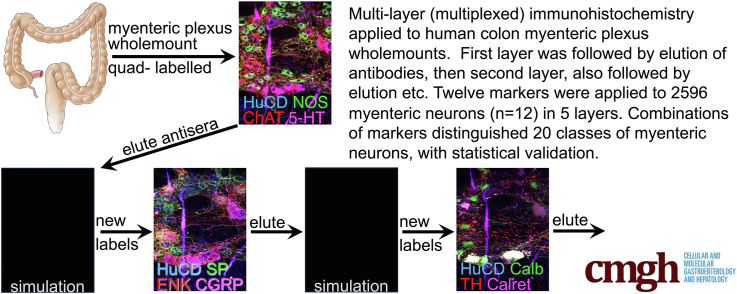
Abstract
Background and Aims
Gut functions including motility, secretion, and blood flow are largely controlled by the enteric nervous system. Characterizing the different classes of enteric neurons in the human gut is an important step to understand how its circuitry is organized and how it is affected by disease.
Methods
Using multiplexed immunohistochemistry, 12 discriminating antisera were applied to distinguish different classes of myenteric neurons in the human colon (2596 neurons, 12 patients) according to their chemical coding. All antisera were applied to every neuron, in multiple layers, separated by elutions.
Results
A total of 164 combinations of immunohistochemical markers were present among the 2596 neurons, which could be divided into 20 classes, with statistical validation. Putative functions were ascribed for 4 classes of putative excitatory motor neurons (EMN1–4), 4 inhibitory motor neurons (IMN1–4), 3 ascending interneurons (AIN1–3), 6 descending interneurons (DIN1–6), 2 classes of multiaxonal sensory neurons (SN1–2), and a small, miscellaneous group (1.8% of total). Soma-dendritic morphology was analyzed, revealing 5 common shapes distributed differentially between the 20 classes. Distinctive baskets of axonal varicosities surrounded 45% of myenteric nerve cell bodies and were associated with close appositions, suggesting possible connectivity. Baskets of cholinergic terminals and several other types of baskets selectively targeted ascending interneurons and excitatory motor neurons but were significantly sparser around inhibitory motor neurons.
Conclusions
Using a simple immunohistochemical method, human myenteric neurons were shown to comprise multiple classes based on chemical coding and morphology and dense clusters of axonal varicosities were selectively associated with some classes.
Keywords: Enteric Nervous System, Antisera, Immunofluorescence, Classes
Graphical abstract
Summary.
Multiplexed immunohistochemistry for 12 markers was used to classify myenteric neurons of human colon. Twenty classes were distinguished with statistical validation. Cell morphology, soma size, and associations with axon terminals were quantified, providing a wide-ranging account of human myenteric plexus.
The enteric nervous system plays a major role in coordinating and controlling gut motility, secretion, blood flow, and immune function.1, 2, 3, 4 This is well illustrated by Hirschsprung’s disease in which there is aganglionosis of part of the colon, which is amotile.5 Characterizing the different classes of neurons in human gut is an important step in understanding how its circuitry is organized, how it changes in disease and how it may be targeted therapeutically. Early approaches to distinguish classes were based on soma-dendritic morphology and axonal projections.6 Physiological characteristics have also been used in animal preparations, measuring firing properties and responses to current or voltage steps enabling several types to be distinguished.7 A third broad approach studies combinations of molecular features including neurotransmitters, receptors, enzymes, structural proteins, etc. detected by immunohistochemical methods. This chemical coding approach has discriminated major functional classes of neurons in the enteric nervous system of small laboratory animals.8 In recent times, transcriptomic analysis at the single-cell level has greatly expanded chemical coding. Large numbers of individual cell bodies or nuclei can be isolated from human colon and analyzed for expression of neuron-specific messenger RNA (mRNA) markers9 with several thousand genes per neuron being assayed.
The attraction of using immunohistochemical coding to classify neurons is that it can be combined with studies of cell location, morphology, size, and projection without transgenic manipulation or use of viruses. In the past, immunohistochemical labeling was typically limited to 3–4 markers per preparation. This required data from many preparations to be combined to identify different classes, with attendant problems of summing errors. Nevertheless, this approach successfully distinguished 14 functional classes in the myenteric plexus of guinea pig small intestine8 based on numerous combinations of 8 antisera. In the present study, we tested whether larger numbers of discriminating immunohistochemical markers could be applied to individual cells to distinguish more classes without having to combine data from multiple preparations. A multiplexed labeling strategy was adapted with antibody elution between layers and a statistical method was developed to test for associations between markers based on a divisive hierarchical clustering. Cell body size and morphology were recorded as were visible baskets of axonal varicosities closely surrounding some nerve cell bodies. Published morphological, retrograde tracing, and immunohistochemical studies were then used to assign putative functional roles to each class. This wide-ranging account distinguished 20 different types of nerve cells in the human colonic myenteric plexus, based on a small number of discriminating immunohistochemical markers.
Results
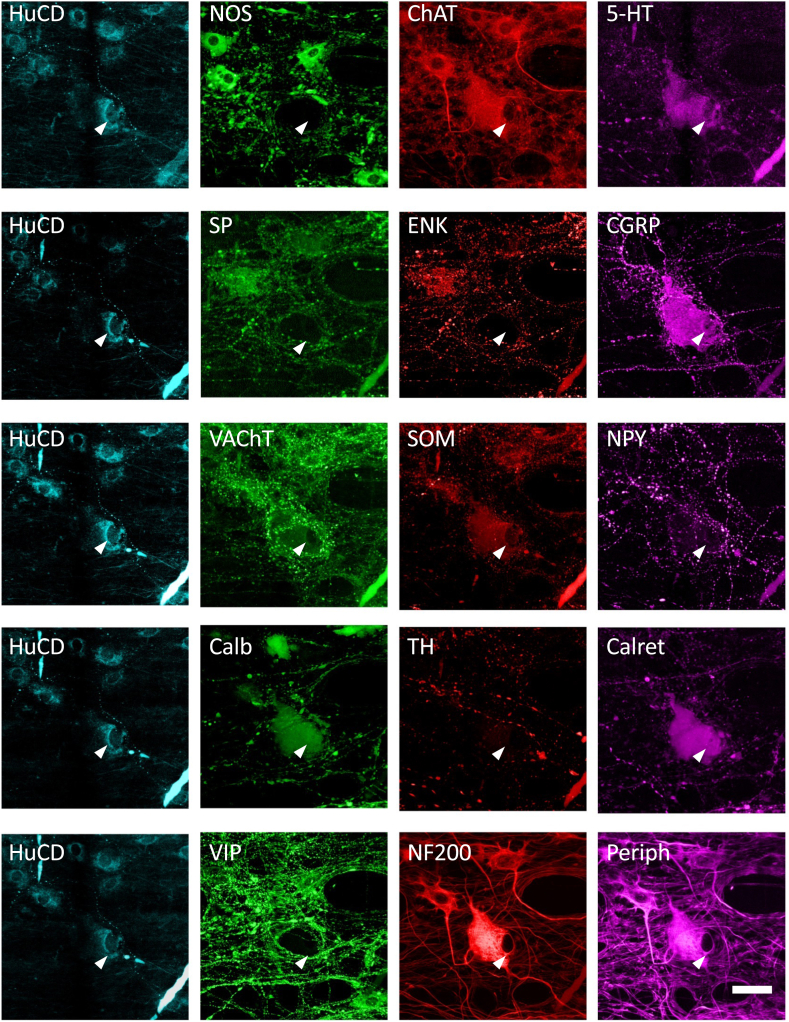
A total of 2596 myenteric neurons were subjected to multiplexed immunohistochemistry in ascending colon (n = 6 patients, 4 women, mean age 67.8 years [range, 60–73 years], 1303 cells) and descending colon (n = 6 patients, 2 women, mean age 71.8 years [range, 63–80 years], 1293 cells) (Table 1). All specimens were obtained from patients undergoing surgery for removal of non-obstructing cancers of the large bowel. Data from the 2 regions were initially pooled but treated separately when comparing between regions. Five layers of staining were applied, separated by antibody elutions, allowing every neuron to be scored as immunoreactive or nonimmunoreactive for 12 discriminating cell body markers: (layer 1: neuronal nitric oxide synthase [NOS], choline acetyltransferase [ChAT], 5-hydroxytryptamine [5-HT]; layer 2: substance P [SP], leu enkephalin [ENK], calcitonin gene-related peptide [CGRP]; layer 3: somatostatin [SOM], neuropeptide Y [NPY], Layer 4: calbindin [Calb], calretinin [Calret], Layer 5: vasoactive intestinal polypeptide [VIP], neurofilament 200 kD [NF200]). VAChT antisera were used for axonal labeling (in layer 3) but were not reliable for nerve cell bodies. Hu C or D protein (HuCD) was present in all 5 layers as a pan-neuronal marker10 to distinguish every myenteric nerve cell body. Additional markers (tyrosine hydroxylase and peripherin) were included for some preparations but were not part of the analysis. The elution process (see Materials and Methods) completely removed immunohistochemical labeling by primary antisera coupled to fluorophore-linked secondary antisera (Figure 1). However primary antisera bound to biotinylated secondary antisera (revealed by fluorophore-linked streptavidin) were highly resistant to elution.11 Biotinylated secondary antisera were reserved for HuCD, visualized with biotin-streptavidin-AMCA (see Figure 1). Based on the shape, size, and position in ganglia, each HuCD-labeled myenteric nerve cell body could be numbered and reliably reidentified in each layer of staining, obviating the need for precision alignment of layers. HuCD labeling was mostly confined to the cytoplasm of neural somata. Nuclear translocation of HuCD may be a sign of hypoxia and damage12 but was rarely seen in these specimens.
Table 1.
Details of Patients
| Patient | Region | Total Cells | Age | Sex |
|---|---|---|---|---|
| H2190 | Asc | 210 | 60 | F |
| H2192 | Asc | 227 | 70 | M |
| H2195 | Asc | 221 | 73 | F |
| H2203 | Asc | 214 | 66 | M |
| H2206 | Asc | 224 | 72 | F |
| H2211 | Asc | 207 | 66 | F |
| H2194 | Desc | 246 | 80 | M |
| H2201 | Desc | 213 | 63 | F |
| H2202 | Desc | 204 | 70 | M |
| H2208 | Desc | 210 | 66 | F |
| H2210 | Desc | 214 | 72 | M |
| H2226 | Desc | 206 | 80 | M |
| Total | 2596 | |||
| H2196 | Asc | 0 | 60 | F |
| H2383 | Desc | 0 | 78 | M |
| H2384 | Sigmoid | 0 | 58 | M |
Details of 15 patients from whom specimens were obtained. The top 12 patients provided tissue that was used for quantified multilayer immunohistochemistry; the 3 other patients' specimens were used for less systematic immunohistochemical staining.
Asc, ascending colon; Desc, descending colon; F, female; M, male; Sigmoid, sigmoid colon.
Figure 1.
Effects of antibody elution on immunohistochemical labeling. The top row shows typical multiple labeling with HuCD visualized with a biotinylated secondary and AMCA-labeled tertiary. Primaries for the other 3 markers, NOS, ChAT, and 5-HT, were visualized with fluorophore-labeled secondary antisera. The middle row shows the same ganglion after 60 minutes of elution. Note that the HuCD (with biotin-streptavidin complexes) is unaffected but labeling in the other 3 channels is abolished apart from faint autofluorescence of the ganglion and red blood cells. In the third layer, primary antisera to SP, ENK, and CGRP were applied and visualized with fluorophore-coupled secondary antisera, revealing quite different patterns of immunoreactivity from the top row (although ENK and ChAT largely coexist and the 5-HT cell is also immunoreactive for CGRP). Images were taken at exposures shown with false colors applied but no alterations to brightness or contrast.
Markers
ChAT and NOS are widely studied, functionally important markers because acetylcholine and nitric oxide are major excitatory and inhibitory motor neuron (IMN) transmitters in the gut13,14 and acetylcholine also plays a role in enteric neuro-neuronal transmission.15 Between them, ChAT and NOS labeled nearly all enteric neurons. Of the 2596 somata, 1083 (41.7%) were immunoreactive for NOS without ChAT (NOS+/ChAT–), 1237 (47.7%) were NOS–/ChAT+, 228 (8.8%) were NOS+/ChAT+, and 48 cells (1.8%) were NOS–/ChAT–. We tested whether the proportions of the 4 combinations differed between ascending and descending colon because the motility patterns of the 2 regions are quite distinct.16 No significant differences were detected (χ23= 6.525, P < .089) (Table 2); proportions were comparable to previous published studies on myenteric neurons in human colon.10,17,18
Table 2.
Coexistence of NOS and ChAT in Colonic Myenteric Neurons
| Whole Colon (n = 12) | Ascending Colon (n = 6) | Descending Colon (n = 6) | |
|---|---|---|---|
| NOS+/ChAT+ | 8.8% (228) | 9.8% (128) | 7.7% (100) |
| NOS+/ChAT– | 41.7% (1083) | 42.8% (558) | 40.6% (525) |
| NOS–/ChAT+ | 47.7% (1237) | 45.7% (595) | 49.7% (642) |
| NOS–/ChAT– | 1.8% (48) | 1.7% (22) | 2.0% (26) |
Values are % (n). Combinations of the 2 functionally important markers, ChAT and NOS, defined 4 populations of neurons that did not differ in proportion between ascending colon (n = 6) and descending colon (n = 6).
ChAT, choline acetyltransferase; NOS, neuronal nitric oxide synthase.
Other abundant markers were NF200 (51.7%), VIP (34.4%), and Calb (26.5%) (see Tables 3 and 4), whereas CGRP (1.8%) and SOM (1.7%) were scarcer, despite exposure to colchicine, which enhances neuropeptide immunoreactivity in nerve cell bodies.8 ENK, CGRP, and VIP were significantly more abundant in myenteric neurons in descending compared with ascending colon (Table 3). This may reflect differences in peptidergic neuromodulation between upper and lower colon. One marker (NF200) was expressed more frequently in myenteric neurons from male (n = 6) than female (n = 6) subjects (χ21 = 9.282, P < .044) (see Table 4); the functional significance of differences in this cytoskeletal marker is not clear.
Table 3.
Abundance of Individual Markers in Ascending and Descending Colon Compared
| Marker | In Ascending Colon | In Descending Colon | χ21 | Corrected P |
|---|---|---|---|---|
| NOS | 52.6% | 48.3% | 4.8240 | >.05 |
| ChAT | 55.5% | 57.4% | 0.9515 | >.05 |
| 5-HT | 2.4% | 3.5% | 2.7690 | >.05 |
| SP | 7.3% | 8.6% | 1.4871 | >.05 |
| ENK | 8.2% | 11.9% | 9.8167 | .0208a |
| CGRP | 0.9% | 2.8% | 12.4154 | .0051a |
| SOM | 1.3% | 2.2% | 2.8233 | >.05 |
| Calb | 25.1% | 27.9% | 2.6564 | >.05 |
| Calret | 8.7% | 10.8% | 2.9547 | >.05 |
| NF200 | 50.0% | 53.3% | 2.7426 | >.05 |
| VIP | 31.3% | 37.6% | 11.3170 | .0092a |
| NPY | 8.0% | 5.3% | 7.7754 | >.05 |
Comparison of overall frequency of 12 immunohistochemical markers between ascending (n = 6) and descending colon (n = 6) considered for each marker separately. From the cell counts, chi-square values were calculated. P values are shown after Bonferroni correction for multiple tests, revealing that 3 markers showed significant differences in abundance between upper and lower colon.
5-HT, 5-hydroxytryptamine; Calb, calbindin; Calret, calretinin; CGRP, calcitonin gene-related peptide; ChAT, choline acetyltransferase; ENK, leu enkephalin; NF200, neurofilament 200 kD; NOS, neuronal nitric oxide synthase; NPY, neuropeptide Y; SOM, somatostatin; SP, substance P; VIP, vasoactive intestinal polypeptide.
Significant (P < .05).
Table 4.
Expression of 12 Markers for Nerve Cell Bodies, by Sex
| All Cells in Whole Colon (n = 12) | Female (n = 6) | Male (n = 6) | P | |
|---|---|---|---|---|
| NOS | 50.5% | 51.8% | 49.2% | NS |
| ChAT | 56.4% | 55.9% | 57.0% | NS |
| 5-HT | 2.9% | 2.1% | 3.7% | NS |
| SP | 7.9% | 10.7% | 5.3% | NS |
| ENK | 10.1% | 12.1% | 8.1% | NS |
| CGRP | 1.8% | 1.8% | 1.9% | NS |
| SOM | 1.7% | 2.3% | 1.1% | NS |
| Calb | 26.5% | 25.4% | 27.6% | NS |
| Calret | 9.7% | 10.4% | 9.2% | NS |
| NF200 | 51.7% | 49.9% | 53.4% | .044a |
| VIP | 34.4% | 34.1% | 34.8% | NS |
| NPY | 6.6% | 7.2% | 6.0% | NS |
The percentage of myenteric neurons expressing each of the markers averaged across all subjects (n = 12) combining cells from ascending and descending colon but comparing females (n = 6) and males (n = 6). NF200 showed a small but significant difference in expression between the sexes, with a higher predominance in males (asterisks). Bonferroni corrections were employed to correct for multiple comparisons.
5-HT, 5-hydroxytryptamine; Calb, calbindin; Calret, calretinin; CGRP, calcitonin gene-related peptide; ChAT, choline acetyltransferase; ENK, leu enkephalin; NF200, neurofilament 200 kD; NOS, neuronal nitric oxide synthase; NPY, neuropeptide Y; NS, not significant; SOM, somatostatin; SP, substance P; VIP, vasoactive intestinal polypeptide.
Significant (P < .05).
Combinations of Markers
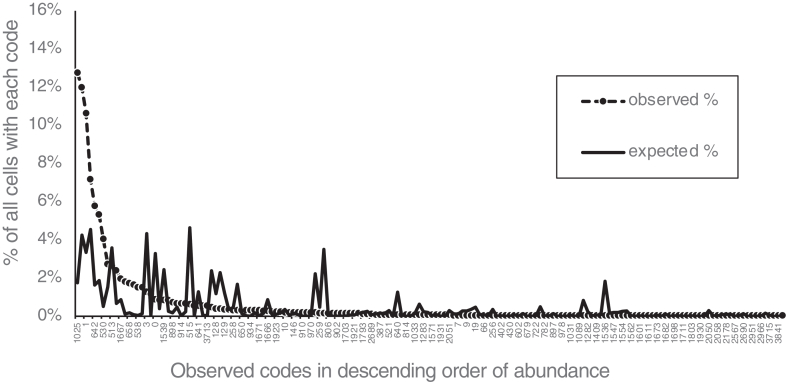
Chemical coding was reflected in the combinations of the 12 cell body markers expressed by each neuron. To keep track of different combinations, a naming system was devised based on a numerical code from 0 to 4095, calculated from the presence or absence of the 12 markers, in the order applied. Thus, NOS+ was given a score of 1, ChAT+ was given a score of 2, 5-HT+ was given a score of 4, SP+ was given a score of 8, ENK+ was given a score of 16, CGRP+ was given a score of 32 etc.; non-immunoreactive markers all scored 0. The scores for all 12 markers expressed by each cell were summed. Thus, a cell immunoreactive for ChAT and ENK (and no other markers) would have a code of 18 (2+16). Code numbers were influenced by the order of antisera used and thus are specific to this study. From the frequencies of each marker (eg, 56.4% of neurons were ChAT+, 10.1% of cells were ENK+), it was calculated that 333 ± 7 different combinations/codes would be expected from a sample of 2596 neurons, if markers were randomly assorted. In fact, 164 different codes were identified, of which 77 were limited to a single neuron (see Table 5). A few codes accounted for very large proportions. For example, cells immunoreactive for just NOS (lacking the 11 other markers: code = #1) accounted for 276 (10.6%) of 2596 neurons. Neurons with NOS+/VIP+ (and no other markers; code: #1025) were the most abundant (331 cells [12.8%]). Based on marker frequency, these 2 codes would be expected to account for 3.3% and 1.8% of all neurons, respectively, if markers were randomly assorted. These data are summarized in Figure 2. On average, each neuron expressed 2.6 ± 1.4 (range, 0–9; n = 2596) of the 12 discriminating markers. Overall, this leads to the conclusion that markers were not independently assorted between cells but tend to be found in specific combinations, as predicted by the chemical coding concept.
Table 5.
Total of 164 Combinations of Markers (Codes) Identified in 2596 Neurons
| Code | Immunoreactivity | No. Cells (n = 2596) | % of Cells | Code | Immunoreactivity | No. Cells of 2596 cells | % of 2596 cells | Code | Immunoreactivity | No. Cells (n = 2596) | % of Cells | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | No markers | 23 | 0.89% | 714 | ChAT+/SP+/SOM+/Calb+/NF200+ | 2 | 0.08% | 1631 | NOS+/ChAT+/5-HT+/SP+/ENK+/SOM+/NF200+/VIP+ | 1 | 0.04% | ||
| 1 | NOS+ | 276 | 10.63% | 722 | ChAT+/ENK+/SOM+/Calb+/NF200+ | 1 | 0.04% | 1665 | NOS+/Calb+/NF200+/VIP+ | 62 | 2.39% | ||
| 2 | ChAT+ | 311 | 11.98% | 771 | NOS+/ChAT+/Calret+/NF200+ | 1 | 0.04% | 1666 | ChAT+/Calb+/NF200+/VIP+ | 7 | 0.27% | ||
| 3 | NOS+/ChAT+ | 33 | 1.27% | 778 | ChAT+/SP+/Calret+/NF200+ | 14 | 0.54% | 1667 | NOS+/ChAT+/Calb+/NF200+/VIP+ | 51 | 1.96% | ||
| 6 | ChAT+/5-HT+ | 1 | 0.04% | 782 | ChAT+/5-HT+/SP+/Calret+/NF200+ | 1 | 0.04% | 1671 | NOS+/ChAT+/5-HT+/Calb+/NF200+/VIP+ | 8 | 0.31% | ||
| 7 | NOS+/ChAT+/5-HT+ | 1 | 0.04% | 806 | ChAT+/5-HT+/CGRP+/Calret+/NF200+ | 4 | 0.15% | 1673 | NOS+/SP+/Calb+/NF200+/VIP+ | 1 | 0.04% | ||
| 8 | SP+ | 1 | 0.04% | 814 | ChAT+/5-HT+/SP+/CGRP+/Calret+/NF200+ | 2 | 0.08% | 1675 | NOS+/ChAT+/SP+/Calb+/NF200+/VIP+ | 2 | 0.08% | ||
| 9 | NOS+/SP+ | 1 | 0.04% | 842 | ChAT+/SP+/SOM+/Calret+/NF200+ | 4 | 0.15% | 1679 | NOS+/ChAT+/5-HT+/SP+/Calb+/NF200+/VIP+ | 1 | 0.04% | ||
| 10 | ChAT+/SP+ | 5 | 0.19% | 870 | ChAT+/5-HT+/CGRP+/SOM+/Calret+/NF200+ | 1 | 0.04% | 1682 | ChAT+/ENK+/Calb+/NF200+/VIP+ | 1 | 0.04% | ||
| 16 | ENK+ | 1 | 0.04% | 897 | NOS+/Calb+/Calret+/NF200+ | 1 | 0.04% | 1695 | NOS+/ChAT+/5-HT+/SP+/ENK+/Calb+/NF200+/VIP+ | 1 | 0.04% | ||
| 18 | ChAT+/ENK+ | 17 | 0.65% | 898 | ChAT+/Calb+/Calret+/NF200+ | 19 | 0.73% | 1698 | ChAT+/CGRP+/Calb+/NF200+/VIP+ | 1 | 0.04% | ||
| 19 | NOS+/ChAT+/ENK+ | 1 | 0.04% | 902 | ChAT+/5-HT+/Calb+/Calret+/NF200+ | 4 | 0.15% | 1699 | NOS+/ChAT+/CGRP+/Calb+/NF200+/VIP+ | 1 | 0.04% | ||
| 26 | ChAT+/SP+/ENK+ | 5 | 0.19% | 906 | ChAT+/SP+/Calb+/Calret+/NF200+ | 30 | 1.16% | 1703 | NOS+/ChAT+/5-HT+/CGRP+/Calb+/NF200+/VIP+ | 4 | 0.15% | ||
| 34 | ChAT+/CGRP+ | 1 | 0.04% | 910 | ChAT+/5-HT+/SP+/Calb+/Calret+/NF200+ | 5 | 0.19% | 1711 | NOS+/ChAT+/5-HT+/SP+/CGRP+/Calb+/NF200+/VIP+ | 1 | 0.04% | ||
| 66 | ChAT+/SOM+ | 1 | 0.04% | 914 | ChAT+/ENK+/Calb+/Calret+/NF200+ | 17 | 0.65% | 1793 | NOS+/Calret+/NF200+/VIP+ | 3 | 0.12% | ||
| 128 | Calb+ | 10 | 0.39% | 930 | ChAT+/CGRP+/Calb+/Calret+/NF200+ | 2 | 0.08% | 1795 | NOS+/ChAT+/Calret+/NF200+/VIP+ | 3 | 0.12% | ||
| 129 | NOS+/Calb+ | 9 | 0.35% | 934 | ChAT+/5-HT+/CGRP+/Calb+/Calret+/NF200+ | 8 | 0.31% | 1799 | NOS+/ChAT+/5-HT+/Calret+/NF200+/VIP+ | 1 | 0.04% | ||
| 130 | ChAT+/Calb+ | 71 | 2.73% | 938 | ChAT+/SP+/CGRP+/Calb+/Calret+/NF200+ | 1 | 0.04% | 1803 | NOS+/ChAT+/SP+/Calret+/NF200+/VIP+ | 1 | 0.04% | ||
| 138 | ChAT+/SP+/Calb+ | 2 | 0.08% | 942 | ChAT+/5-HT+/SP+/CGRP+/Calb+/Calret+/NF200+ | 5 | 0.19% | 1920 | Calb+/Calret+/NF200+/VIP+ | 4 | 0.15% | ||
| 146 | ChAT+/ENK+/Calb+ | 5 | 0.19% | 970 | ChAT+/SP+/SOM+/Calb+/Calret+/NF200+ | 5 | 0.19% | 1921 | NOS+/Calb+/Calret+/NF200+/VIP+ | 4 | 0.15% | ||
| 211 | NOS+/ChAT+/ENK+/SOM+/Calb+ | 1 | 0.04% | 978 | ChAT+/ENK+/SOM+/Calb+/Calret+/NF200+ | 1 | 0.04% | 1922 | ChAT+/Calb+/Calret+/NF200+/VIP+ | 43 | 1.66% | ||
| 256 | Calret+ | 1 | 0.04% | 999 | NOS+/ChAT+/5-HT+/CGRP+/SOM+/Calb+/Calret+/NF200+ | 1 | 0.04% | 1923 | NOS+/ChAT+/Calb+/Calret+/NF200+/VIP+ | 6 | 0.23% | ||
| 257 | NOS+/Calret+ | 9 | 0.35% | 1025 | NOS+/VIP+ | 331 | 12.75% | 1927 | NOS+/ChAT+/5-HT+/Calb+/Calret+/NF200+/VIP+ | 1 | 0.04% | ||
| 258 | ChAT+/Calret+ | 8 | 0.31% | 1026 | ChAT+/VIP+ | 5 | 0.19% | 1930 | ChAT+/SP+/Calb+/Calret+/NF200+/VIP+ | 1 | 0.04% | ||
| 259 | NOS+/ChAT+/Calret+ | 4 | 0.15% | 1027 | NOS+/ChAT+/VIP+ | 10 | 0.39% | 1931 | NOS+/ChAT+/SP+/Calb+/Calret+/NF200+/VIP+ | 2 | 0.08% | ||
| 386 | ChAT+/Calb+/Calret+ | 7 | 0.27% | 1031 | NOS+/ChAT+/5-HT+/VIP+ | 1 | 0.04% | 1959 | NOS+/ChAT+/5-HT+/CGRP+/Calb+/Calret+/NF200+/VIP+ | 1 | 0.04% | ||
| 387 | NOS+/ChAT+/Calb+/Calret+ | 2 | 0.08% | 1033 | NOS+/SP+/VIP+ | 2 | 0.08% | 1966 | ChAT+/5-HT+/SP+/CGRP+/Calb+/Calret+/NF200+/VIP+ | 2 | 0.08% | ||
| 394 | ChAT+/SP+/Calb+/Calret+ | 1 | 0.04% | 1061 | NOS+/5-HT+/CGRP+/VIP+ | 1 | 0.04% | 2049 | NOS+/NPY+ | 17 | 0.65% | ||
| 402 | ChAT+/ENK+/Calb+/Calret+ | 1 | 0.04% | 1089 | NOS+/SOM+/VIP+ | 1 | 0.04% | 2050 | ChAT+/NPY+ | 1 | 0.04% | ||
| 426 | ChAT+/SP+/CGRP+/Calb+/Calret+ | 1 | 0.04% | 1153 | NOS+/Calb+/VIP+ | 2 | 0.08% | 2051 | NOS+/ChAT+/NPY+ | 2 | 0.08% | ||
| 430 | ChAT+/5-HT+/SP+/CGRP+/Calb+/Calret+ | 1 | 0.04% | 1155 | NOS+/ChAT+/Calb+/VIP+ | 1 | 0.04% | 2057 | NOS+/SP+/NPY+ | 1 | 0.04% | ||
| 512 | NF200+ | 4 | 0.15% | 1281 | NOS+/Calret+/VIP+ | 8 | 0.31% | 2058 | ChAT+/SP+/NPY+ | 1 | 0.04% | ||
| 513 | NOS+/NF200+ | 68 | 2.62% | 1282 | ChAT+/Calret+/VIP+ | 1 | 0.04% | 2177 | NOS+/Calb+/NPY+ | 1 | 0.04% | ||
| 514 | ChAT+/NF200+ | 186 | 7.16% | 1283 | NOS+/ChAT+/Calret+/VIP+ | 2 | 0.08% | 2178 | ChAT+/Calb+/NPY+ | 1 | 0.04% | ||
| 515 | NOS+/ChAT+/NF200+ | 16 | 0.62% | 1291 | NOS+/ChAT+/SP+/Calret+/VIP+ | 1 | 0.04% | 2179 | NOS+/ChAT+/Calb+/NPY+ | 1 | 0.04% | ||
| 519 | NOS+/ChAT+/5-HT+/NF200+ | 2 | 0.08% | 1409 | NOS+/Calb+/Calret+/VIP+ | 1 | 0.04% | 2561 | NOS+/NF200+/NPY+ | 22 | 0.85% | ||
| 521 | NOS+/SP+/NF200+ | 2 | 0.08% | 1410 | ChAT+/Calb+/Calret+/VIP+ | 1 | 0.04% | 2567 | NOS+/ChAT+/5-HT+/NF200+/NPY+ | 1 | 0.04% | ||
| 522 | ChAT+/SP+/NF200+ | 22 | 0.85% | 1536 | NF200+/VIP+ | 1 | 0.04% | 2578 | ChAT+/ENK+/NF200+/NPY+ | 1 | 0.04% | ||
| 530 | ChAT+/ENK+/NF200+ | 105 | 4.04% | 1537 | NOS+/NF200+/VIP+ | 138 | 5.32% | 2689 | NOS+/Calb+/NF200+/NPY+ | 3 | 0.12% | ||
| 538 | ChAT+/SP+/ENK+/NF200+ | 39 | 1.50% | 1538 | ChAT+/NF200+/VIP+ | 12 | 0.46% | 2690 | ChAT+/Calb+/NF200+/NPY+ | 1 | 0.04% | ||
| 546 | ChAT+/CGRP+/NF200+ | 1 | 0.04% | 1539 | NOS+/ChAT+/NF200+/VIP+ | 22 | 0.85% | 2946 | ChAT+/Calb+/Calret+/NF200+/NPY+ | 1 | 0.04% | ||
| 578 | ChAT+/SOM+/NF200+ | 5 | 0.19% | 1543 | NOS+/ChAT+/5-HT+/NF200+/VIP+ | 6 | 0.23% | 2951 | NOS+/ChAT+/5-HT+/Calb+/Calret+/NF200+/NPY+ | 1 | 0.04% | ||
| 586 | ChAT+/SP+/SOM+/NF200+ | 14 | 0.54% | 1545 | NOS+/SP+/NF200+/VIP+ | 1 | 0.04% | 2963 | NOS+/ChAT+/ENK+/Calb+/Calret+/NF200+/NPY+ | 1 | 0.04% | ||
| 594 | ChAT+/ENK+/SOM+/NF200+ | 2 | 0.08% | 1546 | ChAT+/SP+/NF200+/VIP+ | 2 | 0.08% | 2966 | ChAT+/5-HT+/ENK+/Calb+/Calret+/NF200+/NPY+ | 1 | 0.04% | ||
| 602 | ChAT+/SP+/ENK+/SOM+/NF200+ | 1 | 0.04% | 1547 | NOS+/ChAT+/SP+/NF200+/VIP+ | 1 | 0.04% | 3073 | NOS+/VIP+/NPY+ | 47 | 1.81% | ||
| 640 | Calb+/NF200+ | 2 | 0.08% | 1553 | NOS+/ENK+/NF200+/VIP+ | 1 | 0.04% | 3075 | NOS+/ChAT+/VIP+/NPY+ | 4 | 0.15% | ||
| 641 | NOS+/Calb+/NF200+ | 14 | 0.54% | 1554 | ChAT+/ENK+/NF200+/VIP+ | 1 | 0.04% | 3585 | NOS+/NF200+/VIP+/NPY+ | 39 | 1.50% | ||
| 642 | ChAT+/Calb+/NF200+ | 150 | 5.78% | 1555 | NOS+/ChAT+/ENK+/NF200+/VIP+ | 1 | 0.04% | 3586 | ChAT+/NF200+/VIP+/NPY+ | 1 | 0.04% | ||
| 643 | NOS+/ChAT+/Calb+/NF200+ | 8 | 0.31% | 1562 | ChAT+/SP+/ENK+/NF200+/VIP+ | 1 | 0.04% | 3587 | NOS+/ChAT+/NF200+/VIP+/NPY+ | 6 | 0.23% | ||
| 650 | ChAT+/SP+/Calb+/NF200+ | 8 | 0.31% | 1571 | NOS+/ChAT+/CGRP+/NF200+/VIP+ | 2 | 0.08% | 3713 | NOS+/Calb+/NF200+/VIP+/NPY+ | 14 | 0.54% | ||
| 656 | ENK+/Calb+/NF200+ | 1 | 0.04% | 1573 | NOS+/5-HT+/CGRP+/NF200+/VIP+ | 1 | 0.04% | 3715 | NOS+/ChAT+/Calb+/NF200+/VIP+/NPY+ | 1 | 0.04% | ||
| 658 | ChAT+/ENK+/Calb+/NF200+ | 45 | 1.73% | 1575 | NOS+/ChAT+/5-HT+/CGRP+/NF200+/VIP+ | 4 | 0.15% | 3759 | NOS+/ChAT+/5-HT+/SP+/CGRP+/Calb+/NF200+/VIP+/NPY+ | 1 | 0.04% | ||
| 666 | ChAT+/SP+/ENK+/Calb+/NF200+ | 8 | 0.31% | 1601 | NOS+/SOM+/NF200+/VIP+ | 1 | 0.04% | 3841 | NOS+/Calret+/NF200+/VIP+/NPY+ | 1 | 0.04% | ||
| 679 | NOS+/ChAT+/5-HT+/CGRP+/Calb+/NF200+ | 1 | 0.04% | 1610 | ChAT+/SP+/SOM+/NF200+/VIP+ | 1 | 0.04% | 3843 | NOS+/ChAT+/Calret+/NF200+/VIP+/NPY+ | 1 | 0.04% | ||
| 711 | NOS+/ChAT+/5-HT+/SOM+/Calb+/NF200+ | 1 | 0.04% | 1611 | NOS+/ChAT+/SP+/SOM+/NF200+/VIP+ | 1 | 0.04% |
A total of 164 unique combinations (codes) of the 12 cell body markers were identified, with only the immunoreactive markers shown together with the number of cells (out of 2596 sampled, n = 12) and the percentage that each combination counts for. The numerical value of codes depends on the order in which antibodies were applied and thus are only relevant to the present study: they are not a system of nomenclature for enteric neurons. Most combinations were only present in 1 or 2 cells in the sample of 2596; however, some were very abundant (eg, 1, 1025, 2, 514).
5-HT, 5-hydroxytryptamine; Calb, calbindin; Calret, calretinin; CGRP, calcitonin gene-related peptide; ChAT, choline acetyltransferase; ENK, leu enkephalin; NF200, neurofilament 200 kD; NOS, neuronal nitric oxide synthase; NPY, neuropeptide Y; SOM, somatostatin; SP, substance P; VIP, vasoactive intestinal polypeptide.
Figure 2.
Markers of myenteric neurons are far from randomly assorted. Each combination of markers was given a numerical code from 0 to 4095 (see Materials and Methods and Results and Table 5). Percentages of cells expressing codes were ordered by descending frequency (dashed line) and compared with expected frequencies calculated if markers randomly combined in their original proportions (solid line). Note that a few classes were far more abundant than expected; these included 2 classes of IMNs (codes 1025 and 1) (n = 12 patients).
Clustering of Myenteric Neurons
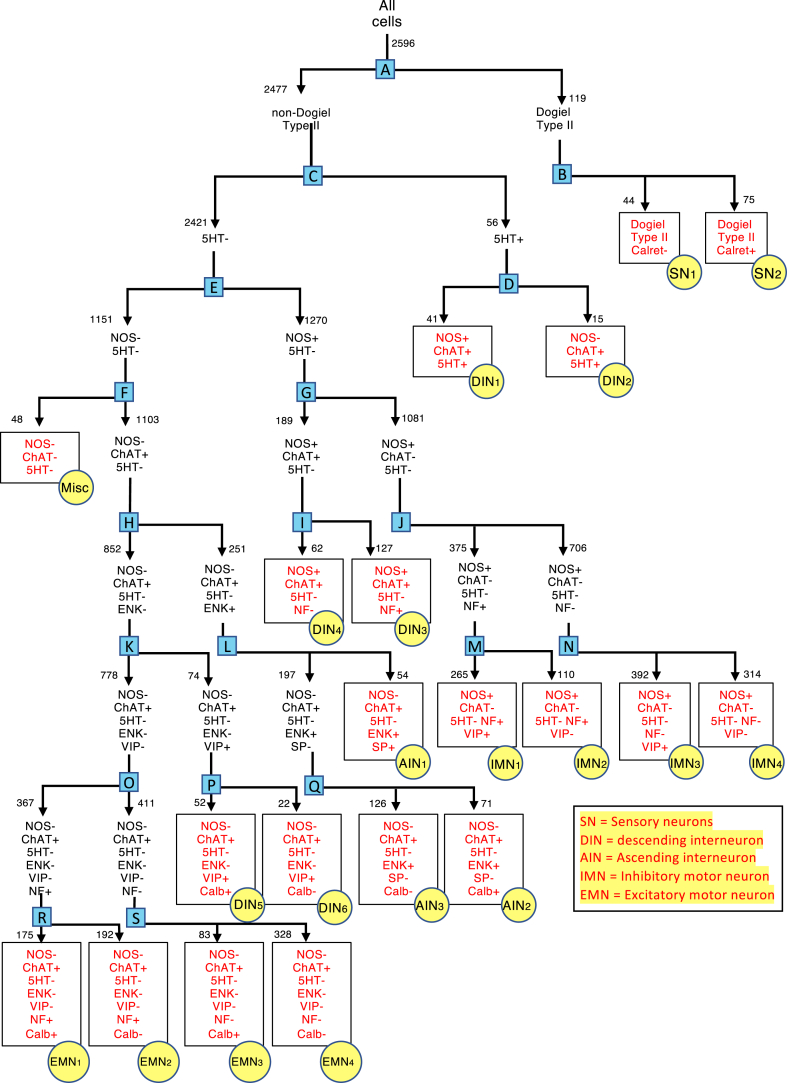
As described in the Materials and Methods, divisive hierarchical clustering was used to group myenteric neurons on the basis of their coding (see Figure 3). Two well-established groups of neurons were targeted first; (1) Dogiel type II neurons with their distinctive multiaxonal morphology and (2) 5-HT immunoreactive nerve cell bodies, which accumulate 5-HT from solution.19, 20, 21 With Dogiel type II neurons and 5-HT–immunoreactive neurons accounted for, the remaining 2421 neurons were divided, first using NOS as the discriminating marker, followed by ChAT (the functionally important markers for IMNs and EMNs, respectively).10,17,18 The 4 resulting groups of neurons (NOS+/ChAT+, NOS+/ChAT–, NOS–/ChAT+, NOS–/ChAT–), were then each divided by the next most discriminating marker, in an iterative process with statistical testing (see Materials and Methods and refer to Figure 3). This continued until a group of neurons could not be divided by any marker into significantly different subgroups. The statistical basis for the 19 division points (A-S in blue) of the dendrogram in Figure 3 is summarized in Table 6.
Figure 3.
Dendrogram summarizing the divisive hierarchical analysis of 2596 cells that distinguished 20 types of neurons in the myenteric plexus of human colon (n = 12). A series of binary divisions was undertaken, based on statistical identification of the most discriminating marker for each population (see Materials and Methods). Red font and yellow circles denote endpoints (classes). Division points are letters in blue boxes. Numbers next to arrows indicate the number of cells at each branch point. The statistical validation of each branch point (blue boxes A–S) is summarized in Table 6.
Table 6.
Statistical Basis of Division Points in Divisive Hierarchical Clustering
| Pools of Cells | Most Discriminating Marker | Class Defined | Results of Individual Marker Distribution Tests |
Cell Size Comparisons |
Analysis Across All Markers |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NOS | ChAT | 5-HT | SP | ENK | CGRP | SOM | Calb | Calret | NF200 | VIP | NPY | Mean ± SD | t | df | P Value | Omnibus χ2 | df | Probability | |||
| A. 2596—all cells | Dogiel type II morphology | <.0001 | <.0001 | <.0001 | <.0001 | .0023 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | NS | 1616 ± 785 vs 610 ± 347 | 64.40 | 2594 | <.0001 | 2122.32 | 11 | <.0001 | |
| B. 119 Dogiel type II neurons | Calret | NS | NS | .0007 | NS | NS | .0060 | .0329 | <.0001 | NS | NS | NS | NS | 1788 ± 757 vs 1322 ± 743 | 3.24 | 117 | .0016 | 42.10 | 3 | <.0001 | |
| 44 Dogiel type II neurons/Calret– not divisible | — | SN1 | |||||||||||||||||||
| 75 Dogiel type II neurons/Calret+—not divisible | — | SN2 | |||||||||||||||||||
| C. 2477 Non-Dogiel type II neurons | 5-HT | .0230 | <.0001 | NS | <.0001 | NS | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | .0003 | NS | 1264 ± 501 vs 595 ± 328 | 14.87 | 2475 | <.0001 | 857.58 | 10 | <.0001 | |
| D. 56 5-HT+/non-Dogiel type II neurons | NOS | — | NS | NS | NS | NS | NS | NS | NS | NS | <.0001 | NS | <.0001 | NS | 1198 ± 501 vs 1445 ± 454 | 1.65 | 54 | NS | 29.98 | 1 | <.0001 |
| 41 5-HT+NOS+/non-Dogiel type II neurons cells—not divisible | — | DIN1 | |||||||||||||||||||
| 15 5-HT+NOS–/non-Dogiel type II neurons—not divisible | — | DIN2 | |||||||||||||||||||
| E. 2421 5-HT–/Non Dogiel type II neurons | NOS | — | NS | <.0001 | NS | <.0001 | <.0001 | NS | NS | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | 628 ± 327 vs 559 ± 325 | 5.20 | 2419 | <.0001 | 1850.01 | 8 | <.0001 |
| F. 1151 NOS–/5-HT–/Non Dogiel type II neurons | ChAT | — | NS | NS | NS | NS | .0070 | NS | NS | NS | NS | <.0001 | NS | NS | 563 ± 325 vs 462 ± 304 | 2.10 | 1149 | .0358 | 16.69 | 2 | .00024 |
| 48 NOS–/ChAT–/5-HT–/non-Dogiel type II neurons—not divisible | — | Misc | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |||||||
| G. 1270 NOS+/5-HT–/Non Dogiel type II neurons | ChAT | — | NS | NS | NS | NS | NS | NS | NS | <.0001 | <.0001 | <.0001 | NS | NS | 763 ± 433 vs 604 ± 298 | 6.24 | 1268 | <.0001 | 187.69 | 4 | <.0001 |
| H. 1103 NOS–/ChAT+/5-HT–/NonDII | ENK | — | NS | NS | NS | <.0001 | NS | NS | NS | NS | NS | <.0001 | .0002 | NS | 793 ± 374 vs 495 ± 275 | 13.76 | 1101 | <.0001 | 182.56 | 4 | <.0001 |
| I. 189 NOS+/ChAT+/5-HT–/non-Dogiel type II neurons | NF200 | — | NS | NS | NS | NS | NS | NS | NS | <.0001 | NS | NS | <.0001 | NS | 868 ± 466 vs 546 ± 236 | 5.10 | 187 | <.0001 | 42.16 | 3 | <.0001 |
| 62 NOS+/ChAT+/NF200–/5-HT–/NonDogiel type II—not divisible | — | DIN4 | |||||||||||||||||||
| 127 NOS+/ChAT+/NF200+/5-HT–/NonDogiel type II—not divisible | — | DIN3 | |||||||||||||||||||
| J. 1081 NOS+/ChAT–/5-HT–/non-Dogiel type II neurons | NF200 | — | NS | NS | NS | NS | NS | NS | NS | <.0001 | NS | NS | <.0001 | <.0001 | 744 ± 384 vs 530 ± 206 | 11.88 | 1079 | <.0001 | 176.90 | 3 | <.0001 |
| K. 852 NOS–/ChAT+/ENK–/5-HT–/non-Dogiel type II neurons | VIP 74 | — | NS | NS | NS | NS | NS | NS | NS | <.0001 | <.0001 | <.0001 | NS | NS | 800 ± 487 vs 466 ± 225 | 10.60 | 850 | <.0001 | 263.91 | 2 | <.0001 |
| L. 251 NOS–/ChAT+/ENK+/5-HT–/non-Dogiel type II neurons | SP | — | NS | NS | NS | NS | NS | NS | NS | .0059 | NS | NS | NS | NS | 997 ± 438 vs 737 ± 334 | 4.71 | 249 | <.0001 | 6.10 | 1 | .01356 |
| 54 NOS–/ChAT+/ENK+/SP+/5-HT–/non-Dogiel type II neurons—not divisible | — | AIN1 | |||||||||||||||||||
| M. 375 NOS+/ChAT–/NF200+/5-HT–/non-Dogiel type II neurons | VIP | — | NS | NS | NS | NS | NS | NS | NS | .0090 | NS | NS | NS | NS | 814 ± 412 vs 575 ± 232 | 5.70 | 373 | <.0001 | 6.14 | 1 | .01319 |
| 265 NOS+/ChAT–/NF200+/VIP+/5-HT–/non-Dogiel type II neurons—not divisible | — | IMN1 | |||||||||||||||||||
| 110 NOS+/ChAT–/NF200+/VIP–/5-HT–/non-Dogiel type II neurons—not divisible | — | IMN2 | |||||||||||||||||||
| N. 706 NOS+/ChAT–/NF200–/5-HT–/non-Dogiel type II neurons | VIP | — | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | .0212 | 577 ± 218 vs 472 ± 172 | 6.94 | 704 | <.0001 | 12.34 | 2 | .00209 |
| 392 NOS+/ChAT–/NF200–/VIP+/5-HT–/non-Dogiel type II neurons—not divisible | — | IMN3 | |||||||||||||||||||
| 314 NOS+/ChAT–/NF200/VIP–/5-HT–/non-Dogiel type II neurons—not divisible | — | IMN4 | |||||||||||||||||||
| O. 778 NOS–/ChAT+/ENK–/VIP–/5-HT–/non-Dogiel type II neurons | NF200 | — | NS | NS | NS | NS | NS | NS | NS | <.0001 | NS | NS | NS | NS | 544 ± 259 vs 397 ± 160 | 9.58 | 776 | <.0001 | 48.54 | 2 | |
| P. 74 NOS–/ChAT+/ENK–/VIP+/5-HT–/non-Dogiel type II neurons | Calb | — | NS | NS | NS | NS | NS | NS | NS | NS | <.0001 | .0013 | NS | NS | 886 ± 529 vs 597 ± 277 | 2.39 | 72 | .0194 | 17.40 | 1 | <.0001 |
| 52 NOS–/ChAT+/ENK–/VIP+/Calb+/5-HT–/non-Dogiel type II neurons—not divisible | — | DIN5 | |||||||||||||||||||
| 22 NOS–/ChAT+/ENK–/VIP+/Calb–/5-HT–/non-Dogiel type II neurons—not divisible | — | DIN6 | |||||||||||||||||||
| Q. 197 NOS–/ChAT+/ENK–/SP–/5-HT–/non-Dogiel type II neurons | Calb | — | NS | NS | NS | NS | NS | NS | NS | NS | <.0001 | NS | NS | NS | 647 ± 223 vs 787 ± 373 | 2.86 | 195 | .0046 | 33.85 | 1 | <.0001 |
| 126 NOS–/ChAT+/ENK+/SP–/Calb–/5-HT–/non-Dogiel type II neurons—not divisible | — | AIN3 | |||||||||||||||||||
| 71 NOS–/ChAT+/ENK+/SP–/Calb+/5-HT–/non-Dogiel type II neurons—not divisible | — | AIN2 | |||||||||||||||||||
| R. 367 NOS–/ChAT+/ENK–/VIP–/NF200+/5-HT–/non-Dogiel type II neurons | Calb | — | NS | NS | NS | NS | NS | NS | NS | NS | <.0001 | NS | NS | NS | 574 ± 262 vs 517 ± 254 | 2.118 | 365 | .0349 | 24.14 | 1 | 8.97E-07 |
| 175 NOS–/ChAT+/ENK–/VIP–/NF200+/Calb+/5-HT–/non-Dogiel type II neurons—not divisible | — | EMN1 | |||||||||||||||||||
| 192 NOS–/ChAT+/ENK–/VIP–/NF200+/Calb–/5-HT–/non-Dogiel type II neurons—not divisible | — | EMN2 | |||||||||||||||||||
| S. 411 NOS–/ChAT+/ENK–/VIP–/NF200–/5-HT–/non-Dogiel type II neurons | Calb | — | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | 496 ± 188 vs 373 ± 142 | 6.583 | 409 | <.0001 | |||
| 83 NOS–/ChAT+/ENK–/VIP–/NF200–/Calb+/5-HT–/non-Dogiel type II neurons—not divisible | — | EMN3 | |||||||||||||||||||
| 328 NOS–/ChAT+/ENK–/VIP–/NF200–/Calb–/5-HT–/non-Dogiel type II neurons—not divisible | — | EMN4 | |||||||||||||||||||
This should be read with reference to dendrogram of Figure 3 in which 18 division points (A-S) in the hierarchical analysis are shown as blue squares—and at start of cells in column 1. The pool of cells at each branch point is summarized in the first column; the second column identifies the most discriminating marker to divide that pool of cells (into 2 subpools—one immunoreactive for the marker, the other non-immunoreactive). The third column shows the endpoints of the analysis with pools of cells that could not be further divided; these are given class names (eg, DIN1, IMN). The next 12 columns show probabilities associated with the number of cells expressing each marker compared between the 2 subpools of cells calculated from chi-square tests with Bonferroni corrections. The 4 columns labeled cell size comparisons show average ± SD of the size of cells in the 2 subpools with t value, degrees of freedom, and probability. The 3 columns on the right called chi-square analysis across all markers show an omnibus chi-square value summed across all 12 markers.
AIN, ascending interneuron; Calb, calbindin; Calret, calretinin; CGRP, calcitonin gene-related peptide; ChAT, choline acetyltransferase; DIN, descending interneuron; EMN, excitatory motor neuron; ENK, leu enkephalin; IMN, inhibitory motor neuron; NF200, neurofilament 200 kD; NOS, neuronal nitric oxide synthase; NPY, neuropeptide Y; NS, nonsignificant; SN, sensory neuron; SOM, somatostatin; SP, substance P; VIP, vasoactive intestinal polypeptide.
Details of Clustering: Multiaxonal Neurons (Class SN1 and SN2)
Dogiel type II neurons are a major functional type of enteric SNs, readily identified by having 2 or more axons and a large smooth soma readily visible with NF200 immunoreactivity.22 However, the density of NF200-labeled fibers in ganglia obscured some neurons' morphology. To overcome this, 55 definite Dogiel type II neurons (see Figure 4) with 13 different codes were identified. It was assumed that all neurons with these 13 codes were Dogiel type II neurons, identifying 119 (4.6%) Dogiel type II neurons out of the 2596 neurons. Of these 119, 100% were ChAT immunoreactive, 92.4% were SP immunoreactive, and a majority were immunoreactive for Calb (52.9%) and/or Calret (63.0%), with 16.7% immunoreactive for 5-HT, 12.6% for CGRP, and 19.3% for SOM. Other markers were sparse: NOS 0%, ENK 0%, VIP 0.8%, NPY 0.8% (see Table 7). Overall, 11 of the 12 immunohistochemical markers were non-uniformly distributed between Dogiel type II and non-Dogiel type II cells (omnibus χ211 = 2122, P < .0001) (Table 6). As reported in guinea pigs,8 Dogiel type II neurons were larger than other nerve cells (1616 ± 785 μm2 [n = 119 cells] vs 610 ± 347 μm2 [n = 12 cells]; t2594 = 64.8, P < .0001). The most discriminating marker to subdivide the 119 Dogiel type II was Calret (decision point B, Figure 3), which split them into 75 Calret+ (SN1 class) and 44 Calret– neurons (SN2 class), with significant nonuniform partitioning of CGRP, SOM, and Calb (see Figure 4 and Table 6). These 2 classes (SN1 and SN2) could not be further divided in a statistically validated manner.
Figure 4.
Identification of Dogiel type II neurons and their coding. A large multiaxonal Dogiel type II cell is clearly identified by NF200 immunoreactivity (fifth row, white arrowhead indicates nucleus). The same cell was immunoreactive for HuCD, ChAT, 5-HT (top row), CGRP (second row), SOM (3rd row), Calret, Calb (fourth row), and peripherin (fifth row) but not for NOS, SP, ENK, NPY, TH, or VIP. The code for this cell was calculated (ChAT = 2, 5-HT = 4, CGRP = 32, SOM = 64, Calb = 128, Calret = 256, NF200 = 512, with all other nonimmunoreactive markers set to 0; this summed to give this cell a code of 998). Micrographs were manually realigned between layers of immunohistochemistry. Similar data were obtained in the 12 patients studied with the full multilayer protocol). Calibration bar (bottom right) = 50 μm.
Table 7.
Percentage of Each of the 20 Classes That Expressed 12 Discriminating Markers
| Class | NOS | ChAT | 5-HT | SP | ENK | CGRP | SOM | Calb | Calret | NF200 | VIP | NPY |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AIN1 | 0.0%a | 100.0% | 0.0%a | 100.0%a | 100.0%a | 0.0% | 1.9% | 14.8% | 0.0% | 90.7% | 1.9% | 0.0% |
| AIN2 | 0.0%a | 100.0% | 0.0%a | 0.0%a | 100.0%a | 0.0% | 2.8% | 100.0%a | 26.8% | 91.5% | 1.4% | 0.0% |
| AIN3 | 0.0%a | 100.0% | 0.0%a | 0.0%a | 100.0%a | 0.0% | 1.6% | 0.0%a | 0.0% | 86.5% | 0.8% | 0.8% |
| DIN1 | 100.0%a | 95.1% | 100.0%a | 12.2% | 4.9% | 36.6% | 7.3% | 53.7% | 12.2% | 92.7% | 80.5% | 7.3% |
| DIN2 | 0.0%a | 100.0%a | 100.0%a | 26.7% | 6.7% | 53.3% | 6.7% | 53.3% | 93.3% | 86.7% | 13.3% | 6.7% |
| DIN3 | 100.0%a | 100.0%a | 0.0%a | 5.5% | 1.6% | 2.4% | 0.8% | 56.7% | 11.8% | 100.0%a | 79.5% | 7.1% |
| DIN4 | 100.0%a | 100.0%a | 0.0%a | 1.6% | 3.2% | 0.0% | 1.6% | 8.1% | 14.5% | 0.0%a | 29.0% | 11.3% |
| DIN5 | 0.0%a | 100.0%a | 0.0%a | 0.0% | 0.0%a | 1.9% | 0.0% | 100.0%a | 84.6% | 98.1% | 100.0%a | 0.0% |
| DIN6 | 0.0%a | 100.0%a | 0.0%a | 13.6% | 0.0%a | 0.0% | 4.5% | 0.0%a | 4.5% | 72.7% | 100.0%a | 4.5% |
| EMN1 | 0.0% | 100.0% | 0.0%a | 1.7% | 0.0% | 1.7% | 1.1% | 100.0%a | 12.6% | 100.0%a | 0.0% | 0.6% |
| EMN2 | 0.0% | 100.0% | 0.0%a | 0.0% | 0.0% | 0.5% | 2.6% | 0.0% | 0.0% | 100.0%a | 0.0% | 0.0% |
| EMN3 | 0.0% | 100.0% | 0.0%a | 4.8% | 0.0% | 1.2% | 0.0% | 100.0%a | 10.8% | 0.0% | 0.0% | 1.2% |
| EMN4 | 0.0% | 100.0% | 0.0%a | 1.8% | 0.0% | 0.3% | 0.3% | 0.0% | 2.4% | 0.0% | 0.0% | 0.6% |
| IMN1 | 100.0%a | 0.0%a | 0.0%a | 0.8% | 0.4% | 0.0% | 0.4% | 30.6% | 3.0% | 100.0%a | 100.0%a | 20.4% |
| IMN2 | 100.0%a | 0.0%a | 0.0%a | 1.8% | 0.0% | 0.0% | 0.0% | 16.4% | 0.9% | 100.0%a | 0.0%a | 22.7% |
| IMN3 | 100.0%a | 0.0%a | 0.0%a | 0.5% | 0.0% | 0.0% | 0.3% | 0.8% | 2.3% | 0.0%a | 100.0%a | 12.0% |
| IMN4 | 100.0%a | 0.0%a | 0.0%a | 0.6% | 0.0% | 0.0% | 0.0% | 3.2% | 2.9% | 0.0%a | 0.0%a | 6.1% |
| Misc | 0.0%a | 0.0%a | 0.0% | 2.1% | 4.2% | 0.0% | 0.0% | 35.4% | 10.4% | 25.0% | 10.4% | 0.0% |
| SN1 | 0.0% | 100.0% | 26.7% | 88.0% | 0.0% | 20.0% | 12.0% | 73.3% | 100.0%a | 100.0%a | 1.3% | 1.3% |
| SN2 | 0.0% | 100.0% | 0.0% | 100.0% | 0.0% | 0.0% | 31.8% | 18.2% | 0.0%a | 100.0%a | 0.0% | 0.0% |
| All cells | 50.5% | 56.4% | 2.9% | 7.9% | 10.1% | 1.8% | 1.7% | 26.5% | 9.7% | 51.7% | 34.4% | 6.6% |
Twelve immunohistochemical markers expressed by 20 identified classes of neurons in human colon. Percentages refer to the proportion of cells of that class which expressed each of the markers.
5-HT, 5-hydroxytryptamine; AIN, ascending interneuron; Calb, calbindin; Calret, calretinin; CGRP, calcitonin gene-related peptide; ChAT, choline acetyltransferase; DIN, descending interneuron; EMN, excitatory motor neuron; ENK, leu enkephalin; IMN, inhibitory motor neuron; Misc, miscellaneous; NF200, neurofilament 200 kD; NOS, neuronal nitric oxide synthase; NPY, neuropeptide Y; NS, nonsignificant; SN, sensory neuron; SOM, somatostatin; SP, substance P; VIP, vasoactive intestinal polypeptide.
Values of 0% or 100% that were set as discriminating markers during the divisive hierarchical clustering process (see Materials and Methods).
Details of Clustering: 5-HT–Immunoreactive Neurons
5-HT–immunoreactive neurons have been characterized in guinea pigs,21,23 mice,24,25 pigs,26 and humans,27 in which they comprise a type of cholinergic descending interneuron (DIN).27 After excluding 119 Dogiel type II neurons, 56 (of 2477) myenteric nerve cell bodies were immunoreactive for 5-HT (2.2% of all 2596 neurons). Of these, 54 (96.4%) were immunoreactive for ChAT, 51 (91.1%) were immunoreactive for NF200, and 41 (73.2%) were immunoreactive for NOS. Other markers included SP (16.1%), CGRP (41.1%), SOM (7.1%), Calb (53.6%), Calret (33.9%), VIP (62.5%), NPY (7.1%), and ENK (5.4%).
5-HT–immunoreactive neurons divided into 2 clusters when discriminated by NOS immunoreactivity (branch point D, Figure 3). NOS+/ChAT+/5-HT+ cells were mostly Calret– with many VIP+ (class: DIN1, 41 cells) whereas NOS–/ChAT+/5-HT+ cells were largely Calret+ and VIP– (class: DIN2; omnibus χ21 = 29.98, P < .001) (see Figure 5 and Table 6). 5-HT–immunoreactive neurons were large uniaxonal cells (DIN1: 1198 ± 501 μm2, n = 41; DIN2: 1445 ± 454 μm2, n = 15).
Figure 5.
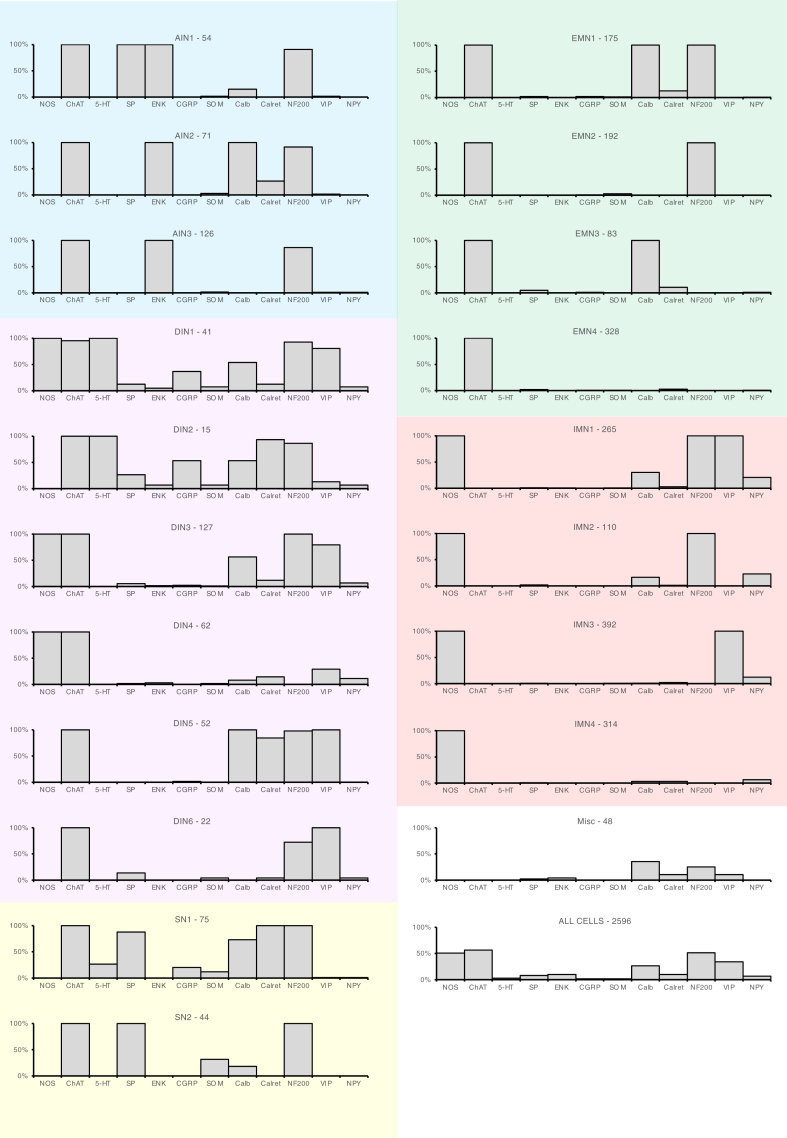
The 20 types of neurons distinguished in the study differed in the combinations of markers that they expressed. Each graph shows one type of neuron. The x-axis shows the 12 cell body markers and the y-axis shows the proportion of neurons immunoreactive for each marker. In general, 100% or 0% indicates that a marker had been used to discriminate subtypes further up the dendrogram. There were 3 types of AINs (AIN1–3), 6 types of DINs (DIN1–6), 2 intrinsic SNs (multiaxonal Dogiel type II neurons: SN1, SN2), 4 types of EMNs (EMN1–4), 4 IMNs (IMN1–4), and a small population (1.8%) that lacked both NOS and ChAT immunoreactivity (Misc). n = 2596 cells from 12 patients.
Discrimination by NOS Immunoreactivity
After accounting for 5-HT+ and multiaxonal neurons, 2421 remained. These were first divided by NOS immunoreactivity; present in 1270 (52.5%) neurons. Eight markers had significant nonuniform distributions between NOS+ and NOS– subgroups (ChAT, SP, ENK, Calb, Calret, NF200, VIP, and NPY; omnibus χ28 = 1850.0, P < .001) (Table 6). NOS+ cells were slightly larger than NOS– cells (628 ± 327 μm2 vs 559 ± 325 μm2; t2419 = 5.199, P < .0001). The NOS+ and NOS– groups were then both divided by ChAT immunoreactivity.
A series of similar steps were iteratively carried out to divide each remaining group of neurons according to its most discriminating marker (for details, see Materials and Methods). The statistically supported decision points are summarized in Figure 3 and Table 6. This yielded a further 16 classes, none of which could be subdivided in a statistically valid way.
Thus, a total of 20 groups of neurons were distinguished (Figure 3). Marker frequency for each class is summarized in Figure 5 and Table 7. Putative functions were ascribed largely from published retrograde tracing and immunohistochemical studies.27, 28, 29, 30, 31, 32 In brief, the scheme identified 3 classes of cholinergic AINs (AIN1–3) distinguished by ENK immunoreactivity, which is abundant in varicose axons projecting orally in myenteric ganglia but not in EMNs.27 There were 6 classes of uniaxonal neurons tentatively identified as DINs (DIN1–6) which were all ChAT+ (ie, cholinergic) and hence were unlikely to be descending IMNs.29 There were 4 classes of putative cholinergic EMNs with ChAT immunoreactivity but NOS–, small cell bodies, some SP+, sparse Calret, and little to no VIP or NPY, as reported previously.28,29,32 There were 4 classes of nitrergic IMN, all lacking ChAT (and hence noncholinergic) with abundant VIP and NPY, previously described as markers for IMN axons.33 Two classes of distinctive multiaxonal cholinergic SNs (SN1–2, Dogiel type II neurons) made up the remainder, together with a small heterogenous miscellaneous group of non-cholinergic/non-nitrergic neurons (Misc). We compared the percentages of cells in each of the 20 classes compared with proportions expected if markers were randomly assorted (see Table 8). Markers show strong non-random patterns of coexistence within classes.
Table 8.
Expected Abundance of Classes: Random Markers vs Observed
| Cell Type | No. Codes in the Class | Expected Probability Cell Belongs to Class | Expected No. Cells in Class (of 2596) | Observed No. Cells Among the 2596 |
|---|---|---|---|---|
| AIN1 | 5 | .0012 | 3.2 | 54 |
| AIN2 | 7 | .0049 | 12.8 | 71 |
| AIN3 | 5 | .0129 | 33.6 | 126 |
| DIN1 | 22 | .0047 | 12.2 | 41 |
| DIN2 | 8 | .0014 | 3.5 | 15 |
| DIN3 | 19 | .1139 | 295.6 | 127 |
| DIN4 | 12 | .0941 | 244.3 | 62 |
| DIN5 | 4 | .0106 | 27.4 | 52 |
| DIN6 | 6 | .0524 | 136.0 | 22 |
| EMN1 | 6 | .0194 | 50.3 | 175 |
| EMN2 | 3 | .0471 | 122.2 | 192 |
| EMN3 | 6 | .0195 | 50.7 | 83 |
| EMN4 | 7 | .0556 | 144.3 | 328 |
| IMN1 | 11 | .0349 | 90.7 | 265 |
| IMN2 | 6 | .0566 | 147.0 | 110 |
| IMN3 | 7 | .0296 | 76.8 | 392 |
| IMN4 | 7 | .0555 | 144.1 | 314 |
| Misc | 10 | .1230 | 319.2 | 48 |
| SN1 | 10 | .0008 | 2.1 | 75 |
| SN2 | 3 | .0054 | 14.0 | 44 |
Cells are not randomly distributed between classes. Each of the 20 classes comprised 3–22 codes (164 in total); each code is represented a unique combination of markers. We calculated the theoretic probability for each of the 164 codes by assuming that markers were assorted independently. As shown in Figure 2, the proportions of most codes were very different to those predicted by random assortment. These data were then used to calculate the proportion of each class that would be expected if markers assorted independently (shown above). As can be seen, in 15 of the 20 cases, there were more cells in a class than predicted by random assortment; in the other 5, there were fewer cells. Again, this reinforces that the chemical coding of classes is unlikely to arise from random assortment of markers.
AIN, ascending interneuron; DIN, descending interneuron; EMN, excitatory motor neuron; IMN, inhibitory motor neuron; SN, sensory neuron.
Distribution of Classes by Region and Sex
Because the motility of ascending and descending colon differ significantly, it was of interest to determine whether the innervation reflected this functional diversity. The abundance of several classes differed significantly between ascending and descending bowel (omnibus χ219 = 115.3, P < .0001) (see Table 6). 5-HT–immunoreactive DINs (DIN2) (see Table 9) were more abundant in descending colon, as were IMN1, whereas IMN4 were significantly more abundant in ascending colon. The proportion of cells in each class was also compared between males (n = 6) and females (n = 6); small differences were detected (χ219 = 70.3, P < .0001). DIN6 (DINs with ChAT+/VIP+/NOS) were more abundant in males, as were IMN2 (NOS+/NF+). In contrast, IMN4 (small IMNs with NOS and no other markers) were more abundant in females (Table 10).
Table 9.
Abundance of Classes in Ascending and Descending Colon Compared
| Class Name | No. Cells Whole Colon | % Cells Whole Colon | No. Ascending Colon | % Asc ending Colon | No. Desc ending Colon | % Desc ending Colon |
|---|---|---|---|---|---|---|
| AIN1 | 54 | 2.1% | 20 | 1.5% | 34 | 2.6% |
| AIN2 | 71 | 2.7% | 29 | 2.2% | 42 | 3.2% |
| AIN3 | 126 | 4.9% | 55 | 4.2% | 71 | 5.5% |
| DIN1 | 41 | 1.6% | 23 | 1.8% | 18 | 1.4% |
| DIN2 | 15a | 0.6%a | 2a | 0.2%a | 13a | 1.0%a |
| DIN3 | 127 | 4.9% | 66 | 5.1% | 61 | 4.7% |
| DIN4 | 62 | 2.4% | 39 | 3.0% | 23 | 1.8% |
| DIN5 | 52 | 2.0% | 31 | 2.4% | 21 | 1.6% |
| DIN6 | 22 | 0.8% | 10 | 0.8% | 12 | 0.9% |
| EMN1 | 175 | 6.7% | 91 | 7.0% | 84 | 6.5% |
| EMN2 | 192 | 7.4% | 111 | 8.5% | 81 | 6.3% |
| EMN3 | 83 | 3.2% | 32 | 2.5% | 51 | 3.9% |
| EMN4 | 328 | 12.6% | 151 | 11.6% | 177 | 13.7% |
| IMN1 | 265a | 10.2%a | 98a | 7.5%a | 167a | 12.9%a |
| IMN2 | 110 | 4.2% | 60 | 4.6% | 50 | 3.9% |
| IMN3 | 392 | 15.1% | 185 | 14.2% | 207 | 16.0% |
| IMN4 | 314a | 12.1%a | 215a | 16.5%a | 99a | 7.7%a |
| SN1 | 75 | 2.9% | 33 | 2.5% | 42 | 3.2% |
| SN2 | 44 | 1.7% | 30 | 2.3% | 14 | 1.1% |
| Misc | 48 | 1.8% | 22 | 1.7% | 26 | 2.0% |
| Totals | 2596 | 100.0% | 1303 | 100.0% | 1293 | 100.0% |
The abundance of the 20 classes between ascending (n = 6, 1303 cells) and descending colon (n = 6, 1293 cells) differed significantly (χ219 = 115.3, P < .001) with analysis of standard residuals indicating that 3 classes differed significantly between regions.
AIN, ascending interneuron; DIN, descending interneuron; EMN, excitatory motor neuron; IMN, inhibitory motor neuron; SN, sensory neuron.
Significant difference (P < .05).
Table 10.
Abundance of Classes Compared Between Sexes
| Class Name | No. Cells Whole Colon | % Cells Whole Colon | No. Cells Male | % Cells Male | No. Cells Female | % Cells Female |
|---|---|---|---|---|---|---|
| AIN1 | 54 | 2.1% | 18 | 1.4% | 36 | 2.8% |
| AIN2 | 71 | 2.7% | 38 | 2.9% | 33 | 2.6% |
| AIN3 | 126 | 4.9% | 63 | 4.8% | 63 | 4.9% |
| DIN1 | 41 | 1.6% | 29 | 2.2% | 12 | 0.9% |
| DIN2 | 15 | 0.6% | 9 | 0.7% | 6 | 0.5% |
| DIN3 | 127 | 4.9% | 67 | 5.1% | 60 | 4.7% |
| DIN4 | 62 | 2.4% | 31 | 2.4% | 31 | 2.4% |
| DIN5 | 52 | 2.0% | 22 | 1.7% | 30 | 2.3% |
| DIN6 | 22a | 0.8%a | 20a | 1.5%a | 2a | 0.2%a |
| EMN1 | 175 | 6.7% | 100 | 7.6% | 75 | 5.8% |
| EMN2 | 192 | 7.4% | 108 | 8.2% | 84 | 6.5% |
| EMN3 | 83 | 3.2% | 50 | 3.8% | 33 | 2.6% |
| EMN4 | 328 | 12.6% | 159 | 12.1% | 169 | 13.2% |
| IMN1 | 265 | 10.2% | 139 | 10.6% | 126 | 9.8% |
| IMN2 | 110a | 4.2%a | 71a | 5.4%a | 39a | 3.0%a |
| IMN3 | 392 | 15.1% | 188 | 14.3% | 204 | 15.9% |
| IMN4 | 314a | 12.1%a | 120a | 9.2%a | 194a | 15.1%a |
| SN1 | 75 | 2.9% | 34 | 2.6% | 41 | 3.2% |
| SN2 | 44 | 1.7% | 19 | 1.4% | 25 | 1.9% |
| Misc | 48 | 1.8% | 26 | 2.0% | 22 | 1.7% |
| Totals | 2596 | 100.0% | 1311 | 100.0% | 1285 | 100.0% |
Abundance of 20 classes compared between male (n = 6) and female (n = 6) patients. An omnibus chi-square test between classes and sex gave χ219 = 70.3 (P < .0001) with standard residuals indicating that 3 classes were differentially distributed; DIN6 and IMN2 were more abundant in males, whereas IMN4 was more abundant in females.
AIN, ascending interneuron; DIN, descending interneuron; EMN, excitatory motor neuron; IMN, inhibitory motor neuron; SN, sensory neuron.
Significant difference (P < .05).
Baskets of Axonal Varicosities Were Selectively Associated With Some Myenteric Nerve Cell Bodies
Immunohistochemically labeled varicose axons sometimes appeared to form denser rings or baskets around a proportion of myenteric nerve cell bodies. In previous studies, these have been shown to reflect local accumulations of synaptic inputs.34, 35, 36 Identifiable baskets were prominent in axons immunoreactive for 7 of 13 markers tested (ENK, SP, CGRP, SOM, VAChT, VIP, and NPY). Other markers (HuCD, NOS, ChAT, 5-HT, Calb, Calret) labeled varicose axons in ganglia, but these rarely formed distinctive baskets. Identifying baskets involved some subjectivity; to minimize bias, this process was carried out on anonymous nerve cell bodies identified only by HuCD labeling without information about other markers. It was beyond the scope of the study to test whether baskets actually represent functional synaptic connections between neurons.
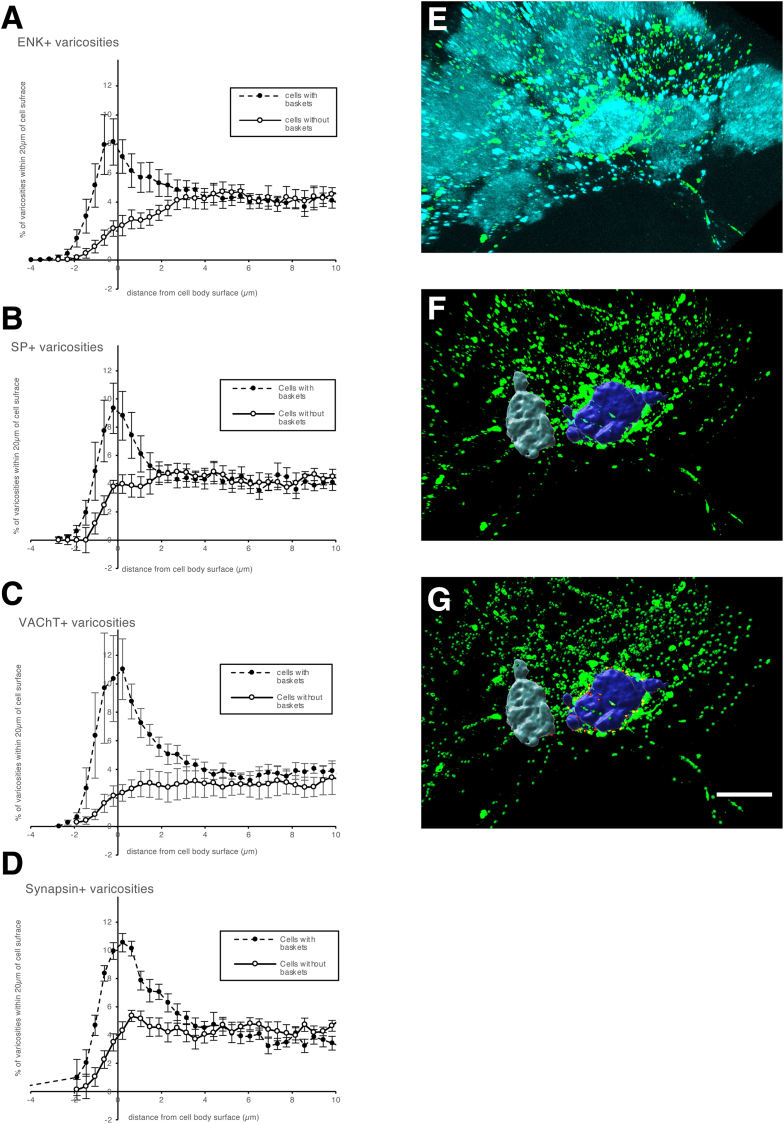
The distribution of varicosities from the surface of HuCD-labeled cell bodies was plotted for cells surrounded by baskets, compared with cells without baskets. SP, ENK, VAChT, and synapsin 1 baskets all had a peak in varicosities within 2 μm of the surface of cells with baskets, which was absent for cells without baskets. This suggests that visually identified baskets represent close associations between some axons and nerve cell bodies in the myenteric plexus of human colon (see Figure 6A–D), possibly indicating a degree of connectivity.
Figure 6.
Distribution of varicosities relative to cell body surface compared between cells with baskets and with no baskets. (A–D) Distribution of varicosities immunoreactive for ENK, SP, VAChT, and synapsin 1a/1b as 3-dimensional (3D) distance from nerve cell body, for baskets (dashed line) and nonbasket (solid line). Note the large peak of varicosities within 2 μm of the cell surface in baskets that was absent in cells that did not receive baskets (y-axis data are normalized). (E–G) 3D rendering of myenteric nerve cell bodies (HuCD+) and ENK+ varicosities (green), with 2 cells rendered in 3D (F) with closely apposing varicosities (within 2 μm) shown by red spheres (G); these are numerous around the purple soma (surrounded by a basket of varicosities) but less so around the gray cell (no basket). This shows that visually identified baskets make a higher proportion of close appositions to myenteric nerve cell bodies than is the case for cells not surrounded by identifiable baskets. (A–D) Data from 24 cells from 2 patients. (E–G) Calibration bar = 20 μm.
A total of 2247 baskets (with 1 of the 7 axonal markers listed previously) were identified among the 2596 cells; 1174 (45.2%) of 2596 neurons received 1 or more baskets. The most abundant were SP immunoreactive (surrounding 26.1% of 2596 neurons). Other abundant baskets were VAChT+ (24.7%) or ENK+ (surrounding 12.7% of nerve cell bodies) (see Figure 7).
Figure 7.
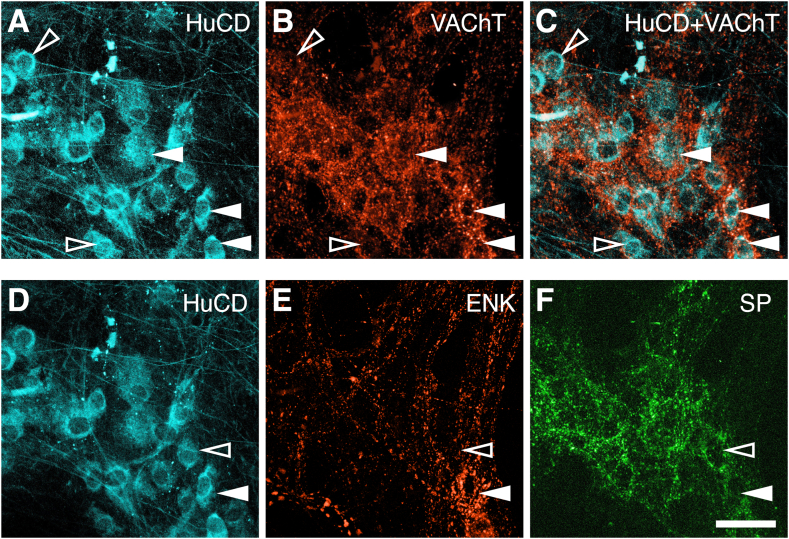
Baskets of labeled axonal varicosities surround some but not all myenteric nerve cell bodies. (A, D) Left micrographs show HuCD somata (cyan) that persisted through the elution process. (B) shows immunoreactivity for VAChT revealing dense rings of varicosities that in the merged image (C) are shown to surround nerve cell bodies (solid arrowheads); other neurons had fewer juxtaposed varicosities (open arrowheads). (D–F) Two types of baskets surrounded different myenteric nerve cell bodies. (E) A cell that has an ENK-immunoreactive basket (white arrowhead) is not targeted by an SP basket, (F) whereas a cell that was surrounded by an SP basket does not receive an ENK basket (open arrowhead) Calibration bar = 50 μm.
We identified whether any of the 20 classes of cell bodies were preferentially surrounded by baskets (see Table 11). Four classes of IMNs (IMN1–4) mostly received fewer baskets than expected if baskets were randomly distributed. Exceptions were VIP baskets onto IMN4 and CGRP baskets onto IMN1, which were more abundant than expected. In contrast, EMNs (EMN1–4) and AINs (AIN1–3) generally received more baskets than expected, especially those immunoreactive for ENK, SP, and VAChT. SP baskets and VAChT baskets colocated more often than predicted by their individual occurrences (χ21 = 300.3, P < .001). Likewise, ENK baskets and VAChT baskets were associated (χ21 = 241.5, P < .001). SP and ENK baskets also preferentially innervated the same neurons (χ21 = 93.7, P < .001). Both SP and ENK myenteric nerve cell bodies are nearly always ChAT immunoreactive (see Table 7); unsurprisingly, these 2 markers showed significant overlap with VAChT. Thus, visually identified baskets of axons, distinguished by ENK, SP, CGRP, SOM, VAChT, VIP, or NPY, are preferentially associated with particular classes of myenteric nerve cell bodies. We speculate that this reflects a degree of functional connectivity in enteric neural circuitry.
Table 11.
Abundance of Baskets of Varicosities Surrounding Each Class of Myenteric Neuron
| Class | No. Cells in Class | Cells With SP Baskets | Cells With ENK Baskets | Cells With CGRP Baskets | Cells With VAChT Baskets | Cells With SOM Baskets | Cells With VIP Baskets | Cells With NPY Baskets | Cells With Any Baskets |
|---|---|---|---|---|---|---|---|---|---|
| AIN1 | 54 | 11.1%a | 18.5%b | 16.7%b | 42.6%b | 7.4%b | 7.4%b | 5.6%b | 57.4%b |
| AIN2 | 71 | 38.0%c | 31.0%c | 9.9%b | 49.3%c | 8.5%b | 0.0%b | 5.6%b | 62.0%c |
| AIN3 | 126 | 31.7%b | 12.7%b | 4.8%b | 62.7%c | 16.7%c | 0.8%b | 12.7%c | 71.4%c |
| DIN1 | 41 | 9.8%a | 31.7%c | 7.3%b | 24.4%b | 7.3%b | 2.4%b | 4.9%b | 61.0%c |
| DIN2 | 15 | 20.0%b | 53.3%b | 6.7%b | 66.7%b | 26.7%b | 20.0%b | 33.3%b | 86.7%c |
| DIN3 | 127 | 29.9%b | 9.4%b | 4.7%b | 25.2%b | 8.7%b | 4.7%b | 3.9%b | 50.4%b |
| DIN4 | 62 | 25.8%b | 8.1%b | 3.2%b | 19.4%b | 6.5%b | 4.8%b | 1.6%b | 41.9%b |
| DIN5 | 52 | 23.1%b | 11.5%b | 0.0%b | 15.4%b | 7.7%b | 0.0%b | 3.8%b | 38.5%b |
| DIN6 | 22 | 54.5%c | 27.3%b | 0.0%b | 31.8%b | 13.6%b | 0.0%b | 0.0%b | 72.7%c |
| EMN1 | 175 | 39.4%c | 61.1%c | 0.6%b | 49.1%c,d | 9.1%b | 0.0%b | 8.0%b | 76.6%c |
| EMN2 | 192 | 58.3%c | 22.4%c | 2.1%b | 46.4%c,d | 31.8%c | 1.0%b | 22.4%c | 82.8%c |
| EMN3 | 83 | 32.5%b | 33.7%c | 1.2%b | 50.6%c,d | 14.5%b | 2.4%b | 7.2%b | 74.7%c |
| EMN4 | 328 | 59.1%c | 7.6%a | 0.9%a | 35.4%c,d | 28.4%c | 1.8%b | 21.3%c | 75.0%c |
| IMN1 | 265 | 17.0%a | 0.4%a | 6.0%c | 8.3%a,d | 0.8%a | 0.0%a | 1.1%a | 24.9%a |
| IMN2 | 110 | 10.9%a | 1.8%a | 1.8%b | 8.2%a,d | 2.7%a | 9.1%b | 1.8%a | 23.6%a |
| IMN3 | 392 | 3.6%a | 0.3%a | 1.5%b | 2.6%a,d | 0.5%a | 0.3%a | 1.3%a | 6.9%a |
| IMN4 | 314 | 4.8%a | 0.3%a | 1.0%a | 3.8%a,d | 1.0%a | 8.3%c | 1.0%a | 17.5%a |
| SN1 | 75 | 13.3%a | 13.3%b | 1.3%b | 28.0%b | 9.3%b | 2.7%b | 6.7%b | 41.3%b |
| SN2 | 44 | 15.9%b | 13.6%b | 0.0%b | 22.7%b | 2.3%b | 0.0%b | 0.0%b | 29.5%a |
| Misc | 48 | 31.3%b | 14.6%b | 4.2%b | 18.8%b | 8.3%b | 0.0%b | 10.4%b | 54.2%b |
| total | 2596 | 678 | 329 | 73 | 642 | 264 | 67 | 194 |
AIN, ascending interneuron; CGRP, calcitonin gene-related peptide; DIN, descending interneuron; EMN, excitatory motor neuron; IMN, inhibitory motor neuron; NPY, neuropeptide Y; SN, sensory neuron; SOM, somatostatin; SP, substance P; VIP, vasoactive intestinal polypeptide.
Significant negative association (P < .05)
Nonsignificant proportion.
Significant positive association (P < .05).
Note difference between EMNs and IMNs and their innervation by VAChT-immunoreactive varicosities.
Size and Morphology of Nerve Cell Bodies
The sizes of nerve cell bodies (vertical projection area) were measured from HuCD labeling (see Table 12). The largest neurons were Dogiel type II neurons (SN1: 1788 ± 757 μm2, n = 75; SN2: 1322 ± 743 μm2) and 5-HT–containing DINs (DIN1: 1198 ± 501 μm2; DIN2: 1445 ± 454 μm2). Putative motor neurons made up many of the smaller cell bodies (EMN1–4; 778 cells: 466 ± 225 μm2). IMNs were significantly larger (IMN1–4: 604 ± 298 μm2, n = 1081; t1857 =10.84, P < .0001). Retrograde tracing studies have previously reported that motor neurons generally have small cell bodies.28,29
Table 12.
Sizes of Nerve Cell Bodies in Each Class
| Class | AIN1 | AIN2 | AIN3 | DIN1 | DIN2 | DIN3 | DIN4 | DIN5 | DIN6 | EMN1 | EMN2 | EMN3 | EMN4 | IMN1 | IMN2 | IMN3 | IMN4 | SN1 | SN2 | Misc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. cells | 54 | 71 | 126 | 41 | 15 | 127 | 62 | 52 | 22 | 175 | 192 | 83 | 328 | 265 | 110 | 392 | 314 | 75 | 44 | 48 |
| Average | 997 | 647 | 787 | 1198 | 1445 | 868 | 546 | 886 | 597 | 574 | 517 | 496 | 373 | 814 | 575 | 577 | 472 | 1788 | 1322 | 462 |
| SD | 442 | 225 | 374 | 508 | 470 | 468 | 238 | 534 | 283 | 263 | 255 | 189 | 142 | 413 | 233 | 218 | 173 | 762 | 752 | 308 |
| Median | 896 | 659 | 743 | 1058 | 1524 | 725 | 483 | 753 | 534 | 529 | 458 | 472 | 352 | 684 | 546 | 533 | 444 | 1905 | 1189 | 422 |
| Quartile 1 | 639 | 463 | 491 | 810 | 1121 | 575 | 378 | 483 | 380 | 425 | 349 | 374 | 271 | 521 | 416 | 421 | 355 | 1228 | 709 | 314 |
| Quartile 3 | 1210 | 785 | 928 | 1544 | 1826 | 1063 | 645 | 1037 | 795 | 649 | 596 | 561 | 432 | 1057 | 648 | 693 | 565 | 2251 | 1895 | 505 |
| Largest | 2722 | 1182 | 2109 | 2728 | 2189 | 2786 | 1282 | 2445 | 1183 | 2401 | 1782 | 1425 | 971 | 2842 | 1486 | 2025 | 1145 | 3623 | 3098 | 2306 |
| Smallest | 387 | 191 | 214 | 389 | 442 | 180 | 210 | 243 | 211 | 179 | 136 | 164 | 141 | 206 | 179 | 166 | 139 | 543 | 251 | 190 |
All measurements were in units of μm2. Soma-dendritic area was measured from HuCD immunoreactivity for each cell as its vertical projection. Data are displayed graphically in Figure 8. The largest cells were Dogiel type II putative SNs (SN1 and SN2) and the 5-HT–containing descending interneurons (DIN1, DIN2). Most of the smallest classes of cells were EMNs or IMNs.
AIN, ascending interneuron; DIN, descending interneuron; EMN, excitatory motor neuron; HuCD, Hu C or D protein; IMN, inhibitory motor neuron; SN, sensory neuron.
We also classified the soma-dendritic morphology because this has been widely used historically to distinguish types of human enteric nerve cells37,38 using a scheme modified from a recent study38 using NF200 immunoreactivity, to distinguish 7 morphological types: (1) cells without NF200 labeling (NF200–); (2) simple cells with a single axon, small oval cell bodies with small or absent dendrites; (3) spiny cells with a single axon and longer dendrites, sometimes branching, with sharp spiny ends; (4) stubby cells with short club-like dendrites and a single axon; (5) Dogiel type II cells with 2 or more tapering axons (including pseudounipolar neurons) usually with large ovoid cell bodies; (6) type III cells, uniaxonal cells with extended long thin radiating dendrites; and (7) type V cells, with a single axon and filamentous dendrites arising from the axon base.
A total of 7 type III cells and 2 type V cells were encountered; these were excluded from subsequent statistical analysis, as were Dogiel type II neurons, which had been defined by their multiaxonal morphology.
Just over half of all cells were NF200– (51.9%), for which morphological details could not be determined adequately. Simple, spiny, and stubby cells (see Figure 8) comprised approximately equal proportions, which did not differ between ascending and descending colon (χ23 = 4.83, P < .305 [not significant]). However, there were significant nonuniform distributions of 5 morphological types between all 20 classes (omnibus χ276 = 5038, P < .0001) (see Table 13). In brief, spiny and stubby morphologies tended to be associated with ascending and descending interneurons, whereas motor neurons were more likely to be simple or NF200– cells. Consistent with this, simple and NF200– cells were the smallest morphological types (Figure 8A–E).
Figure 8.
Morphological types of myenteric neurons in human colon labeled with NF200 and distribution of their soma size measured as vertical projection in μm2. (A) Filled arrowheads show NF200– or faint cells; open arrows show NF200+ cells. (B) Simple cells without visible dendrites are indicated. (C) Arrowheads point to spiny cells with single axons and short narrow dendrites: (D) Arrowheads indicated stubby cells with short blunt dendrites. (E) Arrowheads indicate multiaxonal Dogiel type II neurons with large smooth cell bodies. Calibration bar = 50 μm.
Table 13.
Distribution of Morphological Types of Cells Between Classes
| NF200– | Simple | Spiny | Stubby | Total cells per Class | |
|---|---|---|---|---|---|
| AIN1 | 5 (9.4%)a | 0 (0.0%)a | 33 (62.3%)b | 15 (28.3%)b | 53 |
| AIN2 | 8 (11.4%)a | 8 (11.4%)c | 21 (30.0%)b | 33 (47.1%)b | 70 |
| AIN3 | 17 (13.6%)a | 3 (2.4%)a | 66 (52.8%)b | 39 (31.2%)b | 125 |
| DIN1 | 5 (12.2%)a | 3 (7.3%)c | 20 (48.8%)b | 13 (31.7%)b | 41 |
| DIN2 | 1 (7.1%)a | 0 (0.0%)c | 9 (64.3%)b | 4 (28.6%)c | 14 |
| DIN3 | 10 (7.9%)a | 19 (15.1%)c | 46 (36.5%)b | 51 (40.5%)b | 126 |
| DIN4 | 59 (95.2%)b | 0 (0.0%)a | 1 (1.6%)a | 2 (3.2%)a | 62 |
| DIN5 | 3 (6.0%)a | 6 (12.0%)c | 26 (52.0%)b | 15 (30.0%)b | 50 |
| DIN6 | 9 (40.9%)c | 3 (13.6%)c | 4 (18.2%)c | 6 (27.3%)c | 22 |
| EMN1 | 19 (10.9%)a | 42 (24.1%)b | 54 (31.0%)b | 59 (33.9%)b | 174 |
| EMN2 | 26 (13.5%)a | 100 (52.1%)b | 30 (15.6%)c | 36 (18.8%)c | 192 |
| EMN3 | 82 (98.8%)b | 0 (0.0%)a | 0 (0.0%) | 1 (1.2%)a | 83 |
| EMN4 | 322 (98.2%)b | 4 (1.2%)a | 1 (0.3%) | 1 (0.3%)a | 328 |
| IMN1 | 28 (10.6%)a | 81 (30.7%) | 103 (39.0%)b | 52 (19.7%)c | 264 |
| IMN2 | 16 (14.5%)a | 55 (50.0%) | 15 (13.6%)c | 24 (21.8%)c | 110 |
| IMN3 | 388 (99.0%)b | 3 (0.8%)a | 1 (0.3%)a | 0 (0.0%)a | 392 |
| IMN4 | 313 (99.7%)b | 1 (0.3%)a | 0 (0.0%)a | 0 (0.0%)a | 314 |
| Misc | 36 (75.0%)b | 5 (10.4%)c | 3 (6.3%)c | 4 (8.3%)c | 48 |
| SN1 | — | — | — | — | — |
| SN2 | — | — | — | — | — |
| All cells | 1347 (54.6%) | 333 (13.5%) | 433 (17.5%) | 355 (14.4%) | 2468 (100%) |
Values are n and (% of cells of that class). Morphological types of neurons defined by NF200 immunoreactivity; distribution between classes. After 119 Dogiel type II neurons, 7 type 3 cells and 2 type 5 cells were excluded, 2468 neurons belonging to 18 classes remained. A forced-choice paradigm was used to classify each cell as NF200–, simple, spiny or stubby modified from a previous study.38 Seventeen of 18 classes showed significant associations with specific morphological types; 1 class (DIN6) had too few cells to analyze reliably. This suggests that chemical coding is often associated with specific morphological types, but soma-dendritic morphology may not be a good predictor of class.
AIN, ascending interneuron; DIN, descending interneuron; EMN, excitatory motor neuron; IMN, inhibitory motor neuron; NF200, neurofilament 200 kD; SN, sensory neuron.
significant negative association with morphological type (P < .05)
significant positive association with morphological type (P < .05)
no significant association with morphological type
Discussion
Using a multiplexed immunohistochemical process with 12 antisera applied to 2596 neurons (n = 12), human colonic myenteric neurons were subdivided by coexistence of markers (ie, their chemical coding) into 20 classes. These classes varied in soma-dendritic morphology, soma size, and the extent to which they were associated with dense baskets of varicose axons . Comparisons between ascending and descending colon, and between males and females were assessed statistically. Many of the 12 cell body markers have previously been used in ex vivo retrograde tracing and immunohistochemical studies, allowing targets and probable functions to be identified. This approach complements recent studies based on single-cell RNA sequencing (RNA-seq).9,39 Evidence was provided for 8 classes of excitatory and IMNs and 9 classes of interneurons, suggesting considerable integrative capability of human myenteric circuitry.
Multiplexed Immunohistochemistry
In conventional immunohistochemical studies, the 3–4 antisera that can be applied simultaneously are often insufficient to assign all neurons to classes. Several methods allow more than 3–4 markers to be assayed,11,40, 41, 42 including antibody elution.11,43 Here, this approach allowed 5 layers of quadruple labeling to be applied successively without interference from previous layers. Modifications from the original published method11 included use on wholemount tissues rather than sections, increased elution time (60 minutes) and use of an indelible pan-neuronal marker (HuCD visualized with biotinylated secondary antisera) for registration of nerve cell bodies between layers. The order of staining, combinations of primary antisera in each layer, concentrations of primary and secondary antisera, and choice of fluorophore were optimized before data gathering started. Micrographs were taken after every bout of elution to confirm complete removal of labeling (Figure 1). Using these methods, each nerve cell body could be judged as immunoreactive or nonimmunoreactive for all 12 cell body markers.
Combinations of neurochemical markers denote a chemical coding of neurons.44,45 In the guinea pig small intestine, 16 classes of myenteric neurons were distinguished using 8 selective immunohistochemical markers, applied in multiple combinations.8 Similar approaches have been used in guinea pig stomach,46 guinea pig distal colon,47 and small and large intestine of the mouse.25,48 A recent study in human stomach, small intestine, and colon combined immunohistochemical markers and cell morphology.38 A potential weakness of all of these studies was a lack of statistical validation. In the present study, a statistical approach was used to determine whether markers showed significant coexistence. This worked as follows. A group of cells could be divided into 2 subgroups by immunoreactivity for a marker; X (ie, into X+ and X-). If marker Y coexists significantly with X, then the X+ group will contain a higher proportion of Y+ cell than the X- group (ie, a nonuniform distribution). This can be tested with a chi-square test (with Bonferroni corrections for multiple tests). For each group of neurons, every marker was tested to identify which was most discrminating when used to divide the group (ie, created the most non-uniform distribution of other markers).
Comparison With Single-Cell RNA-Seq
A recent study sequenced 1445 nuclei identified as belonging to human enteric neurons (1347 of myenteric origin, 98 submucosal)9 and grouped them into 14 clusters assaying 4300 genes per nucleus. They tentatively identified 4 types of EMN, 5 types of IMN, 2 interneurons, 1 type of SN, and 2 vasomotor/secretomotor neurons. The present study gave somewhat comparable results, distinguishing 4 classes of EMNs, 4 classes of IMNs, and 2 classes of SNs. However, many more classes of DINs (n = 6) and AINs (n = 3) were distinguished, comparable to retrograde tracing studies which identified 8 classes of interneuron.27,30,32 The same RNA-seq study reported more classes of enteric neurons in mouse colon (18 or 21) than human.9 Another single cell RNA- analysis reported 14 clusters of mouse colon myenteric neurons49 demonstrating variability between RNA-seq studies using different methods.
This raises the question of how 12 immunohistochemical cell body markers could distinguish similar numbers of classes to single-cell RNA-seq, which typically measures thousands of mRNA species. Unsupervised clustering of single-cell RNA-seq is powerful but has limitations. It is susceptible to significant variation arising from tissue handling, batch effects, variations in sequencing depth, uneven coverage, dropout of low-frequency transcripts, and high sample heterogeneity.50 Cluster analysis of transcriptomic data usually requires initial reduction of dimensionality with principal component analysis followed by t-distributed stochastic neighbor embedding or Uniform Manifold Approximation and Projection routines. Assumptions made at this stage, for example, the values of k and setting manually defined parameters for resolution, influence subsequent clustering.51 Linear reduction of dimensionality by principle component analysis yields highly variable results.52 In addition, there is no accepted standard to validate the results of the clustering process.50 Transcriptome analysis, on its own, may not identify all classes reliably. For example, in mouse primary visual cortex, previously overlooked classes were detected when axonal projections were combined with gene expression profiles.53 Unsupervised RNA-seq analysis in well-characterized crustacean pyloric and cardiac ganglia also did not distinguish all known neuron types.54 However, combining transcriptomic analysis with other data modalities can lead to better discrimination.55 An extensive, systematic analysis of mammalian primary motor cortex shows what can be achieved with multimodal approaches.56 However, at present it may be difficult to apply these methods to human enteric neurons, which are dispersed, embedded in connective tissue, with multiple target tissues (eg, smooth muscle) nearby.57 We suggest that for the time being, immunohistochemical coding and single-cell RNA-seq data on human enteric neurons may be considered as complementary. This could be tested by determining whether the mRNAs corresponding to the 12 cell body markers (NOS1, CHAT/SLC5A7, TPH2/SLC6A4, TAC1, PENK, CALCA/CALCB, SST, CALB1, CALB2, NEFH, VIP, NPY) are expressed in similar combinations to the cognate antigens, in a cell-by-cell analysis. In future, it may be possible to test whether signature genes identified in single-cell RNA-seq studies are selectively expressed in immunohistochemically defined classes using selective antisera or in situ hybridization chain reaction.49
Species and Regional Differences
Myenteric neurons of human colon share many features with enteric neurons in other species and other regions of gut. Nearly all human myenteric neurons express either NOS or ChAT, with a small number of presumed interneurons30 expressing both.10,17,18 Multiaxonal (Dogiel type II) neurons comprise a minority of myenteric nerve cells and nearly all are cholinergic (ChAT immunoreactive), but they rarely express NOS.8 Interneurons that express 5-HT are cholinergic and all have aborally directed projections.27 However, there are some striking differences between animal and human colonic neurons. Calretinin is mostly a marker of DINs and multiaxonal myenteric neurons in human colon27 but is almost absent in motor neurons.27 However, it is a marker for EMNs in mice.48 In human small intestine, 93% of myenteric multiaxonal neurons were immunoreactive for SOM,58 compared with just 19% in the colon. The functional significance of such differences is unclear, but they emphasize that chemical coding can be region- and species-specific.59
Sex Differences
Sex differences in adult human colonic function are well established: women have slower colonic transit than men and have a higher prevalence of constipation and functional disorders, such as irritable bowel syndrome.60 We tested whether there were differences in the myenteric plexus between the sexes. The cytoskeletal protein marker NF200 was slightly more abundant in males compared with females and the proportions of 3 classes also differed between the sexes; males had more DIN6 and IMN2 and fewer IMN4. Differences in mRNA expression in myenteric ganglia have previously been reported between human males and females.49 Immunohistochemically, sex differences have been reported in animals; coexistence of CGRP with SP and VAChT differs between male and female pigs.61 Female mice show changes in NOS immunoreactivity in the enteric nervous system throughout the estrous cycle.62 In the present study, all female tissue donors were 60 years or older and likely to be in postmenopause, making it less likely that cyclic endocrine influences were operating. Thus the present study suggests that there may be constitutive sex differences in colonic neuronal circuitry.
Influence of Age
Specimens came from patients ranging from 58 to 80 years who were undergoing surgery for removal of colon cancer. It is possible, but currently untestable, that results would be different if the study had included specimens from younger disease-free individuals. Regression analysis did not detect significant age-associated changes in markers or classes, but given the restricted age range, this study was not well structured to detect an association. Total nerve cell number may decrease with age in human colonic myenteric plexus,63, 64, 65, 66 leading to cavities in the ganglia. Such cavities65 were observed in specimens in the present study, consistent with the age of patients. Whether chemical coding changes with age is less clear. It has been reported that colonic mRNAs for NOS1 and ChAT differ significantly between infants and adults,67 and ChAT immunoreactivity increased with age in human ascending colon, with no changes in NOS1.68 The small cohort of patients studied in the present study did not have the statistical power to test whether NOS and ChAT levels change in ageing adults.
Morphology of Enteric Neurons
Soma-dendritic features of enteric neurons were studied by Dogiel6; his foundational work has been expanded37 and made more robust by using immunohistochemical labeling of cytoskeletal filaments.38 In the present study, immunoreactivity for NF200 clearly distinguished several morphological types; most with short dendritic processes, confirmed by intracellular dye fills.69 However, the density of labeled neurites made it difficult to determine detailed morphology of some neurons. In addition, nearly half of the neurons were labeled at low intensity or not at all by NF200. For these reasons, we did not use soma-dendritic morphology for clustering myenteric neurons. Irrespective, there were highly significant differences in morphological types in nearly all of the twenty classes (table 13), consistent with recent reports.38 The functional significance of enteric soma-dendritic morphology is not clear, however many SNs in the body are multiaxonal or pseudo-unipolar, reflecting axonal specialization for transduction and neurotransmitter release. This was a defining feature of Dogiel type II neurons (SN1 and SN2). It has been reported for sympathetic neurons that larger dendritic trees are associated with large numbers of preganglionic synaptic inputs70; whether this holds true for human enteric neurons remains to be determined.
Classes Not Identified
Several known classes of enteric neurons are missing from the current account. Viscerofugal neurons project from the myenteric plexus to sympathetic prevertebral ganglia71 and secretomotor and vasomotor neurons project to the mucosa. It was not possible to distinguish them by the antisera used in the present study; but it has been suggested that CART or GLP1-receptors may be selective markers in mice.72,73 Secretomotor and vasomotor neurons are abundant in submucosal plexuses, but whether they are present in human colonic myenteric plexus is unclear. In human colon, 79% of submucous neurons have VIP and Calret coexisting,74 but this population only accounted for 3.4% of myenteric neurons, and many of these are probably DINs (DIN5).27 Using retrograde tracing, myenteric neurons projecting to the mucosa were not detected in adult small and large intestine31 but may exist in infant small intestine.75 In a single cell sequencing study, 2 classes of secretomotor/vasomotor neurons were described in human colonic neurons, but it is not clear whether these were from the 1347 myenteric neurons or the 98 submucosal neurons included in the samples.9
Associations of Myenteric Nerve Cell Bodies With Baskets of Varicosities
Seven of 12 markers made dense, basket-like clusters of varicosities around some HuCD+ nerve cell bodies. Baskets, identified at light microscopy, contain abundant ultrastructural synapses34, 35, 36,76,77 and may represent a concentration of synaptic inputs. Consistent with this, there was a peak in varicosity density close (<2 μm) to the surface of cells with baskets (Figure 6) but not in cells without baskets. Deciding whether a cluster of varicosities forms a 3-dimensional basket has some subjectivity. This potential source of bias was minimized by having observers record whether each anonymous HuCD-immunoreactive cell body was surrounded by a basket before other information about markers was available. Three markers formed very dense baskets: VAChT, SP, and ENK (Figure 7). VAChT varicosities could arise from any of the 14 classes of cholinergic neurons, or from extrinsic cholinergic parasympathetic efferents.78 SP-immunoreactive varicosities may come from cholinergic Dogiel type II SNs (92.4% SP immunoreactive) or from cholinergic AINs (21% of AINs were immunoreactive for SP).27 Enkephalin immunoreactive baskets probably represent inputs from 3 classes of cholinergic AINs (AIN1–3). Because ChAT, ENK, and SP frequently coexist in myenteric nerve cell bodies, it is unsurprising that their baskets also overlapped significantly. These 3 types of baskets were abundant around AINs and EMNs (Table 11) but were under-represented around IMNs. Thus these baskets are likely to be involved in pathways mediating ascending excitatory reflexes, identified over 100 years ago,79 which have cholinergic and non-cholinergic components in human colon.80 Interestingly, baskets of extrinsic sympathetic axons innervating human colon also preferentially surround cholinergic rather than nitrergic neurons.81
Types of Neurons: Functional Implications
Large multiaxonal neurons, (Dogiel type II) formed 2 classes (SN1, SN2). From animal studies, these are likely to be primary afferent neurons,82 activated by mechanical stimuli, which excite many classes of enteric neurons,83,84 initiating motor responses. In several species, type II cells are immunoreactive for ChAT85 and tachykinins.86 At least 2 subgroups of Dogiel type II neurons exist in guinea pig small intestine myenteric plexus, based on soma-dendritic morphology and projections.87 In humans, SN1 and SN2 were distinguished by Calret immunoreactivity and differed significantly in expression of 5-HT, SOM, Calb, CGRP, and soma size. Whether they have different physiology or connectivity remains to be determined.
Three classes of AINs (AINs 1–3) were distinguished. Retrograde labeling ex vivo suggested 4 types, but without statistical validation.27 AIN1–3 were all cholinergic (ChAT+) and ENK immunoreactive, consistent with the dense baskets of varicosities observed around AINs and EMNs. Strikingly, 6 types of DINs were distinguished (DIN1–6). Two of these were cholinergic with 5-HT immunoreactivity, subdivided by NOS immunoreactivity. 5-HT is restricted to neurons with aborally directed axons.27 Two other classes were immunoreactive for both NOS and ChAT, making it unlikely that they were motor neurons. Another 2 classes had ChAT and VIP without NOS. The connectivity and roles of these putative interneurons are currently unknown but it can be speculated that they play roles in descending inhibitory and excitatory reflexes and in generating the multiple patterns of motility present in human colon.16
Eight classes of putative motor neurons were identified. Retrograde tracing studies have shown that longitudinal and circular muscle layers are innervated by separate populations of both excitatory cholinergic and inhibitory (nitrergic) motor neurons, accounting for 4 classes. Subtypes of circular muscle EMNs have been distinguished by baskets of inputs in mouse colon88 and retrograde tracing distinguished long and short motor neurons to circular muscle of guinea pig small intestine.89 Some enteric motor neurons may use neuropeptides as transmitters to smooth muscle. Here, VIP discriminated 2 IMN populations, consistent with retrograde tracing of VIP-containing IMNs.28,29 A proportion of EMNs express tachykinins,32 which contribute to colonic peristaltic contractility.90 The physiological significance of multiple types of motor neurons is not clear but raises the intriguing possibility that there may be parallel final common pathways in the neural circuits controlling motility.
Conclusions
Using an immunohistochemical approach, 8 classes of putative motor neurons, 3 AINs, 6 DINs, and 2 classes of multiaxonal intrinsic SNs were identified in human colon. The number of classes may reflect the complex repertoire of motor patterns observed in human colon with at least 5 different propagating motor patterns identified.16 Motor neurons are likely to be the final common pathway underlying these patterns; interneurons may play a role in generating and switching between them. The classes of human colonic myenteric neurons identified are comparable to those of laboratory animals but show distinct combinations of features and neurochemical markers.
Materials and Methods
For the main part of the study using the multilayer immunohistochemistry protocol, colonic tissue was obtained from 12 patients undergoing elective large bowel surgery for cancer, with prior written informed consent (Southern Adelaide Clinical Human Research Ethics approval 207.17; 6 women; 6 men) (see Table 1). Tissue from a further 3 patients was analyzed using other protocols (2 male, 1 ascending, 1 descending, 1 sigmoid colon, age 65 ± 9 years). In all cases, surgery was for removal of nonobstructing tumors; specimens (2 × 2 cm to 8 cm × full circumference) were taken, under the surgeon's supervision, from uninvolved ends of resected bowel. Tissue was transported to the laboratory in room-temperature oxygenated Krebs solution (NaCl 118mM, KCl 4.8mM, CaCl2 2.5mM, MgSO4 1.2mM, NaHCO3 25mM, NaH2PO4 1.0mM, glucose 11mM, bubbled with 95% O2, 5% CO2, pH 7.4, containing 1 μM nicardipine and 1 μM hyoscine).
The specimen was pinned under tension and mucosa and submucosa were removed by sharp dissection in a SYLGARD-lined petri dish (Dow Corning, Midland, MI). The serosa was then dissected off, the preparation was washed in sterile Krebs solution and incubated overnight in Dulbecco’s Modified Eagle Medium/F12 medium with 10% v/v fetal bovine serum, 100 IU/mL penicillin, 100 μg/mL streptomycin, 20 μg/mL gentamicin, 2.5 μg/mL amphotericin, 1 μM nicardipine, and 1μM hyoscine (all from Sigma-Aldrich/Merck, Bayswater, Australia) at 37 °C in 5% CO2. Colchicine was added at a final concentration of 83 μM. In the last 30 minutes, preparations were treated with a monoamine oxidase inhibitor, pargyline (50 μM) then 10 μM 5-HT mesylate, to load 5-HT–accumulating neurons.20
The preparation was then rinsed in phosphate-buffered saline (PBS) (0.15 M NaCl with 0.01 M phosphate buffer, pH 7.2), repinned under maximal tension, and immersion-fixed in 4% formaldehyde for 24 hours at 4 °C, then for a further 24 hours in at room temperature with agitation. Preparations were rinsed in PBS and dissected to remove circular muscle, exposing the myenteric plexus. A sample was cut from the thin intertaenial region (approximately 8–10 mm2) to create wholemounts of myenteric plexus. They were immersed in PBS with 0.5% Triton X100 for 48 hours at room temperature. Each layer of immunohistochemical staining used mixed primary antisera diluted in hypertonic PBS at room temperature for 3 days, followed by PBS washes, followed by secondary antisera overnight, then further PBS washes. Primary and secondary antisera are summarized in Table 14. The preparation was immersed in bicarbonate buffered glycerol (pH 8.6) then mounted between 2 coverslips mounted on a plastic frame, to allow viewing from either side.
Table 14.
Antisera Used for Multilayer Immunohistochemistry
| Antigen | Species | Source | Code | RRID | Dilution | Secondary Antisera | |
|---|---|---|---|---|---|---|---|
| Layer 1 | HuCD | Mouse | Molecular Probes | A-21271 | AB_221448 | 1:200 | Donkey-mouse-biotin, Streptavidin-AMCA |
| NOS | Sheep | P. Emson | K205 | AB_2314957 | 1:5000 | Donkey-sheep-AF488 | |
| ChAT | Rabbit | M. Schemann | P3/Yeb | AB_2314176 | 1:1000 | Donkey-rabbit-Cy3 | |
| 5-HT | Rat | C. Cuello | YC5/45/HL | AB_449517 | 1:300 | Donkey-rat-Cy5 | |
| Layer 2 | HuCD | ||||||
| SP | Rabbit | ImmunoStar | 20064 | AB_572266 | 1:1000 | Donkey-rabbit-DTAF | |
| L-ENK | Mouse | SeraLab | MAS 083c | AB_572247 | 1:200 | Donkey-mouse-Cy3 | |
| CGRP | Goat | Biogenesis | 1720-9007 | AB_2290729 | 1:500 | Donkey-goat-AF647 | |
| Layer 3 | HuCD | ||||||
| VAChT | Rabbit | Synaptic Syst | 139 103 | AB_887864 | 1:1000 | Donkey-rabbit-DTAF | |
| Somatostatin | Rat | Abcam | ab30788 | AB_778010 | 1:200 | Donkey-rat-Cy3 | |
| NPY | Sheep | WW Blessing | E2210 | AB_2783534 | 1:2000 | Donkey-sheep-Cy5 | |
| Layer 4 | HuCD | ||||||
| Calbindin | Rabbit | SWANT | CB-38 | AB_2721225 | 1:5000 | Donkey-rabbit-DTAF | |
| Calretinin | Goat | SWANT | CG1 | AB_10000320 | 1:1000 | Donkey-goat-Cy3 | |
| TH | Mouse | Diasorin | 136032 | AB_572268 | 1:500 | Donkey-mouse-Cy5 | |
| Layer 5 | HuCD | ||||||
| VIP | Rabbit | J. Walsh | 7913 | AB_2783533 | 1:2000 | Donkey-rabbit-DTAF | |
| NF200 | Mouse | Sigma | N0142 | AB_477257 | 1:1000 | Donkey-mouse-Cy3 | |
| Other antisera | Peripherin | Chicken | Abcam | ab39374 | AB_777207 | 1:200 | Donkey-chicken-AF647 |
| Synapsin1a & 1b | Rabbit | Biogenesis | 8478-9007 | AB_618496 | 1:200 | Donkey-rabbit-DTAF |
Primary and secondary antisera used for multilayer immunohistochemistry are summarized. Between each of the 5 layers, staining was eluted (see Materials and Methods). Note that HuCD was visualized with a biotinylated secondary antiserum followed by streptavidin-AMCA: this staining persisted through the elution process while other antisera, revealed with fluorophore-coupled secondary antisera, were removed.
CGRP, calcitonin gene-related peptide; ChAT, choline acetyltransferase; ENK, leu enkephalin; HuCD, Hu C or D protein; NF200, neurofilament 200 kD; NOS, neuronal nitric oxide synthase; NPY, neuropeptide Y; SP, substance P; VIP, vasoactive intestinal polypeptide.
After viewing, analysis, and photography, preparations were unmounted and rinsed in PBS. Then antisera were eluted using 50 mL of a solution containing 2% w/v sodium dodecyl sulfate, 62.5 mM Tris-HCl (pH 6.8), and 0.8% mercaptoethanol, at 56 °C for 60 minutes11 with agitation. Destained preparations were remounted and photographed with bracketed exposures to check that antisera had been fully removed. Preparations were then washed in PBS and the next layer of primary and secondary antisera was applied followed by mounting in buffered glycerol (see Figure 1) This process was repeated for a total of 5 layers of immunohistochemical staining on each of the 12 preparations. A total of 14 antisera were applied (HuCD [a pan-neuronal marker] with 12 type-specific cell body markers: NOS, ChAT, 5-HT, SP, ENK, CGRP, SOM, NPY, Calb, Calret, VIP, and NF200). VAChT was also included and primarily labeled axonal varicosities. Additional markers (tyrosine hydroxylase and peripherin) were added in some preparations but were not included in the analysis.
Preparations were viewed on an Olympus IX71 microscope (Olympus, Tokyo, Japan) fitted with epifluorescence and appropriate filter sets (Chroma Color Corporation, McHenry, IL). Images were captured by a monochrome CoolSNAP ES digital camera (Roper Scientific; Photometerics, Tucson, AZ) using analySIS 5.0 (Soft Imaging System, Adelaide, Australia). Confocal images were taken on an LSM 880 microscope (Zeiss, Oberkochen, Germany). Between 2 and 6 ganglia were chosen for analysis; ganglia with adhering circular muscle were rejected because this interfered with penetration of antisera.
Nomenclature
Cells were scored as immunoreactive (eg, ENK+) or non-immunoreactive (eg, ENK–) for each marker, based on labeling, compared with background. The cell body markers are listed in the order in which 12 antisera were applied; NOS, ChAT, 5-HT, SP, ENK, CGRP, SOM, Calb, Calret, NF200, VIP, NPY. We created a naming system for each combination of markers by assigning a binary value to each immunoreactive marker, in this order; NOS+ = 1, ChAT+ = 2, 5-HT+ = 4, SP+ = 8, ENK+ = 16, CGRP+ = 32, SOM+ = 64, Calb+ = 128, Calret+ = 256, NF200+ = 512, VIP+ = 1024, NPY+ = 2048). Markers that showed no immunoreactivity were assigned a value of 0. By adding all values, a unique code number (from 0 to 4095) was generated for each combination of markers. Thus a cell with ChAT+/ENK+ (but no other markers) would have a code of 18 (ie, 2+16). A cell expressing ChAT, ENK, and Calb would have a code of 146 (2+16+128). Codes were simply used to keep track of the 164 combinations of cells and were useful for data handling (codes are spelled out in Table 5). However, they depend on order of markers studied: thus the codes only apply to the present study; they are not intended as a general system of nomenclature of enteric neurons.
Analysis of Classes
Several methods were tried to cluster the data. These included a mixture model, where K mixtures (clusters) of M Bernoulli variables (one per marker) were fitted. Hamiltonian Monte Carlo with the Stan probabilistic programming language (v2.19.11 - https://mc-stan.org/) was used to perform the fit, for K = 2 to K = 12. Using this approach, only 6–7 clusters were robustly detected, and for K > 7, the remaining clusters were of negligible size, with Bernoulli parameters corresponding to priors, and thus uninformed by the data. Adding cell size to the mixture, as a log-normally distributed factor, only increased the cluster count to 7–8. As this was fewer classes than are known to exist, this approach was discarded.
A divisive hierarchical analysis was then used to identify classes of cells. The first decision point divided cells into multiaxonal (Dogiel type II) and uniaxonal types. The second division point separated out a distinct group of uniaxonal cells characterized by soma 5-HT immunoreactivity. The third and fourth divisions were by NOS and ChAT immunoreactivities, to distinguish major excitatory and inhibitory cell lines. After that, an iterative process was followed for subdividing each group of neurons by the marker which divided the remaining cells with the most nonuniform distribution of other markers.
To work through an example, the most discriminating marker to divide 1081 NOS+/ChAT– cells (without Dogiel type II and 5-HT+ neurons) was identified as follows) (see Figure 3, refer to division point G). First, we divided these 1081 cells by expression of Calb, producing 2 subgroups: 112 NOS+/ChAT–/Calb+ cells and 969 NOS+/ChAT–/Calb–. We then tested the extent to which other markers (SP, ENK, CGRP, SOM, Calret, NF200, VIP, NPY) distributed nonuniformly between Calb+ and Calb– subgroups, using chi-square tests (with Bonferroni corrections). For example, VIP was expressed by 657 (60.8%) of the 1081 cells. The null hypothesis would be that 60.8% of Calb+ cells and 60.8% of Calb– cells would be VIP immunoreactive. In reality, VIP significantly coexisted with Calb (75% of 112 Calb+ cells were VIP+). We tested whether other cell body markers (SP, ENK, CGRP, SOM, Calret, NF200, or NPY) also codistributed with Calb, giving a quantitative measure of how discriminating Calb was for these 1081 cells. We also tested whether cell body size differed significantly between the 2 subgroups (see Table 6). We then repeated the whole process using Calret to divide the 1081 NOS+/ChAT– cells. Each remaining marker was tested in this way and the most discriminating one chosen. This was then finally used to divide the group (see Figure 3, division point J). In fact, for the 1081 NOS+/ChAT– cells, the most discriminating marker was NF200 (ie, neither VIP nor Calb) and the 1081 cells were divided into 375 NOS+/ChAT–/NF200+ and 706 NOS+/ChAT–/NF200– subgroups. Dividing the cells by NF200 revealed disproportionate expression of Calb, VIP, and NPY between NF200+ and NF200– subgroups. This process was then applied iteratively to each subgroup until no marker led to statistically significant divisions. Thus cells were divided according to their chemical coding with statistical validation. This is summarized in the dendrogram of Figure 3 and the statistical basis for each decision point is shown in Table 6.
Recording Data
HuCD immunoreactivity was used to identify all nerve cell bodies in the first layer of immunohistochemistry. HuCD immunoreactivity was photographed and each myenteric nerve cell body was numbered on the micrograph. The position in the ganglion, size, and shape of HuCD-labeled cell bodies was used to reidentify them in subsequent layers. Micrographs of the other 3 markers in the layer were then overlaid in a stack. An experienced observer recorded whether each numbered cell body was immunoreactive for the other 3 markers in that layer and results were recorded directly in a spreadsheet. Cells were classified as either immunoreactive or nonimmunoreactive; intensity of staining was not recorded. The preparation was then unmounted, eluted, rephotographed (to show that elution was successful), then stained with the next layer of antisera. HuCD did not elute due to the use of a biotinylated secondary antiserum.11 Thus 12 markers could be distinguished in every numbered Hu/CD-labeled cell across 5 layers of immunohistochemical triple labeling.
Analysis of Varicosities in Baskets
Some but not all nerve cell bodies were surrounded by dense baskets or rings of varicosities. We tested whether basket were associated with close appositions (ie, varicosities within 2 μm of their cell surface). Six confocal images per preparation were captured, each with one nerve cell body with a basket and one cell body without (63X NA: 1.4, 0.4 μm Z-step). Using the Imaris software package (Oxford Instruments, Abingdon, United Kingdom; version 9.8.0), surfaces were created (0.5 μm surface detail) from HuCD-labeled cell bodies. Varicosities (ENK+, SP+, VAChT+, or synapsin1+) were detected using the spots function. For the spots-surface correlation, the number of spots data were exported, giving numbers of varicosities within prescribed distances of HuCD cell surfaces. Values were normalized as percentage of the total number of varicosities within 20 μm of the surface.
Statistical Analysis
Nominal data (immunoreactive/nonimmunoreactive) were analyzed by nonparametric Pearson's chi-square tests; cell sizes were compared using Student's t test for independent variables. Where multiple tests were used, Bonferroni corrections were applied. A value of P < .05 is taken as significant.
All authors had access to the data and have reviewed and approved the final manuscript.
Acknowledgments
The authors are very grateful to the patients who gave consent for the use of their tissues for research. They thank the staff of the Department of Surgery of Flinders Medical Centre for their invaluable assistance in obtaining surgical specimens. Image processing was carried out in Flinders Microscopy and Microanalysis, part of the National Collaborative Research Infrastructure Strategy. Upon publication, data will be available via Pennsieve DOI:10.26275/k11r-zl51.
CRediT Authorship Contributions
Bao Nan Chen, MSc (Investigation: Equal; Methodology: Equal; Writing – review and editing: Supporting)
Adam Humenick, BSc (Honours) (Investigation: Equal; Methodology: Equal; Writing – review and editing: Supporting)
Wai Ping Yew, PhD (Data curation: Supporting; Methodology: Supporting; Writing – review and editing: Supporting)
Rochelle A Peterson, PhD (Investigation: Supporting; Methodology: Supporting; Writing – review and editing: Supporting)
Lukasz Wiklendt, PhD (Conceptualization: Supporting; Data curation: Equal; Formal analysis: Supporting; Software: Lead; Writing – review and editing: Supporting)
Phil G Dinning, PhD (Conceptualization: Equal; Funding acquisition: Equal; Investigation: Supporting; Supervision: Equal; Writing – review and editing: Equal)
Nicholas J Spencer, PhD (Investigation: Supporting; Supervision: Supporting; Writing – review and editing: Equal)
David A Wattchow, BMBS, PhD (Conceptualization: Supporting; Resources: Lead; Supervision: Supporting; Writing – original draft: Equal; Writing – review and editing: Equal)
Marcello Costa, MD (Conceptualization: Equal; Investigation: Equal; Supervision: Supporting; Writing – original draft: Equal; Writing – review and editing: Equal)
Simon JH Brookes, PhD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Funding acquisition: Lead; Investigation: Lead; Project administration: Lead; Supervision: Lead; Writing – original draft: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by National Institutes of Health SPARC award OT2OD24899 to Y. Taché, University of California, Los Angeles (UCLA).
References
- 1.Muller P.A., Kocscó B., Rajani G.M., et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furness J.B. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 3.Poole D.P., Furness J.B. In: Physiology of the Gastrointestinal Tract. Johnson L.R., editor. Elsevier; Amsterdam, the Netherlands: 2012. Enteric nervous systems structure and neurochemistry related to function and neuropathology; pp. 557–582. [Google Scholar]
- 4.Wood J.D. In: Physiology of the Gastrointestinal Tract. Johnson L.R., editor. Elsevier; Amsterdam, the Netherlands: 2012; 671–688. Integrative functions of the enteric nervous system. [Google Scholar]
- 5.Swenson O., Rheinlander H., Diamond I. Hirschsprung’s disease: a new concept of the etiology. N Eng J Med. 1949;241:551–556. doi: 10.1056/NEJM194910132411501. [DOI] [PubMed] [Google Scholar]
- 6.Dogiel A.S. Über den Bau der Ganglien in den Geflechten des Darmes und der Gallenblase des Menschen und der Säugethiere. Arch Anat Physiol. 1899;1899:130–158. [Google Scholar]
- 7.Migliore M., Shepherd G.M. Opinion: an integrated approach to classifying neuronal phenotypes. Nat Rev Neurosci. 2005;6:810–818. doi: 10.1038/nrn1769. [DOI] [PubMed] [Google Scholar]
- 8.Costa M., Brookes S.J., Steele P.A., et al. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- 9.Drokhlyansky E., Smillie C.S., Van Wittenberghe N., et al. The human and mouse enteric nervous system at single-cell resolution. Cell. 2020;182:1606–1622.e23. doi: 10.1016/j.cell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy E.M.A., Defontgalland D., Costa M., et al. Quantification of subclasses of human colonic myenteric neurons by immunoreactivity to Hu, choline acetyltransferase and nitric oxide synthase. Neurogastroenterol Motil. 2007;19:126–134. doi: 10.1111/j.1365-2982.2006.00843.x. [DOI] [PubMed] [Google Scholar]
- 11.Gendusa R., Scalia C.R., Buscone S., et al. Elution of high-affinity (>10–9 KD) antibodies from tissue sections: clues to the molecular mechanism and use in sequential immunostaining. J Histochem Cytochem. 2014;62:519–531. doi: 10.1369/0022155414536732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desmet A.S., Cirillo C., Vanden Berghe P. Distinct subcellular localization of the neuronal marker HuCD reveals hypoxia-induced damage in enteric neurons. Neurogastroenterol Motil. 2014;26:1131–1143. doi: 10.1111/nmo.12371. [DOI] [PubMed] [Google Scholar]
- 13.Harrington A.M., Hutson J.M., Southwell B.R. Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Prog Histochem Cytochem. 2010;44:173–202. doi: 10.1016/j.proghi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Sanders K.M., Ward S.M. Nitric oxide and its role as a non-adrenergic, non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract. Br J Pharmacol. 2019;176:212–227. doi: 10.1111/bph.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galligan J.J., North R.A. Pharmacology and function of nicotinic acetylcholine and P2X receptors in the enteric nervous system. Neurogastroenterol Motil. 2004;16:64–70. doi: 10.1111/j.1743-3150.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- 16.Dinning P.G., Wiklendt L., Maslen L., et al. Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high-resolution fiber-optic manometry. Neurogastroenterol Motil. 2014;26:1443–1457. doi: 10.1111/nmo.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck M., Schlabrakowski A., Schrödl F., et al. ChAT and NOS in human myenteric neurons: co-existence and co-absence. Cell Tissue Res. 2009;338:37–51. doi: 10.1007/s00441-009-0852-4. [DOI] [PubMed] [Google Scholar]
- 18.Ng K.S., Montes-Adrian N.A., Mahns D.A., et al. Quantification and neurochemical coding of the myenteric plexus in humans: No regional variation between the distal colon and rectum. Neurogastroenterol Motil. 2018;30 doi: 10.1111/nmo.13193. [DOI] [PubMed] [Google Scholar]
- 19.Anlauf M., Schäfer M.K.-H., Eiden L., et al. Chemical coding of the human gastrointestinal nervous system: cholinergic, VIPergic, and catecholaminergic phenotypes. J Compr Neurol. 2003;459:90–111. doi: 10.1002/cne.10599. [DOI] [PubMed] [Google Scholar]
- 20.Costa M., Furness J.B., Cuello A.C., et al. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their visualization and reactions to drug treatment. Neuroscience. 1982;7:351–363. doi: 10.1016/0306-4522(82)90272-x. [DOI] [PubMed] [Google Scholar]
- 21.Meedeniya A.C., Brookes S.J., Hennig G.W., et al. The projections of 5-hydroxytryptamine-accumulating neurones in the myenteric plexus of the small intestine of the guinea-pig. Cell Tissue Res. 1998;291:375–384. doi: 10.1007/s004410051007. [DOI] [PubMed] [Google Scholar]
- 22.Brehmer A., Croner R., Dimmler A., et al. Immunohistochemical characterization of putative primary afferent (sensory) myenteric neurons in human small intestine. Auton Neurosci. 2004;112:49–59. doi: 10.1016/j.autneu.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Furness J.B., Costa M. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their projections in the guinea-pig small intestine. Neuroscience. 1982;7:341–349. doi: 10.1016/0306-4522(82)90271-8. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto T., Barton M.J., Hennig G.W., et al. Extensive projections of myenteric serotonergic neurons suggest they comprise the central processing unit in the colon. Neurogastroenterol Motil. 2014;26:556–570. doi: 10.1111/nmo.12302. [DOI] [PubMed] [Google Scholar]
- 25.Sang Q., Williamson S., Young H.M. Projections of chemically identified myenteric neurons of the small and large intestine of the mouse. J Anat. 1997;190:209–222. doi: 10.1046/j.1469-7580.1997.19020209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbiers M., Timmermans J.P., Adriansen D., et al. Projections of neurochemically specified neurons in the porcine colon. Histochem Cell Biol. 1995;103:115–126. doi: 10.1007/BF01454008. [DOI] [PubMed] [Google Scholar]
- 27.Humenick A., Chen B.N., Wattchow D.A., et al. Characterization of putative interneurons in the myenteric plexus of human colon. Neurogastroenterol Motil. 2021;33 doi: 10.1111/nmo.13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humenick A., Chen B.N., Lauder C.I.W., et al. Characterisation of projections of longitudinal muscle motor neurons in human colon. Neurogastroenterol Motil. 2019;31 doi: 10.1111/nmo.13685. [DOI] [PubMed] [Google Scholar]
- 29.Porter A.J., Wattchow D.A., Brookes S.J., et al. The neurochemical coding and projections of circular muscle motor neurons in the human colon. Gastroenterology. 1997;113:1916–1923. doi: 10.1016/s0016-5085(97)70011-8. [DOI] [PubMed] [Google Scholar]
- 30.Porter A.J., Wattchow D.A., Brookes S.J.H., et al. Cholinergic and nitrergic interneurones in the myenteric plexus of the human colon. Gut. 2002;51:70–75. doi: 10.1136/gut.51.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wattchow D.A., Brookes S.J., Costa M. The morphology and projections of retrogradely labeled myenteric neurons in the human intestine. Gastroenterology. 1995;109:866–875. doi: 10.1016/0016-5085(95)90396-8. [DOI] [PubMed] [Google Scholar]
- 32.Wattchow D.A., Porter A.J., Brookes S.J., et al. The polarity of neurochemically defined myenteric neurons in the human colon. Gastroenterology. 1997;113:497–506. doi: 10.1053/gast.1997.v113.pm9247469. [DOI] [PubMed] [Google Scholar]
- 33.Wattchow D.A., Furness J.B., Costa M. Distribution and coexistence of peptides in nerve fibers of the external muscle of the human gastrointestinal tract. Gastroenterology. 1988;95:32–41. doi: 10.1016/0016-5085(88)90287-9. [DOI] [PubMed] [Google Scholar]
- 34.Mann P.T., Southwell B.R., Young H.M., et al. Appositions made by axons of descending interneurons in the guinea-pig small intestine, investigated by confocal microscopy. J Chem Neuroanat. 1997;12:151–164. doi: 10.1016/s0891-0618(96)00189-5. [DOI] [PubMed] [Google Scholar]
- 35.Pompolo S., Furness J.B. Sources of inputs to longitudinal muscle motor neurons and ascending interneurons in the guinea-pig small intestine. Cell Tissue Res. 1995;280:549–560. doi: 10.1007/BF00318359. [DOI] [PubMed] [Google Scholar]
- 36.Young H.M., Furness J.B. Ultrastructural examination of the targets of serotonin-immunoreactive descending interneurons in the guinea pig small intestine. J Compr Neurol. 1995;356:101–114. doi: 10.1002/cne.903560107. [DOI] [PubMed] [Google Scholar]
- 37.Brehmer A. Structure of Enteric Neurons. Advances in Anatomy, Embryology, and Cell Biology. Vol. 186. Berlin, Germany: Springer-Verlag; 2006. [PubMed]
- 38.Brehmer A. Classification of human enteric neurons. Histochem Cell Biol. 2021;156:95–108. doi: 10.1007/s00418-021-02002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morarach K., Michailova A., Knoflach V., et al. Diversification of molecularly defined myenteric neuron classes revealed by single-cell RNA sequencing. Nat Neurosci. 2021;24:34–46. doi: 10.1038/s41593-020-00736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsurui H., Nishimura H., Hattori S., et al. Seven-color fluorescence imaging of tissue samples based on Fourier spectroscopy and singular value decomposition. J Histochem Cytochem. 2000;48:653–662. doi: 10.1177/002215540004800509. [DOI] [PubMed] [Google Scholar]
- 41.Gerdes M.J., Sevinsky C.J., Sood A., et al. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc Natl Acad Sci U S A. 2013;110:11982–11987. doi: 10.1073/pnas.1300136110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedenberger M., Bode M., Krusche A., et al. Fluorescence detection of protein clusters in individual cells and tissue sections by using toponome imaging system: sample preparation and measuring procedures. Nat Protoc. 2007;2:2285–2294. doi: 10.1038/nprot.2007.320. [DOI] [PubMed] [Google Scholar]
- 43.van den Brand M., Hoevenaars B.M., Sigmans J.H.M., et al. Sequential immunohistochemistry: a promising new tool for the pathology laboratory. Histopathology. 2014;65:651–657. doi: 10.1111/his.12446. [DOI] [PubMed] [Google Scholar]
- 44.Costa M., Furness J.B., Gibbins I.L. Chemical coding of enteric neurons. Prog Brain Res. 1986;68:217–240. doi: 10.1016/s0079-6123(08)60241-1. [DOI] [PubMed] [Google Scholar]
- 45.Furness J.B., Morris J.L., Gibbins I.L., Costa M. Chemical coding of neurons and plurichemical transmission. Annu Rev Pharmacol Toxicol. 1989;29:289–306. doi: 10.1146/annurev.pa.29.040189.001445. [DOI] [PubMed] [Google Scholar]
- 46.Schemann M., Schaaf C., Mader M. Neurochemical coding of enteric neurons in the guinea pig stomach. J Compr Neurol. 1995;353:161–178. doi: 10.1002/cne.903530202. [DOI] [PubMed] [Google Scholar]
- 47.Lomax A.E., Furness J.B. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res. 2000;302:59–72. doi: 10.1007/s004410000260. [DOI] [PubMed] [Google Scholar]
- 48.Qu Z.-D., Thacker M., Castelucci P., et al. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147–161. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- 49.May-Zhang A.A., Tycksen E., Southard-Smith A.N., et al. Combinatorial transcriptional profiling of mouse and human enteric neurons identifies shared and disparate subtypes in situ. Gastroenterology. 2021;160:755–770.e26. doi: 10.1053/j.gastro.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petegrosso R., Li Z., Kuang R. Machine learning and statistical methods for clustering single-cell RNA-sequencing data. Brief Bioinform. 2020;21:1209–1223. doi: 10.1093/bib/bbz063. [DOI] [PubMed] [Google Scholar]
- 51.Kiselev V.Y., Andrews T.S., Hemberg M. Challenges in unsupervised clustering of single-cell RNA-seq data. Nat Rev Genet. 2019;20:273–282. doi: 10.1038/s41576-018-0088-9. [DOI] [PubMed] [Google Scholar]
- 52.Elhaik E. Principal component analyses (PCA)-based findings in population genetic studies are highly biased and must be reevaluated. Sci Rep. 2022;12 doi: 10.1038/s41598-022-14395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim E.J., Zhang Z., Huang L., et al. Extraction of distinct neuronal cell types from within a genetically continuous population. Neuron. 2020;107:274–282.e6. doi: 10.1016/j.neuron.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Northcutt A.J., Kick D.R., Otopalik A.G., et al. Molecular profiling of single neurons of known identity in 2 ganglia from the crab Cancer borealis. Proc Natl Acad Sci U S A. 2019;5:5. doi: 10.1073/pnas.1911413116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hao Y., Hao S., Andersen-Nissen E., et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.BRAIN Initiative Cell Census Network A multimodal cell census and atlas of the mammalian primary motor cortex. Nature. 2021;598:86–102. doi: 10.1038/s41586-021-03950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brookes S.J.H. Retrograde tracing of enteric neuronal pathways. Neurogastroenterol Motil. 2001;13:1–18. doi: 10.1046/j.1365-2982.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- 58.Weidmann S., Schrödl F., Neuhuber W., et al. Quantitative estimation of putative primary afferent neurons in the myenteric plexus of human small intestine. Histochem Cell Biol. 2007;128:399–407. doi: 10.1007/s00418-007-0335-1. [DOI] [PubMed] [Google Scholar]
- 59.Brookes S.J.H., Costa M., editors. Innervation of the Gastrointestinal Tract. Harwood; London, United Kingdom: 2002. Cellular organisation of the mammalian enteric nervous system. 393-467. [Google Scholar]
- 60.Prusator D.K., Chang L. Sex-related differences in GI disorders. Handb Exp Pharmacol. 2017;239:177–192. doi: 10.1007/164_2016_121. [DOI] [PubMed] [Google Scholar]
- 61.Makowska K., Gonkowski S. Age and sex-dependent differences in the neurochemical characterization of calcitonin gene-related peptide-like immunoreactive (CGRP-LI) nervous structures in the porcine descending colon. Int J Mol Sci. 2019;20:27. doi: 10.3390/ijms20051024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balasuriya G.K., Nugapitiya S.S., et al. Nitric oxide regulates estrus cycle dependent colonic motility in mice. Fronti Neurosci. 2021;15 doi: 10.3389/fnins.2021.647555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernard C.E., Gibbons S.J., Gomez-Pinilla P.J., et al. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil. 2009;21 doi: 10.1111/j.1365-2982.2008.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomes O.A., de Souza R.R., Liberti E.A. A preliminary investigation of the effects of aging on the nerve cell number in the myenteric ganglia of the human colon. Gerontology. 1997;43:210–217. doi: 10.1159/000213852. [DOI] [PubMed] [Google Scholar]
- 65.Hanani M., Fellig Y., Udassin R., et al. Age-related changes in the morphology of the myenteric plexus of the human colon. Auton Neurosci. 2004;113:71–78. doi: 10.1016/j.autneu.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 66.Michel K., Kuch B., Dengler S., et al. How big is the little brain in the gut? Neuronal numbers in the enteric nervous system of mice, Guinea pig, and human. Neurogastroenterol Motil. 2022;34 doi: 10.1111/nmo.14440. [DOI] [PubMed] [Google Scholar]
- 67.Hetz S., Acikgoez A., Moll C., et al. Age-related gene expression analysis in enteric ganglia of human colon after laser microdissection. Front Aging Neurosci. 2014;6:276. doi: 10.3389/fnagi.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broad J., Kung V.W.S., Palmer A., et al. Changes in neuromuscular structure and functions of human colon during ageing are region-dependent. Gut. 2019;68:1210–1223. doi: 10.1136/gutjnl-2018-316279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yew W.P., Humenick A., Chen B.N., et al. Electrophysiological and morphological features of myenteric neurons of human colon revealed by intracellular recording and dye fills. Neurogastroenterol Motil. 2023;35 doi: 10.1111/nmo.14538. [DOI] [PubMed] [Google Scholar]
- 70.Purves D., Snider W.D., Voyvodic J.T. Trophic regulation of nerve cell morphology and innervation in the autonomic nervous system. Nature. 1988;336:123–128. doi: 10.1038/336123a0. [DOI] [PubMed] [Google Scholar]
- 71.Kuntz A., Saccomanno G. Reflex inhibition of intestinal motility mediated through decentralized prevertebral ganglia. J Neurophysiol. 1944;7:163–170. [Google Scholar]
- 72.Muller P.A., Matheis F., Schneeberger M., et al. Microbiota-modulated CART+ enteric neurons autonomously regulate blood glucose. Science. 2020;370:314–321. doi: 10.1126/science.abd6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang T., Perkins M.H., Chang H., et al. An inter-organ neural circuit for appetite suppression. Cell. 2022;185:2478–2494.e28. doi: 10.1016/j.cell.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beuscher N., Jabari S., Strehl J., et al. What neurons hide behind calretinin immunoreactivity in the human gut? Histochem Cell Biol. 2014;141:393–405. doi: 10.1007/s00418-013-1163-0. [DOI] [PubMed] [Google Scholar]
- 75.Hens J., Vanderwinden J.M., De Laet M.H., et al. Morphological and neurochemical identification of enteric neurones with mucosal projections in the human small intestine. J Neurochem. 2001;76:464–471. doi: 10.1046/j.1471-4159.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 76.Li Z.S., Furness J.B. Inputs from intrinsic primary afferent neurons to nitric oxide synthase-immunoreactive neurons in the myenteric plexus of guinea pig ileum. Cell Tissue Res. 2000;299:1–8. doi: 10.1007/s004419900125. [DOI] [PubMed] [Google Scholar]
- 77.Llewellyn Smith I.J., Furness J.B., Costa M. Ultrastructural analysis of substance P-immunoreactive nerve fibers in myenteric ganglia of guinea pig small intestine. J Neurosci. 1989;9:167–174. doi: 10.1523/JNEUROSCI.09-01-00167.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar D., Phillips S.F. Human myenteric plexus: confirmation of unfamiliar structures in adults and neonates. Gastroenterology. 1989;96:1021–1028. doi: 10.1016/0016-5085(89)91619-3. [DOI] [PubMed] [Google Scholar]
- 79.Bayliss W.M., Starling E.H. The movements and the innervation of the large intestine. J Physiol. 1900;26:107–118. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carbone S.E., Dinning P.G., Costa M., et al. Ascending excitatory neural pathways modulate slow phasic myogenic contractions in the isolated human colon. Neurogastroenterol Motil. 2013;25:670–676. doi: 10.1111/nmo.12129. [DOI] [PubMed] [Google Scholar]
- 81.Parker D.R., Wiklendt L., Humenick A., et al. Brookes SJH Sympathetic pathways target cholinergic neurons in the human myenteric plexus. Front Neurosci. 2022;16 doi: 10.3389/fnins.2022.863662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Furness J.B., Jones C., Nurgali K., et al. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol. 2004;72:143–164. doi: 10.1016/j.pneurobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 83.Bornstein J.C., Hendriks R., Furness J.B., et al. Ramifications of the axons of AH-neurons injected with the intracellular marker biocytin in the myenteric plexus of the guinea pig small intestine. J Comp Neurol. 1991;314:437–451. doi: 10.1002/cne.903140303. [DOI] [PubMed] [Google Scholar]
- 84.Kunze W.A., Furness J.B., Bornstein J.C. Simultaneous intracellular recordings from enteric neurons reveal that myenteric AH neurons transmit via slow excitatory postsynaptic potentials. Neuroscience. 1993;55:685–694. doi: 10.1016/0306-4522(93)90434-h. [DOI] [PubMed] [Google Scholar]
- 85.Steele P.A., Brookes S.J., Costa M. Immunohistochemical identification of cholinergic neurons in the myenteric plexus of guinea-pig small intestine. Neuroscience. 1991;45:227–239. doi: 10.1016/0306-4522(91)90119-9. [DOI] [PubMed] [Google Scholar]
- 86.Song Z.M., Brookes S.J., Costa M. Identification of myenteric neurons which project to the mucosa of the guinea-pig small intestine. Neurosci Lett. 1991;129:294–298. doi: 10.1016/0304-3940(91)90484-b. [DOI] [PubMed] [Google Scholar]
- 87.Brookes S.J., Song Z.M., Ramsay G.A., et al. Long aboral projections of Dogiel type II, AH neurons within the myenteric plexus of the guinea pig small intestine. J Neurosci. 1995;15:4013–4022. doi: 10.1523/JNEUROSCI.15-05-04013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smolilo D.J., Costa M., Hibberd T.J., et al. Distribution, projections, and association with calbindin baskets of motor neurons, interneurons, and sensory neurons in guinea-pig distal colon. J Compr Neurol. 2019;527:1140–1158. doi: 10.1002/cne.24594. [DOI] [PubMed] [Google Scholar]
- 89.Brookes S.J.H., Steele P.A., Costa M. Identification and immunohistochemistry of cholinergic and non-cholinergic circular muscle motor neurons in the guinea-pig small intestine. Neuroscience. 1991;42:863–878. doi: 10.1016/0306-4522(91)90050-x. [DOI] [PubMed] [Google Scholar]
- 90.Tonini M., Spelta V., De Ponti F., et al. Tachykinin-dependent and -independent components of peristalsis in the guinea pig isolated distal colon. Gastroenterology. 2001;120:938–945. doi: 10.1053/gast.2001.22526. [DOI] [PubMed] [Google Scholar]