Abstract
As major depressive disorder (MDD) is such a diverse condition, there are currently no clear ways for determining its severity, endophenotype, or therapy response. The distinctive nature of depression, the variability of analysis in literature and the large number of conceptually complicated biomarkers are some of the many reasons for the lack of progress. Markers are involved in the process of neurotrophic, metabolic, and inflammation as well as neuroendocrine and neurotransmitter systems’ components. Some clinical indicators are strong enough so that can be measured using assessments of proteomic, genetic, metabolomics, neuroimaging, epigenetic and transcriptomic. Markers of oxidative stress, endocrine, inflammatory, proteomic, and growth indicators are currently among the promising biologic systems/markers identified in this analysis. This narrative review examines succinct studies which investigated cytokines of inflammatory factors, peripheral factors of development, metabolic and endocrine markers as pathophysiological biomarkers of MDD, and treatment responses. Endocrine and metabolic alterations have also been linked to MDD in various studies. So, this study summarizes all of the numerous biomarkers that are significant in the detection or treatment of MDD patients. The paper also provides an overview of various biomarkers which are important for the regulation and its effects on MDD.
Keywords: Major depressive disorder, Insulin-like growth factor I, Brain-derived neurotrophic factor, Mood disorders, IL-1b
1. Introduction
One of the most common and recurring mental illnesses that disabled people is known as Major Depressive Disorder [[1], [2], [3], [4], [5]] Antidepressant medication is one example of a therapeutic strategy that is available, but more than half of the responses from the patients show ineffectiveness so the results are quite unsatisfactory [6,7]. It's critical to look at the cardinal predispositions that make people vulnerable to the onset of depression and also recurrence to improve treatment techniques that will prevent a recurrence. Prospective research into the MDD's Biological predictors' recurrence, relapse, and development will help gain an insight into the possible causes of MDD. To understand this aetiology of MDD along with developing advanced clinical methods, it is quite necessary to study the effects of biomarkers.
In several studies, MDD was shown to be in association with interchanges in the diversification of physio-biological systems [8,9] for example gastrointestinal factors, endocrinology(hormones), immunology, oxidative stress, neurotrophic factors along with diversity in the structure and function of the brain [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]]. Several scientific explanations for the origin of MDD have been established based on these often-reported biomarker abnormalities. Although cross-sectional studies are the source for the majority of evidence for these theories, causality cannot be proven from these, and as such secondary effects cannot be distinguished from causes of illness [21]. The biomarkers must be examined before the onset of MDD or a recurring episode to establish the potential responsibility of an etiological mechanism for MDD development. Prospective investigations of biomarkers before the emergence or recurrence of MDD are thus required. There are also studies showing the existence of a distinct biological profile underlying suicidal behavior with inflammatory dysregulation that may be significantly involved in the physiopathology of depression with suicidality [109]. MDD and it's correlation with suicidal behavior may also be related with relevant neurobiological dysfunctions, in particular immune-inflammatory abnormalities. Furthermore, some studies point towards the distinction between the mechanism of the first onset and that of recurrence which coincides with suicidal behaviour [22,23]. As a result, looking at biomarkers of MDD for recurrence and onset independently may help enhance estimated models.
As a result, this study will be a narrative overview of prospective research looking into the primary biological ideas on MDD's origin. The initial goal is to determine the strengths and weaknesses of the evidence regarding the viability of biomarkers in predicting MDD occurrence or recurrence. To explore prospective studies, a systematic search will be conducted. We concentrate on research that uses clinical approaches which successfully determine the occurrence and recurrence of severe depressive episodes. Gastrointestinal, immunological, neurotrophic, neurotrophic, oxidative stress, neurotransmitters and hormones are among the biological topics covered by the search. The second goal will be to determine each biomarker's robustness and compare the effect sizes of other biomarkers.
2. An insight into depression
For a thorough knowledge of the molecular route and its role in psychiatric diseases, various biological “levels” are needed to be assessed [24]. The best measuring sources differ even when the systems may be investigated at the omics level. For example, brain anatomy or activity is assessed by neuroimaging indirectly, whereas blood protein assays assess indicators. Both bacteria and genetic components are intended to be categorized in The Human Microbiome Project [25]. Modern technologies allow us so that we can measure more than only blood, for example, cerebrospinal fluid, cortisol which is a chronic indicator, saliva, and urine [26,27] Hair and nails are now being analyzed for hormones like cortisol [28].
3. Biomarkers benefits
In addition to being simpler and less expensive to analyze, biomarkers may be evaluated repeatedly and, in less time [29,30]. Creating biomarker panels that could identify multiple proteomic markers, metabolic markers, growth factors, cytokines, and hormonal markers may be a feasible alternative to a single biomarker technique [31]. Biomarkers have shown improvement in patients who are suffering from MDD when compared to controls who are healthy [32,33]. Thus, it shows a lack of clear connections between MDD and other concomitant depressive illnesses is a major flaw in depression biomarker collection. There are some latest indicators of MDD which would allow reliable discernment of factors of growth, hormones, cytokines, along with proteomic markers present in plasma samples. For the same predictions, biomarker platforms can identify hormones, regulators of growth, and other protein indicators. This allows a more thorough review, revealing characteristics that could better identify disease states along with symptoms. It can also be utilized to modify or counteract levels of b cytokine or growth factor. While single biomarkers cannot reliably distinguish between troubled and non-distressed subjects, panels of biomarkers may.
4. Testing biomarker accuracy
A person who has a favorable biomarker outcome is more likely to have depression, than one with a negative predictive value [34]. Because a patient's depression propensity is associated with the biomarkers, the potential will be predictive. A further warning is that when psychological issues like dependency are implicated, the biomarker that is discovered above the level of normal comparison was taken from data that were used as controls not exposed to the disease. So, even when healing is applicable in psychology, it is more particular than in other medical fields. To combine imperfect biomarker analytics, latent analysis of classes is better in these cases [35]. This will be a panel of biomarkers already acknowledgement and have undergone clinical trials study groups [36].
5. Methods
We used the terms "MDD", "biomarker" to search the PubMed database, Google Scholar, PsychInfo, Scopus, and ScienceDirect for articles published in English. The investigation lasted from October 2021 to December 2021. We also looked through the reference lists of the publications we got to see if there was any further information. A systematic examination of human clinical literature relating to MDD biomarkers was also performed. (Supplementary file S1 [1]. original studies that are of peer-review style with case-control designs and reviews where the relationship between MDDs and possible biomarkers was studied; [2]. patients who had MDD at least once; 3). participants are 18 years of age; [4]. valid assessment instrument of MDD; [5]. biomarker measurements that are standardized were among the inclusion criteria. Duplicate papers that contained the same data along with studies having insufficient data have been eliminated. The first, second, and fourth investigators reviewed and examined all of the articles. The third and fourth investigators double-checked the accuracy of the results that had been received. If there were any disagreements, they were handled by discussion and consensus.
6. Proteomic biomarkers
Current research examining various proteins and amino acids as biomarkers for the identification of MDD has yielded positive results. Some of the primary markers identified by current research include a variety of excitatory and inhibitory amino acids such as: glutamic acid, aspartic acid, GABA, and glycine [37]. While glutamic and the aspartic acid act as excitatory neurotransmitters within CNS, glycine and GABA which are some of the commonest inhibitory neurotransmitters found within the CNS [38]. A matched case-control study observed that the plasma levels of several amino acids, including glutamic acid, aspartic acid, and GABA which were predominantly lower in patients with Major Depressive Disorder [39].
Additionally, it has been found that the pathway involving glutamic acid, or glutamate, can be manipulated. One study observed that a 96-h infusion containing a glutamate receptor antagonist, ketamine, with molecules that mitigate the psychotomimetic effects led to 40% of subject investigated the use of ketamine, a glutamate receptor antagonist, as a potential treatment for depression. The study involved a 96-h infusion of ketamine, combined with other molecules to mitigate its psychotomimetic effects, in a group of individuals with depression. The study found that 40% of the subjects still observed a good response to the treatment 8 weeks after the infusion. This suggests that ketamine, when used in a controlled setting and with appropriate modifications to mitigate its side effects, may be a promising treatment option for depression [40]. While this does not directly address the potential of glutamic acid as a biomarker for MDD, it is indicative that glutamate takes a major role in Major Depressive Disorder pathophysiology, instead of being elevated as a result of some derivative process associated with MDD [41].
With regards to inhibitory neurotransmitters, further investigations conducted by Altamura et al. measured the levels of a variety of amino acids, both excitatory and inhibitory, in unmedicated MDD patients compared to a control group [41]. They observed lower glycine levels and a greater serine or glycine ratio in the group with MDD patients [41]. Similar to glutamic acid, studies have also observed significant links between glycine and the onset of MDD. For instance, an investigation conducted by Ji et al. observed that in the gene coding, the presence of a single nucleotide polymorphism for the terminating enzyme of glycine, glycine dehydrogenase, was associated with MDD treatment outcomes [42].
Finally, the last biomarker of interest elucidated by our research was N-acetyl aspartate, a derivative of aspartic acid which is an indicator of mitochondrial dysfunction found in high concentrations within neurons, acting as a marker of neuronal viability [43]. An investigation by Chen et al. employed the CUMS model (chronic unpredictable mild stress). to evaluate changes in metabolic states of rats when responding to stress in the prefrontal cortex. They observed that compared to healthy control, CUMS rats contained higher levels of N-acetyl aspartate [37,44,45].
Current research regarding the potential for proteomic biomarkers for MDD has elucidated a variety of fascinating conclusions, however, there remain flaws in the current assortment of literature that reduce the generalizability of the study conclusions. First and foremost, current studies regarding biomarkers have often lacked study designs that accommodate for a variety of comorbidities associated with MDD. These comorbidities may be responsible for the elevated levels of particular biomarkers that were associated with MDD. For instance, an investigation by Thaipisuttikul et al. revealed that psychotic issues, panic disorders, and OCD are all common comorbidities associated with MDD, yet current literature does not provide a conclusive answer as to whether or not these proteomic correlations are a result of MDD or these aforementioned comorbidities [46]. It is depicted in Figure-1.
Fig. 1.
The diagram above exhibits the current status of research from the proteomic biomarkers lens. It also demonstrates the key markers that compose excitatory and inhibitory amino acids. Glutamic acid, aspartic acid, GABA, and glycine levels have been found to be lower in patients with MDD in comparison to serline levels which have been determined to have a relatively greater ratio.
7. Growth factors
MDD patients have various growth factors which alter gene expression as well as peripheral levels, but treatments are frequently ineffectual. BDNF which is a growth factor resembling insulin is the most vehemently studied growth factor of MDD [47,48]. This is generally released from neurons, then transported by platelets, leukocytes, and cells [49,50]. It is very important in responding to stress and protecting against brain stimulation alterations, according to research. In animal studies, physical or behavioral stress reduced hippocampus BDNF expression. Chronic stress reduces neurogenesis and resilience by inhibiting BDNF pathways in animals [51,52]. Reduced hippocampus BDNF activity has been associated to stress disturbance and MDD pathogenesis. Various studies and meta-analyses show that depressed people have higher BDNF levels in their blood. Therefore MDD is highly associated with higher BDNF levels in depressed population. Higher BDNF levels are also seen in Bipolar disorder which may indicate an association between MDD and bipolar disorder even though BDNF levels are lower in Bipolar disorder when compared to MDD [53,54]. Antidepressants and ECT have been shown in studies to counteract the decline of BDNF associated with depression. Also, those with Major Depressive Disorder giving a positive response towards treatment had greater inceptive BDNF levels. One meta-analysis study indicated that levels of BDNF might be useful to predict antidepressant response [55].
BDNF, VGF, FGF, and FGF cooperate in neuronal differentiation, development, synaptic plasticity, and repair. They are affected by medications and serve as indicators of peripheral circulation and brain owing to their presence [[56], [57], [58], [59]]. In locus coeruleus, hippocampal corpus, anterior cingulate cortex, along with dorsolateral prefrontal cortex, MDD patients showed dysregulation of FGFR 1, FGF2, FGFR2, FGFR3, FGF 1 when compared with controls who are healthy [[60], [61], [62], [63]].
Cytokines and peripheral growth factors are proven to control behavioral responses in animal models. The endocytic drug-receptor successfully transmits IGF-1 secretion from the liver and then it reached the brain. IGF-1 promotes antidepressants and neurogenesis is promoted by IGF-1. the amount of BDNF in the blood varies depending on secondary issues such as the kidney, brain, and liver.
8. Inflammation biomarkers
The inflammation biomarkers associated with Major Depressive Disorder (MDD) are IL-2, IL-6, IL-8, IL-10 (TNF) α, interleukin (IL)-1b, and serum FAM19A5 [[64], [65], [66], [67], [68], [69]]. From the biomarkers stated above, two studies found greater level of the cytokine TNF- α which is pro-inflammatory [66,67]. One study observed that MDD patients with greater TNF- α levels had lower levels of BDNF leading to MDD patients having a greater likelihood of Val66Met polymorphism occurring [66]. Another study revealed that greater levels of TNF- α was in a positive correlation with white matter changes with the study suggesting that the white matter demyelination is a possible effect of higher levels of TNF- α [67]. Both these studies show how higher levels of TNF- α disrupt other processes among MDD patients and future studies should target TNF- α for treatment responses.
Three studies looked at potential treatments affecting pro-inflammatory cytokines [64,65,69] One of the studies looked at the effects of prebiotic and probiotic supplementation and found that inflammatory cytokine levels between the control and the MDD patient group were the same despite probiotic supplementation significantly improving the Beck Depression Index scores for MDD patients [65]. The other two studies evaluated antidepressant treatments’ effect on pro-inflammatory cytokines on cognition and how those biomarkers may potentially affect treatment. One study found the varying response to antidepressant treatment is associated with defects in cognition and MPO expression [64]. For example, the study noticed greater levels of IL-8 were in association with a defect in cognition in learning and memory [64]. In order to provide better suggestions from a clinical standpoint, the study saw benefits in using a Stroop Colour Test and Continuous Performance Test before prescribing an antidepressant treatment for MDD patients [64]. The other study revealed patients who had higher IL-1B levels did not respond well to antidepressant drug treatment and TNF- α was not a good predictor of outcomes of antidepressant drugs [69]. These two studies relate to each other as the study done by Zhou et al. (2021) did not assess IL-1b however the varying responses of TNF- α to the drug treatment support the idea of how different varying results may be caused by different cytokine MPO expression and cognitive defects [64,69]. One study looked at a unique potential inflammation biomarker. The study saw a potential biomarker serum FAM19A5 which was associated with neuroinflammation and neuron degradation in MDD patients [68]. The study saw that serum FA19A5 was greater in MDD patients and lead to a significantly lower cortical thickness. This study provides a different scope of inflammation biomarkers as it was different compared to the other but was associated with inflammation in the body [68]. It is depicted in Fig. 2.
Fig. 2.
The figure above depicts the key markers of MDD from the Inflammation domain. Patients with MDD exhibit greater levels of IL-8 and experience defects in cognition, learning and memory. The diagram further illustrates that high levels of serum FAT19A5 can lead to lower cortisol thickness whereas high levels of TNF-a can result in white matter degradation and decreased BDNF levels.
9. Endocrine biomarkers
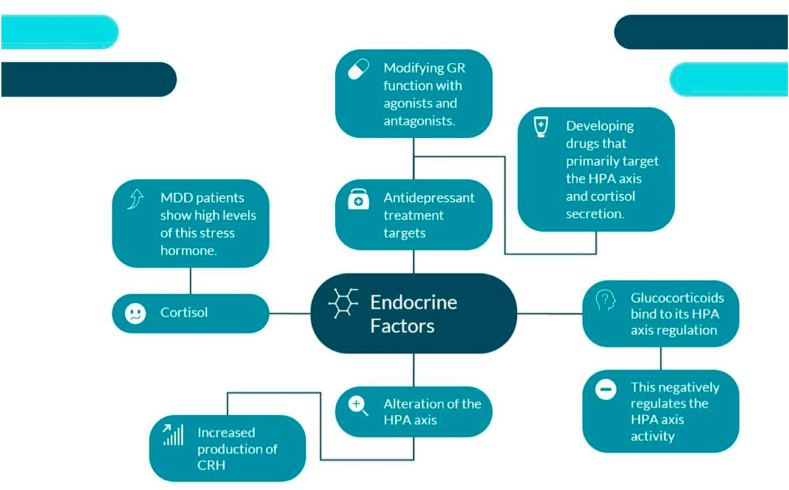
Psychiatry and endocrinology have long been interconnected with psychological and endocrine disorders [70]. Patients diagnosed with depression do not overtly exhibit major endocrine disorders, however, changes in hormones are prevalent among this population, and thus by extension, researchers have identified endocrine disturbances to be a therapeutic target in this illness [70]. For starters, cortisol, also known as the stress hormone, is one of the most commonly studied hypothalamic-pituitary-adrenal (HPA) axis biomarkers associated with depression. Chronic cortisol levels were reported as hyperactive among most Major Depressive Disorder patients, and its elevated degree tends to showcase a deficient response to cognitive and antidepressant treatments [71]. Specifically, depression alters the regulation of the HPA axis which leads to the increased production of CRH and high concentrations of cortisol. Glucocorticoids or cortisol in humans bind to its HPA axis regulation, then negatively regulate the HPA axis activity [72]. Panels of biomarkers that observe disruptions in cortisol, therefore, provide critical information for the identification of MDD subtypes [72]. A strength of the paper by Pariante et al. (2010) is that it sheds light on the high levels of glucocorticoid hormones being the cause of impairment in the glucocorticoid receptors (GRs) and serving as a compensatory mechanism. Evidence shows that modifying GR function with agonists and antagonists can act as a therapeutic mechanism as well as developing other drugs that primarily target the HPA axis and cortisol secretion can also have an antidepressant impact on patients suffering from MDD [73]. The study reveals that individuals diagnosed with depression exhibit irregular responses to TSH, TRH, and excessive levels of TRH [73]. Therefore, previous research indicates that CRH, thyroid hormones, glucocorticoids, gonadal steroids, as well as somatostatin are all endocrine-related factors that largely contribute to the pathophysiologic expression of MDD [74]. These are depicted in Fig. 3.
Fig. 3.
The figure above showcases the primary markers and treatment options for MDD from an endocrine perspective. MDD patients experience an altered HPA axis as glucocorticoids negatively regulates HPA axis activity. This alteration increases CRH and cortisol levels in MDD patients. Treatment options that can combat these markers include the developmentation of drugs targeting cortisol secretion and modifying GR function with agonists and antagonists.
10. Hpa axis
The most extensively studied biomarker of the HPA axis for depression is cortisol. Various HPA indices of HPA have been examined; in general, which indicate the association of stress with hypercortisolemia and a recurring reduction of awakening responses by cortisol [75,76]. Another study corroborates the hyperactivity of cortisol theory in patients with depression but not with specific diseases like panic disorder. This study was done on levels of persistent hair cortisol. Whereas, increased levels of cortisol may imply a lower amount of psychological reactions [77]. along with increased medication for depression [78,79]. Historically, dexamethasone inhibition studies have established the gold standard neuroendocrine tracking of prospective therapeutic progress, since non-suppression of cortisol was associated with a lwithser likelihood of recurrence which is eventual. Additional biomarker possibilities showing biological actions have been rigorously evaluated utilizing genetics or neuroimaging. Given the small and insignificant genomic differences between disturbed and non-depressed populations [80]., A greater beneficial method may be to use modern genetic processes for example- Circadian processes, telomere length [81], chronobiological biomarkers, and polygenic scores [26]. are currently being explored using a variety of methodologies. Through the use of an accelerometer, an exact measurement of sleep and wake operation and sleep can be offered by actigraphy, while instruments can analyze environmental factors, for example, the intensity of light. It could be more efficacious than routinely utilized subjective reports of the patients for detection while giving novel determinants of therapy outcome [82].
11. Metabolic biomarkers
The primary biomarkers which are associated with this type of metabolic state are ghrelin, leptin, high-density lipoprotein, adiponectin, insulin albumin triglycerides, and creatine are the preliminary biomarkers that are associated with the metabolic state of MDD [83]. For the context of depression, these relationships have been explored in previous works of research. When compared with controls, leptin [84] and ghrelin [85] levels are predominantly lower in patients suffering from depression and prescription of antidepressants may help with improved levels along with recovery. In depression, there may be alleviated levels of insulin resistance but it is presented to a lesser extent [86]. In patients with poor physical health and some cases, depression, lipid levels, particularly HDL cholesterol, wavers [87]. Additionally, hyperglycemia [88] and hypoalbuminemia depression have been described. In clinical conditions, molecular metabolomics panels may be used to demonstrate an important biochemical signature for the investigation of common metabolic diseases. Raised levels of glucose lipid signals found in a metabolites sequence can firmly predict a diagnosis of Major Depressive Disorder, correlating with prior research [89], when AI models are utilized [90,91]. There is a hypothesis that when antioxidant defenses and oxidative stress are enhanced in MDD patients, these specific components of oxidative stress can influence the depression pathophysiology [92].
Recent investigations have revealed that people who experience depression frequently have a higher level of malondialdehyde than those who experience it only once. In patients with Major Depressive Disorder, the activity of superoxide dismutase is another indicator of oxidative stress being investigated. As a result, in patients suffering from depression, serum SOD levels may be decreased or erythrocyte SOD levels may be increased [93].
To establish a connection between 8-Oxo-2′-deoxyguanosine and 8-hydroxy-2-deoxyguanosine numerous cross-sectional investigations have been conducted. Serum concentrations and urinary rates [94] of 8-Oxo-2′-deoxyguanosine were significantly greater among people suffering from severe depression when compared to control groups who were stable [95]. Additionally, the degree and severity of depression are positively correlated with levels of 8-hydroxy-2-deoxyguanosine [96], and those suffering from a recurrence of MDD were more likely to have elevated levels of 8-hydroxy-2-deoxyguanosine than those with single episodes [97]. As no differences were observed in groups with depressive symptoms, the 8-hydroxy-2-deoxyguanosine level increase may be recognized as a clinical feature of depression [98,99]. Levels of isoprostane are also increased among depressed people, as seen by increased urine concentrations of 8-iso-PGF2a. An analysis was conducted to determine how antidepressant medication can affect the rates of isoprostane. In Major Depressive Disorder (MDD), isoprostanes have been found to be elevated in the serum
and cerebrospinal fluid of patients, indicating increased oxidative stress in these individuals.
Elevated levels of isoprostanes have been associated with a number of other psychiatric and
neurological conditions as well, including bipolar disorder and Alzheimer's disease.
Isoprostanes may contribute to the development of MDD by damaging cellular membranes and disrupting normal cellular processes. They may also contribute to the neuroinflammation that is often seen in individuals with MDD. Additionally, isoprostanes have been shown to decrease the levels of neurotransmitters such as serotonin and dopamine, which are known to play a role in the development of MDD. After sertraline or bupropion treatment for eight weeks, F2 isoprostane excretion was shown to improve significantly among patients with severe depression. Increases in F2 have also been linked to improvements in depression severity [77].
12. Immunological biomarker
There are several markers for immunology such as C-Reactive Protein, Interleukin-6 (IL-6), IL-1β, Tumor Necrosis Factor-α (TNFα), Soluble Urokinase Plasminogen Activator Receptor.
(suPAR), 3-nitrotyrosine, and heat-shock protein 70 (HSP70) in blood or serum [[100], [101], [102], [103], [104], [105], [106]].
One study investigated the hazard ratio and showed that CRP significantly predicted earlier time to onset or relapse/recurrence of depression [104]. In three studies (of which two investigated the same sample) TNFα was not found to predict non-significance were also reported [100,106,107]. A protein marker for inflammation SuPAR was found to predict reduced time to MDD [102]. In addition, three-nitrotyrosine and HSP70 were higher at baseline in participants that develop vs that do not develop MDD [105].
13. Gastrointestinal biomarkers
Only one study investigated gastrointestinal biomarkers that showed children reporting
symptoms of abdominal discomfort (e.g. nausea or vomiting) in response to tryptophan (L-
5HTP) infusion have a higher risk of developing MDD than children who do not report these
symptoms [108].
14. Discussion
Many of the biomarkers are connected with depression through different pathways. Such identifiers are constantly interwoven in with a dynamic and strenuous way. As most of the evidence is presented in a mixed way when some of this could be epiphenomena of particular variables, while others in a specific category of patients are substantial. Biomarkers can be utilized in a lot of different situations. Modern methods are needed if we are to increase therapeutic utilization and homogeneity of psychiatric communities' biological processes.
The previous investigations’ findings have been replicated in big studies. New biomarkers, such as the tyrosine kinase 2 growth factor, activation regulated chemokines, and thymus chemokines are yet to be examined in clinically stressed and stable test samples, as far as we know. In clinical and non-clinical populations, big-data studies will evaluate entire panels of a biomarker for extensive exploration of relationships with markers along with more enhancements using opulent analysis tools. Furthermore, significant component study replications may identify biomarker classes that are substantially clustered, and improve the uniformity of future tests.
Consistently recommended treatments of depression must undergo careful evaluation in terms of their biological impact, taking into account the success of medical research. It creates structures around biomarkers and indicators to foresee effects along a more tailored path where a broad range of therapeutic drugs are used to treat both unipolar and depressive disorders. This has the potential to be advantageous to current and futuristic medicines.
Prospective studies, on the other hand, will take on a consolidative perspective towards the research of biomarkers to satisfy the goals of RDoC's and focus on a variety of sub-types. Experiments must involve a large number of people to truly portray society. The amount of data that can be obtained through both exploration and guided research. The greater portion of the assets listed above is fully operative, allowing for the discovery of new MDD hits. The previously implicated markers which are discussed should be duplicated and possibly further investigated.
The above methods' practical application will help to improve therapeutic resistance modeling. To effectively treat people who did not react, or responded only partially, to existing antidepressant medication and are more susceptible to adverse clinical consequences, innovative techniques are needed. Evidence also revealed that even in patients with treatment-resistant depression linked to immune-inflammatory dysfunctions, some psychoactive compounds are effective, well-tolerated, and safe options for reducing depressive symptoms and serious suicidal ideation at low doses [110]. Other relevant metrics of patient well-being, for example: the quality of life may be assessed more accurately, and biomarkers may be examined more precisely. Even though biological actions may be unable to differentiate between response and non-response of treatment, analytic model development and its measurement using data of biomarkers from inadequate patient responses may be able to combine demographic and psychological parameters. If such models could be established and validated in a larger sample, the development of architecture translation can take place. The results of the biochemical process of all commonly used depressed drugs were thoroughly examined, with the validity of the therapeutic findings also taken into account. It can be used to predict the outcomes of various antidepressant medications in a more personal fashion using constructs associated with biomarkers along with symptoms, as well as bipolar or unipolar depression. It could be useful for future therapies as well as the ones that have previously been offered.
As a result, there was no single biomarker or collection of biomarkers that could be used to diagnose depression or guide therapy selection. Despite the ability to incorporate other biomarkers which are essential(e.g a biosignature), depression of heterogeneous nature poses a special hurdle for the identification of biomarkers. Biomarker analysis and analytical instruments must continue to be developed, as these are dependable procedures. These are critical for personalizing depression treatments to specific individuals; these technological advancements, together with the discovery of potential biomarkers, could lead to a faster and more accurate diagnosis. It would get better.
15. Conclusion
We outline all of the different biomarkers which are important in diagnosing or treating MDD in this review. This extensive study regarding depression has revealed a great variety of biomarkers that may aid the diagnosis and treatment of people suffering from depression. The use of the aforementioned biomarkers will almost certainly increase the ability to predict treatment resistance in the future. More genuine and long-term therapeutic response metrics can bolster this. Other legitimate patient wellness measures of patient well-being may be used to give a more holistic evaluation of outcomes of treatment, which might also assess biomarkers. Even though biological actions may be unable to differentiate between response and non-response of treatment, analytic model development and its measurement using data of biomarkers from inadequate patient responses may be able to combine demographic and psychological parameters for poor treatment response. In a controlled trial with a larger sample, a design of a translational model can be utilized for response prediction once a reliable model is constructed and verified. Recent research has highlighted the inflammatory response along with receptors of neuroendocrine and neurotransmitter growth factors, metabolism, and the immune system which have been studied extensively for years on end. However, the abundance of contradictory evidence implies that before biomarker testing can be applied, several factors must be investigated if we want to modify the diagnosing and treating process of patients suffering from depression. Such markers have the greatest promise in predicting treatment reactions in a small group of patients. A combined evaluation of psychological and physiobiological information can improve the quality of life among patients at risk of the ability of people at risk of fragile therapy practices to recognize imminently.
Authors’ contributions
Conceptualization, methodology, and supervision: Fahmida Hoque Rimti; Resources: Reemal Shahbaz Data curation: Kunj Bhatt, Alex Wang, Reemal Shahbaz, Fahmida Hoque Rimti; Writing original draft: All the authors participated equally; Editing: Fahmida Hoque Rimti; Correspondence: Fahmida Hoque Rimti.
Funding
None.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
N/A.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e18909.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e18909.
Abbreviations Full form
- MDD
Major Depressive Disorder
- BDNF
Brain-Derived Neurotrophic Factor
- VGF
VGF Nerve Growth Factor Inducible
- FGF
Fibroblast Growth Factor
- FGF2
Fibroblast Growth Factor 2
- FGFR 1
Fibroblast Growth Factor Receptor 1
- FGF2
Fibroblast Growth Factor 2
- FGFR2
Fibroblast Growth Factor Receptor 2
- FGFR3
Fibroblast Growth Factor Receptor 3
- FGF1
Fibroblast Growth Factor 1
- ECT
Electroconvulsive Therapy
- IGF-1
Insulin-Like Growth Factor 1
- IL-2
Interleukin-2
- IL-6
Interleukin-6
- FGFR2
Fibroblast Growth Factor Receptor 2
- Serum FAM19A5
Family with sequence similarity 19 (chemokine (C–C motif)-like) member A5
- VAL66MET
A genetic variant in the BDNF gene that results in the substitution of the amino acid valine (Val) with methionine (Met) at position 66
- MPO
Myeloperoxidase
- FAT19A5
Fatty acid translocase/CD36 antigen-like 5
- HPA
Hypothalamic-Pituitary-Adrenal Axis
- CRH
Corticotropin-Releasing Hormone
- TSH
Thyroid-Stimulating Hormone
- TRH
Thyrotropin-Releasing Hormone
- SOD
Superoxide Dismutase
- 8-iso-PGF2a
8-Isoprostane, a biomarker of oxidative stress and lipid peroxidation
- RDoC
Research Domain Criteria
Appendix B. Supplementary data
The following is the supplementary data related to this article:
Appendix BSupplementary data
The following is the supplementary data related to this article:
Multimedia component 1
References
- 1.Kessler R.C., Wai T.C., Demler O., Walters E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatr. 2005 doi: 10.1001/archpsyc.62.6.617. https://jamanetwork.com/journals/jamapsychiatry/fullarticle/208671 [Internet] Jun 1 [cited 2021 Nov 27];62(6):617–27. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moussavi S., Chatterji S., Verdes E., Tandon A., Patel V., Ustun B. 2007. Depression, Chronic Diseases, and Decrements in Health: Results from the World Health Surveys.http://www.thelancet.com/article/S0140673607614159/fulltext Lancet [Internet] Sep 8 [cited 2021 Nov 27];370(9590):851–8. Available from: [DOI] [PubMed] [Google Scholar]
- 3.Moffitt T.E., Caspi A., Taylor A., Kokaua J., Milne B.J., Polanczyk G., et al. How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychol. Med. 2010 Jun doi: 10.1017/S0033291709991036. https://www.cambridge.org/core/journals/psychological-medicine/article/abs/how-common-are-common-mental-disorders-evidence-that-lifetime-prevalence-rates-are-doubled-by-prospective-versus-retrospective-ascertainment/E0698943DD3432F8C9F294CB074FDEA4 [cited 2021 Nov 27];40(6):899–909. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardeveld F., Spijker J., De Graaf R., Hendriks S.M., Licht C.M.M., Nolen W.A., et al. Recurrence of major depressive disorder across different treatment settings: results from the NESDA study. J. Affect. Disord. 2013;147(1–3):225–231. doi: 10.1016/j.jad.2012.11.008. May 1. [DOI] [PubMed] [Google Scholar]
- 5.Mueller T.I., Leon A.C., Keller M.B., Solomon D.A., Endicott J., Coryell W., et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999 doi: 10.1176/ajp.156.7.1000. https://pubmed.ncbi.nlm.nih.gov/10401442/ Oct 15 [cited 2021 Nov 28];156(7):100–6. Available from: [DOI] [PubMed] [Google Scholar]
- 6.Undurraga J., Baldessarini R.J. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacol. 2012;37(4):851–864. doi: 10.1038/npp.2011.306. https://www.nature.com/articles/npp2011306 374 [Internet]. 2011 Dec 14 [cited 2021 Nov 28]; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cipriani A., Furukawa T.A., Salanti G., Chairman A., Atkinson L.Z., Ogawa Y., et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet (London, England) 2018 doi: 10.1016/S0140-6736(17)32802-7. https://pubmed.ncbi.nlm.nih.gov/29477251/ Apr 7 [cited 2021 Nov 28];391(10128):1357–66. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan V., Nestler E.J. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010 Nov 1 doi: 10.1176/appi.ajp.2009.10030434. https://ajp.psychiatryonline.org/doi/abs/10.1176/appi.ajp.2009.10030434 [Internet] [cited 2021 Nov 27];167(11):1305–20. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nestler E.J., Barrot M., DiLeone R.J., Eisch A.J., Gold S.J., Monteggia L.M. Neurobiology of depression. Neuron. 2002 doi: 10.1016/s0896-6273(02)00653-0. http://www.cell.com/article/S0896627302006530/fulltext Mar 28 [cited 2021 Nov 28];34(1):13–25. Available from: [DOI] [PubMed] [Google Scholar]
- 10.Schlaepfer T.E., Cohen M.X., Frick C., Kosel M., Brodesser D., Axmacher N., et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacol. 2008;33(2):368–377. doi: 10.1038/sj.npp.1301408. https://www.nature.com/articles/1301408 332 [Internet]. 2007 Apr 11 [cited 2021 Nov 28] Available from: [DOI] [PubMed] [Google Scholar]
- 11.Wallace C.J.K., Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry. 2017 doi: 10.1186/s12991-017-0138-2. https://annals-general-psychiatry.biomedcentral.com/articles/10.1186/s12991-017-0138-2 Feb 20 [cited 2021 Nov 28];16(1):1–10. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clapp M., Aurora N., Herrera L., Bhatia M., Wilen E., Wakefield S. Gut microbiota's effect on mental health: the gut-brain Axis. Clin. Pract. 2017;7:131–136. doi: 10.4081/cp.2017.987. https://www.mdpi.com/2039-7283/7/4/987 [Internet]. 2017 Sep 15 [cited 2021 Nov 28];7(4):131–6. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2015 161 doi: 10.1038/nri.2015.5. https://www.nature.com/articles/nri.2015.5 [Internet]. 2015 Dec 29 [cited 2021 Nov 28];16(1):22–34. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price J.L., Drevets W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. Jan 1. [DOI] [PubMed] [Google Scholar]
- 15.Krystal J.H., Sanacora G., Blumberg H., Anand A., Charney D.S., Marek G., et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;71 doi: 10.1038/sj.mp.4001021. https://www.nature.com/articles/4001021 [Internet]. 2002 Mar 18 [cited 2021 Nov 28];7(1):S71–80. Available from: [DOI] [PubMed] [Google Scholar]
- 16.Brunoni A.R., Lopes M., Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int. J. Neuropsychopharmacol. 2008 Dec 1 doi: 10.1017/S1461145708009309. https://academic.oup.com/ijnp/article/11/8/1169/696736 [cited 2021 Nov 28];11(8):1169–80. Available from: [DOI] [PubMed] [Google Scholar]
- 17.Andrews P.W., Bharwani A., Lee K.R., Fox M., Thomson J.A. Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response. Neurosci. Biobehav. Rev. 2015 Apr 1;51:164–188. doi: 10.1016/j.neubiorev.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacol. 2000 235 doi: 10.1016/S0893-133X(00)00159-7. https://www.nature.com/articles/1395567 [Internet]. 2000 [cited 2021 Nov 28];23(5):477–501. Available from: [DOI] [PubMed] [Google Scholar]
- 19.Duman R.S., Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc B Biol Sci [Internet. 2012 doi: 10.1098/rstb.2011.0357. https://royalsocietypublishing.org/doi/abs/10.1098/rstb.2011.0357 [cited 2021 Nov 28];367(1601):2475–84. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black C.N., Bot M., Scheffer P.G., Penninx B.W.J.H. Psychol Med; 2017. Oxidative Stress in Major Depressive and Anxiety Disorders, and the Association with Antidepressant Use; Results from a Large Adult Cohort.https://www.cambridge.org/core/journals/psychological-medicine/article/abs/oxidative-stress-in-major-depressive-and-anxiety-disorders-and-the-association-with-antidepressant-use-results-from-a-large-adult-cohort/290134BC6EDFE19312ADFBCDF6AD67CE Apr 1 [cited 2021 Nov 28];47(5):936–948. [DOI] [PubMed] [Google Scholar]
- 21.Mayeux R. Biomarkers: potential uses and limitations. NeuroRx. 2004 doi: 10.1602/neurorx.1.2.182. https://link.springer.com/article/10.1602/neurorx.1.2.182 12 [Internet]. 2004 Apr [cited 2021 Nov 28];1(2):182–8. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belmaker R.H., Agam G. 2009. Major Depressive Disorder. Jun 6 [cited 2021 Nov 28];358(1):55–68. Available fro. [DOI] [PubMed] [Google Scholar]
- 23.Van Loo H.M., Aggen S.H., Gardner C.O., Kendler K.S. Multiple risk factors predict the recurrence of major depressive disorder in women. J. Affect. Disord. 2015;180:52–61. doi: 10.1016/j.jad.2015.03.045. Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtzheimer P.E., Mayberg H.S. Deep brain stimulation for psychiatric disorders. Annu. Rev. Neurosci. 2011 doi: 10.1146/annurev-neuro-061010-113638. https://pubmed.ncbi.nlm.nih.gov/21692660/ Jul 21 [cited 2021 Nov 28];34:289–307. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menke A. Gene expression: biomarker of antidepressant therapy? Int Rev Psychiatry. 2013 doi: 10.3109/09540261.2013.825580. https://pubmed.ncbi.nlm.nih.gov/24151803/ [cited 2021 Nov 28];25(5):579–91. Available from: [DOI] [PubMed] [Google Scholar]
- 26.Peng B., Li H., Peng X.X. 2015. Functional Metabolomics: from Biomarker Discovery to Metabolome Reprogramming.https://pubmed.ncbi.nlm.nih.gov/26135925/ Protein Cell [Internet] Sep 17 [cited 2021 Nov 28];6(9):628–37. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonner Z., Wilder E., Heikenfeld J., Kasting G., Beyette F., Swaile D., et al. Biomicrofluidics [Internet]; 2015. The Microfluidics of the Eccrine Sweat Gland, Including Biomarker Partitioning, Transport, and Biosensing Implications.https://pubmed.ncbi.nlm.nih.gov/26045728/ May 1 [cited 2021 Nov 28];9(3). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendrickx H., McEwen B.S., Ouderaa F Van Der. Neurobiol Aging; 2005. Metabolism, Mood and Cognition in Aging: the Importance of Lifestyle and Dietary Intervention.https://pubmed.ncbi.nlm.nih.gov/16290269/ [cited 2021 Nov 28];26 Suppl 1(SUPPL.):1–5. Available from: [DOI] [PubMed] [Google Scholar]
- 29.Pt T., Ys C., Yw C., Ck W., Py L. Increased levels of vascular endothelial growth factor in patients with major depressive disorder: a meta-analysis. Eur. Neuropsychopharmacol. 2015 doi: 10.1016/j.euroneuro.2015.06.001. https://pubmed.ncbi.nlm.nih.gov/26123242/ Oct 1 [cited 2021 Oct 18];25(10):1622–30. Available from: [DOI] [PubMed] [Google Scholar]
- 30.Kaufman J., DeLorenzo C., Choudhury S., Parsey R.V. Eur Neuropsychopharmacol; 2016. The 5-HT1A Receptor in Major Depressive Disorder. Mar 1 [cited 2021 Nov 28];26(3):397–410. Available from: https://pubmed.ncbi.nlm.nih.gov/26851834/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jd C., S G., Cg A., Br B. A neurobiological hypothesis of treatment-resistant depression - mechanisms for selective serotonin reuptake inhibitor non-efficacy. Front. Behav. Neurosci. 2014 doi: 10.3389/fnbeh.2014.00189. https://pubmed.ncbi.nlm.nih.gov/24904340/ May 20 [cited 2021 Oct 18];8(MAY). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiles S.A., Attia J., Baker A.L. Brain Behav Immun; 2010 Aug. Changes in Interleukin-6, C-Reactive Protein and Interleukin-10 in People with Depression Following Antidepressant Treatment: A Meta-Analysis.https://www.infona.pl//resource/bwmeta1.element.elsevier-37f8d087-0f93-332e-836c-3baaf5ef77a4 [cited 2021 Nov 28];Supplement 1(24):S44. Available from: [DOI] [PubMed] [Google Scholar]
- 33.Hasler G., Drevets W.C., Manji H.K., Charney D.S. Neuropsychopharmacology; 2004 Oct. Discovering Endophenotypes for Major Depression. [cited 2021 Nov 28];29(10):1765–81. Available from: https://pubmed.ncbi.nlm.nih.gov/15213704/ [DOI] [PubMed] [Google Scholar]
- 34.Horowitz M.A., Zunszain P.A. Ann N Y Acad Sci; 2015 Sep 1. Neuroimmune and Neuroendocrine Abnormalities in Depression: Two Sides of the Same Coin.https://pubmed.ncbi.nlm.nih.gov/25943397/ [cited 2021 Nov 28];1351(1):68–79. Available from: [DOI] [PubMed] [Google Scholar]
- 35.Juruena M.F., Cleare A.J. 2007 May. [Overlap between Atypical Depression, Seasonal Affective Disorder and Chronic Fatigue Syndrome. Rev Bras Psiquiatr [Internet] [cited 2021 Nov 28];29 Suppl 1(SUPPL. 1). Available from: https://pubmed.ncbi.nlm.nih.gov/17546343/ [DOI] [PubMed] [Google Scholar]
- 36.Beauchaine T.P. 2009. The Role of Biomarkers and Endophenotypes in Prevention and Treatment of Psychopathological Disorders.https://pubmed.ncbi.nlm.nih.gov/19727417/ Biomark Med [Internet] [cited 2021 Nov 28];3(1):1–3. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacDonald K., Krishnan A., Cervenka E., Hu G., Guadagno E., Trakadis Y. Biomarkers for major depressive and bipolar disorders using metabolomics: a systematic review. Am. J. Med. Genet. Part B Neuropsychiatr Genet [Internet] 2019 doi: 10.1002/ajmg.b.32680. https://onlinelibrary.wiley.com/doi/full/10.1002/ajmg.b.32680 Mar 1 [cited 2021 Nov 28];180(2):122–37. Available from: [DOI] [PubMed] [Google Scholar]
- 38.Petroff O.A.C. Neuroscientist; 2002 Dec 29. GABA and Glutamate in the Human Brain.https://journals.sagepub.com/doi/abs/10.1177/1073858402238515 [cited 2021 Nov 28];8(6):562–73. Available from: [DOI] [PubMed] [Google Scholar]
- 39.Lu Y.R., Fu X.Y., Shi L.G., Jiang Y., Wu J.L., Weng X.J., et al. Decreased plasma neuroactive amino acids and increased nitric oxide levels in melancholic major depressive disorder. BMC Psychiatr. 2014 doi: 10.1186/1471-244X-14-123. https://link.springer.com/articles/10.1186/1471-244X-14-123 [Internet] Apr 27 [cited 2021 Nov 28];14(1):1–7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang W., Meng X., Huang Y., Liu S., Zhu A., Li P., et al. Med Sci Monit; 2019. Nitric Oxide and Cyclic Guanosine Monophosphate Signaling Mediates the Antidepressant Effects of Acupuncture in the Rat Model of Chronic Unpredictable Mild Stress. Nov 30 [cited 2021 Nov 28];25:9112. Available from:/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altamura C., Maes M., Dai J., Meltzer H.Y. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur. Neuropsychopharmacol. 1995;5(SUPPL. 1):71–75. doi: 10.1016/0924-977x(95)00033-l. Jan 1. [DOI] [PubMed] [Google Scholar]
- 42.Ji Y., Hebbring S., Zhu H., Jenkins G.D., Biernacka J., Snyder K., et al. Glycine and a Glycine dehydrogenase (GLDC) SNP as citalopram/escitalopram response biomarkers in depression: pharmacometabolomics-informed pharmacogenomics. Clin. Pharmacol. Ther. 2011 doi: 10.1038/clpt.2010.250. Jan 1 [cited 2021 Nov 28];89(1):97–104. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen R.H., Yang L.J., Hamdoun S., Chung S.K., Lam C.W., Zhang K.X., et al. 1,2,3,4,6-Pentagalloyl glucose, a RBD-ACE2 binding inhibitor to prevent SARS-CoV-2 infection. Front. Pharmacol. 2021:150. doi: 10.3389/fphar.2021.634176. Mar 4;0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen G., Yang D., Yang Y., Li J., Cheng K., Tang G., et al. Amino acid metabolic dysfunction revealed in the prefrontal cortex of a rat model of depression. Behav. Brain Res. 2015;278:286–292. doi: 10.1016/j.bbr.2014.05.027. Feb 1. [DOI] [PubMed] [Google Scholar]
- 45.Zhong S., Lai S., Yue J., Wang Y., Shan Y., Liao X., et al. The characteristic of cognitive impairments in patients with bipolar II depression and its association with N-acetyl aspartate of the prefrontal white matter. Ann. Transl. Med. 2020 Nov doi: 10.21037/atm-20-7098. pmc/articles/PMC7723520/ [cited 2021 Nov 28];8(21):1457–1457. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitriol V., Cancino A., Leiva-Bianchi M., Serrano C., Ballesteros S., Asenjo A., et al. 2016. Childhood Trauma and Psychiatric Comorbidities in Patients with Depressive Disorder in Primary Care in Chile.https://www.tandfonline.com/doi/abs/10.1080/15299732.2016.1212449 Mar 15 [cited 2021 Nov 28];18(2):189–205. Available from: [DOI] [PubMed] [Google Scholar]
- 47.SR D., Nm C., Tl R., S P. 2008 Apr. Research Applications of Magnetic Resonance Spectroscopy to Investigate Psychiatric Disorders.https://pubmed.ncbi.nlm.nih.gov/19363431/ [cited 2021 Oct 18];19(2):81–96. Available from: Top Magn Reson Imaging [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feyissa A.M., Chandran A., Stockmeier C.A., Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2009;(1):70. doi: 10.1016/j.pnpbp.2008.10.005. pmc/articles/PMC2655629/ [Internet] Feb 1 [cited 2021 Nov 28];33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.dos Santos M.C.T., Barreto-Sanz M.A., Correia B.R.S., Bell R., Widnall C., Perez L.T., et al. miRNA-based signatures in cerebrospinal fluid as potential diagnostic tools for early stage Parkinson's disease. Oncotarget. 2018;9(25) doi: 10.18632/oncotarget.24736. pmc/articles/PMC5915128/ Apr 1 [cited 2021 Nov 28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duman R.S. Depress Anxiety; 2014. Neurobiology of Stress, Depression, and Rapid Acting Antidepressants: Remodeling Synaptic Connections. [cited 2021 Nov 28];31(4):291. Available from:/ pmc/articles/PMC4432471/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emamzadeh F.N. Alpha-synuclein structure, functions, and interactions. J Res Med Sci [Internet. 2016 doi: 10.4103/1735-1995.181989. [cited 2021 Nov 28];21(2). Available from:/ pmc/articles/PMC5122110/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunlop B.W., Mayberg H.S. Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues Clin. Neurosci. 2014 doi: 10.31887/DCNS.2014.16.4/bdunlop. pmc/articles/PMC4336918/ [cited 2021 Nov 28];16(4):479. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Felger J.C., Lotrich F.E. Neuroscience; 2013. Inflammatory Cytokines in Depression: Neurobiological Mechanisms and Therapeutic Implications. Aug 29 [cited 2021 Nov 28];246:199. Available from:/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernandes B.S., Gama C.S., Kauer-Sant’Anna M., Lobato M.I., Belmonte-de-Abreu P., Kapczinski F. Serum brain-derived neurotrophic factor in bipolar and unipolar depression: a potential adjunctive tool for differential diagnosis. J. Psychiatr. Res. 2009 Oct doi: 10.1016/j.jpsychires.2009.04.010. https://pubmed.ncbi.nlm.nih.gov/19501841/ [cited 2021 Nov 28];43(15):1200–4. Available from: [DOI] [PubMed] [Google Scholar]
- 55.Castrén E., Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol. 2010 Apr doi: 10.1002/dneu.20758. [cited 2021 Nov 28];70(5):289–97. Available from: https://pubmed.ncbi.nlm.nih.gov/20186711/ [DOI] [PubMed] [Google Scholar]
- 56.Clark-Raymond A., Halaris A. 2013. VEGF and Depression: a Comprehensive Assessment of Clinical Data.https://pubmed.ncbi.nlm.nih.gov/23684549/ J Psychiatr Res [Internet] [cited 2021 Nov 28];47(8):1080–7. Available from: [DOI] [PubMed] [Google Scholar]
- 57.Malberg J.E., Monteggia L.M. 2008. VGF, a New Player in Antidepressant Action? Sci Signal. May 6 [cited 2021 Nov 28];1(18):pe19. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernard R., Kerman I.A., Thompson R.C., Jones E.G., Bunney W.E., Barchas J.D., et al. Altered expression of glutamate signaling, growth factor and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry. 2011 Jun;(6):634. doi: 10.1038/mp.2010.44. pmc/articles/PMC2927798/ [cited 2021 Nov 28];16. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turner C.A., Akil H., Watson S.J., Evans S.J. The fibroblast growth factor system and mood disorders. Biol. Psychiatr. 2006 doi: 10.1016/j.biopsych.2006.02.026. https://pubmed.ncbi.nlm.nih.gov/16631131/ Jun 15 [cited 2021 Nov 28];59(12):1128–35. Available from: [DOI] [PubMed] [Google Scholar]
- 60.Evans S.J., Choudary P.V., Neal C.R., Li J.Z., Vawter M.P., Tomita H., et al. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci U S A. 2004;101(43) doi: 10.1073/pnas.0406788101. Oct 26 [cited 2021 Nov 28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Irwin M.R., Miller A.H. Brain Behav Immun; 2007 May. Depressive Disorders and Immunity: 20 Years of Progress and Discovery.https://pubmed.ncbi.nlm.nih.gov/17360153/ [cited 2021 Nov 28];21(4):374–83. Available from: [DOI] [PubMed] [Google Scholar]
- 62.Smith R.S. 1991. The Macrophage Theory of Depression.https://pubmed.ncbi.nlm.nih.gov/1943879/ Med Hypotheses [Internet] [cited 2021 Nov 28];35(4):298–306. Available from: [DOI] [PubMed] [Google Scholar]
- 63.Gaughran F., Payne J., Sedgwick P.M., Cotter D., Berry M. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res. Bull. 2006 doi: 10.1016/j.brainresbull.2006.04.008. Jul 31 [cited 2021 Nov 28];70(3):221–7. Available from: https://pubmed.ncbi.nlm.nih.gov/16861106/ [DOI] [PubMed] [Google Scholar]
- 64.Zhou Q., Lv X., Zhou S., Liu Q., Tian H., Zhang K., et al. Inflammatory cytokines, cognition, and response to antidepressant treatment in patients with major depressive disorder. Psychiatry Res. 2021 Nov 1;305 doi: 10.1016/j.psychres.2021.114202. [DOI] [PubMed] [Google Scholar]
- 65.Kazemi A., Noorbala A.A., Azam K., Djafarian K. Effect of prebiotic and probiotic supplementation on circulating pro-inflammatory cytokines and urinary cortisol levels in patients with major depressive disorder: a double-blind, placebo-controlled randomized clinical trial. J. Funct.Foods. 2019;52:596–602. Jan 1. [Google Scholar]
- 66.Caldieraro M.A., McKee M., Leistner-Segal S., Vares E.A., Kubaski F., Spanemberg L., et al. 2017. Val66Met Polymorphism Association with Serum BDNF and Inflammatory Biomarkers in Major Depression. Jul 4 [cited 2021 Nov 28];19(5):402–9. Available f. [DOI] [PubMed] [Google Scholar]
- 67.Lim J., Sohn H., Kwon M.S., Kim B. Clin Psychopharmacol Neurosci; 2021. White Matter Alterations Associated with Pro-inflammatory Cytokines in Patients with Major Depressive Disorder. Aug 31 [cited 2021 Nov 28];19(3):449–58. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han K.M., Tae W.S., Kim A., Kang Y., Kang W., Kang J., et al. Serum FAM19A5 levels: a novel biomarker for neuroinflammation and neurodegeneration in major depressive disorder. Brain Behav. Immun. 2020;87:852–859. doi: 10.1016/j.bbi.2020.03.021. Jul 1. [DOI] [PubMed] [Google Scholar]
- 69.Mosiołek A., Pięta A., Jakima S., Zborowska N., Mosiołek J., Szulc A. Effects of antidepressant treatment on peripheral biomarkers in patients with major depressive disorder (MDD) J. Clin. Med. 2021;10 doi: 10.3390/jcm10081706. https://www.mdpi.com/2077-0383/10/8/1706/htm Page 1706 [Internet]. 2021 Apr 15 [cited 2021 Nov 28];10(8):1706. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chávez-Castillo M., Núñez V., Nava M., Ortega Á., Rojas M., Bermúdez V., et al. Depression as a neuroendocrine disorder: emerging neuropsychopharmacological approaches beyond monoamines. Adv Pharmacol Sci. 2019;2019 doi: 10.1155/2019/7943481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strawbridge R., Young A.H., Cleare A.J. Neuropsychiatr Dis Treat [Internet; 2017. Biomarkers for Depression: Recent Insights, Current Challenges and Future Prospects. May 10 [cited 2021 Nov 28];13:1245–62. Available from:/record/2017-21535-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmidt H.D., Shelton R.C., Duman R.S. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacol. 2011 doi: 10.1038/npp.2011.151. https://www.nature.com/articles/npp2011151 3612 [Internet]. 2011 Aug 3 [cited 2021 Nov 28];36(12):2375–94. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pariante C.M. Ann N Y Acad Sci; 2009. Risk Factors for Development of Depression and Psychosis: Glucocorticoid Receptors and Pituitary Implications for Treatment with Antidepressant and Glucocorticoids. [cited 2021 Nov 28];1179:144. Available from:/ pmc/articles/PMC2982725/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gold P.W. Endocrine factors in key structural and intracellular changes in depression. Trends Endocrinol Metab. 2021 doi: 10.1016/j.tem.2021.01.003. http://www.cell.com/article/S1043276021000199/fulltext Apr 1 [cited 2021 Nov 28];32(4):212–23. Available from: [DOI] [PubMed] [Google Scholar]
- 75.C S., Ge M. 2011 Feb. Depression and Hypothalamic-Pituitary-Adrenal Activation: a Quantitative Summary of Four Decades of Research.https://pubmed.ncbi.nlm.nih.gov/21257974/ Psychosom Med [Internet] [cited 2021 Nov 6];73(2):114–26. Available from: [DOI] [PubMed] [Google Scholar]
- 76.A H.V., V D.A., A P R.S., T W., Ah Y., et al. The relationship between cortisol, stress and psychiatric illness: new insights using hair analysis. J. Psychiatr. Res. 2015 doi: 10.1016/j.jpsychires.2015.08.007. https://pubmed.ncbi.nlm.nih.gov/26424422/ Nov 1 [cited 2021 Nov 6];70:38–49. Available from: [DOI] [PubMed] [Google Scholar]
- 77.Cp C., D S., Cm S., Jd M., Rm S. Increased oxidative stress in patients with depression and its relationship to treatment. Psychiatry Res. 2013 doi: 10.1016/j.psychres.2012.10.018. https://pubmed.ncbi.nlm.nih.gov/23245537/ Apr 30 [cited 2021 Nov 6];206(2–3):213–6. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.S F R.S., Ah V., Aj C. Cortisol as a predictor of psychological therapy response in depressive disorders: systematic review and meta-analysis. Br. J. Psychiatry. 2017 doi: 10.1192/bjp.bp.115.180653. Feb 1 [cited 2021 Nov 6];210(2):105–9. Available from: https://pubmed.ncbi.nlm.nih.gov/27908897/ [DOI] [PubMed] [Google Scholar]
- 79.Kj P., Af S., Dm L. 2003. Neuroendocrine Aspects of Hypercortisolism in Major Depression.https://pubmed.ncbi.nlm.nih.gov/12614635/ [cited 2021 Nov 6];43(1):60–6. Available from: [DOI] [PubMed] [Google Scholar]
- 80.C A., Pa Z., LA C., Cm P. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011 Apr doi: 10.1016/j.psyneuen.2010.03.007. [cited 2021 Nov 6];36(3):415–25. Available from: https://pubmed.ncbi.nlm.nih.gov/20399565/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aagaard K., Petrosino J., Keitel W., Watson M., Katancik J., Garcia N., et al. The Human Microbiome Project strategy for comprehensive sampling of the human microbiome and why it matters. FASEB J. 2013 doi: 10.1096/fj.12-220806. Mar [cited 2021 Nov 28];27(3):1012. Available from:/ pmc/articles/PMC3574278/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rs D., B V. Trends Neurosci; 2012 Jan. Signaling Pathways Underlying the Pathophysiology and Treatment of Depression: Novel Mechanisms for Rapid-Acting Agents. [cited 2021 Nov 6];35(1):47–56. Available from: https://pubmed.ncbi.nlm.nih.gov/22217452/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Da W M.K. Psychoneuroendocrinology; 2015. Ghrelin in Psychiatric Disorders - A Review.https://pubmed.ncbi.nlm.nih.gov/25459900/ [cited 2021 Nov 6];52(1):176–94. Available from: [DOI] [PubMed] [Google Scholar]
- 84.C K N.S., Sh G., U R., M T D.S., et al. 2013 Feb. A Systematic Review and Meta-Analysis of the Association between Depression and Insulin Resistance. Diabetes Care [Internet] [cited 2021 Nov 6];36(2):480–9. Available from: https://pubmed.ncbi.nlm.nih.gov/23349152/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.X L., J L., P Z., X Z., C Z., C H., et al. Plasma lipidomics reveals potential lipid markers of major depressive disorder. Anal Bioanal Chem [Internet. 2016 doi: 10.1007/s00216-016-9768-5. https://pubmed.ncbi.nlm.nih.gov/27457104/ Sep 1 [cited 2021 Oct 18];408(23):6497–507. Available from: [DOI] [PubMed] [Google Scholar]
- 86.Pj L., Rj A., Ke F., M de G., Rm C., Re C. 2000. Depression and Poor Glycemic Control: a Meta-Analytic Review of the Literature.https://pubmed.ncbi.nlm.nih.gov/10895843/ Diabetes Care [Internet] [cited 2021 Nov 6];23(7):934–42. Available from: [DOI] [PubMed] [Google Scholar]
- 87.M M. Evidence for an immune response in major depression: a review and hypothesis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1995 doi: 10.1016/0278-5846(94)00101-m. https://pubmed.ncbi.nlm.nih.gov/7708925/ [cited 2021 Oct 18];19(1):11–38. Available from: [DOI] [PubMed] [Google Scholar]
- 88.Pan A., Keum N., Okereke O.I., Sun Q., Kivimaki M., Rubin R.R., et al. Bidirectional association between depression and metabolic syndrome. Diabetes Care. 2012 doi: 10.2337/dc11-2055. May 1 [cited 2021 Nov 6];35(5):1171–80. Available from: https://care.diabetesjournals.org/content/35/5/1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.H Z., P Z., L Z., J J., S T., P X., et al. Predictive diagnosis of major depression using NMR-based metabolomics and least-squares support vector machine. Clin. Chim. Acta. 2017 Jan 1 doi: 10.1016/j.cca.2016.11.039. https://pubmed.ncbi.nlm.nih.gov/27931880/ [cited 2021 Nov 6];464:223–7. Available from: [DOI] [PubMed] [Google Scholar]
- 90.F L N.V., Kr M., de J P., At B., Bw P. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. 2013 Jun doi: 10.1038/mp.2012.144. [cited 2021 Nov 6];18(6):692–9. Available from: https://pubmed.ncbi.nlm.nih.gov/23089630/ [DOI] [PubMed] [Google Scholar]
- 91.Carvalho A.F., Rocha D.Q.C., McIntyre R.S., Mesquita L.M., Köhler C.A., Hyphantis T.N., et al. Adipokines as emerging depression biomarkers: a systematic review and meta-analysis. J. Psychiatr. Res. 2014;59:28–37. doi: 10.1016/j.jpsychires.2014.08.002. Dec 1. [DOI] [PubMed] [Google Scholar]
- 92.T L., S Z., X L., J C., T H., S L., et al. PLoS One; 2015. A Meta-Analysis of Oxidative Stress Markers in Depression.https://pubmed.ncbi.nlm.nih.gov/26445247/ Oct 7 [cited 2021 Nov 6];10(10). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pg F., Jd M., A P., R T., G G., P D., et al. Phosphorylated α-synuclein can be detected in blood plasma and is potentially a useful biomarker for Parkinson's disease. FASEB J. 2011 Dec doi: 10.1096/fj.10-179192. [cited 2021 Nov 6];25(12):4127–37. Available from: https://pubmed.ncbi.nlm.nih.gov/21865317/ [DOI] [PubMed] [Google Scholar]
- 94.R F S.O., Sy L., Im H., J van P., Sg van D., et al. 2007 Jun. Hypocretin (Orexin) Loss in Parkinson's Disease.https://pubmed.ncbi.nlm.nih.gov/17470494/ Brain [Internet] [cited 2021 Oct 18];130(Pt 6):1577–85. Available from: [DOI] [PubMed] [Google Scholar]
- 95.Maes M., Twisk F.N.M., Ringel K. Inflammatory and cell-mediated immune biomarkers in myalgic encephalomyelitis/chronic fatigue syndrome and depression: inflammatory markers are higher in myalgic encephalomyelitis/chronic fatigue syndrome than in depression. Psychother. Psychosom. 2012 Aug;81(5):286–295. doi: 10.1159/000336803. [DOI] [PubMed] [Google Scholar]
- 96.Mj F., Ge M. Psychosom Med; 2006 Jan. Increased Serum Levels of 8-Hydroxy-2’-Deoxyguanosine in Clinical Depression.https://pubmed.ncbi.nlm.nih.gov/16449405/ [cited 2021 Oct 18];68(1):1–7. Available from: [DOI] [PubMed] [Google Scholar]
- 97.A J., J K K.M., Tg B., Lv K., A F.-J., et al. Systemic oxidatively generated DNA/RNA damage in clinical depression: associations to symptom severity and response to electroconvulsive therapy. J. Affect. Disord. 2013 Jul doi: 10.1016/j.jad.2013.02.011. https://pubmed.ncbi.nlm.nih.gov/23497793/ [cited 2021 Nov 6];149(1–3):355–62. Available from: [DOI] [PubMed] [Google Scholar]
- 98.T I., C C., K I., Y I., H I R.T., et al. Association of STAI and SDS scores with 8-hydroxydeoxyguanosine and serotonin levels in young women with depressive symptoms. J. Neuropsychiatry Clin. Neurosci. 2011 doi: 10.1176/jnp.23.1.jnpe10. https://pubmed.ncbi.nlm.nih.gov/21304109/ [Internet] [cited 2021 Nov 6];23(1). Available from: [DOI] [PubMed] [Google Scholar]
- 99.S Y., A N., Y M., H K., K K., T M. Psychiatry Res; 2012. Depressive Symptoms and Oxidative DNA Damage in Japanese Municipal Employees.https://pubmed.ncbi.nlm.nih.gov/22732398/ Dec 30 [cited 2021 Nov 6];200(2–3):318–22. Available from: [DOI] [PubMed] [Google Scholar]
- 100.Chocano-Bedoya P.O., Mirzaei F., O'Reilly E.J., Lucas M., Okereke O.I., Hu F.B., et al. C- reactive protein, interleukin-6, soluble tumor necrosis factor α receptor 2 and incident clinicaldepression. J. Affect. Disord. 2014;163:25–32. doi: 10.1016/j.jad.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Copeland W.E., Shanahan L., Worthman C., Angold A., Costello E.J. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol. Psychiatr. 2012;71:15–21. doi: 10.1016/j.biopsych.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haastrup E., Grau K., Eugen-Olsen J., Thorball C., Kessing L.V., Ullum H. Soluble urokinase plasminogen activator receptor as a marker for use of antidepressants. PLoS One. 2014 doi: 10.1371/journal.pone.0110555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khandaker G.M., Pearson R.M., Zammit S., Lewis G., Jones P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatr. 2014;71:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pasco J.A., Nicholson G.C., Williams L.J., Jacka F.N., Henry M.J., Kotowicz M.A., et al. Association of high-sensitivity C-reactive protein with de novo major depression. Br. J. Psychiatry. 2010;197:372–377. doi: 10.1192/bjp.bp.109.076430. [DOI] [PubMed] [Google Scholar]
- 105.Pasquali M.A., Harlow B.L., Soares C.N., Otto M.W., Cohen L.S., Minuzzi L., et al. A longitudinal study of neurotrophic, oxidative, and inflammatory markers in first-onset depression in midlife women. Eur Arch Psychiatry Clin Neurosci. 2017;268:771–781. doi: 10.1007/s00406-017-0812-z. [DOI] [PubMed] [Google Scholar]
- 106.Rudaz D.A., Vandeleur C.L., Gebreab S.Z., Gholam-Rezaee M., Strippoli M.-P.F., Lasserre A.M., et al. Partially distinct combinations of psychological, metabolic and inflammatory risk factors are prospectively associated with the onset of the subtypes of Major Depressive Disorder inmidlife. J. Affect. Disord. 2017;222:195–203. doi: 10.1016/j.jad.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 107.Glaus J., von Känel R., Lasserre A.M., lok M.P.F., Vandeleur C.L., Castelao E., et al. Mood disorders and circulating levels of inflammatory markers in a longitudinal population-based study. Psychol. Med. 2018;48:961–973. doi: 10.1017/S0033291717002744. [DOI] [PubMed] [Google Scholar]
- 108.Campo J.V., Dahl R.E., Williamson D.E., Birmaher B., Perel J.M., Ryan N.D. Gastrointestinaldistress to serotonergic challenge: a risk marker for emotional disorder? J. Am. Acad. Child Adolesc. Psychiatry. 2003;42:1221–1226. doi: 10.1097/00004583-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 109.Serafini G., Parisi V.M., Aguglia A., Amerio A., Sampogna G., Fiorillo A., et al. A specific inflammatory profile underlying suicide risk? Systematic review of the main literature findings. Int. J. Environ. Res. Publ. Health. 2020;17:2393. doi: 10.3390/ijerph17072393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Serafini G., Adavastro G., Canepa G., De Berardis D., Valchera A., Pompili M., et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int. J. Mol. Sci. 2018;19:2410. doi: 10.3390/ijms19082410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1
Data Availability Statement
Data included in article/supp. material/referenced in article.