ABSTRACT
Background
Patients on B-cell-depleting agents may have a suboptimal response to vaccination, placing them at a higher risk of contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or suffering from a more severe prognosis. Indeed, available data on pre-exposure prophylaxis with tixagevimab/cilgavimab (Evusheld) in subjects with glomerular diseases (GDs) who received rituximab are limited.
Methods
We conducted a prospective study analysing the safety and efficacy of tixagevimab/cilgavimab for pre-exposure prophylaxis in patients with GDs who received rituximab in the previous 12 months. The rates of symptomatic infections and hospitalizations were compared with those for patients with GD treated with rituximab who refused to receive tixagevimab/cilgavimab.
Results
Tixagevimab/cilgavimab was administered to 22 patients (12 females, mean age 58.4 ± 19.6 years) with GD diagnoses including membranous nephropathy, lupus nephritis, anti-neutrophil cytoplasmic antibody–associated vasculitis and focal segmental glomerulosclerosis. No patient treated with tixagevimab/cilgavimab experienced symptomatic infection with SARS-CoV-2 during the follow-up (mean observation time of follow-up was 112 ± 23 days), while 11 of 28 controls (39.3%) reported a symptomatic infection (P = .0001), requiring hospitalization in 2 cases. Reported adverse events were mild, namely self-limiting headache [4], discomfort at the injection site [3], flu-like symptoms/myalgia [3] and fever [1]. No serious adverse events (e.g. cardiac events, anaphylaxis) were reported.
Conclusion
Pre-exposure prophylaxis with tixagevimab/cilgavimab seems safe and lowered the risk of symptomatic SARS-CoV-2 infection by ≈40% in vaccinated subjects with GD who received anti-CD20 therapy. Possible applications in the subset of patients who need immunosuppressive therapy, especially with rituximab, in a pandemic setting might be envisaged.
Keywords: Evusheld, glomerular diseases, glomerulonephritis, monoclonal antibodies, rituximab, tixagevimab/cilgavimab
INTRODUCTION
The main defence against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is vaccination. However, it has been demonstrated that some patients, such as those on B-cell-depleting drugs, may have a suboptimal response to vaccination, placing them at a higher risk of contracting SARS-CoV-2 or suffering from a more severe prognosis [1–6]. In fact, past use of rituximab therapy has been linked to unfavourable outcomes and higher morbidity and mortality rates in individuals with chronic disorders who become infected with SARS-CoV-2 [4–6].
In these patient populations, pre-exposure prophylaxis against SARS-CoV-2 with tixagevimab and cilgavimab (Evusheld) could be considered [7]. Tixagevimab and cilgavimab are monoclonal antibodies with neutralizing activity recognizing different epitopes of the receptor-binding domain of the SARS-CoV-2 spike protein [8]. In the PROVENT study (NCT04625725) [9], tixagevimab and cilgavimab significantly reduced symptomatic infection with SARS-CoV-2 in high-risk patients, who were characterized as those >60 years of age, obese or with major comorbidities, including an immunocompromised state. However, the real-world efficacy of tixagevimab and cilgavimab in avoiding symptomatic SARS-CoV-2 infection in immunocompromised patients, such as those with glomerular disorders (GDs) treated with rituximab, has not yet been documented.
In this prospective study, we reported our experience on the use of pre-exposure prophylaxis with tixagevimab/cilgavimab in patients with GDs who received rituximab in the previous 12 months, focusing on the safety and efficacy.
MATERIALS AND METHODS
In this single-centre prospective study, individuals ≥18 years of age with GD who had been treated with rituximab therapy during the previous 12 months and who agreed to administration of tixagevimab and cilgavimab were followed longitudinally.
Patients were all given the standard dosage of 150 mg/150 mg [10]. Patients’ reports, record reviews and telephone interviews were used to identify patients with symptoms of SARS-CoV-2 infection. SARS-CoV-2 infection confirmation needed a positive test (either polymerase chain reaction or antigen test). On the day when tixagevimab and cilgavimab were administered, adverse events were evaluated and thereafter post-administration occurrences were followed by phone interviews and chart review.
Patients ≥18 years of age with GD who had been treated with rituximab therapy during the previous 12 months but did not agree to administration of tixagevimab and cilgavimab were enrolled as controls and followed longitudinally.
The significance of baseline differences and outcomes was determined by the χ2 test, Fisher's exact test or unpaired t-test, as appropriate. A two-sided P-value <.05 was considered statistically significant. All statistical analyses were performed using SPSS version 28.0 (IBM, Armonk, NY, USA).
Tixagevimab and cilgavimab were obtained from the local hospital pharmacy. The study was conducted according to the rules for the management of rare diseases (Piedmont, Northwest Italy). All patients provided informed consent. No commercial sponsor was involved.
RESULTS
Twenty-two patients (12 females, mean age 58.4 ± 19.6 years) received tixagevimab/cilgavimab (from early August 2022 through the end of September 2022), with GD diagnoses including membranous nephropathy [7], lupus nephritis [4], anti-neutrophil cytoplasmic antibody–associated vasculitis [4] and focal segmental glomerulosclerosis [5], minimal change disease [1] and monoclonal immunoglobulin deposits [1]. Clinical and demographic characteristics are detailed in Table 1. Fifteen (68.2%) patients were receiving oral glucocorticoids (mean dosage of prednisone 7.5 mg/day). Ten (45%) were still B-cell depleted at the time of tixagevimab/cilgavimab administration. Fifteen (68.2%) subjects had confirmed SARS-CoV-2 infection before the administration of tixagevimab and cilgavimab, with a mean 271 ± 91 days (range 101–507) before receiving tixagevimab and cilgavimab. Before receiving tixagevimab and cilgavimab, 18 (81.2%) patients were given three doses of messenger RNA (mRNA) SARS-CoV-2 vaccine and 4 (18.8%) patients had four doses.
Table 1:
Main demographic and clinical characteristics of cases and controls at study entry and the end of follow-up.
| Characteristics | Cases (n = 22) | Controls (n = 28) |
|---|---|---|
| Patients | ||
| Female, n (%) | 12 (54.5) | 13 (46.4) |
| Age (years), median (range) | 58.39 (38–78) | 63.2 (22–81) |
| Vaccinations, n (%) | ||
| 2 doses | 0 (0) | 6 (21.4) |
| 3 doses | 18 (81.2) | 17 (60.7) |
| 4 doses | 4 (18.8) | 5 (17.9) |
| B-cell depleted at study enrolment | 10 (45) | 12 (42.9) |
| IgG level (U/L), mean ± SD at study enrolment | 871 ± 67 | 907 ± 57 |
| eGFR (ml/min/1.73 m2), mean ± SD at study enrolment | 57 ± 21 | 61 ± 26 |
| Diagnosis, n (%) | ||
| Membranous glomerulonephritis | 7 (31.8) | 7 (25.0) |
| ANCA-associated vasculitis | 4 (18.2) | 4 (14.3) |
| Focal segmental glomerulosclerosis | 5 (22.7) | 3 (10.7) |
| Lupus nephritis | 4 (18.2) | 1 (3.6) |
| IgAN | 1 (4.5) | 2 (7.1) |
| Other glomerulopathiesa | 1 (4.5) | 11 (39.3) |
| Outcomes, n (%) | ||
| Symptomatic COVID-19 infection | 0 (0) | 11 (39.3) |
| Hospitalisation | 0 (0) | 2 (18.2) |
| Mortality | 0 (0) | 0 (0) |
aThrombotic thrombocytopenic purpura, minimal change disease, immunoglobulin M glomerulonephritis, membranoproliferative glomerulonephritis, immunotactoid glomerulonephritis, cryoglobulinaemic vasculitis.
IgG: immunoglobulin G; eGFR: estimated glomerular filtration rate; IgAN: immunoglobulin A nephropathy.
All patients received vaccination at least 30 days before being treated with anti-CD20 agents. Adverse events related with the administration of tixagevimab and cilgavimab were mild and temporally limited (<72 hours after the injection): self-limiting headache [4], discomfort at the injection site [3], flu-like symptoms/myalgia [3] and fever [1]. No serious adverse events (e.g. cardiac events, anaphylaxis) were reported. During the observation time (mean 112 ± 23 days), no symptomatic infection with SARS-CoV-2 occurred.
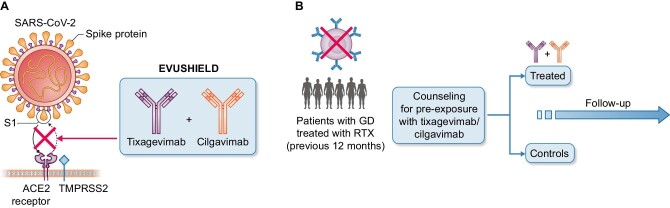
After tailored counselling, 28 patients meeting the inclusion criteria for this study did not agree to receive tixagevimab and cilgavimab and were enrolled as controls (Fig. 1).
Figure 1:
(A) Schematic representation of the mechanism of action of tixagevimab and cilgavimab. (B) Study design.
Eighteen (64.3%) controls were receiving oral glucocorticoids (mean dosage of prednisone 5.5 mg/day). Twelve (42.9%) were still B-cell depleted at the time of tixagevimab/cilgavimab counselling. The main characteristics are described in Table 1.
During the observation time (mean 121 ± 31 days), we observed a significantly higher occurrence of symptomatic infection with SARS-CoV-2 in the controls (11 of 28 [39.3%], P = .0001), requiring hospitalization for pneumonia, with high-flow nasal oxygen therapy in 2 cases. No symptomatic infection was observed in the cases. Six of 12 controls who were B-cell depleted at study inclusion were still depleted 30 days before the symptomatic coronavirus disease 2019 (COVID-19) infection. The main SARS-CoV-2 strains dominant in Italy during the study period are detailed in Supplementary Table 1S.
DISCUSSION
To date, the safety and efficacy of tixagevimab/cilgavimab (Evusheld) in subjects with GDs who received rituximab have not been explicitly examined, despite evidence that pre-exposure prophylaxis might lower the risk for symptomatic infection with SARS-CoV-2. In a real-world prospective study of GD subjects receiving rituximab treatment, we found a very good efficacy of tixagevimab and cilgavimab in avoiding symptomatic SARS-CoV-2 infection. When compared with controls, we observed in subjects treated with tixagevimab/cilgavimab a significant reduction in the occurrence of symptomatic infection with SARS-CoV-2 (two of those requiring hospitalization) as high as 39.3%. Our observations were further supported by the fact that throughout the study period, the Piedmont area (4356 million inhabitants) experienced a relatively high incidence of SARS-CoV-2 transmission, with active cases ranging from 33 498 to 55 468 and a rate of positive tests of at least 8.13% [11]. In addition, since public health authorities might not be informed of the results of home antigen tests, it is almost certain that the real rate of infection was underestimated in this analysis.
Our study has some limitations. Data on humoral response to vaccine (e.g. anti-S titres) were not accessible for all patients. Second, the investigation was conducted at a single centre with a small sample size. Third, six controls received only two doses of vaccine, in line with the general hesitancy towards preventive anti-COVID-19 strategies observed in this group despite adequate counselling. Also, the mean age was higher in the controls, although the difference was not statistically significant when compared with the cases (P = .55). Finally, data on the variants affecting patients with COVID-19 during the study were not available. For these reasons, the results of this study should be regarded cautiously and further research is required to validate these findings in larger cohorts.
Nevertheless, our study is the first that we are aware of that reports on the real-life use of tixagevimab/cilgavimab in patients with GDs and provides preliminary evidence of therapeutic benefit. Second, and most importantly, neither cardiac nor anaphylactic episodes were seen, and no patients reported any clinically significant adverse events. Third, up to 45% of the patients were still B-cell depleted at the time of tixagevimab/cilgavimab administration.
Our observations have the potential to support a paradigmatic change in pre-exposure prophylaxis strategies against SARS-CoV-2, suggesting that tixagevimab and cilgavimab can be systematically considered in patients with GDs when receiving rituximab. Indeed, anti-CD20 therapy is especially implicated in COVID/SARS exposure due to patients’ long-term inability to protect themselves with a consistent adaptive immune response.
Despite the short duration of our follow-up, we showed that tixagevimab and cilgavimab may be efficacious and tolerable in this particular demographic, in the hopes that this may lead to an increased use of these treatments. In fact, even though >80% of our patients were given at least three doses of vaccine, anti-CD20 agents are recognized to dramatically reduce the humoral response to mRNA vaccinations, posing a higher risk of severe infections [1–6].
Our research supports the prompt pre-exposure use of tixagevimab and cilgavimab in GD patients who have substantial immunosuppression from prior anti-CD20 use [12, 13], in order to avoid severe SARS-CoV-2. In this high-risk category, a multilayered approach to risk reduction is strongly recommended.
Supplementary Material
Contributor Information
Savino Sciascia, University Center of Excellence on Nephrologic, Rheumatologic and Rare Diseases (ERK-net, ERN-Reconnect and RITA-ERN Member) with Nephrology and Dialysis Unit and Center of Immuno-Rheumatology and Rare Diseases (CMID), Coordinating Center of the Interregional Network for Rare Diseases of Piedmont and Aosta Valley, San Giovanni Bosco Hub Hospital, Turin, Italy.
Maria Letizia Antonietta Rilat, University Center of Excellence on Nephrologic, Rheumatologic and Rare Diseases (ERK-net, ERN-Reconnect and RITA-ERN Member) with Nephrology and Dialysis Unit and Center of Immuno-Rheumatology and Rare Diseases (CMID), Coordinating Center of the Interregional Network for Rare Diseases of Piedmont and Aosta Valley, San Giovanni Bosco Hub Hospital, Turin, Italy.
Roberta Fenoglio, University Center of Excellence on Nephrologic, Rheumatologic and Rare Diseases (ERK-net, ERN-Reconnect and RITA-ERN Member) with Nephrology and Dialysis Unit and Center of Immuno-Rheumatology and Rare Diseases (CMID), Coordinating Center of the Interregional Network for Rare Diseases of Piedmont and Aosta Valley, San Giovanni Bosco Hub Hospital, Turin, Italy.
Silvia Grazietta Foddai, University Center of Excellence on Nephrologic, Rheumatologic and Rare Diseases (ERK-net, ERN-Reconnect and RITA-ERN Member) with Nephrology and Dialysis Unit and Center of Immuno-Rheumatology and Rare Diseases (CMID), Coordinating Center of the Interregional Network for Rare Diseases of Piedmont and Aosta Valley, San Giovanni Bosco Hub Hospital, Turin, Italy.
Massimo Radin, University Center of Excellence on Nephrologic, Rheumatologic and Rare Diseases (ERK-net, ERN-Reconnect and RITA-ERN Member) with Nephrology and Dialysis Unit and Center of Immuno-Rheumatology and Rare Diseases (CMID), Coordinating Center of the Interregional Network for Rare Diseases of Piedmont and Aosta Valley, San Giovanni Bosco Hub Hospital, Turin, Italy.
Irene Cecchi, University Center of Excellence on Nephrologic, Rheumatologic and Rare Diseases (ERK-net, ERN-Reconnect and RITA-ERN Member) with Nephrology and Dialysis Unit and Center of Immuno-Rheumatology and Rare Diseases (CMID), Coordinating Center of the Interregional Network for Rare Diseases of Piedmont and Aosta Valley, San Giovanni Bosco Hub Hospital, Turin, Italy.
Giacoma Cinnirella, Pharmacy Department, S. Giovanni Bosco Hospital, Turin, Italy.
Paola Crosasso, Pharmacy Department, S. Giovanni Bosco Hospital, Turin, Italy.
Maria Gabriella Guidetti, University Center of Excellence on Nephrologic, Rheumatologic and Rare Diseases (ERK-net, ERN-Reconnect and RITA-ERN Member) with Nephrology and Dialysis Unit and Center of Immuno-Rheumatology and Rare Diseases (CMID), Coordinating Center of the Interregional Network for Rare Diseases of Piedmont and Aosta Valley, San Giovanni Bosco Hub Hospital, Turin, Italy.
Alice Barinotti, University Center of Excellence on Nephrologic, Rheumatologic and Rare Diseases (ERK-net, ERN-Reconnect and RITA-ERN Member) with Nephrology and Dialysis Unit and Center of Immuno-Rheumatology and Rare Diseases (CMID), Coordinating Center of the Interregional Network for Rare Diseases of Piedmont and Aosta Valley, San Giovanni Bosco Hub Hospital, Turin, Italy.
Simone Baldovino, University Center of Excellence on Nephrologic, Rheumatologic and Rare Diseases (ERK-net, ERN-Reconnect and RITA-ERN Member) with Nephrology and Dialysis Unit and Center of Immuno-Rheumatology and Rare Diseases (CMID), Coordinating Center of the Interregional Network for Rare Diseases of Piedmont and Aosta Valley, San Giovanni Bosco Hub Hospital, Turin, Italy.
Elisa Menegatti, University Center of Excellence on Nephrologic, Rheumatologic and Rare Diseases (ERK-net, ERN-Reconnect and RITA-ERN Member) with Nephrology and Dialysis Unit and Center of Immuno-Rheumatology and Rare Diseases (CMID), Coordinating Center of the Interregional Network for Rare Diseases of Piedmont and Aosta Valley, San Giovanni Bosco Hub Hospital, Turin, Italy.
Dario Roccatello, University Center of Excellence on Nephrologic, Rheumatologic and Rare Diseases (ERK-net, ERN-Reconnect and RITA-ERN Member) with Nephrology and Dialysis Unit and Center of Immuno-Rheumatology and Rare Diseases (CMID), Coordinating Center of the Interregional Network for Rare Diseases of Piedmont and Aosta Valley, San Giovanni Bosco Hub Hospital, Turin, Italy.
FUNDING
None declared.
AUTHORS’ CONTRIBUTIONS
Study Design: SS, DR Data collection and Data analysis: SS, MLAR, RF, SGF, MR, IC, GC, PC, MGG, AB, SB, EM, DR. All authors approved the final version.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared upon reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
This manuscript is not under consideration elsewhere. The authors declare no conflicts of interest, no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
REFERENCES
- 1. Smith JB, Gonzales EG, Li BHet al. Analysis of rituximab use, time between rituximab and SARS-CoV-2 vaccination, and COVID-19 hospitalization or death in patients with multiple sclerosis. JAMA Netw Open 2022;5:e2248664. 10.1001/jamanetworkopen.2022.48664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schultz K, Jannat-Khah D, Spiera R. B cell reconstitution is associated with COVID-19 booster vaccine responsiveness in patients previously seronegative treated with rituximab. J Rheumatol 2023;50:420–5. 10.3899/jrheum.220475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh N, Madhira V, Hu Cet al. Rituximab is associated with worse COVID-19 outcomes in patients with rheumatoid arthritis: a retrospective, nationally sampled cohort study from the U.S. National COVID Cohort Collaborative (N3C). Semin Arthritis Rheum. 2023;58:152149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magliulo D, Wade SD, Kyttaris VC. Immunogenicity of SARS-CoV-2 vaccination in rituximab-treated patients: effect of timing and immunologic parameters. Clin Immunol 2022;234:108897. 10.1016/j.clim.2021.108897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tolf A, Wiberg A, Muller Met al. Factors associated with serological response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with rituximab. JAMA Netw Open 2022;5:e2211497. 10.1001/jamanetworkopen.2022.11497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Avouac J, Drumez E, Hachulla Eet al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol 2021;3:e419–26. 10.1016/S2665-9913(21)00059-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis ofCOVID-19. JAMA 2022;327:384–5. 10.1001/jama.2021.24931 [DOI] [PubMed] [Google Scholar]
- 8. Loo Y-M, McTamney PM, Arends RHet al. The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans. Sci Transl Med 2022;14:eabl8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levin MJ, Ustianowski A, De Wit Set al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of COVID-19. N Engl J Med 2022;386:2188–200. 10.1056/NEJMoa2116620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agenzia Italiana del Farmaco . Inserimento del medicinale «Evusheld» (associazione di anticorpi monoclonali tixagevimab e cilgavimab) nell'elenco dei medicinali ai sensi della legge 23 dicembre 1996, n. 648. (Determina n. DG/344/2022). https://www.aifa.gov.it/documents/20142/961234/Determina_DG-344-2022_Evusheld.pdf [accessed 3 January 2023]. [Google Scholar]
- 11. Regione Piemonte . Covid-19: la mappa del Piemonte. https://www.regione.piemonte.it/web/covid-19-mappa-piemonte [accessed 3 January 2023]. [Google Scholar]
- 12. Xue C, Yang B, Xu Jet al. Efficacy and safety of rituximab in adult frequent-relapsing or steroid-dependent minimal change disease or focal segmental glomerulosclerosis: a systematic review and meta-analysis. Clin Kidney J 2021;14:1042–54. 10.1093/ckj/sfaa191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rojas-Rivera JE, Carriazo S, Ortiz A. Treatment of idiopathic membranous nephropathy in adults: KDIGO 2012, cyclophosphamide and cyclosporine a are out, rituximab is the new normal. Clin Kidney J 2019;12:629–38. 10.1093/ckj/sfz127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.