ABSTRACT
Background
Kidney transplant patients with glomerulonephritis (GN) as their native disease may receive significant amounts of pre-transplant immunosuppression (PTI), which could increase the risk for development of malignancy post-transplant.
Methods
We conducted a single-center, retrospective study of kidney transplant recipients from January 2005 until May 2020. Patients with GN as their native kidney disease who received PTI for treatment of GN (n = 184) were compared with a control cohort (n = 579) of non-diabetic, non-PTI-receiving kidney transplant patients. We calculated hazard ratios (HR) with 95% confidence intervals (95% CI) for outcomes of first occurrence of solid or hematologic malignancy, non-melanoma skin cancer (NMSC) and post-transplant lymphoproliferative disorder (PTLD).
Results
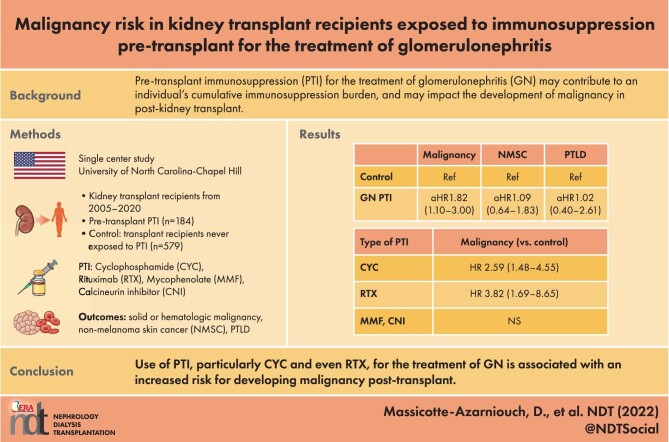
Over a median follow-up of 5.7 years, PTI for GN was associated with significantly increased risk for malignancy compared with controls [13.0% vs 9.7%, respectively; adjusted HR 1.82 (95% CI 1.10–3.00)], but not for NMSC [10.3% vs 11.4%, respectively; adjusted HR 1.09 (95% CI 0.64–1.83)] or PTLD [3.3% vs 3.1%, respectively; adjusted HR 1.02 (95% CI 0.40–2.61)]. The risk for malignancy was significantly increased in those who received cyclophosphamide [HR 2.59 (95% CI 1.48–4.55)] or rituximab [HR 3.82 (95% CI 1.69–8.65)] pre-transplant, and particularly in those who received both cyclophosphamide and rituximab, but not for calcineurin inhibitors or mycophenolate.
Conclusion
The use of PTI for treatment of GN, especially cyclophosphamide or even with rituximab, is associated with increased risk for development of solid or hematologic malignancy post-transplant. These data highlight potential risks with treatment of GN and underscore the importance of post-transplant malignancy surveillance in this patient population.
Keywords: glomerulonephritis, immunosuppression, kidney transplant, malignancy, non-melanomatous skin cancer
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Immunosuppression for the treatment of glomerular disease pre-kidney transplant may impact adverse outcomes post-transplant by contributing to overall immunosuppressive burden. Notably, this could influence the development of malignancy post-transplant. Few studies have examined this association.
A better understanding of this possible risk from the treatment of glomerular disease pre-transplant, particularly in the era of lower dose immunosuppression and with newer agents felt to be less associated with malignancy, could help guide clinicians and patients in decision-making.
What this study adds?
The use of pre-transplant immunosuppression for the treatment of glomerulonephritis is associated with a greater risk for developing post-transplant malignancy.
This was true for solid and hematologic malignancies, but not for non-melanomatous skin cancers.
The increased risk was mostly seen with the use of cyclophosphamide and of rituximab pre-transplant, but not with mycophenolate mofetil or with calcineurin inhibitors.
What impacts this may have on practice or policy?
The risks for adverse outcomes post-transplant are often overlooked or given little consideration when deciding on treatment of glomerulonephritis pre-transplant.
Patients receiving a significant amount of immunosuppression pre-transplant should be counseled on these risks and monitored for malignancy post-transplant.
Transplant registries should capture the use of pre-transplant immunosuppression to further improve our understanding of this problem, particularly as treatments for glomerular diseases continue to evolve.
INTRODUCTION
Kidney transplant is the preferred method of renal replacement therapy as it offers increased survival and quality of life compared with dialysis [1–4]. However, the need for intense, life-long immunosuppression after kidney transplant puts recipients at high risk for complications, notably malignancy [5, 6]. The cumulative amount of immunosuppression received post-transplant, as well as the intensity of induction and maintenance therapies, confer an increased risk for the development of malignancy post-transplant [7–10]. An often overlooked but potentially important contributor to a kidney transplant patient's immunosuppressive burden, and thus subsequent malignancy risk, is treatment received prior to kidney transplant.
Glomerulonephritis (GN) is a leading cause of end-stage kidney disease (ESKD), and seen frequently among individuals receiving a kidney transplant [11, 12]. Patients with GN who eventually undergo kidney transplantation have often been exposed to immunosuppression for treatment of their underlying GN prior to transplant. The types, amount and duration of immunosuppression often vary based on the type of glomerular disease [13]. These therapies may include corticosteroids, calcineurin inhibitors, cytotoxic therapies, anti-proliferative anti-metabolites and, more recently, anti-CD20 therapy. Combinations of any number of these agents may also be used for management of the underlying GN. The duration of immunosuppression may vary greatly from a few months to many years [14]. These prospective candidates with underlying GN can accrue significant immunosuppression exposure prior to transplant which, added to further immunosuppression post-transplant, could potentially increase risk for malignancy after transplant.
The goal of this study was to examine the risks for malignancy post-kidney transplant in individuals treated with immunosuppression for GN prior to kidney transplant. We examined an era of pre-transplant immunosuppression (PTI) including those receiving more modern lower dose immunosuppression regimens and agents such as anti-CD20 therapy, thought to be less associated with malignancy risks [15]. We hypothesized that receipt of PTI would be associated with an increased risk for malignancy post-transplant.
MATERIALS AND METHODS
Study design, setting and participants
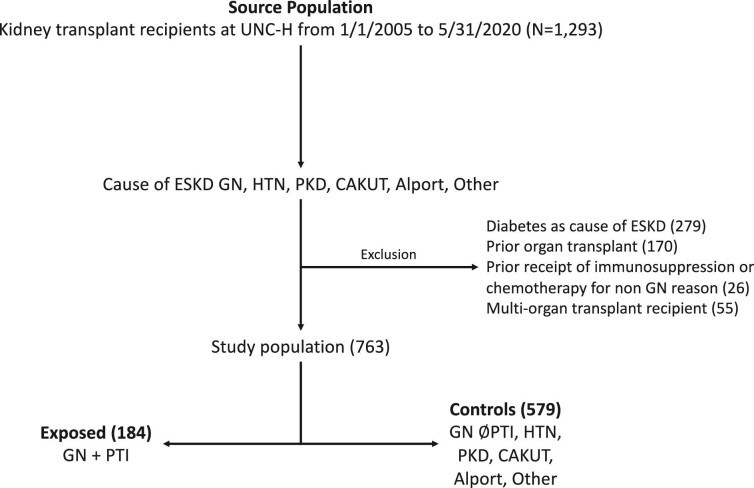
We conducted a single-center, retrospective study at the University of North Carolina (UNC) after obtaining local institutional review board approval. Study participants consisted of all individuals (pediatric and adult) who received a kidney transplant at UNC Hospitals between 1 January 2005 and 31 May 2020. These individuals were identified through the solid organ transplant registry at UNC; patients are followed from time of transplant until either loss to follow-up (e.g. moving to another center/state), graft failure or death. All kidney transplant patients are seen at least twice a year, even if their primary follow-up occurs with another provider outside UNC, and outcome data are captured during these visits. We excluded individuals who had diabetes designated as their cause of kidney failure, those who had received prior organ transplant (because of exposure to prior immunosuppression), those who received multi-organ transplant, and those who received chemotherapy or immunosuppression prior to transplant for indications other than treatment of GN (e.g. malignancy pre-transplant treated with chemotherapy or treatment of a non-GN autoimmune disease such as Crohn's disease). Exclusions were ascertained through chart review and implemented to produce comparable exposure and control groups in terms of comorbidities and inherent risks for adverse outcomes, and to ensure the exposure of interest was not contaminated by immunosuppression used for non-GN diseases.
The exposure of interest was receipt of PTI among patients with GN as the primary cause of kidney failure. Patients included those who had the following GN diagnoses: focal segmental glomerulosclerosis (FSGS), immunoglobulin A nephropathy (IgAN), lupus nephritis (LN), anti-neutrophil cytoplasmic antibody (ANCA) vasculitis, membranous nephropathy (MN), membranoproliferative glomerulonephritis, cryoglobulinemia, C3 glomerulopathy, anti-glomerular basement membrane disease, atypical hemolytic uremic syndrome, fibrillary GN and immunotactoid GN. The cause of kidney failure was captured in the patient registry, as required by the United Network for Organ Sharing, and was confirmed and further specified (for patients with GN) by direct chart review. The receipt of PTI for treatment of GN was obtained from chart review. We defined a patient as exposed to PTI if any of the following occurred pre-transplant for treatment of GN: at least one dose of intravenous cyclophosphamide or rituximab were administered, or prescriptions had been provided for oral cyclophosphamide, mycophenolate (MMF), azathioprine (AZA), calcineurin inhibitor (CNI) or oral corticosteroid of ≥20 mg prednisone equivalent per day for ≥4 weeks. We quantified PTI exposure for each patient and each drug group by ascertaining total grams of cyclophosphamide (intravenous and oral) and rituximab used, and total months of MMF, AZA and CNI use. We were not able to quantify the exact duration of exposure to high-dose prednisone because dosage and duration were not consistently captured in the patient record. Supplementary File 1 explains how we proceeded when we did not have information on dosage or duration of PTI.
Those with GN who were exposed to PTI were compared with a control population that consisted of ESKD due to: (i) GN and never exposed to PTI; (ii) hypertension; (iii) polycystic kidney disease; (iv) congenital anomalies of the kidneys and urinary tracts; (v) Alport syndrome; and (vi) any other cause of ESKD where the individual would not have received PTI or chemotherapy for treatment of that disease. These patients were chosen since they would be expected to be similar by demographics and comorbidities to transplant patients with GN as a cause of ESKD but would not have received PTI for their primary disease.
Covariates
The following variables were collected through chart review, and were selected a priori, based on what were felt to be potentially important confounders: year of transplant, age at transplant, sex, race, cause of ESKD, type of transplant (living donor, donation after brain death, donation after cardiac death), time on dialysis (dialysis vintage, including pre-emptive transplants where vintage was considered zero), Epstein–Barr virus (EBV) and cytomegalovirus donor–recipient status, pre-transplant panel reactive antibodies (most recent value pre-transplant), type of induction therapy (T-cell-depleting or not) and type of T-cell-depleting therapy [either alemtuzumab or anti-thymocyte globulin (ATG)], maintenance therapy initially prescribed after transplant (tacrolimus or other), presence of diabetes as a comorbidity (without being cause of ESKD, based on chart review), smoking history pre-transplant and presence of delayed graft function post-transplant. Supplementary File 2 details the usual immunosuppression protocol at our institution during the study period.
Outcomes
Our main study outcomes were: first occurrence of non-melanomatous skin cancer (NMSC) (basal cell carcinoma or squamous cell carcinoma), solid or hematologic malignancy, or post-transplant lymphoproliferative disorder (PTLD). All were ascertained through chart review. As long as a patient was followed at our institution, these outcomes would have been identified during their follow-up visit or through keyword search in the electronic record.
Statistical analysis
For both study groups (GN exposed to PTI vs no PTI), descriptive statistics were calculated for each covariate [medians with interquartile ranges (IQR) and numbers with percentages, where appropriate]. Differences between groups were evaluated using Wilcoxon two sample test and Fisher's exact test. P-values <.05 were considered significant. We calculated the median with IQR of grams of cyclophosphamide and rituximab received pre-transplant, as well as months of MMF, AZA and CNI use.
Counts with percentages, cumulative incidence rates at 1, 5 and 10 years, and Kaplan–Meier survival curves were determined for each outcome: NMSC, solid or hematologic malignancy, and PTLD. Hazard ratios (HR) with 95% confidence intervals (95% CI) for outcomes based on presence or absence of PTI were estimated using Cox proportional hazards models. Multivariable Cox regression models were used to generate adjusted HR (aHR), accounting for the following covariates: age, sex, race, donor type, year of transplant surgery, dialysis vintage and receipt of T-cell-depleting induction, and age for PTLD. These covariates were felt to be most clinically important, and their selection kept an event-to-variable ratio of 5–10. The index date (time zero) for start of follow-up was the date of kidney transplant. The end of follow-up was the earliest of either occurrence of an outcome, death, graft loss (permanent return to dialysis or re-transplantation), loss to follow-up (transfer to another program and no longer followed at the UNC transplant clinic), or end of study period (1 June 2021, to allow minimum 1-year follow-up for each study participant). We also calculated aHRs for outcomes based on whether PTI was cyclophosphamide, rituximab, MMF or CNI. We did not perform these analyses for AZA due to the small number of patients who received AZA as PTI. All analyses and plots were conducted with SAS software (Version 9.4 SAS Institute, Cary, NC, USA).
Additional analyses
We examined the risk for malignancy after excluding any individual who had malignancy pre-transplant which was not treated with chemotherapy (for example, renal cell carcinoma treated solely with nephrectomy). After noting post hoc an increased risk for malignancy with rituximab, we determined risk for malignancy in those who received rituximab but never cyclophosphamide, those who received both rituximab and cyclophosphamide, and those who received cyclophosphamide but never rituximab. We also examined occurrence of malignancy based on exposure to only one single type of PTI. Given the differing effects on lasting lymphopenia from alemtuzumab compared with ATG [16], we calculated HRs for outcomes in those who received alemtuzumab and those who received ATG separately. We also calculated the risks for the adverse outcomes of interest restricting to only adults (≥18 years old at time of transplant) given differing risks for malignancy in children compared with adults. We examined risk for rejection associated with PTI since this could lead to further immunosuppression which could potentially impact subsequent malignancy. Finally, we analysed the risk for malignancy restricting to a study population consisting only of individuals with GN as their native disease (GN PTI vs GN no PTI).
RESULTS
Population characteristics
A total of 763 kidney transplant patients were followed for a median 5.7 years. The median age was 46 years, 44.4% were female, 43.9% were white, 42.9% Black, 35.8% were recipients of a living donor transplant and 85.9% received T-cell-depleting induction, the vast majority of which (86.6%) was alemtuzumab (Table 1). There were 331 who had GN as a cause of ESKD, of which 127 (38.4%) had FSGS, 55 (16.6%) IgAN, 36 (10.9%) LN, 33 (10.0%) ANCA vasculitis, 19 (5.7%) MN and 61 (18.4%) other type of GN. There were 184 who had GN and PTI (of which 31.0% had FSGS, 13.6% IgA, 18.0% LN, 17.4% ANCA vasculitis, 7.6% membranous and 12.5% other type of GN), and 579 controls (see Fig. 1). Patients who had GN and PTI were more likely to be female and younger, and have a shorter dialysis vintage compared with controls.
Table 1:
Baseline characteristics.
| Characteristics | Total population (n = 763) | GN PTI (n = 184) | Control (n = 579) | P-value |
|---|---|---|---|---|
| Sex female, n (%) | 336 (44.4) | 93 (50.5) | 243 (41.6) | .0498 |
| Pediatric, n (%) | 90 (11.8) | 24 (13.0) | 66 (11.4) | .5997 |
| Age (years), median (IQR) | 46 (31–57) | 37.5 (24.5–52) | 49 (35–58) | <.0001 |
| Age category, n (%) | .695 | |||
| <12 years | 43 (5.6) | 11 (6.0) | 32 (5.5) | |
| 12–17 years | 47 (6.2) | 13 (7.7) | 34 (5.8) | |
| 18–65 years | 604 (79.2) | 147 (79.9) | 457 (78.9) | |
| >65 years | 69 (9.0) | 13 (7.1) | 56 (9.7) | |
| Race/ethnicity, n (%) | .152 | |||
| White | 335 (43.9) | 89 (48.4) | 246 (42.5) | |
| Black | 327 (42.9) | 66 (35.8) | 261 (45.1) | |
| Hispanic | 63 (8.3) | 18 (9.8) | 45 (7.8) | |
| Other | 38 (5.0) | 11 (6.0) | 27 (4.7) | |
| Year of transplant, n (%) | .0128 | |||
| 2005–09 | 222 (29.1) | 38 (20.7) | 184 (31.8) | |
| 2010–14 | 234 (30.7) | 64 (34.8) | 170 (29.4) | |
| 2015–20 | 307 (40.2) | 82 (44.6) | 225 (38.9) | |
| Native kidney disease, n (%) | <.0001 | |||
| GN | 331 (43.4) | 184 (100) | 147 (25.4) | |
| Hypertension | 154 (20.2) | 154 (26.6) | ||
| PKD | 81 (10.6) | 81 (14.0) | ||
| CAKUT | 80 (10.5) | 80 (13.8) | ||
| Unknown/other | 117 (15.3) | 117 (20.2) | ||
| Dialysis vintage months, median (IQR) | 44.7 (17.1–78.3) | 29.3 (12.5–69.3) | 51.1 (19.9–81.3) | .0002 |
| Pre-emptive transplant, n (%) | 148 (19.4) | 36 (19.6) | 112(19.3) | 1 |
| Donor type, n (%) | .0425 | |||
| Living | 273 (35.8) | 79 (42.9) | 194 (33.5) | |
| DBD | 398 (52.2) | 89 (48.4) | 309 (53.4) | |
| DCD | 92 (12.1) | 16 (8.7) | 76 (13.1) | |
| PRA %, mean (SD) | 6.0 (19.1) | 5.1 (17.2) | 6.3 (19.7) | .8104 |
| History of diabetes pre-transplant, n (%) | 35 (4.6) | 10 (5.4) | 25 (4.3) | .5451 |
| Smoking history pre-transplant, n (%) | 232 (30.4) | 44 (23.9) | 188 (32.5) | .0277 |
| EBV transplant status, n (%) | .1643 | |||
| Donor–/recipient– | 19 (2.5) | 8 (4.4) | 11 (1.9) | |
| Recipient+ | 699 (91.6) | 164 (89.1) | 535 (92.4) | |
| Donor+/recipient– | 45 (5.9) | 12 (6.5) | 33 (5.7) | |
| CMV transplant status, n (%) | .1288 | |||
| Donor–/recipient– | 160 (21.0) | 48 (26.1) | 112 (19.3) | |
| Recipient+ | 463 (60.7) | 102 (55.4) | 361 (62.4) | |
| Donor+/recipient– | 140 (18.4) | 34 (18.5) | 106 (18.3) | |
| T-cell-depleting induction therapy, n (%)a | 655 (85.9) | 152 (82.6) | 503 (86.9) | .1474 |
| Alemtuzumab | 567 (74.3) | 128 (69.6) | 439 (75.8) | |
| Thymoglobulin | 88 (11.5) | 24 (13.0) | 64 (11.1) | |
| Maintenance at time of transplant, n (%) | .1549 | |||
| Tacrolimus | 757 (99.2) | 181 (98.4) | 576 (99.5) | |
| Other | 6 (0.8) | 3(1.6) | 3 (0.5) | |
| Delayed graft function, n (%) | 169 (22.2) | 29 (15.8) | 140 (24.2) | .0188 |
aThirty-six individuals were imputed the type of depleting induction therapy (31 alemtuzumab, 5 thymoglobulin) when the exact depleting agent was not available through chart review, considering alemtuzumab only started being used in 2006 at our center.
CMV: cytomegalovirus; PKD: polycystic kidney disease; CAKUT: congenital anomalies of the kidney and urinary tract: PRA: panel reactive antibodies; DBD: donation after brain death; DCD: donation after cardiac death.
P-values were calculated using Fisher's exact test for categorical variables and Wilcoxon two samples test for continuous variable.
Figure 1:
Study flow chart for cohort creation. CAKUT: congenital anomalies of the kidneys and urinary tracts; HTN: hypertension; PKD: polycystic kidney disease; UNC-H: University of North Carolina Hospital.
Pre-transplant immunosuppression in patients with GN
Out of 184 patients with GN who received PTI, 81 (44.0%) had received cyclophosphamide, 31 (16.9%) rituximab, 83 (45.1%) MMF, 18 (9.8%) AZA, 60 (32.6%) CNI and 170 (92.4%) a course of high-dose prednisone at some point pre-transplant for treatment of GN. The PTI median cumulative dose of cyclophosphamide was 6 g and for rituximab it was 2 g. The median duration of MMF was 12 months, for AZA it was 17 months and for CNI it was 20.5 months (Table 2).
Table 2:
Pre-transplant immunosuppression use.
| Immunosuppression | GN PTI (n = 184) |
|---|---|
| Cyclophosphamide used, n (%) | 81 (44.0) |
| 0 to <10 g | 53 (28.8) |
| 10 to 25 g | 17 (9.2) |
| >25 g | 11 (6.0) |
| Cumulative dose (g), median (IQR) | 6.0 (3.0–13.5) |
| Rituximab used, n (%) | 31 (16.9) |
| 0 to <3 | 22 (12.0) |
| ≥3 | 9 (4.9) |
| Cumulative dose (g), median (IQR) | 2.0 (1.8–3.4) |
| MMF used, n (%) | 83 (45.1) |
| Total duration (months), median (IQR) | 12.0 (6.0–47.0) |
| AZA used, n (%) | 18 (9.8) |
| Total duration (months), median (IQR) | 17.0 (3.0–30.0) |
| CNI used, n (%) | 60 (32.6) |
| Total duration (months), median (IQR) | 20.5 (6.0–41.0) |
| High-dose prednisone used, n (%) | 170 (92.4) |
Percentages do not total 100 because a given participant could have received more than one type of immunosuppressant pre-transplant for the treatment of GN.
Risk for malignancies
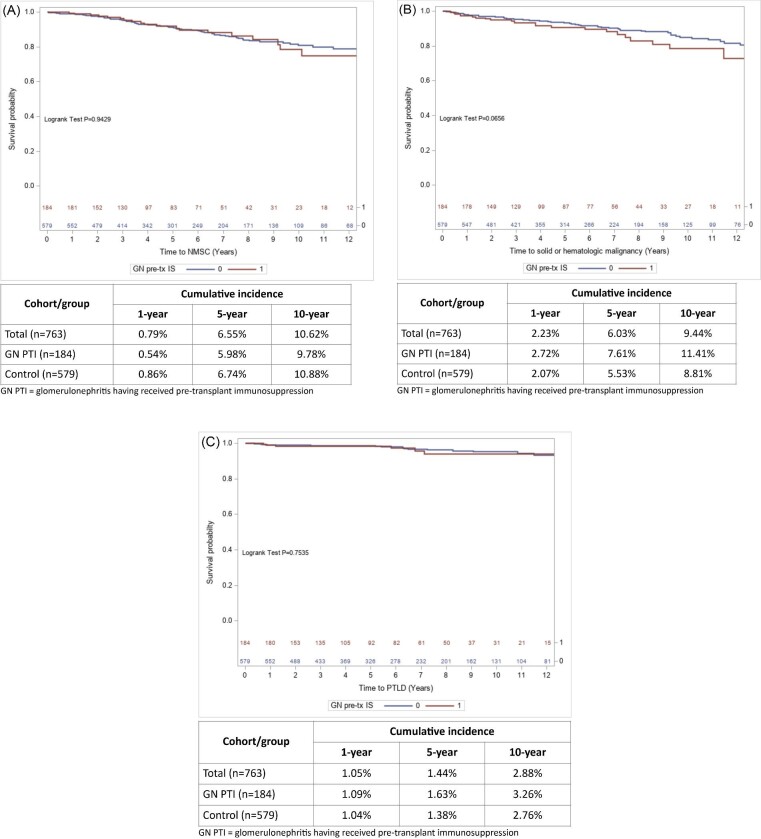
Eighty-five individuals developed a NMSC (11.1%); the first occurrence of NMSC was squamous cell carcinoma in 77.6%, and basal cell carcinoma in 22.4%. For patients with GN and PTI, compared with controls, time to occurrence of NSMC was similar (Fig. 2A) and there was no significant difference in risk for NMSC [10.3% vs 11.4%, respectively; aHR 1.09 (0.64–1.83); Table 3]. A solid tumor or hematologic malignancy occurred in 80 individuals (10.5%); the most frequent were renal cell carcinoma (20/80, 25.0%), PTLD (20/80, 25.0%) and lung cancer (11/80, 13.8%). Other types of first malignancies are shown in Supplementary data, Table S1. There was a trend for these malignancies to develop more quickly (Fig. 2B) and the risk was significantly greater in patients with GN and PTI compared with controls [13.0% vs 9.7%, respectively; aHR 1.82 (1.10–3.00); Table 3]. There were 24 individuals (3.2%) who developed PTLD; there was no difference in time-to-occurrence nor in risk for PTLD in patients with GN and PTI compared with controls (Fig. 2C, Table 3).
Figure 2:
Crude cumulative incidence for malignancies observed after kidney transplantation. (A) Kaplan–Meier curves of time to NMSC, with cumulative incidences. (B) Kaplan–Meier curves of time to solid or hematologic malignancy observed after kidney transplantation, with cumulative incidences. (C) Kaplan–Meier curves of time to PTLD, with cumulative incidences.
Table 3:
Occurrence of malignancy outcomes after kidney transplantation.
| Outcomes | Total population (n = 763) | GN PTI (n = 184) | Control (n = 579) |
|---|---|---|---|
| Follow-up time (years), median (IQR) | 5.7 (3.0–9.2) | 5.1 (2.9–8.6) | 5.8 (3.0–9.7) |
| Loss-to-follow-up, n (%) | 36 (4.7) | 6 (3.3) | 30 (5.2) |
| NMSC, n (%) | 85 (11.1) | 19 (10.3) | 66 (11.4) |
| Days to event, median (IQR) | 1407 (849–2389) | 1407 (873–2683) | 1390 (823–2389) |
| Univariate HR (95% CI) | 02 (0.61–1.70) | Reference | |
| Adjusted HR* (95% CI) | 1.1.09 (0.64–1.83) | Reference | |
| Malignancy, n (%) | 80 (10.5) | 24 (13.0) | 56 (9.7) |
| Days to event, median (IQR) | 1412 (470–2638) | 1384 (470–2759) | 1442 (473–2622) |
| Univariate HR (95% CI) | 1.54 (0.97–2.53) | Reference | |
| Adjusted HRa (95% CI) | 1.82 (1.10–3.00) | Reference | |
| PTLD, n (%) | 24 (3.2) | 6 (3.3) | 18 (3.1) |
| Days to event, median (IQR) | 1932 (306–2532) | 1283 (294–2464) | 1932 (317–2644) |
| Univariate HR (95% CI) | 1.16 (0.46–2.93) | Reference | |
| Adjusted HRb (95% CI) | 1.02 (0.40–2.61) | Reference |
aAdjusted for age, sex, race, donor type, year of transplant, dialysis vintage and receipt of T-cell-depleting induction.
bAdjusted for age at transplant.
Malignancy risk by type of immunosuppressant
Among the 81 who received cyclophosphamide, 10 (12.3%) developed a NMSC and 16 (22.2%) developed a solid or hematologic malignancy (Table 4). There was no significant difference in risk for NMSC among those who were exposed to cyclophosphamide [aHR 1.22 (0.61–2.42)], but risk for malignancy was significantly greater [aHR 2.59 (1.48–4.55)] compared with recipients who had no PTI. There were 31 in the GN PTI group who received rituximab; 4 (12.9%) developed NMSC and 7 (22.6%) a malignancy. Compared with recipients without PTI, there was no significant association between receipt of rituximab and NMSC risk [HR 0.79 (0.27–2.28)]; however, there was significantly greater risk for malignancy [HR 3.82 (1.69–8.65)]. Use of MMF and CNI pre-transplant were not associated with greater risk for NMSC nor malignancy.
Table 4:
NMSC and malignancy risk, by type of immunosuppressant.
| NMSC | Malignancy | |||
|---|---|---|---|---|
| Immunosuppression (N) | N (%) | aHR (95% CI)a | N (%) | aHR (95% CI)a |
| CYC | ||||
| Controls (579) | 66 (11.4) | Reference | 56 (9.7) | Reference |
| CYC PTI (81) | 10 (12.3) | 1.22 (0.61–2.42) | 16 (19.8) | 2.59 (1.48–4.55) |
| Controls (579) | 66 (11.4) | Reference | 56 (9.7) | Reference |
| 0 to <10 g (53) | 5 (9.4) | 0.96 (0.38–2.41) | 10 (18.9) | 2.47 (1.22–5.02) |
| 10 to 25 g (17) | 2 (11.8) | 0.80 (0.18–3.50) | 3 (17.6) | 3.18 (0.94–10.71) |
| >25 g (11) | 3 (27.3) | 4.45 (1.33–14.91) | 3 (27.3) | 2.57 (0.78–8.46) |
| RTX | ||||
| Controls (579) | 66 (11.4) | Reference | 56 (9.7) | Reference |
| RTX PTI (31) | 4 (12.9) | 0.79 (0.27–2.28) | 7 (22.6) | 3.82 (1.69–8.65) |
| 0 g (579) | 66 (11.4) | Reference | 56 (9.7) | Reference |
| 0 to <3 g (22) | 4 (18.2) | 0.97 (0.34–2.75) | 6 (27.3) | 4.11 (1.70–9.93) |
| ≥3 g (9) | 0 | N/A | 1 (11.1) | 2.02 (0.27–15.20) |
| MMF | ||||
| Controls (579) | 66 (11.4) | Reference | 56 (9.7) | Reference |
| MMF PTI (83) | 10 (12.0) | 1.24 (0.61–2.49) | 9 (10.8) | 1.73 (0.83–3.60) |
| CNI | ||||
| Controls (579) | 66 (11.4) | Reference | 56 (9.7) | Reference |
| CNI PTI (60) | 3 (5.0) | 1.08 (0.34–3.49) | 3 (5.0) | 0.96 (0.29–3.21) |
aAdjusted for age, sex, race, donor type, year of transplant, dialysis vintage and receipt of T-cell-depleting induction.
CYC: cyclophosphamide; RTX: rituximab.
Additional analyses
Thirty-six study participants had a diagnosis of malignancy pre-transplant for which chemotherapy was not used. Excluding these 36 individuals (Supplementary data, Table S2) did not lead to major changes in risk for malignancy in patients with GN and PTI [aHR 1.82 (1.10–3.02)]. Sixty-three individuals with GN received cyclophosphamide without ever getting rituximab and the risk for malignancy was non-statistically significantly higher [aHR 1.87 (0.95–3.66)]. Only 13 individuals were exposed to rituximab without ever getting cyclophosphamide, and only 18 individuals were exposed to both cyclophosphamide and rituximab during their treatment course; assessing risk for malignancy was limited in these groups, but those exposed to both cyclophosphamide and rituximab seemed to have the greatest risk [aHR 1.70 (0.28–12.67) for rituximab without cyclophosphamide; aHR 4.44 (1.88–10.84) for both cyclophosphamide and rituximab] (Supplementary data, Table S3). There were few patients who were exposed to only a single type of PTI, and therefore assessing malignancy risk was limited in these individuals (Supplementary data, Table S4). When stratifying our analyses by type of T-cell-depleting induction therapy used (alemtuzumab or thymoglobulin), there were no major changes in our results (Supplementary data, Table S5). Our results were also similar when restricting our analyses to adults ≥18 years old at time of transplantation) (Supplementary data, Table S6). The rates of rejection were similar between the two groups and risk for rejection was not significantly greater in patients with GN and PTI compared with controls [30.4% vs 27.8%, respectively; aHR 1.12 (0.82–1.53)]. We also found overall similar results for malignancy risks when restricting the study groups to those with GN as their native, by comparing GN PTI vs GN no PTI (Supplementary data, Table S7).
DISCUSSION
In this retrospective, single-center study of kidney transplant patients with GN as the native kidney disease, the use of PTI for treatment of GN was associated with greater risk for developing post-transplant malignancy compared with transplant recipients who had not received PTI. This was true for solid and hematologic malignancies, where the adjusted risk was 1.8-fold in those with GN and PTI but was not seen for NMSC or for PTLD only. The increased risk for malignancy was mediated mostly by use of cyclophosphamide or rituximab but was not seen with MMF or CNI use pre-transplant. These findings highlight possible risks from PTI for treatment of GN and possibly previously unrecognized risks from the use of rituximab.
The impact of PTI on risks for adverse outcomes post-kidney transplant have not been extensively studied. The role the immune system plays on cancer surveillance is undeniable. Innate and adaptive immune cells are crucial for the recognition of tumor antigens and subsequent cytotoxic effects against tumor cells [17, 18]. Immunosuppression leads to increased susceptibility to viral infections, including EBV, a major cause of PTLD in kidney transplant recipients [19, 20]. Therefore, it is biologically plausible that greater immunosuppression burden conferred from the treatment of underlying GN pre-transplant could increase the risk for post-transplant malignancy. Hibberd et al. examined the impact of any PTI on malignancy post-transplant in patients transplanted between the years 1982 and 1997, and similarly found an increased risk for non-NMSC malignancy with PTI [21]. While we were limited in the ability to examine risks for specific types of malignancy, our findings exhibit some important differences. Our study reflects a more modern era of practice for treatment of GN, where there is a greater focus on minimization of immunosuppression. With the relative granularity of our data, we were able to determine risks associated with specific types of PTI, many of which have only recently started to be used for treatment of GN. In particular, malignancy risk with rituximab use as PTI has not been previously observed, and would have been unlikely to be included in the aforementioned study given its years of inclusion. We demonstrated that greatest risk from PTI lies with use of cyclophosphamide or rituximab, but not with CNI or MMF. This could have been expected for cyclophosphamide, given its well-recognized risk for malignancy in the non-transplant population [22–25]. For rituximab, it came as a surprise since malignancy is not a recognized consequence of therapy [15, 26]. Because some patients in our cohort may have been exposed to both cyclophosphamide and rituximab during their GN treatment course, it is difficult to ascertain which of these agents carries the greatest malignancy risk in our study. Interestingly, PTI exposure to rituximab without cyclophosphamide, and to cyclophosphamide without rituximab, did not seem to be associated with malignancy risk, whereas PTI exposure to both cyclophosphamide and rituximab during a patient's GN treatment course seemed to carry the greatest risk (4.4-fold). The interpretation of this finding is limited by the small number of such patients. Prior studies in non-transplant populations treated for ANCA vasculitis do not consistently show an increased risk for malignancy with the use of cyclophosphamide combined with rituximab [27, 28]. The possible association between rituximab use as PTI and development of malignancy post-transplant is hypothesis generating, as is the possible interaction between cyclophosphamide and rituximab for malignancy risk. Our findings could be explained by the compounded risk from induction and maintenance immunosuppression after transplant on top of PTI, but require further study.
We did not find an increased risk for NMSC with use of PTI for treatment of GN. This was unexpected since one would think that cumulative immunosuppression exposure would have an impact on this as well. A prior study by Jorgenson et al. found that use of cyclophosphamide pre-transplant for treatment of GN was associated with an increased risk for skin cancer [29]. It is difficult to determine in our study why solid and hematologic malignancy risk was increased but not NMSC risk. There was no clear signal for a specific type of PTI medication to be associated with NMSC in our study. Neither MMF nor CNI receipt prior to transplant were associated with NMSC; however, a median 1–2 years of MMF or CNI use pre-transplant may be superfluous in the context of cumulative doses from continuous use post-transplant. Not even cyclophosphamide use was associated with NMSC. Interestingly, we found a greater than 4-fold risk for NMSC in patients who received the highest doses of cyclophosphamide pre-transplant (>25 g). Although this was limited to a very small number of patients, it may suggest that a significant enough amount of PTI may predispose to NMSC post-transplant.
The major strength of our study was that we were able to obtain information on types and amounts of PTI used for treatment of GN, allowing us to analyze the effects of different types of immunosuppressants. Furthermore, using chart review to confirm our exposure group and outcomes decreases risk for misclassification of these. Some limitations should be mentioned. This was a single-centre study where many transplant patients with GN as their native disease would have had their GN treated at our center. Our results may therefore not be generalizable since both induction and maintenance therapy for GN treatment may vary between centers. Also, non-T-cell-depleting induction is used infrequently at our center (14% in our study). A center which predominantly uses basiliximab for transplant induction therapy may not find the same risks associated with GN PTI since it is a less potent immunosuppressive compared with alemtuzumab or thymoglobulin, and therefore may not contribute as much to immunosuppression burden. Despite the relative granularity in our data, doses and duration of corticosteroid use were not ascertainable, precluding detailed examination of the intensity of its use on risk for development of malignancy and NMSC. Although our overall sample size was substantial, some of our sub-analyses regarding specific immunosuppressant risk were hindered by small final sample size. In these sub-analyses, confidence intervals were wide and drawing conclusions is difficult. Patients also could have been exposed to more than one type of PTI, making it difficult to isolate the effect on malignancy risk of a single type of PTI. Further, although <1% of our study cohort received non-tacrolimus initial maintenance immunosuppression, we did not identify patients who could have switched to a mammalian (mechanistic) target of rapamycin (mTOR) inhibitor. These agents may reduce the incidence of cancer in solid organ transplant recipients, mostly driven by reduction in NMSC [30]. However, since a switch from CNI to mTOR inhibitor would mostly have occurred once a NMSC was diagnosed, and since our outcomes were only looking at first events, this is unlikely to have influenced our results. Finally, as an observational study, the risk of unmeasured confounding remains. Our results were consistent across multiple sub-analyses, including restricting to those who had GN as their native disease, thus potentially accounting for unmeasured confounders inherent to individuals with GN pre-transplant. However, if patients with GN and PTI represent a group of patients theoretically more adherent to post-transplant follow-up, more malignancy diagnoses may be made due to more opportunity for investigation. For example, renal cell carcinoma (the most frequent malignancy post-transplant in our study) diagnosis could be influenced by the frequency of post-transplant renal ultrasound testing.
In this cohort of kidney transplant recipients, use of PTI for treatment of GN was associated with an increased risk for solid or hematologic malignancy post-transplant, but not for NMSC. This may be especially true for cyclophosphamide or rituximab use. Clinicians should be mindful of these risks, in addition to infection risks and medication toxicity, when deciding to continue immunosuppression for GN, particularly if patients have long-standing impaired kidney function or marked chronicity and fibrosis by kidney biopsy. Patients with significant receipt of any type of immunosuppression pre-transplant should also be counseled on the importance of post-transplant monitoring for malignancy. Existing transplant registries should capture type and duration of PTI, as well as transplant induction therapy, to ascertain changes in risk of malignancy, how induction therapy may modulate this risk and other relevant outcomes, particularly as immunosuppression regimens for GN continue to evolve and anti-CD20 therapy, including rituximab, has become more widespread.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Paula Steele and Allan Wagner for their assistance in compiling data from our institution's transplant registry data, and Caroline Poulton for her help with regulatory management of the study.
Contributor Information
David Massicotte-Azarniouch, UNC Kidney Center, Division of Nephrology and Hypertension, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Division of Nephrology, Department of Medicine, University of Ottawa, Ottawa, Ontario, Canada.
Randal K Detwiler, UNC Kidney Center, Division of Nephrology and Hypertension, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Yichun Hu, UNC Kidney Center, Division of Nephrology and Hypertension, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Ronald J Falk, UNC Kidney Center, Division of Nephrology and Hypertension, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Manish K Saha, UNC Kidney Center, Division of Nephrology and Hypertension, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Susan L Hogan, UNC Kidney Center, Division of Nephrology and Hypertension, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Vimal K Derebail, UNC Kidney Center, Division of Nephrology and Hypertension, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
FUNDING
This work was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (R01DK125350).
AUTHORS’ CONTRIBUTIONS
D.M.-A., R.K.D., S.L.H. and V.K.D. were responsible for the study conception, design and analysis plan. D.M.-A. carried out the data retrieval. Y.H. conducted the statistical analyses. D.M.-A. and V.K.D. drafted the manuscript and all authors contributed to the interpretation and editing of the final manuscript.
DATA AVAILABILITY STATEMENT
Deidentified individual data that supports the results will be shared beginning 9 to 36 months following publication provided the investigator who proposes to use the data has approval from an Institutional Review Board, Independent Ethics Committee or Research Ethics Board, as applicable, and executes a data use/sharing agreement with UNC.
CONFLICT OF INTEREST STATEMENT
V.K.D. reports consultancy agreements with Forma Therapeutics and Novartis; honoraria from UpToDate; and serving in an advisory role for Travere, Merck and Bayer. R.J.F. reports serving on an advisory board for Vertex Pharmaceuticals. M.K.S. reports honoraria from Calliditas, Elsevier, Chemocentryx and Travere therapeutics.
REFERENCES
- 1. Ferguson TW, Tangri N, Rigatto Cet al. Cost-effective treatment modalities for reducing morbidity associated with chronic kidney disease. Expert Rev Pharmacoecon Outcomes Res 2015;15:243–52. 10.1586/14737167.2015.1012069 [DOI] [PubMed] [Google Scholar]
- 2. Czyżewski Ł, Sańko-Resmer J, Wyzgał Jet al. Assessment of health-related quality of life of patients after kidney transplantation in comparison with hemodialysis and peritoneal dialysis. Ann Transplant 2014;19:576–85. 10.12659/AOT.891265 [DOI] [PubMed] [Google Scholar]
- 3. Wolfe RA, Ashby VB, Milford ELet al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999;341:1725–30. 10.1056/NEJM199912023412303 [DOI] [PubMed] [Google Scholar]
- 4. Winkelmayer WC, Weinstein MC, Mittleman MAet al. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Making 2002;22:417–30. 10.1177/027298902236927 [DOI] [PubMed] [Google Scholar]
- 5. Vajdic CM, McDonald SP, McCredie MREet al. Cancer incidence before and after kidney transplantation. JAMA 2006;296:2823–31. 10.1001/JAMA.296.23.2823 [DOI] [PubMed] [Google Scholar]
- 6. Villeneuve PJ, Schaubel DE, Fenton SSet al. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant 2007;7:941–8. 10.1111/J.1600-6143.2007.01736.X [DOI] [PubMed] [Google Scholar]
- 7. Bustami RT, Ojo AO, Wolfe RAet al. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant 2004;4:87–93. 10.1046/J.1600-6135.2003.00274.X [DOI] [PubMed] [Google Scholar]
- 8. Opelz G, Döhler B.. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant 2004;4:222–30. 10.1046/J.1600-6143.2003.00325.X [DOI] [PubMed] [Google Scholar]
- 9. Dantal J, Hourmant M, Cantarovich Det al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet North Am Ed 1998;351:623–8. 10.1016/S0140-6736(97)08496-1 [DOI] [PubMed] [Google Scholar]
- 10. Carroll RP, Ramsay HM, Fryer AAet al. Incidence and prediction of nonmelanoma skin cancer post-renal transplantation: a prospective study in Queensland, Australia. Am J Kidney Dis 2003;41:676–83. 10.1053/AJKD.2003.50130 [DOI] [PubMed] [Google Scholar]
- 11. Lentine KL, Smith JM, Hart Aet al. OPTN/SRTR 2020 Annual Data Report: Kidney. Am J Transplant 2022;22:21–136. 10.1111/AJT.16982 [DOI] [PubMed] [Google Scholar]
- 12. Johansen KL, Chertow GM, Gilbertson DTet al. US Renal Data System 2021 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2022;79:A8–12. 10.1053/J.AJKD.2022.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barbour S, Lo C, Espino-Hernandez Get al. The population-level costs of immunosuppression medications for the treatment of glomerulonephritis are increasing over time due to changing patterns of practice. Nephrol Dial Transplant 2018;33:626–34. 10.1093/NDT/GFX185 [DOI] [PubMed] [Google Scholar]
- 14. Rovin BH, Adler SG, Barratt Jet al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int 2021;100:S1–276. 10.1016/J.KINT.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 15. Emery P, Furst DE, Kirchner Pet al. Risk of malignancies in patients with rheumatoid arthritis treated with rituximab: analyses of global postmarketing safety data and long-term clinical trial data. Rheumatol Ther 2020;7:121–31. 10.1007/S40744-019-00183-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hanaway MJ, Woodle ES, Mulgaonkar Set al. Alemtuzumab induction in renal transplantation. N Engl J Med 2011;364:1909–19. 10.1056/NEJMOA1009546 [DOI] [PubMed] [Google Scholar]
- 17. Lakshmi Narendra B, Eshvendar Reddy K, Shantikumar Set al. Immune system: a double-edged sword in cancer. Inflamm Res 2013;62:823–34. 10.1007/S00011-013-0645-9 [DOI] [PubMed] [Google Scholar]
- 18. Hiam-Galvez KJ, Allen BM, Spitzer MH.. Systemic immunity in cancer. Nat Rev Cancer 2021;21:345–59. 10.1038/S41568-021-00347-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kotton CN, Fishman JA.. Viral infection in the renal transplant recipient. J Am Soc Nephrol 2005;16:1758–74. 10.1681/ASN.2004121113 [DOI] [PubMed] [Google Scholar]
- 20. Dierickx D, Habermann TM.. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med 2018;378:549–62. 10.1056/NEJMRA1702693 [DOI] [PubMed] [Google Scholar]
- 21. Hibberd AD, Trevillian PR, Wlodarczyk JHet al. Effect of immunosuppression for primary renal disease on the risk of cancer in subsequent renal transplantation: a population-based retrospective cohort study. Transplantation 2013;95:122–7. 10.1097/TP.0b013e3182782f59 [DOI] [PubMed] [Google Scholar]
- 22. Bernatsky S, Clarke AE, Suissa S.. Hematologic malignant neoplasms after drug exposure in rheumatoid arthritis. Arch Intern Med 2008;168:378–81. 10.1001/ARCHINTERNMED.2007.107 [DOI] [PubMed] [Google Scholar]
- 23. Radis CD, Kahl LE, Baker GLet al. Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis. A 20-year followup study. Arthritis Rheum 1995;38:1120–7. 10.1002/ART.1780380815 [DOI] [PubMed] [Google Scholar]
- 24. Faurschou M, Sorensen IJ, Mellemkjaer Let al. Malignancies in Wegener's granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol 2008;35:100–5. [PubMed] [Google Scholar]
- 25. Heijl C, Westman K, Höglund Pet al. Malignancies in patients with antineutrophil cytoplasmic antibody-associated vasculitis: a population-based cohort study. J Rheumatol 2020;47:1229–37. 10.3899/JRHEUM.181438 [DOI] [PubMed] [Google Scholar]
- 26. Van Daalen EE, Rizzo R, Kronbichler Aet al. Effect of rituximab on malignancy risk in patients with ANCA-associated vasculitis. Ann Rheum Dis 2017;76:1064–9. 10.1136/ANNRHEUMDIS-2016-209925 [DOI] [PubMed] [Google Scholar]
- 27. McGregor JAG, Hogan SL, Kotzen ESet al. Rituximab as an immunosuppressant in antineutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant 2015;30 Suppl 1:i123–31. 10.1093/NDT/GFV076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Daalen EE, Rizzo R, Kronbichler Aet al. Effect of rituximab on malignancy risk in patients with ANCA-associated vasculitis. Ann Rheum Dis 2017;76:1064–9. 10.1136/ANNRHEUMDIS-2016-209925 [DOI] [PubMed] [Google Scholar]
- 29. Jorgenson MR, Descourouez JL, Singh Tet al. Malignancy in renal transplant recipients exposed to cyclophosphamide prior to transplantation for the treatment of native glomerular disease. Pharmacotherapy 2018;38:51–7. 10.1002/phar.2059 [DOI] [PubMed] [Google Scholar]
- 30. Yanik EL, Siddiqui K, Engels EA.. Sirolimus effects on cancer incidence after kidney transplantation: a meta-analysis. Cancer Med 2015;4:1448. 10.1002/CAM4.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified individual data that supports the results will be shared beginning 9 to 36 months following publication provided the investigator who proposes to use the data has approval from an Institutional Review Board, Independent Ethics Committee or Research Ethics Board, as applicable, and executes a data use/sharing agreement with UNC.