Abstract
Growth in the biomedical and biotechnology sectors requires a highly trained and highly skilled workforce to answer the next great scientific questions. Undergraduate laboratory courses incorporating hands-on training based in authentic research position soon-to-be graduates to learn in environments that mirror that of academic, industrial, and government laboratories. Mass spectrometry is one of the most broadly applied analyses carried out in the biomedical and pharmaceutical sciences and thus it is essential that upper-division students gain hands-on experience in techniques and analytical workflows in mass spectrometry. Our pre-course assessments identified weaknesses in student experience and knowledge in the fundamentals of mass spectrometry, supporting that it was a necessary area for improvement. We incorporated a laboratory experiment focused on tandem mass spectrometry and database searching into a preexisting mini-semester project devoted to identifying metabolites from medicinal plants. Implementation of the experiment allowed students to make more confident metabolite identifications, introduced them to a cutting-edge database analysis platform (GNPS: Global Natural Products Social Molecular Networking), and increased student experience and knowledge of mass spectrometry in addition to the principle of dereplication of samples derived from nature.
Keywords: Upper-Division Undergraduate, Analytical Chemistry, Laboratory Instruction, Hands-On Learning, Internet/Web-Based Learning, Mass Spectrometry, Medicinal Chemistry, Natural Products
Graphical Abstract

Upper-division undergraduate laboratory courses provide students with a framework for hands-on training and knowledge acquisition to cultivate essential research skills. These skills and competencies are key to developing a highly trained and talented workforce to fill high-skill and often high-wage jobs post-graduation. Universities can play a pivotal role in workforce development by integrating research-driven teaching laboratories supported by biotechnology infrastructure and instruction from faculty experts. The creation of classroom-based research courses and modules, which allow students to perform authentic research activities, gives students the opportunity to gain independent research skills, enhance information literacy, and perform data collection using the latest resources.1–3
Liquid chromatography-mass spectrometry (LC-MS) has become a widely used analytical technique in the pharmaceutical industry. Incorporation of mass spectrometry in the teaching laboratory is highly relevant to student training, especially in the areas of metabolomics and metabolite profiling. As with other ‘omics approaches such as genomics and proteomics, which deal with the collective characterization of specific biological molecules, a metabolomics study focuses on the comprehensive analysis of metabolites in a biological specimen.5,6 Metabolite profiling is a focused approach to analyze and annotate molecules that are produced via a specific metabolic pathway or in the case of medicinal plants, specific classes of molecules such as polyketides, terpenes, or shikimates.6,7 Additionally, liquid chromatography coupled with tandem mass spectrometry (MS/MS) allows for increased confidence in metabolite identification in untargeted analysis by comparing MS/MS fragmentation patterns of unknown analytes to those of authentic samples found in the literature or to standards analyzed independently by the researcher.8
LC-MS/MS-based molecular networking is an untargeted metabolite profiling approach whereby metabolites present in extracts and chromatography fractions can be annotated following LC-MS/MS analysis, ultimately linking molecules into clusters based on common MS/MS fragmentation ions in individual analytes.9 These networks can be created using the online platform available freely to users via the University of California San Diego Global Natural Products Social Molecular Networking (GNPS) website.10 The GNPS platform also contains a collection of MS/MS libraries which can be compared to the MS/MS spectra of unknown metabolites in a user’s sample. This matching feature aids in performing the essential function of dereplication (limiting compound rediscovery) in metabolite profiling and drug discovery.
Dereplication is a key approach in natural products and pharmacognosy drug discovery research. Dereplication strategies enable the researcher to focus time and resources on new metabolites of interest rather than exhausting precious effort purifying and characterizing metabolites that have been studied extensively. The costs associated with compound rediscovery in the late stages of the isolation and characterization of a potential therapeutic in part led to the reduction of natural product drug discovery programs in the pharmaceutical industry.11 Early dereplication at the level of the extract or chromatography fraction is necessary to limit the potential for compound rediscovery and sensitive, high information techniques (e.g., LC-MS/MS) can aid in this effort.12
There are several effective examples of teaching LC-MS analysis and metabolomics in the upper-division undergraduate laboratory. Boyce and coworkers developed a hands-on module using liquid-chromatography paired to high resolution mass spectrometry (LC-HRMS) to analyze the metabolite differences in horse blood subjected to hemolysis.13 Students used the open-source online platform available at XCMS Online from The Scripps Research Institute, and differences were visualized using principal component analysis (PCA).13 In another study, LC-MS/MS with multiple reaction monitoring (MRM) was used to investigate cocaine and its metabolites from simulated human serum.14 This approach is especially effective in terms of quantification of metabolites. In terms of metabolite profiling and identification based on specialized metabolite composition, Manninen et al. (2021) showed that LC-MS could be used to identify birch trees species by the defensive compounds.15 There are many examples of targeted LC-MS and LC-MS/MS approaches that have been published in this journal including laboratory experiments on multiple reaction monitoring (MRM) development,16 analyzing amoxicillin in river waters,17 and quantifying drugs of abuse on paper currency.18
Our experiment departs from those previously published by pairing the LC-MS/MS technique with database analysis to introduce students to important workflows not limited only to natural products research, but commonly used in untargeted metabolomics approaches studying human and animal cells and body fluids.19 The goal of this laboratory experiment was to enhance the skills of students in LC-MS/MS operation, analysis, and metabolite database searching, which improved their ability to dereplicate natural product extracts. To accomplish this, a tandem mass spectrometry component was added to the existing framework of a mini-semester classroom-based research project.20 The recent acquisition of a Thermo Fisher Scientific LTQ XL mass spectrometer devoted to undergraduate student learning allowed for the creation of the experiment. Students analyzed botanical samples using LC-MS/MS and performed database analysis to dereplicate the samples.
RATIONALE FOR THE LABORATORY EXPERIMENT
Rationale for the LC-MS/MS analysis module
The mini-semester research project was carried out in an upper-division undergraduate course (BPS451, Techniques in Medicinal Chemistry and Molecular Biology). The class is comprised of 4th year Pharmaceutical Sciences majors with experience in basic laboratory techniques, organic chemistry, and pharmacology. The specific focus of this Laboratory Experiment was on LC-MS/MS operation and data analysis with an emphasis on database searching. We began the course with a voluntary pre-quiz comprised of questions designed to gauge student understanding of extractions, chromatography, and analytical chemistry with an emphasis on LC-MS (approximately 3 questions per category). Twenty-six out of a total of twenty-nine students completed the pre-quiz. The results clearly showed that students had a limited understanding of basic LC-MS knowledge. The three mass spectrometry-related questions posed to the students resulted in fewer than 50% correct answers (Figure 1). Thus, the development of this mass spectrometry and database searching experimental module was designed specifically to be responsive to improving students’ knowledge of mass spectrometry, particularly tandem mass spectrometry. Moreover, due to the SARS-CoV-2 pandemic, an emphasis was put on web-based exercises that could be completed outside of the classroom, which did not require any specific software packages that could have potentially limited student engagement.
Figure 1.

Student pre-quiz results. Questions 4, 6, and 8 (red bars) were all related to mass spectrometry.
METHODS AND FRAMEWORK
The framework for a research-based course was previously established in 2019 with the implementation of a mini-semester project focused on determining antioxidant metabolites found in medicinal plants.20 During the 2019 mini-semester project, students utilized the LC-MS technique on a single quadrupole Thermo Fisher Scientific ISQ to make putative identifications of metabolites, and they deposited their plant extracts into a developing extract library called PRISM. PRISM is continually being used in research laboratories at the University of Rhode Island for drug discovery in a wide variety of therapeutic areas. The plant specimens, which were collected from the URI Heber W. Youngken Jr. Medicinal Garden, included the following: rosemary (Rosmarinus officinalis syn. Salvia rosmarinus), aloe (Aloe vera syn. Aloe barbadensis), echinacea (Echinacea purpurea and E. angustifolia), elderberry (Sambucus nigra), hops (Humulus lupulus), St. John’s wort (Hypericum perforatum), ashwagandha (Withania sominfera), black cohosh (Acetaea racemosa syn. Cimicifuga racemosa), and red barberry (Berberis thunbergii). Ashwagandha was a new specimen added into the course in 2021, therefore different plant parts were analyzed by the students (aerial and roots).
For the in-person portion of the 8-week mini-semester course (2019–2020), students first extracted their plant specimen using either water or acetone for 48 h, vacuum filtered the extracts, removed solvent in vacuo, and assessed antioxidant activity using a standard DPPH assay.20 Approximately three students were assigned to a single specimen for rigor and replication assessment. The active extract was fractionated using an SPE C18 cartridge, and a subsequent DPPH assay was completed to identify an active antioxidant fraction.20 Next this fraction was subjected to HPLC-UV analysis to develop a chromatographic method for LC-MS, and finally LC-MS was used for identification of putative bioactive metabolites.20 Students completed the first part of the course individually (extractions, bioassays, chromatography, and subsequent bioassays). In the final three weeks, students worked in groups of three operating HPLC-UV systems and LC-MS systems.
In the Fall 2021 semester, a new MS/MS laboratory experiment was added to the 8-week mini-semester project. Students completed the experiments described above (extraction, bioassay, SPE fractionation); however, instead of using a single quadruple mass spectrometer, the students used a mass spectrometer capable of MS/MS fragmentation (Thermo Fisher Scientific LTQ XL). Students first received a lecture on the theory of tandem mass spectrometry and the instrument specifications that differentiate LC-MS/MS from LC-MS. The generation of the MS/MS spectra would be key to utilizing the GNPS library search platform. Furthermore, students received lecture material on the concept of dereplication and how the GNPS platform works for library searching and metabolite identification. In a departure from the previous iteration of the course (2019–2020), video lectures and video experiment tutorials were included to create a ‘hybrid’ version of the course in which lectures would be carried out in an asynchronous and virtual manner. This was to accommodate students considering the ongoing SARS-CoV-2 pandemic. The major benefits gained from moving lecture materials online included more in-person laboratory time for the new LC-MS/MS experiment, greater opportunities for one-on-one instruction, and increased instruction from watching videos prior to the hands-on LC-MS/MS experiments.
For the experiment, students subjected their antioxidant fractions to LC-Diode Array Detector (DAD)-MS/MS analysis by operating the LTQ XL mass spectrometer. The LC-MS/MS method and operations SOP can be found in the Notes for Instructors. Students first identified prominent MS1 peaks in their total ion chromatograms (TIC). Next, students accessed the MS/MS spectrum for their analyte of interest. Since the MS/MS spectra were acquired in data-dependent mode, there were MS/MS spectra for all the most intense precursor m/z values (generally > 100 MS/MS spectra). Students utilized these spectrometric data to make putative metabolite identifications, emphasizing the MS/MS fragmentation patterns as key data in identifications. If possible, students compared these fragmentation patterns to literature values or speculated as to potential key losses in spectra, which indicated specific functional groups, such as the loss of 162 Da for a hexose sugar.
In the database searching portion of the experiment, .raw files were converted to .mgf files by the laboratory instructor, and students uploaded their individual .mgf files to the molecular networking platform available at gnps.ucsd.edu. Students followed a standard operating procedure (SOP) for library searching (see Notes for Instructors). The GNPS website also contains informative documentation encompassing the variety of tools available on the platform. Students then integrated the information gained from mining their raw data with the results from the library analysis and spectra available in the scientific literature to putatively identify potential antioxidant metabolites. The database searching offered another avenue for confidence in metabolite identification in addition to the raw data analysis and the literature reviews.
Research Questions
We began the Fall 2021 laboratory experiment with two research questions that would hopefully be answered with our LC-MS/MS experiment in dereplication accomplished by metabolite profiling and database analysis: 1) Can antioxidant compounds or specialized metabolites from botanical extracts be identified and matched by their MS/MS spectra to literature or GNPS library MS/MS spectra? 2) Do the plant samples with strong antioxidant activity have the same antioxidant compounds in them, or are there specialized compounds found in specific plant specimens?
FINDINGS
Out of the 29 students who took the course, 28 of them (97%) were able to make a putative identification of a metabolite and dereplicate samples with limited instructor input (Table 1). Nineteen out of the 29 students (66%) were able to identify their metabolite in the GNPS libraries to carry out the dereplication process (Table 1). However, the students who did not find metabolites in the GNPS libraries were still able to make strong putative identifications and dereplicate their samples based on literature searching. In most cases, the students had identified well-known metabolites in their source (e.g., the alkylamides from echinacea, withaferin analogs from ashwagandha, and fukinolic acid from black cohosh),21–23 but not all of these specific molecules were in the GNPS libraries. Regardless of the presence of a metabolite in the GNPS library, analysis of the raw data was essential in the students’ rationale for putative identification. An example of raw data is included in Figure 2. The student with ashwagandha-6 (aerial portion) identified withaferin A with reference to the MS/MS fragmentation data present in the raw data file. The major fragments at m/z 281 and m/z 299 were identical to those discussed in multiple reports of withaferin A analysis.24,25 Following the raw data interpretation, the student then used the database searching tool to identify withaferin A in the ashwagandha chromatography fraction, even comparing the MS/MS fragmentation patterns of their query to the fragmentation pattern of the library MS/MS (Figure 3). Every student was tasked with MS/MS interpretation through either comparing their raw data to that found in the literature or identifying putative fragments from neutral losses, such as 162 Da in the MS/MS spectra of the putative withanoside II identification in ashwagandha-5 (Figure S1). The students then detailed their investigations to the rest of the class and instructor via both an oral presentation and a laboratory report.
Table 1.
Plant Metabolite Identifications Made by Students Using GNPS Database and Examination of Raw LC-DAD-MS/MS Data
| Sample | Putative ID (LC-DAD-MS/MS) | GNPS Library ID |
|---|---|---|
| Rosemary-1 | Rosmarinic Acida | Yes |
| Rosemary-2 | Rosmarinic Acida | Yes |
| Rosemary-3 | Rosmanola | No |
| Aloe-1 | Aloin A/Ba | Yes |
| Aloe-2 | Aloin A/B | Yes |
| Aloe-3 | Aloin A/B | Yes |
| Echinacea-1 | Alkylamide m/z 230 | No |
| Echinacea-2 | No Identification | N/A |
| Echinacea-3 | Alkylamide m/z 244 | No |
| Elderberry-1 | Cyanidin 763896–30-6b | Yes |
| Elderberry-2 | Malvidin-3-O-galactoside | Yes |
| Elderberry-3 | Malvidin | No |
| Hops-1 | Quercetin | Yes |
| Hops-2 | Xanthohumol | Yes |
| Hops-3 | Xanthohumol | Yes |
| SJWc-1 | Isovitexin | Yes |
| SJW-2 | Quercetin | Yes |
| SJW-3 | Calycosin | Yes |
| Ashwagandha-1 (aerial) | Dihydrowithaferin A | No |
| Ashwagandha-2 (aerial) | Withaferin A | Yes |
| Ashwagandha-3 (roots) | Withaferin A | Yes |
| Ashwagandha-4 (roots) | Withaferin A | Yes |
| Ashwagandha-5 (aerial) | Withanoside II | No |
| Ashwagandha-6 (aerial) | Withaferin A | Yes |
| Black Cohosh-1 | Fukinolic Acida | No |
| Black Cohosh-2 | Fukinolic Acida | No |
| Black Cohosh-3 | 23-epi-26-Deoxyactein | No |
| Red Barberry-1 | Berberine | Yes |
| Red Barberry-1 | Berberine | Yes |
Identifications made with help from LC-MS data generated in negative mode
PubChem ID number;
St. John’s wort
Figure 2.
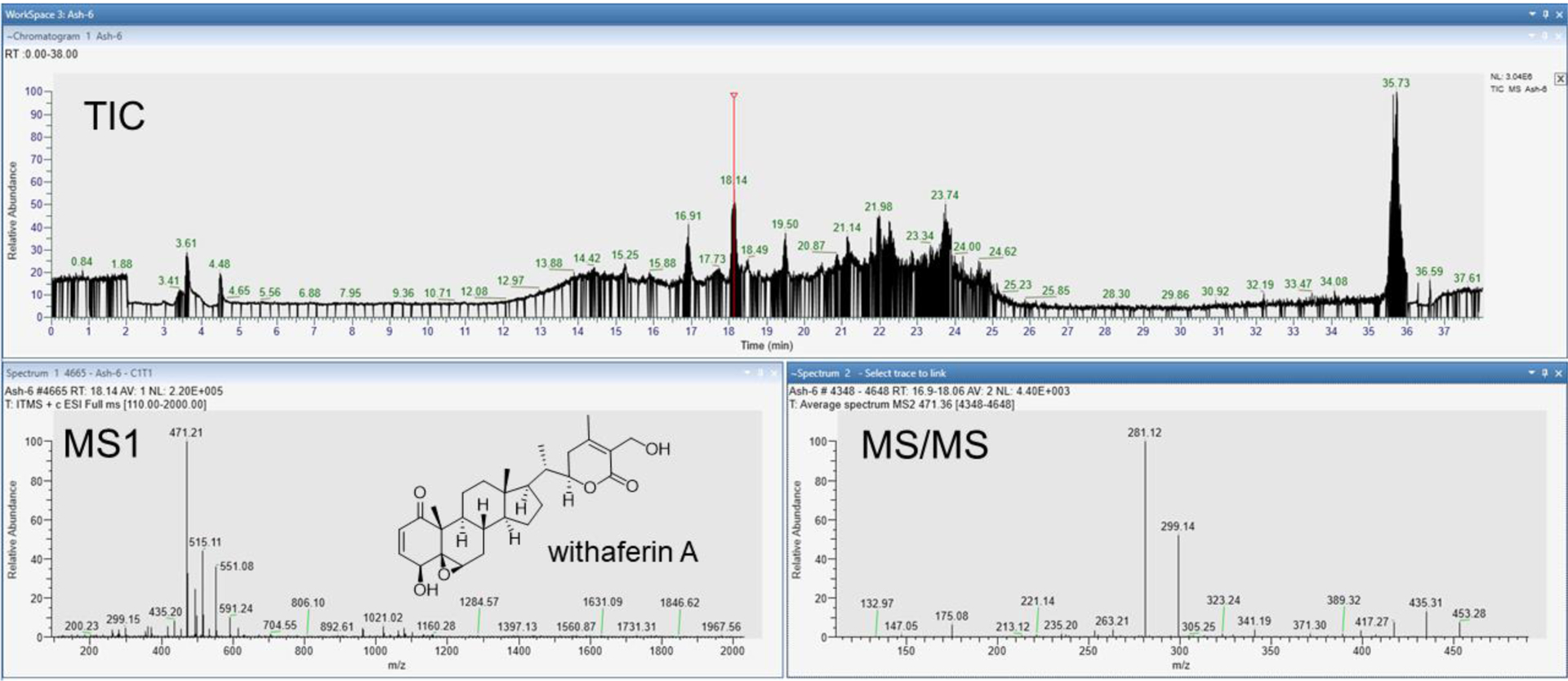
Student-generated raw LC-MS/MS data. MS/MS data were acquired in data-dependent mode. Student example is ashwagandha-6 (aerial). The TIC was examined for metabolites and m/z 471 (tR 18.14 min) was chosen for identification.
Figure 3.

GNPS library search result of ashawagandha-6. The library search identified withaferin A in the student’s chromatography fraction. A ‘mirror match’ of the MS/MS between the query and library spectra shows an identical match with fragments at m/z 299 [M+H-C9H13O3-2H] and m/z 281 [M+H-C9H13O3-2H-H2O]. The structure of the molecule (shown above) is also available on the GNPS platform.
Students were unequivocally able to identify metabolites in their samples with the assistance of the GNPS database and mining through raw MS/MS data, which answered our first research question (Antioxidant compounds in botanical samples can be identified using MS/MS fragmentation patterns and GNPS) (cf. Table 1 and Figure 2). Table 1 shows that most of the plants investigated have specialized metabolites specifically sequestered to them (e.g., aloin in aloe, rosmarinic acid in rosemary, the alkylamides in echinacea, berberine in red barberry, etc.) This enabled students to answer the second research question (Plant samples with strong antioxidant activity have specialized compounds found in specific plant specimens). In the future, a learning module on biosynthesis could complement a focus on specialized plant metabolites.
ASSESSMENT, IMPLICATIONS, AND LIMITATIONS
Comparing pre-quiz performance to post-quiz performance, we saw a large increase in correct responses to mass spectrometry-related questions. While 48%, 41%, and 37% of students answered questions 4, 6, and 8 correctly in the pre-quiz, 86% of students answered these questions correctly in the post-quiz assessment. In future iterations of the course, we will move toward formative self-assessment throughout the mini-semester so that students can have more time to reflect on their experiments and alter approaches (e.g., LC methods, putative metabolite identifications) for better overall learning outcomes.
The files from the analysis in this report are available as a MassIVE dataset and can be accessed by searching for MSV000088444 at https://massive.ucsd.edu/ProteoSAFe/static/massive.jsp. Thus, these data are publicly available and can be directly used in other courses and learning modules eliminating the need to acquire specific mass spectrometry equipment. Data files are designated by plant portion (e.g., aerial or root). The GNPS website has the Dashboard LC-MS data browser that students can use to mine data on their own computer without the need for specific software packages. Comparing the students’ raw data to that of the data presented in the Dashboard shows how effective this tool is for data analysis since the total ion chromatogram, MS1 spectra, and MS/MS for an analyte of interest can be viewed (cf. Figure 2 and Figure S2).26 While utilizing the publicly available data would remove the hands-on operation of the LC-MS/MS described in this experiment, the availability of the files greatly enhances potential accessibility and student engagement without the need for software purchases. Therefore, one could envision a subsequent class in which the data generated in this report could be accessed through GNPS, mined, and analyzed without the need for an actual mass spectrometer, which we feel is an important contribution of this report. This reduces the ‘barrier to entry’ for LC-MS/MS analysis, which includes the financial commitment of acquiring the mass spectrometer. The file type chosen for student database searching was .mgf. This file type is the smallest in terms of size, which facilitated transfer between instructors and students, and also abrogated the need for an ftp transfer of larger file types such as .mzXML. However, .mzXML files have been deposited in the open access MassIVE for greater integration with the GNPS Dashboard platform. Those working with open access GNPS data may want to conduct some initial ‘trial and error’ to determine which file type will work best for their course. In fact, there are intriguing use cases described in the GNPS Dashboard documentation in which the Dashboard was used in teaching settings (see Notes for Instructors for link). These involved the LC-MS analysis of pure antibiotics and culture extracts incorporated into the Tiny Earth course, which is a course-based undergraduate research experience.27
New open-access tools have been developed that can be applied to teaching and classroom-based research. Many of these public data deposition sources have been recently reviewed.28 The development of more publicly available datasets will increase opportunities for students to mine metadata and carry out procedures such as quality control, comparative metabolomics, and data re-analysis. ReDU allows for the re-analysis of public mass spectrometry datasets, co-analysis of user-generated data with public data sets, and additional comparative platforms such as principal component analysis (PCA) and the chemical explorer tool.29 Multi-omics tools are available to integrate metabolomics, transcriptomics, proteomics, etc. and the acquisition or analysis of these data sets can be incorporated into courses that are also teaching students advanced aspects of coding languages and bioinformatics.30 The continued evolution of hands-on LC-MS teaching approaches and the move toward open access data will create amazing new opportunities for dynamic, multi-disciplinary classroom-based research experiences.
FUTURE WORK
While the incorporation of this module was successful in improving students’ understanding of mass spectrometry and the dereplication of natural product extracts, there are ample opportunities to enhance the experiment. Students focused on searching the GNPS libraries for known metabolites, but a separate module or exercise examining the actual molecular networks generated would be illuminating. For instance, the identification of dihydrowithaferin A could be further supported by noting a network connection to withaferin A, which was identified in the library search. This would reinforce the concepts of MS/MS fragmentation and its utility in structure characterization. It would also allow students to create interactive and visually informative data pieces. Future iterations of this approach may shift from discovery to an emphasis on hypothesis-driven research in which student teams conduct experiments to test specific hypotheses with respect to medicinal chemistry and pharmacognosy. One could certainly envision a set of experiments assessing growth parameter alterations causing altered metabolite composition and quantity in plant specimens for example. Furthermore, comparing putatively identified metabolites that were not found in the GNPS library to authentic standards could add a new aspect to the course. The spectra of these metabolites could then be added to the GNPS libraries, which would directly engage the students, cultivate an appreciation for open-access data, and illustrate the connectedness of the scientific community.
CONCLUSIONS
Adding a new LC-MS/MS laboratory experiment into a pre-existing mini-semester project was successful in teaching students operational and analytical skills with respect to tandem mass spectrometry. The previous mini-semester project used LC-MS and UV absorbance data to putatively identify components in bioactive natural product extracts in order to dereplicate these samples. This new laboratory experiment allowed students to acquire MS/MS fragmentation data, which they analyzed and compared to literature values. Furthermore, these MS/MS data were uploaded and analyzed in the GNPS database to afford students additional confidence in identifications and to become proficient in a publicly available web-based tool that has become essential in dereplication workflows.12 Students learned to integrate key pieces of spectrometric data to make metabolite identifications, and they learned about the specialized secondary metabolism of medicinal plants such as aloe, ashwagandha, elderberry and other plants that are widely used in traditional medicine and the herbal supplement market.
HAZARDS AND SAFETY PRECAUTIONS
The majority of the LC-MS/MS module was conducted online and did not require specific safety precautions. However, for active LC-MS/MS acquisition, students wore laboratory coats and safety glasses. Solvents were collected in appropriate hazardous waste containers in accordance with URI Environmental Health and Safety.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully thank the developers of GNPS and GNPS Dashboard. Additionally, we thank the University of Rhode Island, The URI College of Pharmacy, and the Department of Biomedical and Pharmaceutical Sciences for funding to purchase the LTQ XL mass spectrometer. We gratefully acknowledge funding from the Rhode Island IDeA Network of Biomedical Research Excellence program (P20GM103430) in the form of an Enhanced Virtual Education, Research, and Training award to M.B. Data were collected from pre-quizzes and course surveys as approved by URI IRB (#1678632–1).
Footnotes
Supporting Information
The Supporting Information is available on the ACS Publications website at DOI: 10.1021/acs.jchemed.
Notes for Instructors (DOC): Includes LC-MS/MS methods and LC-MS/MS and GNPS database searching SOPs
Supporting Information (PDF): Includes pre-quiz questions, student raw LC-MS/MS spectra, data from GNPS library searches, and data from the GNPS LC-MS Dashboard.
REFERENCES
- 1.Harrison M; Dunbar D; Ratmansky L; Boyd K; Lopatto D Classroom-based research at the introductory level: changes in career choices and attitude. CBE Life Sci. Educ 2011, 10, 279–286. doi: 10.1187/cbe.10-12-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corwin LA; Graham MJ; Dolan EL Modeling course-based undergraduate research experiences: an agenda for future research and evaluation. CBE Life Sci. Educ 2015, 14, es1. doi: 10.1187/cbe.14-10-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mordacq JC; Drane DL; Swarat SL; Lo SM Development of course-based undergraduate research experiences using a design-based approach. J. Coll. Sci. Teach 2017, 46, 64–75. doi: 10.2505/4/JCST17_046_04_64 [DOI] [Google Scholar]
- 4.De Vijlder T; Valkenborg D; Lemière F; Romijn EP; Laukens K; Cuyckens F A tutorial in small molecule identification via electrospray ionization-mass spectrometry: The practical art of structural elucidation. Mass Spectrom. Rev 2018, 37, 607–629. doi: 10.1002/mas.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clish CB Metabolomics: an emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud 2015, 1, a000588. doi: 10.1101/mcs.a000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson CH; Ivanisevic J; Siuzdak G; Metabolomics: beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol 2016, 17, 451–459. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfender J-L; Marti G; Thomas A; Bertrand S Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. doi: 10.1016/j.chroma.2014.10.091. [DOI] [PubMed] [Google Scholar]
- 8.Park J; Suh DH; Singh D; Lee S; Lee JS; Lee CH Systematic metabolic profiling and bioactivity assays for bioconversion of Aceraceae family. PLoS ONE 2018, 13, e0198739. doi: 10.1371/journal.pone.0198739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watrous J; Roach P; Alexandrov T; Heath BS; Yang JY; Kersten RD; van der Voort M; Pogliano K; Gross H; Raaijmakers JM; Moore BS; Laskin J; Bandeira N; Dorrestein PC Mass spectral molecular networking of living microbial colonies. Proc. Natl. Acad. Sci. U. S. A 2012, 109, E1743–E1752. doi: 10.1073/pnas.1203689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M; Carver JJ; Phelan VV; Sanchez LM; Garg N; Peng Y; Nguyen DD; Watrous J; Kapono CA; Luzzatto-Knaan T; Porto C; Bouslimani A; Melnik AV; Meehan MJ; Liu W-T; Crüsemann M; Boudreau PD; Esquenazi E; Sandoval-Calderón M; Kersten RD; Pace LA; Quinn RA; Duncan KR; Hsu C-C; Floros DJ; Gavilan RG; Kleigrewe K; Northen T; Dutton RJ; Parrot D; Carlson EE; Aigle B; Michelsen CF; Jelsbak L; Sohlenkamp C; Pevzner P; Edlund A; McLean J; Piel J; Murphy BT; Gerwick L; Liaw C-C; Yang Y-L; Humpf H-U; Maansson M; Keyzers RA; Sims AC; Johnson AR; Sidebottom AM; Sedio BE; Klitgaard A; Larson CB; Boya P CA; Torres-Mendoza D; Gonzalez DJ; Silva DB; Marques LM; Demarque DP; Pociute E; O’Neill EC; Briand E; Helfrich EJN; Granatosky EA; Glukhov E; Ryffel F; Houson H; Mohimani H; Kharbush JJ; Zeng Y; Vorholt JA; Kurita KL; Charusanti P; McPhail KL; Nielsen KF; Vuong L; Elfeki M; Traxler MF; Engene N; Koyama N; Vining OB; Baric R; Silva RR; Mascuch SJ; Tomasi S; Jenkins S; Macherla V; Hoffman T; Agarwal V; Williams PG; Dai J; Neupane R; Gurr J; Rodríguez AMC; Lamsa A; Zhang C; Dorrestein K; Duggan BM; Almaliti J; Allard P-M; Phapale P; Nothias L-F; Alexandrov T; Litaudon M; Wolfender J-L; Kyle JE; Metz TO; Peryea T; Nguyen D-T; VanLeer D; Shinn P; Jadhav A; Müller R; Waters KM; Shi W; Liu X; Zhang L; Knight R; Jensen PR; Palsson BØ; Pogliano K; Linington RG; Gutiérrez M; Lopes NP; Gerwick WH; Moore BS; Dorrestein PC; Bandeira N Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol 2016, 34 (8), 828–837. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beutler JA; Natural products as a foundation for drug discovery. Curr. Protoc. Pharmacol 2009, 46, 1–9. doi: 10.1002/0471141755.ph0911s46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang JY; Sanchez LM; Rath CM; Liu X; Boudreau PD; Bruns N; Glukhov E; Wodtke A; de Felicio R; Fenner A; Wong WR; Linington RG; Zhang L; Debonsi HM; Gerwick WH; Dorrestein PC Molecular networking as a dereplication strategy. J. Nat. Prod 2013, 76, 1686–1699. doi: 10.1021/np400413s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyce MC; Lawler NG; Tu Y; Reinke SN Introducing undergraduate students to metabolomics using liquid chromatography-high resolution mass spectrometry analysis of horse blood. J. Chem. Ed 2019, 96, 745–750. doi: 10.1021/acs.jchemed.8b00625. [DOI] [Google Scholar]
- 14.Vergne MJ Crime scene investigation: simulated post-mortem LC-MS/MS analysis of cocaine and cocaine metabolites in synthetic human serum. J. Chem. Ed 2021, 98, 3567–3571. doi: 10.1021/acs.jchemed.1c00161. [DOI] [Google Scholar]
- 15.Manninen M; Vesterinen V-M; Vainio A-K; Korhonen H; Karonen M; Salminen J-P Identification of tree species by their defensive compounds: a study with leaf buds of white and silver birches. J. Chem. Ed 2021, 98, 973–981. doi: 10.1021/acs.jchemed.0c00589. [DOI] [Google Scholar]
- 16.Betts TA; Palkendo JA Teaching undergraduates LC-MS/MS theory and operation via multiple reaction monitoring (MRM development). J. Chem. Ed 2018, 95, 1035–1039. doi: 10.1021/acs.jchemed.7b00914. [DOI] [Google Scholar]
- 17.Homem V; Alves A; Santos L Development and validation of a fast procedure to analyze amoxicillin in river waters by direct-injection LC-MS/MS. J. Chem. Ed 2014, 91, 1961–1965. doi: 10.1021/ed500482j. [DOI] [Google Scholar]
- 18.Parker PD; Beers B; Vergne MJ What is in your wallet? Quantitation of drugs of abuse on paper currency with a rapid LC-MS/MS method. J. Chem. Ed 2017, 94, 1522–1526. doi: 10.1021/acs.jchemed.7b00196. [DOI] [Google Scholar]
- 19.Chaleckis R; Meister I; Zhang P; Wheelock CE Challenges, progress and promise of metabolite annotation for LC-MS-based metabolomics. Curr. Opin. Biotechnol 2019, 55, 44–50. doi: 10.1016/j.copbio.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Kirk RD; Carro MA; Wu C; Aldine MJ; Wharton AM; Goldstein DG; Rosario ME; Gallucci GM Zhao Y; Leibovitz E; Bertin MJ Integrating natural product chemistry workflows into medicinal chemistry laboratory training: building the PRISM library and cultivating independent research. J. Chem. Ed 2020, 98, 410–415. doi: 10.1021/acs.jchemed.0c00396. [DOI] [Google Scholar]
- 21.Cech NB; Kandhi V; Davis JM; Hamilton A; Eads D; Laster SM Echinacea and its alkylamides: effects on the influenza A-induced secretion of cytokines, chemokines, and PGE(2) from RAW 246.7 macrophage-like cells. Int Immunopharmacol. 2010, 10, 1268–1278. doi: 10.1016/j.intimp.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Girme A; Saste G; Pawar S; Balasubramaniam AK; Musande K; Darji B; Satti NK; Verma MK; Anand R; Singh R; Vishwakarma RA; Hingorani L Investigating 11 withanosides and withanolides by UHPLC-PDA and mass fragmentation studies from ashwagandha (Withania somnifera). ACS Omega 2020, 5, 27933–27943. doi: 10.1021/acsomega.0c03266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang B; Ma C; Motley T; Kronenberg F; Kennelly EJ Phytochemical fingerprinting to thwart black cohosh adulteration: a 15 Actaea species analysis. Phytochem. Anal 2011, 22, 339–351. doi: 10.1002/pca.1285. [DOI] [PubMed] [Google Scholar]
- 24.Grime A; Saste G; Pawar S; Balasubramaniam AK; Musande K; Darji B; Satti NK; Verma MK; Anand R; Singh R; Vishwakarma RA; Hingorani L Investigating 11 withanosides and withanolides by UHPLC-PDA and mass fragmentation studies from Ashwagandha (Withania somnifera). ACS Omega 2020, 5, 27933–27943. doi: 10.1021/acsomega.0c03266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varsha T; Till B; Debabrata S A validated HPTLC method for the quantifications of three phenolic acids and three withanolides from Withania somnifera plants and its herbal products. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 2019, 1124, 154–160. doi: 10.1016/j.jchromb.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Petras D; Phelan VV; Acharya D; Allen AE; Aron AT; Bandeira N; Bowen BP; Belle-Oudry D; Boecker S; Cummings DA; Deutsch JM; Fahy E; Garg N; Gregor R; Handelsman J; Navarro-Hoyos M; Jarmusch AK; Jarmusch SA; Louie K; Maloney KN; Marty MT; Meijler MM; Mizrahi I; Neve RL; Northen TR; Molina-Santiago C; Panitchpakdi M; Pullman B; Puri AW; Schmid R; Subramaniam S; Thukral M; Vasquez-Castro F; Dorrestein PC; Wang M GNPS Dashboard: Collaborative Exploration of Mass Spectrometry Data in the Web Browser. Nat. Methods 2021. doi: 10.1038/s41592-021-01339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurley A; Chevrette MG; Acharya DD; Lozano GL; Garavito M; Heinritz J; Balderrama L; Beebe M; DenHartog ML; Corinaldi K; Engels R; Gutierrez A; Jona O; Putnam JHI; Rhodes B; Tsang T; Hernandez S; Bascom-Slack C; Blum JE; Price PA; Davis D; Klein J; Pultorak J; Sullivan NL; Mouncey NJ; Dorrestein PC; Miller S; Broderick NA; Handelsman J Tiny Earth: A Big Idea for STEM Education and Antibiotic Discovery. mBio 2021, 12 (1), e03432–20. doi: 10.1128/mBio.03432-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarmusch SA; van der Hooft JJJ; Dorrestein PC; Jarmusch AK Advancements in capturing and mining mass spectrometry data are transforming natural products research. Nat. Prod. Rep 2021, 38, 2066–2082. doi: 10.1039/D1NP00040C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarmusch AK; Wang M; Aceves CM; Advani RS; Aguirre S; Aksenov AA; Aleti G; Aron AT; Bauermeister A; Bolleddu S; Bouslimani A; Caraballo Rodriguez AM; Chaar R; Coras R; Elijah EO; Ernst M; Gauglitz JM; Gentry EC; Husband M; Jarmusch SA; Jones II KL; Kamenik Z; Le Gouellec A; Lu A; McCall LI; McPhail KL; Meehan MJ; Melnik AV; Menezes RC; Montoya Giraldo YA; Nguyen NH; Nothias LF; Nothias-Esposito M; Panitchpakdi M; Petras D; Quinn RA; Sikora N; van der Hooft JJJ; Vargas F; Vrbanac A; Weldon KC; Knight R; Bandeira N; Dorrestein PC ReDU: a framework to find and reanalyze public mass spectrometry data. Nat. Methods 2020, 17, 901–904. doi: 10.1038/s41592-020-0916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li F; Chen F; Liang H; Yan J MoNET: an R package for multi-omic network analysis. Bioinformatics 2021, btab722. doi: 10.1093/bioinformatics/btab722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


