Abstract
Sex-based differences in animal microbiota are increasingly recognized as of biological importance. While most animal biomass is found in aquatic ecosystems and many water-dwelling species are of high economic and ecological value, biological sex is rarely included as an explanatory variable in studies of the aquatic animal microbiota. In this opinion piece, we argue for greater consideration of host sex in studying the microbiota of aquatic animals, emphasizing the many advancements that this information could provide in the life sciences, from the evolution of sex to aquaculture.
Keywords: Microbiome, Aquatic animals, Sex
Introduction
Among sexually reproducing animals, males and females typically exhibit distinct physiological and morphological traits driven at least in part by differences in sex-specific selection pressures. These sex-specific asymmetries can in turn have evolutionarily important consequences, for example by driving speciation (Darwin, 1871; West-Eberhard, 1983; Gavrilets, 2000; Panhuis et al., 2001; Servedio & Boughman, 2017). In recent years, sexual differences in host biology have been shown to be associated with differences in resident microbial communities (the microbiota) across animal hosts, including humans (Mueller et al., 2006; Markle et al., 2013; Yurkovetskiy et al., 2013; Bolnick et al., 2014; de la Cuesta-Zuluaga et al., 2019; Ma & Li, 2019; Sinha et al., 2019; Janiak et al., 2021). Animal-associated microbiota also play vital roles in host health (Ottman et al., 2012), impacting metabolism (Fan & Pedersen, 2021), behaviour (Johnson & Foster, 2018), development (Shin et al., 2011), and response to infection (Hooper et al., 2012; Stevens et al., 2021). In many instances, these processes are moderated by host sex (Jašarević et al., 2016; Baars et al., 2018; Elderman et al., 2018; Weger et al., 2019).
Although the majority of animal biomass is found in the oceans (Bar-On et al., 2018) and despite the ecological/economic importance of aquatic ecosystems (Geist, 2011; Food and Agriculture Organisation of the United Nations [FAO], 2016), the microbiota of aquatic animals is often overlooked compared to terrestrial taxa (Fig. 1). This is an important knowledge gap in light of the fact that aquatic and terrestrial environments differ in ways likely to impact host biology and microbial ecology (Grummer et al., 2019). Water is at least 40 times more viscous and ~ 800 times denser than air. Water also has substantially higher thermal conductivity and capacity. Oxygen solubility exhibits an inverse relationship with water temperature, a property that likely drives adaptation in aquatic animals (Chen et al., 2018b; Sandoval‐Castillo et al., 2018) and influences microbial communities (Spietz et al., 2015; Sunagawa et al., 2015; Ullah Khan et al., 2021). The variable flow/current of water in aquatic environments further impacts microbial locomotion and ecology (Rusconi et al., 2014) as well as shapes host microbial communities (Lee et al., 2017). Finally, aquatic ecosystems typically have high connectivity, meaning that organisms often encounter a range of habitats over their lifetime, each with their own unique stressors (Grummer et al., 2019). When examining fundamental biological questions, such as the association between the microbiota and host sex, it is therefore essential that we include the aquatic environment. Doing so will help us gain a holistic view of the biological processes underpinning the ecology and evolution of animal life.
Fig. 1.
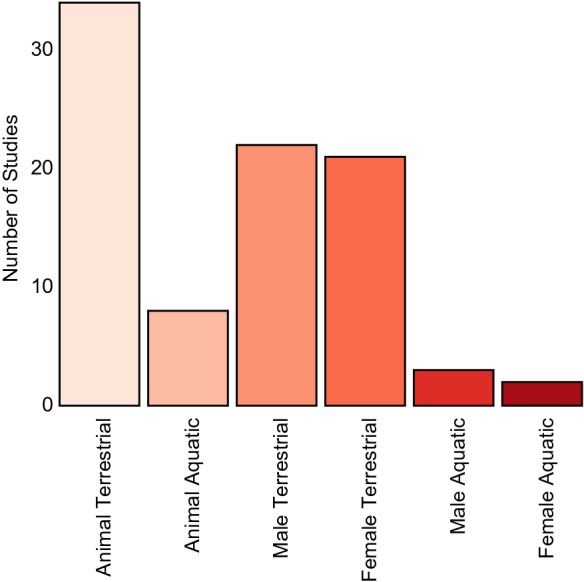
Bar plot of number of Earth Microbiome Project Database (The Earth Microbiome Project Consortium et al., 2017) studies based on terrestrial or aquatic environment and host sex in animals. Sample metadata were searched using the tool rediom (McDonald et al., 2019) with the terms “animal & terrestrial/aquatic & male/female” within the context “Deblur-Illumina-16S-V4-90nt-99d1d8” which was selected based on having the highest number of samples
In this opinion piece, we discuss how host sex shapes the microbiota of aquatic animals. We demonstrate how the diversity of sexual systems exhibited by aquatic animals provide powerful models for examining how sex might structure host microbiotas and vice versa. We also discuss the strengths that aquatic animal systems offer when it comes to studying the intersection between the microbiota and the ecology/evolution of animals. Finally, we highlight how consideration of sex-specific microbiotas may benefit species conservation and aquaculture.
Sexual differences in the microbiota of aquatic animals
Sexual signatures in the microbial communities of aquatic animals have been found across diverse host taxa including invertebrates, fish, and marine mammals (Table 1). Although very few studies have investigated how host sex influences the microbiota of aquatic invertebrates, sexual differences have been reported in coral (Wessels et al., 2017), intertidal crustaceans (Wenzel et al., 2018; Clarke et al., 2019), cephalopods (Iehata et al., 2015), and gastropods (Takacs-Vesbach et al., 2016). In a study of intertidal isopods (Jaera albifrons Leach, 1814), sex was attributed to 14% of variation in microbial beta diversity, with higher alpha diversity also reported in males (Wenzel et al., 2018). The authors hypothesize that these sexual differences in microbial communities could be linked to sexual size dimorphism (Veuille, 1980) or to sex-specific differences in habitat selection (Merilaita & Jormalainen, 1997). Microbial community functional differences have also been observed based on host sex. For example, bacterial community structure and nutritional enzyme activity in the digestive tract of the Chilean octopus (Octopus mimus Gould,1852) were shown to differ between males and females, with males also showing higher bacterial alpha diversity (Iehata et al., 2015).
Table 1.
Examples of observed sexual differences in the microbiota of selected aquatic animals
| Phylum | Species | Sexual or Asexual | Microbial associations as a function of sex |
|---|---|---|---|
| Mollusca |
New Zealand Mud Snail; (Potamopyrgus antipodarum (Gray, 1843)) |
Sexual or asexual | Sexual and asexual animals had a mean beta dissimilarity of 90% (Takacs-Vesbach et al., 2016) |
| Mollusca | Chilean Octopus (Octopus mimus Gould, 1852) | Sexual | Digestive tract bacterial community structure and nutritional enzyme activity differed between males and females, with males showing higher alpha diversity (Iehata et al., 2015) |
| Cnidaria |
Octocoral (Lobophytum pauciflorum (Ehrenberg, 1834)) |
Sexual | Some suggestion that males and females differed in community structure: Spirochaetes- and Rhodobacteraceae-related sequences more abundant in males than in female corals (1.4 × and 4x, respectively) (Wessels et al., 2017) |
| Arthropoda |
Intertidal isopod (Jaera albifrons Leach, 1814) |
Sexual | 14.1% of variation in bacterial beta diversity could be attributed to host sex (Wenzel et al., 2018) |
| Vertebrata | Three-spined stickleback; (Gasterosteus aculeatus Linnaeus, 1758) | Sexual | Among-individual diet variation was correlated with individual differences in gut microbiota in a sex-dependent fashion (Bolnick et al., 2014) |
| Vertebrata | Eurasian perch; (Perca fluviatilis Linnaeus, 1758) | Sexual | Among-individual diet variation was correlated with individual differences in gut microbiota in a sex-dependent fashion (Bolnick et al., 2014). Gut microbial community reacts to predation stress and food rationing in sex-dependent manner (Zha et al., 2018) |
| Vertebrata | Zebrafish (Danio rerio (Hamilton, 1822)) | Sexual | Exposure to titanium dioxide in combination with bisphenol A shifted gut microbiota and host physiology differently between males and females (Chen et al., 2018a) |
| Vertebrata | Fathead minnow (Pimephales promelas Rafinesque, 1820) | Sexual | Females exhibited higher gut bacterial Shannon diversity, differences in beta diversity, taxon abundance, and predicted functional pathways relative to males. Low-dose exposure of the polycyclic hydrocarbon BaP (PAH benzo[a]pyrene), disturbed the gut microbiota structure of females, but not males (DeBofsky et al., 2020) |
| Vertebrata | Elephant Seal (Mirounga leonina (Linnaeus, 1758)) | Sexual | Significant difference in gut microbial community of males and females (Nelson et al., 2013b) |
| Vertebrata | Beluga whales (Delphinapterus leucas (Pallas, 1776)) | Sexual | Significant differences in epidermal microbiota of males and females (Van Cise et al., 2020) |
Among marine mammals, which differ in their gut microbiota compared to terrestrial relatives (Nelson et al., 2013a), the majority of studies indicate that the microbiota is not strongly influenced by host sex. Studies in leopard seals (Hydrurga leptonyx (Blainville, 1820)) (Nelson et al., 2013b), dugongs (Dugong dugon (Müller, 1776)) (Eigeland, 2012), manatees (Trichechus manatus Linnaeus, 1758) (Merson et al., 2014), bottle nose dolphins (Tursiops truncatus (Montagu, 1821)) (Bik et al., 2016), and kogiid whales (Kogia sima (Owen 1866) & Kogia breviceps (de Blainville, 1838)) (Erwin et al., 2017; Denison et al., 2020) have all not found a significant association between host sex and gut microbiota. By contrast, elephant seals (Mirounga leonina (Linnaeus, 1758)) do exhibit pronounced differences in the microbial communities of males and females. This distinction is thought to potentially reflect sexual size dimorphism that may drive prey shifts (altering diet) as well as metabolic differences that are not evident in the other marine mammal species studied from this perspective (Nelson et al., 2013b). Similar evaluations of cetacean epidermal microbiota include one study reporting no significant differences between sexes in microbiota structure of Humpback Whales (Megaptera novaeangliae (Borowski, 1781)) (Apprill et al., 2014), but another finding significant sex differences in Beluga whales (Delphinapterus leucas (Pallas, 1776)) (Van Cise et al., 2020). The latter is thought to potentially be driven by either endogenous differences (e.g. hormone levels, group associations, dietary differences) or sex-specific habitat preference (e.g. males and females may differ in preferences for shore proximity and ice concentration) (Hauser et al., 2017; Van Cise et al., 2020).
Fish represent the most diverse vertebrate Class, with an estimated 35,934 described species compared to the next most speciose Class, Reptilia (estimated 11,570 species) (IUCN, 2021). The high species diversity of fish makes this clade especially important for studying the microbiota, and in particular, determining how host-microbe associations might impact evolutionary trajectories that shape biodiversity. Fish are also of high importance both in terms of the global economy and food security (Food and Agriculture Organisation of the United Nations [FAO], 2016), with improvements in aquaculture benefiting both. Studies to date have demonstrated an important link between the gut microbiota and fish sex, with males and females differing in both alpha and beta diversity (Li et al., 2016; DeBofsky et al., 2020).
In addition to the evidence for innate sexual differences in the microbiota of fish, several studies have demonstrated sex-specific microbial responses to environmental factors. In zebrafish (Danio rerio (Hamilton, 1822)), exposure to titanium dioxide in combination with bisphenol A shifted the gut microbiota, neurotransmission, epithelial permeability, inflammation, and oxidative stress in a sex-specific manner (Chen et al., 2018a). Similarly, in fathead minnows (Pimephales promelas Rafinesque, 1820), females exhibited higher gut bacterial alpha diversity, differences in beta diversity, taxa abundance, and predicted functional pathways relative to males (DeBofsky et al., 2020). The gut microbiota of males and females of this species also responded differently to low-dose exposure of the polycyclic hydrocarbon BaP (PAH benzo[a]pyrene), with exposure disturbing the gut microbial community structure of females but not males (DeBofsky et al., 2020). In three-spined stickleback (Gasterosteus aculeatus Linnaeus, 1758) and Eurasian perch (Perca fluviatilis Linnaeus, 1758), among-individual diet variation was correlated with individual differences in gut microbiota in a sex-dependent fashion, a result further confirmed by experimental dietary manipulation (Bolnick et al., 2014). In another study of Eurasian perch, elements of the gut microbial community were found to react to predation stress and food rationing in a sex-dependent manner (Zha et al., 2018). The sex-dependent response of the microbiota to environmental changes observed across fish taxa (and other vertebrate classes) poses important questions in terms of our approach to microbial manipulation to manage host health, demonstrating the importance of considering the effect of sex in any such intervention.
Case Study: Studying the effect of sex on the microbiota using Potamopyrgus antipodarum
Some animal taxa exist in both sexual and asexual forms (e.g. Neiman et al., 2014), providing a powerful model to examine microbial differences between males, females, and asexual individuals while controlling for host lineage. One such species is Potamopyrgus antipodarum (Gray, 1843)—an aquatic snail native to New Zealand freshwater lakes and streams (Winterbourn, 1973). P. antipodarum is characterized by the existence of multiple triploid and tetraploid asexual lineages that are separately derived from diploid sexual conspecifics (Lively, 1987; Neiman et al., 2011). This reproductive mode and ploidy variation, combined with the ability to easily collect from the field and maintain and culture in the laboratory, has led to these snails achieving prominence as a model system for the evolution of sex (Lively, 1987; Neiman et al., 2011). These same strengths are now being leveraged in microbiota research, where P. antipodarum is being used to assess the impact of reproductive mode in shaping host microbial communities.
Recent studies have shown that snail microbiota composition varies substantially among native New Zealand populations (Takacs-Vesbach et al., 2016), between native and invasive populations (Bankers et al., 2021), and between sexual and asexual forms (Takacs-Vesbach et al., 2016; Bankers et al., 2021). Although ploidy is a confounding factor in the latter comparison, the variance between the bacterial communities of sexual vs. asexual populations (representing multiple lakes) was more than two times greater than between those of triploid and tetraploid populations. While these data hint that reproductive mode is a more important factor than ploidy in determining P. antipodarum microbiota, ploidy is nevertheless worth exploring more broadly. Triploid and tetraploid P. antipodarum did tend to harbour different microbial communities (Takacs-Vesbach et al., 2016), and ploidy level can influence immune function and host resistance (King et al., 2012). For this latter reason, the role of ploidy in host control of the microbiota (Foster et al., 2017) is an especially interesting avenue going forward. With few exceptions (e.g. Cavé-Radet et al., 2019; Forrester and Ashman, 2018), links between animal ploidy level and microbial community composition are unclear and may shed light on how host biological differences can drive microbiota variation.
Specific bacterial taxa also differ in their prevalence between sexual and asexual P. antipodarum across New Zealand lakes (Takacs-Vesbach et al., 2016; Bankers et al., 2021). Perhaps, the most intriguing difference was reported by Takacs-Vesbach et al. (2016), who found that Rickettsiales were absent in asexual snails but present in sexual males and females, regardless of lake origin. Members of Rickettsiales have a wide host range and operate across the parasite-mutualist continuum (Perlman et al., 2006), with some members driving sex ratio distortion (Lawson et al., 2001; von der Schulenburg et al., 2001). Colonization of Rickettsiales in male and female sexual snails from both field and lab cultures suggests that these symbionts might be inherited (Takacs-Vesbach et al., 2016). Conversely, asexual snails across field populations and lab cultured lineages were enriched for bacteria closely related to the Proteobacteria genus Rhodobacter. Members of the Rhodobacter genus are phototrophic in aquatic environments and have been found to be symbionts of marine sponges (Althoff et al., 1998) and Daphnia (Qi et al., 2009). That Rhodobacter was found in both adults and juveniles from one lake suggests that this bacterium might also be inherited and potentially of functional importance in asexual animals.
A more recent study found that even within the same New Zealand lake, P. antipodarum microbial community structure differed by reproductive strategy and sex. Ten amplicon sequence variants (ASVs) (all Xanthomonadaceae) were significantly more abundant in asexuals than sexuals (Bankers et al., 2021), and fifty ASVs (over-represented by Niabella, Bacillus, and OM60) were significantly more abundant in male than female snails. Overall, the differences in microbiota structure identified between male and female or sexual and asexual P. antipodarum demonstrate how host sexual systems can greatly influence the microbiota. The relevance of these findings will be enhanced through future work examining whether sexual differences in the microbiota of P. antipodarum are of functional significance to host health or host evolutionary trajectories.
A role of the microbiota in sex differentiation?
The broad diversity of sex-determining systems makes aquatic animals especially good models for research. Simultaneous or sequential hermaphroditism occurs across a range of aquatic invertebrates including sponges, arthropods, echinoderms, and molluscs, while among vertebrates occurs only in fish (Policansky, 1982). Animals that are simultaneous hermaphrodites have both male and female gonads, while sequential hermaphrodites change from one sex to another within an individual’s lifetime (Warner, 1975; Munday et al., 2006). Sequential hermaphroditism is broadly classified into three categories based on the modality of transition: (1) female to male (protogynous), (2) male to female (protandrous), or (3) serial sex change (bi-directional). In sequential hermaphroditism, sex change is typically driven by body size, age, or community social structure and results in changes in reproductive behaviour, gonadal anatomy, and external morphology (Warner, 1975; Munday et al., 2006; Godwin, 2009; Todd et al., 2016; Liu et al., 2017).
As the only vertebrate group known to exhibit sequential hermaphroditism, teleost fish offer a unique insight into the biological basis of sex. Mechanistically, sex change in teleost fish appears to be governed by a complex interplay of factors including host neurology, hormone balance, stress pathways, and epigenetics (reviewed in Gemmell et al., 2019; Todd et al., 2016). The Hypothalamic–Pituitary–Gonadal (HPG) and Hypothalamic–Pituitary–Interrenal (HPI) axes are involved in oestrogen/androgen balance and release of stress hormones (glucocorticoid steroids), respectively, and are considered the major neuroendocrine system components underpinning sex change in fish (Todd et al., 2016; Goikoetxea et al., 2017; Liu et al., 2017). Of particular importance are 11-ketotestosterone (11-KT) and 17β-estradiol (E2), which respectively promote testicular and ovarian function (Devlin & Nagahama, 2002; Godwin, 2010; Todd et al., 2016; Gemmell et al., 2019). When levels of 11-KT and E2 are altered experimentally, the result is promotion of masculinization or feminization in fish (Chang et al., 1995; Higa et al., 2003; Yeh et al., 2003).
There is some evidence that the microbiota is important in modulating the HPG/HPI axes in fish (Avella et al., 2012; Davis et al., 2016) and could be a missing link in our understanding of sequential hermaphroditism and sex determination. Some of the most convincing evidence for a microbial role in sex determination comes from studies of zebrafish (Danio rerio). D. rerio is a juvenile protogynous hermaphrodite species (Takahashi, 1977) that first develops ovary-like gonads before some individuals undergo bisexual differentiation, whereby ovaries enter an intermediate phase termed “altered ovary” before finally forming testes (Maack & Segner, 2003). Chronic administration of the probiotic Lactobacillus rhamnosus to juvenile D. rerio from the time of first feeding up until 9 weeks post-fertilization has been found to result in 93% females and 7% males in the control group, compared to 55% females and 45% males in the probiotic group (Avella et al., 2012). This study also reported increased expression of gonadotropin-releasing hormone 3 (GnRH3), which is thought to elicit gonadotropin release (which acts as an upstream regulator of sex steroids) (Kuo et al., 2005) and sexual differentiation in this species (Abraham et al., 2009). A possible mechanistic basis for this finding has been deduced by demonstrating that L. rhamnosus activates the HPG axis of D. rerio via increased production of the hormone leptin, which in turn is correlated with a rise in brain gene expression of kiss1 and kiss2 and an increase in GnRH3 expression (Gioacchini et al., 2010). Activation of the HPG axis and GnRH transcription critically depends on adequate host energy stores (Hill et al., 2008), the magnitude of which are signalled to the hypothalamus by neuropeptide hormones and metabolic signals such as kiss1, kiss2, and leptin (Fernandez-Fernandez et al., 2006; Castellano et al., 2009; Kitahashi et al., 2009) that subsequently moderate GnRH expression (Smith et al., 2002; Barb et al., 2005). The link between host energy stores, leptin, kiss1, kiss2, and GnRH expression may, therefore, be applicable more broadly across sequentially hermaphroditic fish, where sex change may be dependent on host size (Ross et al., 1983).
The action of the products of microbial metabolism such as short-chain fatty acids (SCFAs) has also been shown to impact the HPG axis in other fish taxa. For example, dietary modification by addition of the SCFA butyrate reversed the androgenic effects of a plant-based diet in gilthead sea bream (Sparus aurata Linnaeus, 1758) (Simó-Mirabet et al., 2018), while another study demonstrated changes in the microbiota associated with age and sex in this species (Piazzon et al., 2019). These findings warrant further investigation, indicating a possible mechanistic link between host environmental cues (in this case diet), microbial metabolism, and subsequent host hormonal changes that may initiate or contribute to the sex change process.
There is a relatively large body of research on the interaction between the microbiota, the HPG axis, and sexual phenotypes in other vertebrates. This work could also be of broader relevance to fish and other sequentially hermaphroditic animals. For example, the level of Gonadotropin Releasing Hormone (GnRH), which stimulates release of Leuteinizing Hormone (LH) and Follicle-stimulating Hormone (FSH), can be impacted by the presence of certain microbes in both birds and mammals (Wang et al., 2017; Haziak et al., 2018; Lee et al., 2019). Similarly, the gut microbiota has been directly linked with circulating levels of gonadotropins (LH and FSH) and sex steroids (testosterone, oestrogen, progesterone) across several mammalian taxa (Markle et al., 2013; Al-Asmakh et al., 2014; Poutahidis et al., 2014; Lindheim et al., 2017; Shin et al., 2019; Xu et al., 2020). Of particular relevance are experimental mammalian studies that have demonstrated that gut microbiota influence both sex hormone levels and other host phenotypes. For example, a study in mice found that microbiota transplantation from males to females increased circulating testosterone to a sufficient degree to modify autoimmune disease risk (Markle et al., 2013). Moreover, male mice supplemented with Lactobacillus reuteri have been shown to have increased circulating testosterone and testicular weight relative to controls (Poutahidis et al., 2014). Similar results have been demonstrated in a study of germ-free mice colonized with a microbial community (Al-Asmakh et al., 2014).
Microbial sex distortion in aquatic animals
In extreme cases, microbial symbionts have evolved mechanisms to skew the sex ratio of host populations to favour their own fitness (Hurst & Frost, 2015). This phenomenon is particularly prevalent among arthropods, with approximately 52% of aquatic insects estimated to carry the feminizing bacterial symbiont Wolbachia (Sazama et al., 2017). Microbe-driven sex distortion has also been reported in aquatic crustaceans (Bouchon et al., 1998; Terry et al., 1998; Ironside et al., 2003). For example, in the aquatic isopod Gammarus deubeni Lilljeborg, 1852, two species of eukaryotic microbes belonging to the Microspora phylum have been shown to drive host feminization by inhibiting development of the androgenic gland (Jahnke et al., 2013). Sex-distorting symbionts have been proposed to be important drivers of the evolution of sex-determination systems (Cordaux et al., 2011), with evidence from terrestrial isopods (the common pillbug Armadillidium vulgare (Latreille, 1804)) that horizontal gene transfer from the endosymbiont Wolbachia drives the evolution of novel sex chromosomes (Leclercq et al., 2016). Examining sex-distorting symbionts in aquatic hosts, with their diversity of sex-determining mechanisms, could yield new discoveries and advance our understanding of evolutionary transitions in animal sex-determining systems.
Importance in aquaculture and conservation
Aquatic ecosystems are of great importance for food security, the economy, and biodiversity (Geist, 2011; Food and Agriculture Organisation of the United Nations [FAO], 2016). While the microbiota has been shown to be important in growth rate (Lopez Cazorla et al., 2015; Zuo et al., 2019) and disease susceptibility (Boutin et al., 2013; Sha et al., 2016) in aquatic animals, the overall factors governing microbiota structure (including host sex) are poorly understood.
The uses of probiotics (microbes conferring health benefits to their hosts) and prebiotics (substrates utilized by microbes that confer host health benefits) (Sanders et al., 2019) have become a key focus in aquaculture and aquatic species conservation (Robertson et al., 2000; Refstie et al., 2010; Dias et al., 2012; Akhter et al., 2015; Hai, 2015; Mehrim et al., 2015; Ringø, 2020). Despite the recognition that the effects of pre/probiotics can vary between sexes in mammals (Shastri et al., 2015; Christoforidou et al., 2019), few studies have taken sex into account in aquaculture research (Mehrim et al., 2015). Addressing this oversight is critically important given that optimal host responses to probiotic treatment can differ between sexes (Mehrim et al., 2015) and that accounting for sexual differences in species is important for successful species conservation outcomes (Gantchoff et al., 2019).
Conclusion and future directions
As studies examining the importance of host sex and the microbiota garner increasing attention in the terrestrial realm, our understanding of this topic in aquatic hosts remains limited. We emphasize that sexual differences in the microbiota exist across diverse aquatic animal hosts yet much remains unknown in terms of what drives these differences and why sexual differences in the microbiota occur in some species but not others.
Crucially, extending studies on microbe associations across sexes in aquatic animals offers much more than simply “filling in the gaps” in our understanding of host-associated microbial diversity. That many aquatic animals have such diverse sex-determining mechanisms and sexual plasticity, makes these organisms powerful models to examine how the microbiota interacts with, or even shapes host sex. Although experimental work has demonstrated a role of microbes in sequential hermaphroditism in fish, much more research is needed encompassing wider host taxa.
A key question is how often microbes play a role in initiating the sex change process, which in itself will require more advanced studies on microbial sex distortion, as well as the gut-brain axis and neuroendocrine pathways of teleosts and other sequentially hermaphroditic taxa. Ultimately, this avenue of research will be valuable in our understanding of the intersection of biological sex determination and microbiology and more broadly yield key models to advance our knowledge of vertebrate development (including in humans), particularly in terms of normal and atypical sexual development.
Future studies investigating the microbiota and aquatic animal health should also include sex as a variable (where appropriate). The information regarding sexual differences in the response to stressors or in the efficacy of pre/probiotic therapies will undoubtedly have potentially long-term pay-offs in terms of managing aquatic animal health.
Acknowledgements
K.A.B. is funded by a Junior Research Fellowship from St Hilda’s College, University of Oxford. K.C.K. is funded by an ERC Starting Grant (COEVOPRO 802242).
Data availability
Data used to generate Fig. 1 are available upon request from the authors.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Guest editors: Isa Schön, Diego Fontaneto & Elena L. Peredo / Aquatic Microbiomes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abraham E, Palevitch O, Gothilf Y, Zohar Y. The zebrafish as a model system for forebrain GnRH neuronal development. General and Comparative Endocrinology. 2009;164:151–160. doi: 10.1016/j.ygcen.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Akhter N, Wu B, Memon AM, Mohsin M. Probiotics and prebiotics associated with aquaculture: A review. Fish & Shellfish Immunology. 2015;45:733–741. doi: 10.1016/j.fsi.2015.05.038. [DOI] [PubMed] [Google Scholar]
- Al-Asmakh, M., J.-B. Stukenborg, A. Reda, F. Anuar, M.-L. Strand, L. Hedin, S. Pettersson, & O. Söder, 2014. The gut microbiota and developmental programming of the testis in mice. PLoS ONE 9: e103809. [DOI] [PMC free article] [PubMed]
- Althoff K, Schütt C, Steffen R, Batel R, Müller WEG. Evidence for a symbiosis between bacteria of the genus Rhodobacter and the marine sponge Halichondria panicea : harbor also for putatively toxic bacteria? Marine Biology. 1998;130:529–536. doi: 10.1007/s002270050273. [DOI] [Google Scholar]
- Apprill, A., J. Robbins, A. M. Eren, A. A. Pack, J. Reveillaud, D. Mattila, M. Moore, M. Niemeyer, K. M. T. Moore, & T. J. Mincer, 2014. Humpback whale populations share a core skin bacterial community: towards a health index for marine mammals? PLoS ONE 9: e90785. [DOI] [PMC free article] [PubMed]
- Avella, M. A., A. Place, S.-J. Du, E. Williams, S. Silvi, Y. Zohar, & O. Carnevali, 2012. Lactobacillus rhamnosus accelerates zebrafish backbone calcification and gonadal differentiation through effects on the GnRH and IGF systems. PLoS ONE 7: e45572. [DOI] [PMC free article] [PubMed]
- Baars A, Oosting A, Lohuis M, Koehorst M, El Aidy S, Hugenholtz F, Smidt H, Mischke M, Boekschoten MV, Verkade HJ, Garssen J, van der Beek EM, Knol J, de Vos P, van Bergenhenegouwen J, Fransen F. Sex differences in lipid metabolism are affected by presence of the gut microbiota. Scientific Reports. 2018;8:13426. doi: 10.1038/s41598-018-31695-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankers L, Dahan D, Neiman M, Adrian-Tucci C, Frost C, Hurst GDD, King KC. Invasive freshwater snails form novel microbial relationships. Evolutionary Applications. 2021;14:770–780. doi: 10.1111/eva.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barb CR, Hausman GJ, Czaja K. Leptin: A metabolic signal affecting central regulation of reproduction in the pig. Domestic Animal Endocrinology. 2005;29:186–192. doi: 10.1016/j.domaniend.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Bar-On YM, Phillips R, Milo R. The biomass distribution on Earth. Proceedings of the National Academy of Sciences. 2018;115:6506–6511. doi: 10.1073/pnas.1711842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bik EM, Costello EK, Switzer AD, Callahan BJ, Holmes SP, Wells RS, Carlin KP, Jensen ED, Venn-Watson S, Relman DA. Marine mammals harbor unique microbiotas shaped by and yet distinct from the sea. Nature Communications. 2016;7:10516. doi: 10.1038/ncomms10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick DI, Snowberg LK, Hirsch PE, Lauber CL, Org E, Parks B, Lusis AJ, Knight R, Caporaso JG, Svanbäck R. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nature Communications. 2014;5:4500. doi: 10.1038/ncomms5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchon, D., T. Rigaud, & P. Juchault, 1998. Evidence for widespread Wolbachia infection in isopod crustaceans: molecular identification and host feminization. Proceedings of the Royal Society of London. Series B: Biological Sciences 265: 1081–1090. [DOI] [PMC free article] [PubMed]
- Boutin S, Audet C, Derome N. Probiotic treatment by indigenous bacteria decreases mortality without disturbing the natural microbiota of Salvelinus fontinalis. Canadian Journal of Microbiology. 2013;59:662–670. doi: 10.1139/cjm-2013-0443. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Roa J, Luque RM, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. KiSS-1/kisspeptins and the metabolic control of reproduction: Physiologic roles and putative physiopathological implications. Peptides. 2009;30:139–145. doi: 10.1016/j.peptides.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Cavé-Radet, A., C. Monard, A. El-Amrani, A. Salmon, M. Ainouche, & É. Yergeau, 2019. Phenanthrene contamination and ploidy level influence the rhizosphere microbiome of Spartina. Microbiology, http://biorxiv.org/lookup/doi/10.1101/625657. [DOI] [PubMed]
- Chang C-F, Lau E-L, Lin B-Y. Stimulation of spermatogenesis or of sex reversal according to the dose of exogenous Estradiol-17β in juvenile males of protandrous Black Porgy, Acanthopagrus schlegeli. General and Comparative Endocrinology. 1995;100:355–367. doi: 10.1006/gcen.1995.1166. [DOI] [PubMed] [Google Scholar]
- Chen L, Guo Y, Hu C, Lam PKS, Lam JCW, Zhou B. Dysbiosis of gut microbiota by chronic coexposure to titanium dioxide nanoparticles and bisphenol A: Implications for host health in zebrafish. Environmental Pollution. 2018;234:307–317. doi: 10.1016/j.envpol.2017.11.074. [DOI] [PubMed] [Google Scholar]
- Chen Z, Farrell AP, Matala A, Narum SR. Mechanisms of thermal adaptation and evolutionary potential of conspecific populations to changing environments. Molecular Ecology. 2018;27:659–674. doi: 10.1111/mec.14475. [DOI] [PubMed] [Google Scholar]
- Christoforidou Z, Mora Ortiz M, Poveda C, Abbas M, Walton G, Bailey M, Lewis MC. Sexual dimorphism in immune development and in response to nutritional intervention in neonatal piglets. Frontiers in Immunology. 2019;10:2705. doi: 10.3389/fimmu.2019.02705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LJ, Suter L, King R, Bissett A, Deagle BE. Antarctic krill are reservoirs for distinct southern ocean microbial communities. Frontiers in Microbiology. 2019;9:3226. doi: 10.3389/fmicb.2018.03226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaux R, Bouchon D, Grève P. The impact of endosymbionts on the evolution of host sex-determination mechanisms. Trends in Genetics. 2011;27:332–341. doi: 10.1016/j.tig.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Darwin C. The descent of man and selection in realtion to sex. London: John Murray; 1871. [Google Scholar]
- Davis DJ, Bryda EC, Gillespie CH, Ericsson AC. Microbial modulation of behavior and stress responses in zebrafish larvae. Behavioural Brain Research. 2016;311:219–227. doi: 10.1016/j.bbr.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cuesta-Zuluaga, J., S. T. Kelley, Y. Chen, J. S. Escobar, N. T. Mueller, R. E. Ley, D. McDonald, S. Huang, A. D. Swafford, R. Knight, & V. G. Thackray, 2019. Age- and sex-dependent patterns of gut microbial diversity in human adults. MSystems. 10.1128/mSystems.00261-19. [DOI] [PMC free article] [PubMed]
- DeBofsky A, Xie Y, Grimard C, Alcaraz AJ, Brinkmann M, Hecker M, Giesy JP. Differential responses of gut microbiota of male and female fathead minnow (Pimephales promelas) to a short-term environmentally-relevant, aqueous exposure to benzo[a]pyrene. Chemosphere. 2020;252:126461. doi: 10.1016/j.chemosphere.2020.126461. [DOI] [PubMed] [Google Scholar]
- Denison ER, Rhodes RG, McLellan WA, Pabst DA, Erwin PM. Host phylogeny and life history stage shape the gut microbiome in dwarf (Kogia sima) and pygmy (Kogia breviceps) sperm whales. Scientific Reports. 2020;10:15162. doi: 10.1038/s41598-020-72032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208:191–364. doi: 10.1016/S0044-8486(02)00057-1. [DOI] [Google Scholar]
- Dias DC, Leonardo AFG, Tachibana L, Corrêa CF, Bordon ICAC, Romagosa E, Ranzani-Paiva MJT. Effect of incorporating probiotics into the diet of matrinxã (Brycon amazonicus) breeders. Journal of Applied Ichthyology. 2012;28:40–45. doi: 10.1111/j.1439-0426.2011.01892.x. [DOI] [Google Scholar]
- Eigeland K. Bacterial community structure in the hindgut of wild and captive dugongs (Dugong dugon) Aquatic Mammals. 2012;38:402–411. doi: 10.1578/AM.38.4.2012.402. [DOI] [Google Scholar]
- Elderman M, de Vos P, Faas M. Role of microbiota in sexually dimorphic immunity. Frontiers in Immunology. 2018;9:1018. doi: 10.3389/fimmu.2018.01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin PM, Rhodes RG, Kiser KB, Keenan-Bateman TF, McLellan WA, Pabst DA. High diversity and unique composition of gut microbiomes in pygmy (Kogia breviceps) and dwarf (K. sima) sperm whales. Scientific Reports. 2017;7:7205. doi: 10.1038/s41598-017-07425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nature Reviews Microbiology. 2021;19:55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez R, Martini AC, Navarro VM, Castellano JM, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Novel signals for the integration of energy balance and reproduction. Molecular and Cellular Endocrinology. 2006;254–255:127–132. doi: 10.1016/j.mce.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organisation of the United Nations [FAO], 2016. The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All. FAO, Rome.
- Forrester NJ, Ashman T-L. The direct effects of plant polyploidy on the legume–rhizobia mutualism. Annals of Botany. 2018;121:209–220. doi: 10.1093/aob/mcx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KR, Schluter J, Coyte KZ, Rakoff-Nahoum S. The evolution of the host microbiome as an ecosystem on a leash. Nature. 2017;548:43–51. doi: 10.1038/nature23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantchoff M, Conlee L, Belant J. Conservation implications of sex-specific landscape suitability for a large generalist carnivore. Diversity and Distributions. 2019;25:1488–1496. doi: 10.1111/ddi.12954. [DOI] [Google Scholar]
- Gavrilets S. Rapid evolution of reproductive barriers driven by sexual conflict. Nature. 2000;403:886–889. doi: 10.1038/35002564. [DOI] [PubMed] [Google Scholar]
- Geist J. Integrative freshwater ecology and biodiversity conservation. Ecological Indicators. 2011;11:1507–1516. doi: 10.1016/j.ecolind.2011.04.002. [DOI] [Google Scholar]
- Gemmell, N. J., E. V. Todd, A. Goikoetxea, O. Ortega-Recalde, & T. A. Hore, 2019. Natural sex change in fish. Current Topics in Developmental Biology. Elsevier: 71–117. https://linkinghub.elsevier.com/retrieve/pii/S0070215318301145. [DOI] [PubMed]
- Gioacchini G, Maradonna F, Lombardo F, Bizzaro D, Olivotto I, Carnevali O. Increase of fecundity by probiotic administration in zebrafish (Danio rerio) Reproduction. 2010;140:953–959. doi: 10.1530/REP-10-0145. [DOI] [PubMed] [Google Scholar]
- Godwin J. Social determination of sex in reef fishes. Seminars in Cell & Developmental Biology. 2009;20:264–270. doi: 10.1016/j.semcdb.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Godwin J. Neuroendocrinology of sexual plasticity in teleost fishes. Frontiers in Neuroendocrinology. 2010;31:203–216. doi: 10.1016/j.yfrne.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goikoetxea A, Todd EV, Gemmell NJ. Stress and sex: does cortisol mediate sex change in fish? Reproduction. 2017;154:R149–R160. doi: 10.1530/REP-17-0408. [DOI] [PubMed] [Google Scholar]
- Grummer JA, Beheregaray LB, Bernatchez L, Hand BK, Luikart G, Narum SR, Taylor EB. Aquatic landscape genomics and environmental effects on genetic variation. Trends in Ecology & Evolution. 2019;34:641–654. doi: 10.1016/j.tree.2019.02.013. [DOI] [PubMed] [Google Scholar]
- Hai NV. The use of probiotics in aquaculture. Journal of Applied Microbiology. 2015;119:917–935. doi: 10.1111/jam.12886. [DOI] [PubMed] [Google Scholar]
- Hauser DDW, Laidre KL, Stern HL, Moore SE, Suydam RS, Richard PR. Habitat selection by two beluga whale populations in the Chukchi and Beaufort seas. PLoS ONE. 2017;12:e0172755. doi: 10.1371/journal.pone.0172755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haziak K, Herman AP, Wojtulewicz K, Pawlina B, Paczesna K, Bochenek J, Tomaszewska-Zaremba D. Effect of CD14/TLR4 antagonist on GnRH/LH secretion in ewe during central inflammation induced by intracerebroventricular administration of LPS. Journal of Animal Science and Biotechnology. 2018;9:52. doi: 10.1186/s40104-018-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa M, Ogasawara K, Sakaguchi A, Nagahama Y, Nakamura M. Role of steriod hormones in sex change of protogynous wrasse. Fish Physiology and Biochemistry. 2003;28:149–150. doi: 10.1023/B:FISH.0000030505.28138.d1. [DOI] [Google Scholar]
- Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. American Journal of Physiology-Endocrinology and Metabolism. 2008;294:E827–E832. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst, G. D. D., & C. L. Frost, 2015. Reproductive parasitism: Maternally inherited symbionts in a biparental world. Cold Spring Harbor Perspectives in Biology 7: a017699. [DOI] [PMC free article] [PubMed]
- Iehata S, Valenzuela F, Riquelme C. Analysis of bacterial community and bacterial nutritional enzyme activity associated with the digestive tract of wild Chilean octopus (Octopus mimus Gould, 1852) Aquaculture Research. 2015;46:861–873. doi: 10.1111/are.12240. [DOI] [Google Scholar]
- Ironside JE, Smith JE, Hatcher MJ, Sharpe RG, Rollinson D, Dunn AM. Two species of feminizing microsporidian parasite coexist in populations of Gammarus duebeni. Journal of Evolutionary Biology. 2003;16:467–473. doi: 10.1046/j.1420-9101.2003.00539.x. [DOI] [PubMed] [Google Scholar]
- IUCN, 2021. The IUCN Red List of Threatened Species. Version 2021–2. https://www.iucnredlist.org.
- Jahnke M, Smith JE, Dubuffet A, Dunn AM. Effects of feminizing microsporidia on the masculinizing function of the androgenic gland in Gammarus duebeni. Journal of Invertebrate Pathology. 2013;112:146–151. doi: 10.1016/j.jip.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Janiak MC, Montague MJ, Villamil CI, Stock MK, Trujillo AE, DePasquale AN, Orkin JD, Bauman Surratt SE, Gonzalez O, Platt ML, Martínez MI, Antón SC, Dominguez-Bello MG, Melin AD, Higham JP. Age and sex-associated variation in the multi-site microbiome of an entire social group of free-ranging rhesus macaques. Microbiome. 2021;9:68. doi: 10.1186/s40168-021-01009-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E, Morrison KE, Bale TL. Sex differences in the gut microbiome-brain axis across lifespan. Philosophical Transactions of the Royal Society b: Biological Sciences. 2016;371:20150122. doi: 10.1098/rstb.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KVA, Foster KR. Why does the microbiome affect behaviour? Nature Reviews Microbiology. 2018;16:647–655. doi: 10.1038/s41579-018-0014-3. [DOI] [PubMed] [Google Scholar]
- King KC, Seppälä O, Neiman M. Is more better? Polyploidy and parasite resistance. Biology Letters. 2012;8:598–600. doi: 10.1098/rsbl.2011.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahashi T, Ogawa S, Parhar IS. Cloning and expression of kiss2 in the zebrafish and medaka. Endocrinology. 2009;150:821–831. doi: 10.1210/en.2008-0940. [DOI] [PubMed] [Google Scholar]
- Kuo M-W, Lou S-W, Postlethwait J, Chung B-C. Chromosomal organization, evolutionary relationship, and expression of zebrafish GnRH family members. Journal of Biomedical Science. 2005;12:629–639. doi: 10.1007/s11373-005-7457-z. [DOI] [PubMed] [Google Scholar]
- Lawson ET, Mousseau TA, Klaper R, Hunter MD, Werren JH. Rickettsia associated with male-killing in a buprestid beetle. Heredity. 2001;86:497–505. doi: 10.1046/j.1365-2540.2001.00848.x. [DOI] [PubMed] [Google Scholar]
- Leclercq S, Thézé J, Chebbi MA, Giraud I, Moumen B, Ernenwein L, Grève P, Gilbert C, Cordaux R. Birth of a W sex chromosome by horizontal transfer of Wolbachia bacterial symbiont genome. Proceedings of the National Academy of Sciences. 2016;113:15036–15041. doi: 10.1073/pnas.1608979113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Li S, Li XF, Stalker DAE, Cooke C, Shao B, Kelestimur H, Henry BA, Conductier G, O’Byrne KT, Clarke IJ. Lipopolysaccharide reduces gonadotrophin-releasing hormone (GnRH) gene expression: role of RFamide-related peptide-3 and kisspeptin. Reproduction, Fertility and Development. 2019;31:1134. doi: 10.1071/RD18277. [DOI] [PubMed] [Google Scholar]
- Lee STM, Davy SK, Tang S-L, Kench PS. Water flow buffers shifts in bacterial community structure in heat-stressed Acropora muricata. Scientific Reports. 2017;7:43600. doi: 10.1038/srep43600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yan Q, Ringø E, Wu X, He Y, Yang D. The influence of weight and gender on intestinal bacterial community of wild largemouth bronze gudgeon (Coreius guichenoti, 1874) BMC Microbiology. 2016;16:191. doi: 10.1186/s12866-016-0809-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindheim, L., M. Bashir, J. Münzker, C. Trummer, V. Zachhuber, B. Leber, A. Horvath, T. R. Pieber, G. Gorkiewicz, V. Stadlbauer, & B. Obermayer-Pietsch, 2017. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): A pilot study. PLOS ONE 12: e0168390. [DOI] [PMC free article] [PubMed]
- Liu H, Todd EV, Lokman PM, Lamm MS, Godwin JR, Gemmell NJ. Sexual plasticity: A fishy tale. Molecular Reproduction and Development. 2017;84:171–194. doi: 10.1002/mrd.22691. [DOI] [PubMed] [Google Scholar]
- Lively CM. Evidence from a New Zealand snail for the maintenance of sex by parasitism. Nature. 1987;328:519–521. doi: 10.1038/328519a0. [DOI] [Google Scholar]
- Lopez Cazorla A, Sica MG, Brugnoni LI, Marucci PL, Cubitto MA. Evaluation of Lactobacillus paracasei subsp. tolerans isolated from Jenyn’s sprat (Ramnogaster arcuata) as probiotic for juvenile rainbow trout Oncorhynchus mykiss (Walbaum, 1792) Journal of Applied Ichthyology. 2015;31:88–94. doi: 10.1111/jai.12496. [DOI] [Google Scholar]
- Ma Z, Li, W. How and why men and women differ in their microbiomes: medical ecology and network analyses of the microgenderome. Advanced Science. 2019;6:1902054. doi: 10.1002/advs.201902054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maack G, Segner H. Morphological development of the gonads in zebrafish. Journal of Fish Biology. 2003;62:895–906. doi: 10.1046/j.1095-8649.2003.00074.x. [DOI] [Google Scholar]
- Markle JGM, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- McDonald, D., B. Kaehler, A. Gonzalez, J. DeReus, G. Ackermann, C. Marotz, G. Huttley, & R. Knight, 2019. redbiom: a rapid sample discovery and feature characterization system. mSystems 4: e00215–19, /msystems/4/4/mSys.00215–19.atom. [DOI] [PMC free article] [PubMed]
- Mehrim AI, Khalil FF, Hassan ME. Hydroyeast Aquaculture® as a reproductive enhancer agent for the adult Nile tilapia (Oreochromis niloticus Linnaeus, 1758) Fish Physiology and Biochemistry. 2015;41:371–381. doi: 10.1007/s10695-014-9989-5. [DOI] [PubMed] [Google Scholar]
- Merilaita S, Jormalainen V. Evolution of sex differences in microhabitat choice and colour polymorphism in Idotea baltica. Animal Behaviour. 1997;54:769–778. doi: 10.1006/anbe.1996.0490. [DOI] [PubMed] [Google Scholar]
- Merson SD, Ouwerkerk D, Gulino L-M, Klieve A, Bonde RK, Burgess EA, Lanyon JM. Variation in the hindgut microbial communities of the Florida manatee, Trichechus manatus latirostris over winter in Crystal River, Florida. FEMS Microbiology Ecology. 2014;87:601–615. doi: 10.1111/1574-6941.12248. [DOI] [PubMed] [Google Scholar]
- Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC, Clavel T, Koebnick C, Zunft H-JF, Doré J, Blaut M. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Applied and Environmental Microbiology. 2006;72:1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday P, Buston P, Warner R. Diversity and flexibility of sex-change strategies in animals. Trends in Ecology & Evolution. 2006;21:89–95. doi: 10.1016/j.tree.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Neiman M, Paczesniak D, Soper DM, Baldwin AT, Hehman G. Wide variation in ploidy level and genome size in a New Zealand freshwater snail with coexisting sexual and asexual lineages. Evolution. 2011;65:3202–3216. doi: 10.1111/j.1558-5646.2011.01360.x. [DOI] [PubMed] [Google Scholar]
- Neiman M, Sharbel TF, Schwander T. Genetic causes of transitions from sexual reproduction to asexuality in plants and animals. Journal of Evolutionary Biology. 2014;27:1346–1359. doi: 10.1111/jeb.12357. [DOI] [PubMed] [Google Scholar]
- Nelson, T. M., T. L. Rogers, & M. V. Brown, 2013a. The gut bacterial community of mammals from marine and terrestrial habitats. PLoS ONE 8: e83655. [DOI] [PMC free article] [PubMed]
- Nelson TM, Rogers TL, Carlini AR, Brown MV. Diet and phylogeny shape the gut microbiota of Antarctic seals: a comparison of wild and captive animals. Environmental Microbiology. 2013;15:1132–1145. doi: 10.1111/1462-2920.12022. [DOI] [PubMed] [Google Scholar]
- Ottman N, Smidt H, de Vos WM, Belzer C. The function of our microbiota: who is out there and what do they do? Frontiers in Cellular and Infection Microbiology. 2012;2:104. doi: 10.3389/fcimb.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panhuis TM, Butlin R, Zuk M, Tregenza T. Sexual selection and speciation. Trends in Ecology & Evolution. 2001;16:364–371. doi: 10.1016/S0169-5347(01)02160-7. [DOI] [PubMed] [Google Scholar]
- Perlman SJ, Hunter MS, Zchori-Fein E. The emerging diversity of Rickettsia. Proceedings of the Royal Society b: Biological Sciences. 2006;273:2097–2106. doi: 10.1098/rspb.2006.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzon MC, Naya-Català F, Simó-Mirabet P, Picard-Sánchez A, Roig FJ, Calduch-Giner JA, Sitjà-Bobadilla A, Pérez-Sánchez J. Sex, age, and bacteria: How the intestinal microbiota is modulated in a protandrous hermaphrodite fish. Frontiers in Microbiology. 2019;10:2512. doi: 10.3389/fmicb.2019.02512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policansky D. Sex change in plants and animals. Annual Review of Ecology and Systematics. 1982;13:471–495. doi: 10.1146/annurev.es.13.110182.002351. [DOI] [Google Scholar]
- Poutahidis, T., A. Springer, T. Levkovich, P. Qi, B. J. Varian, J. R. Lakritz, Y. M. Ibrahim, A. Chatzigiagkos, E. J. Alm, & S. E. Erdman, 2014. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS ONE 9: e84877. [DOI] [PMC free article] [PubMed]
- Qi W, Nong G, Preston JF, Ben-Ami F, Ebert D. Comparative metagenomics of Daphnia symbionts. BMC Genomics. 2009;10:172. doi: 10.1186/1471-2164-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refstie S, Baeverfjord G, Seim RR, Elvebø O. Effects of dietary yeast cell wall β-glucans and MOS on performance, gut health, and salmon lice resistance in Atlantic salmon (Salmo salar) fed sunflower and soybean meal. Aquaculture. 2010;305:109–116. doi: 10.1016/j.aquaculture.2010.04.005. [DOI] [Google Scholar]
- Ringø E. Probiotics in shellfish aquaculture. Aquaculture and Fisheries. 2020;5:1–27. doi: 10.1016/j.aaf.2019.12.001. [DOI] [Google Scholar]
- Robertson PAW, O’Dowd C, Burrells C, Williams P, Austin B. Use of Carnobacterium sp. as a probiotic for Atlantic salmon (Salmo salar L.) and rainbow trout (Oncorhynchus mykiss, Walbaum) Aquaculture. 2000;185:235–243. doi: 10.1016/S0044-8486(99)00349-X. [DOI] [Google Scholar]
- Ross RM, Losey GS, Diamond M. Sex change in a coral-reef fish: dependence of stimulation and inhibition on relative size. Science. 1983;221:574–575. doi: 10.1126/science.221.4610.574. [DOI] [PubMed] [Google Scholar]
- Rusconi R, Garren M, Stocker R. Microfluidics expanding the frontiers of microbial ecology. Annual Review of Biophysics. 2014;43:65–91. doi: 10.1146/annurev-biophys-051013-022916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nature Reviews Gastroenterology & Hepatology. 2019;16:605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- Sandoval-Castillo J, Robinson NA, Hart AM, Strain LWS, Beheregaray LB. Seascape genomics reveals adaptive divergence in a connected and commercially important mollusc, the greenlip abalone (Haliotis laevigata), along a longitudinal environmental gradient. Molecular Ecology. 2018;27:1603–1620. doi: 10.1111/mec.14526. [DOI] [PubMed] [Google Scholar]
- Sazama EJ, Bosch MJ, Shouldis CS, Ouellette SP, Wesner JS. Incidence of Wolbachia in aquatic insects. Ecology and Evolution. 2017;7:1165–1169. doi: 10.1002/ece3.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servedio MR, Boughman JW. The role of sexual selection in local adaptation and speciation. Annual Review of Ecology, Evolution, and Systematics. 2017;48:85–109. doi: 10.1146/annurev-ecolsys-110316-022905. [DOI] [Google Scholar]
- Sha Y, Wang L, Liu M, Jiang K, Xin F, Wang B. Effects of lactic acid bacteria and the corresponding supernatant on the survival, growth performance, immune response and disease resistance of Litopenaeus vannamei. Aquaculture. 2016;452:28–36. doi: 10.1016/j.aquaculture.2015.10.014. [DOI] [Google Scholar]
- Shastri P, McCarville J, Kalmokoff M, Brooks SPJ, Green-Johnson JM. Sex differences in gut fermentation and immune parameters in rats fed an oligofructose-supplemented diet. Biology of Sex Differences. 2015;6:13. doi: 10.1186/s13293-015-0031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J-H, Park Y-H, Sim M, Kim S-A, Joung H, Shin D-M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Research in Microbiology. 2019;170:192–201. doi: 10.1016/j.resmic.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Shin SC, Kim S-H, You H, Kim B, Kim AC, Lee K-A, Yoon J-H, Ryu J-H, Lee W-J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 2011;334:670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- Simó-Mirabet P, Felip A, Estensoro I, Martos-Sitcha JA, V. de las Heras, J. Calduch-Giner, M. Puyalto, V. Karalazos, A. Sitjà-Bobadilla, & J. Pérez-Sánchez, Impact of low fish meal and fish oil diets on the performance, sex steroid profile and male-female sex reversal of gilthead sea bream (Sparus aurata) over a three-year production cycle. Aquaculture. 2018;490:64–74. doi: 10.1016/j.aquaculture.2018.02.025. [DOI] [Google Scholar]
- Sinha T, Vich Vila A, Garmaeva S, Jankipersadsing SA, Imhann F, Collij V, Bonder MJ, Jiang X, Gurry T, Alm EJ, D’Amato M, Weersma RK, Scherjon S, Wijmenga C, Fu J, Kurilshikov A, Zhernakova A. Analysis of 1135 gut metagenomes identifies sex-specific resistome profiles. Gut Microbes. 2019;10:358–366. doi: 10.1080/19490976.2018.1528822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Jackson LM, Foster DL. Leptin regulation of reproductive function and fertility. Theriogenology. 2002;57:73–86. doi: 10.1016/S0093-691X(01)00658-6. [DOI] [PubMed] [Google Scholar]
- Spietz, R. L., C. M. Williams, G. Rocap, & M. C. Horner-Devine, 2015. A dissolved oxygen threshold for shifts in bacterial community structure in a seasonally hypoxic estuary. PLOS ONE 10: e0135731. [DOI] [PMC free article] [PubMed]
- Stevens, E. J., K. A. Bates, & K. C. King, 2021. Host microbiota can facilitate pathogen infection. PLOS Pathogens 17: e1009514. [DOI] [PMC free article] [PubMed]
- Sunagawa, S., L. P. Coelho, S. Chaffron, J. R. Kultima, K. Labadie, G. Salazar, B. Djahanschiri, G. Zeller, D. R. Mende, A. Alberti, F. M. Cornejo-Castillo, P. I. Costea, C. Cruaud, F. d’Ovidio, S. Engelen, I. Ferrera, J. M. Gasol, L. Guidi, F. Hildebrand, F. Kokoszka, C. Lepoivre, G. Lima-Mendez, J. Poulain, B. T. Poulos, M. Royo-Llonch, H. Sarmento, S. Vieira-Silva, C. Dimier, M. Picheral, S. Searson, S. Kandels-Lewis, Tara Oceans coordinators, C. Bowler, C. de Vargas, G. Gorsky, N. Grimsley, P. Hingamp, D. Iudicone, O. Jaillon, F. Not, H. Ogata, S. Pesant, S. Speich, L. Stemmann, M. B. Sullivan, J. Weissenbach, P. Wincker, E. Karsenti, J. Raes, S. G. Acinas, P. Bork, E. Boss, C. Bowler, M. Follows, L. Karp-Boss, U. Krzic, E. G. Reynaud, C. Sardet, M. Sieracki, & D. Velayoudon, 2015. Structure and function of the global ocean microbiome. Science 348: 1261359–1261359. [DOI] [PubMed]
- Takacs-Vesbach, C., K. King, D. Van Horn, K. Larkin, & M. Neiman, 2016. Distinct bacterial microbiomes in sexual and asexual Potamopyrgus antipodarum, a New Zealand freshwater snail. PLOS ONE 11: e0161050. [DOI] [PMC free article] [PubMed]
- Takahashi H. Juvenile hermaphroditism in the zebrafish, Brachydanio rerio. Bulletin of the Faculty of Fisheries Hokkaido University. 1977;28:57–65. [Google Scholar]
- Terry RS, Smith JE, Dunn AM. Impact of a novel, feminising microsporidium on its crustacean host. The Journal of Eukaryotic Microbiology. 1998;45:497–501. doi: 10.1111/j.1550-7408.1998.tb05106.x. [DOI] [Google Scholar]
- The Earth Microbiome Project Consortium. Thompson LR, Sanders JG, McDonald D, Amir A, Ladau J, Locey KJ, Prill RJ, Tripathi A, Gibbons SM, Ackermann G, Navas-Molina JA, Janssen S, Kopylova E, Vázquez-Baeza Y, González A, Morton JT, Mirarab S, Zech Xu Z, Jiang L, Haroon MF, Kanbar J, Zhu Q, Jin Song S, Kosciolek T, Bokulich NA, Lefler J, Brislawn CJ, Humphrey G, Owens SM, Hampton-Marcell J, Berg-Lyons D, McKenzie V, Fierer N, Fuhrman JA, Clauset A, Stevens RL, Shade A, Pollard KS, Goodwin KD, Jansson JK, Gilbert JA, Knight R. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature. 2017;551:457–463. doi: 10.1038/nature24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd EV, Liu H, Muncaster S, Gemmell NJ. Bending genders: The biology of natural sex change in fish. Sexual Development. 2016;10:223–241. doi: 10.1159/000449297. [DOI] [PubMed] [Google Scholar]
- Ullah Khan F, Shang Y, Chang X, Kong H, Zuberi A, Fang JKH, Liu W, Peng J, Zhang X, Hu M, Wang Y. Effects of ocean acidification, hypoxia, and warming on the gut microbiota of the thick shell mussel Mytilus coruscus through 16S rRNA gene sequencing. Frontiers in Marine Science. 2021;8:736338. doi: 10.3389/fmars.2021.736338. [DOI] [Google Scholar]
- Van Cise AM, Wade PR, Goertz CEC, Burek-Huntington K, Parsons KM, Clauss T, Hobbs RC, Apprill A. Skin microbiome of beluga whales: spatial, temporal, and health-related dynamics. Animal Microbiome. 2020;2:39. doi: 10.1186/s42523-020-00057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veuille M. Sexual behaviour and evolution of sexual dimorphism in body size in Jaera (Isopoda Asellota) Biological Journal of the Linnean Society. 1980;13:89–100. doi: 10.1111/j.1095-8312.1980.tb00072.x. [DOI] [Google Scholar]
- von der Schulenburg JHG, Habig M, Sloggett JJ, Webberley KM, Bertrand D, Hurst GDD, Majerus MEN. Incidence of Male-Killing Rickettsia spp. (α-Proteobacteria) in the Ten-Spot Ladybird Beetle Adalia decempunctata L. (Coleoptera: Coccinellidae) Applied and Environmental Microbiology. 2001;67:270–277. doi: 10.1128/AEM.67.1.270-277.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Du W, Lei K, Wang B, Wang Y, Zhou Y, Li W. Effects of dietary Bacillus licheniformis on gut physical barrier, immunity, and reproductive hormones of laying hens. Probiotics and Antimicrobial Proteins. 2017;9:292–299. doi: 10.1007/s12602-017-9252-3. [DOI] [PubMed] [Google Scholar]
- Warner RR. The adaptive significance of sequential hermaphroditism in animals. The American Naturalist. 1975;109:61–82. doi: 10.1086/282974. [DOI] [Google Scholar]
- Weger BD, Gobet C, Yeung J, Martin E, Jimenez S, Betrisey B, Foata F, Berger B, Balvay A, Foussier A, Charpagne A, Boizet-Bonhoure B, Chou CJ, Naef F, Gachon F. The mouse microbiome is required for sex-specific diurnal rhythms of gene expression and metabolism. Cell Metabolism Elsevier. 2019;29:362–382.e8. doi: 10.1016/j.cmet.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel MA, Douglas A, Piertney SB. Microbiome composition within a sympatric species complex of intertidal isopods (Jaera albifrons) PLoS ONE. 2018;13:e0202212. doi: 10.1371/journal.pone.0202212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels W, Sprungala S, Watson S-A, Miller DJ, Bourne DG. The microbiome of the octocoral Lobophytum pauciflorum: minor differences between sexes and resilience to short-term stress. FEMS Microbiology Ecology. 2017 doi: 10.1093/femsec/fix013. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Sexual Selection, Social Competition, and Speciation. The Quarterly Review of Biology. 1983;58:155–183. doi: 10.1086/413215. [DOI] [Google Scholar]
- Winterbourn MJ. A guide to the freshwater mollusca of New Zealand. Tuatara. 1973;20:141–159. [Google Scholar]
- Xu K, Bai M, Liu H, Duan Y, Zhou X, Wu X, Liao P, Li T, Yin Y. Gut microbiota and blood metabolomics in weaning multiparous sows: associations with oestrous. Journal of Animal Physiology and Animal Nutrition. 2020;104:1155–1168. doi: 10.1111/jpn.13296. [DOI] [PubMed] [Google Scholar]
- Yeh S-L, Kuo C-M, Ting Y-Y, Chang C-F. Androgens stimulate sex change in protogynous grouper, Epinephelus coioides: spawning performance in sex-changed males. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2003;135:375–382. doi: 10.1016/s1532-0456(03)00136-4. [DOI] [PubMed] [Google Scholar]
- Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha Y, Eiler A, Johansson F, Svanbäck R. Effects of predation stress and food ration on perch gut microbiota. Microbiome. 2018;6:28. doi: 10.1186/s40168-018-0400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z, Shang B, Shao Y, Li W, Sun J. Screening of intestinal probiotics and the effects of feeding probiotics on the growth, immune, digestive enzyme activity and intestinal flora of Litopenaeus vannamei. Fish Shellfish Immunology. 2019;86:160–168. doi: 10.1016/j.fsi.2018.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used to generate Fig. 1 are available upon request from the authors.


