Abstract
Herein, we reported the fabrication of a magnesium vanadate-reduced graphene oxide (Mg3V2O8-rGO) composite. Further, the structural morphology of the as-prepared Mg3V2O8-rGO composite was studied by scanning electron microscopy. Powder X-ray diffraction and energy-dispersive X-ray spectroscopy techniques were also adopted to check the phase purity and elemental composition of the prepared Mg3V2O8-rGO composite. Mg3V2O8-rGO possesses a band gap of 2.98 eV, which prompted us to explore its photocatalytic activity for hydrogen (H2) evolution reaction. The Mg3V2O8-rGO composite demonstrated the generation of a reasonable amount of H2 evolution (97.45 μmol g–1), which is relatively higher than that of pristine Mg3V2O8 (17.45 μmol g–1). This may be attributed to the presence of synergism between Mg3V2O8 and rGO. In addition, Mg3V2O8-rGO also showed good stability and suggested its potential application for photocatalytic H2 evolution applications. So far, no report is available on the use of Mg3V2O8-rGO as a photocatalyst for H2 evolution. We propose the potential role of the Mg3V2O8-rGO composite for photocatalytic H2 evolution applications.
1. Introduction
In the past few years, a rapid surge in energy consumption has been observed.1 This surge in energy consumption may be responsible for the energy crisis.2 Currently, energy consumption relies on conventional energy resources such as coal, natural gas, waste, biomass, sun, wind, hydropower, nuclear power, and geothermal power.3−5 However, these resources are limited and lead to increasing global warming-related issues.6−9 Therefore, researchers have been attracted toward the design and development of energy-related devices and techniques such as solar cells, energy storage devices, batteries, and supercapacitors.7
Hydrogen (H2) evolution or production has received enormous attention because of its eco-friendly nature and cost-effectiveness.10,11 In particular, photocatalytic H2 production has been considered the most efficient technique to produce H2.12 H2 may be an alternative green energy source to overcome energy-related issues.13 Therefore, it is of great importance to design or develop technologies for H2 production. Fujishima et al.14 reported the catalytic activities of titanium dioxide (TiO2) for H2 production applications. This review explores the potential applications of transition metal oxides/semiconducting metal oxides for H2 production applications. The photocatalytic breakdown of water molecules into H2 and oxygen requires semiconducting metal oxide-based photocatalyst.15,16 In previous years, various photocatalysts such as tin oxide (SnO2), nickel oxide (NiO), manganese oxide (MnO2), graphitic carbon nitride (g-C3N4), copper oxide (CuO), cobalt oxide (Co3O4), perovskite-like materials, zinc oxide (ZnO), tungsten oxide (WO3), etc. have been widely used for H2 production applications.17−24 However, many efficient photocatalysts have been reported for H2 production but still there is a chance to further design and develop new photocatalysts for H2 production applications.23
Recently, metal vanadate has received great interest from the scientific community because of its excellent chemical stability, electronic properties, narrow optical band gap, and cost-effectiveness.25−32 In previous years, various metal vanadate materials, which include MVO4, M2V2O7, MV2O6, M3V2O8, MV2O4, and MV3O8 (herein, M= Zn, Ni, Fe or Mn), have received significant attention of the researchers due to their reasonable optoelectronic properties and environment-friendly nature.33−37 Particularly, M3V2O8 has been extensively used as a suitable electrode material for various optoelectronic applications.38 Mg3V2O8 has various advantageous features and characteristics, which make it a desirable and promising material for photocatalytic applications.38 In a previous study, Mg3V2O8 was adopted as a suitable active electrode material for water oxidation using visible-light irradiation.38 Therefore, it is clear from the above reports that Mg3V2O8 possesses a reasonable potential for photocatalytic applications.
Herein, our group has obtained a Mg3V2O8-rGO composite via simple strategies and approaches. Furthermore, the photocatalytic behavior of the as-synthesized Mg3V2O8-rGO composite was investigated toward the production of H2 using a photocatalytic approach. So far, no report has been found on the use of Mg3V2O8-rGO as a photocatalyst for H2 production. For the first time, we propose the photocatalytic H2 production activities of the Mg3V2O8-rGO composite. Mg3V2O8-rGO demonstrated good photocatalytic activities toward H2 evolution under visible light.
2. Experimental Section
2.1. Chemicals and Reagents
In this study, magnesium nitrate hexahydrate was purchased from Merck. Ammonium vanadate (AV) was purchased from Merck. Graphene oxide (GO) was purchased from Sigma. 2-Methyl imidazole (MIM) was purchased from Alfa-Aesar.
2.2. Synthesis of rGO, Mg3V2O8, and Mg3V2O8-rGO
For the synthesis of rGO, 50 wt % GO was dispersed in 100 mL of deionized water (DI) and sonicated for 2 h. Further, this dispersion was transferred to a Teflon cup covered with a stainless steel autoclave and heated at 200 °C for 6 h. The obtained product was washed with DI water and ethanol and dried in an oven for 6 h at 60 °C. For the preparation of Mg3V2O8, 0.55 mmol of magnesium nitrate hexahydrate (ACS reagent, 99%) was dissolved in 10 mL of DI using magnetic stirring at room temperature (RT) for 10 min. In another beaker, 0.35 mmol of AV (99.95% trace metals basis) was dissolved in 10 mL of DI water using magnetic stirring at room temperature (RT) for 10 min. Further, the AV solution was slowly added to the above-prepared magnesium nitrate hexahydrate solution. Further, an aqueous solution of MIM (4.0 mmol) was also added to the above reaction mixture and stirred for 30 min at RT. Finally, this reaction mixture was transferred to the Teflon cup, and this Teflon cup was kept in a stainless steel autoclave. The autoclave was heated at 180 °C for 24 h and the precipitate was collected by centrifugation and dried at 70 °C for 6 h, which was further calcinated for 3 h at 450 °C. The obtained product was found to be Mg3V2O8.
For the preparation of Mg3V2O8-rGO, the obtained Mg3V2O8 was dispersed in 20 mL of DI water using ultrasonication for 20 min. Further, an aqueous dispersion of GO was added to the Mg3V2O8 dispersion solution and transferred to a Teflon cup, which was covered with the stainless steel autoclave. This autoclave was heated at 200 °C for 6 h, and the obtained product was washed with DI water and ethanol and dried in an oven for 6 h at 70 °C. This product was found to be Mg3V2O8-rGO.
2.3. H2 Production Process
Airtight quartz tube has been used as a photocatalytic reactor system for photocatalytic H2 evolution studies. Twenty milliliters of lactic acid was added to 100 mL of water and 250 mg of photocatalyst (Mg3V2O8 or Mg3V2O8-rGO) was added to the above-prepared lactic acid solution. Further, nitrogen gas (N2) was purged into the above solution for 30–40 min to extract the dissolved gases in the prepared solution. A 300 W LED (visible-light source) with a wavelength of 420 nm has been used for H2 evolution studies. The produced H2 was studied by a gas chromatograph.
2.4. Apparatus
To characterize the physiochemical characteristics of the fabricated samples, various techniques such as scanning electron microscopy (SEM; Zeiss), powder X-ray diffraction (PXRD, Rigaku, Japan), energy-dispersive X-ray spectroscopy (EDX; Oxford), photoluminescence (PL) spectroscopy, X-ray photoelectron spectroscopy (XPS), and ultraviolet–visible (UV–vis) spectroscopy (Agilent Cary Instrument) have been used.
3. Results and Discussion
3.1. Physiochemical Properties of Mg3V2O8-rGO
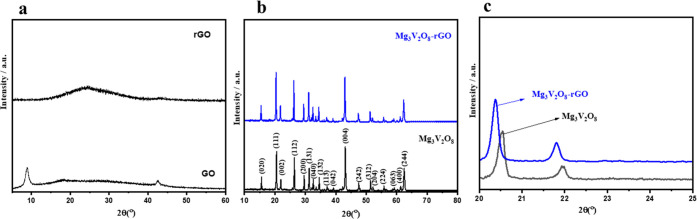
The obtained PXRD patterns of the synthesized samples (GO, rGO, Mg3V2O8, and Mg3V2O8-rGO) are shown in Figure 1a,1b. The obtained PXRD of GO exhibits the existence of a well-defined diffraction peak at ∼9.8°, which confirms the presence of the (002) diffraction plane of GO (Figure 1a). On the other side, rGO exhibits a characteristic broad diffraction peak related to the (002) diffraction plane of rGO, which suggested the transformation of GO to rGO (Figure 1a). The PXRD pattern of the synthesized Mg3V2O8 shows various strong diffraction peaks and authenticated presence of (020), (111), (002), (112), (200), (131), (040), (132), (113), (042), (004), (242), (312), (204), (224), (063), (400), and (224) diffraction planes (Figure 1b). The obtained PXRD pattern of Mg3V2O8 was matched with the JCPDS number 37-0351 and confirmed its formation.36
Figure 1.
(a) PXRD patterns of GO and rGO (a) and Mg3V2O8 and Mg3V2O8-rGO composites (b). (c) Enlarged view showing the change in the diffraction peak.
The PXRD pattern of the synthesized Mg3V2O8-rGO is also displayed in Figure 1b. The PXRD pattern of Mg3V2O8-rGO shows the presence of (020), (111), (002), (112), (200), (131), (040), (132), (113), (042), (004), (242), (312), (204), (224), (063), (400), and (224) diffraction planes of Mg3V2O8. The presence of rGO could not be observed by PXRD analysis, which may be due to the amorphous nature or low content of rGO in the Mg3V2O8-rGO sample. However, the introduction of rGO to the Mg3V2O8 material shifted the diffraction peak, as shown in Figure 1c (enlarged view of Figure 1b). The electronic properties of rGO may significantly affect the X-ray with Mg3V2O8, which may have shifted the PXRD peak (Figure 1c).
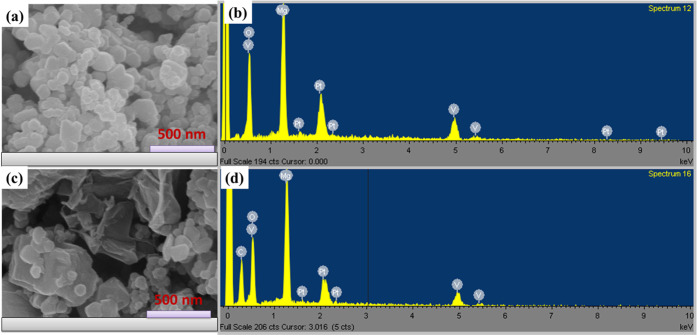
The surface morphology and structural properties of Mg3V2O8 and Mg3V2O8-rGO samples were also studied by employing the SEM technique. Figure 2a shows an SEM image of the prepared Mg3V2O8, indicating that Mg3V2O8 nanoparticles are formed. The Mg3V2O8 nanoparticles were interconnected and formed an agglomeration of Mg3V2O8 nanoparticles. The obtained SEM image of Mg3V2O8-rGO is depicted in Figure 2c. The observations show that Mg3V2O8 is embedded in the rGO sheets (Figure 2c).
Figure 2.
SEM images of Mg3V2O8 (a) and Mg3V2O8-rGO (c). EDX spectrum of Mg3V2O8 (b) and the Mg3V2O8-rGO composite (d).
It confirms the presence of Mg3V2O8 in the prepared Mg3V2O8-rGO sample. Furthermore, we have also recorded the EDX spectrum of the prepared Mg3V2O8 and Mg3V2O8-rGO samples to further characterize and verify the elemental composition. Figure 2b shows the EDX spectrum of Mg3V2O8 and indicated the presence of Mg, V, and O elements. This suggested that Mg3V2O8 has been formed. The obtained EDX spectrum of Mg3V2O8-rGO is shown in Figure 2d. The obtained EDX spectrum exhibits the presence of C, Mg, V, and O elements. This revealed the successful synthesis of Mg3V2O8-rGO. There was no other impurity element detected in the EDX spectrum of Mg3V2O8-rGO, which confirmed its good phase purity.
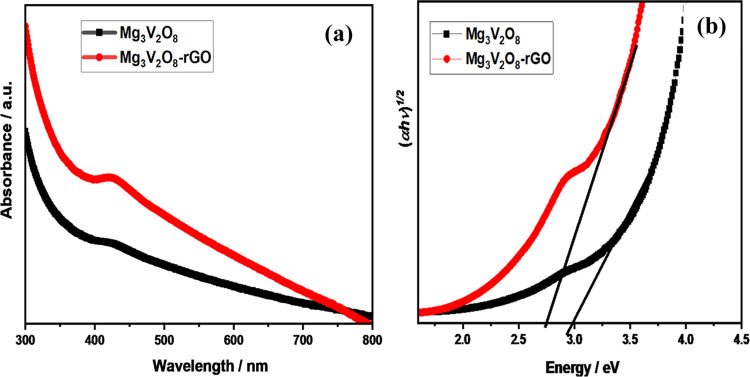
The optical features such as the optical band gap of the prepared Mg3V2O8 and Mg3V2O8-rGO samples were determined by using UV–vis absorption spectroscopy. The obtained UV–vis spectra of Mg3V2O8 and Mg3V2O8-rGO are presented in Figure 3.
Figure 3.
UV–vis absorption spectra of Mg3V2O8 (black) and Mg3V2O8-rGO (red) composite (a). Tauc plot of Mg3V2O8 (black) and Mg3V2O8-rGO (red) composite (b).
According to Figure 3a, a broad absorption band is observed around 423 nm. In the case of Mg3V2O8-rGO, the absorption appeared at 429 nm. The band gaps of Mg3V2O8 and Mg3V2O8-rGO were calculated by utilizing the Tauc method. The optical study suggested that Mg3V2O8 and Mg3V2O8-rGO possess band gaps of 2.77 and 2.98 eV, respectively (Figure 3b).
Further, photoluminescence (PL) spectroscopy was also used to characterize the prepared Mg3V2O8 and Mg3V2O8-rGO samples. The PL spectra of the Mg3V2O8 and Mg3V2O8-rGO samples are shown in Figure S1. The PL spectrum of Mg3V2O8 and Mg3V2O8-rGO exhibited a PL peak between 550 to 600 nm. X-ray photoelectron spectroscopy (XPS) was also adopted to examine the elemental composition of the prepared Mg3V2O8-rGO. The XPS survey spectrum of Mg3V2O8-rGO is shown in Figure S2a. The survey spectrum confirmed the presence of Mg, V, O, and C elements, which indicated the formation of Mg3V2O8-rGO. The high-resolution C 1s, Mg 1s, V 2p, and O 1s of Mg3V2O8-rGO are shown in Figures S2b–e.39 The C 1s scan revealed the presence of binding energies of 284.27 and 288.7 eV for graphitic carbon atoms and C=O groups, respectively. This indicated the presence of rGO in the prepared Mg3V2O8-rGO sample.
The binding energy value of 1303.5 eV can be assigned to Mg 1s, as shown in Figure S2c. The binding energy value of 524.73 and 517.22 eV may be assigned to the presence of 2p1/2 and 2p3/2, respectively (Figure S2d). The O 1s scan of Mg3V2O8-rGO exhibited binding energy values of 531.5 and 529.4 eV, which can be ascribed to the presence of Mg–O and V–O bonds, respectively (Figure S2e).40 These excellent physiochemical and optical properties of Mg3V2O8 and Mg3V2O8-rGO suggested their potential for photocatalytic applications. Thus, we have adopted the use of Mg3V2O8 and Mg3V2O8-rGO as effective and efficient photocatalysts for H2 evolution.
3.2. H2 Production Activities of Mg3V2O8 and Mg3V2O8-rGO
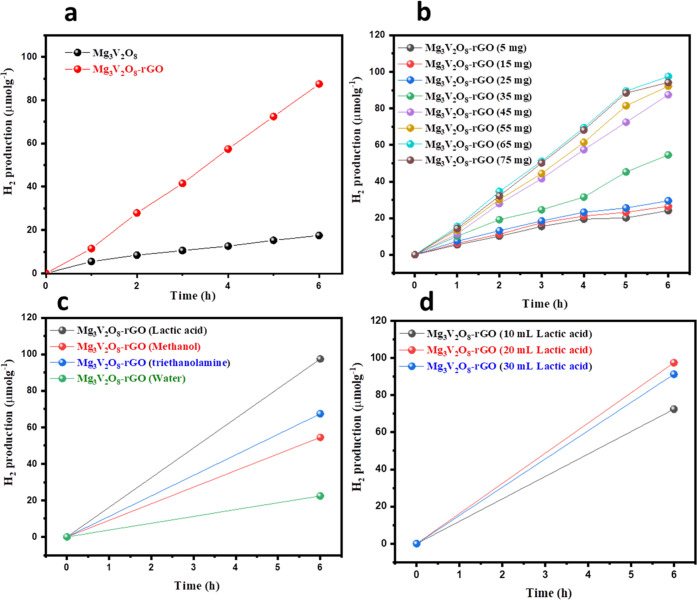
The photocatalytic properties of the fabricated Mg3V2O8 and Mg3V2O8-rGO catalysts were evaluated in the presence of 20 mL of lactic acid in 100 mL of water. The H2 evolution activities of Mg3V2O8 and Mg3V2O8-rGO were studied under visible-light irradiation. In this investigation, 25 mg of photocatalyst (Mg3V2O8 or Mg3V2O8-rGO) was used. Further, nitrogen (N2) gas was passed in the solution prepared above for 30 min. The above solution was irradiated with LED light (xenon lamp) for different times (1, 2, 3, 4, 5, and 6 h). At a particular time interval, generated H2 was collected by syringe and examined by a gas chromatograph. The obtained results are summarized in Figure 4a. The reasonable H2 evolution of 17.54 μmol g–1 appeared for Mg3V2O8 catalysts. However, enhanced H2 production of 87.45 μmol g–1 has been obtained using the Mg3V2O8-rGO catalyst as shown in Figure 4a. This excellent photocatalytic property of the Mg3V2O8-rGO photocatalyst may be attributed to the presence of synergistic effects between rGO and Mg3V2O8. The amounts of Mg3V2O8 and Mg3V2O8-rGO catalysts were also optimized in 20 mL of lactic acid. Different amounts (5, 15, 25, 35, 45, 55, 65, and 75 mg) of Mg3V2O8 or Mg3V2O8-rGO have been used for photocatalytic H2 evolution studies. The obtained results are presented in Figure 4b. It can be observed from Figure 4b that the H2 evolution amount increases with the increasing number of catalysts (Mg3V2O8 or Mg3V2O8-rGO) from 5 to 65 mg (Figure 4b). However, relatively lower H2 was generated with 75 mg of photocatalyst, which suggested that 65 mg of photocatalyst is the optimized amount for the photocatalytic H2 evolution process. Thus, we have used this optimized amount of 65 mg of catalyst for further photocatalytic studies. Subsequently, we have examined the photocatalytic properties of Mg3V2O8 or Mg3V2O8-rGO in various solvents (methanol, lactic acid, triethanolamine, and water). The obtained photocatalytic results are summarized in Figure 4c. It can be seen that Mg3V2O8 or Mg3V2O8-rGO possesses poor photocatalytic properties in pure water. However, relatively higher photocatalytic H2 generation was observed for triethanolamine and methanol solvents. The highest amount of H2 was generated for lactic acid using Mg3V2O8 or Mg3V2O8-rGO catalysts.
Figure 4.
Photocatalytic H2 production amount of Mg3V2O8 and Mg3V2O8-rGO catalysts (a). Photocatalytic H2 production amount of the Mg3V2O8-rGO catalyst with different doses (b). Photocatalytic H2 production activity of Mg3V2O8-rGO in different solvents (c). Photocatalytic H2 production activity of Mg3V2O8-rGO in different volumes of lactic acid (d).
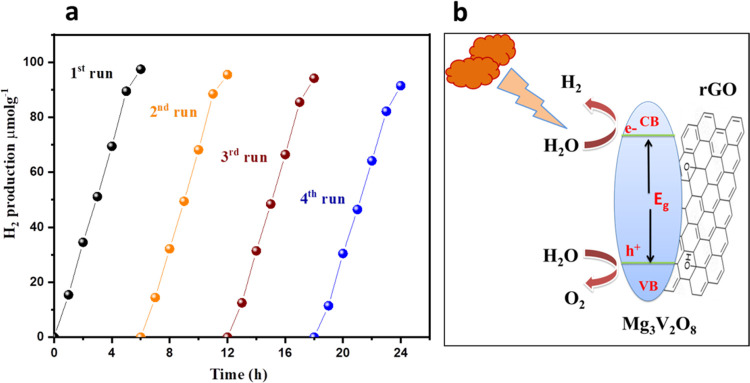
It can be observed that Mg3V2O8-rGO has shown excellent improvements in photocatalytic H2 evolution compared to Mg3V2O8 (Figure 4c). We have also checked the photocatalytic properties of Mg3V2O8 or Mg3V2O8-rGO by varying the amount of lactic acid. Figure 4d shows the photocatalytic H2 production activities of Mg3V2O8 or Mg3V2O8-rGO in the presence of different amounts (10, 20, and 30 mL) of lactic acid. The highest amount of H2 was generated for 20 mL of lactic acid compared to 10 or 30 mL of lactic acid. The highest H2 production of 72.45 and 97.45 μmol g–1 were observed for 20 mL of lactic acid using Mg3V2O8 and Mg3V2O8-rGO, respectively (Figure 4d). The obtained results are impressive for the Mg3V2O8-rGO catalyst compared to Mg3V2O8. The reusability of the photocatalysts is a challenging task and plays a vital role in large-scale H2 production applications. We have studied the reusability study of the Mg3V2O8-rGO catalyst for 20 mL of lactic acid. Figure 5a demonstrates the reusability study of the Mg3V2O8-rGO catalyst. There was insignificant degradation in the performance of the Mg3V2O8-rGO catalyst observed up to four cycles.
Figure 5.
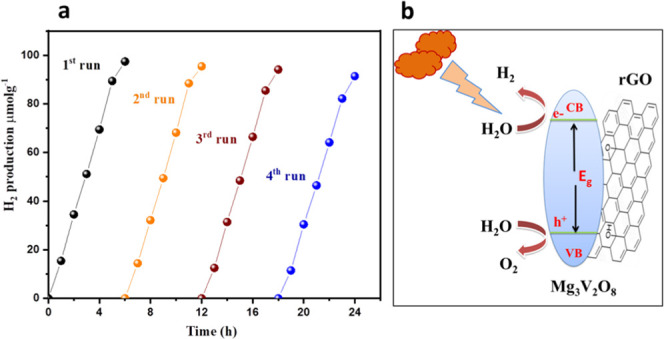
Photocatalytic H2 production reusability study of the Mg3V2O8-rGO catalyst (a). Probable mechanism of the Mg3V2O8-rGO catalyst for H2 production (b).
The probable mechanism for H2 production using the Mg3V2O8-rGO catalyst has been schematically presented in Figure 5b. The visible light irradiated over the Mg3V2O8-rGO catalyst generated electron–hole pairs as shown in eq i(41,42)
| i |
| ii |
| iii |
| iv |
The generated e– in Mg3V2O8 may move toward the conduction band of rGO and can be trapped due to the resonance effect. Therefore, it can be assumed that the synergistic interactions between Mg3V2O8 and rGO may assist the charge separation between the generated e––h+ pairs. The remaining h+ at the valence band interacts with the surrounding H2O molecules to form the H+ ions and reactive hydroxyl radicals (•OH) as shown in eq ii. The H+ ions can move to the conduction band and thereby interact with e– on the rGO surface and result in the formation of H2 as described in eq iii. The sacrificial reagent (lactic acid) can also interact with •OH to form oxidized products and H+ ions (eq iv), which can further reduce to H2 gas on the surface of rGO. Therefore, the combined efforts of photocatalytic water splitting and the lactic acid reforming process work together to enhance H2 production.
4. Conclusions
In this work, a new photocatalyst has been developed by merging the physiochemical properties of Mg3V2O8 and rGO. Simple strategies have been adopted to fabricate Mg3V2O8 and Mg3V2O8-rGO. The photocatalytic properties of Mg3V2O8-rGO were studied toward the evolution of H2 using a photocatalytic approach. Mg3V2O8-rGO showed an H2 production amount of 97.45 μmol g–1 and an H2 production rate of 16.24 μmol g–1 h–1, which are relatively higher than that of the pristine Mg3V2O8. Mg3V2O8-rGO also demonstrated excellent reusability up to four cycles. This superior photocatalytic performance of Mg3V2O8-rGO can be assigned to the presence of synergistic effects and improved charge-carrier transport properties. We proposed a low-cost and environmentally friendly photocatalyst for the H2 evolution application.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number (IFKSUDR_E172).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c04476.
PL spectra of Mg3V2O8 (black) and Mg3V2O8-rGO (red) composite (Figure S1). XPS survey scan of the Mg3V2O8-rGO (a) composite; high-resolution XPS C 1s (b), Mg 1s (c), V 2p (d), and O 1s (e) of the Mg3V2O8-rGO composite (Figure S2) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ahmad K.; Khan M. Q.; Khan R. A.; Kim H. Numerical Simulation and Fabrication of Pb-free Perovskite Solar Cells (FTO/TiO2/Cs3Bi2I9/spiro-MeOTAD/Au). Opt. Mater. 2022, 128, 112458 10.1016/j.optmat.2022.112458. [DOI] [Google Scholar]
- Ahmad K.; Song G.; Kim H. Fabrication of Tungsten Oxide/Graphene Quantum Dot (WO3@GQD) Thin Films on Indium Tin Oxide-Based Glass and Flexible Substrates for the Construction of Electrochromic Devices for Smart Window Applications. ACS Sustainable Chem. Eng. 2022, 10 (36), 11948–11957. 10.1021/acssuschemeng.2c03229. [DOI] [Google Scholar]
- Ahmad K.; Kim H. Enhanced Stability of MAPbI3 based Perovskite Solar Cells. Mater. Lett. 2022, 318, 132187 10.1016/j.matlet.2022.132187. [DOI] [Google Scholar]
- Dhabarde N.; Selvaraj J.; Yuda A.; Kumar A.; Subramanian V. R. Review of Photocatalytic and Photo-electrocatalytic Reduction of CO2 on Carbon Supported Films. Int. J. Hydrogen Energy 2022, 47, 30908–30936. 10.1016/j.ijhydene.2022.02.124. [DOI] [Google Scholar]
- Ahmad K.Bismuth Halide Perovskites for Photovoltaic Applications. In Bismuth—Fundamentals and Optoelectronic Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Allured B.; DelaCruz S.; Darling T.; Huda M. N.; Subramanian V. R. Enhancing the visible light absorbance of Bi2Ti2O7 through Fe-substitution and its effects on photocatalytic hydrogen evolution. Appl. Catal., B 2014, 144, 261–268. 10.1016/j.apcatb.2013.07.019. [DOI] [Google Scholar]
- Ragsdale W.; Gupta S.; Conard K.; Delacruz S.; Subramanian V. R. Photocatalytic Activity of Fe-modified Bismuth Titanate Pyrochlores: Insights into its Stability, Photoelectrochemical, and Optical responses. Appl. Catal., B 2016, 180, 442–450. 10.1016/j.apcatb.2015.06.016. [DOI] [Google Scholar]
- Khanal V.; Ragsdale W.; Gupta S.; Subramanian V. R. Insights into the Photoactivity of Iron Modified Bismuth Titanate (Fe_BTO) Nanoparticles. Catal. Today 2018, 300, 81–88. 10.1016/j.cattod.2017.07.017. [DOI] [Google Scholar]
- Mondal A.; Prabhakaran A.; Gupta S.; Subramanian V. R. Boosting Photocatalytic Activity Using Reduced Graphene Oxide (RGO)/Semiconductor Nanocomposites: Issues and Future Scope. ACS Omega 2021, 6, 8734–8743. 10.1021/acsomega.0c06045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza W.; Ahmad K.. Visible Light-Driven Photocatalysts for Environmental Applications Based on Graphitic Carbon Nitride. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova O.; Martínez L.; Kharisov B., Eds.; Springer: Cham, 2020. [Google Scholar]
- Raza W.; Ahmad K.; Kim H. Fabrication of Defective Graphene Oxide for Efficient Hydrogen Production and Enhanced 4-nitro-phenol Reduction. Nanotechnology 2021, 32, 495404 10.1088/1361-6528/ac1dd4. [DOI] [PubMed] [Google Scholar]
- Ahmad K.; Khan M. Q.; Alsalme A.; Kim H. Sulfur-doped graphitic-carbon nitride (S@g-C3N4) as bi-functional catalysts for hydrazine sensing and hydrogen production applications. Synth. Met. 2022, 288, 117100 10.1016/j.synthmet.2022.117100. [DOI] [Google Scholar]
- Tong H.; Ouyang S.; Bi Y.; Umezawa N.; Oshikiri M.; Ye J. Nano-photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. 10.1002/adma.201102752. [DOI] [PubMed] [Google Scholar]
- Fujishima A.; Honda K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- Jiang W. J.; Zhu Y. F.; Zhu G. X.; Zhang Z. J.; Chen X. J.; Yao W. Q. Three-dimensional photocatalysts with a network structure. J. Mater. Chem. A 2017, 5, 5661–5679. 10.1039/C7TA00398F. [DOI] [Google Scholar]
- Low J.; Yu J.; Jaroniec M.; Wageh S.; Al-Ghamdi A. A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694 10.1002/adma.201601694. [DOI] [PubMed] [Google Scholar]
- Sun S.; Ren D.; Yang M.; Cui J.; Yang Q.; Liang S. In-situ construction of direct Z-scheme sea-urchin-like ZnS/SnO2 heterojunctions for boosted photocatalytic hydrogen production. Int. J. Hydrogen Energy 2022, 47, 9201–9208. 10.1016/j.ijhydene.2021.12.249. [DOI] [Google Scholar]
- Saldaña-Ramírez A.; Alfaro Cruz M. R.; Juárez-Ramírez I.; Torres-Martínez L. M. Influence of the power density and working pressure in the magnetron co-sputtering deposition of ZnO–SnO2 thin films and their effect in photocatalytic hydrogen production. Opt. Mater. 2020, 110, 110501 10.1016/j.optmat.2020.110501. [DOI] [Google Scholar]
- Yu-Cheng C.; Yu-Wen L. MoS2@SnO2 core-shell sub-microspheres for high efficient visible-light photodegradation and photocatalytic hydrogen production. Mater. Res. Bull. 2020, 129, 110912 10.1016/j.materresbull.2020.110912. [DOI] [Google Scholar]
- Li B.; Wang Y.; Zeng Y.; Wang R. Synthesis of CuO micro-sphere combined with g-C3N4 using Cu2O as precursor for enhanced photocatalytic hydrogen evolution. Mater. Lett. 2016, 178, 308–311. 10.1016/j.matlet.2016.05.026. [DOI] [Google Scholar]
- Huang S.; Bao R.; Wang J.; Yi J.; Zhang Z.; Liu L.; Han Y.; Li Z.; Min D.; Zhang W.; Ge Z.; Zhang X. Synergistic effect of oxygen vacancy defects and TiO2/WO3 heterostructures in photocatalytic hydrogen production and dye degradation. J. Alloys Compd. 2023, 961, 170945 10.1016/j.jallcom.2023.170945. [DOI] [Google Scholar]
- Dong X.; Wang H.; Li X.; Fatehi P.; Wang S.; Wu Q.; Liu K.; Kong F. In-situ sulfidation to fabricate NiSx modified g-C3N4/NiO composite for efficient photocatalytic hydrogen production under visible-light. Appl. Surf. Sci. 2023, 610, 155570 10.1016/j.apsusc.2022.155570. [DOI] [Google Scholar]
- Chen L.; Xie X.; Su T.; Ji H.; Qin Z. Co3O4/CdS p-n heterojunction for enhancing photocatalytic hydrogen production: Co-S bond as a bridge for electron transfer. Appl. Surf. Sci. 2021, 567, 150849 10.1016/j.apsusc.2021.150849. [DOI] [Google Scholar]
- Wei D.; Ding Y.; Li Z. Noble-metal-free Z-Scheme MoS2–CdS/WO3–MnO2 nanocomposites for photocatalytic overall water splitting under visible light. Int. J. Hydrogen Energy 2020, 45, 17320–17328. 10.1016/j.ijhydene.2020.04.160. [DOI] [Google Scholar]
- Dhabarde N.; Carrillo-Ceja O.; Tian S.; Xiong G.; Raja K.; Ravi Subramanian V. Bismuth Vanadate Encapsulated with Reduced Graphene Oxide: A Nanocomposite for Optimized Photocatalytic Hydrogen Peroxide Generation. J. Phys. Chem. C 2021, 125, 23669–23679. 10.1021/acs.jpcc.1c05315. [DOI] [Google Scholar]
- Wang D.; Tang J.; Zou Z.; Ye J. Photophysical and Photocatalytic Properties of a New Series of Visible-Light-Driven Photocatalysts M3V2O8 (M = Mg, Ni, Zn). Chem. Mater. 2005, 17, 5177. 10.1021/cm051016x. [DOI] [Google Scholar]
- Butt F. K.; Cao C.; Ahmed R.; Khan W. S.; Cao T.; Bidin N.; Li P.; Wan Q.; Qu X.; Tahir M.; Idrees F. Synthesis of novel ZnV2O4 spinel oxide nanosheets and their hydrogen storage properties. CrystEngComm 2014, 16, 894–899. 10.1039/C3CE41859F. [DOI] [Google Scholar]
- Ma H.; Zhang S.; Ji W.; Tao Z.; Chen J. a-CuV2O6 nanowires: hydrothermal synthesis and primary lithium battery application. J. Am. Chem. Soc. 2008, 130, 5361–5367. 10.1021/ja800109u. [DOI] [PubMed] [Google Scholar]
- Xiao L.; Zhao Y.; Yin J.; Zhang L. Clewlike ZnV2O4 hollow spheres: nonaqueous sol–gel synthesis, formation mechanism, and lithium storage properties. Chem. - Eur. J. 2009, 15, 9442–9450. 10.1002/chem.200901328. [DOI] [PubMed] [Google Scholar]
- Shi R.; Wang Y.; Zhou F.; Zhu Y. Zn3V2O7(OH)2(H2O)2 and Zn3V2O8 nanostructures: controlled fabrication and photocatalytic performance. J. Mater. Chem. 2011, 21, 6313–6320. 10.1039/c0jm04451b. [DOI] [Google Scholar]
- Mondal C.; Ganguly M.; Sinha A. K.; Pal J.; Sahoo R.; Pal T. Robust cubooctahedron Zn3V2O8 in gram quantity: a material for photocatalytic dye degradation in water. CrystEngComm 2013, 15, 6745–6751. 10.1039/c3ce40852c. [DOI] [Google Scholar]
- Sambandam B.; Soundharrajan V.; Song J.; Kim S.; Jo J.; Pham D. T.; Kim S.; Mathew V.; Kim J. Zn3V2O8 porous morphology derived through a facile and green approach as an excellent anode for high-energy lithium ion batteries. Chem. Eng. J. 2017, 328, 454–463. 10.1016/j.cej.2017.07.050. [DOI] [Google Scholar]
- Vijayakumar S.; Lee S. H.; Ryu K. S. Synthesis of Zn3V2O8 nanoplatelets for lithium-ion battery and supercapacitor applications. RSC Adv. 2015, 5, 91822–91828. 10.1039/C5RA13904J. [DOI] [Google Scholar]
- Ye J.; Zou Z.; Arakaw H.; Oshikiri M.; Shimoda M.; Matsushita A.; Shishido T. Correlation of crystal and electronic structures with photophysical properties of water splitting photocatalysts InMO4 (M = V5+, Nb5+, Ta5+). J. Photochem. Photobiol., A 2002, 148, 79–83. 10.1016/S1010-6030(02)00074-6. [DOI] [Google Scholar]
- Kudo A.; Omori K.; Kato H. A Novel Aqueous Process for Preparation of Crystal Form-Controlled and Highly Crystalline BiVO4 Powder from Layered Vanadates at Room Temperature and Its Photocatalytic and Photophysical Properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. 10.1021/ja992541y. [DOI] [Google Scholar]
- Li P.; Zhou Y.; Tu W.; Liu Qi.; Yan S.; Zou Z. Direct Growth of Fe2V4O13 Nanoribbons on a Stainless-Steel Mesh for Visible-Light Photoreduction of CO2 into Renewable Hydrocarbon Fuel and Degradation of Gaseous Isopropyl Alcohol. ChemPlusChem 2013, 78, 274–278. 10.1002/cplu.201200289. [DOI] [Google Scholar]
- Konta R.; Kato H.; Kobayashi H.; Kudo A. Photophysical properties and photocatalytic activities under visible light irradiation of silver vanadates. Phys. Chem. Chem. Phys. 2003, 5, 3061–3065. 10.1039/b300179b. [DOI] [PubMed] [Google Scholar]
- Mirsadeghi S.; Ghoreishian S. M.; Zandavar H.; Behjatmanesh-Ardakani R.; Naghian E.; Ghoreishian M.; Mehrani A.; Abdolhoseinpoor N.; Reza Ganjali M.; Huh Y. S.; Pourmortazavi S. M. In-depth insight into the photocatalytic and electrocatalytic mechanisms of Mg3V2O8@Zn3V2O8@ZnO ternary heterostructure toward linezolid: Experimental and DFT studies. J. Environ. Chem. Eng. 2023, 11, 109106 10.1016/j.jece.2022.109106. [DOI] [Google Scholar]
- Low W. H.; Khiew P. S.; Lim S. S.; Siong C. W.; Chia C. H.; Ezeigwe E. R. Facile synthesis of graphene-Zn3V2O8 nanocomposite as a high performance electrode material for symmetric supercapacitor. J. Alloys Compd. 2019, 784, 847–858. 10.1016/j.jallcom.2019.01.137. [DOI] [Google Scholar]
- Ma X. F.; Li H. Y.; Gao D.; Ren W.; Diao J.; Xie B.; Huang G.; Wang J.; Pan F.. Mg3V2O8: A Promising Cathode Material for Aqueous Mg-ion Battery. In Magnesium Technology; Barela S.; Leonard A.; Maier P.; Neelameggham N. R.; Miller V. M., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, 2023. [Google Scholar]
- Kondarides D. I.; Daskalaki V. M.; Patsoura A.; Verykios X. E. Hydrogen Production by Photo-Induced Reforming of Biomass Components and Derivatives at Ambient Conditions. Catal. Lett. 2008, 122, 26–32. 10.1007/s10562-007-9330-3. [DOI] [Google Scholar]
- Wang Y.; Liu T.; Tian W.; Zhang Y.; Shan P.; Chen Y.; Wei W.; Yuan H.; Cui H. Mechanism for Hydrogen Evolution from Water Splitting Based on a MoS2/WSe2 Heterojunction Photocatalyst: A First-Principle Study. RSC Adv. 2020, 10, 41127–41136. 10.1039/D0RA06939F. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.