Abstract
Consistent laterality is a crucial aspect of embryonic development, physiology, and behavior. While strides have been made in understanding unilaterally expressed genes and the asymmetries of organogenesis, early mechanisms are still poorly understood. One popular model centers on the structure and function of motile cilia and subsequent chiral extracellular fluid flow during gastrulation. Alternative models focus on intracellular roles of the cytoskeleton in driving asymmetries of physiological signals or asymmetric chromatid segregation, at much earlier stages. All three models trace the origin of asymmetry back to the chirality of cytoskeletal organizing centers, but significant controversy exists about how this intracellular chirality is amplified onto cell fields. Analysis of specific predictions of each model and crucial recent data on new mutants suggest that ciliary function may not be a broadly conserved, initiating event in left–right patterning. Many questions about embryonic left–right asymmetry remain open, offering fascinating avenues for further research in cell, developmental, and evolutionary biology.
Keywords: left–right asymmetry, chirality, laterality, cilia, nodal flow
INTRODUCTION
Consistent left–right asymmetry of the internal organs and human behavior (i.e., handedness) has fascinated mankind for centuries if not millennia (McManus, 2002). In the past 2 decades, significant progress has been made in understanding the genetic pathways that result in the specific arrangement and morphogenesis of the heart, viscera, and brain of vertebrate and invertebrate model systems (Levin, 2005; Speder et al., 2007). In particular, extensive cascades of asymmetric gene expression have been described that ultimately give the left and right side their unique morphology. However, genes are “agnosic” with respect to laterality (Morgan, 1991); transcription does not know left from right, and a most interesting aspect of this field concerns the biophysics of how the left–right (LR) axis first becomes oriented with respect to the anterior–posterior and dorsal–ventral axes of the organism (Okumura et al., 2008; Vandenberg and Levin, 2009).
Other active areas of investigation include the asymmetric morphogenesis of the organs (Kurpios et al., 2008), the asymmetry of the brain and central nervous system (Sun and Walsh, 2006; Sagasti, 2007; Taylor et al., 2010), the conservation of asymmetry mechanisms throughout phyla (Levin, 2006; Speder et al., 2007; Raya and Izpisua Belmonte, 2008), and various curious presentations of asymmetry in the health and disease of human patients that are not easily dissected in tractable model systems (Aw and Levin, 2008).
Despite the many questions that have significance not only for developmental biology but also for biomedicine (Morelli et al., 2001; Ramsdell, 2005; Tabin, 2005; Peeters and Devriendt, 2006), there is a belief outside of this field that the fundamental question is solved. Most cell biology textbooks and reviews state as fact, in the chapter on motile cilia, language to the effect of: “chiral ciliary motion, and the resulting leftward flow, initiates LR asymmetry.” Both students and grant reviewers increasingly believe that the most important issue in this area (the origin of LR asymmetry) has been resolved and that the movement of cilia during neurulation is definitively a conserved, “first step” of asymmetry.
The ciliary model posits that asymmetry is initiated by a coordinated flow of extra-embryonic fluid at the ciliated node during gastrulation (Tabin, 2006). This fluid flow is net unidirectional because of the tilt and chiral nature of the cilia (Cartwright et al., 2004; Nonaka et al., 2005). This motion generates asymmetric signaling at the node through redistribution of extracellular morphogens or direct transduction of fluid flow by sensory cilia (McGrath et al., 2003; Tabin and Vogan, 2003). The ciliary models were first derived from data indicating that mutations in ciliary proteins that alter the motion, sensory abilities, or assembly of cilia are accompanied by laterality disorders (Brueckner, 2001; Basu and Brueckner, 2008).
Previously, we suggested alternative interpretations of existing data and presented a different view on LR asymmetry (Levin and Nascone, 1997; Levin and Palmer, 2007), as have others (Brown and Wolpert, 1990; Bock and Marsh, 1991; Yost, 1991). Since then, while papers associating ciliary function with asymmetry continue to mount (see Basu and Brueckner, 2008, for an excellent review), several important studies now reveal additional crucial data that require a reappraisal of the field. Here, we argue that, despite the popularity and universal appeal of the ciliary paradigm, the community should be aware of competing models and their relative fit to recent and classical data.
An alternative model (Fig. 1) derives consistent asymmetry from the orientation of a chiral intracellular component, which distributes cytoplasmic ion transporter proteins in a biased manner, thus driving a variety of physiological and biophysical asymmetries at very early stages (i.e., during initial cleavages; Levin and Palmer, 2007). It is now known that the cytoskeleton of some very early vertebrate embryos has inherent chirality (Danilchik et al., 2006; Aw et al., 2008). In the frog embryo, the asymmetry of the cytoskeleton drives differential localization of maternal protein cargo along the LR axis during the first cleavages. These proteins include two potassium channels (Aw et al., 2008; Morokuma et al., 2008) and two proton pumps (Levin et al., 2002; Adams et al., 2006). The asymmetric fluxes and transmembrane potential gradients resulting from the biased localization of these transporters, together with a network of open gap junctions, allows LR morphogens such as serotonin to be distributed to the right- and ventral-most blastomere (Fukumoto et al., 2005a,b). All of these steps are required for asymmetry of subsequent gene expression and organ situs (Levin, 2006). While this scheme has been studied in most detail in Xenopus embryos, analysis of a wide variety of phyla reveals surprising conservation in the pathways involved (Levin, 2006; Oviedo and Levin, 2007).
Fig. 1.

Schematic of cytoplasmic model of left–right (LR) asymmetry. This schematization, based on events characterized in Xenopus laevis, shows how intracellular chirality can be imposed upon multicellular cell fields, and initiate asymmetric gene expression relative to the midline, by a physiological mechanism driven by maternal, biophysical events. A: Eggs are initially loaded with maternal mRNAs and proteins (blue) that are symmetrically (radially) distributed around the animal–vegetal axis. B: When the egg is fertilized, the location of the sperm entry point dictates the location of the first cleavage plane, which usually coincides with the midline of the developing animal (Scharf and Gerhart, 1983; Ubbels et al., 1983; Klein, 1987; Masho, 1990; Marrari et al., 2004) . The crucial event at this stage is the complex set of known cytoskeletal rearrangements that include the hypothetical orientation of an organizing center (e.g., the centrioles existing at right angles to each other) with respect to the animal–vegetal and dorsal–ventral axes (the enantiomeric “F-molecule,” here represented by a hand). Maternal cargo proteins/mRNAs can then begin to be distributed in a consistently-asymmetric manner by cytoplasmic motor transport (dependent on kinesin, dynein, and microtubule arrays). C: By the four-cell stage, maternal mRNAs are now largely localized to the right ventral blastomere. These mRNAs encode ion transporters including two potassium channels (Aw et al., 2008; Morokuma et al., 2008) and two proton pumps, the H+-K+-ATPase (Levin et al., 2002) and the H+-V-ATPase (Adams et al., 2006). D: Together, the asymmetric localization of these transporters (blue circles) leads to a circuit establishing physiological asymmetries such as an increased pumping of positively charged ions out of the right side cell (+), leading to a difference in transmembrane potential between the L and R blastomeres across the ventral midline. The blastomeres of the early embryo next become connected by means of open gap junctions (orange channels), with the exception of the cells between the left and right ventral cells (Levin and Mercola, 1998b), which are junctionally-isolated (dark line). E: Serotonin and perhaps other small charged morphogens (yellow dots) are initially present in all blastomeres. F: Subject to selectivity of the gap junctions, some are then driven toward the right-most ventral cell by an electrophoretic force maintained by the battery at the ventral edge. G,H: Through an as-yet uncharacterized pathway, the localization of morphogens such as serotonin to the right side of the embryo suppresses downstream expression of genes (i.e., Nodal), which are thus expressed only on the left side (G), which eventually leads to asymmetric organ morphogenesis (H). This model can be readily extended to bodyplans where the midline is defined after the initial cleavages are complete by the spread of LR orientation information from coordinator cell(s) by means of planar cell polarity pathways (Aw and Levin, 2009; Vandenberg and Levin, 2010).
A third model proposes segregation of differentially-imprinted chromatids as the origin of asymmetry during very early development (Klar, 1994; Armakolas and Klar, 2007; Armakolas et al., 2010). Just as mRNAs and proteins have been shown to be differentially distributed to daughter cells, this model proposes that selective imprinting of chromosomes is a means of achieving asymmetries on the left and right side. Differential chromatid segregation is well-studied in yeast (Armakolas et al., 2010), and early cleavages in some animals, such as snails, are determined by the asymmetric structure of a contractile ring carrying out cytokinesis (Meshcheryakov and Beloussov, 1975). The discovery of a role for the motor protein left–right Dynein in this process is also extremely suggestive (Armakolas and Klar, 2007). Because the centrosome interacts with cortical membrane cues to achieve biased DNA segregation (Carmena and Earnshaw, 2003; Tajbakhsh and Gonzalez, 2009), this model is compatible with a variety of proposals about how the centrosomes orient cytokinesis with respect to the other two axes to achieve consistent segregation bias along the LR axis.
All three models rest fundamentally on the chirality of the microtubule organizing center (MTOC)/centrosomes, which ultimately control the directionality of intracellular transport, ciliary rotation, and chromatid segregation (Fig. 2). Thus, although it is rarely stated explicitly, there appears to be fundamental agreement that centrioles are the ultimate source of asymmetry (Beisson and Jerka-Dziadosz, 1999). However, there is vigorous argument about how and when this intracellular chirality is amplified and transmitted across multicellular fields, as well as the evolutionary conservation and relative importance of downstream mechanisms. Table 1 summarizes the key features of each of the three major models of LR asymmetry initiation.
Fig. 2.
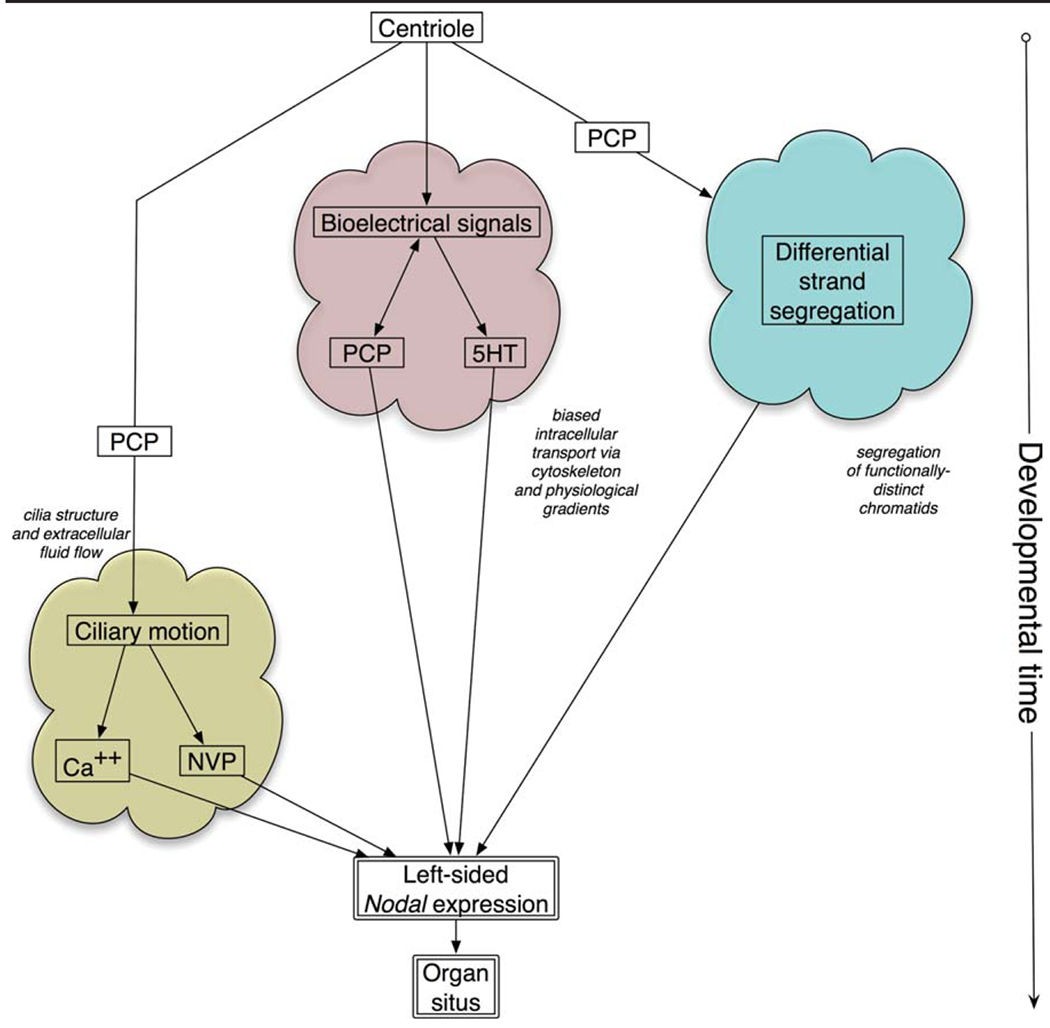
Logical schematization of the three major models of left–right (LR) asymmetry. The three fundamental models of asymmetry generation all derive the initial chirality from the centrosome or other cytoskeletal organizing center. The ciliary models propose that, having formed a cilium with a molecular chirality determined by its physical structure and the nucleating element, planar polarity aligns its rotary motion with the anterior–posterior (AP) and dorsal–ventral (DV) axes. Thereupon, calcium fluxes generated by mechanosensory cilia or specific receptors for bulk transport of some extracellular morphogen provide the first difference between the left and right sides; this would occur on the Node during gastrulation, and shortly thereafter turned into asymmetric gene expression of Nodal on the left side. The differential strand segregation model proposes that the two DNA strands during the earliest cell divisions are not identical in terms of their imprinting or in terms of the mRNAs associating with them (Lambert and Nagy, 2002). The cytokinesis machinery, coupled to planar polarity or another mechanism for orienting DNA segregation with respect to the existing two axes, ensures that the right and left sides acquire DNA with distinct gene expression profiles in subsequent stages. The intracellular/physiological model proposes that early asymmetries of the cytoskeleton allow ion channels and pumps to be localized by intracellular motor proteins to one side of blastomeres also oriented within the anatomical polarity of the embryo during the first few cleavages. These bioelectrical asymmetries result in the redistribution of a small molecule morphogen (maternal serotonin) that subsequently initiates repression of Nodal on the right side. All three models feed into the less controversial steps of the asymmetric gene cascade that ultimately controls the patterning of the heart and visceral organs.
TABLE 1.
Fundamental Features of Three Main Models of LR Symmetry Determinationa
| Feature | Predictions |
||
|---|---|---|---|
| Ciliary model | Cytoplasmic model | Chromatid segregation model | |
| Origin of asymmetry | Centriole | Centriole | Mitotic apparatus |
| Timing for initiation step | Gastrulation (late) | Cleavage (early) | Cleavage (early) |
| Amplification by | Cilia-driven fluid flow in node during gastrulation | Intracellular localization of bioelectric machinery at cleavage stages | PCP-aligned differential chromatid segregation |
| Evolutionary conservation | Mouse is typical vertebrate; other examples that don’t use cilia are outliers | Mouse may be an outlier; asymmetry is fundamentally very widely conserved | Mouse may be an outlier; asymmetry is fundamentally very widely conserved |
All three models agree on an intracellular fundamental origin of asymmetry. Although the cilia model focuses on ciliary motion, the biochemical structure of cilia (and thus their unique chirality) derives from the centriole—the same microtubule organizing center that is proposed to orient asymmetric intracellular transport in the cytoplasmic model. The chromatid segregation and cytoplasmic models propose that fundamental steps of left–right (LR) patterning occur very early, during cleavage stages, and propose planar cell polarity (PCP) or physiological systems for amplifying asymmetry on the single-cell level onto multicellular cell fields. The ciliary model proposes fluid flow in the node during gastrulation—a fundamentally multicellular process—to be the origin of consistent asymmetry. The cilia model proposes nodal flow to be a fundamental feature of vertebrate laterality, holding the mouse embryo as a prototypical model for this process, and suggests that vertebrates that pattern the LR axis prior to (or without) cilia are evolutionary outliers. In contrast, the early models suggest that the rodent embryo may instead be atypical, with chirality being a fundamental, ancient, well-conserved property of individual cells.
It is crucial for the health of the field to examine tacit assumptions inherent in existing work, and avoid giving students, workers in other fields, and funding bodies the idea that everything fundamental about asymmetry has already been discovered. Below, we present a comparative analysis of the existing models and highlight data that, we believe, suggests a revision of the currently mainstream view of the origins and evolution of asymmetry. (Table 2 summarizes how well each model does with respect to matching its predictions to specific findings.) The available data reveal many unresolved questions in this field, suggesting that the puzzle of the origins of left–right asymmetry is far from solved.
TABLE 2.
Specific Predictions and the Fit of the Three Main Models to These Dataa
| Experimental question | Cilia model predicts | Cytoplasmic model predicts | Chromatid segregation model predicts | Experimental result |
|---|---|---|---|---|
| Should mutation of kinesin, dynein, MTOC, and PCP proteins randomize LR? | YES | YES | YES | YES |
| Should viscosity changes at the node randomize LR? | YES | no, unless cilia amplify | no, unless cilia amplify | YES |
| Should chick embryos have asymmetric gene expression before node forms? | NO | YES | YES | YES |
| Should frog embryos establish asymmetric gradients long before cilia form? | NO | YES | YES | YES |
| Should disruption of cytoskeleton or of physiological asymmetries only during the first couple of cleavages randomize LR? | NO | YES | YES | YES |
| Should chick node receive LR information from lateral tissue? | NO | YES | NO | YES |
| Should embryos (human & newt) randomize if split at the two-cell stage? | NO | YES | YES | YESb |
| Should the brains of primary ciliary dyskinesia patients have normal laterality? | NO | YES | YES | YES |
| Should any mutants exist with abnormal ciliary flow but normal LR asymmetry? | NO | YES | YES | YES |
| Should animals and plants with no cilia and no node be able to establish asymmetry using some of the same molecules as ciliated vertebrates? | NO | YES | YES | YES |
| Should organizers induced past the first few cleavages be randomized? | NO | YES | YES | YES |
The three main models make distinct predictions as to the outcomes of a number of experimental questions. Most work on cilia has focused on mutations of ciliary genes, which does not distinguish between these models (since many of these same components also play important roles in intracellular polarity determination and are present in non-ciliated cells). However, a variety of extant data do distinguish between the models; with the exception of the two studies that have shown that fluid flow per se can affect asymmetry (clearly supporting the ciliary model), the majority of the data support the early models, not the ciliary model. Green = consistent and predicted by model. Yellow = not predicted by model but does not contradict it. Red = contradicts the predictions of the model; LR, left–right.
Human twins show subtle bookending (opposite asymmetry of unilateral defects), not real laterality disturbances.
CILIA AND NODAL FLOW:A CLOSE LOOK AT THE DOMINANT MODEL
Does Ciliary Motion Per Se Determine LR Asymmetry?
PubMed contains approximately 100 papers describing ciliary proteins’ associations with LR phenotypes, giving the impression of a solid causal link in the literature. However, only a couple of these studies functionally tested ciliary motion itself (in the absence of genetic perturbations that also affect intracellular functions) for a role in LR patterning. Direct changes of viscosity in the interciliary space (Schweickert et al., 2007) and reversal/rescue of nodal flow (Nonaka et al., 2002) suggest that ciliary motion can in fact control LR patterning. In contrast, the majority of studies suggesting a role for cilia in LR asymmetry have involved genetic deletions/mutations of proteins that possess nonciliary (intracellular) functions that are never tested (reviewed in Levin, 2003; Levin and Palmer, 2007); even “ciliary” dynein (i.e., LRD) is expressed outside of cilia, for instance in the limbs and headfolds of the developing mouse (Supp et al., 1997), and plays a role in segregation of differentially imprinted chromatids (Armakolas and Klar, 2007). Thus, the vast majority of data does not actually distinguish between ciliary function and intracellular transport or chromatid segregation models, as all three are consistent with a requirement for proteins involved in cytoskeletal assembly, motor protein function, and planar polarity. We proposed previously that alterations in proteins with pleiotropic roles in both intracellular transport and ciliary structure/function cause LR phenotypes and ciliary defects (including kidney cysts, etc.) as parallel downstream consequences (Levin, 2003, 2006). This model, in contrast to the model that ciliary motion causes LR asymmetry, predicts that mutations will be found that dissociate ciliary defects from errors of laterality.
Mutants That Do Not Show Expected Concordance Between Ciliary Defects and LR Randomization
Crucially, recent analyses reveal that, as predicted by the cytoplasmic model, there are mutants in which LR phenotypes and ciliary motion can be dissociated; these provide data that are difficult to reconcile with ciliary motion as the primary cause of LR asymmetry. One example is the knockdown of foxj1b, a ciliary gene, in zebrafish dorsal forerunner cells (Tian et al., 2009), i.e., the cells that contribute to the ciliated nodelike structure—Kupffer’s vesicle (KV)—in the fish. The loss of foxj1b in only this node-like structure has no effect on LR patterning, but knockdown in all cells induces randomization of the LR axis.
Another study, examining mutations in two alleles of the zebrafish gene seahorse, also provides evidence that ciliary defects and laterality errors can be genetically separated (Serluca et al., 2009). One mutant, seatg238a-C, produces LR defects in only 28% of the fish examined, despite the 80% of fish with reduced or absent ciliary flow at the node. Another mutant examined, Seafa20r-M21, produces only 2% with laterality defects, and 38% with reduced or absent ciliary flow at the node. A second study of Seahorse mutant zebrafish found that 52% of seahorsehi3308 mutants have laterality defects, yet the cilia of these mutants were found to be normal (Kishimoto et al., 2008).
Several other mutants identified in zebrafish genetic screens show little correlation between alterations in cilia at the KV and LR phenotypes. For instance, the mutant locke has abnormal cilia but a low incidence of laterality defects, whereas the mutant garbus has normal KV cilia but a high incidence of laterality defects (Zhao and Malicki, 2007). Further weakening the causal connection between ciliary function and LR asymmetry are studies in the mouse model (where the cilia link is expected to be the strongest) on genes such as Inturned, which regulate cilia formation but do not randomize asymmetry when disrupted (Zeng et al., 2010). Mutations in TMEM216 also result in ciliopathies but no LR defects in both mice and zebrafish (Valente et al., 2010). While it has been suggested that the dissociation of ciliary mutants and LR phenotypes could be due to a maternal protein that can somehow rescue early defects (Serluca et al., 2009), together these examples demonstrate that ciliary defects can indeed be separated from LR patterning errors, and cast considerable doubt on the idea that ciliary motion is a required component of vertebrate asymmetry; these examples also confirm that LR phenotypes observed in genetic mutations of “ciliary” genes do not establish a causal link.
Many Phyla Establish LR Asymmetry Without Ciliary Flows
Among single-celled invertebrates such as protozoa, it has long been known that chirality can be established and maintained by means of an epigenetic inheritance mechanism (Frankel, 1991; Shi et al., 1991) similarly to what occurs in plants (Doss, 1978; Nelsen et al., 1989). Importantly, this is a true example of left–right asymmetry, not simply rotational chirality: when mirror-image doublets of Tetrahymena are subjected to stressful conditions, it is always the right half that is absorbed (Bell et al., 2008). Strikingly, a recent study suggests that even single cells of vertebrate origin define a consistent LR axis (Xu et al., 2007). In culture, unciliated neutrophil-like differentiated HL60 cells possess one axis of polarization due to the fact that one surface is in contact with the coverslip while the other is exposed to the free medium (similar to the dorsal–ventral axis of amniote embryos’ blastodiscs, which have highly distinct environments facing their dorsal and ventral surfaces). A second axis (similar to the anterior–posterior axis) can be drawn as a line connecting the nucleus and centrosome. Remarkably, it was observed that the vast majority of cells display leftward biased movements and pseudopodia formation in response to exogenous attractants, revealing a third (LR) axis along which these cells display a consistent asymmetry. In many cells, such directional movement is controlled by a biased cytoskeleton, oriented by the centriole (Albrecht-Buehler and Bushnell, 1979; Albrecht-Buehler, 1981). Similarly, cultured vertebrate neurites (Heacock and Agranoff, 1976) and even bacteria (Mendelson, 1976) exhibit strong and consistent asymmetries of behavior along the LR axis; in colonies of Bacillus mycoides, the asymmetry can be reversed by ion-dependent signals (Mendelson and Karamata, 1982). Thus, consistent LR asymmetry may be a fundamental property of most individual cells that requires neither nodes nor fluid flows across multicellular structures.
LR asymmetry is established in some animals during early developmental stages, long before cilia are present or in some cases, with no cilia present at all; these include snails (Meshcheryakov and Beloussov, 1975; Shibazaki et al., 2004), sea urchin (Kitazawa and Amemiya, 2007), Drosophila (Hozumi et al., 2006; Coutelis et al., 2008), Arabidopsis (Hashimoto, 2002; Thitamadee et al., 2002; Abe et al., 2004), and C. elegans (Priess, 1994; Hutter and Schnabel, 1995), for example. Other animals establish the LR axis later in development, when thousands of cells are present, but also without using cilia. For instance, the chick node exists at the end of the primitive streak, i.e., a continuous sheet of cells where no fluid flow is likely to be established (Manner, 2001); nor has any chiral fluid flow in chick been described. Even though monociliated cells are present at the node (Essner et al., 2002), they are not confined to this area, they are short and immotile, and they are found on endodermal cells, not mesodermal cells (Gros et al., 2009). Additionally, the Talpid3 mutant chick lacks cilia (Yin et al., 2009) yet has normal LR asymmetry (Cheryll Tickle, personal communication), further indicating that cilia are not required to establish laterality in the chick.
A recent study finds that, similar to the chick, the pig also does not require cilia for proper LR asymmetry (Gros et al., 2009). In fact, the pig notochordal plate completely lacks cilia, as well as a space where fluid flow could be generated. The pig and chick both have morphologically asymmetric nodes that form following leftward biased cell movements, and asymmetric gene expression domains form in both animals several hours before the asymmetric appearance of Nodal, the first gene that is known to be asymmetrically expressed in Xenopus, mice, and rabbits. Thus, many phyla, including some birds and mammals, establish asymmetry without ciliary flow.
Do Cilia Initiate, or Transmit, LR Information?
In several vertebrates, consistent asymmetries exist long before formation of a ciliated node, indicating that whatever the role of cilia, they cannot be the initial symmetry-breaking steps in these animals. Such consistent asymmetries include expression of mRNAs such as Syndecan-2 (Fukumoto and Levin, 2005) and activin receptors in chick (Stern et al., 1995; Levin et al., 1997). At the protein level, mainly investigated in Xenopus, these include the K+ channel KCNQ1 (Morokuma et al., 2008), H+-V-ATPase (Adams et al., 2006), H+-K+-ATPase (Levin et al., 2002; Aw et al., 2008), 14-3-3E polarity protein (Bunney et al., 2003), and a series of “ciliary” proteins with intracellular roles like Kinesin 3B (Qiu et al., 2005). At the level of physiology, the right ventral blastomere in the four-cell frog embryo pumps twice as many protons out as does the left ventral blastomere (Adams et al., 2006), the L and R blastomeres of ascidian embryos exhibit asymmetric calcium signaling (Albrieux and Villaz, 2000), and left-sided depolarization is observed to the left of the early (prenode) primitive streak in the chick (Levin et al., 2002). Early biophysical asymmetries include the discovery that the Xenopus egg has consistently chiral microfilament organization (Danilchik et al., 2006), as well as a cytoskeleton that has a net right-ward orientation for the guidance of intracellular transport by means of kinesin motors (Aw et al., 2008). At the level of protein modification, asymmetric syndecan phosphorylation occurs in gastrulating Xenopus embryos before the time when the node has formed and motile cilia are present (Kramer et al., 2002; Kramer and Yost, 2002); in a pathway controlled by PKCγ, syndecan-2 is phosphorylated in right, but not left, ectodermal cells. At the cellular level, classical data describe asymmetric, centripetal, chiral, not mirror-symmetric counterclockwise movement of cells in avian embryos during early (prenode) gastrulation (Lepori, 1969). Functionally, it is known that sea urchin (Kitazawa and Amemiya, 2007) and mouse (Gardner, 2010) blastomeres are not equivalent with respect to their LR identity from very early cleavage stages. Together, these data further confirm that embryos of numerous phyla know their left from their right well before (or indeed, without) the appearance of cilia.
The ciliary model predicts that ciliated organs with unperturbed nodal flow ought to be sufficient for establishment of LR asymmetry ab initio. Two recent studies show that this is in fact not the case. Newt blastomeres separated at early cleavage stages give rise to animals with otherwise normal axial development and organogenesis, yet exhibit reversals in heart laterality in one of the twins (Takano et al., 2007). Because it is a true visceral laterality reversal, this phenotype is a more striking example than the long-known bookending (mirroring) of hair whorls and craniofacial defects in human monozygotic twins (Levin, 1999), and shows that in amphibia, later ciliary motions and nodal flow cannot overcome the disruption of LR information at early cleavage stages. The second line of evidence comes from recent work analyzing the ability of late-induced organizers to pattern the LR axis in Xenopus (Vandenberg and Levin, 2010). When the endogenous organizer is ablated and embryonic patterning is rescued by induction of an organizer as early as the 32-cell stage, embryos are normal except that their LR axis is destabilized, showing that an organizer deprived of the orienting events occurring during the first few cleavages cannot orient asymmetry properly, despite the lack of any disruption at the later ciliated node. Furthermore, there are several examples from zebrafish studies indicating that molecular genetic alterations in the cells contributing to the ciliated KV are not sufficient to induce laterality defects (Yamauchi et al., 2009) or produce diminished LR phenotypes compared with genetic knockdown of targets in all cells (Bisgrove et al., 2005).
Finally, it has been shown in the chick that LR identity is transferred to the sides of the node from adjacent tissue (Pagan-Westphal and Tabin, 1998; Yuan and Schoenwolf, 1998; Levin and Mercola, 1999), and does not arise de novo in the node as predicted by the ciliary model; this lateral imprinting is, however, predicted by the gap-junction-mediated morphogen gradient proposed to amplify cytoskeletal asymmetries in the intracellular model (see Fig. 1; Levin and Mercola, 1999). Collectively, the data indicate that consistent LR asymmetries are present in embryos of several vertebrate species before the time when the node is formed. This suggests that nodal flow is not a broadly conserved mechanism used to establish the LR axis; while no earlier mechanisms have been described in mice, it is seen from the chick and pig data that even some amniotes can pattern asymmetry without nodal flow, and Xenopus embryos establish asymmetry shortly after fertilization.
While cilia are involved in some aspects of patterning the LR axis in mouse and frog, and could even be an originating element for asymmetry in the rodent bodyplan, which has a very atypical architecture compared with other mammals, cilia may instead be useful as a means of amplifying signals that are already present in earlier stages of most other types of embryos. For example, in the mouse, cilia may be used to amplify signals downstream of Shh (Huangfu et al., 2003; Corbit et al., 2005; Liu et al., 2005).
INTRACELLULAR MECHANISMS
Origins and Evolution of Early Cytoplasmic Models of LR Asymmetry
Consistent with the classic F-molecule theory of Brown and Wolpert (Brown and Wolpert, 1990), we previously suggested a model in which consistent LR asymmetry is generated when a chiral cytoskeletal component becomes tethered (oriented) with respect to the anterior–posterior and dorsal–ventral axes during fertilization. Specifically, we proposed that this component was the MTOC, which nucleates cytoskeletal assembly and thus can set up asymmetric tracks for directed intracellular transport by means of cytoplasmic motor proteins (Levin and Nascone, 1997; Levin and Mercola, 1998a), as it does in Drosophila embryo cells (Theurkauf et al., 1992). This model was first proposed by Spemann as the “microstructure hypothesis” (Spemman, 1906): “this structure surrounds the main axis of the egg in a manner that would cause a primary difference between the right and left sides of the ensuing embryo … is probably formed before or during fertilization” (Wehrmaker, 1969). A closely related proposal was made in the early 1990s by H. Joseph Yost, whose results implicated rearrangements of the cytoplasm during the first cell cycle after fertilization in asymmetry (Yost, 1991).
In the most modern formulation of the model, a chiral cytoskeleton results in the asymmetric localization of maternal mRNAs and proteins that subsequently amplify the asymmetry and impose it on multicellular fields. This pathway has been most clearly elucidated in Xenopus, and involves a system of ion transporters, gap junctions, and serotonergic pathway elements that use electrophoresis to amplify intracellular chirality into multicellular gradients of a small molecule morphogen (Levin and Palmer, 2007; Aw and Levin, 2009). While the MTOC has not yet been molecularly dissected in this role, the other components (i.e., the asymmetric orientation and function of the cytoskeleton, transport proteins, ion transporters, and LR morphogens) are known in significant detail (Fig. 1). The specifics (e.g., spatial distribution, timing) differ among model species with divergent early embryonic architectures, these and the other biophysical components elucidated in Xenopus have now also been shown to be required for asymmetry in sea urchin (Duboc et al., 2005; Hibino et al., 2006), Ciona (Shimeld and Levin, 2006), chick (Levin et al., 2002; Raya et al., 2004; Adams et al., 2006), zebrafish (Adams et al., 2006), C. elegans (Chuang et al., 2007), and snails (Shibazaki et al., 2004).
Amplification: Bioelectric Redistribution of Morphogens and Planar Cell Polarity
Once asymmetric pH and voltage gradients arise in blastomeres during early embryogenesis, how are these signals propagated through the blastoderm and transduced into asymmetric gene expression? Three non–mutually exclusive models have been proposed for how this may occur in vertebrates. The first involves control of calcium signaling which activates Notch (Raya et al., 2004; Raya and Belmonte, 2006). This appears to occur in chick, but not Xenopus (Levin et al., 2002; Adams et al., 2006). In frog, the bioelectrical LR gradient appears to redistribute serotonin, a small molecule morphogen, which subsequently induces asymmetric gene expression (Fukumoto et al., 2005a,b; Adams et al., 2006; Levin et al., 2006). The rapid redistribution of serotonin among blastomeres (Fukumoto et al., 2005b) leads to stable, specific gene expression cascades on the left side; a similar situation occurs in C. elegans, where the NSY-5 gap junction protein establishes transient connections for physiological signals that specify the permanent asymmetry in the structure and function of the AWC olfactory neurons (Chuang et al., 2007). Similarly, as very early cytoskeletal biases in Xenopus ultimately result in asymmetries of visceral organs (Aw et al., 2008), the transduction of chiral cytoskeletal activity into asymmetric Nodal induction has recently been demonstrated in snail embryos; alterations in spindle orientations by means of mechanical manipulations in early cleavage stage embryos alter the Nodal signaling pathway as well as handedness of shell coiling (Kuroda et al., 2009). Thus, while details of the early steps by which physiological asymmetries result in consistently different genetic programs on the left and right sides differ among phyla, a remarkably wide conservation is seen in the logic and molecular nature of the control systems by which biophysical, epigenetic signals are transformed into stable transcriptional states.
We recently proposed that the early blastoderm’s planar polarization (Wang and Nathans, 2007; Zallen, 2007) also plays a key role in coordinating LR asymmetry (Aw and Levin, 2009; Vandenberg and Levin, 2009). We note that planar cell polarity (PCP) and LR orientation have many similarities, including the need to amplify a patterning signal across large numbers of cells, and the ability of proteins to segregate and orient themselves with respect to each other in a consistently biased manner, generating a large scale signal that originates intracellularly. PCP also enables orientation of cell polarity within the large-scale axes of a host; for example, when grafts of embryonic skin were transplanted into adults, the skin cells realigned their planar hair polarity to match that of the hosts (Devenport and Fuchs, 2008). PCP is an attractive mechanism for LR asymmetry because it can impose the initial orientation of cells or small groups of blastomeres to entire cell fields (Amonlirdviman et al., 2005), as was originally proposed in the “LR Coordinator” model (Hyatt and Yost, 1998). A similar mechanism was recently proposed to explain the instructive influence by means of which primary organizers (existing during the initial cytoskeletal rearrangements) can impose correct asymmetry upon secondary organizers induced later in development (which can otherwise correctly pattern all of their axes except the LR; Vandenberg and Levin, 2010). Consistently, it is known that reversal of the blastocoel roof can randomize asymmetry (Yost, 1992), as predicted by the proposal that the embryonic blastoderm polarizes long before neurulation.
Several recent papers suggest a role for PCP in LR asymmetry (Ross et al., 2005; Antic et al., 2010; Song et al., 2010). Not surprisingly, this has been suggested to be due to the actions of PCP in setting up the positioning of cilia in the node (Okada et al., 2005; Hashimoto et al., 2010). Our alternative proposal, of PCP as primary to the spread of LR orientation throughout the blastoderm in a manner unrelated to nodal cilia, is supported by the requirement for the PCP protein Vangl2 in the asymmetry of the chick embryo, where cilia play no role in asymmetry (Zhang and Levin, 2009). PCP has also recently been shown to be required for asymmetry in Xenopus embryos (Antic et al., 2010), but the crucial experiment distinguishing between nodal cilia and early blastoderm roles has not yet been done because only dorsal microinjection of Vangl morpholino was reported. One prediction distinguishing the ciliary model from the cytoplasmic→ blastoderm model concerns the outcome of ventral injections, because the frog ciliated organ (the GRP) receives no contribution from ventral blastomeres (Schweickert et al., 2007; Blum et al., 2009). A LR phenotype arising from ventral microinjection of PCP-targeting morpholinos would support a role for PCP in coordinating LR orientation throughout the blastoderm, because a ciliary positioning function in the GRP would be ruled out. We are currently testing this directly.
The proposal linking PCP to the elaboration of intracellular LR chirality makes another prediction: that the physiological asymmetries observed in early blastomeres be associated with cell polarity machinery. Indeed this is known not only in Xenopus (Bunney et al., 2003; Qiu et al., 2005), but has recently been shown in Drosophila (Simons et al., 2009) and even yeast (Minc and Chang, 2010), raising the possibility that the linkage between physiological and anatomical polarity is truly fundamental and wide-spread, existing not only at the level of embryos and cell sheets but also that of single cells. A similar mechanism is predicted by the chromatid segregation model, as linking the axial orientation of cells at the earliest cleavages to the mitotic machinery is required for differential chromatid segregation to be consistently aligned with respect to the LR axis.
Data Gaps in the Early Models
The early physiological mechanisms are less well-known than the ciliary model for LR patterning. One misconception that sometimes appears in the literature is that the data implicating ion flows, gap junctions, and serotonergic signaling derive solely from drug-based inhibition experiments, and thus are subject to all the usual caveats inherent in pharmacological perturbation. Indeed, chemical genetic strategies (Smukste and Stockwell, 2005; Adams and Levin, 2006a,b; Wheeler and Brandli, 2009) are a rapid and inexpensive method for initially implicating targets for further characterization, as well as allowing dissection of the timing of different mechanisms (which is often impossible with genetic manipulations). However, it is crucial to note that the drug screens that first suggested the involvement of physiological early signals and helped narrow their timing of activity were accompanied by experiments using molecular loss-of-function (and sometimes also gain-of-function) gene-specific reagents (Levin and Mercola; Levin and Mercola, 1999; Kramer et al., 2002; Levin et al., 2002; Bunney et al., 2003; Raya et al., 2004; Fukumoto et al., 2005a,b; Adams et al., 2006; Morokuma et al., 2008) in every major study, rendering concerns about pharmacological specificity irrelevant and firmly implicating several ion transport and neurotransmitter pathways in LR patterning.
A true area of ignorance in the early cytoplasmic models however is an understanding of how asymmetric gradients of voltage and serotonin content (Fukumoto et al., 2005b; Adams et al., 2006) result in left-sided Nodal expression. Mechanisms converting intracellular serotonin levels at blastula and gastrula stages into changes in transcription at much later stages remain to be identified. The gap junctional communication phase of the early intracellular model (Fig. 1) predicts a novel intracellular serotonin-binding protein that directly modifies transcription. Thus, the linkage between events occurring between stage 5 and stage 17 are largely a black box, although some components such as asymmetric syndecan phosphorylation have now been characterized (Kramer et al., 2002; Kramer and Yost, 2002), and others are currently being characterized in our lab.
A second puzzle reveals itself when the physiological pathways are compared between chick and frog. The electric circuit that establishes asymmetric voltage gradients has been well characterized in Xenopus; it remains to be worked out to the same level of detail in the chick. The same molecular components have been implicated in both organisms: the H+-V-ATPase and H+-K+-ATPase, gap junctions, serotonin, etc. (Levin and Mercola, 1998b; Levin and Mercola, 1999; Levin et al., 2002; Fukumoto et al., 2005b). Yet, the chick expresses these targets and drives asymmetric ion flows at much later stages than does the frog, when tens of thousands of cells are present (Levin et al., 2002; Raya et al., 2004). Moreover, while serotonin and associated signaling pathways have been shown to play a role in both model systems, no asymmetric distribution of serotonin has been observed in chick as it has in frog; this suggests that while similar components are used in amphibia and avians, the pathway has undergone significant modifications in adaptation to a very different bodyplan architecture. Extension of these data to zebrafish and mammals represent additional key areas for future work.
CAN THE CILIA AND INTRACELLULAR MODELS BE COMBINED?
As a possible reconciliation of the two datasets, it may be suggested that the ion transport events important for LR patterning take place on the cilia themselves. Cilia do indeed possess ion channels (Teilmann et al., 2005) and even neurotransmitter receptors (Brailov et al., 2000; Berbari et al., 2008), so perhaps gradients other than calcium (McGrath et al., 2003; Schneider et al., 2008) also function during ciliary flow at the node. The very early presence of the relevant ion transporter proteins does not rule out this model, because, like ciliary proteins themselves which can also be expressed long before ciliogenesis, they could be present but not functioning in LR asymmetry until cilia appear. This proposal is however not consistent with the timing of the earliest consistently-asymmetrical physiological gradients (Levin et al., 2002; Adams et al., 2006), cytoskeletal structures (Danilchik et al., 2006), and protein localizations (Bunney et al., 2003; Adams et al., 2006; Aw et al., 2008; Morokuma et al., 2008), which clearly show that at least in Xenopus, embryos know their left from their right within an hour or two of fertilization. This model is also not consistent with the timing of perturbations that are known to disrupt asymmetry far upstream of unilateral gene expression such as left-sided Nodal (Yost, 1991; Fukumoto et al., 2005a,b; Adams et al., 2006; Danilchik et al., 2006; Vandenberg and Levin, 2010). Early windows of activity indicated by the LR phenotypes of pharmacological blockades initiated at early vs. later time periods are supported by data showing that some gene-specific ion transporter constructs randomize LR asymmetry when injected at the 1 cell stage but lose the ability to do so by the four-cell stage (Aw et al., 2010).
Most decisively, such a model is refuted by the fact that genetic reagents disrupting specific physiological signals can randomize laterality when targeted to cells outside the ciliated organ. The elegant recent mapping of the origin and function of the frog ciliated node by Blum et al. shows that it is only the dorsal cells that contribute to the frog GRP (Blum et al., 2009); moreover, it is only the cells on the left side that are important for LR-relevant flow (Vick et al., 2009). The “channels on cilia” model predicts that constructs functionally disruptive of these pathways should only be effective when targeted to the cells that give rise to the ciliated organ. Yet reagents disrupting these early events that are injected into right ventral blastomeres, which never contribute to the ciliated organ, significantly disrupt the LR axis (Table 3), while targeting those same serotonergic and bioelectrical gradients in cells that contribute to the GRP has much less effect on asymmetry. Most of the physiological activity during early LR steps takes place in the right ventral blastomere and its descendants (Levin, 2006). Importantly, even “ciliary” proteins like Inv exert LR-randomizing effects on the right side, not on the left (Yasuhiko et al., 2001) as predicted by the GRP model.
TABLE 3.
Examples of Molecular-Genetic Perturbations That Influence LR Asymmetry When Not Targeted to GRP (Ciliated Organ) Precursorsa
| Construct or reagent | Blastomeres’ injection penetrance (total N examined) | Reference |
|---|---|---|
| Connexin43 gap junction mRNA | Dorsal cells → 5% heterotaxia (176) Ventral cells → 22% heterotaxia (149) |
(Levin and Mercola, 1998) |
| Connexin26 gap junction mRNA | Ventral cells → 32% heterotaxia | (Levin and Mercola, 1998) |
| Planarian (Smed) innexin11 gap junction mRNA | Dorsal cells → 12% heterotaxia (17) Ventral cells → 35% heterotaxia (72) |
(Oviedo and Levin, 2007) |
| Planarian (D.j.) innexin11 gap junction mRNA | Ventral cells → 35% heterotaxia (39) | (Oviedo and Levin, 2007) |
| SERT mutant D98G mRNA | Dorsal left cells → 7% heterotaxia Right ventral cells → 18% heterotaxia (179) |
(Fukumoto et al., 2005a) |
| LY-278,584 | Dorsal left cells → 4% heterotaxia | (Fukumoto et al., 2005b) |
| (5HT-R3 antagonist) | Right ventral cells → 18% heterotaxia | |
| Serotonin binding protein mRNA | Dorsal left cells → 7% heterotaxia Right ventral cells → 20% heterotaxia (201) |
(Fukumoto et al., 2005b) |
| Inversin protein overexpression | Right side cells → 46% (205) Left side cells → 4% (126) |
(Yasuhiko et al., 2001) |
| PKC-γ morpholinos (PCP loss-of-function) | Right ventral cells → 43% (>60) Left ventral cells → 4% (>60) |
(Kramer et al., 2002) |
One way of reconciling the proposal of nodal cilia as the initiator of asymmetry with the data set implicating ion transporters in LR patterning is a model in which ion transporters and serotonergic pathway members function on cilia or at the node. This model predicts that constructs functionally disruptive of these pathways should have the most effect when targeted to the cells that give rise to the ciliated organ. The GRP has been reverse fate-mapped (Schweickert et al., 2007; Blum et al., 2009) and is known to arise from dorsal cells; moreover, ciliary function on the right side of the node is dispensable for asymmetry (Vick et al., 2009). In contrast, a number of constructs (including the “ciliary” protein Inversin) have their maximum effect when injected on the right and/or ventral side, which according to the GRP model should have no consequence for cilia-dependent events. This proposal is also not consistent with published data on the timing of involvement of physiological events in the LR pathway (Yost, 1991; Fukumoto et al., 2005a; Fukumoto et al., 2005b; Adams et al., 2006; Danilchik et al., 2006; Vandenberg and Levin, 2010).
The Mouse as a Model for Mammalian Asymmetry
Which model is best supported by rodent data? The embryonic architecture of mice is quite unlike most amniotes, being a cylinder rather than a flat blastodisc, and in other mammals, it is not a node per se that is ciliated but an area on the posterior notochord (Blum et al., 2007). Yet data from this model system are often taken as prototypical of all mammals, making it important to ask which model of LR asymmetry best fits the data from rodent embryos. Subject to the caveat mentioned above (intracellular roles for proteins that are normally thought to have cilia-dependent LR phenotypes), the data in mice almost exclusively address (and seem to support) the ciliary model. The question is actually still wide open because the difficulty of early experiments in mice has prevented any tests of physiological models during prenode stages, or of examination of possible early roles for characterized “ciliary” proteins.
Single gene knockouts for the various channels, pumps, gap junctions, serotonergic proteins, etc. known to be important from chick and frog studies have not shown any specific laterality phenotypes in mice; this is not unexpected for physiological control systems, because a large degree of compensation ensures that multiple family gene members (of which there are usually many, for each kind of small molecule flux) can often fulfill the role of any single protein removed. A true test for the role of these ion transporters in establishing the LR axis of mice would involve the knock-in of relevant dominant-negatives or constitutively active mutants, which could be used to specifically affect transmembrane potential during early embryonic stages. At least in humans, one exception may be the connexin43 mutation found in heterotaxia patients. DNA collected from severely affected individuals was compared with normal subjects, and mutations in connexin43 were only found in patients with visceroatrial heterotaxia (Britz-Cunningham et al., 1995). Although this mutation was not found in bigger studies (Casey and Ballabio, 1995; Splitt et al., 1995; Gebbia et al., 1996), the Britz-Cunningham study specifically examined a subclass of heterotaxia patients: those with pulmonary stenosis, a malformation that was also observed in connexin43 null mice (Britz-Cunningham et al., 1995). This was not specifically selected for in later studies, and may account for the difference in results.
Like in frog (Hilton et al., 2007; Sakano et al., 2010), BCoR mutations randomize LR asymmetry in human embryos (Ng et al., 2004; Hilton et al., 2007), but this does not occur in mouse (Ye et al., 1997; Yoshida et al., 1999). The loss of brain asymmetry observed in mouse mutants with ciliary dyskinesia (Kawakami et al., 2008) is not observed in human patients (Kennedy et al., 1999; Tanaka et al., 1999; McManus et al., 2004; Afzelius and Stenram, 2006). Another way in which mouse developmental genetics seems to differ with that of human embryos concerns the LR-bilateral separation of pigmentation patterns in CHILD syndrome that occurs in man (Happle, 2002, 2006) but is highly mosaic in mice, even though all of the other important features of this disease are present (Konig et al., 2000). The difference here may be profound precisely because a clean midline separation resulting from a nondisjunction/inactivation event at very early cleavage stages (as observed in bird gynandromorphs [Hutt, 1949; Agate et al., 2003; Levin, 2006]) suggests an extremely early origin of the midline, which may be true in many amniotes but not in mice.
Significant difficulties in working with early mouse embryos have prevented substantial investigation of prestreak LR mechanisms in mice, but a telling recent study (Gardner, 2010) found that when individual blastomeres from eight-cell stage mouse embryos were rearranged, reversal of the direction of axial rotation was induced. Consistent with earlier proposals about the importance of the positioning of early blastomeres and the possible determination of the midline long before the initiation of the primitive streak (Gardner, 1997, 2001), this work shows that the mouse embryo is not exempt from the importance of very early events for LR asymmetry. Importantly, this is the same outcome as seen in the experiments involving micromanipulation of blastomeres in snail (Kuroda et al., 2009) and C. elegans (Wood and Kershaw, 1991) embryos, providing more evidence that there are conserved mechanisms for establishing LR asymmetry across phyla. While much work remains to be done to characterize other aspects of this laterality phenotype, it is clear that events in the mouse taking place 7 days before the appearance of the ciliated node can influence aspects of asymmetry.
An unfortunate aspect of the field is that most labs perform work uniquely on one model species, with very little crossover. Two targets are promising entry points that may help clarify the differences, and similarities, between rodent asymmetry and that in other model systems. One is the NOD mouse, which develops diabetes mellitus with age. Those mice that became pregnant before the onset of diabetes give rise to offspring with normal organ situs (Morishima et al., 1991). In contrast, offspring born to dams with diabetes have randomized organ placement; these pups were exposed to higher levels of glucose during embryogenesis, causing a down-regulation of glucose transporters, likely altering glucose-coupled ion transport. These data from the NOD mouse, therefore, suggest that laterality disturbances in rodents can be caused by some aspect of ion-related physiology. Thus, future studies may provide interesting insights into the influence of altered pH and membrane voltage on LR development in the mouse, an area that has been widely studied in frog but not yet addressed in mammals. Another promising target that may help unify the mouse and frog models is ZIC3: this molecule is important for LR asymmetry in both mouse and Xenopus embryos, and is known to act before the node and cilia in frog (Kitaguchi et al., 2000; Purandare et al., 2002). ZIC3 is expressed as early as gastrula stages in the mouse and ZIC3-deficient embryos display asymmetries in normally symmetrical structures (Ware et al., 2006). An investigation of even earlier action in mouse should be most informative with respect to the developmental timing of conserved steps.
MAJOR OPEN ISSUES
An analysis of basic physical principles (Brown and Wolpert, 1990; Henley, 2009) as well as data developed over the last 20 years converges on the cytoskeletal organizer as the fundamental source of chirality. Significant disagreement exists in the field about how this is converted to multicellular asymmetry. Each of the three major models of LR asymmetry elaboration still contains significant areas of ignorance. In the case of cilia, it is clear that in two vertebrate model systems, nodal flow per se can affect asymmetry (Nonaka et al., 2002; Schweickert et al., 2007), but a lot of work remains to understand the etiology of true LR phenotypes in “ciliary” mutants, and those in which asymmetry is normal despite cilia defects. Furthermore, the relationship between nodal cilia and upstream LR cues (especially in species like Xenopus where both early and ciliary mechanisms have been demonstrated) must be dissected.
In the case of cytoplasmic early mechanisms (Levin and Palmer, 2007), the nature of the oriented MTOC and the means by which it is tethered with respect to the other two axes remains to be investigated. In the case of the differential chromatid model (Klar, 1994), it likewise remains to be uncovered how the chromatid segregation machinery links to the dorsal–ventral and anterior–posterior axes, to allow consistent distribution of the correctly-marked DNA strand to the correct blastomere during first cleavage. As discussed previously (Armakolas et al., 2010), machinery involved in asymmetric cell division, such as Numb, is a promising avenue for future research. The chromatid segregation model initially arose from the discovery of the chromosome-specific segregation phenomenon among mouse chromosome 7 homologs (Klar, 2008; Armakolas et al., 2010). This model is then especially interesting considering a recently identified mouse with a mutation on Chromosome 7 and LR asymmetry defects, yet apparently normal cilia (Aune et al., 2008); although tracheal, not nodal, cilia were examined in this study, their phenotypes are quite often linked (e.g., in Kartageners syndrome). If the node ciliary function indeed mirrors tracheal in these animals, the data would fit predictions made by the cytoplasmic model (which presumes the existence of mutants in which ciliary flow problems are dissociated from LR defects). Future studies are needed to determine whether biased chromatid segregation is affected in this mutant.
One of the key barriers to progress in the LR field has been the partitioning of models to specific species: early mechanisms have not been specifically tested in mammals, while the role of chromatid segregation has not yet been checked in the LR pathway of any animal. Early zebrafish development has not yet been sufficiently probed, despite the recent discovery of interesting “orphan” pathway members such as endoplasmic reticulum ion transporters (Kreiling et al., 2008) and evidence indicating the dorsal–ventral axis is established at the second cleavage (Gore et al., 2005). Moving forward will require increased collaboration among labs with expertise in specific pathways and model systems to cross-check their pathways in a wider variety of taxa, and remaining open to the idea that the origin problem is not yet solved.
In addition to questions raised by human clinical data that are not tractable in any of the available model systems (Aw and Levin, 2008), specific major directions include the following: How do upstream mechanisms functioning in chick, mouse, sea urchin, etc. all converge on asymmetric Nodal expression during much later development? For example, Sonic hedgehog is a crucial early asymmetric gene in the chick node (Levin et al., 1995), but is not necessary in mouse node for normal asymmetry (Tsiairis and McMahon, 2009). Strongly related to the nexus in which epigenetic events become converted to transcriptional responses is the question of “coordination.” Despite the near-universal presence of heterotaxia (randomization and independent sidedness of each organ) in LR phenotypes, we still have very little understanding of the mechanisms underlying concordance among organs in wild-type or situs inversus individuals, or the differences underlying heterotaxia, situs inversus, and isomerisms (which, oddly, are never seen in Xenopus).
Other important questions remain: What is the nature of the maternal chirality-determining factor in snails (Freeman and Lundelius, 1982)? The data suggest strongly that the cytoskeleton is crucial in determining very early events of asymmetry but what is the role of the factor that has been demonstrated in cytoplasm transfer experiments but not molecularly identified? And, is it related to the epigenetic control of laterality in the duckweed Lemna? The development of individual fronds toward L- or R-handedness during vegetative propagation is not controlled by either nuclear or cytoplasmic genes, but handedness can be altered by modulators of the auxin/serotonin pathway, resulting in the formation of an inheritably unstable frond capable of giving rise to both L and R stable clones (Kasinov, 1973; Doss, 1978). A similar effect occurs in the colonial sea-squirt Botryllus: experimental reversal of visceral asymmetry is propagated vegetatively in all buds that descend from one that is experimentally reversed (Sabbadin et al., 1975).
The relationship of brain asymmetry to body asymmetry remains a fascinating area. While some models, such as zebrafish (Halpern et al., 2003; McManus, 2005; Aizawa et al., 2007), have resulted in significant progress on the molecular basis of brain asymmetry, the fundamental question in mammals remains open. Mice with the iv mutation exhibit brain isomerisms, not 50/50 incidence of situs inversus as is observed in their viscera (Kawakami et al., 2008). Although humans with situs inversus exhibit reversal of some anatomical brain asymmetries, they do not show reversals in functional asymmetries, and establish language dominance on the left cerebral hemisphere and a strong bias for right-handedness, just like in the general population (Kennedy et al., 1999; Tanaka et al., 1999; McManus et al., 2004).
CONCLUSIONS AND PERSPECTIVES
We have presented here an overview of the strengths and weaknesses of three models for the establishment of LR asymmetry. While the ciliary model is widely accepted as “fact” in cell biology textbooks and by students and grant reviewers alike, there are many examples of data that are at odds with this model (Table 2). First, the ciliary model cannot explain results from several studies suggesting that consistent LR asymmetry can be produced in the absence of a node, confined fluid flow, or even multicellularity (Xu et al., 2007; Gros et al., 2009). Additionally, just as the cytoplasmic model predicts, we have identified at least six examples where ciliary phenotypes can be dissociated from problems with LR asymmetry, indicating that a causal link between cilia and LR asymmetry is less than solid (Zhao and Malicki, 2007; Aune et al., 2008; Kishimoto et al., 2008; Serluca et al., 2009; Tian et al., 2009; Zeng et al., 2010). Finally, a role for cilia as initiators of laterality is inconsistent with the existence of asymmetries that are consistent, and both physiological and morphological during early cleavage stages (Adams et al., 2006; Danilchik et al., 2006; Levin and Palmer, 2007).
Bringing the state of the field (and its dominant models) into sharper focus is critical for continued progress. This is important not only from the perspective of funding, biomedical implications, and drawing new talent to the field, but also because an exclusive focus on ciliary, late mechanisms functions as a positive-feedback loop. Many studies on novel players in the LR pathway analyze their late roles only, assuming that pregastrulation functions are unlikely to be important; this is particularly true in Zebrafish, where many studies examine only KV roles, never analyzing potentially important processes occurring early and leaving the field with the impression that nothing interesting happens before the KV forms (which in turn makes it less likely that future studies examining early roles, especially in mice, are ever performed).
The links between intracellular (cytoskeleton-driven) polarity and LR asymmetry have grown stronger: recent data from snails, frogs and mice all suggest that a chiral cytoskeleton is established extremely early during development (Danilchik et al., 2006; Aw et al., 2008; Kuroda et al., 2009; Gardner, 2010), a remarkable example of a widely-conserved system that has relevance to invertebrate and even plant systems (Doss, 1978; Hashimoto, 2002; Abe et al., 2004; Levin, 2006; Oviedo and Levin, 2007). Interestingly, the role of a MTOC as the initiator of LR asymmetry is common to all three main models in the field, a largely unacknowledged common ground among the many interesting controversies about downstream amplification and coordination mechanisms.
LR asymmetry may be fundamental to most (if not all) cells, evolutionarily ancient, likely driven by the inherent chirality of cytoskeletal organizing centers, manifest at the single-cell level, and imposed across multicellular cell fields early in embryogenesis by a combination of physiological, biophysical, and epigenetic pathways upstream of asymmetric gene expression. This field thus offers some of the richest opportunities for novel biology that spans boundaries of scale and discipline, and there is truly much still to be learned.
ACKNOWLEDGMENTS
We thank Richard Palmer, Heide Schatten, Janine Beisson, Dany Adams, and other members of the Levin lab and the LR community for many helpful discussions. M.L. gratefully acknowledges the support of the American Heart Association and the National Institute of Health. L.N.V. is also supported by the National Institutes of Health.
Grant sponsor:
American Heart Association; Grant number: Established Investigator Grant 0740088N; Grant sponsor: The National Institutes of Health; Grant numbers: R01-GM077425, 1F32GM087107-01.
REFERENCES
- Abe T, Thitamadee S, Hashimoto T. 2004. Microtubule defects and cell morphogenesis in the lefty1lefty2 tubulin mutant of Arabidopsis thaliana. Plant Cell Physiol 45:211–220. [DOI] [PubMed] [Google Scholar]
- Adams DS, Levin M. 2006a. Inverse drug screens: a rapid and inexpensive method for implicating molecular targets. Genesis 44:530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Levin M. 2006b.Strategies and techniques for investigation of biophysical signals in patterning. In:Whitman M,Sater AK, editors. Analysis of growth factor signaling in embryos. London: Taylor and Francis Books. p 177–262. [Google Scholar]
- Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, Yelick P, Kuo L, McSweeney M, Levin M. 2006. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development 133:1657–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzelius BA, Stenram U. 2006. Prevalence and genetics of immotile-cilia syndrome and left-handedness. Int J Dev Biol 50:571–573. [DOI] [PubMed] [Google Scholar]
- Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. 2003. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proc Natl Acad Sci U S A 100:4873–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Goto M, Sato T, Okamoto H. 2007. Temporally regulated asymmetric neurogenesis causes left-right difference in the zebrafish habenular structures. Dev Cell 12:87–98. [DOI] [PubMed] [Google Scholar]
- Albrecht-Buehler G. 1981. Does the geometric design of centrioles imply their function? Cell Motility 1:237–245. [DOI] [PubMed] [Google Scholar]
- Albrecht-Buehler G, Bushnell A. 1979. The orientation of centrioles in migrating 3T3 cells. Exp Cell Res 120:111–118. [DOI] [PubMed] [Google Scholar]
- Albrieux M, Villaz M. 2000. Bilateral asymmetry of the inositol trisphosphate-mediated calcium signaling in two-cell ascidian embryos. Biol Cell 92:277–284. [DOI] [PubMed] [Google Scholar]
- Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. 2005. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science 307:423–426. [DOI] [PubMed] [Google Scholar]
- Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. 2010. Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS One 5:e8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armakolas A, Klar AJ. 2007. Left-right dynein motor implicated in selective chromatid segregation in mouse cells. Science 315:100–101. [DOI] [PubMed] [Google Scholar]
- Armakolas A, Koutsilieris M, Klar AJ. 2010. Discovery of the mitotic selective chromatid segregation phenomenon and its implications for vertebrate development. Curr Opin Cell Biol 22:81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune CN, Chatterjee B, Zhao XQ, Francis R, Bracero L, Yu Q, Rosenthal J, Leatherbury L, Lo CW. 2008. Mouse model of heterotaxy with single ventricle spectrum of cardiac anomalies. Pediatr Res 63:9–14. [DOI] [PubMed] [Google Scholar]
- Aw S, Levin M. 2008. What’s left in asymmetry? Dev Dyn 237:3453–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Levin M. 2009. Is left-right asymmetry a form of planar cell polarity? Development 136:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Adams DS, Qiu D, Levin M. 2008. H,K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech Dev 125:353–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Koster J, Pearson W, Nichols C, Shi NQ, Carneiro K, Levin M. 2010. The ATP-sensitive K(+)-channel (K(ATP)) controls early left-right patterning in Xenopus and chick embryos. Dev Biol 346:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu B, Brueckner M. 2008. Cilia: multifunctional organelles at the center of vertebrate left-right asymmetry. Curr Top Dev Biol 85:151–174. [DOI] [PubMed] [Google Scholar]
- Beisson J, Jerka-Dziadosz M. 1999. Polarities of the centriolar structure: morphogenetic consequences. Biol Cell 91:367–378. [PubMed] [Google Scholar]
- Bell AJ, Satir P, Grimes GW. 2008. Mirror-imaged doublets of Tetmemena pustulata: implications for the development of left-right asymmetry. Dev Biol 314:150–160. [DOI] [PubMed] [Google Scholar]
- Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. 2008. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell 19:1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgrove BW, Snarr BS, Emrazian A, Yost HJ. 2005. Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer’s vesicle are required for specification of the zebrafish left-right axis. Dev Biol 287:274–288. [DOI] [PubMed] [Google Scholar]
- Blum M, Andre P, Muders K, Schweickert A, Fischer A, Bitzer E, Bogusch S, Beyer T, van Straaten HW, Viebahn C. 2007. Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation 75:133–146. [DOI] [PubMed] [Google Scholar]
- Blum M, Beyer T, Weber T, Vick P, Andre P, Bitzer E, Schweickert A. 2009. Xenopus, an ideal model system to study vertebrate left-right asymmetry. Dev Dyn 238:1215–1225. [DOI] [PubMed] [Google Scholar]
- Bock G, Marsh J. 1991. Biological asymmetry and handedness. New York: Wiley. ix, 327 pp. [Google Scholar]
- Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, Verge D. 2000. Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res 872:271–275. [DOI] [PubMed] [Google Scholar]
- Britz-Cunningham S, Shah M, Zuppan C, Fletcher W. 1995. Mutations of the connexin-43 gap-junction gene in patients with heart malformations and defects of laterality. N Engl J Med 332:1323–1329. [DOI] [PubMed] [Google Scholar]
- Brown NA, Wolpert L. 1990. The development of handedness in left/right asymmetry. Development 109:1–9. [DOI] [PubMed] [Google Scholar]
- Brueckner M. 2001. Cilia propel the embryo in the right direction. Am J Med Genet 101:339–344. [DOI] [PubMed] [Google Scholar]
- Bunney TD, De Boer AH, Levin M. 2003. Fusicoccin signaling reveals 14–3-3 protein function as a novel step in left-right patterning during amphibian embryogenesis. Development 130:4847–4858. [DOI] [PubMed] [Google Scholar]
- Carmena M, Earnshaw WC. 2003. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol 4:842–854. [DOI] [PubMed] [Google Scholar]
- Cartwright JH, Piro O, Tuval I. 2004. Fluid-dynamical basis of the embryonic development of left-right asymmetry in vertebrates. Proc Natl Acad Sci U S A 101:7234–7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B, Ballabio A. 1995. Connexin43 mutations in sporadic and familial defects of laterality. N Engl J Med 333:941. [DOI] [PubMed] [Google Scholar]
- Chuang CF, Vanhoven MK, Fetter RD, Verselis VK, Bargmann CI. 2007. An innexin-dependent cell network establishes left-right neuronal asymmetry in C. elegans. Cell 129:787–799. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. 2005. Vertebrate Smoothened functions at the primary cilium. Nature 437:1018–1021. [DOI] [PubMed] [Google Scholar]
- Coutelis JB, Petzoldt AG, Speder P, Suzanne M, Noselli S. 2008. Left-right asymmetry in Drosophila. Semin Cell Dev Biol 19:252–262. [DOI] [PubMed] [Google Scholar]
- Danilchik MV, Brown EE, Riegert K. 2006. Intrinsic chiral properties of the Xenopus egg cortex: an early indicator of left-right asymmetry? Development 133:4517–4526. [DOI] [PubMed] [Google Scholar]
- Devenport D, Fuchs E. 2008. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol 10:1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss RP. 1978. Handedness in duckweed - double flowering fronds produce right-handed and left-handed lineages. Science 199:1465–1466. [DOI] [PubMed] [Google Scholar]
- Duboc V, Rottinger E, Lapraz F, Besnardeau L, Lepage T. 2005. Left-right asymmetry in the sea urchin embryo is regulated by nodal signaling on the right side. Dev Cell 9:147–158. [DOI] [PubMed] [Google Scholar]
- Essner J, Vogan K, Wagner M, Tabin C, Yost H, Brueckner M. 2002. Conserved function for embryonic nodal cilia. Nature 418:37–38. [DOI] [PubMed] [Google Scholar]
- Frankel J. 1991. Intracellular handedness in ciliates. CIBA Found Symp 162:73–88. [DOI] [PubMed] [Google Scholar]
- Freeman G, Lundelius JW. 1982. The developmental genetics of dextrality and sinistrality in the gastropod lymnaeaperegra. Wilhelm Rouxs Arch Dev Biol 191:69–83. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Blakely R, Levin M. 2005a. Serotonin transporter function is an early step in left-right patterning in chick and frog embryos. Dev Neurosci 27:349–363. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Kema IP, Levin M. 2005b. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol 15:794–803. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Levin M. 2005. Asymmetric expression of Syndecan-2 in early chick embryogenesis. Gene Expr Patterns 5:525–528. [DOI] [PubMed] [Google Scholar]
- Gardner RL. 1997. The early blastocyst is bilaterally symmetrical and its axis of symmetry is aligned with the animal-vegetal axis of the zygote in the mouse. Development 124:289–301. [DOI] [PubMed] [Google Scholar]
- Gardner RL. 2001. Specification of embryonic axes begins before cleavage in normal mouse development. Development 128:839–847. [DOI] [PubMed] [Google Scholar]
- Gardner RL. 2010. Normal bias in the direction of fetal rotation depends on blastomere composition during early cleavage in the mouse. PLoS One 5:e9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbia M, Towbin JA, Casey B. 1996. Failure to detect connexin43 mutations in 38 cases of sporadic and familial heterotaxy. Circulation 94:1909–1912. [DOI] [PubMed] [Google Scholar]
- Gore AV, Maegawa S, Cheong A, Gilligan PC, Weinberg ES, Sampath K. 2005. The zebrafish dorsal axis is apparent at the four-cell stage. Nature 438:1030–1035. [DOI] [PubMed] [Google Scholar]
- Gros J, Feistel K, Viebahn C, Blum M, Tabin CJ. 2009. Cell movements at Hensen’s node establish left/right asymmetric gene expression in the chick. Science 324:941–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern ME, Liang JO, Gamse JT. 2003. Leaning to the left: laterality in the zebrafish forebrain. Trends Neurosci 26:308–313. [DOI] [PubMed] [Google Scholar]
- Happle R. 2002. Dohi Memorial Lecture. New aspects of cutaneous mosaicism. J Dermatol 29:681–692. [DOI] [PubMed] [Google Scholar]
- Happle R. 2006. X-chromosome inactivation: role in skin disease expression. Acta Paediatr Suppl 95:9–10. [DOI] [PubMed] [Google Scholar]
- Hashimoto T. 2002. Molecular genetic analysis of left-right handedness in plants. Philos Trans R Soc Lond B Biol Sci 357:799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A, Hamada H. 2010. Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol 12:170–176. [DOI] [PubMed] [Google Scholar]
- Heacock AM, Agranoff BW. 1977. Clockwise growth of neurites from retinal explants. Science 198:64–66. [DOI] [PubMed] [Google Scholar]
- Henley CL. 2009.Possible mechanisms for initiating macroscopic left-right asymmetry in developing organisms. In:Lebedev V,Feigelman M, editors. Advances in theoretical physics. Mellville, NY: American Institute of Physics. p 54–62. [Google Scholar]
- Hibino T, Ishii Y, Levin M, Nishino A. 2006. Ion flow regulates left-right asymmetry in sea urchin development. Dev Genes Evol 216:265–276. [DOI] [PubMed] [Google Scholar]
- Hilton EN, Manson FD, Urquhart JE, Johnston JJ, Slavotinek AM, Hedera P, Stattin EL, Nordgren A, Biesecker LG, Black GC. 2007. Left-sided embryonic expression of the BCL-6 corepressor, BCOR, is required for vertebrate laterality determination. Hum Mol Genet 16:1773–1782. [DOI] [PubMed] [Google Scholar]
- Hozumi S, Maeda R, Taniguchi K, Kanai M, Shirakabe S, Sasamura T, Speder P, Noselli S, Aigaki T, Murakami R, Matsuno K. 2006. An unconventional myosin in Drosophila reverses the default handedness in visceral organs. Nature 440:798–802. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. 2003. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426:83–87. [DOI] [PubMed] [Google Scholar]
- Hutt FB. 1949. Genetics of the fowl. New York: McGraw Hill. [Google Scholar]
- Hutter H, Schnabel R. 1995. Establishment of left-right asymmetry in the Caenorhabditis elegans embryo: a multistep process involving a series of inductive events. Development 121:3417–3424. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Yost HJ. 1998. The left-right coordinator: the role of Vg1 in organizing left-right axis formation. Cell 93:37–46. [DOI] [PubMed] [Google Scholar]
- Kasinov VB. 1973. Handedness in Lemnaceae: on the determination of left and right types of development in Lemna clones and on its alteration by means of external influences. Beitr Biol Pflanzen 49:321–337. [Google Scholar]
- Kawakami R, Dobi A, Shigemoto R, Ito I. 2008. Right isomerism of the brain in inversus viscerum mutant mice. PLoS One 3:e1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D, O’Craven K, Ticho B, Goldstein A, Makris N, Henson J. 1999. Structural and functional brain asymmetries in human situs inversus totalis. Neurology 53:1260–1265. [DOI] [PubMed] [Google Scholar]
- Kishimoto N, Cao Y, Park A, Sun Z. 2008. Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev Cell 14:954–961. [DOI] [PubMed] [Google Scholar]
- Kitaguchi T, Nagai T, Nakata K, Aruga J, Mikoshiba K. 2000. Zic3 is involved in the left-right specification of the Xenopus embryo. Development 127:4787–4795. [DOI] [PubMed] [Google Scholar]
- Kitazawa C, Amemiya S. 2007. Micromere-derived signal regulates larval left-right polarity during sea urchin development. J Exp Zool Part A Ecol Genet Physiol 307:249–262. [DOI] [PubMed] [Google Scholar]
- Klar AJ. 1994. A model for specification of the left-right axis in vertebrates. Trends Genet 10:392–396. [DOI] [PubMed] [Google Scholar]
- Klar AJ. 2008. Support for the selective chromatid segregation hypothesis advanced for the mechanism of left-right body axis development in mice. Breast Dis 29:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL. 1987. The first cleavage furrow demarcates the dorsal-ventral axis in Xenopus embryos. Dev Biol 120:299–304. [DOI] [PubMed] [Google Scholar]
- Konig A, Happle R, Bornholdt D, Engel H, Grzeschik KH. 2000. Mutations in the NSDHL gene, encoding a 3beta-hydroxysteroid dehydrogenase, cause CHILD syndrome. Am J Med Genet 90:339–346. [PubMed] [Google Scholar]
- Kramer KL, Yost HJ. 2002. Ectodermal syndecan-2 mediates left-right axis formation in migrating mesoderm as a cell-nonautonomous Vg1 cofactor. Dev Cell 2:115–124. [DOI] [PubMed] [Google Scholar]
- Kramer KL, Barnette JE, Yost HJ. 2002. PKCgamma regulates syndecan-2 inside-out signaling during xenopus left-right development. Cell 111:981–990. [DOI] [PubMed] [Google Scholar]
- Kreiling JA, Balantac ZL, Crawford AR, Ren Y, Toure J, Zchut S, Kochilas L, Creton R. 2008. Suppression of the endoplasmic reticulum calcium pump during zebrafish gastrulation affects left-right asymmetry of the heart and brain. Mech Dev 125:396–410. [DOI] [PubMed] [Google Scholar]
- Kuroda R, Endo B, Abe M, Shimizu M. 2009. Chiral blastomere arrangement dictates zygotic left-right asymmetry pathway in snails. Nature 462:790–794. [DOI] [PubMed] [Google Scholar]
- Kurpios NA, Ibanes M, Davis NM, Lui W, Katz T, Martin JF, Belmonte JC, Tabin CJ. 2008. The direction of gut looping is established by changes in the extracellular matrix and in cell:cell adhesion. Proc Natl Acad Sci U S A 105:8499–8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JD, Nagy LM. 2002. Asymmetric inheritance of centrosomally localized mRNAs during embryonic cleavages. Nature 420:682–686. [DOI] [PubMed] [Google Scholar]
- Lepori NG. 1969. Sur la genese des structures asymetriques chez l’embryon des oiseaux. Monit Zool Ital (NS) 3:33–53. [Google Scholar]
- Levin M. 1999. Twinning and embryonic left-right asymmetry. Laterality 4:197–208. [DOI] [PubMed] [Google Scholar]
- Levin M. 2003. Motor protein control of ion flux is an early step in embryonic left-right asymmetry. Bioessays 25:1002–1010. [DOI] [PubMed] [Google Scholar]
- Levin M. 2005. Left-right asymmetry in embryonic development: a comprehensive review. Mech Dev 122:3–25. [DOI] [PubMed] [Google Scholar]
- Levin M. 2006. Is the early left-right axis like a plant, a kidney, or a neuron? The integration of physiological signals in embryonic asymmetry. Birth Defects Res C Embryo Today 78:191–223. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. 1998a. The compulsion of chirality: toward an understanding of left-right asymmetry. Genes Dev 12:763–769. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. 1998b. Gap junctions are involved in the early generation of left-right asymmetry. Dev Biol 203:90–105. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. 1999. Gap junction-mediated transfer of left-right patterning signals in the early chick blastoderm is upstream of Shh asymmetry in the node. Development 126:4703–4714. [DOI] [PubMed] [Google Scholar]
- Levin M, Nascone N. 1997. Two molecular models of initial left-right asymmetry generation. Med Hypotheses 49:429–435. [DOI] [PubMed] [Google Scholar]
- Levin M, Palmer AR. 2007. Left-right patterning from the inside out: widespread evidence for intracellular control. Bioessays 29:271–287. [DOI] [PubMed] [Google Scholar]
- Levin M, Johnson RL, Stern CD, Kuehn M, Tabin C. 1995. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell 82:803–814. [DOI] [PubMed] [Google Scholar]
- Levin M, Pagan S, Roberts DJ, Cooke J, Kuehn MR, Tabin CJ. 1997. Left/right patterning signals and the independent regulation of different aspects of Situs in the chick embryo. Dev Biol 189:57–67. [DOI] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. 2002. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell 111:77–89. [DOI] [PubMed] [Google Scholar]
- Levin M, Buznikov GA, Lauder JM. 2006. Of minds and embryos: left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci 28:171–185. [DOI] [PubMed] [Google Scholar]
- Liu A, Wang B, Niswander LA. 2005. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132:3103–3111. [DOI] [PubMed] [Google Scholar]
- Manner J. 2001. Does an equivalent of the “ventral node” exist in chick embryos? A scanning electron microscopic study. Anat Embryol (Berl) 203:481–490. [DOI] [PubMed] [Google Scholar]
- Marrari Y, Rouviere C, Houliston E. 2004. Complementary roles for dynein and kinesins in the Xenopus egg cortical rotation. Dev Biol 271:38–48. [DOI] [PubMed] [Google Scholar]
- Masho R. 1990. Close correlation between the 1st cleavage plane and the body axis in early Xenopus embryos. Dev Growth Differ 32:57–64. [DOI] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. 2003. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114:61–73. [DOI] [PubMed] [Google Scholar]
- McManus IC. 2002. Right hand, left hand: the origins of asymmetry in brains, bodies, atoms, and cultures. Cambridge, MA: Harvard University Press. xix,412 pp. [Google Scholar]
- McManus C. 2005. Reversed bodies, reversed brains, and (some) reversed behaviors: of zebrafish and men. Dev Cell 8:796–797. [DOI] [PubMed] [Google Scholar]
- McManus IC, Martin N, Stubbings GF, Chung EM, Mitchison HM. 2004. Handedness and situs inversus in primary ciliary dyskinesia. Proc R Soc Lond B Biol Sci 271:2579–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson NH. 1976. Helical growth of Bacillus subtilis: a new model of cell growth. Proc Natl Acad Sci U S A 73:1740–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson NH, Karamata D. 1982. Inversion of helix orientation in Bacillus subtilis macrofibers. J Bacteriol 151:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshcheryakov VN, Beloussov LV. 1975. Asymmetrical rotations of blastomeres in early cleavage of gastropoda. Wilhelm Roux Arch Entwickl Org 177:193–203. [DOI] [PubMed] [Google Scholar]
- Minc N, Chang F. 2010. Electrical control of cell polarization in the fission yeast schizosaccharomyces pombe. Curr Biol [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli SH, Young L, Reid B, Ruttenberg H, Bamshad MJ. 2001. Clinical analysis of families with heart, midline, and laterality defects. Am J Med Genet 101:388–392. [DOI] [PubMed] [Google Scholar]
- Morgan MJ. 1991. The asymmetrical genetic determination of laterality: flatfish, frogs and human handedness. CIBA Found Symp 162:234–247. [DOI] [PubMed] [Google Scholar]
- Morishima M, Ando M, Takao A. 1991. Visceroatrial heterotaxy syndrome in the NOD mouse with special reference to atrial situs. Teratology 44:91–100. [DOI] [PubMed] [Google Scholar]
- Morokuma J, Blackiston D, Levin M. 2008. KCNQ1 and KCNE1 K+ channel components are involved in early left-right patterning in Xenopus laevis embryos. Cell Biochem Biophys 21:357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen EM, Frankel J, Jenkins LM. 1989. Non-genic inheritance of cellular handedness. Development 105:447–456. [DOI] [PubMed] [Google Scholar]
- Ng D, Thakker N, Corcoran CM, Donnai D, Perveen R, Schneider A, Hadley DW, Tifft C, Zhang L, Wilkie AO, van der Smagt JJ, Gorlin RJ, Burgess SM, Bardwell VJ, Black GC, Biesecker LG. 2004. Oculofaciocardiodental and Lenz microphthalmia syndromes result from distinct classes of mutations in BCOR. Nat Genet 36:411–416. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Shiratori H, Saijoh H, Hamada H. 2002. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 418:96–99. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Yoshiba S, Watanabe D, Ikeuchi S, Goto T, Marshall WF, Hamada H. 2005. De novo formation of left-right asymmetry by posterior tilt of nodal cilia. PLoS Biol 3:e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Takeda S, Tanaka Y, Belmonte JC, Hirokawa N. 2005. Mechanism of nodal flow: a conserved symmetry breaking event in left-right axis determination. Cell 121:633–644. [DOI] [PubMed] [Google Scholar]
- Okumura T, Utsuno H, Kuroda J, Gittenberger E, Asami T, Matsuno K. 2008. The development and evolution of left-right asymmetry in invertebrates: lessons from Drosophila and snails. Dev Dyn 237:3497–3515. [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Levin M. 2007. Gap junctions provide new links in left-right patterning. Cell 129:645–647. [DOI] [PubMed] [Google Scholar]
- Pagan-Westphal SM, Tabin CJ. 1998. The transfer of left-right positional information during chick embryogenesis. Cell 93:25–35. [DOI] [PubMed] [Google Scholar]
- Peeters H, Devriendt K. 2006. Human laterality disorders. Eur J Med Genet 49:349–362. [DOI] [PubMed] [Google Scholar]
- Priess JR. 1994. Establishment of initial asymmetry in early Caenorhabditis elegans embryos. Curr Opin Genet Dev 4:563–568. [DOI] [PubMed] [Google Scholar]
- Purandare SM, Ware SM, Kwan KM, Gebbia M, Bassi MT, Deng JM, Vogel H, Behringer RR, Belmont JW, Casey B. 2002. A complex syndrome of left-right axis, central nervous system and axial skeleton defects in Zic3 mutant mice. Development 129:2293–2302. [DOI] [PubMed] [Google Scholar]
- Qiu D, Cheng SM, Wozniak L, McSweeney M, Perrone E, Levin M. 2005. Localization and loss-of-function implicates ciliary proteins in early, cytoplasmic roles in left-right asymmetry. Dev Dyn 234:176–189. [DOI] [PubMed] [Google Scholar]
- Ramsdell AF. 2005. Left-right asymmetry and congenital cardiac defects: getting to the heart of the matter in vertebrate left-right axis determination. Dev Biol 288:1–20. [DOI] [PubMed] [Google Scholar]
- Raya A, Belmonte JC. 2006. Left-right asymmetry in the vertebrate embryo: from early information to higher-level integration. Nat Rev Genet 7:283–293. [DOI] [PubMed] [Google Scholar]
- Raya A, Izpisua Belmonte JC. 2008. Insights into the establishment of left-right asymmetries in vertebrates. Birth Defects Res C Embryo Today 84:81–94. [DOI] [PubMed] [Google Scholar]
- Raya A, Kawakami Y, Rodriguez-Esteban C Ibanes M, Rasskin-Gutman D, Rodriguez-Leon J, Buscher D, Feijo JA, Izpisua Belmonte JC. 2004. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature 427:121–128. [DOI] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL. 2005. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet 37:1135–1140. [DOI] [PubMed] [Google Scholar]
- Sabbadin A, Zaniolo G, Majone F. 1975. Determination of polarity and bilateral asymmetry in palleal and vascular buds of ascidian Botryllus-schlosseri. Dev Biol 46:79–87. [DOI] [PubMed] [Google Scholar]
- Sagasti A. 2007. Three ways to make two sides: genetic models of asymmetric nervous system development. Neuron 55:345–351. [DOI] [PubMed] [Google Scholar]
- Sakano D, Kato A, Parikh N, McKnight K, Terry D, Stefanovic B, Kato Y. 2010. BCL6 canalizes Notch-dependent transcription, excluding Mastermind-like1 from selected target genes during left-right patterning. Dev Cell 18:450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf SR, Gerhart JC. 1983. Axis determination in eggs of Xenopus laevis: a critical period before first cleavage, identified by the common effects of cold, pressure and ultraviolet irradiation. Dev Biol 99:75–87. [DOI] [PubMed] [Google Scholar]
- Schneider I, Houston DW, Rebagliati MR, Slusarski DC. 2008. Calcium fluxes in dorsal forerunner cells antagonize beta-catenin and alter left-right patterning. Development 135:75–84. [DOI] [PubMed] [Google Scholar]
- Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M. 2007. Cilia-driven leftward flow determines laterality in Xenopus. Curr Biol 17:60–66. [DOI] [PubMed] [Google Scholar]
- Serluca FC, Xu B, Okabe N, Baker K, Lin SY, Sullivan-Brown J, Konieczkowski DJ, Jaffe KM, Bradner JM, Fishman MC, Burdine RD. 2009. Mutations in zebrafish leucine-rich repeat-containing six-like affect cilia motility and result in pronephric cysts, but have variable effects on left-right patterning. Development 136:1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XB, Qiu ZI, He W, Frankel J. 1991. Microsurgically generated discontinuities provoke heritable changes in cellular handedness of a ciliate, Stylonychia mytilus. Development 111:337–356. [DOI] [PubMed] [Google Scholar]
- Shibazaki Y, Shimizu M, Kuroda R. 2004. Body handedness is directed by genetically determined cytoskeletal dynamics in the early embryo. Curr Biol 14:1462–1467. [DOI] [PubMed] [Google Scholar]
- Shimeld SM, Levin M. 2006. Evidence for the regulation of left-right asymmetry in Ciona intestinalis by ion flux. Dev Dyn 235:1543–1553. [DOI] [PubMed] [Google Scholar]
- Simons M, Gault WJ, Gotthardt D, Rohatgi R, Klein TJ, Shao Y, Lee HJ, Wu AL, Fang Y, Satlin LM, Dow JT, Chen J, Zheng J, Boutros M, Mlodzik M. 2009. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat Cell Biol 11:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smukste I, Stockwell BR. 2005. Advances in chemical genetics. Annu Rev Genomics Hum Genet 6:261–286. [DOI] [PubMed] [Google Scholar]
- Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. 2010. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature 466:378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speder P, Petzoldt AG, Suzanne M, Noselli S. 2007. Strategies to establish left/right asymmetry in vertebrates and invertebrates. Curr Opin Genet Dev 17:351–358. [DOI] [PubMed] [Google Scholar]
- Spemman H. 1906. Uber eine neue methode der embryonalen transplantation. Verh Dtsch Zool Ges 195–202. [Google Scholar]
- Splitt MP, Burn J, Goodship J. 1995. Connexin43 mutations in sporadic and familial defects of laterality. N Engl J Med 333:941; author reply 941–942. [PubMed] [Google Scholar]
- Stern C, Yu R, Kakizuka A, Kintner C, Mathews L, Vale W, Evans R, Umesono K. 1995. Activin and its receptors during gastrulation and the later phases of mesoderm development in the chick embryo. Dev Biol 172:192–205. [DOI] [PubMed] [Google Scholar]
- Sun T, Walsh CA. 2006. Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci 7:655–662. [DOI] [PubMed] [Google Scholar]
- Supp DM, Witte DP, Potter SS, Brueckner M. 1997. Mutation of an axonemal dynein affects left-right asymmetry in inversus viscerum mice. Nature 389:963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C. 2005. Do we know anything about how left-right asymmetry is first established in the vertebrate embryo? J Mol Histol 36:317–323. [DOI] [PubMed] [Google Scholar]
- Tabin CJ. 2006. The key to left-right asymmetry. Cell 127:27–32. [DOI] [PubMed] [Google Scholar]
- Tabin CJ, Vogan KJ. 2003. A two-cilia model for vertebrate left-right axis specification. Genes Dev 17:1–6. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Gonzalez C. 2009. Biased segregation of DNA and centrosomes: moving together or drifting apart? Nat Rev Mol Cell Biol 10:804–810. [DOI] [PubMed] [Google Scholar]
- Takano K, Ito Y, Obata S, Oinuma T, Komazaki S, Nakamura H, Asashima M. 2007. Heart formation and left-right asymmetry in separated right and left embryos of a newt. Int J Dev Biol 51:265–272. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kanzaki R, Yoshibayashi M, Kamiya T, Sugishita M. 1999. Dichotic listening in patients with situs inversus: brain asymmetry and situs asymmetry. Neuropsychologia 37:869–874. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Hsieh YW, Gamse JT, Chuang CF. 2010. Making a difference together: reciprocal interactions in C. elegans and zebrafish asymmetric neural development. Development 137:681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teilmann SC, Byskov AG, Pedersen PA, Wheatley DN, Pazour GJ, Christensen ST. 2005. Localization of transient receptor potential ion channels in primary and motile cilia of the female murine reproductive organs. Mol Reprod Dev 71:444–452. [DOI] [PubMed] [Google Scholar]
- Theurkauf WE, Smiley S, Wong ML, Alberts BM. 1992. Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development 115:923–936. [DOI] [PubMed] [Google Scholar]
- Thitamadee S, Tuchihara K, Hashimoto T. 2002. Microtubule basis for left-handed helical growth in Arabidopsis. Nature 417:193–196. [DOI] [PubMed] [Google Scholar]
- Tian T, Zhao L, Zhang M, Zhao X, Meng A. 2009. Both foxj1a and foxj1b are implicated in left-right asymmetric development in zebrafish embryos. Biochem Biophys Res Commun 380:537–542. [DOI] [PubMed] [Google Scholar]
- Tsiairis CD, McMahon AP. 2009. An Hh-dependent pathway in lateral plate mesoderm enables the generation of left/right asymmetry. Curr Biol 19:1912–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubbels GA, Hara K, Koster CH, Kirschner MW. 1983. Evidence for a functional role of the cytoskeleton in determination of the dorsoventral axis in Xenopus laevis eggs. J Embryol Exp Morphol 77:15–37. [PubMed] [Google Scholar]
- Valente EM, Logan CV, Mougou-Zerelli S, Lee JH, Silhavy JL, Brancati F, Iannicelli M, Travaglini L, Romani S, Illi B, Adams M, Szymanska K, Mazzotta A, Lee JE, Tolentino JC, Swistun D, Salpietro CD, Fede C, Gabriel S, Russ C, Cibulskis K, Sougnez C, Hildebrandt F, Otto EA, Held S, Diplas BH, Davis EE, Mikula M, Strom CM, Ben-Zeev B, Lev D, Sagie TL, Michelson M, Yaron Y, Krause A, Boltshauser E, Elkhartoufi N, Roume J, Shalev S, Munnich A, Saunier S, Inglehearn C, Saad A, Alkindy A, Thomas S, Vekemans M, Dallapiccola B, Katsanis N, Johnson CA, Attie-Bitach T, Gleeson JG. 2010. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat Genet 42:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. 2009. Perspectives and open problems in the early phases of left-right patterning. Semin Cell Dev Biol 20:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. 2010. Consistent left-right asymmetry cannot be established by late organizers in Xenopus unless the late organizer is a conjoined twin. Development 137:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick P, Schweickert A, Weber T, Eberhardt M, Mencl S, Shcherbakov D, Beyer T, Blum M. 2009. Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Dev Biol 331:281–291. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nathans J. 2007. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development 134:647–658. [DOI] [PubMed] [Google Scholar]
- Ware SM, Harutyunyan KG, Belmont JW. 2006. Zic3 is critical for early embryonic patterning during gastrulation. Dev Dyn 235:776–785. [DOI] [PubMed] [Google Scholar]
- Wehrmaker. 1969. Right-left asymmetry and situs invertus in Triturus alpestria. Wilhem Roux Arch 163:1–32. [DOI] [PubMed] [Google Scholar]
- Wheeler GN, Brandli AW. 2009. Simple vertebrate models for chemical genetics and drug discovery screens: lessons from zebrafish and Xenopus. Dev Dyn 238:1287–1308. [DOI] [PubMed] [Google Scholar]
- Wood WB, Kershaw D. 1991. Handed asymmetry, handedness reversal and mechanisms of cell fate determination in nematode embryos. CIBA Found Symposium 162:143–159; discussion 159–164. [DOI] [PubMed] [Google Scholar]
- Xu J, Van Keymeulen A, Wakida NM, Carlton P, Berns MW, Bourne HR. 2007. Polarity reveals intrinsic cell chirality. Proc Natl Acad Sci U S A 104:9296–9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi H, Miyakawa N, Miyachi A, Itoh N. 2009. Fgf4 is required for left-right patterning of visceral organs in zebrafish. Dev Biol 332:177–185. [DOI] [PubMed] [Google Scholar]
- Yasuhiko Y, Imai F, Ookubo K, Takakuwa Y, Shiokawa K, Yokoyama T. 2001. Calmodulin binds to inv protein: implication for the regulation of inv function. Dev Growth Differ 43:671–681. [DOI] [PubMed] [Google Scholar]
- Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, de Waard R, Leung C, Nouri-Shirazi M, Orazi A, Chaganti RS, Rothman P, Stall AM, Pandolfi PP, Dalla-Favera R. 1997. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet 16:161–170. [DOI] [PubMed] [Google Scholar]
- Yin Y, Bangs F, Paton IR, Prescott A, James J, Davey MG, Whitley P, Genikhovich G, Technau U, Burt DW, Tickle C. 2009. The Taplin3 gene (KIAA0586) encodes a centrosomal protein that is essential for primary cilia formation. Development 136:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fukuda T, Hatano M, Koseki H, Okabe S, Ishibashi K, Kojima S, Arima M, Komuro I, Ishii G, Miki T, Hirosawa S, Miyasaka N, Taniguchi M, Ochiai T, Isono K, Tokuhisa T. 1999. The role of Bcl6 in mature cardiac myocytes. Cardiovasc Res 42:670–679. [DOI] [PubMed] [Google Scholar]
- Yost HJ. 1991. Development of the left-right axis in amphibians. Ciba Found Symp 162:165–176. [DOI] [PubMed] [Google Scholar]
- Yost HJ. 1992. Regulation of vertebrate left–right asymmetries by extracellular matrix. Nature 357:158–161. [DOI] [PubMed] [Google Scholar]
- Yuan S, Schoenwolf GC. 1998. De novo induction of the organizer and formation of the primitive streak in an experimental model of notochord reconstitution in avian embryos. Development 125:201–213. [DOI] [PubMed] [Google Scholar]
- Zallen JA. 2007. Planar polarity and tissue morphogenesis. Cell 129:1051–1063. [DOI] [PubMed] [Google Scholar]
- Zeng H, Hoover AN, Liu A. 2010. PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev Biol 339:418–428. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Levin M. 2009. Left-right asymmetry in the chick embryo requires core planar cell polarity protein Vangl2. Genesis 47:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Malicki J. 2007. Genetic defects of pronephric cilia in zebrafish. Mech Dev 124:605–616. [DOI] [PubMed] [Google Scholar]


