ABSTRACT
Background
Chronic kidney disease (CKD) is a common complication of type 2 diabetes (T2D). Glucagon-like peptide-1 receptor agonists (GLP-1RAs) improve glycaemic control and lower body weight in people with T2D, and some reduce the risk of cardiovascular (CV) events in those with high CV risk. GLP-1RAs might also have kidney-protective effects. We report the design and baseline data for FLOW (NCT03819153), a trial investigating the effects of semaglutide, a once-weekly (OW) GLP-1RA, on kidney outcomes in participants with CKD and T2D.
Methods
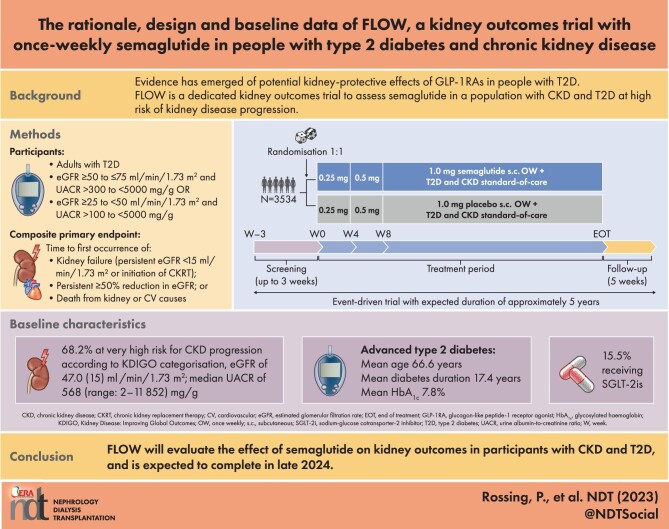
FLOW is a randomised, double-blind, parallel-group, multinational, phase 3b trial. Participants with T2D, estimated glomerular filtration rate (eGFR) ≥50‒≤75 ml/min/1.73 m2 and urine albumin:creatinine ratio (UACR) >300‒<5000 mg/g or eGFR ≥25‒<50 ml/min/1.73 m2 and UACR >100‒<5000 mg/g were randomised 1:1 to OW semaglutide 1.0 mg or matched placebo, with renin–angiotensin–aldosterone system blockade (unless not tolerated/contraindicated). The composite primary endpoint is time to first kidney failure (persistent eGFR <15 ml/min/1.73 m2 or initiation of chronic kidney replacement therapy), persistent ≥50% reduction in eGFR or death from kidney or CV causes.
Results
Enrolled participants (N = 3534) had a baseline mean age of 66.6 years [standard deviation (SD) 9.0], haemoglobin A1c of 7.8% (SD 1.3), diabetes duration of 17.4 years (SD 9.3), eGFR of 47.0 ml/min/1.73 m2 (SD 15.2) and median UACR of 568 mg/g (range 2‒11 852). According to Kidney Disease: Improving Global Outcomes guidelines categorisation, 68.2% were at very high risk for CKD progression.
Conclusion
FLOW will evaluate the effect of semaglutide on kidney outcomes in participants with CKD and T2D, and is expected to be completed in late 2024.
Keywords: albuminuria, cardiovascular disease, diabetic kidney disease, glomerular filtration rate, glucagon-like peptide-1 receptor agonist
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Evidence has emerged of the potential kidney-protective effects of glucagon-like peptide-1 receptor agonists (GLP-1RAs) in people with type 2 diabetes (T2D).
To date, data have mostly been derived from cardiovascular (CV) outcome or glycaemic control trials featuring populations not selected for chronic kidney disease (CKD) and/or with kidney disease events as secondary outcomes.
Reduction of CKD progression by GLP-1RAs is yet to be confirmed and requires dedicated trials of kidney outcomes with GLP-1RAs.
What this study adds?
FLOW (NCT03819153) is a dedicated kidney outcomes trial to assess semaglutide, a once-weekly GLP-1RA, in a population with CKD and T2D at high risk of kidney disease progression.
The trial is designed to assess whether treatment with once-weekly subcutaneous semaglutide delays the progression of kidney disease and lowers the risk of kidney failure, as well as kidney and CV disease mortality, compared with placebo in people with CKD and T2D.
Baseline data from the FLOW trial, which is ongoing, show that enrolled participants are nearly all classified as high or very high risk for CKD progression according to Kidney Disease: Improving Global Outcomes guidelines categorisation, which assesses risk based on estimated glomerular filtration rate and urine albumin:creatinine ratio.
What impact this may have on practice or policy?
The FLOW trial will provide evidence on the effects of semaglutide on kidney outcomes, potentially expanding treatment options for patients with T2D to slow the progression of CKD and reduce kidney failure.
INTRODUCTION
Chronic kidney disease (CKD) is associated with increased morbidity and mortality, including an increased risk of both kidney failure and cardiovascular (CV) events [1]. CKD is a common complication of type 2 diabetes (T2D), affecting ≈40% of those with T2D, and the risk increases with diabetes duration [1]. The presence of CKD causes average healthcare costs to increase by almost 50% compared with costs for those with diabetes alone [2].
Clinical practice guidelines recommend multifactorial interventions to reduce the risk of CKD developing or progressing in people with T2D [3]. Recommendations include optimising blood glucose levels towards an individualised target, lowering blood pressure, moderating dietary protein intake and weight loss. Guidelines on CKD and diabetes management, such as the 2022 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for patients with CKD and the 2022 American Diabetes Association (ADA) standards-of-care (SoC) guidelines for patients with T2D recommend treatment with renin–angiotensin–aldosterone system (RAAS) blocking agents and sodium–glucose co-transporter 2 inhibitors (SGLT2is) in most patients [3, 4]. Both guidelines also recommend a non-steroidal mineralocorticoid receptor antagonist (finerenone) for patients with CKD at increased risk for CV events, or CKD progression, or unable to use SGLT2is [4, 5]. The 2022 KDIGO guidelines recommend long-acting glucagon-like peptide-1 receptor agonists (GLP-1RAs) with proven CV benefits as the preferred second-line treatment for glucose lowering and CV event risk reduction in individuals either with CV disease (CVD) or at high risk of CVD who are not achieving individualised glycaemic targets despite the use of metformin and an SGLT2i or who are unable to use those medications [3]. For patients with confirmed atherosclerotic CVD or at high risk of CV events, the 2022 ADA SoC guidelines recommend either a GLP-1RA or an SGLT2i with proven CV benefits [4].
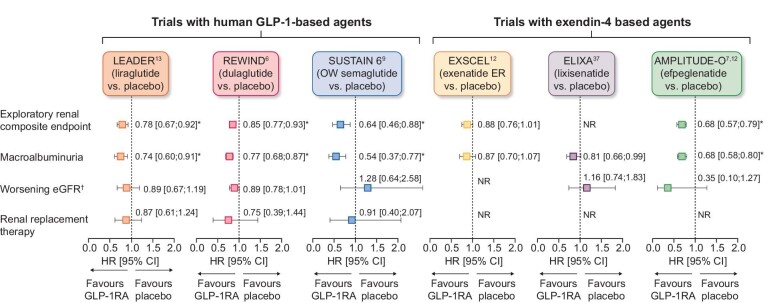
GLP-1RAs are an effective treatment for glycaemic control and weight reduction in people with T2D and have a good safety and tolerability profile (generally transient gastrointestinal adverse events being most common), including in those with CKD [3, 4]. Some GLP-1RAs [albiglutide, efpeglenatide, dulaglutide, liraglutide and once-weekly (OW) semaglutide] reduce the risk of major adverse CV events (MACE) in people with T2D with established CVD or at high risk of CVD [6–10]. Meta-analyses of CV outcomes trials (CVOTs), which included >40 000 participants, suggest potential kidney-protective effects of GLP-1RAs, with some (liraglutide, dulaglutide, semaglutide and efpeglenatide) associated with reductions in secondary composite kidney outcomes (macroalbuminuria, substantial loss of kidney function, kidney failure and death due to kidney disease; Fig. 1) [11, 12]. However, these benefits were driven by a lower risk of persistent macroalbuminuria [6, 10, 13].
Figure 1:
Results of exploratory kidney analyses from GLP-1RA CV outcomes trials. *P < .05. †Defined as doubling of serum creatinine in LEADER, SUSTAIN 6 and ELIXA and sustained eGFR decline of ≥30% in REWIND. Direct comparisons not possible due to differing study designs. Secondary kidney composite endpoints were new-onset persistent macroalbuminuria/very high albuminuria, persistent doubling of the serum creatinine or end-stage kidney disease in LEADER; first occurrence of new macroalbuminuria/very high albuminuria (UACR >33.9 mg/mmol), a sustained decline in eGFR of ≥30% or chronic KRT in REWIND; new or worsening nephropathy (defined as persistent macroalbuminuria/very high albuminuria, persistent doubling of serum creatinine and a creatinine clearance of <45 ml/min/1.73 m2 or the need for continuous KRT) in SUSTAIN 6; a 40% decline in eGFR, KRT, kidney death or incident macroalbuminuria/very high albuminuria in EXSCEL; and incident macroalbuminuria (UACR >33.9 mg/mmol) plus an increase in UACR of ≥30% from baseline, sustained decrease in eGFR of ≥40% for ≥30 days, renal replacement therapy for ≥90 days and sustained eGFR <15 ml/min/1.73 m2 for ≥30 days in AMPLITUDE-O [6, 7, 10, 13, 38, 43]. exenatide ER, exenatide extended release; KRT, kidney replacement therapy; NR, not reported.
Despite improvements in kidney outcomes with available pharmacotherapies, the risk of kidney failure in people with CKD and T2D remains high [14, 15]. Given the promising preliminary findings with GLP-1RAs, further exploration of this drug class as agents to improve kidney outcomes in people with CKD and T2D is a priority. FLOW (NCT03819153) is a dedicated kidney outcomes trial designed to determine the kidney-protective effects of semaglutide in participants with CKD and T2D, as well as assessing the effect on CVD mortality. Here we describe the study design and report the baseline characteristics of the FLOW population.
MATERIALS AND METHODS
Overall trial design and treatment
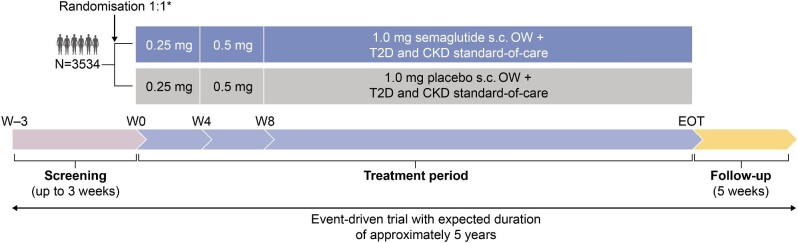
FLOW is a randomised, double-blind, parallel-group, multinational, phase 3b trial comparing semaglutide with matched placebo. Both arms received the SoC when the trial began (2019), including RAAS blockade for delaying the progression of CKD in participants with CKD and T2D.
An overview of the FLOW trial design is shown in Fig. 2. Participants were randomly assigned 1:1, using a central interactive web response system, to receive OW subcutaneous (s.c.) injections of semaglutide 1.0 mg or visually identical placebo, in addition to the maximum labelled or tolerated dose of a RAAS blocking agent (unless contraindicated or not tolerated). SGLT2i use is permitted, and randomisation was stratified by use at baseline. Participants, investigators and all trial personnel (except the independent data monitoring committee) are blinded to treatment assignment. An 8-week dose escalation regimen was employed at the start of treatment, with dose escalation (as tolerated) from 0.25 mg/week for 4 weeks to 0.5 mg for 4 weeks, followed by a maintenance dose of 1.0 mg/week throughout the remainder of the treatment period. Participants were trained in handling the pen injector when dispensed for the first time and instructed to inject the trial product s.c. OW in the abdomen or thigh. Training was repeated at week 4, week 26 and yearly. If a participant experiences unacceptable adverse events (AEs), extensions of dose escalation intervals, dose reductions and treatment pauses are allowed at the discretion of the investigator. Optimisation of glucose-lowering, CKD and CVD SoC medications, in accordance with local practice guidelines, is permitted throughout the trial. A specific guidance document on the management of CKD and CVD in T2D was provided to support the trial investigators.
Figure 2:
FLOW trial design. *Stratified by SGLT-2i use (yes/no). EOT, end of treatment; N, number of participants; W, week.
FLOW is an event-driven trial with an expected duration of ≈5 years; participants are anticipated to receive trial medication for 3–5 years depending on recruitment time.
The trial is being conducted in accordance with the International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice guidelines and the Declaration of Helsinki. The trial protocol is approved by the institutional review board and ethics committee at each participating centre. All participants provided written informed consent before any trial-related activity. The trial is sponsored by Novo Nordisk.
During the coronavirus disease 2019 (COVID-19) pandemic, protocol amends were made and local guidance adjusted to ensure no undue risk of COVID-19 exposure for participants and staff and to maintain data integrity, recruitment and retention. Face-to-face site visits could be replaced with phone and home visits, remote monitoring and alternative trial drug dispensing and co-participation in COVID-19 trials was permitted. Guidance documents were issued to trial sites and along with local support from global expert panel members. Data on COVID events, vaccination and co-occurrence with study outcomes were added to the data collection.
FLOW includes sites in Ukraine and Russia, which are being monitored continuously to ensure the safety of investigators, site staff and subjects in these countries, as well as protocol compliance, trial product supply, data integrity and trial oversight. No protocol adjustments have been required, due to the built-in flexibility of the protocol.
Population
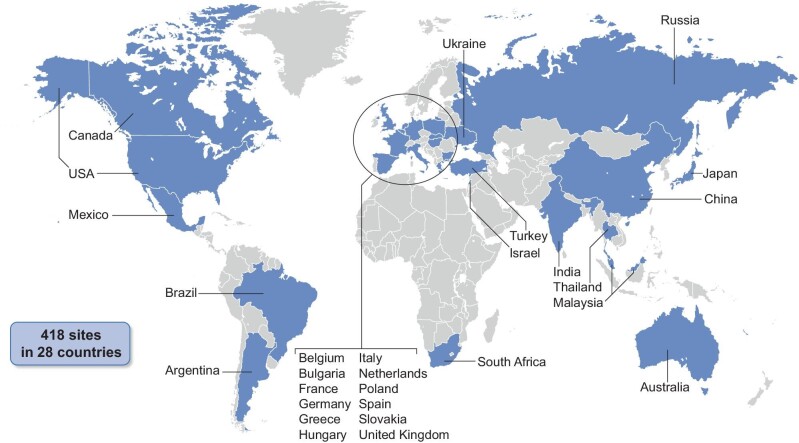
Participants were enrolled from 418 trial locations across 28 countries (Fig. 3 and Supplementary Table 1). Adults (≥18 years or ≥20 years in Japan) with pre-existing CKD with high albuminuria, low estimated glomerular filtration rate (eGFR), T2D, haemoglobin A1c (HbA1c) ≤10% (<86 mmol/mol) and on stable treatment with the maximum labelled or tolerated dose of a RAAS blocking agent (unless contraindicated or not tolerated) were eligible for enrolment. Key inclusion and exclusion criteria are shown in Table 1; eligibility criteria were designed to select a broad population with both CKD and T2D and at high risk for progression of CKD.
Figure 3:
Map of trial site locations.
Table 1:
Inclusion and exclusion criteria for the FLOW trial.
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Informed consent • Male or female • Age ≥18 years (except for Japan where participants must be ≥20 years) • Diagnosis of T2D • HbA1c ≤10% (≤86 mmol/mol) • Renal impairment, defined as either: eGFRa ≥50 and ≤75 ml/min/1.73 m2 and UACR >300 and <5000 mg/gb,c or eGFRa ≥25 and <50 ml/min/1.73 m2 and UACR >100 and <5000 mg/gb • Stable treatment with maximum labelled or tolerated dose of a RAAS blocking agentd |
• Known or suspected hypersensitivity to trial product(s) or related
products • Pregnancy, breastfeeding or intention to become pregnant; child-bearing potential and not using a highly effective contraceptive method • Participation in any clinical trial of an approved or non-approved investigational medicinal product within 30 days before screening • Any disorder which, in the investigator's opinion, might jeopardise a subject's safety or compliance with the protocol • Congenital or hereditary kidney diseasese or autoimmune kidney diseasesf • MI, stroke, hospitalisation for unstable angina or TIA within 60 days prior to the day of screening; NYHA class IV; or planned coronary, carotid or peripheral artery revascularisation • Use of any GLP-1RA within 30 days prior to screening • Personal or first-degree relative(s) with a history of MEN2 or MTC • Chronic or intermittent haemodialysis or peritoneal dialysis within ≤90 days • Uncontrolled and potentially unstable diabetic retinopathy or maculopathyg • Presence or history of malignant neoplasm within 5 years prior to the day of screeningh • Prior solid organ transplant or awaiting solid organ transplant • Combination use of ACE inhibitor and ARB |
aBased on the CKD-EPI formula.
bMeasurements taken ≤90 days before screening with subject in usual health condition.
cNumber of participants with eGFR ≥60 ml/min/1.73 m2 capped at 20% of randomised participants.
dACE inhibitor or ARB, unless such treatment is contraindicated or not tolerated.
eIncluding PKD.
fIncluding glomerulonephritis or congenital urinary tract malformations.
gVerified by a fundus examination performed within the past 90 days prior to screening or in the period between screening and randomisation; eye examination performed by a suitably qualified healthcare provider (e.g. optometrist or ophthalmologist).
hBasal and squamous cell skin cancer and any carcinoma in situ were allowed.
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; MEN2, multiple endocrine neoplasia type 2; MI, myocardial infarction; MTC, medullary thyroid carcinoma; NYHA, New York Heart Association; PKD, polycystic kidney disease; TIA, transient ischaemic attack.
The number of participants with eGFR ≥60 ml/min/1.73 m2 at randomisation was capped at 20% to ensure the predominance of participants with moderate–severe CKD. Laboratory-based inclusion and exclusion criteria were based on historical values recorded within 90 days of the screening visit, values recorded at an optional pre-screening visit or central laboratory values recorded at screening. Baseline data for eGFR and urine albumin:creatinine ratio (UACR) came from central laboratory assessments only.
Outcome measures
All primary, confirmatory secondary and supportive secondary endpoints (Table 2) are assessed from randomisation to the end of the trial. The composite primary endpoint is time to first occurrence of kidney failure [persistent eGFR <15 ml/min/1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula or initiation of chronic kidney replacement therapy (dialysis or kidney transplantation)], persistent ≥50% reduction in eGFR (CKD-EPI) when compared with baseline, or death due to kidney or CV causes. For the eGFR components of the primary endpoint, persistent is defined as two consecutive central laboratory assessments at least 4 weeks apart that meet the criteria. Confirmatory secondary endpoints are annual rate of change of eGFR (total eGFR slope), time to first occurrence of three-point MACE (comprising non-fatal myocardial infarction, non-fatal stroke or death from CV causes) and time to occurrence of all-cause death. Additional secondary and exploratory endpoints compare the effect of semaglutide versus placebo on a range of CV, CKD, clinical and metabolic outcomes, as well as health outcomes and quality of life (Table 2).
Table 2:
FLOW trial endpoints.
| Study endpoints | Time frame |
|---|---|
| Primary endpoint | |
| Time to first occurrence of a composite endpoint consisting of • Onset of kidney failure, defined as initiation of chronic kidney replacement therapy (dialysis or kidney transplantation) or persistent eGFR <15 ml/min/1.73 m2 for at least 4 weeks • Death from kidney failure • CV death • Onset of persistent ≥50% reduction in eGFR (CKD-EPI) versus baseline |
Randomisation to EOT |
| Confirmatory secondary endpoints | |
| Annual rate of change in eGFR (CKD-EPI) (total eGFR slope) | Randomisation to EOT |
| Time to first occurrence of a composite CV MACE endpoint consisting
of: • Non-fatal myocardial infarction • Non-fatal stroke • CV death |
Randomisation to EOT |
| Time to occurrence of all-cause death | Randomisation to EOT |
| Supportive secondary endpoints | |
| Time to occurrence of each of the individual components of the primary
composite endpoint and of the confirmatory secondary MACE endpoint |
Randomisation to EOT |
| Time to first occurrence of MALE, a composite endpoint consisting of: • Acute limb ischaemia hospitalisation • Chronic limb ischaemia hospitalisation |
Randomisation to EOT |
| Annual rate of change in eGFR (CKD-EPI) (chronic eGFR slope) | Week 12 to EOT |
| Change in eGFR (CKD-EPI) | Randomisation to week 12 |
| Change in eGFR (cystatin C CKD-EPI) | Randomisation to year 3 |
| Relative change in UACR | Randomisation to year 3 |
| Change in body weight | Randomisation to year 3 |
| Change in HbA1c | Randomisation to year 3 |
| Change in systolic blood pressure | Randomisation to year 3 |
| Change in diastolic blood pressure | Randomisation to year 3 |
| Number of severe hypoglycaemic episodes | Randomisation to EOT |
| Exploratory endpoints | |
| Change in EQ-5D-5L index score | Randomisation to year 3 |
| Change in EQ-5D-5L visual analogue scale score | Randomisation to year 3 |
Randomisation = week 0; end of trial = a period expected to be ≥61 months for the individual participant; persistent = two consecutive central laboratory assessments that meet criteria, at least 4 weeks apart. Events including deaths, those leading to kidney replacement therapy, acute coronary syndrome, stroke or transient ischaemic attack, and MALE are reviewed by an independent external EAC in a blinded manner.
EOT, end of trial; EQ-5D-5L, EuroQol five-dimension, five-level questionnaire; MALE, major adverse limb events.
Statistical considerations
FLOW is an event-driven trial; a priori a minimum of 3508 participants were expected to be randomised to provide 90% power, using a one-sided type I error rate of 0.025, to detect the likelihood of semaglutide 1.0 mg versus placebo reaching a 20% relative risk reduction [corresponding to a hazard ratio (HR) of 0.8] for the primary endpoint. Assuming a true HR of 0.8, a total of 854 primary endpoint events are required and the trial will be complete when this number of primary endpoints is collected.
The trial uses a group sequential design. Interim testing evaluating the primary endpoint for superiority will be performed based on a locked snapshot of the study database at the time-point of interim testing. Interim testing will be performed by an external statistician independent of trial conduct. An independent data monitoring committee (DMC) will evaluate unblinded interim testing using group sequential stopping boundaries as guidance; to ensure type I error rate control, the alpha spending function developed by Lan and DeMets will be used to provide stopping boundaries. Stopping the trial for superiority is allowed if a stopping boundary is crossed and the DMC makes the decision to recommend early trial termination.
The primary efficacy estimand for all endpoints is based on the intention-to-treat principle, evaluating the effect of the randomised treatment intervention, irrespective of adherence and changes to background medication.
The treatment effect will be expressed as an HR with corresponding 95% confidence interval (CI) and will be estimated using a stratified Cox proportional hazards model with treatment (semaglutide, placebo) as a fixed factor together with the two-sided 95% CI and one-sided P-value for hypothesis testing; stratification is by use of SGLT2is (yes/no) at baseline. If superiority is confirmed for the primary endpoint, then superiority will be tested for the confirmatory secondary endpoints adjusted to account for the group sequential design through a hierarchical testing strategy:
Annual rate of change in eGFR (total eGFR slope)
Time to first occurrence of MACE
Time to occurrence of all-cause death.
Since the recruitment of FLOW trial participants, a new CKD-EPI formula without race as a factor has been recommended; a pre-specified supplementary analysis has now been added to evaluate the impact of the updated formula on the results [16].
Study oversight
FLOW was designed and organised by the sponsor in collaboration with regulatory agencies, a steering committee and a global expert panel. The steering committee provides academic and scientific leadership and ensures that conduct of this study conforms to protocols, while the global expert panel provides scientific, medical and operational input at a country level. The independent DMC reviews safety and efficacy data as defined in the protocols and makes recommendations for additions or adjustments. Planned interim testing for superiority will also be performed by the DMC. An external, independent and blinded events adjudication committee (EAC) is in charge of adjudicating predefined events. The management structure of the trial and relationships between the trial committees can be found in Supplementary Fig. S1.
RESULTS
A total of 3534 participants have been enrolled and randomised to treatment. Baseline and demographic characteristics for the overall population are shown in Table 3 and Supplementary Table 1. Participants had a mean age of 66.6 years (SD 9.0), 69.7% were male and 65.7% were white. The mean HbA1c was 7.8% (SD 1.3) or 61.5 mmol/mol (SD 14.1) and a high proportion of participants (68.6%) had a baseline HbA1c >7%. The mean baseline diabetes duration was 17.4 years (SD 9.3), body mass index was 32.0 kg/m2 (SD 6.3) and systolic and diastolic blood pressure were 138.6 mmHg (SD 15.8) and 76.4 mmHg (SD 10.0), respectively.
Table 3:
Baseline characteristics and demographics.
| Characteristics | Values |
|---|---|
| Total randomised population, N | 3534 |
| Age (years) | 66.6 (9.0) |
| Male, n (%) | 2464 (69.7) |
| Race, n (%) White Black or African American Asian American Indian or Alaska Native Hawaiian or other Pacific Islander Other Not reported |
2323 (65.7) 160 (4.5) 846 (23.9) 25 (0.7) 5 (0.1) 96 (2.7) 79 (2.2) |
| Ethnicity, n (%) Hispanic or Latino Not Hispanic or Latino Not reported |
556 (15.7) 2833 (80.2) 145 (4.1) |
| Region, n (%) Europe Asia North America South America Africa Other |
962 (27.2) 913 (25.8) 865 (24.5) 252 (7.1) 101 (2.9) 441 (12.5) |
| Systolic BP (mmHg) | 138.6 (15.8) |
| Diastolic BP (mmHg) | 76.4 (10.0) |
| Pulse (bpm) | 73.1 (11.3) |
| Body mass index (kg/m2) | 32.0 (6.3) |
| Body weight (kg) | 89.6 (20.5) |
| HbA1c (%) | 7.8 (1.3) |
| HbA1c (mmol) | 61.5 (14.1) |
| HbA1c >7%, n (%) | 2424 (68.6) |
| T2D duration, years | 17.4 (9.3) |
| eGFR (ml/min/1.73 m2)a | 47.0 (15.2) |
| eGFR <60 ml/min/1.73 m2, n (%) | 2814 (79.6) |
| UACR (mg/g), median (range) | 568 (2–11 852) |
| Macroalbuminuria (UACR ≥300 mg/g), n (%) | 2419 (68.4) |
| Diabetic neuropathy, n (%)b | 1521 (43.0) |
| Diabetic retinopathy in worst eye, n (%)c Mild non-proliferative Moderate–severe non-proliferative Proliferative |
1585 (44.9) 943 (26.7) 436 (12.3) 204 (5.8) |
| Diabetic macular oedema in worst eye, n (%)c | 240 (6.8) |
| Previous cardiovascular diseasec Previous MI Previous stroke Heart failure |
1838 (52.0) 513 (14.5) 367 (10.4) 675 (19.1) |
| Very high CKD progression risk (KDIGO criteria), n (%)d | 2413 (68.2) |
| Oral anti-diabetes drugs at baseline, n
(%) Metformine Sulphonylurea DPP-4i SGLT2i Thiazolidinedione Alpha-glucosidase inhibitors |
1825 (51.6) 879 (24.9) 898 (25.4) 548 (15.5) 147 (4.2) 109 (3.1) |
| Insulin at baseline, n (%) Long acting Short acting Premixed |
2172 (61.5) 1616 (45.7) 1293 (36.6) 224 (6.3) |
| RAAS inhibitors, n (%) | 3368 (95.3) |
| Cardiovascular medications, n (%) Beta blockers ACE inhibitors Angiotensin receptor blockers Calcium channel blockers Angiotensin receptor–neprilysin inhibitor |
3511 (99.3) 1823 (51.6) 1242 (35.1) 2127 (60.2) 1992 (56.4) 24 (0.68) |
| Lipid-lowering drugs, n (%) Statins Bile acid sequestrants Fibrates Ezetimibe PCSK-9 inhibitors Eicosapentaenoic acid ethyl ester Omega-3-acid ethyl ester Other omega 3-triglycerides |
2799 (79.2) 2655 (75.1) 1 (0.03) 302 (8.5) 248 (7.0) 9 (0.3) 22 (0.6) 12 (0.3) 64 (1.8) |
All values are mean (SD) unless stated otherwise.
As the trial is ongoing, data may be subject to minor changes until database lock.
aeGFR was estimated using the CKD-EPI equation.
bBased on medical history.
cBased on eye examination at baseline, verified by a fundus examination performed within the past 90 days prior to screening or in the period between screening and randomisation; eye examination performed by a suitably qualified healthcare provider (e.g. optometrist or ophthalmologist).
dDefined as (i) UACR (mg/g) <30 and eGFR (mL/min/1.73 m2) ≤15 to <30, (ii) UACR (mg/g) ≤30 to <300 and eGFR (mL/min/1.73 m2) ≤30 to <45, (iii) UACR (mg/g) ≤30 to <300 and eGFR (mL/min/1.73 m2) ≤15 to <30, (iv) UACR (mg/g) ≤300 and eGFR (mL/min/1.73 m2) ≤45 to <60, (v) UACR (mg/g) ≤300 and eGFR (mL/min/1.73 m2) ≤30 to <45 or (vi) UACR (mg/g) ≤300 and eGFR (mL/min/1.73 m2): ≤15 to <30.
eRelatively low proportion of patients due to metformin being contraindicated in participants with a low eGFR.
ACE, angiotensin-converting enzyme; BP, blood pressure; bpm, beats per minute; DPP-4i, dipeptidyl peptidase-4 inhibitor; MI, myocardial infarction; n, number of participants; PCSK-9, proprotein convertase subtilisin/kexin type 9.
Regarding kidney function, the mean baseline eGFR was 47.0 ml/min/1.73 m2 (SD 15.2) and the median UACR was 568 mg/g (range 2‒11 852); baseline laboratory assessments may differ slightly from eligibility criteria required at screening (see the Population section of the Methods where this is described). Macroalbuminuria (UACR ≥300 mg/g) was evident in 68.4% of participants. According to KDIGO categories [3], the majority of participants (68.2%) were classified as very high risk for CKD progression (Fig. 4).
Figure 4:
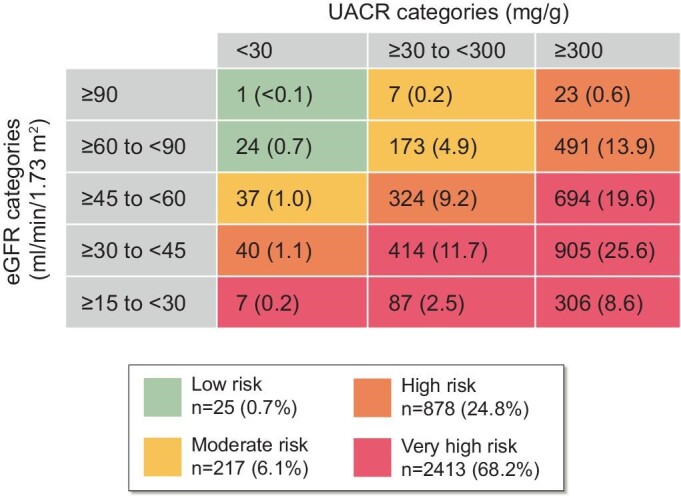
KDIGO risk categories among FLOW participants. Data are n (%) for each category. To facilitate recruitment to the FLOW trial and increase generalisability of the outcomes by reflecting typical clinical practice, eligibility in terms of laboratory results could be based on historical values. Adapted from the KDIGO Diabetes Work Group, 2020 [3].
Many participants had comorbidities such as neuropathy and retinopathy (43.0% and 44.9%, respectively) at baseline. A medical history of CVD was also common (52.0%) and 19.1% of participants had previous heart failure. The most frequently used glucose-lowering medications at baseline were insulin (61.5%) and metformin (51.6%), and 15.5% of participants were receiving SGLT2is. Participants were required to be on RAAS blockade unless treatment was contraindicated or not tolerated; overall, 95.3% were receiving RAAS inhibitors at baseline. Most participants were also receiving lipid-lowering medication (79.2%) (Table 3).
DISCUSSION
Despite the availability of established and newer pharmacotherapies to delay CKD progression in people with T2D, the risk of progression and the associated morbidity and mortality remains substantial [14, 15]. With a projected global increase in diabetes prevalence from an estimated 537 million in 2021 to 780 million by 2045 [1], there is a clear need for additional treatment options to help mitigate the residual risk in those individuals with concomitant CKD.
Secondary analyses, primarily of CVOTs, suggest a potential kidney-protective effect of GLP-1RAs in people with T2D and at risk of CVD [6, 9–12, 17–28]. Given the relatively low proportion of participants with CKD at baseline (≈20–25%), the variability in the severity of CKD exhibited by participants and the nature of secondary endpoints in CVOTs, available data can only be considered hypothesis generating. Improved albuminuria is the most consistent kidney outcome with GLP-1RAs to date, although this might largely be due to the relatively low occurrence of kidney events and the relatively low prevalence of existing CKD in CVOT populations selected for CV risk. GLP-1RA-associated preservation of eGFR in participants with T2D and CKD has also been demonstrated in CVOTs and non-CVOTs [17, 20, 29]. Trials that are both specifically designed and statistically powered are needed to clearly define the effects of GLP-1RAs on clinically meaningful kidney outcomes.
FLOW is a dedicated kidney outcomes trial designed to assess the kidney-protective effects of semaglutide, a GLP-1RA, and its potential to improve treatment options in people with CKD and T2D. The composite endpoint components selected for FLOW are widely recognised by health authorities and regulatory bodies as robust measures for the assessment of kidney protection [30]. CV death is included in the composite endpoint, alongside death from kidney failure, because of the multiple pathways and risk factors that are common to these two conditions and the resulting competing risks [31].
To facilitate recruitment to the FLOW trial and increase generalisability of the outcomes by reflecting typical clinical practice, eligibility in terms of laboratory results could be based on historical values. However, baseline data were based on central laboratory-derived values, resulting in data from a small number of participants not fulfilling all inclusion criteria at this specific visit. Permitting use of the broad spectrum of concomitant glucose-lowering medications inherent in a SoC approach, as well as treatments for comorbidities and CKD/CV risk factors (including SGLT2is), enables a pragmatic approach to treatment comparisons and for secondary analyses of treatment combinations depending on patient characteristics/baseline therapy. There is substantial overlap between the FLOW population and the populations studied in the dedicated kidney outcome studies with SGLT2is (CREDENCE, DAPA-CKD and EMPA-KIDNEY) [15, 30, 32], which have led to recommendations for the use of SGLT2i for patients with T2D and CKD [3]. Similarly, FLOW will allow for a rigorous evaluation of the potential of semaglutide for the management of CKD in T2D.
The significant CKD burden of the FLOW trial population is reflected in the baseline data showing that 68.2% of participants classified according to KDIGO guidelines criteria are at very high risk of CKD progression. A high proportion of participants had HbA1c >7%, coupled with a mean diabetes duration of 17.4 years, reflecting a population with a substantial diabetes burden. As such, participants in the FLOW trial represent a population that is likely to benefit from additional treatment options that go beyond glycaemic control to address metabolic risk and comorbidities [3].
Two ongoing CVOTs with secondary kidney outcomes, SOUL (oral semaglutide in participants with T2D and CVD or CKD; NCT03914326) and SELECT (s.c. semaglutide 2.4 mg in participants with obesity and established CVD; NCT03574597), will also provide insights on the effect of semaglutide in kidney disease [33, 34].
The mechanism of action for GLP-1RAs in the kidney is not yet fully elucidated, although several hypotheses have been proposed. Mediation analyses of SUSTAIN 6 (semaglutide), REWIND (dulaglutide) and LEADER (liraglutide) CVOTs suggest that indirect effects such as improved glycaemic control, weight loss and reduced blood pressure cannot fully account for the kidney-protective effects and indicate that other factors also play a role [6, 35–37]. Potential direct effects include reduced inflammation, oxidative stress or haemodynamic effects [38–40]. Indeed, reductions in oxidative stress and inflammation have been demonstrated with semaglutide in murine studies showing antioxidative activity and suppression of inflammatory cytokines specifically in the kidney [40, 41]. Evidence for a direct mechanism is limited and will be more thoroughly examined in the mechanistic REMODEL trial (NCT04865770). REMODEL will utilise magnetic resonance imaging to assess kidney oxygenation, inflammation and global kidney perfusion; kidney biopsies to evaluate gene expression via single-nucleus RNA sequencing and morphometric parameters; and blood and urinalysis to assess biomarkers of kidney function and kidney damage [42].
Existing data from CVOTs and real-world evidence suggest that GLP-1RAs have the potential to slow the progression of CKD in people with T2D at high risk of CV events [6, 9–11, 17–28], although no dedicated kidney outcomes trials in populations with CKD and T2D have yet been conducted. The FLOW trial is fully recruited with a population of participants with a T2D mean baseline eGFR of 47.0 ml/min/1.73 m2, a median UACR of 568 mg/g and a mean baseline HbA1c of 7.8%. FLOW is the first dedicated kidney outcomes trial with a GLP-1RA that includes people with T2D at high or very high risk for CKD progression and is specifically designed to provide evidence on the effects of semaglutide on kidney outcomes. We anticipate that FLOW will complete in late 2024.
Supplementary Material
ACKNOWLEDGEMENTS
This study was funded by Novo Nordisk A/S. We thank all the participants, investigators and study-site staff, as well as Henrik Ravn from Novo Nordisk, for their review and input to the manuscript and Sarah White (AXON Communications) for medical writing and editorial assistance (funded by Novo Nordisk A/S).
Contributor Information
Peter Rossing, Complication Research, Steno Diabetes Center Copenhagen, Herlev, Denmark; Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Florian M M Baeres, Novo Nordisk A/S, Søborg, Denmark.
George Bakris, Department of Medicine, AHA Comprehensive Hypertension Center, University of Chicago Medicine, Chicago, IL, USA.
Heidrun Bosch-Traberg, Novo Nordisk A/S, Søborg, Denmark.
Mette Gislum, Novo Nordisk A/S, Søborg, Denmark.
Stephen C L Gough, Novo Nordisk A/S, Søborg, Denmark.
Thomas Idorn, Novo Nordisk A/S, Søborg, Denmark.
Jack Lawson, Novo Nordisk A/S, Søborg, Denmark.
Kenneth W Mahaffey, Department of Medicine, Stanford Center for Clinical Research, Stanford School of Medicine, Palo Alto, CA, USA.
Johannes F E Mann, Outpatients Clinic, KfH Kidney Center, München, Germany.
Henriette Mersebach, Novo Nordisk A/S, Søborg, Denmark.
Vlado Perkovic, Faculty of Medicine and Health, University of New South Wales, Sydney, NSW, Australia.
Katherine Tuttle, Division of Nephrology, University of Washington/Providence Health Care, Spokane, WA, USA.
Richard Pratley, Translational Research Institute, AdventHealth, Orlando, FL, USA.
FUNDING
Funding was provided by Novo Nordisk A/S.
AUTHORS’ CONTRIBUTIONS
All authors were involved in the conceptualisation of the article and its development and critical revision and have read and agreed to the published version. M.G. provided data analysis.
DATA AVAILABILITY STATEMENT
The data sets analysed during the current study are available from the corresponding author upon reasonable request.
CONFLICT OF INTEREST STATEMENT
P.R. has received grants from Bayer, AstraZeneca and Novo Nordisk; received honoraria to the Steno Diabetes Centre Copenhagen from AstraZeneca, Astellas, Boehringer Ingelheim, Gilead, Novo Nordisk, Merck, Mundipharma, Sanofi and Bayer; and consulting fees from AstraZeneca, Astellas, Boehringer Ingelheim, Gilead, Novo Nordisk, Merck, Mundipharma, Sanofi and Bayer. M.G., F.M.M.B., H.B.-T., H.M., J.L., S.G. and T.I. are employees of Novo Nordisk A/S. F.M.M.B., H.B.-T., H.M., S.G. and T.I. also hold stock in Novo Nordisk A/S. G.B. reports consulting fees from Bayer, KBP Biosciences, Ionis, Alnylam, AstraZeneca, Quantum Genomics, Novo Nordisk and Dia Medica Therapeutics. K.W.M. has received consulting fees from Amgen, Applied Therapeutics, AstraZeneca, Bayer, CSL Behring, Elsevier Fibrogen, Inova, Johnson & Johnson, Lexicon Myokardia, Novartis, Novo Nordisk, Otsuka Phasebio, Portola Sanofi and Theravance. J.M. reports grants from Novo Nordisk, the European Union and McMaster University Hamilton, Canada; consulting fees from Novo Nordisk, AstraZeneca, Bayer and Boehringer Ingelheim; honoraria from Novo Nordisk, AstraZeneca, Bayer, Fresenius and Novartis; and has participated on a data safety monitoring board or advisory board for AstraZeneca, Bayer, Sanofi and Boehringer Ingelheim as well as a leadership role in the KDIGO group. V.P. has received honoraria for steering committee, data monitoring committee or advisory board roles or for scientific presentations from AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, Dimerix, GlaxoSmithKline, Janssen, Medimmune, Mitsubishi Tanabe, Mundipharma, Novo Nordisk, Novartis, Otsuka, Travere, Tricida and Vifor Pharma; is a Board Director for George Clinical, St. Vincents Health Australia and several independent medical research institutes. K.T. reports grants/contracts from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)/National Institutes of Health (NIH), National Heart, Lung, and Blood Institute/NIH, National Center for Advancing Translational Sciences/NIH, Centers for Disease Control and Travere; consulting fees from Eli Lilly, Boehringer Ingelheim, Gilead, Goldfinch Bio, Bayer, Novo Nordisk and AstraZeneca; honoraria from Eli Lilly, AstraZeneca, Gilead, Goldfinch Bio and Bayer; support for travel and meetings from Eli Lilly and Novo Nordisk; has participated on a data safety monitoring board/advisory board for NIDDK/NIH and George Clinical Institute; was Chair of the Diabetic Kidney Disease Collaborative Task Force, American Society of Nephrology and was on the Board of Directors for Kidney Health Initiative, US Food and Drug Administration and the American Society of Nephrology.
REFERENCES
- 1. International Diabetes Federation . Diabetes Atlas, 10th edn.https://diabetesatlas.org/en/resources (15 August 2022, date last accessed). [Google Scholar]
- 2. Li R, Bilik D, Brown MBet al. Medical costs associated with type 2 diabetes complications and comorbidities. Am J Manag Care 2013;19:421–30. [PMC free article] [PubMed] [Google Scholar]
- 3. Kidney Disease: Improving Global Outcomes Diabetes Work Group . KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020;102(5 Suppl):S1–127. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . Standards of medical care in diabetes–2022. Diabetes Care 2022;45(Suppl 1):S1–264. [DOI] [PubMed] [Google Scholar]
- 5. McDermid E. ADA/KDIGO consensus: ‘Speaking the same language’ on CKD management. https://diabetes.medicinematters.com/en-GB/ada-2022/guidelines/ada-kdigo-consensus-guidelines-ckd-dkd-diabetes/23134706 (18 February 2023, date last accessed). [Google Scholar]
- 6. Gerstein HC, Colhoun HM, Dagenais GRet al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 2019;394:131–8. [DOI] [PubMed] [Google Scholar]
- 7. Gerstein HC, Sattar N, Rosenstock Jet al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med 2021;385:896–907. [DOI] [PubMed] [Google Scholar]
- 8. Hernandez AF, Green JB, Janmohamed Set al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–29. [DOI] [PubMed] [Google Scholar]
- 9. Marso SP, Daniels GH, Brown-Frandsen Ket al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marso SP, Bain SC, Consoli Aet al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44. [DOI] [PubMed] [Google Scholar]
- 11. Kristensen SL, Rorth R, Jhund PSet al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019;7:776–85. [DOI] [PubMed] [Google Scholar]
- 12. Sattar N, Lee MMY, Kristensen SLet al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021;9:653–62. [DOI] [PubMed] [Google Scholar]
- 13. Mann JFE, Orsted DD, Brown-Frandsen Ket al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017;377:839–48. [DOI] [PubMed] [Google Scholar]
- 14. Perkovic V, Jardine MJ, Neal Bet al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–306. [DOI] [PubMed] [Google Scholar]
- 15. Heerspink HJL, Stefansson BV, Correa-Rotter Ret al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. [DOI] [PubMed] [Google Scholar]
- 16. Inker LA, Eneanya ND, Coresh Jet al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021;385:1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mann JFE, Hansen T, Idorn Tet al. Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: a post-hoc analysis of the SUSTAIN 1–7 randomised controlled trials. Lancet Diabetes Endocrinol 2020;8:880–93. [DOI] [PubMed] [Google Scholar]
- 18. Mosenzon O, Blicher TM, Rosenlund Set al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol 2019;7:515–27. [DOI] [PubMed] [Google Scholar]
- 19. Tuttle KR, Bosch-Traberg H, Cherney DZIet al. Post hoc analysis of SUSTAIN 6 and PIONEER 6 trials suggests that people with type 2 diabetes at high cardiovascular risk treated with semaglutide experience more stable kidney function compared with placebo [published online ahead of print, 2023 Feb 2]. Kidney Int 2023;S0085-2538(23)00056-X. doi:10.1016/j.kint.2022.12.028. [DOI] [PubMed] [Google Scholar]
- 20. Tuttle KR, Lakshmanan MC, Rayner Bet al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 2018;6:605–17. [DOI] [PubMed] [Google Scholar]
- 21. Pasternak B, Wintzell V, Eliasson Bet al. Use of glucagon-like peptide 1 receptor agonists and risk of serious renal events: Scandinavian cohort study. Diabetes Care 2020;43:1326–35. [DOI] [PubMed] [Google Scholar]
- 22. Xie Y, Bowe B, Gibson AKet al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care 2020;43:2859–69. [DOI] [PubMed] [Google Scholar]
- 23. Mann JFE, Fonseca VA, Poulter NRet al. Safety of liraglutide in type 2 diabetes and chronic kidney disease. Clin J Am Soc Nephrol 2020;15:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mann JFE, Fonseca V, Mosenzon Oet al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation 2018;138:2908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mosenzon O, Bain SC, Heerspink HJLet al. Cardiovascular and renal outcomes by baseline albuminuria status and renal function: results from the LEADER randomized trial. Diabetes Obes Metab 2020;22:2077–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perkovic V, Bain S, Bakris Get al. Effects of semaglutide and liraglutide on urinary albumin-to-creatinine ratio (UACR) – a pooled analysis of SUSTAIN 6 and LEADER. Nephrol Dial Transplant 2019;34:i205–20. [Google Scholar]
- 27. Perkovic V, Bain S, Bakris Get al. Effects of the glucagon-like peptide-1 (GLP-1) analogues semaglutide and liraglutide on renal outcomes – a pooled analysis of the SUSTAIN 6 and LEADER trials. Nephrol Dial Transplant 2019;34:i338–9. [Google Scholar]
- 28. Persson F, Bain SC, Mosenzon Oet al. Changes in albuminuria predict cardiovascular and renal outcomes in type 2 diabetes: a post hoc analysis of the LEADER trial. Diabetes Care 2021;44:1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tuttle KR, Rayner B, Lakshmanan MCet al. Clinical outcomes by albuminuria status with dulaglutide versus insulin glargine in participants with diabetes and CKD: AWARD-7 exploratory analysis. Kidney360 2020;2:254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jardine MJ, Mahaffey KW, Neal Bet al. The canagliflozin and renal endpoints in diabetes with established nephropathy clinical evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol 2017;46:462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bello AK, Hemmelgarn B, Lloyd Aet al. Associations among estimated glomerular filtration rate, proteinuria, and adverse cardiovascular outcomes. Clin J Am Soc Nephrol 2011;6:1418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herrington WG, Preiss D, Haynes Ret al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018;11:749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Novo Nordisk . A heart disease study of semaglutide in patients with type 2 diabetes (SOUL). https://clinicaltrials.gov/ct2/show/NCT03914326 (15 August 2022, date last accessed). [Google Scholar]
- 34. Ryan DH, Lingvay I, Colhoun HMet al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am Heart J 2020;229:61–9. [DOI] [PubMed] [Google Scholar]
- 35. Buse JB, Bain SC, Mann JFEet al. Cardiovascular risk reduction with liraglutide: an exploratory mediation analysis of the LEADER trial. Diabetes Care 2020;43:1546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mann JFE, Buse JB, Idorn Tet al. Potential kidney protection with liraglutide and semaglutide: exploratory mediation analysis. Diabetes Obes Metab 2021;23:2058–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mann JFE, Muskiet MHA.. Incretin-based drugs and the kidney in type 2 diabetes: choosing between DPP-4 inhibitors and GLP-1 receptor agonists. Kidney Int 2021;99:314–8. [DOI] [PubMed] [Google Scholar]
- 38. Muskiet MHA, Tonneijck L, Smits MMet al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol 2017;13:605–28. [DOI] [PubMed] [Google Scholar]
- 39. Rakipovski G, Rolin B, Nohr Jet al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE−/− and LDLr−/− mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci 2018;3:844–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alicic RZ, Cox EJ, Neumiller JJet al. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat Rev Nephrol 2021;17:227–44. [DOI] [PubMed] [Google Scholar]
- 41. Fujita H, Morii T, Fujishima Het al. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int 2014;85:579–89. [DOI] [PubMed] [Google Scholar]
- 42. Novo Nordisk A/S . Research study to find out how semaglutide works in the kidneys compared to placebo, in people with type 2 diabetes and chronic kidney disease (REMODEL). https://clinicaltrials.gov/ct2/show/NCT04865770 (27 August 2021, date last accessed). [Google Scholar]
- 43. Bethel MA, Mentz RJ, Merrill Pet al. Microvascular and cardiovascular outcomes according to renal function in patients treated with once-weekly exenatide: insights from the EXSCEL trial. Diabetes Care 2020;43:446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets analysed during the current study are available from the corresponding author upon reasonable request.