (1.63–2.28) | 0.001* | 0.83 (0.39–0.96) | 0.06 | 1.38 (1.05–1.58) | 0.003* |
| ANCA-associated vasculitis (n = 6) | 1.49 (1.22–2.04) | 0.002* | 1.30 (0.68–2.10) | 0.09 | 0.13 (0.00–1.04) | 0.99 | 1.33 (0.77–1.67) | 0.02* |
| Thrombotic microangiopathy (n = 2) | 1.29 (0.90–1.69) | –a | 1.66 (1.43–1.89) | –a | 0.20 (0.13–0.27) | –a | 1.32 (1.00–1.64) | –a |
| Otherb (n = 9) | 1.57 (1.13–2.08) | 0.0006* | 1.33 (0.74–1.69) | 0.03* | 0.30 (0.10–0.49) | 0.02* | 1.14 (0.96–1.52) | 0.0006* |
P-value for comparison between specific renal disease and control specimens.
aBecause of the small group size, no statistical analysis was performed here.
bAmyloidosis, cast nephropathy, diabetic nephropathy, lupus nephritis, membranoproliferative glomerulonephritis, minimal change nephropathy and renal sarcoidosis.
*P < .05 (Mann–Whitney test).
IHC: immunohistochemistry; IQR: interquartile range.
Renal localization of TIMP-2 and IGFBP7 by fluorescence microscopy
To further characterize the presence of IGFBP7 and TIMP-2 in the kidney, immunofluorescence double staining was performed. In the tubular compartment, IGFBP7 could be detected predominantly in megalin-positive proximal tubules (Fig. 4A) with a vesicular staining pattern (Fig. 4B) and some distal tubules positive for the sodium/calcium exchanger (Fig. 4C). In contrast, uromodulin-positive parts of the thick ascending limb did not stain for IGFBP7 (Fig. 4D). In agreement with the results of the immunohistochemical studies, IGFBP7 was detectable to a lesser extent in the glomerulus and could only be detected in a few podocytes (Fig. 4E), and especially in areas of the glomerulus that already showed podocyte loss (Fig. 4F). Co-localization of IGFBP7 could not be detected in alpha smooth muscle actin (SMA)-positive vascular muscle cells (Fig. 4G) or in CD31-positive endothelial cells (Fig. 4H).
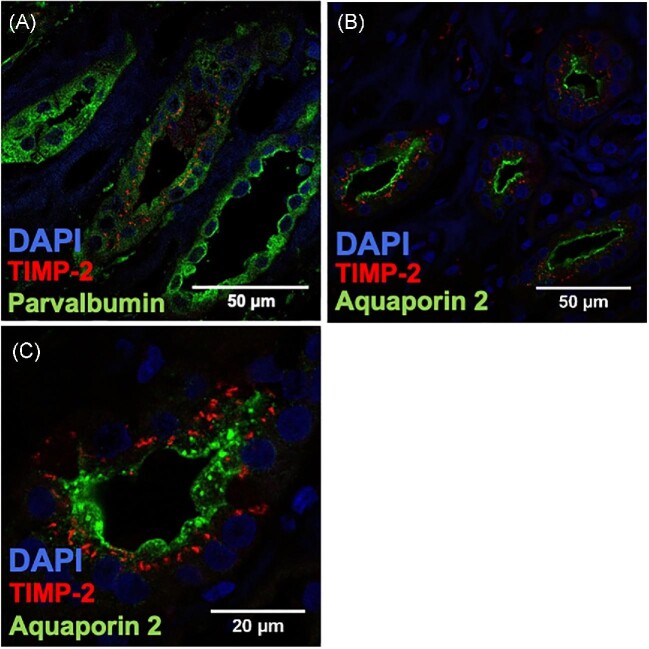
In contrast, TIMP-2 expression was too low in the glomerular compartment for detection by immunofluorescence. Expression of TIMP-2 was predominantly seen in the distal tubules (Fig. 5A; parvalbumin) and the collecting duct (Fig. 5B and C; aquaporin 2).
Figure 5:
Merged images of TIMP-2 and cell-specific markers are shown using immunofluorescence microscopy. TIMP-2 staining was combined with the marker parvalbumin for (A) distal tubules and collecting duct and (B, C) aquaporin 2 for collecting duct cells.
Association of TIMP-2/IGFBP7 and urinary [TIMP-2]*[IGFBP7]
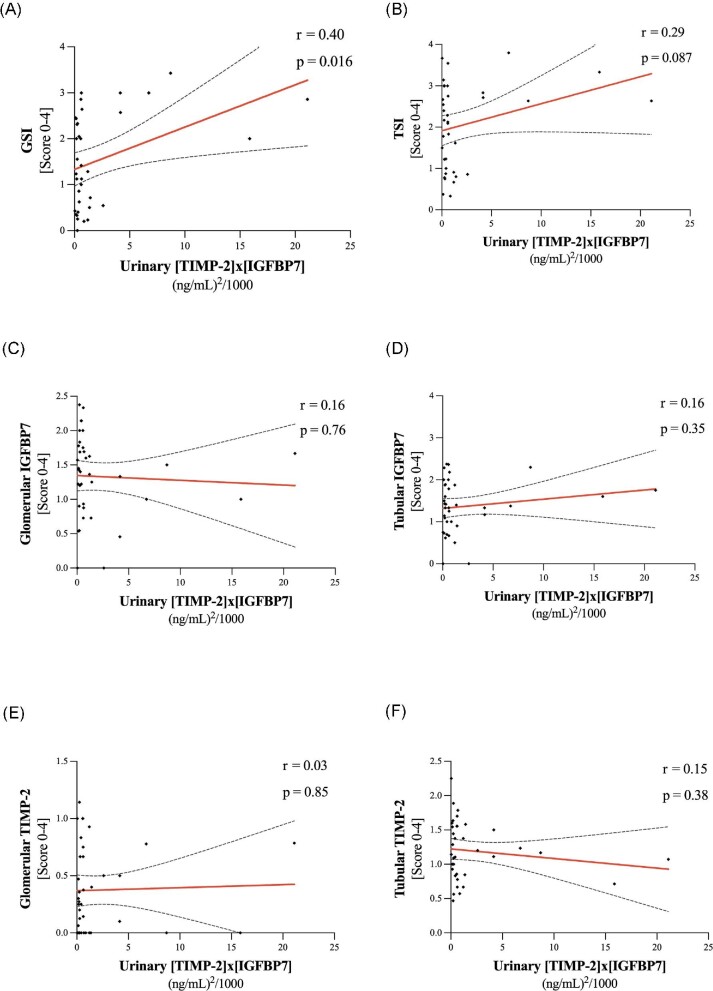
Regarding urinary [TIMP-2]*[IGFBP7] taken simultaneously with the biopsy, only GSI showed a significant association with the urinary marker (r = 0.40, P = .016; Fig. 6A) and TSI showed a trend (r = 0.29, P = .087; Fig. 6B) towards a positive correlation. In contrast, we could not show a significant association of glomerular/tubular IGFBP7 and TIMP-2 quantified by immunohistochemical staining with urinary [TIMP-2]*[IGFBP7] (Fig. 6C–F).
Figure 6:
Association of urinary [TIMP-2]*[IGFBP7] and (A) GSI, (B) TSI, (C, D) glomerular and tubular IGFBP7 as well as (E, F) glomerular and tubular TIMP-2. Spearman's r and P-values for correlations.
DISCUSSION
Our results provide remarkable insights into the presence and localization of TIMP-2 and IGFBP7 in some human renal disease.
Looking at previous data on the expression of TIMP-2 and IGFBP7 assessed by immunohistochemistry, there are only a few data for human kidney tissue. Evidence is mainly derived from cell culture or animal models. Emlet et al. [23] showed the expression and secretion of TIMP-2 and IGBP7 in primary tubular cells. In vitro ischaemia/reperfusion AKI leads to suppression of TIMP-2 and IGFBP7 followed by a burst of TIMP-2 and IGFBP7 secretion after restoration of oxygen and nutrients, as demonstrated in immunoblots [23]. Expression in human kidney transplants of deceased organ donors was variable, with only a fraction of tubules staining for both markers or co-localization with other injury markers such as kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin. In contrast, in experimental AKI in mice, immunohistochemistry of TIMP-2 and IGFBP7 staining showed a progressive decrease in damaged proximal tubule cells, whereas TIMP-2 and IGFBP7 messenger RNA (mRNA) remained at normal levels [10].
Looking at our data, there is a clear association between tissue expression of TIMP-2 and IGFBP7 and histomorphological findings: higher renal injury scores (TSI and GSI) were significantly associated with higher staining scores of TIMP-2 and IGBPP7. The association was present in both glomerular and tubular areas for these two protein markers.
According to our data, TIMP-2/IGFBP7 expression in renal disease is higher than in control tissue. In the tubular compartment, TIMP-2/IGFBP7 was expressed significantly more in biopsies with renal disease, whereas in the glomerulus only IGFBP7 showed statistically significantly higher expression levels. The compartment-specific differences (tubular versus glomerular) are not surprising, as both markers are primarily considered tubular markers. Data on TIMP-2/IGFBP7 expression and localization in healthy human kidneys and renal disease are limited. Nevertheless, increased mRNA expression of both markers is described in several renal diseases (e.g. focal segmental glomerulosclerosis, IgA nephropathy, lupus nephritis, diabetic nephropathy, vasculitis) and CKD [22, 27–31] (see Supplementary data from Nephroseq.org).
Therefore the increased expression of TIMP-2 and IGFBP7 in renal disease appears to be consistent with the existing literature.
It is postulated that various forms of AKI may be associated with transient cell cycle arrest [32]. TIMP-2 and IGFBP7 appear to harbour cell cycle arrest properties [5], although it is not clear whether the proteins are actually causative of arrest themselves or are merely surrogate markers. Our results show significantly lower proliferative activity in biopsies with renal disease than in control kidneys, especially for tubular and interstitial cells. This could support the hypothesis that in our group of renal diseases, acute damage was still present, so cell cycle arrest has occurred and the proliferation stage of the repair process has not yet started.
Considering the tubular localization of TIMP-2 and IGFBP7 in our study, a predominant localization of expression in the area of the collecting duct/distal tubule can be observed for TIMP-2 and for IGFBP7 mainly the proximal tubules, the collecting duct/distal tubules and the podocytes.
Against this background, previous theories describing TIMP-2 and IGFBP7 primarily as markers of proximal tubules seem inconsistent at first glance, as we could not detect TIMP-2 in the proximal tubules. In the light of previous findings, however, this is not contradictory. In a mouse model, Johnson et al. [9] hypothesized that the proteins could be ‘used up’ and secreted upon tubular damage, in the sense of tubular cell leakage. This may be the case in our specimens, where IGFBP7 staining in proximal tubular cells was relatively weak.
Slightly contrary, Emlet et al. [23] showed, in human kidneys of deceased organ donors, IGFBP7 expression almost exclusively and only in a fraction of the proximal tubules. They speculated that it may be the subfraction of cells responding to the injury, as the staining pattern was found to be similar to the heterogeneous/patchy histology of AKI. Indeed, IGFBP7 cannot be detected in all megalin-positive proximal tubules even in our study. Although tubular IGFBP7 correlates with tubular damage, we could not detect differences in tubular damage between IGFBP7-positive and negative tubules, but this could also be due to the reasons already mentioned by Johnson et al. [10].
Looking at TIMP-2 expression, Emlet et al. [23] found quite similar localization with preference to distal tubule cells.
Surprisingly, no significant differences in TIMP-2/IGFBP7 expression were found between the AKI and non-AKI groups and no correlation was found between urinary [TIMP-2]*[IGFBP7] expression and tissue TIMP-2/IGFBP7 expression. One explanation for this observation could be the fact that the diagnosis of AKI was based solely on serum creatinine without including urine excretion. The lack of inclusion of urinary excretion generally complicates the interpretation of the urinary [TIMP-2]*[IGFBP7] score, as the test has only been evaluated and validated in cohorts with documented urinary excretion [5]. Therefore the correlation of our immunohistochemical results with the documented clinical serum creatinine–based AKI diagnosis seems questionable. Although we were able to clearly show that expression was clearly dependent on histological tissue damage, correlation of tissue expression with measured urinary marker concentrations is difficult, as both factors are secreted and accumulation in tissue is not expected. Furthermore, in the presence of underlying renal disease, a large proportion of patients have chronic renal insufficiency, in which the acute event may be attenuated in the presence of acute–chronic renal injury and may merely reflect a fluctuation in serum creatinine. Baseline renal function was significantly different in the AKI and non-AKI groups, which complicates comparison of both groups. Our cohort showed a significant correlation of immunohistochemically detected expression of TIMP-2/IGFBP7 or [TIMP-2]*[IGFBP7] in patients with well-established histological renal damage scores. We would consider these correlations to be much more crucial than clinical serum creatinine–based diagnosis, making the limitations of solely serum creatinine–based AKI diagnosis all the more obvious.
We have to acknowledge the limitation that due to the formalin-fixed, paraffin-embedded tissue, accurate gene expression analyses were not feasible. However, against the background of the results of Johnson et al. [10], no short-term gene expression increases were expected. In addition, our cohort of mixed samples with different renal diseases makes the interpretation of a single disease entity difficult.
In conclusion, TIMP-2 and IGFBP7 expression is significantly higher in renal biopsies with renal disease than in non-diseased control tissue, especially in tubules. Correlation of tissue expression and urinary [TIMP-2]*[IGFBP7] with established histological renal injury scores underscores its properties as injury markers. Tubular localization was found primarily in selected proximal and distal tubules as well as collecting ducts, which reinforces the theory of marker depletion following injury to the proximal tubule.
Supplementary Material
ACKNOWLEDGEMENTS
The technical assistance of S. Söllner, M. Reutelshöfer, D. Biegger, A. Schwab and B. Rettenmaier is gratefully acknowledged.
Contributor Information
Moritz Schanz, Department of Internal Medicine, Division of General Internal Medicine and Nephrology, Robert-Bosch Hospital Stuttgart, Germany.
Martin Kimmel, Department of Internal Medicine, Division of Nephrology, Hypertension and Autoimmune Disorders, Alb-Fils Kliniken, Göppingen, Germany.
Mark Dominik Alscher, Department of Internal Medicine, Division of General Internal Medicine and Nephrology, Robert-Bosch Hospital Stuttgart, Germany.
Kerstin Amann, Department of Nephropathology, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany.
Christoph Daniel, Department of Nephropathology, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany.
FUNDING
The study was funded by DFG and Robert-Bosch Foundation. We acknowledge support from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation; project no. 387509280, SFB 1350).
AUTHORS’ CONTRIBUTIONS
C.D. and M.S. collected and analyzed data, performed experiments and wrote the manuscript, K.A. and M.D.A. collected data and edited the manuscript, M.K. initiated the study, collected and analyzed clinical data and edited the manuscript.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared upon reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
M.K. received lecture honoraria from Abbott, Roche and Astute Medical. M.D.A. received lecture honoraria from Abbott and Roche. M.S. received lecture honoraria from BioMerieux. The remaining authors have no conflicts of interest to declare.
REFERENCES
- 1. Mao H, Katz N, Ariyanon Wet al. Cardiac surgery-associated acute kidney injury. Cardiorenal Med 2013;3:178–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol 2014;10:193–207. [DOI] [PubMed] [Google Scholar]
- 3. Chertow GM, Burdick E, Honour Met al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]
- 4. Palevsky PM, Liu KD, Brophy PDet al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 2013;61:649–72. [DOI] [PubMed] [Google Scholar]
- 5. Kashani K, Al-Khafaji A, Ardiles Tet al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013;17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bihorac A, Kellum JA. Acute kidney injury in 2014: a step towards understanding mechanisms of renal repair. Nat Rev Nephrol 2015;11:74–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kimmel M, Shi J, Latus Jet al. Association of renal stress/damage and filtration biomarkers with subsequent AKI during hospitalization among patients presenting to the emergency department. Clin J Am Soc Nephrol 2016;11:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koyner JL, Shaw AD, Chawla LSet al. Tissue Inhibitor metalloproteinase-2 (TIMP-2)·IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol 2015;26:1747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kimmel M, Shi J, Wasser Cet al. Urinary [TIMP-2]·[IGFBP7] – novel biomarkers to predict acute kidney injury. Am J Nephrol 2016;43:375–82. [DOI] [PubMed] [Google Scholar]
- 10. Johnson ACM, Zager RA. Mechanisms underlying increased TIMP2 and IGFBP7 urinary excretion in experimental AKI. J Am Soc Nephrol 2018;29:2157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seo DW, Li H, Qu CKet al. Shp-1 mediates the antiproliferative activity of tissue inhibitor of metalloproteinase-2 in human microvascular endothelial cells. J Biol Chem 2006;281:3711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pitiyage GN, Lim KP, Gemenitzidis Eet al. Increased secretion of tissue inhibitors of metalloproteinases 1 and 2 (TIMPs -1 and -2) in fibroblasts are early indicators of oral sub-mucous fibrosis and ageing. J Oral Pathol Med 2012;41:454–62. [DOI] [PubMed] [Google Scholar]
- 13. Vizioli MG, Sensi M, Miranda Cet al. IGFBP7: an oncosuppressor gene in thyroid carcinogenesis. Oncogene 2010;29:3835–44. [DOI] [PubMed] [Google Scholar]
- 14. Amemiya Y, Yang W, Benatar Tet al. Insulin like growth factor binding protein-7 reduces growth of human breast cancer cells and xenografted tumors. Breast Cancer Res Treat 2011;126:373–84. [DOI] [PubMed] [Google Scholar]
- 15. Severino V, Alessio N, Farina Aet al. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis 2013;4:e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rupp C, Scherzer M, Rudisch Aet al. IGFBP7, a novel tumor stroma marker, with growth-promoting effects in colon cancer through a paracrine tumor-stroma interaction. Oncogene 2015;34:815–25. [DOI] [PubMed] [Google Scholar]
- 17. Asanuma K, Shirato I, Ishidoh Ket al. Selective modulation of the secretion of proteinases and their inhibitors by growth factors in cultured differentiated podocytes. Kidney Int 2002;62:822–31. [DOI] [PubMed] [Google Scholar]
- 18. Sohn SJ, Kim SY, Kim HSet al. In vitro evaluation of biomarkers for cisplatin-induced nephrotoxicity using HK-2 human kidney epithelial cells. Toxicol Lett 2013;217:235–42. [DOI] [PubMed] [Google Scholar]
- 19. Kugler A, Hemmerlein B, Thelen Pet al. Expression of metalloproteinase 2 and 9 and their inhibitors in renal cell carcinoma. J Urol 1998;160:1914–8. [PubMed] [Google Scholar]
- 20. Wang Z, Famulski K, Lee Jet al. TIMP2 and TIMP3 have divergent roles in early renal tubulointerstitial injury. Kidney Int 2014;85:82–93. [DOI] [PubMed] [Google Scholar]
- 21. Phillips AO, Steadman R, Morrisey Ket al. Exposure of human renal proximal tubular cells to glucose leads to accumulation of type IV collagen and fibronectin by decreased degradation. Kidney Int 1997;52:973–84. [DOI] [PubMed] [Google Scholar]
- 22. Czech KA, Bennett M, Devarajan P. Distinct metalloproteinase excretion patterns in focal segmental glomerulosclerosis. Pediatr Nephrol 2011;26:2179–84. [DOI] [PubMed] [Google Scholar]
- 23. Emlet DR, Pastor-Soler N, Marciszyn Aet al. Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: differential expression and secretion in human kidney tubule cells. Am J Physiol Renal Physiol 2017;312:F284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hainz N, Thomas S, Neubert Ket al. The proteasome inhibitor bortezomib prevents lupus nephritis in the NZB/W F1 mouse model by preservation of glomerular and tubulointerstitial architecture. Nephron Exp Nephrol 2012;120:e47–58. [DOI] [PubMed] [Google Scholar]
- 25. Solez K, Colvin RB, Racusen LCet al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 2008;8:753–60. [DOI] [PubMed] [Google Scholar]
- 26. Kidney Disease: Improving Global Outcomes Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138. [Google Scholar]
- 27. Nakagawa S, Nishihara K, Miyata Het al. Molecular markers of tubulointerstitial fibrosis and tubular cell damage in patients with chronic kidney disease. PLoS One 2015;10:e0136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ju W, Greene CS, Eichinger Fet al. Defining cell-type specificity at the transcriptional level in human disease. Genome Res 2013;23:1862–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ju W, Nair V, Smith Set al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 2015;7:316ra193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reich HN, Tritchler D, Cattran DCet al. A molecular signature of proteinuria in glomerulonephritis. PLoS One 2010;5:e13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sampson MG, Robertson CC, Martini Set al. Integrative genomics identifies novel associations with APOL1 risk genotypes in black NEPTUNE subjects. J Am Soc Nephrol 2016;27:814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson ACM, Zager RA. Mechanisms and consequences of oxidant-induced renal preconditioning: an Nrf2-dependent, P21-independent, anti-senescence pathway. Nephrol Dial Transplant 2018;33:1927–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.