Abstract
Introduction
The advent of biological and targeted synthetic therapies has revolutionized rheumatoid arthritis (RA) treatment. However, this has come at the price of an increased risk of infections. The aim of this study was to present an integrated overview of both serious and non-serious infections, and to identify potential predictors of infection risk in RA patients using biological or targeted synthetic drugs.
Methods
We systematically reviewed available literature from PubMed and Cochrane and performed multivariate meta-analysis with meta-regression on the reported infections. Randomized controlled trials and prospective and retrospective observational studies including patient registry studies were analyzed, combined as well as separately.
We excluded studies focusing on viral infections only.
Results
Infections were not reported in a standardized manner. Meta-analysis showed significant heterogeneity that persisted after forming subgroups by study design and follow-up duration. Overall, the pooled proportions of patients experiencing an infection during a study were 0.30 (95% CI, 0.28–0.33) and 0.03 (95% CI, 0.028–0.035) for any kind of infections or serious infections only, respectively. We found no potential predictors that were consistent across all study subgroups.
Conclusions
The high heterogeneity and the inconsistency of potential predictors between studies show that we do not yet have a complete picture of infection risk in RA patients using biological or targeted synthetic drugs. Besides, we found non-serious infections outnumbered serious infections by a factor 10:1, but only a few studies have focused on their occurrence. Future studies should apply a uniform method of infectious adverse event reporting and also focus on non-serious infections and their impact on treatment decisions and quality of life.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-023-00571-z.
Keywords: Biological, Infection, Heterogeneity, Meta-analysis, Meta-regression, Rheumatoid arthritis, Targeted synthetic drugs
Key Summary Points
| Of all patients with rheumatoid arthritis (RA) that use biological or targeted synthetic therapies, approximately 30% are affected by an infection at some point during their treatment; only 2–3% are affected by a serious infection. |
| Serious infections as described in case reports are generally rare infections; they have hardly been observed in trials and observational studies, meaning the incidence of such infections is indeed very low. |
| There is high variability in the way infectious adverse events are reported in clinical trials and observational studies in RA patients using biological and targeted synthetic therapies, leading to considerable heterogeneity when pooling infection proportions. |
| That heterogeneity is particularly apparent for non-serious infections as opposed to serious infections, due to a lack of a standardized definition for non-serious infections. |
| Using meta-regression, we could not consistently corroborate any prior established predictive factor for infectious adverse events during biological or targeted synthetic therapy use. |
Introduction
Patients with rheumatoid arthritis (RA) have an increased risk of contracting serious infections which are defined as infections that are life-threatening or fatal, result in significant disability or require hospitalization [1, 2]. Aside from RA itself, which impairs an effective immune response to pathogens [3], the immunomodulating therapies used to treat RA also contribute to this risk.
Biologicals are monoclonal antibodies that target essential components in immune pathways related to RA pathogenesis. TNF-alpha inhibitors were introduced first, in the late 1990s (infliximab, etanercept, adalimumab, certolizumab pegol, golimumab). Other biologicals soon followed: anti-CD20 (B-cell) agents (rituximab), T-cell co-stimulation inhibitors (abatacept) and interleukin-(IL)-antagonists (most notably tocilizumab (anti-IL6) and anakinra (anti-IL1)). Existing categories are still expanding, and many other biological classes are being investigated as potential RA treatments. Targeted synthetic drugs are the most recent addition to the RA treatment options. These are small molecules that inhibit proinflammatory cytokine production through interference with intracellular signaling pathways, for example Janus Kinase-Signal Transducer and Activator of Transcription (JAK-STAT) inhibitors (tofacitinib, filgotinib). Most RA patients need a combination of different immunomodulators to adequately control their RA.
The current knowledge on infectious adverse events following treatment of RA with biologicals originates from several study designs, each with their own strengths and weaknesses [4]. Initially, infectious adverse events were mainly reported in randomized controlled trials (RCTs), which are limited by a relatively short follow-up and the employment of exclusion criteria, such as serious comorbidities or recurrent infections. Post-marketing observational studies may have a longer follow-up and include patients that would be considered ineligible in RCTs, but generally focus on serious infections only. Case reports mostly describe (very) rare, serious infections which are likely to be missed in trials due to the shorter follow-up.
A number of factors have been identified that increase the risk of serious infections in RA, most notably the presence of comorbidities such as diabetes mellitus and cardiovascular disease [5, 6], longer RA disease duration [7], older age, extra-articular RA manifestations, a previous history of recurrent infections, higher disease activity [8–10] and corticosteroid use [3, 11]. Biological use is another risk factor. An increased rate of serious infections as compared to placebo has been reported for several biologicals [12]. Tuberculosis reactivation, for example, is a well-documented adverse effect of TNF-alpha inhibitor use [13–15]. Little attention has been paid to the specific types of infections or the occurrence of non-serious infections during biological therapy [16]. More detailed knowledge would enable clinicians to monitor patients more closely or even prevent infections.
To perform a thorough analysis of infections in biological and targeted synthetic therapy use, we included as many study designs and as many articles as possible to be able to generate a complete overview. To this end, we performed a scoping review and meta-analysis with meta-regression taking disease characteristics, patient characteristics, and comorbidities into account. We determined the pooled proportion of patients that experienced serious and/or non-serious infections during treatment with biologicals or targeted synthetic therapies included in trials, observational studies, and registries. We compared this information with an overview of infections published in case reports of RA patients using biological or targeted synthetic therapies. In addition, we aimed to identify risk factors for developing these infections.
Methods
Ethics approval was waived as this study article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. We systematically collected available literature and performed a descriptive as well as meta-analysis with meta-regression following the PRISMA guidelines [17]. See Supplement 1 for the search terms and strategy. We included a wide variety of article types, as a complete overview of infections can only be obtained when analyzing all available literature. Narrative reviews, meta-analyses, studies on other antirheumatic drugs, studies not reporting infectious complications, not reporting data on RA patients separately, focusing on postoperative complications, or aggregating results of different drug classes were excluded. We also excluded studies focusing on the coronavirus disease that emerged in 2019 (COVID-19) because the lockdowns created a unique situation of decreased exposure, and studies focusing on reactivating viral infections only, as they originate in a different epidemiological setting altogether. Only published, peer-reviewed articles published until January 10, 2021, written in English and collected from PubMed and Cochrane were included. This review was registered at the Open Science Framework (Registration https://doi.org/10.17605/OSF.IO/FWTQE).
Blinded study selection was performed using Rayyan software [18]. EdV, JLM and BB performed the title and abstract selection, JLM and EdV each evaluating half and BB all hits, in order to review each article twice. Discrepancies were resolved by discussion until consensus was reached. BB screened the remaining full-text articles (flowchart in Supplement 2). We discussed beforehand which variables would be extracted (see Supplement 3 and 4). Two databases were constructed: one for RCTs, RCTs with open-label extension studies (RCT + OLE), prospective observational studies, registry studies, open-label studies and retrospective observational studies, and one for the case reports and series.
Randomized Controlled, Prospective, and Retrospective Observational Studies
Studies with multiple study arms examining multiple drugs, multiple different dosages of the same drug, or studies that reported the use of more than one observational study separately were divided into multiple entries in the database. Studies using the same registry or database, and RCTs and associated extension studies were critically reviewed and in- or excluded as described in Supplement 5 and 6 to prevent duplicate data. The following information was extracted per study (arm): year of publication, country of origin, biological or targeted synthetic agent name, class and dosage, follow-up duration, number of included patients, sex, age, and other patient characteristics, laboratory parameters, being: C-Reactive Protein (CRP) or Erythrocyte Sedimentation Rate (ESR), disease activity inventories (Disease Activity Score using CRP or ESR (DAS28-CRP/ESR), Clinical Disease Activity Index for RA (CDAI), Health Assessment Questionnaire – Disability Index (HAQ-DI), Swollen Joint Count in 66 joints (SJC66), Tender Joint Count in 68 Joints (TJC68), Patient’s Global Assessment (Visual Analogue Scale 0–100), Physicians, Global Assessment (Visual Analogue Scale 0–100), Pain score (Visual Analogue Scale 0–100), Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-fatigue)) concomitant immunosuppressant use, concomitant Non-Steroidal Anti-Inflammatory Drug (NSAID) use, use of any prior conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) or biological/targeted synthetic therapies, presence of comorbidities, number and type of infectious events during follow-up, number of serious infectious events and rate of infectious events during follow-up using Medical Dictionary for Regulatory Activities (MedDRA)[19] terminology (details in Supplement 3).
Infection count was extracted either as the total number of infected patients in a population of n patients, the number of infectious events in a population of n patients, an incidence rate (n infected patients per 100 patients) or an event rate (n events per 100 patient-years of exposure to study drug), as reported in the original article. Infections were extracted as either the number and/or rate of the total number of infections, as stated by the authors, or the number and/or rate of serious infections, as stated by the authors. See Table 1 for terms and definitions used throughout this paper. Specific infections were grouped into composite variables by the corresponding organ system (see Supplement 7). Information on the occurrence of specific infections was also extracted, if available, in the manner stated previously.
Table 1.
Terms and definitions used throughout this paper
| Term | Definition |
|---|---|
| Serious infection | A serious adverse event [2] of infectious nature, as defined by the authors |
| Total number of infected patients | Total number of infected patients in the study as stated by the authors (a patient is counted only once even though she/he could have had multiple infections) including both serious and non-serious infections |
| Total number of seriously infected patients | Total number of patients in the study that experienced an infection considered serious, as stated by the authors (a patient is counted only once even though she/he could have had multiple serious infections) |
| Total number of infectious events | Total number of infectious events in the study as stated by the authors (a patient can be counted multiple times as she/he can experience multiple events), including both serious and non-serious infections |
| Total number of serious infectious events | Total number of serious infectious events in the study as stated by the authors (each event a patient experiences is counted, even if the patient experiences multiple serious events) |
| Incidence rate | The number of infected patients per 100 patient-years of follow-up |
| Event rate | The number of infectious events per 100 patient-years of follow-up |
Case Reports and Series
Single case reports and case series from the literature search and from secondary citations were included. We analyzed the case series as separate case reports (they contained 2–15 cases per article). Per case, the following information was registered in the database: country of origin, year of publication, patient characteristics (age, sex, comorbidities), time since RA diagnosis, prescribed biological/targeted therapy and duration of use, comedication, infectious diagnosis using MedDRA [19] terminology, causative micro-organism, affected organ system, diagnostic method and outcome (details in Supplement 4).
Statistical Analysis
Data analyses and visualizations were carried out using R (R Core Team, version 4.2.1, 2022-06-23) and RStudio (version 2021.9.1.372) with the packages metafor [20], dmetar [21], and ggplot2 [22].
Randomized Controlled, Prospective, and Retrospective Observational Studies
We estimated the pooled proportions of the total number of infected patients and the number of seriously infected patients with their 95% confidence intervals (CI) across all studies as well as grouped by study design and biological target. The majority of the studies included in this review contributed two or more study arms and therefore multiple proportions of infections. A multivariate meta-regression model was used to account for this potential dependence between proportions belonging to the same study. Raw proportions were transformed into logit transformed proportions (i.e., the log of the proportion divided by one minus the proportion). Logit transformation was selected for its straightforward back-transformation and to ensure the confidence interval estimates fell between 0 and 1 [23]. Event rates per patient year were also estimated for total and serious infection events using multivariate meta-regression.
We used the rma.mv function of the metafor package [20] with the input of the observed effect sizes and the corresponding sampling variances; see Supplement 8 for details.
The heterogeneity between the reported proportions and event rates was assessed by computing [24] and Cochran's generalized Q test [25, 26]. The statistic describes the percentage of variation in the estimated effects that is explained by differences between the included studies rather than by chance. Similar to Higgins et al. [24], we categorized the level of heterogeneity based on as low (25–50%), moderate (50–75%), and high (≥ 75%). The Cochran's Q test statistic is the weighted sum of squared differences between the observed effects and the overall effects and the pooled effect across studies. The null hypothesis of the test is that all studies have the same effect size, and the alternative hypothesis is that the effect size for at least one study is different. For the Cochran's generalized Q test, 0.05 was regarded as significant heterogeneity.
We followed a two-stage procedure to deal with heterogeneity, if present. In the first stage, we attempted to create mutually exclusive sets of studies that were relatively homogeneous. We grouped studies based on study design (RCT, retrospective study, RCT + OLE, prospective cohort study, registry, open-label trial) and follow-up length (≤ 18 weeks; 19–38 weeks; 39–60 weeks) (see Supplement 8 for details) and assessed the level of heterogeneity separately for each subgroup. In the second stage, a multivariate meta-regression was used to further identify factors that significantly moderated the observed heterogeneity.
A common pitfall of meta-regression is the use of a small number of studies per examined covariate [27]. The Cochrane handbook suggests a minimum of ten studies per covariate [28]. For our multivariate meta-regression model, we first identified potential moderators from the literature (see Supplement 9 for details). Then, all of these features that were reported by at least ten studies were included in the model. Moderator analysis using multivariate meta-regression was performed only for RCT studies as the number of studies per subgroup was below ten for other study designs. In comparison, the multivariate meta-regression model was also fitted for alternative grouping of studies, separately for each study design (results see Supplement 10).
We also created two composite features ourselves. The included studies used a selection of ten different disease activity indices (see above). Each study reported at least two of these indices, however, the specific indices that were used varied greatly across studies. A composite disease activity was computed by scaling each disease activity index using each index’s minimum and maximum values and then taking the mean of the scaled values (details in Supplement 11). Out of all utilized exclusion criteria, we identified 14 unique ones that all studies used in varying combinations. To overcome this, we created a second composite variable: the summed number of exclusion criteria used in each study (details in Supplement 12).
Case Reports and Series
In the case reports database, patient characteristics, infection location and micro-organisms were analyzed using descriptive statistics.
Results
Trials, Registries and Cohort Studies
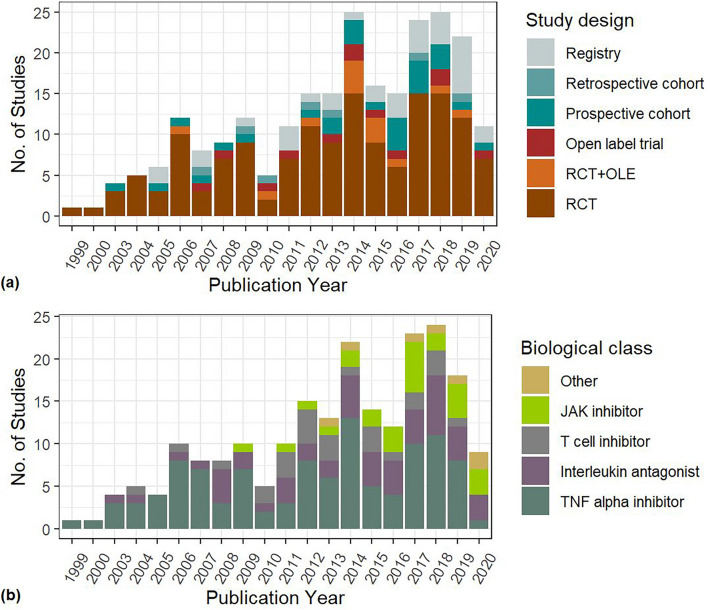
The final selection for this category yielded 242 studies containing 512 study arms and a total of 293,431 patients (descriptive statistics Supplement 13). The largest proportion of studies were RCTs (150, 61.9%), but the highest proportion of patients were supplied by registry studies (172,892 patients, 59%). A high proportion of studies was carried out in Western countries, with 182 (79%) studies taking place in North America and/or Europe (see Supplement 14). Out of all studies reporting on race or ethnicity, 89% of participants was Caucasian. TNF-alpha inhibitors were the most often administered biological class, with 117 studies (44%), 205 study arms (42%) and 170,826 patients (58%). Most patients were female (79.0%). The weighted mean age was 57 years. No causative micro-organisms were specified in trials, registries, or cohort studies. There was a high variability in the methods of infectious adverse event reporting. These were not mutually exclusive, with 129 studies reporting a total number of infected patients, 157 studies reporting a total number of seriously infected patients, 25 studies reporting the total number of infectious events and 38 studies reporting the total number of serious infectious events. Specific infections within the number of infected patients and number of infectious events were reported by 166 and 29 studies, respectively. Incidence and event rates were reported by a minority of studies, see Supplement 13. Figure 1 shows the number of studies by study design and by biological target over the past three decades.
Fig. 1.
Distribution of included studies by year of publication and study design (a) and by year of publication and biological target (b). JAK Janus kinase, OLE open-label extension, RCT randomized controlled trial, TNF tumor necrosis factor
Between-Study Heterogeneity and Subgroups
The between-study heterogeneity was assessed first for all studies together, then for each study design separately, and finally by further grouping studies based on follow-up duration (see Fig. 2). There was substantial between-study heterogeneity when all studies are taken together (both for total as well as seriously infected patients). All subgroups based on study design – except the registry subgroup (which comprised only three studies) – showed significant heterogeneity. Following the pattern observed in the follow-up duration of studies (see Supplement 8 Figs S1 and S2), we defined three subgroups: 18 weeks, 19–38 weeks, and 39–60 weeks. Although the level of heterogeneity was reduced after subgroups were formed, a moderate to higher level of heterogeneity remained, especially among subgroups for the total number of infected patients. For that, we employed a multivariate meta-regression model, see Supplement 15 and 16 for the results.
Fig. 2.
Between-study heterogeneity metrics and subgroup formation. variation (%) explained by differences in studies, n = number of studies/study arms, = variance (between-study), variance (within study), Q(P) = Cochran's Q test p value. Overall, heterogeneity reduced sequentially as studies were grouped first based on study design (stage 2) then based on follow-up duration (stage 3). This was especially true for the number of seriously infected patients
Pooled Proportion of Infected Patients and Event Rate Estimates
The estimated pooled proportions of the total number of infected patients and of the number of seriously infected patients were determined consecutively (1) across all study arms, (2) grouped by study design, and (3) grouped by biological/synthetic therapy target. Across all study arms, this comprised 58,789 (totally infected) and 168,042 (seriously infected) participants, as there were more studies reporting on serious infections only. There was considerable between-study heterogeneity, more so in the pooled proportion of the total number of infected patients than in the pooled proportion of seriously infected patients (Fig. 3). The pooled estimates and heterogeneity metrics for both the proportion of infected patients and event rates are presented in Table 2. In Table 2, we presented results for the categories of biological target and study design that are reported by at least two studies (n ≥ 2) as it is the minimum number (n) required to compute the pooled estimates using multivariate meta-regression.
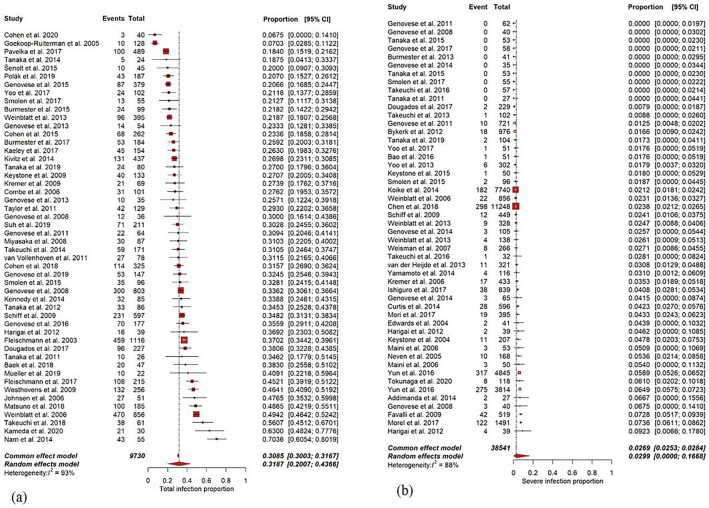
Fig. 3.
Forest plot for 50 randomly selected RCT studies, a for the total number of infected patients, b for seriously infected patients. 95% CI 95% confidence interval. Events number of (seriously) infected patients. Total total number of patients included in the study. A forest plot visually illustrates the relationship between included studies and gives an overview of heterogeneity of individual results. For a complete overview of forest plots, see Supplement 17
Table 2.
a Pooled proportion estimates b Pooled event rate estimates
| Total number of infected patients | Number of seriously infected patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pooled estimate | n | 95% CI | Cochran's Q test (P) | Pooled estimate | n | 95% CI | Cochran's Q test (P) | |||
| a. Proportions | ||||||||||
| All studies | 0.30 | 260 | [0.28, 0.33] | 95.14 | < 0.001 | 0.03 | 332 | [0.03, 0.03] | 91.44 | < 0.001 |
| Study design | ||||||||||
| RCT | 0.35 | 211 | [0.32, 0.37] | 89.79 | < 0.001 | 0.03 | 253 | [0.02, 0.03] | 38.08 | 0.001 |
| Prospective cohort | 0.16 | 19 | [0.10, 0.23] | 98.89 | < 0.001 | 0.05 | 18 | [0.03, 0.06] | 86.67 | < 0.001 |
| Registry | 0.23 | 3 | [0.19, 0.27] | 57.51 | 0.1 | 0.07 | 37 | [0.05, 0.09] | 98.43 | < 0.001 |
| RCT + OLE | 0.36 | 12 | [0.21, 0.53] | 94.17 | < 0.001 | 0.04 | 6 | [0.01, 0.10] | 77.78 | < 0.001 |
| Retrospective cohort | 0.10 | 7 | [0.07, 0.15] | 52.82 | 0.04 | 0.06 | 7 | [0.02, 0.19] | 95.24 | < 0.001 |
| Open-label trial | 0.18 | 8 | [0.10, 0.32] | 96.38 | < 0.001 | 0.03 | 11 | [0.02, 0.05 | 69.62 | < 0.001 |
| Biological target | ||||||||||
| TNF alpha inhibitor | 0.30 | 95 | [0.26, 0.35] | 96.12 | < 0.001 | 0.03 | 113 | [0.03, 0.04] | 94.63 | < 0.001 |
| B-cell inhibitor | 0.37 | 24 | [0.30, 0.45] | 86.50 | < 0.001 | 0.03 | 18 | [0.02, 0.05] | 61.27 | 0.01 |
| Rituximab | 0.29 | 11 | [0.20, 0.40] | 96.16 | < 0.001 | 0.06 | 21 | [0.04, 0.09] | 96.77 | < 0.001 |
| T-cell inhibitor | 0.34 | 12 | [0.21, 0.50] | 99.17 | < 0.001 | 0.03 | 20 | [0.02, 0.05] | 95.33 | < 0.001 |
| JAK1/3 inhibitor | 0.25 | 10 | [0.20, 0.30] | 36.30 | 0.06 | 0.02 | 20 | [0.01, 0.03] | 31.60 | 0.48 |
| JAK1/2 inhibitor | 0.40 | 12 | [0.33, 0.47] | 81.61 | < 0.001 | 0.02 | 15 | [0.02, 0.03] | 0 | 0.96 |
| JAK1 inhibitor | 0.32 | 15 | [0.27, 0.37] | 65.75 | 0.03 | 0.02 | 15 | [0.012, 0.03] | 0 | 0.76 |
| Pan-JAK inhibitor | 0.25 | 4 | [0.17, 0.34] | 53.25 | 0.09 | 0.02 | 14 | [0.01, 0.03] | 25.85 | 0.88 |
| IL6 inhibitor | 0.30 | 48 | [0.24, 0.36] | 95.33 | < 0.001 | 0.04 | 59 | [0.03, 0.04] | 77.29 | < 0.001 |
| IL17 inhibitor | 0.26 | 17 | [0.21, 0.31] | 36.12 | 0.29 | 0.02 | 11 | [0.01, 0.05] | 9.70 | 0.94 |
| IL1 inhibitor | 0.23 | 2 | [0.04, 0.67] | 85.40 | 0.01 | 0.03 | 7 | [0.02, 0.06] | 74.33 | 0.001 |
| IL23 inhibitor | 0.23 | 2 | [ 0.16, 0.32] | 0 | 0.78 | 0.03 | 2 | [0.01, 0.09] | 0 | 0.36 |
| IL 12/IL 23 inhibitor | 0.31 | 2 | [0.18, 0.47] | 62.76 | 0.10 | 0.01 | 2 | [0, 0.07] | 0 | 0.68 |
| BTK inhibitor | 0.11 | 4 | [0.08, 0.16] | 3.69 | 0.39 | 0.01 | 4 | [0.01, 0.04] | 0 | 0.94 |
| SYK inhibitor | – | – | – | – | – | 0.02 | 2 | [0.01, 0.06] | 0 | 0.63 |
| GM-CSF inhibitor | – | – | – | – | – | 0.01 | 7 | [0.01, 0.04] | 0 | 1 |
| Total number of infected patients | Number of seriously infected patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ER | n | 95% CI | Cochran's Q test(P) | ER | n | 95% CI | Cochran's Q test (P) | |||
| b. Event rates | ||||||||||
| All studies | 0.92 | 8 | [0.34, 1.50] | 99.82 | < 0.001 | 0.05 | 44 | [0.03, 0.08] | 99.44 | < 0.001 |
| Study design | ||||||||||
| RCT | – | – | – | – | – | 0.03 | 7 | [0.01, 0.086] | 87.97 | < 0.001 |
| Prospective cohort | – | – | – | – | – | – | – | – | – | – |
| Registry | 0.76 | 3 | [0.67, 0.83] | 79.03 | 0.01 | 0.07 | 32 | [0.03, 0.11] | 99.71 | < 0.001 |
| RCT + OLE | 1.32 | 3 | [0.03, 2.60] | 99.75 | < 0.001 | 0.04 | 4 | [0.03, 0.05] | 43.15 | 0.38 |
| Retrospective cohort | – | – | – | – | – | – | – | – | – | – |
| Open-label trial | – | – | – | – | – | – | – | – | – | |
| Biological target | ||||||||||
| TNF alpha inhibitor | – | – | – | – | – | 0.05 | 19 | [0.00, 0.10] | 99.80 | < 0.001 |
| IL 6 inhibitor | 0.53 | 5 | [0.30, 0.77] | 99.24 | < 0.001 | 0.04 | 9 | [0.03, 0.06] | 94.63 | < 0.001 |
| Rituximab | – | – | – | – | – | 0.09 | 4 | [0.01, 0.18] | 99 | < 0.001 |
| T cell inhibitor | – | – | – | – | – | 0.07 | 6 | [0.0, 0.17] | 99.71 | < 0.001 |
| B cell inhibitor | – | – | – | – | – | 0.04 | 5 | [0.02, 0.07] | 73.98 | 0.01 |
BTK Bruton tyrosine kinase, CI 95% confidence interval, ER pooled event rate, GM-CSF granulocyte–macrophage colony-stimulating factor, IL interleukin, JAK Janus kinase, n number of study arms, RCT randomized controlled trial, RCT + OLE randomized controlled trial and open-label extension, SYK spleen tyrosine kinase, TNF tumor necrosis factor
The estimated pooled proportion of the total number of infected patients was highest in RCT ± OLE and RCT studies. However, the differences between the estimated pooled proportions must be interpreted with caution as the heterogeneity as well as the number of studies per design subgroup varied substantially. The estimated pooled proportion of seriously infected patients was highest in registry and retrospective studies; however, the heterogeneity was also highest in these two study designs.
Fewer studies reported the infection event rates; 37 study arms (25 studies) and 79 study arms (38 studies) reported 7821 infectious events in 13,937 patients and 6100 serious infectious events in 90,204 patients, respectively.
Multivariate Meta-Regression
A multivariate meta-regression model was fitted using one moderator at a time to explore the association between demographic, biological/targeted therapy, and methodological characteristics as potential predictors of the logit of infection prevalence in RCT studies with a follow-up duration of 18 weeks, 19–38 weeks, and 39–60 weeks. For the purpose of readability, we included the results of the meta-regression analyses in Supplement 15 (follow-up duration of 18 weeks and 19–38 weeks) and Supplement 16 (follow-up duration 39–60 weeks). A separate analysis by follow-up duration was carried out with anticipation that different sets of moderators are responsible for the heterogeneity in the three subgroups. No single moderator was found to be significant across all three groups. In both the total number of infected patients as well as in the number of seriously infected patients, the subgroup of 39–60 weeks of follow-up was the smallest and most heterogenous, with results not significantly different from other subgroups. More information on how studies are grouped based on study design and follow-up duration is available in Supplement 8.
Type of Infections
Across all biological/targeted synthetic therapy classes, upper respiratory tract (URT) infections were the most prevalent (12.7%, 95% CI 10.6–15.0), followed by genitourinary tract infections (3.5%, 95% CI 2.9–4.2) and lower respiratory tract (LRT) infections (2.2%, 95% CI 1.9–2.7). See Table 3. Individual infection proportions were comparable across biological classes. TNF-alpha inhibitors were associated with an increased proportion of mycobacterial infections (0.9%, 95% CI 0.6–1.0) compared to other drug classes. Overall, the proportion of seriously infected patients was highest in rituximab users (6.7%, 95% CI 4.5–9.7). The proportion of LRT infections (3.3%, 95% CI 1.6–6.5), and skin and soft tissue infections (2.3%, 95% CI 0.4–13.4) was also relatively high in this group, as was the proportion of Herpes Zoster (11.8%, 95% CI 3.1–35.4), however, only few studies reported on these adverse events. Compared to other drug classes, tofacitinib use was associated with an increased proportion of Herpes Zoster (2.8%, 95% CI 1.3–6.0) and Pneumocystis jirovecii pneumonia (PJP) (1.5%, 95% CI 0.4–4.5).
Table 3.
Estimated pooled proportions of the various infections across different biological classes and targeted synthetic therapies
| Total | TNF-alpha inhibitors | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prop | 95%CI | I2 (%) | k | Events | Observations | Prop | 95%CI | I2 (%) | k | Events | Observations | |
| Total infected patients | 0.3029 | [0.2765–0.3306] | 95.14 | 260 | 15,515 | 58,789 | 0.3031 | [0.2604–0.3494] | 96.12 | 95 | 5725 | 24,088 |
| Seriously infected patients | 0.0308 | [0.0275–0.0345] | 91.45 | 332 | 8969 | 168,042 | 0.0323 | [0.0271–0.0385] | 94.63 | 113 | 4829 | 83,393 |
| URT | 0.1270 | [0.1067–0.1504] | 95.79 | 252 | 6887 | 57,167 | 0.1332 | [0.0997–0.1757] | 97.31 | 82 | 3193 | 170,826 |
| LRT | 0.0229 | [0.0192–0.0274] | 86.12 | 159 | 1607 | 293,431 | 0.0233 | [0.0181–0.0301] | 89.17 | 67 | 959 | 43,271 |
| Genitourinary | 0.0354 | [0.0294–0.0425] | 75.66 | 121 | 859 | 25,599 | 0.0356 | [0.0255–0.0495] | 83.69 | 41 | 341 | 10,020 |
| Gastrointestinal | 0.0127 | [0.0094–0.0172] | 71.08 | 67 | 228 | 19,437 | 0.0098 | [0.0064–0.0151] | 60.49 | 23 | 77 | 8784 |
| Skin-soft tissue | 0.0122 | [0.0092–0.0161] | 78.83 | 73 | 377 | 28,054 | 0.0117 | [0.0070–0.0196] | 82.96 | 27 | 150 | 8943 |
| Bone and joint | 0.0070 | [0.0050–0.0100] | 40.9 | 73 | 81 | 15,212 | 0.0086 | [0.0065–0.0114] | 0 | 16 | 49 | 6205 |
| Sepsis | 0.0057 | [0.0040–0.0082] | 68.20 | 36 | 153 | 39,927 | 0.0055 | [0.0033–0.0094] | 75.88 | 20 | 104 | 28,321 |
| Mycobacterial | 0.0072 | [0.0050–0.0105] | 79.98 | 47 | 254 | 40,844 | 0.0095 | [0.0063–0.0143] | 77.42 | 35 | 206 | 25,426 |
| Neurological infections | 0.0147 | [0.0117–0.0184] | 65.15 | 78 | 371 | 34,681 | 0.0106 | [0.0082–0.0136] | 20.25 | 25 | 121 | 14,436 |
| Fungal | 0.0088 | [0.0043–0.0182] | 77.66 | 17 | 39 | 12,760 | 0.0078 | [0.0025–0.0238] | 83.48 | 9 | 23 | 10,081 |
| Viral | 0.0180 | [0.0138–0.0235] | 7.70 | 24 | 13 | 4693 | 0.0188 | [0.0140–0.0252] | 0 | 11 | 12 | 2554 |
| Other | 0.0119 | [0.0072–0.0198] | 74.69 | 31 | 101 | 11,869 | 0.0081 | [0.0053–0.0124] | 0 | 8 | 21 | 2901 |
| Herpes Zoster | 0.0139 | [0.0111–0.0174] | 60.19 | 97 | 366 | 36,058 | 0.0101 | [0.0078–0.0131] | 18.48 | 29 | 116 | 14,776 |
| Salmonellosis | 0.0024 | [0.0009–0.0065] | 0 | 3 | 4 | 1664 | 0.0025 | [0.0006–0.0100] | 0 | 2 | 2 | 814 |
| PJP | 0.0048 | [0.0029–0.0079] | 63.65 | 31 | 91 | 35,134 | 0.0038 | [0.0024–0.0062] | 50.99 | 16 | 78 | 28,678 |
| Tuberculosis | 0.0042 | [0.0031–0.0056] | 49.51 | 95 | 171 | 53,277 | 0.0061 | [0.0044–0.0084] | 49.48 | 53 | 157 | 29,592 |
| IL-6 inhibitors | Rituximab | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prop | 95%CI | I2 (%) | k | Events | Observations | Prop | 95%CI | I2 (%) | k | Events | Observations | |
| Total infected patients | 0.2971 | [0.2402–0.3610] | 95.34 | 48 | 3111 | 11,225 | 0.2875 | [0.1988–0.3962] | 96.72 | 8 | 772 | 4108 |
| Seriously infected patients | 0.0359 | [0.0295–0.0437] | 77.29 | 59 | 1137 | 24,568 | 0.0665 | [0.0450–0.0973] | 96.59 | 19 | 1323 | 14,285 |
| URT | 0.1515 | [0.0958–0.2314] | 95.68 | 42 | 1067 | 45,379 | 0.1601 | [0.1123–0.2232] | 75.69 | 8 | 180 | 1022 |
| LRT | 0.0158 | [0.0107–0.0233] | 89.17 | 22 | 246 | 12,429 | 0.0327 | [0.0162–0.0652] | 80.21 | 11 | 51 | 2398 |
| Genitourinary | 0.0388 | [0.0278–0.0540] | 50.72 | 19 | 104 | 3472 | 0.0452 | [0.0350–0.0581] | 0 | 6 | 57 | 1306 |
| Gastrointestinal | 0.0154 | [0.0085–0.0278] | 61.72 | 13 | 39 | 3388 | 0.0080 | [0.0014–0.0454] | 38.57 | 2 | 2 | 359 |
| Skin-soft tissue | 0.0150 | [0.0093–0.0241] | 82.24 | 18 | 168 | 11,514 | 0.0231 | [0.0036–0.1349] | 82.01 | 4 | 7 | 569 |
| Bone and joint | 0.0059 | [0.0028–0.0125] | 49.27 | 8 | 23 | 7192 | NA | NA | NA | < 1 | NA | NA |
| Sepsis | 0.0079 | [0.0034–0.0179] | 59.51 | 5 | 32 | 6494 | NA | NA | NA | < 1 | 6 | 2484 |
| Mycobacterial | 0.0055 | [0.0039–0.0076] | 0 | 2 | 35 | 6412 | NA | NA | NA | < 1 | 1 | 2484 |
| Neurological infections | 0.0109 | [0.0067–0.0177] | 61.52 | 11 | 64 | 8941 | NA | NA | NA | < 1 | 2 | 12 |
| Fungal | NA | NA | NA | 0 | 2 | 32 | 0.0035 | [0.0015–0.0085] | 0 | 2 | 5 | 1469 |
| Viral | 0.0124 | [0.0040–0.0377] | 0 | 3 | 0.0109 | [0.0015–0.0761] | 92.03 | 2 | NA | NA | ||
| Other | 0.0060 | [0.0030–0.0119] | 0 | 4 | 8 | 1401 | 0.0038 | [0.0010–0.0145] | 46.01 | 4 | 4 | 1966 |
| Herpes Zoster | 0.0106 | [0.0066–0.0172] | 59.22 | 12 | 64 | 9122 | 0.1176 | [0.0314–0.3538] | 5.97 | 2 | 2 | 25 |
| Salmonellosis | NA | NA | NA | 0 | 2 | 850 | NA | NA | NA | < 1 | NA | NA |
| PJP | 0.0032 | [0.0002–0.0587] | 67.47 | 2 | 1 | 920 | NA | NA | NA | < 1 | NA | NA |
| Tuberculosis | 0.0012 | [0.0006–0.0024] | 0 | 8 | 5 | 8046 | NA | NA | NA | < 1 | 1 | 2484 |
| Abatacept | Tofacitinib | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prop | 95%CI | I2 (%) | k | Events | Observations | Prop | 95%CI | I2 (%) | k | Events | Observations | |
| Total infected patients | 0.3321 | [0.2723–0.3978] | 99.17 | 12 | 2117 | 8423 | 0.2459 | [0.2022–0.2956] | 36.25 | 10 | 141 | 586 |
| Seriously infected patients | 0.0335 | [0.0238–0.0469] | 95.34 | 20 | 1382 | 30,169 | 0.0208 | [0.0144–0.0299] | 31.62 | 20 | 68 | 3576 |
| URT | 0.0887 | [0.0314–0.2264] | 99.34 | 13 | 908 | 40,098 | 0.0840 | [0.0342–0.1919] | 96.71 | 21 | 450 | 3296 |
| LRT | 0.0165 | [0.0076–0.0352] | 92.5 | 13 | 141 | 8606 | 0.0244 | [0.0140–0.0421] | 59.78 | 12 | 48 | 2290 |
| Genitourinary | 0.0140 | [0.0053–0.0361] | 55.7 | 7 | 72 | 3659 | 0.0325 | [0.0185–0.0566] | 65.48 | 6 | 45 | 1550 |
| Gastrointestinal | 0.0037 | [0.0016–0.0083] | 34.56 | 6 | 11 | 3390 | 0.0192 | [0.0061–0.0585] | 58.95 | 7 | 11 | 999 |
| Skin-soft tissue | 0.0058 | [0.0034–0.0099] | 8 | 6 | 13 | 2484 | 0.0097 | [0.0036–0.0264] | 38.40 | 3 | 7 | 778 |
| Bone and joint | 0.0031 | [0.0010–0.0095] | 0 | 2 | 3 | 996 | NA | NA | NA | < 1 | 1 | 201 |
| Sepsis | 0.0034 | [0.0013–0.0090] | 0 | 2 | 4 | 1372 | 0.0057 | [0.0018–0.0176] | 0 | 3 | 3 | 647 |
| Mycobacterial | 0.0037 | [0.0005–0.0261] | 82.23 | 4 | 7 | 4595 | 0.0060 | [0.0016–0.0218] | 20.98 | 2 | 3 | 598 |
| Neurological infections | 0.0297 | [0.0035–0.2087] | 81.76 | 6 | 43 | 4200 | 0.0293 | [0.0135–0.0624] | 80.58 | 11 | 67 | 2174 |
| Fungal | 0.0047 | [0.0018–0.0125] | 0 | 2 | 4 | 881 | NA | NA | NA | < 1 | NA | NA |
| Viral | NA | NA | NA | < 1 | NA | NA | NA | NA | NA | < 1 | NA | NA |
| Other | 0.0087 | [0.0063–0.0119] | 0 | 3 | 38 | 4477 | 0.0257 | [0.0172–0.0384] | 0 | 8 | 23 | 928 |
| Herpes Zoster | 0.0087 | [0.0063–0.0119] | 0 | 3 | 43 | 4356 | 0.0282 | [0.0131–0.0597] | 77.67 | 13 | 67 | 2280 |
| Salmonellosis | 0.0101 | [0.0075–0.0136] | 0 | 3 | 0 | 0 | NA | NA | NA | < 1 | NA | NA |
| PJP | NA | NA | NA | < 1 | 6 | 4719 | 0.0145 | [0.0042–0.0488] | 0 | 5 | 2 | 209 |
| Tuberculosis | 0.0019 | [0.0007–0.0054] | 29.26 | 4 | 3 | 7973 | 0.0032 | [0.0009–0.0110] | 27.13 | 3 | 1869 | |
Events number of infected patients, K selected number of included studies, LRT lower respiratory tract, NA not available, Observations total number of patients in included studies, PJP Pneumocystits jirovecii pneumonia, Prop pooled proportion of (seriously) infected patients, URT upper respiratory tract, 95%CI 95% confidence interval. Herpes Zoster infections are included in neurological infections, salmonellosis in of gastrointestinal infections, PJP in lower respiratory tract infections and tuberculosis in mycobacterial infections
Case Reports and Series
The final selection yielded 372 articles in this category, comprising 503 cases and 509 identified micro-organisms. Most articles originated in the US (83, 22.3%) and Japan (77, 20.7%). Described patients were mostly female (66.8%), with a mean age of 61 years. Most described patients (398, 82%) used TNF-alpha inhibitors (details in Supplement 18). The median onset of an infectious adverse event was 9 months after start of biological/targeted therapy (interquartile range (IQR) 3–24). Of all reported pathogens, 326 (64%) were bacterial (of which 143 mycobacterial infections), 19.4% (99) fungal, 7.9% [40] parasitic and 8.6% [44] viral (see Supplement 19 and 20). Overall, the most frequently reported infections (187, 32%) occurred in the lower respiratory tract (LRT). The most frequently reported pathogens were Mycobacterium tuberculosis (85, 16.7% of pathogens), nontuberculous mycobacteria (50, 9.8%), Pneumocystis jirovecii (28, 5.5%), Salmonella spp (28, 5.5%), Listeria monocytogenes (24, 4.7%), Histoplasma capsulatum (22, 4.3%) and Leishmania spp (21, 4.1%). The largest proportion of these infections were reported in TNF-alpha inhibitor users, who are known to have difficulty clearing mycobacterial and intracellular bacterial infections.
A relatively high number of viral (38%) and fungal (31%) infections were reported in abatacept users, however, absolute numbers were low. Relatively few fungal infections were reported in interleukin antagonist users (2, 3.6%), conversely, a relatively high number of other than intracellular bacterial infections was reported. This was also true for rituximab. Only three infections were reported in JAK-inhibitors, all fungal; see Supplement 20.
Discussion
We performed a scoping review and meta-analysis with meta-regression to create an overview of and identify risk factors for infectious complications of biological and targeted synthetic therapies in RA patients. We report upon the combined results of 242 studies (trials/registries) with in total 293,431 included patients. To the best of our knowledge, this is the largest meta-analysis on infections in biological and targeted synthetic drug use in RA patients to date.
We found a very high between-study heterogeneity across all analyses. A possible cause of this is likely different study methodologies and differences in the follow-up duration. However, significant heterogeneity persisted even after formation of subgroups that took these factors into account. We therefore presume the high heterogeneity may be attributed to differences in infectious adverse event reporting on multiple levels. Firstly, included studies used a multitude of techniques to express infection count (either as the number of infected patients per follow-up duration, the number of infectious events or an incidence- or event rate. See also Table S14). There was also considerable variation in the reporting of serious- versus non-serious infections, some studies reporting on only one type. Standardization of these reporting methods would likely reduce heterogeneity. Secondly, there is currently a lack of standardized definitions for non-serious infections and there are, therefore, various interpretations of observed adverse events. The registration of infectious adverse events during a clinical trial is in itself subject to bias and inconsistency [29–31]. For example, “sinusitis” may be registered as either “sinusitis”, “upper respiratory tract infection” or “upper respiratory tract symptoms”, the latter being non-infectious in nature. Standardized definitions would reduce heterogeneity, as illustrated by a lower value in the pooled proportion of seriously as opposed to total infected patients (see Fig. 2): only a definition of serious infection is currently available. We tried to mitigate this effect by grouping individual infections by the affected organ system. These shortcomings are important to consider when interpreting the results of this review, and of future studies as well.
Our data show non-serious infections to be the most prevalent infectious adverse events during biological or targeted synthetic drug use in RA, outnumbering serious infections in a ratio of approximately 10:1 (30% of patients being affected by any kind of infection at some point in their treatment, vs. 2–3% being affected by a serious infection – see Table 3). Non-serious infections frequently lead to treatment discontinuation, for which their recurrent nature may be more important than their severity [32]. Furthermore, recurrent non-serious infections are associated with a high patient-experienced burden and socioeconomic costs [33–37]. Although the impact of non-serious infections is high, only a few studies have made their occurrence and their potential implications for treatment and quality of life in patients a priority [16, 38].
As for predictive risk factors, we found no moderator that was significant across all three RCT subgroups for the total number of infected patients. However, several moderators which were previously associated with an increased risk of serious infection were significant in one subgroup [10, 39–43]. Similar to the current study, previous studies also show differences in risks for serious infections between different biologicals [12]. Interestingly, while prior literature shows a dose-dependent effect of corticosteroids on the occurrence of serious infections, our findings do not support that effect. This may be explained by the exclusion of patients using high-dose corticosteroids from most RCTs and by the relatively short duration of corticosteroid therapy.
Limitations of our study include the high observed heterogeneity, which may be attributed to variations in methodology as well as differences in the reporting of infectious adverse events in the included studies. Standardized definitions for infections would probably have reduced the heterogeneity and provided more reliable estimates. Furthermore, the registration of infectious adverse events during clinical trials is subject to inherent bias and inconsistencies. This introduces potential limitations in the accuracy and completeness of the reported data. Finally, as most included studies were RCTs (of which a significant number excluded patients with comorbidities or recurrent or chronic infections, see Supplement 12), it is unclear to what extent our findings can be extrapolated to real-world settings in which patients have multiple comorbidities and use many different immunomodulators. The geographical spread of included studies is not equally distributed across the world. This is most likely a reflection of the biological/targeted drug consumption, which is correlated with accessibility [44]. Therefore, little information could be obtained about the risks for infectious complications were these drugs to be used more in populations where other, ‘exotic’, infections are prevalent.
The broad scope is without doubt this study’s greatest strength. That scope enabled us to analyze information on a comprehensive list of aspects of infectious complications of biological/synthetic targeted treatment in RA, using different study designs that complement each other’s limitations. This review includes not only pooled proportions of the total number of (seriously) infected patients, but also proportions of patients contracting specific infections, and an analysis on potential predictors of infectious adverse events. The addition of case reports provides information on various rare serious infections and their causative pathogens, data that are generally not available from RCT studies.
Conclusions
In conclusion, non-serious infections outnumbered serious infections in a ratio of approximately 10:1 in RA patients using biological/targeted synthetic therapies, however, only little attention has been paid to them in existing literature. None of the risk factors for developing infections that were previously identified in separate studies were consistently confirmed in our meta-analysis. Further prospective research is needed using uniform infectious adverse event registration.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This research received no funding. Funding for the publication fee was provided by Tilburg University, Microvida and the Elisabeth-TweeSteden Hospital.
Author Contributions
Barbara JM Bergmans – conception and design, data acquisition, article selection, data analysis, writing of manuscript. Biniyam Y Gebeyehu – data analysis, writing of manuscript. Eugène P van Puijenbroek – critical revision of manuscript. Katrijn van Deun – critical revision of manuscript. Bennett Kleinberg – critical revision of manuscript. Jean-Luc Murk – conception and design, article selection, critical revision of manuscript. Esther de Vries – conception and design, article selection, critical revision of manuscript.
Prior Publication
An abstract of this research was presented in poster form at the European Society for Immunodeficiencies (ESID), 12–15 October 2022, Gothenburg, Sweden. This manuscript has been published on a preprint server MedRxiv on March 9, 2023 (https://doi.org/10.1101/2023.03.09.23287022).
Disclosures
Barbara JM Bergmans has nothing to disclose. Biniyam Y Gebeyehu has nothing to disclose. Eugène P van Puijenbroek has nothing to disclose. Katrijn van Deun has nothing to disclose. Bennett Kleinberg has nothing to disclose. Jean-Luc Murk has nothing to disclose. Esther de Vries has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article as supplementary information files. All included articles are publicly available.
References
- 1.Uddin J, Kraus AS, Kelly HG. Survivorship and death in rheumatoid arthritis. Arthritis Rheum. 1970;13(2):125–130. doi: 10.1002/art.1780130204. [DOI] [PubMed] [Google Scholar]
- 2.ICH Harmonised Tripartite Guideline Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting E2D. 2003.
- 3.Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford) 2013;52(1):53–61. doi: 10.1093/rheumatology/kes305. [DOI] [PubMed] [Google Scholar]
- 4.Leombruno J. The challenges of quantifying the risk of serious infection with tumor necrosis factor antagonist therapy. J Rheumatol. 2010;37(5):887–889. doi: 10.3899/jrheum.100251. [DOI] [PubMed] [Google Scholar]
- 5.Løppenthin K, Esbensen BA, Østergaard M, Ibsen R, Kjellberg J, Jennum P. Morbidity and mortality in patients with rheumatoid arthritis compared with an age- and sex-matched control population: A nationwide register study. J Comorb. 2019 doi: 10.1177/2235042X19853484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabriel SE. Why do people with rheumatoid arthritis still die prematurely? Annals of the rheumatic diseases. 2008;67 Suppl 3(Suppl 3):iii30–iii4. [DOI] [PMC free article] [PubMed]
- 7.Listing J, Kekow J, Manger B, Burmester G-R, Pattloch D, Zink A, et al. Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFα inhibitors and rituximab. Ann Rheum Dis. 2015;74(2):415. doi: 10.1136/annrheumdis-2013-204021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta B, Pedro S, Ozen G, Kalil A, Wolfe F, Mikuls T, et al. Serious infection risk in rheumatoid arthritis compared with non-inflammatory rheumatic and musculoskeletal diseases: a US national cohort study. RMD Open. 2019;5(1):e000935. doi: 10.1136/rmdopen-2019-000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2294–2300. doi: 10.1002/art.10529. [DOI] [PubMed] [Google Scholar]
- 10.Salmon JH, Gottenberg JE, Ravaud P, Cantagrel A, Combe B, Flipo RM, et al. Predictive risk factors of serious infections in patients with rheumatoid arthritis treated with abatacept in common practice: results from the Orencia and Rheumatoid Arthritis (ORA) registry. Ann Rheum Dis. 2016;75(6):1108–1113. doi: 10.1136/annrheumdis-2015-207362. [DOI] [PubMed] [Google Scholar]
- 11.Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol. 2008;35(3):387–393. [PubMed] [Google Scholar]
- 12.Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011(2):Cd008794. [DOI] [PMC free article] [PubMed]
- 13.Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48(8):2122–2127. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 14.Chan MJ, Wen YH, Huang YB, Chuang HY, Tain YL, Lily Wang YC, et al. Risk of tuberculosis comparison in new users of antitumour necrosis factor-α and with existing disease-modifying antirheumatic drug therapy. J Clin Pharm Ther. 2018;43(2):256–264. doi: 10.1111/jcpt.12644. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Fan W, Yang G, Xu Z, Wang J, Cheng Q, et al. Risk of tuberculosis in patients treated with TNF-α antagonists: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2017;7(3):e012567. doi: 10.1136/bmjopen-2016-012567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dao KH, Herbert M, Habal N, Cush JJ. Nonserious infections: should there be cause for serious concerns? Rheum Dis Clin North Am. 2012;38(4):707–725. doi: 10.1016/j.rdc.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 18.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan — a web and mobile app for systematic reviews. . Systematic Reviews. 2016;5(210). [DOI] [PMC free article] [PubMed]
- 19.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA) Drug Saf. 1999;20(2):109–117. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- 20.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package.
- 21.Ebert MHaPCaTFaDD. dmetar: Companion R Package For The Guide 'Doing Meta-Analysis in R'. 2019.
- 22.Wickham H. ggplot2: Elegant Graphics for Data Analysis: Springer-Verlag New York; 2016.
- 23.Warton DI, Hui FKC. The arcsine is asinine: the analysis of proportions in ecology. Ecology. 2011;92(1):3–10. doi: 10.1890/10-0340.1. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cochran WG. The Combination of Estimates from Different Experiments. Biometrics. 1954;10(1):101–129. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 26.Jackson D. Confidence intervals for the between-study variance in random effects meta-analysis using generalised Cochran heterogeneity statistics. Res Synthesis Methods. 2013;4(3):220–229. doi: 10.1002/jrsm.1081. [DOI] [PubMed] [Google Scholar]
- 27.Geissbühler M, Hincapié CA, Aghlmandi S, Zwahlen M, Jüni P, da Costa BR. Most published meta-regression analyses based on aggregate data suffer from methodological pitfalls: a meta-epidemiological study. BMC Med Res Methodol. 2021;21(1):123. doi: 10.1186/s12874-021-01310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JPT, Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., & Welch, V., editor. Cochrane Handbook for Systematic Reviews of Interventions.: Wiley-Blackwell; 2019. [DOI] [PMC free article] [PubMed]
- 29.Schroll JB, Maund E, Gøtzsche PC. Challenges in coding adverse events in clinical trials: a systematic review. PLoS One. 2012;7(7):e41174. doi: 10.1371/journal.pone.0041174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maund E, Tendal B, Hróbjartsson A, Lundh A, Gøtzsche PC. Coding of adverse events of suicidality in clinical study reports of duloxetine for the treatment of major depressive disorder: descriptive study. BMJ. 2014;348:g3555. doi: 10.1136/bmj.g3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown EG. Methods and pitfalls in searching drug safety databases utilising the Medical Dictionary for Regulatory Activities (MedDRA) Drug Saf. 2003;26(3):145–158. doi: 10.2165/00002018-200326030-00002. [DOI] [PubMed] [Google Scholar]
- 32.Du Pan SM, Dehler S, Ciurea A, Ziswiler HR, Gabay C, Finckh A. Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum. 2009;61(5):560–568. doi: 10.1002/art.24463. [DOI] [PubMed] [Google Scholar]
- 33.Wagenlehner F, Wullt B, Ballarini S, Zingg D, Naber KG. Social and economic burden of recurrent urinary tract infections and quality of life: a patient web-based study (GESPRIT) Expert Rev Pharmacoecon Outcomes Res. 2018;18(1):107–117. doi: 10.1080/14737167.2017.1359543. [DOI] [PubMed] [Google Scholar]
- 34.Linder JA, Atlas SJ. Health-related quality of life in patients with sinusitis. Curr Allergy Asthma Rep. 2004;4(6):490–495. doi: 10.1007/s11882-004-0017-1. [DOI] [PubMed] [Google Scholar]
- 35.François M, Hanslik T, Dervaux B, Le Strat Y, Souty C, Vaux S, et al. The economic burden of urinary tract infections in women visiting general practices in France: a cross-sectional survey. BMC Health Serv Res. 2016;16(a):365. [DOI] [PMC free article] [PubMed]
- 36.Birnbaum HG, Morley M, Greenberg PE, Colice GL. Economic burden of respiratory infections in an employed population. Chest. 2002;122(2):603–611. doi: 10.1378/chest.122.2.603. [DOI] [PubMed] [Google Scholar]
- 37.Wahid NW, Smith R, Clark A, Salam M, Philpott CM. The socioeconomic cost of chronic rhinosinusitis study. Rhinology. 2020;58(2):112–125. doi: 10.4193/Rhin19.424. [DOI] [PubMed] [Google Scholar]
- 38.Bechman K, Halai K, Yates M, Norton S, Cope AP, Hyrich KL, et al. Nonserious infections in patients with rheumatoid arthritis: results from the British Society for Rheumatology biologics register for rheumatoid arthritis. Arthritis Rheumatol. 2021;73(10):1800–1809. doi: 10.1002/art.41754. [DOI] [PubMed] [Google Scholar]
- 39.Lang VR, Englbrecht M, Rech J, Nüsslein H, Manger K, Schuch F, et al. Risk of infections in rheumatoid arthritis patients treated with tocilizumab. Rheumatology (Oxford) 2012;51(5):852–857. doi: 10.1093/rheumatology/ker223. [DOI] [PubMed] [Google Scholar]
- 40.Morel J, Constantin A, Baron G, Dernis E, Flipo RM, Rist S, et al. Risk factors of serious infections in patients with rheumatoid arthritis treated with tocilizumab in the French Registry REGATE. Rheumatology (Oxford) 2017;56(10):1746–1754. doi: 10.1093/rheumatology/kex238. [DOI] [PubMed] [Google Scholar]
- 41.Favalli EG, Desiati F, Atzeni F, Sarzi-Puttini P, Caporali R, Pallavicini FB, et al. Serious infections during anti-TNFalpha treatment in rheumatoid arthritis patients2009 2009-Jan-. 266–73 p. [DOI] [PubMed]
- 42.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum. 2006;54(2):628–634. doi: 10.1002/art.21568. [DOI] [PubMed] [Google Scholar]
- 43.Sakai R, Komano Y, Tanaka M, Nanki T, Koike R, Nagasawa H, et al. Time-dependent increased risk for serious infection from continuous use of tumor necrosis factor antagonists over three years in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64(8):1125–1134. doi: 10.1002/acr.21666. [DOI] [PubMed] [Google Scholar]
- 44.Tong X, Li X, Pratt NL, Hillen JB, Stanford T, Ward M, et al. Monoclonal antibodies and Fc-fusion protein biologic medicines: A multinational cross-sectional investigation of accessibility and affordability in Asia Pacific regions between 2010 and 2020. Lancet Reg Health West Pac. 2022;26:100506. doi: 10.1016/j.lanwpc.2022.100506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article as supplementary information files. All included articles are publicly available.