Abstract
The wild poses a multifaceted challenge to the maintenance of cellular function. Therefore, a multistressor approach is essential to predict the cellular mechanisms which promote homeostasis and underpin whole-organism tolerance. The intertidal zone is particularly dynamic, and thus, its inhabitants provide excellent models to assess mechanisms underpinning multistressor tolerance. Here, we critically review our current understanding of the regulation of the cellular stress response (CSR) under multiple abiotic stressors in intertidal organisms and consider to what extent a multistressor approach brings us closer to understanding responses in the wild. The function of the CSR has been well documented in laboratory and field exposures with a view to understanding single-stressor thermal effects. Multistressor studies still remain relatively limited in comparison but have applied three main approaches: (i) laboratory application of multiple stressors in isolation, (ii) multiple stressors applied in combination, and (iii) field-based correlation of multiple stressors against the CSR. The application of multiple stressors in isolation has allowed the identification of putative, shared stress pathways but overlooks non-additive stressor interactions on the CSR. Combined stressor studies are relatively limited in number but already highlight variable effects on the CSR dependent upon stressor type, timing, and magnitude. Field studies have allowed the identification of responsive components of the CSR to various stressors in situ but are correlative, not causative. A combined approach involving laboratory multistressor studies linking the CSR to whole-organism tolerance as well as field studies is required if we are to understand the role of the CSR in the natural environment.
Keywords: Multistressor, Cellular stress response, Heat shock proteins, Cross-tolerance, Ecophysiology, Integrative
Introduction
In her seminal evaluation of the heat shock response, published in 1986, Susan Lindquist clearly laid the foundations for the environmental stress response work carried out today (Lindquist, 1986). The induction of a small number of highly conserved heat shock proteins in response to a range of different stressors that denature the cellular protein pool was already well known. At the time, the importance of these proteins in the CSR was underlined by the high level of conservation of heat shock protein sequences between diverse species and the ubiquity of the response, even though most studies focussed on model organisms (e.g. Escherichia coli, Saccharomyces cerevisiae, Drosophila melanogaster and vertebrate cell lines) (Lindquist, 1986). It would be more than ten years before the much wider role of heat shock proteins in non-model organisms responding to stress in the natural environment would be promoted. In their highly influential review, Feder and Hofmann (1999) acknowledged the utility of heat shock proteins as environmental biomarkers (particularly for toxicology) and also pointed out that in the aquatic environment, habitual exposure to heat shock protein-inducing thermal stress was probably most commonly found in the intertidal zone. Indeed, in the late 1990s and early 2000s, research on intertidal species provided critical information on the natural variability of the environmental heat shock response (HSR).
Much of this work concentrated on evaluating HSP70 (heat shock protein 70kDa) genes and proteins. This was largely because, in the early days of molecular biology, the high sequence conservation of HSP70 genes between species facilitated the then complicated and lengthy process of candidate gene cloning (e.g. Yokoyama et al., 2006) and/or the identification of protein bands in acrylamide gels, produced via the metabolic incorporation of 35S-labelled methionine (e.g. Roberts et al., 1997). This sequence conservation also enabled the use of heterologous hybridization of HSP70 antibodies raised in very distantly related model species (e.g. anti-HSP70 human monoclonal antibody applied to Mytilus edulis) (Smerdon et al., 1995). Studies concentrated on well-known intertidal species such as the bivalves Mytilus sp., Crassostrea gigas and the marine gastropods (Lottia sp. and Tegula sp.) (Dong et al., 2008; Hofmann and Somero, 1996; Tomanek and Somero, 1999; Yokoyama et al., 2006). Several of these species occur across wide latitudinal gradients and at different tidal heights on the foreshore, providing natural model systems for dissecting the HSR associated with the complex environmental stressors experienced in the intertidal (Tomanek, 2002). At the time, work on model species was showing that heat shock proteins operated not only as molecular chaperones but also had multiple functions and engaged in complex cellular interactions (Lindquist, 1986). Similarly, it was becoming clear in environmental studies that these highly conserved proteins were pivotal cellular thermostats (Feder and Hofmann, 1999). Nonetheless, wider investigations were limited by the sequence technologies at the time and were largely restricted to temperature effects on a limited number of candidate HSPs (heat shock proteins).
Since then, it could be argued that the HSR to multifactorial stressors has been well studied in wild intertidal animals, albeit in certain taxa. For example, intertidal molluscs have often been investigated using a combined laboratory and field approach (Dong et al., 2022). We would argue that the field components in these studies have not been aimed at understanding the HSR in dealing with multiple stressors per se, but have typically been temperature-orientated, i.e. looking for differences in the HSR in field organisms with different evolutionary thermal histories (e.g. along vertical and latitudinal gradients) (Dong et al., 2022). Patterns between the laboratory and the wild may match in some cases (Dong et al., 2022), but even the earliest evaluations of the HSP70 response identified significant differences between field-collected and lab-acclimated individuals (Clark and Peck, 2009). Although temperature is a major factor in initiating the HSR, the use of a few candidate HSPs could not provide detail on the contribution of secondary factors, such as nutrient availability, salinity and predator status (Halpin et al., 2002; Sagarin and Somero, 2006). Certainly, the majority of laboratory studies only subjected intertidal animals to a single stressor, and that was usually temperature (Feder and Hofmann, 1999). However, the intertidal is not a single-stressor environment, and these varied stressors have many different effects on physiology (Fig. 1). Recent advances in sequencing techniques and associated bioinformatics have provided opportunities to investigate cellular adaptations to the intertidal more widely in a discovery-led approach (e.g. Clark et al., 2017), which when linked with physiological and ecological studies, are revealing the true complexity of the native intertidal CSR.
Fig. 1.
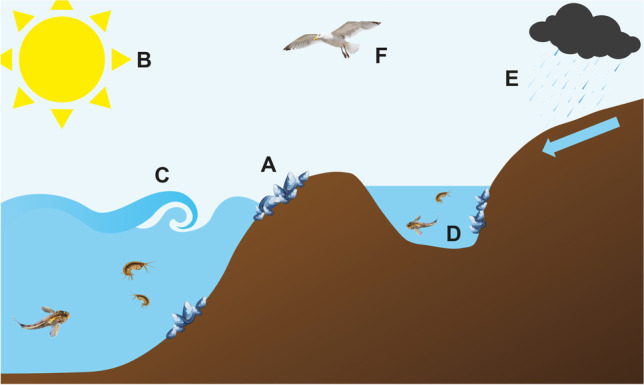
Schematic of the most common stressors in the intertidal region: (A) periodic emersion with potential for desiccation, high salt from sea spray, temperature stress from high temperatures in summer to freezing in winter; (B) heat and UV radiation; (C) wave action; (D) isolated tide pools can be affected by hypoxia, altered salinity and pH (both high and low), temperature fluctuations with the potential to freeze over in winter; (E) rainfall will provide fresh water run-off over the intertidal region and dilute salinity in tide pools. There may also be pollution run-off from the land including agriculture (high nutrients and nitrates/nitrites from fertilisers), pollution from factories, etc.; (F) predation. NB: food supply is much more limited in the intertidal region compared with the subtidal
Box 1. Temperature effects on the HSR – lessons from the intertidal (see main text for references)
|
• The stress response, including the expression of HSPs, may play a role in setting species’ biogeographical boundaries. This is both in terms of their microhabitat (e.g. position on the shore) and their latitudinal distribution. • HSP expression is highly plastic and varies with thermal history. This is exemplified by two phenomena that apply over different times scales: (1) evolutionary variation in the heat shock response exists so that organisms adapted to warmer environments have higher induction temperatures than those in cold environments, and (2) threshold induction temperatures are not fixed, but can be modulated as a result of thermal acclimation, over relatively short timescales (e.g. seasonally). • The HSR, characterised by inducible HSP expression, varies with environmental stability. Organisms in highly variable thermal environments induce HSP expression regularly as a cellular protection mechanism, whereas those in stable thermal environments do not often resort to inducible HSP expression. In the former, this results in species with a larger window of thermal tolerance, at least in the short term. • Thermal tolerance can increase upon both chronic exposure to thermal stress and repeated, short-term exposure to non-lethal extreme heat. Exposure to elevated temperatures can lead to elevated constitutive HSP levels. This ‘constitutive frontloading’ confers increased cellular stress resistance, acting as a preparative defence strategy in thermally challenging environments. |
Hence, it is timely for a critical analysis of the responses of the CSR to multiple abiotic stressors with a view to understanding its role in multistressor tolerance in the wild. We use organisms inhabiting the intertidal zone (rocky shores, estuaries, shallow coastal polar and tropical organisms) as models. We first review what is known about the HSR in terms of a focus on a single stressor - environmental temperature. We then focus upon multistressor approaches conducted in the laboratory and field. We conclude by discussing to what extent a multistressor approach brings us closer to understanding the role of the CSR in the wild.
Patterns of HSP expression with a view to understanding responses to a single stressor: temperature
The intertidal environment represents an extreme environment where harsh conditions prevail due to exposure to tides (Leeuwis and Gamperl, 2022). It is characterised by spatial and temporal thermal heterogeneity, whereby temperature fluctuates markedly within hours and over short distances. As a result, intertidal ectotherms experience rapid changes in body temperature over short temporal and spatial scales and therefore are excellent models to understand the mechanisms underpinning temperature-sensitive physiological processes, both acute and chronic (Tomanek, 2010). Early experiments using the intertidal as a model system demonstrated the wide plasticity of the HSR and its correlation with thermal tolerance and thermal history, thus contributing to our understanding of how variation in the HSR contributes to biogeographic patterns (Dong et al., 2022). Intertidal studies have revealed that, firstly, species living at the highest levels on the shore (and therefore with the longest times for aerial exposure) have higher induction temperatures for the HSR than those much closer to the water (Roberts et al., 1997; Tomanek and Somero, 1999; Tomanek and Somero, 2000). Here, the low shore populations, which do not experience thermal extremes as regularly as those on the high shore, need to resort to protective cellular mechanisms at lower environmental temperatures to ensure the maintenance of homeostasis. A similar phenomenon to that observed vertically in the intertidal is also apparent over broad biogeographic scales, whereby populations on the cold edge of a species’ distributional range, and therefore not regularly experiencing thermal extremes, tend to have lower HSP70 induction temperatures compared to those in the warmer edge (Hofmann and Somero, 1996). For example, the more northern occurring M. trossulus was found to be less thermally tolerant and have lower induction temperatures for HSP70 compared with its more southern relative, M. galloprovincialis (Hofmann and Somero, 1996). Secondly, thermal acclimation can modulate the HSR with changes in HSP70 induction temperatures, as well as maximum expression levels. However, there are limits to this plasticity, as the temperature at which HSP synthesis (and protein synthesis more generally) ceases and often remains unchanged following acclimation (Tomanek and Somero, 1999). In the field, seasonal variation in the production of HSP70 occurs in most species with more HSP70 produced constitutively in the summer, as water and air temperatures naturally increase (Buckley et al., 2001; Roberts et al., 1997). This can result in higher thresholds of induction of HSP70 (potentially by 6 °C or more, depending on the habitat) in summer-acclimated animals (Buckley et al., 2001; Hamdoun et al., 2003; Hamer et al., 2004).
Early experiments also catalogued the varying time course and magnitude of the HSR in different species, providing tantalising glimpses into the complexity of the response (Hofmann and Somero, 1996; Tomanek and Somero, 1999). Certainly, there was not the expected correlation with latitudinal gradient, as the response to local site-specific factors and the effect of microhabitats consistently overrode a more global temperature gradient (Halpin et al., 2002; Sagarin and Somero, 2006). Furthermore, it was becoming clear that such local conditions could influence the expression of HSP70 genes or proteins over longer timescales rather than just for the duration of a tidal cycle. Sessile species such as Mytilus and limpets showed temporal regulation of genes in response to intertidal heat stress (Clark et al., 2008; Dong et al., 2008; Gracey et al., 2008; Lesser et al., 2010). This observation led to the suggestion that elevated levels of HSP70 were always produced in these populations, compared with subtidal populations and that these higher levels of HSP70 were protective, acting as a “preparative defence strategy” against sudden stress (Dong et al., 2008). This phenomenon was also called “constitutive frontloading” in some later studies (Barshis et al., 2013) and appears to be restricted to sessile species, as no endogenous rhythms of HSP70 expression were detected in the tidal sculpin Oligocottus maculosus (Todgham et al., 2006). Expression of HSPs is expensive at the cellular level, and energetic trade-offs could be detected in some intertidal studies. Elevated expenditure could also result in lower growth rates in high intertidal species (Tomanek and Sanford, 2003), and their expression could vary with food availability (Lesser et al., 2010).
Role of the CSR under multiple stressors
The tidal cycle results not only in marked spatial and temporal heterogeneity in temperature but also in other abiotic variables, meaning coastal organisms have to cope with both synchronous and asynchronous shifts in multiple abiotic factors with consequences from genes to the whole organism (Leeuwis and Gamperl, 2022). Stressor effects on physiological function can be (i) additive – where the combined stressor effect equals the sum of individual stressor effects, (ii) synergistic – where the combined stressor effect is greater than the sum of individual effects or (iii) antagonistic – where the combined stressor effect is less than the sum of individual effects (Todgham and Stillman, 2013). Studies have tended to apply stressor combinations simultaneously and acutely, resulting in an overemphasis of detrimental synergistic effects in the scientific literature (Côté et al., 2016). However, the temporal dynamics of stressors have recently begun to receive greater attention, i.e. the effects of asynchronous stressors experienced in tidal habitats, such as daytime high temperatures followed by night-time hypoxia (Collins et al., 2021a; Gunderson et al., 2016). More recent studies have considered whether pre-exposure to one stressor can either (i) reduce the ability of an organism to tolerate a sequential, second stressor, termed ‘cross-susceptibility’ (a type of synergistic effect) or (ii) improve the ability to tolerate another, termed ‘cross-tolerance’ (a type of antagonistic effect) (Gunderson et al., 2016; Rodgers and Gomez Isaza, 2021). It should be noted that cross-tolerance has multiple definitions within the scientific literature. If taking a specifically cellular view, ‘cross-tolerance’ is defined as the phenomenon by which exposure to one stressor improves the ability to tolerate another as a result of shared protective mechanisms between stressors (Bueno et al., 2023; Sinclair et al., 2013). In this review, we explore three main types of multistressor investigation of the CSR in intertidal animals: (i) multiple stressors applied in isolation in the laboratory, (ii) multiple stressors applied in combination in the laboratory and (iii) cellular stress responses measured directly in the natural multifactorial environment. Recent studies have noted that the effect of multiple stressors on CSR still remains poorly studied compared to single stressors (Barrett et al., 2022; Dong et al., 2022), so instead, we draw upon selected multistressor case studies to illustrate the advantages and disadvantages of each type of approach.
Multiple stressors in the laboratory applied in isolation
In terms of the CSR, studies have aimed to expose intertidal animals to a range of stressors to identify common pathways of cellular response. Intertidal bivalves have often been the subject of this approach (Gracey et al., 2008; Lockwood et al., 2015; Zhang et al., 2012). Microarray analysis of mussels exposed to temperature and salinity stress separately revealed a shared CSR of 45 genes, which were predominantly related to ion transport, but showed differential regulation in response to these stressors (Lockwood and Somero, 2011; Lockwood et al., 2010). Such differential regulation may reflect different challenges posed by temperature and salinity to membrane permeability (Lockwood et al., 2015). One gene associated with the oxidative stress response was similarly affected by both temperature and salinity, suggesting the importance of maintaining redox balance during cellular stress (Lockwood et al., 2015). Another example comes from oysters, where transcriptome sequencing of organisms subjected to a wide variety of stressors was analysed alongside the genome sequence (Zhang et al., 2012). Transcriptome data showed a shared cellular stress response of 123 genes across four stressors (temperature, salinity, metal and air exposure), but the identity of those genes was not discussed. This approach is a valuable first step in determining the potential mechanisms used, but it cannot necessarily determine what mechanisms are actually realised when stressors are applied in combination as it neglects the potential for unpredictable, non-additive effects on components of the CSR. Thus, there is a continued need to investigate stressors in combination.
Multiple stressors in the laboratory applied in combination
Fully factorial designs involving two (or more) stressors allow the identification of the effects of stressors in both isolation and combination (Todgham and Stillman, 2013). In M. edulis, the CSR was investigated via transcriptomics in response to different temperatures and salinities using a factorial design (Barrett et al., 2022). At a control salinity, no functional enrichment of groups was observed in response to temperature stress, but at low salinity, temperature induced a more wide-ranging response including elements related to the CSR such as the unfolded protein response (UPR) and cell morphogenesis. This was associated with greater upregulation of CSR genes (HSPs, antioxidants and MAP kinases) and increased mortality at low compared to high salinity. Therefore, with the addition of just one simultaneous stressor, the cellular response of an organism to its environment is markedly different. Similarly, there is evidence that sequential stressor exposures may also modify the CSR, although evidence documenting the role of HSPs in cross-tolerance for intertidal organisms remains scarce using either targeted (Todgham et al., 2005) or global approaches (Collins et al., 2021b; Collins et al., 2022). In our opinion, the study of Todgham et al. (2005) on the intertidal sculpin is the most thorough assessment of HSP involvement in promoting tolerance of the multistressor intertidal. The study investigated how pre-exposure to acute heat shock affected mortality rates upon subsequent exposure to osmotic or hypoxic challenge. It was hypothesised that temperature would induce HSP70 expression which would then confer protection to subsequent stress. The priming of HSPs by one stressor is conceptually similar to the aforementioned frontloading/preparative defence strategy, which would then reduce future stress (albeit in response to a stressor of a different a nature). Cross-tolerance was observed at the physiological level but was dependent upon the magnitude of the initial heat shock and the recovery period between stressors, requiring 8–48 h to develop. Interestingly, the key finding that emerged was that HSP70 induction/frontloading by the priming stressor was not clearly related to tolerance of a subsequent stressor. Tolerance was only clearly related to the ability to acutely raise HSP70 levels in response to the second stressor. Thus, the first stressor may prime some other mechanism within the cell that then increases the ability to mount a HSP response to a second stressor. This finding for HSP70 was interestingly mirrored by a recent transcriptomic study investigating cross-tolerance. Collins et al. (2021b) explored whether thermal plasticity improves subsequent hypoxic performance in an intertidal amphipod. Individuals acclimated to increased temperature out-performed cold-acclimated individuals under hypoxia, which was associated with frontloading as the predominant transcriptome-wide mechanism. Interestingly, frontloaded genes included those involved in immune and cytoskeletal responses, but not specifically HSPs. HSP gene expression displayed a greater response under hypoxia in warm acclimated individuals, suggesting that, similar to Todgham et al. (2005), cross-tolerant phenotypes may be associated with the acute response of HSP to the second stressor rather than their priming from a prior stressor. That said, Collins et al.’s (2021b) experiment was conducted at the gene expression level which can be particularly dynamic (Dong et al., 2022). There may be epigenetic regulation (Clark et al., 2018) and/or post-translational modifications (Elowe and Tomanek, 2021), so future validation at the protein level is key. Overall, our knowledge of molecular mechanisms underpinning two-stressor interactions is limited and, from the studies here, reveals marked variation already between taxa (Fig. 2).
Fig. 2.
Conceptual mechanisms of multistressor effects on CSR depicting a two by two stressor design: (A) simultaneous exposure to two stressors – acutely both stressors, S1 and S2, in isolation induce the CSR. In combination, S1+S2 cause increased stress and synergistic effects associated with reduced organism tolerance, e.g. Barrett et al. (2022) in mussels; (B) sequential exposure to two stressors acutely separated by a recovery period – S1 is applied first and does not trigger the CSR compared to control but may prime other mechanisms that allow for a greater CSR upon exposure to subsequent stress (S1+S2 exposed) than organisms exposed to just S2. This results in cross-tolerance, e.g. Todgham et al. (2005) in tidepool sculpins; (C) sequential exposure to two stressors involving chronic exposure to S1, followed by acute S2 exposure – chronic exposure to S1 allows for frontloading of cellular defences which reduces the need for an acute CSR upon exposure to another acute subsequent stressor (S1+S2) than organisms exposed to just S2. This also may result in cross-tolerance, e.g. Collins et al. (2021b) in amphipod crustaceans. Thus, across phyla, there is wide diversity in multistressor responses in terms of CSR as cross-tolerance may be elicited with/without frontloading of defences following S1 or from increased/reduced CSR response to S2. Increased reaction to S2 could reflect either synergism or antagonism dependent on species, stressor timing and duration
In the discussion above, we referred to two stressor studies applied sequentially, but responses can be modified further by prior dual acclimation to two stressors before determining responses to a subsequent stressor. Dual acclimation for 7 days to temperature-pH combinations (temperature = 20 or 24 °C, pH = 8.1 or 7.8) results in markedly different HSP70 responses to subsequent acute thermal stress in intertidal limpets (Wang et al., 2018). Organisms dual acclimated to high temperature and low pH demonstrated the greatest increase in HSP70 gene expression when exposed to an acute warming event, compared to those acclimated to low temperature, high temperature or low pH in isolation. Overall, studies of stressors in combination are essential to understand the intricacies of the CSR, but given the variation observed with such a small number of stressors, extrapolation to the wild will continue to be challenging.
Disentangling the CSR in a multistressor context directly in the field
Gene-network analyses can provide information about whole genomes and transcriptomes from intertidal animals sampled in situ, i.e. directly in the natural multistressor environment. In an impressive study, particularly at the time, Gracey et al. (2008) performed microarray analysis at a high temporal resolution on field-sampled Mytilus exposed to a tidal cycle. The study revealed distinct phases of temporal gene expression over the tidal cycle corresponding with metabolism, cell division and two phases of heat stress associated with HSPs (Gracey et al., 2008). Comparable studies using RNA-Seq are few in number, likely due to sequencing costs, although costs are decreasing all the time. Using RNA-Seq, Ruiz-Jones and Palumbi (2017) provided an elegant study correlating transcriptome profiles of tabletop coral Acropora hyacinthus with multiple stressors experienced in the natural environment over 17 days. They identified groups of genes whose expression was associated with various stressors such as temperature, pH and dissolved oxygen and previous day-night variability in these factors. These environmentally responsive genes were involved in endoplasmic reticulum stress, the UPR (e.g. HSP68, HSP70 and HSP90) and calcium ion binding. Thus, a network approach may allow a formal statistical assessment of the nature of CSR in response to multiple abiotic stressors. The other benefit of network-type analyses is that they facilitate an integrative multilevel approach from genes to the whole organism. Groups of co-regulated genes can be identified, and their expression correlated with whole animal physiological traits. For example, coral reefs inhabiting back-reef pools can experience high thermal variability which may result in bleaching (Barshis et al., 2013). At the molecular level, network analyses revealed bleaching under heat stress may be associated with the expression of genes whose function involves the extracellular matrix and DNA-binding proteins (Rose et al., 2016). Thus, network analyses may provide a clearer understanding of the mechanisms coordinating physiological responses to environmental change directly in the intertidal zone. However, it could be argued that the weakness of such a field-based approach is that it is correlative, and causative relationships cannot be drawn. A combined laboratory and field approach may alleviate this difficulty. For example, Gracey et al. (2008) measured gene expression in situ but also compared it against gene expression profiles of organisms exposed to a range of abiotic stressors in the laboratory. This hybrid approach may help to better understand the environmental drivers of change observed in the wild.
Insights into the CSR from genome analyses
Whilst transcriptomic analyses provide a much more comprehensive overview of the CSR compared to that of candidate genes, they are limited to those genes expressed under particular conditions. This can lead to a lack of appreciation of the underlying genomic ability of the organism to respond to stress. The best example of this to date is that of the oyster genome, which revealed intriguing insights into life in the multifactorial intertidal. The oyster genome contains an over-representation of genes that respond to environmental stress, with enrichment for genes encoding HSP70 and inhibitors of apoptosis (Zhang et al., 2012; Zhang et al., 2016). Whilst the expansion of HSP gene family members had previously been documented in model species and correlated with thermal tolerance (Bettencourt et al., 2008), this was the first time that such an expansion was demonstrated in a non-model species. The evolution of the HSP70 gene expansion in the oyster is rather unusual in that the expansion has occurred in the atypical HSPA12 family members, which in other species are not associated with the stress response (e.g. Han et al., 2003; Hu et al., 2006). This gene family expansion is bivalve-specific with further species-specific tandem duplications (Cheng et al., 2016), resulting in circa 73 copies in C. gigas, 97 in the pearl oyster (Pinctada fucata), 55 in the invasive golden mussel (Limnoperna fortunei), 57 in the scallop (Patinopecten yessoensis) and at least 43 in the blue mussel (M. edulis) (Barrett et al., 2022; Cheng et al., 2016; Clark et al., 2021; Takeuchi et al., 2016; Uliano-Silva et al., 2014; Zhang et al., 2012). The expansion of the HSPA12 family in bivalves raises the question of how other intertidal classes and families have modified their genomes and adapted their stress signalling pathways. It is suggested that the selection and maintenance of this particular gene family was driven by adaptation to a sessile lifestyle in the dynamically changing marine environment with its complex biotic and abiotic stresses (Cheng et al., 2016). Furthermore, the association of specific sub-sets of these HSPA12 genes with different stressors has led to the suggestion that they act as complex intertidal stress regulators (Clark et al., 2021). This clearly needs to be investigated further using gene network-type analyses (Ramsøe et al., 2020). Overall, these studies show that intertidal organisms may have the potential for more robust multistressor responses via genomic expansion of HSP families or shared mechanisms elicited by a suite of abiotic stressors. There is also evidence for divergent mechanisms in terms of HSPs such as stressor-specific HSPA12 genes (Clark et al., 2021). Thus, there is a need to use cutting-edge molecular tools (including whole genome sequencing) alongside physiology experiments and ecological observations/sampling to understand the multistressor CSR.
Conclusions and future perspectives
Understanding CSR in the wild will continue to be challenging, but multistressor approaches are essential, given the simple observation that even with just two stressors, one stressor can alter HSP expression upon exposure to another. This similarly creates the problem of how we extrapolate laboratory exposures to the natural environment where an estimated fifteen stressors can operate in intertidal systems (Côté et al., 2016). The addition of stressors in the laboratory is logistically challenging in terms of sample size. Field approaches avoid this pitfall, but it is difficult to draw causation (Spicer, 2014) and to assess how one stressor has altered the expression in response to another. We argue that a combined laboratory-field approach provides the best ability to disentangle complex patterns of CSR and their role in understanding organism tolerance in dynamic multistressor environments.
Acknowledgements
The authors would like to thank Jamie Oliver (BAS) for producing Fig. 1 and some of the graphics used in Fig. 2.
Funding
MC and MT are funded by the School of Biological and Marine Sciences, University of Plymouth. MSC is funded by NERC core funding to British Antarctic Survey.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Barrett NJ, Thyrring J, Harper EM, Sejr MK, Sørensen JG, Peck LS, Clark MS (2022) Molecular responses to thermal and osmotic stress in arctic intertidal mussels (Mytilus edulis): the limits of resilience. Genes 13:155 [DOI] [PMC free article] [PubMed]
- Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Traylor-Knowles N, Palumbi SR. Genomic basis for coral resilience to climate change. Proc Natl Acad Sci. 2013;110:1387–1392. doi: 10.1073/pnas.1210224110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt BR, Hogan CC, Nimali M, Drohan BW. Inducible and constitutive heat shock gene expression responds to modification of Hsp70 copy number in Drosophila melanogaster but does not compensate for loss of thermotolerance in Hsp70 null flies. BMC Biol. 2008;6:5. doi: 10.1186/1741-7007-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley BA, Owen M, Hofmann GE. Adjusting the thermostat: the threshold induction temperature for the heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J Exp Biol. 2001;204:3571–3579. doi: 10.1242/jeb.204.20.3571. [DOI] [PubMed] [Google Scholar]
- Bueno EM, McIlhenny CL, Chen YH. Cross-protection interactions in insect pests: implications for pest management in a changing climate. Pest Manag Sci. 2023;79:9–20. doi: 10.1002/ps.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Xun X, Kong Y, Wang S, Yang Z, Li Y, Kong D, Wang S, Zhang L, Hu X, et al. Hsp70 gene expansions in the scallop Patinopecten yessoensis and their expression regulation after exposure to the toxic dinoflagellate Alexandrium catenella. Fish Shellfish Immunol. 2016;58:266–273. doi: 10.1016/j.fsi.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Clark MS, Peck LS. Triggers of the HSP70 stress response: environmental responses and laboratory manipulation in an Antarctic marine invertebrate (Nacella concinna) Cell Stress Chap. 2009;14:649–660. doi: 10.1007/s12192-009-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Geissler P, Waller C, Fraser KPP, Barnes DKA, Peck LS (2008) Low heat shock thresholds in wild Antarctic inter-tidal limpets (Nacella concinna). Cell Stress Chap 13:51–58 [DOI] [PMC free article] [PubMed]
- Clark MS, Sommer U, Sihra JK, Thorne MAS, Morley SA, King M, Viant MR, Peck LS. Biodiversity in marine invertebrate responses to acute warming revealed by a comparative multi-omics approach. Glob Chang Biol. 2017;23:318–330. doi: 10.1111/gcb.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Thorne MAS, King M, Hipperson H, Hoffman JI, Peck LS. Life in the intertidal: cellular responses, methylation and epigenetics. Funct Ecol. 2018;32:1982–1994. doi: 10.1111/1365-2435.13077. [DOI] [Google Scholar]
- Clark MS, Peck LS, Thyrring J. Resilience in Greenland intertidal Mytilus: the hidden stress defense. Sci Total Environ. 2021;767:144366. doi: 10.1016/j.scitotenv.2020.144366. [DOI] [PubMed] [Google Scholar]
- Collins M, Truebano M, Verberk WCEP, Spicer JI (2021a) Do aquatic ectotherms perform better under hypoxia after warm acclimation? J Exp Biol 224:jeb232512 [DOI] [PubMed]
- Collins M, Clark MS, Spicer JI, Truebano M. Transcriptional frontloading contributes to cross-tolerance between stressors. Evol Appl. 2021;14:577–587. doi: 10.1111/eva.13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M, Truebano M, Spicer JI. Consequences of thermal plasticity for hypoxic performance in coastal amphipods. Mar Environ Res. 2022;177:105624. doi: 10.1016/j.marenvres.2022.105624. [DOI] [PubMed] [Google Scholar]
- Côté IM, Darling ES, Brown CJ (2016) Interactions among ecosystem stressors and their importance in conservation. Proc R Soc B Biol Sci 283:20152592 [DOI] [PMC free article] [PubMed]
- Dong Y, Miller LP, Sanders JG, Somero GN. Heat-shock protein 70 (Hsp70) expression in four limpets of the genus Lottia: interspecific variation in constitutive and inducible synthesis correlates with in situ exposure to heat stress. Biol Bull. 2008;215:173–181. doi: 10.2307/25470698. [DOI] [PubMed] [Google Scholar]
- Dong Y, Liao M, Han G, Somero GN. An integrated, multi-level analysis of thermal effects on intertidal molluscs for understanding species distribution patterns. Biol Rev. 2022;97:554–581. doi: 10.1111/brv.12811. [DOI] [PubMed] [Google Scholar]
- Elowe C, Tomanek L. Circadian and circatidal rhythms of protein abundance in the California mussel (Mytilus californianus) Mol Ecol. 2021;30:5151–5163. doi: 10.1111/mec.16122. [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Gracey AY, Chaney ML, Boomhower JP, Tyburczy WR, Connor K, Somero GN. Rhythms of gene expression in a fluctuating intertidal environment. Curr Biol. 2008;18:1501–1507. doi: 10.1016/j.cub.2008.08.049. [DOI] [PubMed] [Google Scholar]
- Gunderson AR, Armstrong EJ, Stillman JH. Multiple stressors in a changing world: the need for an improved perspective on physiological responses to the dynamic marine environment. Ann Rev Mar Sci. 2016;8:357–378. doi: 10.1146/annurev-marine-122414-033953. [DOI] [PubMed] [Google Scholar]
- Halpin PM, Sorte CJ, Hofmann GE, Menge BA. Patterns of variation in levels of Hsp70 in natural rocky shore populations from microscales to mesoscales. Integr Comp Biol. 2002;42:815–824. doi: 10.1093/icb/42.4.815. [DOI] [PubMed] [Google Scholar]
- Hamdoun AM, Cheney DP, Cherr GN. Phenotypic plasticity of HSP70 and HSP70 gene expression in the Pacific oyster (Crassostrea gigas): implications for thermal limits and induction of thermal tolerance. Biol Bull. 2003;205:160–169. doi: 10.2307/1543236. [DOI] [PubMed] [Google Scholar]
- Hamer B, Hamer DP, Müller WEG, Batel R. Stress-70 proteins in marine mussel Mytilus galloprovincialis as biomarkers of environmental pollution: a field study. Environ Int. 2004;30:873–882. doi: 10.1016/j.envint.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Han Z, Truong QA, Park S, Breslow JL. Two Hsp70 family members expressed in atherosclerotic lesions. Proc Natl Acad Sci USA. 2003;100:1256–1261. doi: 10.1073/pnas.252764399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann GE, Somero GN. Interspecific variation in thermal denaturation of proteins in the congeneric mussels Mytilus trossulus and M. galloprovincialis: evidence from the heat-shock response and protein ubiquitination. Mar Biol. 1996;126:65–75. doi: 10.1007/BF00571378. [DOI] [Google Scholar]
- Hu G, Tang J, Zhang B, Lin Y, Hanai JI, Galloway J, Bedell V, Bahary N, Han Z, Ramchandran R, et al. A novel endothelial-specific heat shock protein HspA12B is required in both zebrafish development and endothelial functions in vitro. J Cell Sci. 2006;119:4117–4126. doi: 10.1242/jcs.03179. [DOI] [PubMed] [Google Scholar]
- Leeuwis RHJ, Gamperl AK. Adaptations and plastic phenotypic responses of marine animals to the environmental challenges of the high intertidal zone. Oceanogr Mar Biol An Annu Rev. 2022;60:625–680. [Google Scholar]
- Lesser MP, Bailey MA, Merselis DG, Morrison JR. Physiological response of the blue mussel Mytilus edulis to differences in food and temperature in the Gulf of Maine. Comp Biochem Physiol - A Mol Integr Physiol. 2010;156:541–551. doi: 10.1016/j.cbpa.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The Heat-Shock Response. Ann Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lockwood BL, Somero GN. Transcriptomic responses to salinity stress in invasive and native blue mussels (genus Mytilus) Mol Ecol. 2011;20:517–529. doi: 10.1111/j.1365-294X.2010.04973.x. [DOI] [PubMed] [Google Scholar]
- Lockwood BL, Sanders JG, Somero GN. Transcriptomic responses to heat stress in invasive and native blue mussels (genus Mytilus): molecular correlates of invasive success. J Exp Biol. 2010;213:3548–3558. doi: 10.1242/jeb.046094. [DOI] [PubMed] [Google Scholar]
- Lockwood BL, Connor KM, Gracey AY. The environmentally tuned transcriptomes of Mytilus mussels. J Exp Biol. 2015;218:1822–1833. doi: 10.1242/jeb.118190. [DOI] [PubMed] [Google Scholar]
- Ramsøe A, Clark MS, Sleight VA. Gene network analyses support subfunctionalization hypothesis for duplicated hsp70 genes in the Antarctic clam. Cell Stress Chap. 2020;25:1111–1116. doi: 10.1007/s12192-020-01118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DA, Hofmann GE, Somero GN. Heat-shock protein expression in Mytilus californianus: acclimatization (seasonal and tidal-height comparisons) and acclimation effects. Biol Bull. 1997;192:309–320. doi: 10.2307/1542724. [DOI] [PubMed] [Google Scholar]
- Rodgers EM, Gomez Isaza DF. Harnessing the potential of cross-protection stressor interactions for conservation: a review. Conserv Physiol. 2021;9:coab037. doi: 10.1093/conphys/coab037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose NH, Seneca FO, Palumbi SR. Gene networks in the wild: identifying transcriptional. Genome Biol Evol. 2016;8:243–252. doi: 10.1093/gbe/evv258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Jones LJ, Palumbi SR. Tidal heat pulses on a reef trigger a fine-tuned transcriptional response in corals to maintain homeostasis. Sci Adv. 2017;3:e1601298. doi: 10.1126/sciadv.1601298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagarin RD, Somero GN. Complex patterns of expression of heat-shock protein 70 across the southern biogeographical ranges of the intertidal mussel Mytilus californianus and snail Nucella ostrina. J Biogeogr. 2006;33:622–630. doi: 10.1111/j.1365-2699.2005.01403.x. [DOI] [Google Scholar]
- Sinclair BJ, Ferguson LV, Salehipour-Shirazi G, Macmillan HA. Cross-tolerance and cross-talk in the cold: relating low temperatures to desiccation and immune stress in insects. Integr Comp Biol. 2013;53:545–556. doi: 10.1093/icb/ict004. [DOI] [PubMed] [Google Scholar]
- Smerdon GR, Chapple JP, Hawkins AJS. The simultaneous immunological detection of four stress-70 protein isoforms in Mytilus edulis. Mar Environ Res. 1995;40:399–407. doi: 10.1016/0141-1136(95)92645-K. [DOI] [Google Scholar]
- Spicer JI. What can an ecophysiological approach tell us about the physiological responses of marine invertebrates to hypoxia? J Exp Biol. 2014;217:46–56. doi: 10.1242/jeb.090365. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Koyanagi R, Gyoja F, Kanda M, Hisata K, Fujie M, Goto H, Yamasaki S, Nagai K, Morino Y, et al. Bivalve-specific gene expansion in the pearl oyster genome: implications of adaptation to a sessile lifestyle. Zool Lett. 2016;2:3. doi: 10.1186/s40851-016-0039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todgham AE, Stillman JH. Physiological responses to shifts in multiple environmental stressors: relevance in a changing world. Integr Comp Biol. 2013;53:539–544. doi: 10.1093/icb/ict086. [DOI] [PubMed] [Google Scholar]
- Todgham AE, Schulte PM, Iwama GK. Cross-tolerance in the tidepool sculpin: the role of heat shock proteins. Physiol Biochem Zool. 2005;78:133–144. doi: 10.1086/425205. [DOI] [PubMed] [Google Scholar]
- Todgham AE, Iwama GK, Schulte PM. Effects of the natural tidal cycle and artificial temperature cycling on Hsp levels in the tidepool sculpin Oligocottus maculosus. Physiol Biochem Zool. 2006;79:1033–1045. doi: 10.1086/507664. [DOI] [PubMed] [Google Scholar]
- Tomanek L. The heat-shock response: its variation, regulation and ecological importance in intertidal gastropods (genus Tegula) Integr Comp Biol. 2002;42:797–807. doi: 10.1093/icb/42.4.797. [DOI] [PubMed] [Google Scholar]
- Tomanek L. Variation in the heat shock response and its implication for predicting the effect of global climate change on species’ biogeographical distribution ranges and metabolic costs. J Exp Biol. 2010;213:971–979. doi: 10.1242/jeb.038034. [DOI] [PubMed] [Google Scholar]
- Tomanek L, Sanford E. Heat-shock protein 70 (Hsp70) as a biochemical stress indicator: an experimental field test in two congeneric intertidal gastropods (genus: Tegula) Biol Bull. 2003;205:276–284. doi: 10.2307/1543291. [DOI] [PubMed] [Google Scholar]
- Tomanek L, Somero GN. Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J Exp Biol. 1999;202:2925–2936. doi: 10.1242/jeb.202.21.2925. [DOI] [PubMed] [Google Scholar]
- Tomanek L, Somero GN. Time course and magnitude of synthesis of heat-shock proteins in congeneric marine snails (genus Tegula) from different tidal heights. Physiol Biochem Zool. 2000;73:249–256. doi: 10.1086/316740. [DOI] [PubMed] [Google Scholar]
- Uliano-Silva M, Americo JA, Brindeiro R, Dondero F, Prosdocimi F, Rebelo MDF. Gene discovery through transcriptome sequencing for the invasive mussel Limnoperna fortunei. PLoS One. 2014;9:e102973. doi: 10.1371/journal.pone.0102973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Russell B, Ding MW, Dong YW. Ocean acidification increases the sensitivity of and variability in physiological responses of an intertidal limpet to thermal stress. Biogeosciences. 2018;15:2803–2817. doi: 10.5194/bg-15-2803-2018. [DOI] [Google Scholar]
- Yokoyama Y, Hashimoto H, Kubota S, Kuriyama A, Ogura Y, Mizuta S, Yoshinaka R, Toyohara H. cDNA cloning of Japanese oyster stress protein homologous to the mammalian 78-kDa glucose regulated protein and its induction by heatshock. Fish Sci. 2006;72:402–409. doi: 10.1111/j.1444-2906.2006.01163.x. [DOI] [Google Scholar]
- Zhang G, Fang X, Guo X, Li L, Luo R, Xu F, Yang P, Zhang L, Wang X, Qi H, et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature. 2012;490:49–54. doi: 10.1038/nature11413. [DOI] [PubMed] [Google Scholar]
- Zhang G, Li L, Meng J, Qi H, Qu T, Xu F, Zhang L. Molecular basis for adaptation of oysters to stressful marine intertidal environments. Annu Rev Anim Biosci. 2016;4:357–381. doi: 10.1146/annurev-animal-022114-110903. [DOI] [PubMed] [Google Scholar]



